- 1Association of Dutch Burn Centres, Beverwijk, Netherlands
- 2Department of Plastic, Reconstructive and Hand Surgery, Amsterdam Movement Sciences, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 3Department of Pathology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
Antimicrobial peptides (AMPs) or host defense peptides protect the host against various pathogens such as yeast, fungi, viruses and bacteria. AMPs also display immunomodulatory properties ranging from the modulation of inflammatory responses to the promotion of wound healing. More interestingly, AMPs cause cell disruption through non-specific interactions with the membrane surface of pathogens. This is most likely responsible for the low or limited emergence of bacterial resistance against many AMPs. Despite the increasing number of antibiotic-resistant bacteria and the potency of novel AMPs to combat such pathogens, only a few AMPs are in clinical use. Therefore, the current review describes (i) the potential of AMPs as alternatives to antibiotics, (ii) the challenges toward clinical implementation of AMPs and (iii) strategies to improve the success rate of AMPs in clinical trials, emphasizing the lessons we could learn from these trials.
Introduction
Antibiotic resistance is a global concern in health care as (new) resistance mechanisms are emerging and spreading globally. Resistant bacterial strains have been identified for various antibiotics in clinical use. For example, shortly after the emergence of penicillin-resistant Staphylococcus aureus in 1940 (Abraham and Chain, 1940), several pathogenic bacteria became resistant not only to penicillin but also to semi-synthetic penicillin, cephalosporins and newer carbapenems (Kumarasamy et al., 2010). In addition, the decline in the approval of new antibiotics by regulatory bodies has further exacerbated this problem.
As alternatives to antibiotics, AMPs have been at the forefront of international efforts because they are less likely to induce bacterial resistance (Wimley and Hristova, 2011). AMPs are a diverse group of naturally occurring peptides of the innate defense system with activity against various pathogens such as yeast, fungi, viruses and bacteria (Zasloff, 2002; Beisswenger and Bals, 2005). To inactivate these pathogens, AMPs display a multi-hit, non-specific and rapid action, resulting in the slow or limited emergence of resistance (Wimley and Hristova, 2011). Additionally, some AMPs show synergistic interactions with conventional antibiotics (Steenbergen et al., 2009; Wu et al., 2020), which could decrease the selection of antibiotic resistant bacteria.
Around the same time as the discovery of the first antibiotic penicillin in 1928, the first AMP nisin was discovered in milk (Zhang and Zhong, 2015). This AMP was approved by the FDA of the United States as a food preservative in 1988 due to its heat stability and tolerance of low pH (Delves-Broughton et al., 1996; Cleveland et al., 2001). After the discovery of nisin, several other AMPs such as gramicidin, tyrocidine, alamethicin, purothionin, and defensins were isolated from bacteria, fungi, plants, invertebrates and vertebrates (Van Epps, 2006; Bahar and Ren, 2013). However, the clinical application of AMPs as antimicrobials was limited due to toxicity considerations and other problems, such as high production costs as compared to antibiotics (Fry, 2018).
The renewed interest in AMPs as a consequence of the increasing number of antibiotic resistant and tolerant bacteria has resulted in the FDA approval of gramicidin and polymyxin B as constituents in Neosporin® in 1955, colistin (polymyxin E) in 1962 and daptomycin in 2003 (Chen and Lu, 2020). Several naturally occurring and synthetic AMPs have been clinically investigated to combat pathogenic bacteria but since the approval of daptomycin no new AMPs have been approved as antimicrobials. To understand this innovation gap, we reviewed the literature to describe the potential of AMPs as alternative to antibiotics and the challenges toward clinical application of AMPs. Additionally, we provide an overview of the strategies that are currently available to facilitate the successful clinical implementation of AMPs using examples from clinical trials.
Function of AMPs
Physiological Role of AMPs in the Skin
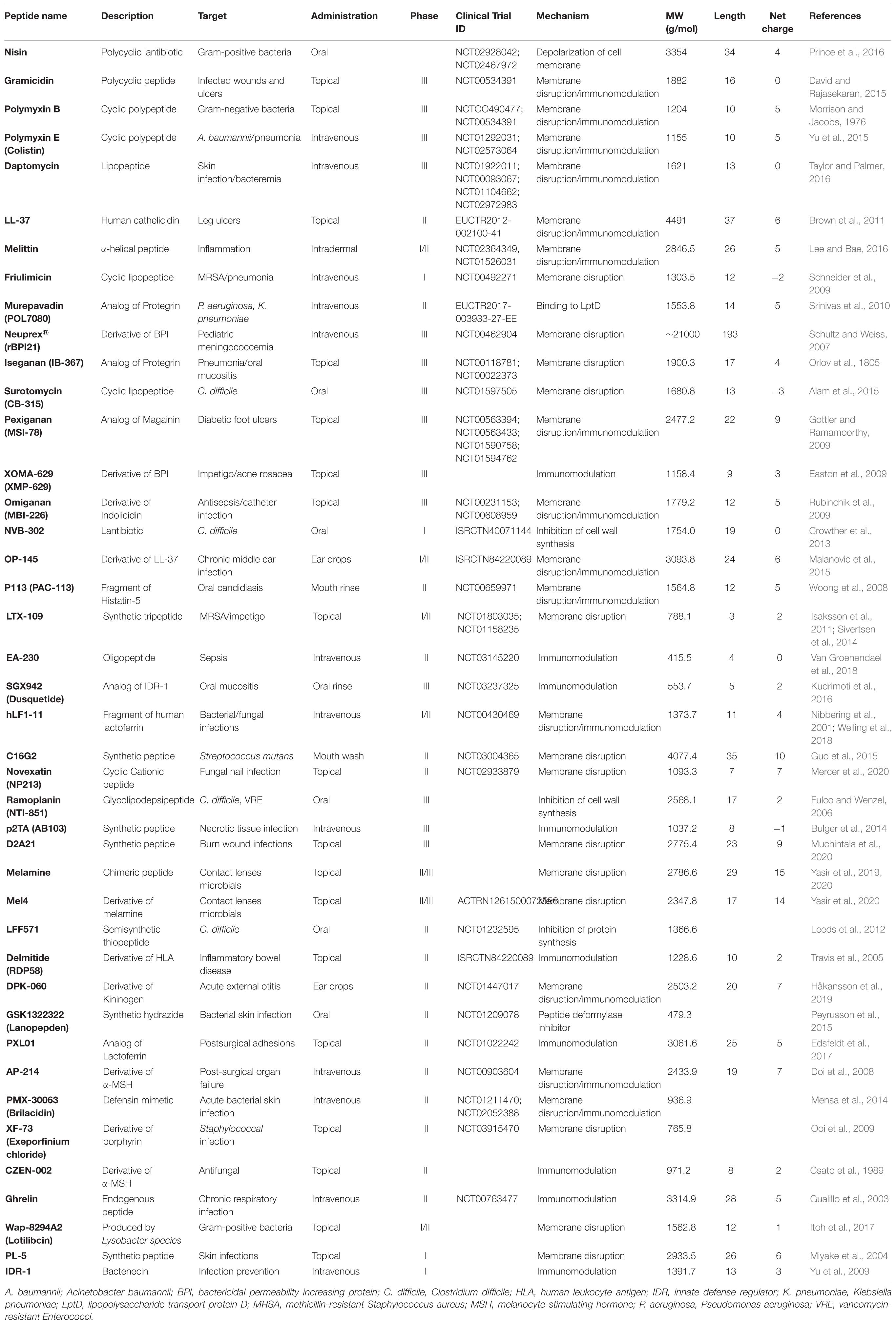
The skin is not only a physical barrier to the external environment. It is an active immune organ protecting the host from harmful toxins and pathogenic organisms (Salmon et al., 1994). The immune response of the skin involves various resident cells in the epidermis such as keratinocytes, melanocytes, Langerhans cells and γδ T cells, and in the dermis such as dendritic cells, macrophages, fibroblasts, mast cells, B and T cells, plasma cells and natural killer cells (Zasloff, 2002; Ryu et al., 2014; Lacey et al., 2016). These skin cells release several pro-inflammatory cytokines such as IL-17 and IL-22 and produce AMPs, which act as the first line of defense against microorganisms (Figure 1) (Liang et al., 2006). AMPs display a broad-spectrum of antimicrobial activity against yeast, fungi, viruses and bacteria. For example, the human cathelicidin LL-37 shows activity against various Gram-positive and Gram-negative bacteria, and antibiotic-resistant bacterial strains (Dean et al., 2011; Shurko et al., 2018). The same AMP also shows activity against fungi and some viruses such as influenza and HIV. Bergman et al. (2007) showed that LL-37 inhibits HIV-1 replication and suggested that this AMP contributes to the protection against HIV-1 infection. Furthermore, Luo et al. (2019) reported that LL-37 inhibits Aspergillus fumigatus infection via direct antifungal activity and reduction of excessive inflammation.
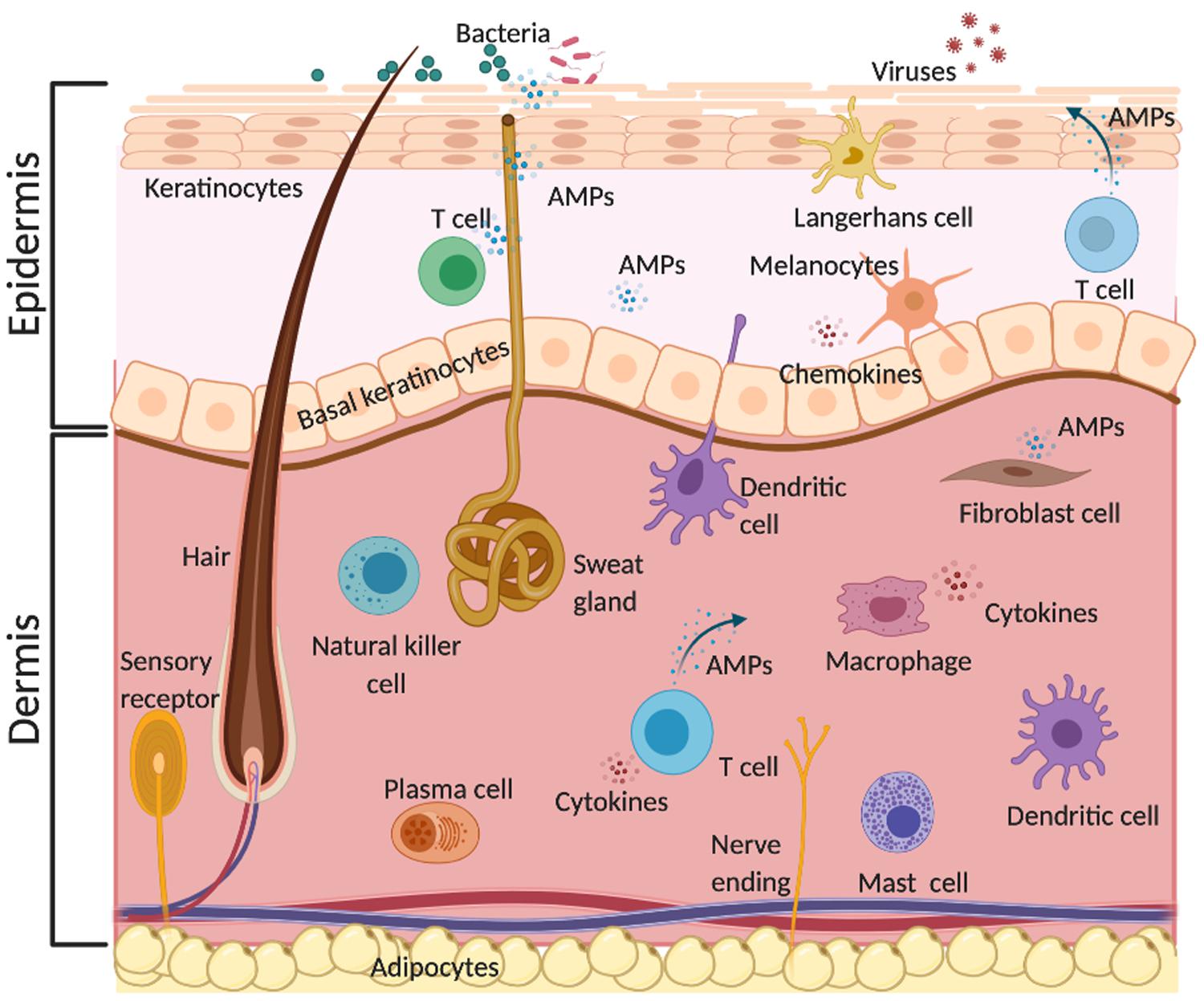
Figure 1. Physiological role of AMPs in the skin. AMPs are produced by different resident skin cells. They act as the first line of innate immune defense against various pathogens such as bacteria, fungi, and viruses via direct and indirect antimicrobial activities and/or immunomodulatory effects. This illustration was created with BioRender.com.
The ability to modulate the immune responses has been reported for several AMPs. LL-37 is a well-studied AMP with such immunomodulatory properties in humans. It acts as a chemoattractant for monocytes and promotes the production and release of various cytokines and chemokines that may direct the course and intensity of inflammation (Agier et al., 2015). Among others, LL-37 can reduce the inflammatory response via interaction with TLR. TLRs are widely expressed receptors on immune cells that recognize pathogenic-associated molecular patterns. LL-37 downregulates signaling through TLR4 by scavenging its ligand LPS (Larrick et al., 1995; Rosenfeld et al., 2006) as well as by disrupting the receptor complex function (Di Nardo et al., 2007; Brown et al., 2011). Furthermore, LL-37 potentially elongates the lifespan of neutrophils via the suppression of neutrophil apoptosis (Nagaoka et al., 2012), thereby enhancing host immunity. Additionally, Carretero et al. (2008) report that LL-37 activates and promotes angiogenesis and migration of keratinocytes, which results in an improved re-epithelialization and granulation tissue formation.
The antimicrobial and immunomodulatory effects of AMPs are necessary to maintain homeostasis of the skin function. Therefore, the production of AMPs is upregulated upon injury and infection (Miller et al., 2005; Sørensen et al., 2006). For example, in acne vulgaris several AMPs such as LL-37 and hBD-2 are upregulated by, e.g., keratinocytes in response to the Propionibacterium acnes (Nagy et al., 2006). As the P. acnes strains vary in their ability to stimulate inflammatory responses, upregulation of AMPs could be beneficial due to their antimicrobial and anti-inflammatory effects. In some skin conditions, for example in diabetic foot ulcers, the upregulation of AMPs such as hBD-2, 3, and 4 is often not sufficient to control the inflammation and wound infection (Rivas-Santiago et al., 2012). Therefore, such skin conditions require specialized care for proper healing.
Structure and Mechanism of Action
Antimicrobial peptides are usually small, consisting of 12–50 amino acids. They are composed of hydrophilic, hydrophobic and cationic residues (net charge +2 to +11). The cationicity and hydrophobicity of AMPs are critical for bactericidal activity. Together with the hydrophilic residues, the hydrophobic residues form an amphipathic structure for insertion into the bacterial membrane (Ebenhan et al., 2014). To form this structure, some AMPs (i.e., α-helical peptides such as melittin) undergo conformational changes upon interaction with bacterial membranes, while others already have a rigid amphipathic structure (i.e., β-sheet peptides such as β-defensins) to target bacterial membranes (Ebenhan et al., 2014). The positive charge of AMPs facilitates the initial binding of AMPs to the membrane surfaces via electrostatic interactions. Bacterial membranes consisting of negatively charged phospholipid headgroups such as phosphatidylglycerol, cardiolipin, or phosphatidylserine show high affinity for cationic AMPs (Matsuzaki, 1999). Contrarily, mammalian cell membranes that are enriched with zwitterionic phospholipids such as phosphatidylethanolamine, phosphatidylcholine, or sphingomyelin show low affinity for cationic AMPs due to their neutral net charge.
Most AMPs cause membrane disruption through non-specific interactions with the membrane surface. They are suggested to form micelles or pores, causing loss of membrane integrity and consequently leakage of intracellular components, resulting in cell death (Chan et al., 2006). Depending on the membrane topology and geometry of pores, pores can be described by three models, i.e., barrel-stave, toroidal and sinking-raft model (Figure 2). In the sinking-raft model, AMPs lie on the membrane surface and cause an increase in membrane curvature. Self-aggregation of the peptide causes the AMPs to sink into the membrane, creating transient pores (Brogden, 2005). In both the barrel-stave pore and toroidal pore, the peptide has a transmembrane topology. The main difference between these two models is that the formation of barrel-stave pores is driven by both hydrophobic and electrostatic interactions, whereas the formation of toroidal pores is mainly driven by electrostatic interactions (Bertelsen et al., 2012). As a result, toroidal pores are covered by phosphate headgroups, initiating changes in membrane curvature. The mechanism of action of AMPs that form micelles is not well-understood. It is believed that these AMPs act by a detergent-like mechanisms causing intrinsic perturbations of the membrane (Li et al., 2017).
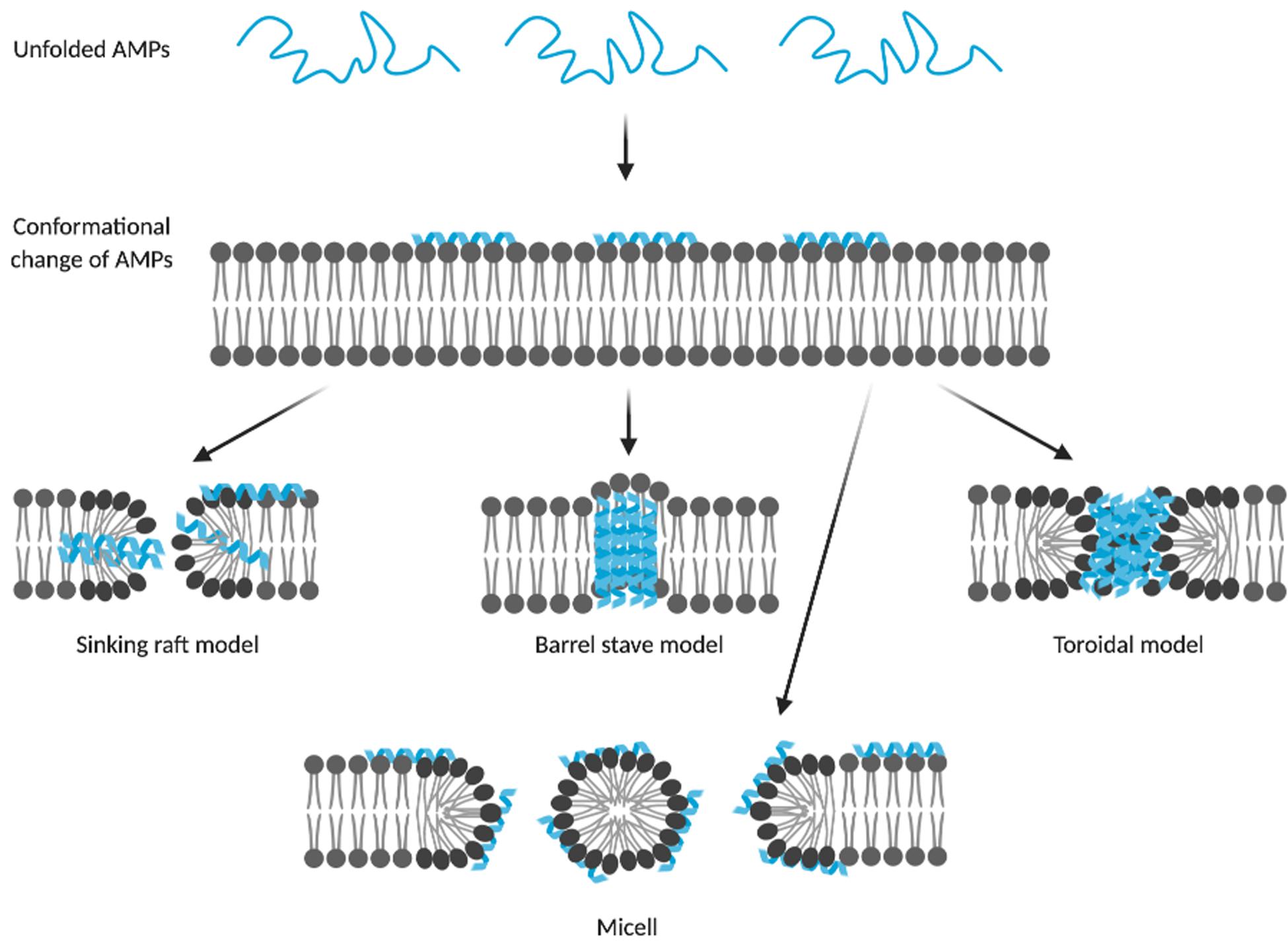
Figure 2. Membrane disruptive models of AMPs. Membrane disruptive AMPs form micelles or pores on the bacterial membrane, resulting in leakage of the intercellular components and cell death. Membrane pores can be categorized by their membrane topology and geometry into the sinking raft, barrel stave and toroidal models. AMPs that form barrel stave and toroidal pores display a transmembrane topology, whereas AMPs of the sinking raft model cause self-aggregation to sink into the membrane, creating transient pores. AMPs that form toroidal pores are mainly driven by electrostatic interactions and as a result of these interactions, the pores are covered by phosphate headgroups. AMPs forming micelles show a detergent-like mechanism of action, causing membrane perturbation. This illustration was created with BioRender.com and Bahar and Ren, 2013.
Membrane disruptive AMPs might also kill bacteria using non-membrane disruptive pathways, and vice versa (Hale and Hancock, 2007). Additionally, they could act independently or in synergy with non-membrane disruptive AMPs. Non-membrane disruptive AMPs are able to transverse membranes to reach their intracellular target components. Such AMPs could inhibit protein-folding, proteases, cell division, the synthesis and metabolism of proteins, nucleic acids and cell walls (Le et al., 2017). Previously, Edgerton et al. (2000) showed that two AMPs with clearly different structures, i.e., histatin 5 and human neutrophil (HNP)-1, act on similar pathways. The role of these AMPs suggests that membrane and non-membrane disruptive AMPs serve as equally important peptides of the innate defense system to inactivate pathogens.
Clinical Trials Using AMPs
The results from pre-clinical studies using AMPs revealed that AMPs could be used for the prevention and treatment of various clinical conditions. For example, peptide coating of catheters using a novel peptide E6 prevented catheter associated infections in mouse models (Yu et al., 2017). PXL150 demonstrated efficacy against Pseudomonas aeruginosa in burn wounds in mouse models (Björn et al., 2015). Both LL-37 and IDR-1 restored pulmonary function in mice with pneumonia (Hou et al., 2013). Also, nisin demonstrated in vitro efficacy against Clostridium difficile (Bartoloni et al., 2004). Furthermore, several AMPs including LL-37 displayed anti-biofilm activity in vivo (Chennupati et al., 2009; Segev-Zarko et al., 2015; Batoni et al., 2016). Based on promising pre-clinical results, numerous AMPs have been investigated in human clinical trials to demonstrate efficacy and safety. Several of these trials are still ongoing, others are completed, discontinued or approved. The successes and flaws of these clinically investigated AMPs are described in the following sections. Structural information, mechanism of action and the intended target of AMPs in clinical trials is provided in Table 1.
AMPs Approved for Clinical Use
Currently, nisin, gramicidin, polymyxins, daptomycin and melittin are in clinical use as alternative to antibiotics because of their antimicrobial potency (Figure 3A). Nisin, also known as nisin A, is composed of 34 amino acids and consists of dehydrated, unsaturated and thioether amino acids, forming five lanthionine rings. It is naturally produced by lactic acid bacteria such as Lactococcus lactis and shows a broad-spectrum of bactericidal activity (Stevens et al., 1991; Severina et al., 1998). L. lactis also produces nisin Z, F and Q, which differ by up to 10 amino acids from nisin A resulting in differences in physiochemical properties and antimicrobial activity (Piper et al., 2011). Among others, nisins inhibit cell wall synthesis through interactions with lipid II, a precursor molecule that is essential for bacterial cell-wall bio-synthesis. Nisins also form membrane pores causing cell lysis (Prince et al., 2016). Nisin A is approved as a food preservative and is GRAS (Delves-Broughton et al., 1996; Cleveland et al., 2001). In clinical trials, the effect of nisin A has been investigated using probiotics, i.e., the consumption of live microorganisms (e.g., L. lactis) that produce nisin A. The results of a systematic review of such trials indicated that these probiotics reduce infectious complications and may subsequently reduce intensive care unit mortality (Petrof et al., 2012). Minor but possible side effects of nisin A are itching (pruritus) and flushing of the skin, and nausea or vomiting. The safety profile together with the broad-spectrum of bactericidal activity, indicated that the application of nisin could extend beyond food-related bacteria (Blay et al., 2007; Shin et al., 2015). Applications of nisins in humans include dental-care and pharmaceutical products such as for the treatment of stomach ulcer and colon infections (Sakamoto et al., 2001; Mitra et al., 2019).
Figure 3. Chemical structure of AMPs in clinical use. (A) A few α-helical (gramicidin and melittin) and cyclic AMPs (nisin A, polymyxin and daptomycin) have been approved for clinical use. (B) Despite the physiochemical similarities of the cyclic AMP friulimicin B with daptomycin, the Phase I clinical trial of friulimicin B was terminated due to unfavorable pharmacokinetics. ChemDraw version 19 was used to draw the chemical structure of AMPs.
Gramicidin or gramicidin D is a mixture of gramicidin A, B and C making up 80, 6, and 14% of the mixture, respectively. These AMPs are hydrophobic linear polypeptides composed of 15 amino acids (Meikle et al., 2016). They are naturally produced by Gram-positive Brevibacillus brevis commonly found in soil (Van Epps, 2006). Gramicidins form ion-channels within the bacterial membrane, allowing the passive diffusion of Na+ and K+ along their concentration gradient (David and Rajasekaran, 2015). This results in membrane depolarization, osmotic swelling and lysis of bacterial cells. Gramicidin is effective against a variety of Gram-positive bacteria and is clinically used for ophthalmic purposes as a constituent in Neosporin®. In a clinical trial, patients suffering from hordeolum who received the ophthalmic solution containing gramicidin (Neosporin®) reported a comparable pain score as those who received a placebo treatment (Hirunwiwatkul and Wachirasereechai, 2005). Additionally, the duration of cure of these treatment groups was not statistically different (p = 0.988). The authors of this study suggested that the lack of statistically significant differences between the placebo and peptide-treated group could be due to the small sample size of 14 patients in each group (Hirunwiwatkul and Wachirasereechai, 2005). In another clinical study using a larger sample size of 91 patients, the effect of this ophthalmic solution on the duration of cure of bacterial-positive corneal ulcers was reported (Bosscha et al., 2004). An average of 12.5 days was required for complete re-epithelialization of these ulcers. This was more favorable compared to the duration of cure of ulcers treated with ofloxacin (13.7 days) and ciprofloxacin (14.4 days) (Prajna et al., 2001). These findings suggest that Neosporin® containing gramicidin could be used as an alternative to conventional antibiotics for such ophthalmic purposes. Another AMP in Neosporin® is polymyxin B.
Polymyxins (A, B, C, D, and E) are a group of cyclic polypeptides naturally produced by Gram-positive Paenibacillus polymyxa. They show activity against MDR Gram-negative bacteria such as P. aeruginosa and Escherichia coli (Zavascki et al., 2007). Polymyxins bind to the lipid A component of LPS on the outer membrane of Gram-negative bacteria (Morrison and Jacobs, 1976), which contributes to the insertion of the AMPs into the membrane. They can increase cell-permeability via a detergent-like mechanism, which causes cell death (Schroder et al., 1992). Polymyxins in clinical use are polymyxin B and E, which differ only by one amino acid from each other (Li et al., 2005; Falagas et al., 2006; Kwa et al., 2007). Polymyxin B is prescribed to treat eye infections, whereas polymyxin E is used to treat wound infections. These AMPs are recognized as crucial but last-resort treatment options because of their ability to induce adverse events. Nephrotoxicity and neurotoxicity are the most common adverse events reported for polymyxins (Falagas and Kasiakou, 2006; Cisneros et al., 2019). To optimize the clinical use of polymyxins without such severe adverse effects, clinical trials are currently being executed, e.g., in combination with different antimicrobial agents. Minor side effects, such as blurred vision, watery eyes, and sensitivity to light, have been reported for Neosporin® containing two AMPs, gramicidin and polymyxin B, and an aminoglycoside antibiotic neomycin. Thus, not one agent but the mixture of three antimicrobial agents are responsible for these minor side effects.
Daptomycin is a cyclic lipopeptide consisting of 13 amino acids, which is naturally produced by the bacterium Streptomyces roseosporus (Ball et al., 2004). It shows bactericidal activity against Gram-positive bacteria, including antibiotic resistant strains (Jorgensen et al., 2003). Daptomycin inhibits cell wall synthesis, causes membrane depolarization and forms membrane pores, eventually causing cell death. A daily treatment of 4-6 mg/kg daptomycin is recommended in critically ill patients. For the treatment of bacteria with reduced susceptibility high dosages of 10 mg/kg can be prescribed, which are also well tolerated. In a phase I clinical trial, 2 of 5 healthy volunteers who received 4 mg/kg per 12 h of daptomycin (8 mg/kg per day) developed reversible myopathy (Dvorchik et al., 2003). Nonetheless, the number of incidence and the severity of myopathy were substantially decreased in healthy volunteers when the total dose of 8 mg/kg was administered once daily. Other studies reported that the development of myopathy was not related to the administration of daptomycin but to other factors such as concomitant medications, comorbidities and the number of surgical interventions (Galar et al., 2019). In a Phase IV clinical trial, the effect of daptomycin on the resolution of skin infections was compared to that of the standard of care, i.e., cloxacillin, nafcillin, oxacillin, flucloxacillin or vancomycin (Arbeit et al., 2004). The success rate of the daptomycin-treated patients was 71.5%, which was clinically and statistically comparable to the standard treatments with a success rate of 71.1%. In another clinical study, efficacy of daptomycin was demonstrated in a placebo-control trial. All patients also received a β-lactam therapy. In this study, the daptomycin treatment resulted in faster clearance of bacteremia than the control treatment (placebo + β-lactam therapy) (Cheng et al., 2018). Hence, combination therapy using daptomycin can improve the clinical success rate.
Melittin is the predominant (40–48%) component of venom from the European honeybee Apis mellifera. It is composed of 26 amino acids and adopts an α-helical conformation upon interaction with the membrane surface (Terwilligert and Eisenbergg, 1982). It possesses anti-inflammatory properties (Lee and Bae, 2016) and is therefore approved by the FDA for relieving pain and swelling associated with rheumatoid arthritis, tendinitis, bursitis and multiple sclerosis (Son et al., 2007; Alves et al., 2011). Melittin also forms membrane toroidal pores to inactivate pathogens. This was shown in several in vitro and animal experiments using cancer cells (Gajski and Garaj-Vrhovac, 2013), viruses (Memariani et al., 2020) and (resistant) bacteria (Park et al., 2006; Van Den Bogaart et al., 2008; Choi et al., 2015). Hence, similar to nisin, the clinical application of melittin could extend beyond the FDA-approved purposes. Side effects of melittin are redness and swelling of the skin at the side of administration, itching, trouble breathing, nausea, sleepiness and low blood pressure.
Flaws of Some AMPs
Some clinical trials using AMPs were discontinued or terminated due to various reasons. For example, the Phase I clinical trial of friulimicin B in healthy volunteers was terminated due to its unfavorable pharmacokinetic profile. The nature of these unfavorable findings remains unknown and unexpected. Friulimicins (A, B, C, D, and E) are a group of naturally occurring peptides produced by Actinoplanes friuliensis and like daptomycin, they are cyclic lipopeptides (Figure 3B). Friulimicin B was clinically investigated and demonstrated efficacy in various murine infection models (Endermann et al., 2007). It has similar physiochemical properties and mechanism of action as daptomycin (Schneider et al., 2009) but has been found to trigger different stress responses in Bacillus subtilis as compared to daptomycin (Wecke et al., 2009). This might be related to differences in the pharmacokinetic profiles of these AMPs. Another AMP, Murepavadin (POL7080) failed unexpectedly in advanced clinical trials. Murepavadin is a 14 amino acid cyclic peptide that targets the LPS transport protein D (LptD) on the bacterial membrane to form pores (Srinivas et al., 2010). It was demonstrated to be safe in Phase I clinical trials in healthy volunteers and in subjects with an impaired renal function (Martin-Loeches et al., 2018). Safety and efficacy of murepavadin were demonstrated in Phase II clinical trials in patients with acute exacerbation of non-cystic fibrosis bronchiectasis or ventilator-associated bacterial pneumonia due to P. aeruginosa (Martin-Loeches et al., 2018). However, Phase III clinical trial of this AMP in patients with nosocomial pneumonia was prematurely ended due to higher than expected acute kidney injuries, i.e., 56% for the murepavadin plus ertapenem treated group versus 25–40% for the meropenem treated control group and according to the literature (BioSpace, 2019).
Besides pharmacokinetic and safety issues, several AMPs have failed Phase III clinical trials because of lack of clear efficacy or lack of superiority over conventional treatments. A clear example is demonstrated for the AMP Neuprex® (rBPI21) which is a recombinant α-helical peptide consisting of the first 193 amino acids of the N-terminus of BPI. Clinical studies showed that patients with meningococcemia or hemorrhage due to trauma who received Neuprex® had no toxic side effects and showed a trend toward improved outcomes, i.e., reduced bone marrow aplasia and deaths (Demetriades et al., 1999; Guinan et al., 2011). However, Neuprex® failed to show clear efficacy (p = 0.07) as compared to the placebo-treated group (Giroir et al., 2001). Similar to Neuprex®, at least five AMP that have completed advanced clinical trials, failed to show clear efficacy (i.e., iseganan and XOMA-629) or superiority over conventional treatments (i.e., surotomycin, pexiganan, and omiganan) (Gordon et al., 2005). Nevertheless, the latter AMPs could be potential alternatives to conventional antibiotics due to their favorable safety profile and low or limited ability to induce bacterial resistance. Since antibiotics are no longer routinely used to treat bacterial infections as a consequence of resistance development, the ability of AMPs to induce bacterial resistance, is a more important parameter to consider during clinical trials. Hence, instead of superiority trials, equivalence or non-inferiority trials in which AMPs cause a similar effect as the standard treatment, should become more common (Committee for Proprietary Medicinal Products, 2001). In two equivalence trials with systemic ofloxacin as the comparator, efficacy of topical pexiganan was determined in patients with diabetic foot ulcers (Lipsky et al., 2008). The combined results of these trials demonstrated that pexiganan was clinically comparable to this antibiotic. However, equivalence to ofloxacin was not acceptable as main evidence of efficacy and FDA approval of pexiganan. Additional clinical trials were required to demonstrate efficacy superior to a topical placebo cream plus standard treatment for diabetic foot ulcers. Of note, clinical trials are often not designed using placebo treatment only as control due to ethical reasons. In the additional trials, pexiganan plus standard treatment failed to meet the primary outcome, i.e., resolution of infection (Genetic Engineering, and Biotechnology News, 2016). Failure of AMPs in such trials may arise from stability issues, inappropriate drug administration or unknown interactions between the peptide and the standard treatment. Currently, the developers of pexiganan continue to evaluate the data to consider this peptide for the treatment of other clinical indications.
Challenges Toward Clinical Application of AMPs
The development of AMPs for clinical use is accompanied by several challenges such as high development and production costs, cytotoxic issues, reduced activity in clinically relevant environments and the emergence of bacterial resistance, despite the initial claims that they may not induce resistance. To begin with, the manufacturing costs of antibiotics are relatively inexpensive. For example, aminoglycoside production costs $0.80 per gram as compared to $50–400 per gram of amino acid for AMPs by solid phase synthesis (Marr et al., 2006). As a consequence, alternative methods are required to promote commercial-scale production.
Furthermore, AMPs acting on membranes are not completely selective to microbial cells and may be toxic for eukaryotic cells as well. Several AMPs cause hemolytic and/or cytotoxic effects at antimicrobial concentrations, limiting their wider utilization (Laverty, 2014; Bacalum and Radu, 2015). Polymyxins are an example of such AMPs: they are crucial antimicrobials to eradicate MDR Gram-negative bacteria but they may cause nephrotoxicity and neurotoxicity at antimicrobial concentrations (Falagas and Kasiakou, 2006).
Another drawback for the clinical implementation of AMPs is the low antimicrobial activity in clinically relevant environments. AMPs may lose their bactericidal activity under physiological salt conditions due to loss of electrostatic interactions between AMPs and cell membranes (Falanga et al., 2016; Mohamed et al., 2016). In the presence of serum, AMPs may bind to proteins such as albumin (Sivertsen et al., 2014; Li et al., 2017). Additionally, AMPs can be susceptible to proteolytic degradation (Perona and Craik, 1997; Thwaite et al., 2006; McCrudden et al., 2014). Also, Starr et al. (2016) suggested that host cells can interfere with the activity of AMPs in a way similar to serum protein binding. This reduces the effective concentration of available AMPs to eradicate bacteria.
Although AMPs do not seem to induce bacterial resistance, resistance to AMPs has been reported. AMPs that require specific recognition molecules such as LPS, Lipid A, Lipid I/II and LptD, on the membrane surface of bacteria most likely develop resistance. For example, resistance to nisin involves mutations in bacterial cells that induce changes in membrane and cell wall composition and eventually prevents the binding of nisin to lipid II (Kramer et al., 2008). Alternatively, bacteria may inactivate nisin using dehydropeptide reductase, also known as nisinase (Bastos et al., 2015). Resistance to polymyxins and cross-resistance to AMPs have also been reported (Li and Nation, 2006; Valencia et al., 2009; Arcilla et al., 2016; Dobias et al., 2017). Resistance to polymyxins is mediated by the mrc-1 gene encoding a phosphoethanolamine modification in lipid A, which prevents the initial binding of polymyxins to the bacterial membranes (Liu et al., 2016). This gene was initially isolated from Chinese livestock animals and has been identified in the human fecal microbiome, indicating that polymyxin resistance is horizontally transferable (Arcilla et al., 2016). Moreover, Li et al. (2007) reported that the aps AMP sensor/regulator system is important for S. aureus virulence in vivo. They show that AMPs may induce resistance mechanisms in MRSA via this system, which involves the D-alanylation of teichoic acids, the incorporation of lysophosphatidylglycerol in the bacterial membrane, the increase of lysine biosynthesis and AMP transport systems (Arcilla et al., 2016). Although the impact of bacterial resistance on the minimal inhibitory concentration of the AMPs (2–30-fold increase) is less dramatic than for antibiotics (100–1000-fold increase) (Andersson et al., 2016), the risk of bacterial resistance should be carefully investigated.
Improvement Strategies
The majority of the AMPs under clinical evaluation are positively charged analogs of naturally occurring AMPs and are limited to topical or intravenous applications for an effective bio-available concentration of the peptides. Importantly, the route of drug administration could markedly affect the efficacy of AMPs as efficacy is dependent on the bio-distribution and stability of the peptides (Benet, 1978). Analogs of naturally occurring AMPs have been prepared to overcome the challenges associated with high production costs, low bio-availability and efficacy, and cytotoxic effects of AMPs (Figure 4). Strategies to improve the performance of AMPs are described in the following sections.
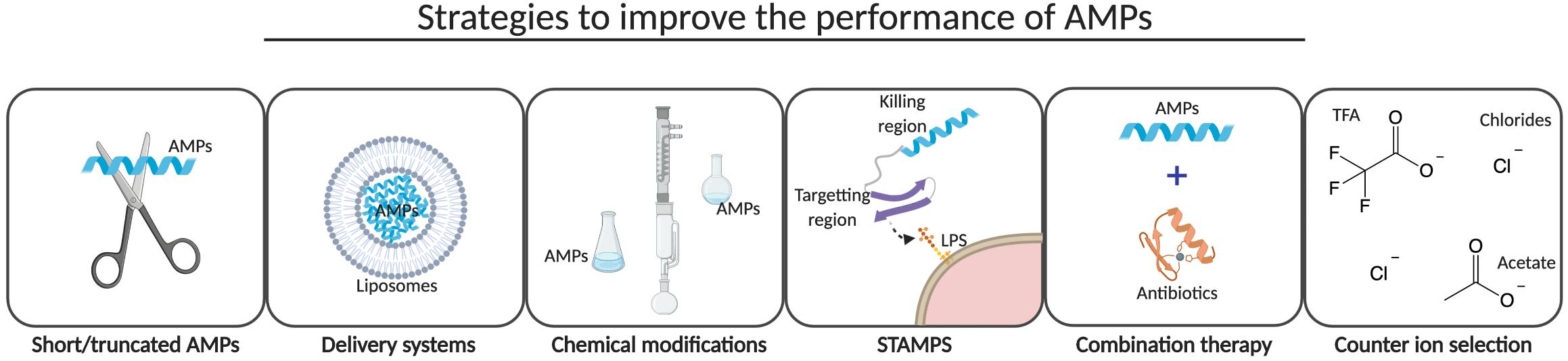
Figure 4. Improvement strategies. Several strategies have been developed to reduce production costs and cytotoxic effects and to improve bio-availability and efficacy of AMPs. Short and/or truncated AMPs have been pursued by several companies to reduce the production costs, whereas the development of a delivery system and the introduction of chemical modifications are more common to improve the bio-availability and efficacy of AMPs in vivo. STAMPS, combination therapy with conventional antibiotics and the selection of a counter ion during the final step of peptide synthesis might not only improve efficacy but may also reduce cytotoxic effects of AMPs. This illustration was created with BioRender.com.
Ultra-Short and/or Truncated AMPs
Efforts to reduce production costs include alternative peptide synthesis methods and the production of ultra-short and/or truncated AMPs. The latter has been pursued by several companies. The AMPs OP-145 (Nell et al., 2006), P113 (Woong et al., 2008), LTX-109 (Midura-Nowaczek and Markowska, 2014), and EA-230 (Van Groenendael et al., 2018) all consist of a lower number of amino acids as compared to their “original” peptide LL-37, histatin-5, bovine lactoferrin and loop-2 of β-hCG, respectively. Beside truncation of AMPs, the synthesis of ultra-short AMPs such as the 5-amino acid linear peptide SGX942 further reduces the production costs of AMPs (Kudrimoti et al., 2016). Alternatively, solution phase synthesis or by chemoenzymatic methods could be used for production of small AMPs (Bray, 2003). This remains challenging for large peptides and therefore a biotechnological approach is often considered, i.e., the production of AMPs in microorganisms (Ingham and Moore, 2007). Magainin, hBD-3, melittin and other AMPs have been synthesized using calmodulin as carrier protein (Ishida et al., 2016; Boto et al., 2018). This protein protects the producing bacterial cells, e.g., E. coli from the toxic effects of the AMPs and prevents degradation of the AMP during the production process. Alternative approaches to obtain AMPs from plants or bacterial ribosomes have been reviewed by Montesinos and Bardají (2008), and Rogers and Suga (2015), respectively.
Delivery Systems
To improve the bio-availability of AMPs, delivery systems can be used to administer the peptides. Nisin is readily degraded by enzymes in the gastrointestinal tract (Heinemann and Williams, 1966). To target C. difficile, a bacterium that can infect the colon (Le Lay et al., 2016), nisin requires a delivery vehicle to reach the colon without being digested and absorbed by the upper gastrointestinal tract. To achieve this, nisin has been encapsulated in pectin/HPMC compression coated tablets to form an enzymatically controlled delivery system (Ugurlu et al., 2007). Alternative systems such as liposomes, nanoparticles, and nisin-controlled gene expression in Lactobacillus gasseri have also shown to be successful delivery systems (Neu and Henrich, 2003; Taylor et al., 2007; Khan and Oh, 2016). Colon-specific delivery approaches such as pro-drugs and conjugates have been reviewed by Fang et al. (2017) and Mishra et al. (2017). These approaches have also been used to improve the in vivo bio-availability of different AMPs, for example Polymyxin E, which is administered as an inactive pro-drug that undergoes hydrolysis to release the active AMPs. Polymyxin E was also successfully integrated into hydrogels for the treatment of burn wound infections (Zhu et al., 2017). Please note that such delivery systems, may not only improve the bio-availability of the AMPs but may also improve the efficacy and reduce cytotoxicity, as a consequence of increased solubility and specificity, respectively (Mahlapuu et al., 2016; Kim et al., 2017; Nordström and Malmsten, 2017).
Chemical Modifications
The order and position of amino acids were found to play an important role in the biological activity of the hLT-1-1 peptide, according to its structure-activity relationship (Welling et al., 2018). Also, the α-helical content, the hydrophobicity and amphipathicity of AMPs may affect their bactericidal activity and cytotoxicity. Schmidtchen et al. (2014) reported that an increase in the hydrophobicity might induce hemolytic activity of AMPs. Another group showed that a reduction in the net positive charge of AMPs may not affect the bactericidal efficacy of AMPs but may reduce cytotoxic effects (Jiang et al., 2008). For higher bactericidal activity and less cytotoxic effects, the introduction of arginine was found to be superior to lysine for providing the positive charge of AMPs (Yang et al., 2018). To improve proteolytic stability, different approaches have been used such as the introduction of D-amino acids, cyclization, amidation or acetylation of the terminal regions (Strömstedt et al., 2009; Gentilucci et al., 2010; Chu et al., 2013). To increase the salt and serum stability of AMPs, tryptophan or β-naphthylalanine end-tagging of the terminal regions of AMPs could be considered (Chu et al., 2013; Pfalzgraff et al., 2018).
Specifically Targeted AMPs (STAMPS)
Due to the broad-spectrum activity and non-specific mechanisms of action of AMPs, these peptides may induce cytotoxic effects as well. To reduce these effects, STAMPS have been designed (Eckert et al., 2012). STAMPS selectively target and kill a specific pathogenic species without affecting the normal flora (Eckert et al., 2006). They consist of at least two regions, i.e., one or multiple targeting regions and a killing region linked by a spacer. The targeting region improves the activity of the AMPs by enhancing the initial binding of the peptide to the specific pathogenic determinants on the membrane (He et al., 2010). Using two are more targeting regions reduces the likelihood of bacterial resistance and improves efficacy (Sarma et al., 2018). Currently, C16G2 which is a synthetic AMP or STAMP, is under clinical investigation for the treatment of tooth decay by Streptococcus mutans. The N-terminus of C16G2, is the targeting region for S. mutans and the C-terminus is the killing region or AMP G2 (Kaplan et al., 2011). C16G2 demonstrated a strong safety profile and efficacy against S. mutans (Todd and Pierre, 2015).
Combination Therapy
To improve treatment outcomes, two or more antibiotics are often used in clinical practice. The same could be done for novel AMPs as many peptides show synergistic interactions with conventional antibiotics. This could not only reduce the amount of peptide needed for effective treatment and thus reduce costs but may also extend the lifetime of current antibiotics (Phee et al., 2015; Kampshoff et al., 2019). There are numerous examples of AMPs demonstrating synergism (Rand and Houck, 2004; Oo et al., 2010; Dosler et al., 2016; Alni et al., 2020). The combination of polymyxins with carbapenems or rifampicin suppresses the development of polymyxin resistance (Rodriguez et al., 2010; Lenhard et al., 2016). Also, Polymyxin B is used in combination with gramicidin and neomycin in Neosporin® due to their synergistic interactions, resulting in reduced resistance development and less cytotoxic effects (Booth et al., 1994; Tempera et al., 2009).
Counter-Ion Selection
The final step of AMP synthesis, which involves the cleavage and deprotection of the peptide chain with, e.g., TFA should be investigated to improve efficacy and reduce cytotoxicity. Counter-ions such as TFA anions are able to interact with positively charged AMPs and affect the hydrogen-bonding network along with the secondary structure (Blondelle et al., 1995; Gaussier et al., 2002). Also, TFA was shown to be cytotoxic for mammalian cells (Cornish et al., 1999). Previously, Sikora et al. (2018) studied the effect of three counter-ions, i.e., TFA anions, acetate and chlorides, on the bactericidal efficacy and cytotoxicity of a set of AMPs. They found that the peptide salts of acetate and chlorides seemed to be more potent antimicrobials than trifluoroacetates. However, trifluoroacetates have greater ability to promote α-helix formation in, e.g., LL-37 (Johansson et al., 1998). Additionally, acetate counter-ions seemed to be associated with high hemolytic activity (Sikora et al., 2018). In contrast, pexiganan acetate showed less cytotoxicity in cell viability assays and was the most stable salt for pexiganan (Desai, 2013; Sikora et al., 2018). Hence, superiority of one salt over another is peptide-dependent and should be taken into account.
Conclusion and Perspectives
As a result of the increasing number of antibiotic resistant bacteria, there has been a renewed interest in AMPs as a potential alternative to conventional antibiotics. AMPs display clear advantages over conventional antibiotics to combat various infectious diseases. In particular, (i) their broad spectrum of activity, (ii) multi-hit, non-specific and rapid mode of action, which results in limited emergence of resistance, (iii) the potential immunomodulatory properties and (iv) synergistic interactions with conventional antibiotics could eliminate the threat of MDR bacteria. Yet, until now, only a few AMPs (e.g., nisin, gramicidin, polymyxins, daptomycin, and melittin) have reached the clinic. Challenges toward clinical application of AMPs include cytotoxic effects, production costs, and problems related to peptide bio-availability and efficacy. To overcome these challenges, several strategies have been designed such as the preparation of ultra-short/truncated AMPs, delivery systems and STAMPS, chemical modifications and the careful selection of a counter-ion in the final step of AMP synthesis. Although not all AMPs in the clinical pipeline will reach the market, these strategies could improve the success rate of AMPs in clinical trials. Nonetheless, several AMPs in clinical trials have failed due to lack of clear efficacy or superiority over conventional antibiotics, while showing a trend toward improved clinical outcomes. Therefore, practical strategies should also be considered in future clinical testing of AMPs as we have learned the following lessons:
(1) The application of AMPs can extend beyond FDA-approved clinical indications;
(2) Defining the most optimal dose and administration regimen might reduce cytotoxic effects of AMPs;
(3) Efficacy of AMPs can be demonstrated in equivalence or non-inferiority trials with an antibiotic as comparator;
(4) Bacterial resistance development should be included as one of the primary outcome parameters in clinical trials of AMPs;
(5) The bio-availability and efficacy of AMPs can be improved using delivery systems and,
(6) The combination AMPs with conventional antibiotics or other compounds (e.g., AMPs) might result in an improved antimicrobial effect in clinical trials.
Taking these lessons into consideration, an increasing number of AMPs could reach the market as multi-functional, potent and long-lasting antimicrobials against various infectious diseases.
Author Contributions
GD wrote a draft version of the manuscript. MU, EM, and BB contributed to the design of the review and revised the manuscript. All authors assisted with the interpretation of the findings, read, and approved the final manuscript.
Funding
This collaborative project was funded by the Ministry of Economic Affairs through two public–private partnership (PPP) allowances, made available by Health-Holland and Top Sector life Sciences & Health. One of the PPP allowances was co-funded by the Dutch Burns Foundation, Madam Therapeutics B.V., Avivia B.V., Leiden University Medical Center, Amsterdam University Medical Center and the Association of Dutch Burn Centers (LSHM17078-SGF) and the other PPP allowance was co-funded by the Dutch Burns Foundation, Madam Therapeutics B.V., Mölnlycke Health Care AB, Leiden University Medical Center, Amsterdam University Medical Center and the Association of Dutch Burn Centers (LSH-TKI40-43100-98-017). These funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AMPs, antimicrobial peptides; BPI, bactericidal permeability-increasing protein; β-hCG, human β-chorionic gonadotropin; FDA, Food and Drug Administration; GRAS, generally recognized as safe; hBD, human β-defensin; hLT-1-1, human lactoferrin; HNP, human neutrophil; HPMC, hydroxypropyl methylcellulose; IL, interleukin; LPS, lipopolysaccharide; LptD, lipopolysaccharide transport protein D; MDR, multi-drug resistant; STAMPS, specifically targeted antimicrobial peptides; TFA, trifluoroacetic acid; TLR, toll-like receptors.
References
Abraham, E. P., and Chain, E. (1940). An enzyme from bacteria able to destroy penicillin. Nature 146:837. doi: 10.1038/146837a0
Agier, J., Efenberger, M., and Brzezińska-Blaszczyk, E. (2015). Cathelicidin impact on inflammatory cells. Cent. Eur. J. Immunol. 40, 225–235. doi: 10.5114/ceji.2015.51359
Alam, M. Z., Wu, X., Mascio, C., Chesnel, L., and Hurdle, J. G. (2015). Mode of action and bactericidal properties of surotomycin against growing and nongrowing Clostridium difficile. Antimicrob. Agents Chemother. 59, 5165–5170. doi: 10.1128/aac.01087-15
Alni, R. H., Tavasoli, F., Barati, A., Badarbani, S. S., Salimi, Z., and Babaeekhou, L. (2020). Synergistic activity of melittin with mupirocin: a study against methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) isolates. Saudi J. Biol. Sci. 27, 2580–2585. doi: 10.1016/j.sjbs.2020.05.027
Alves, E. M., Heneine, L. G. D., Pesquero, J. L., and de Merlo, L. A. (2011). Pharmaceutical Composition Containin an Apitoxin Fraction and Use Thereof. Google Patents No: WO2011041865. International Research Report (Art. 21 (3)).
Andersson, D. I., Hughes, D., and Kubicek-Sutherland, J. Z. (2016). Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 26, 43–57. doi: 10.1016/j.drup.2016.04.002
Arbeit, R. D., Maki, D., Tally, F. P., Campanaro, E., and Eisenstein, B. I. (2004). The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38, 1673–1681. doi: 10.1086/420818
Arcilla, M. S., van Hattem, J. M., Matamoros, S., Melles, D. C., Penders, J., de Jong, M. D., et al. (2016). Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 147–149. doi: 10.1016/s1473-3099(15)00540-x
Bacalum, M., and Radu, M. (2015). Cationic antimicrobial peptides cytotoxicity on mammalian cells: an analysis using therapeutic index integrative concept. Int. J. Pept. Res. Ther. 21, 47–55. doi: 10.1007/s10989-014-9430-z
Bahar, A. A., and Ren, D. (2013). Antimicrobial peptides. Pharmaceuticals 6, 1543–1575. doi: 10.3390/ph6121543
Ball, L. J., Goult, C. M., Donarski, J. A., Micklefield, J., and Ramesh, V. (2004). NMR structure determination and calcium binding effects of lipopeptide antibiotic daptomycin. Org. Biomol. Chem. 2, 1872–1878. doi: 10.1039/b402722a
Bartoloni, A., Mantella, A., Goldstein, B. P., Dei, R., Benedetti, M., Sbaragli, S., et al. (2004). In-vitro activity of nisin against clinical isolates of Clostridium difficile. J. Chemother. 16, 119–121. doi: 10.1179/joc.2004.16.2.119
Bastos, M. D. C. D. F., Coelho, M. L. V., and Santos, O. C. D. S. (2015). Resistance to bacteriocins produced by gram-positive bacteria. Microbiology 161, 683–700. doi: 10.1099/mic.0.082289-0
Batoni, G., Maisetta, G., and Esin, S. (2016). Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 1858, 1044–1060. doi: 10.1016/j.bbamem.2015.10.013
Beisswenger, C., and Bals, R. (2005). Functions of antimicrobial peptides in host defense and immunity. Curr. Protein Pept. Sci. 6, 255–264. doi: 10.2174/1389203054065428
Benet, L. Z. (1978). Effect of route of administration and distribution on drug action. J. Pharmacokinet. Biopharm. 6, 559–585. doi: 10.1007/BF01062110
Bergman, P., Walter-Jallow, L., Broliden, K., Agerberth, B., and Soderlund, J. (2007). The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 5, 410–415. doi: 10.2174/157016207781023947
Bertelsen, K., Dorosz, J., Hansen, S. K., Nielsen, N. C., and Vosegaard, T. (2012). Mechanisms of peptide-induced pore formation in lipid bilayers investigated by oriented 31P solid-state NMR spectroscopy. PLoS One 7:e47745. doi: 10.1371/journal.pone.0047745
BioSpace (2019). Polyphor Temporarily Halts Enrollment in the Phase III Studies of Murepavadin for the Treatment of Patients with Nosocomial Pneumonia.
Björn, C., Noppa, L., Näslund Salomonsson, E., Johansson, A. L., Nilsson, E., Mahlapuu, M., et al. (2015). Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int. J. Antimicrob. Agents 45, 519–524. doi: 10.1016/j.ijantimicag.2014.12.015
Blay, G., Le Lacroix, C., Zihler, A., and Fliss, I. (2007). In vitro inhibition activity of nisin A, nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl. Microbiol. 45, 252–257. doi: 10.1111/j.1472-765X.2007.02178.x
Blondelle, S. E., Ostresh, J. M., Houghten, R. A., and Perez-Paya, E. (1995). Induced conformational states of amphipathic peptides in aqueous/lipid environments. Biophys J. 68, 351–359. doi: 10.1016/S0006-3495(95)80194-3
Booth, J. H., Benrimoj, S. I., and Nimmo, G. R. (1994). In vitro interactions of neomycin sulfate, bacitracin, and polymyxin B sulfate. Int. J. Dermatol. 33, 517–520. doi: 10.1111/j.1365-4362.1994.tb02872.x
Bosscha, M. I., Van Dissel, J. T., Kuijper, E. J., Swart, W., and Jager, M. J. (2004). The efficacy and safety of topical polymyxin B, neomycin and gramicidin for treatment of presumed bacterial corneal ulceration. Br. J. Ophthalmol. 88, 25–28. doi: 10.1136/bjo.88.1.25
Boto, A., De La Lastra, J. M. P., and González, C. C. (2018). The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 23:311. doi: 10.3390/molecules23020311
Bray, B. L. (2003). Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2, 587–593. doi: 10.1038/nrd1133
Brogden, K. A. (2005). Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250. doi: 10.1038/nrmicro1098
Brown, K. L., Poon, G. F. T., Birkenhead, D., Pena, O. M., Falsafi, R., Dahlgren, C., et al. (2011). Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 186, 5497–5505. doi: 10.4049/jimmunol.1002508
Bulger, E. M., Maier, R. V., Sperry, J., Joshi, M., Henry, S., Moore, F. A., et al. (2014). A novel drug for treatment of necrotizing soft-tissue infections: a randomized clinical trial. JAMA Surg. 149, 528–536. doi: 10.1001/jamasurg.2013.4841
Carretero, M., Escámez, M. J., García, M., Duarte, B., Holguín, A., Retamosa, L., et al. (2008). In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Invest. Dermatol. 128, 223–236. doi: 10.1038/sj.jid.5701043
Chan, D. I., Prenner, E. J., and Vogel, H. J. (2006). Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta Biomembr. 1758, 1184–1202. doi: 10.1016/j.bbamem.2006.04.006
Chen, C. H., and Lu, T. K. (2020). Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9:24. doi: 10.3390/antibiotics9010024
Cheng, M. P., Lawandi, A., Butler-Laporte, G., Paquette, K., and Lee, T. C. (2018). Daptomycin versus placebo as an adjunct to beta-lactam therapy in the treatment of Staphylococcus aureus bacteremia: study protocol for a randomized controlled trial. Trials 19, 1–10. doi: 10.1186/s13063-018-2668-6
Chennupati, S. K., Chiu, A. G., Tamashiro, E., Banks, C. A., Cohen, M. B., Bleier, B. S., et al. (2009). Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am. J. Rhinol. Allergy 23, 46–51. doi: 10.2500/ajra.2009.23.3261
Choi, J. H., Jang, A. Y., Lin, S., Lim, S., Kim, D., Park, K., et al. (2015). Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 12, 6483–6490. doi: 10.3892/mmr.2015.4275
Chu, H. L., Yu, H. Y., Yip, B. S., Chih, Y. H., Liang, C. W., Cheng, H. T., et al. (2013). Boosting salt resistance of short antimicrobial peptides. Antimicrob. Agents Chemother. 57, 4050–4052. doi: 10.1128/AAC.00252-13
Cisneros, J. M., Rosso-Fernández, C. M., Roca-Oporto, C., De Pascale, G., Jiménez-Jorge, S., Fernández-Hinojosa, E., et al. (2019). Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): an investigator-driven, open-label, randomized, noninferiority controlled trial. Crit. Care 23:383. doi: 10.1186/s13054-019-2627-y
Cleveland, J., Montville, T. J., Nes, I. F., and Chikindas, M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71, 1–20. doi: 10.1016/s0168-1605(01)00560-8
Committee for Proprietary Medicinal Products (2001). Points to consider on switching between superiority and non-inferiority. Br. J. Clin. Pharmacol. 52:223. doi: 10.1046/J.0306-5251.2001.01397-3.X
Cornish, J., Callon, K. E., Lin, C. Q. X., Xiao, C. L., Mulvey, T. B., Cooper, G. J. S., et al. (1999). Trifluoroacetate, a contaminant in purified proteins, inhibits proliferation of osteoblasts and chondrocytes. Am. J. Physiol. Endocrinol. Metab. 277, E779–E783. doi: 10.1152/ajpendo.1999.277.5.E779
Crowther, G. S., Baines, S. D., Todhunter, S. L., Freeman, J., Chilton, C. H., and Wilcox, M. H. (2013). Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J. Antimicrob. Chemother. 68, 168–176. doi: 10.1093/jac/dks359
Csato, M., Kenderessy, A. S., and Dobozy, A. (1989). Enhancement of Candida albicans killing activity of separated human epidermal cells by α-melanocyte stimulating hormone. Br. J. Dermatol. 121, 145–147. doi: 10.1111/j.1365-2133.1989.tb01415.x
David, J. M., and Rajasekaran, A. K. (2015). Gramicidin A: a new mission for an old antibiotic. J. Kidney Cancer VHL 2, 15–24. doi: 10.15586/jkcvhl.2015.21
Dean, S. N., Bishop, B. M., and van Hoek, M. L. (2011). Susceptibility of Pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front. Microbiol. 2:128. doi: 10.3389/fmicb.2011.00128
Delves-Broughton, J., Blackburn, P., Evans, R. J., and Hugenholtz, J. (1996). Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 69, 193–202. doi: 10.1007/BF00399424
Demetriades, D., Smith, J. S., Jacobson, L. E., Moncure, M., Minei, J., Nelson, B. J., et al. (1999). Bactericidal/permeability-increasing protein (rBPI21) in patients with hemorrhage due to trauma: results of a multicenter phase II clinical trial. J. Trauma Inj. Infect. Crit. Care 46, 667–677. doi: 10.1097/00005373-199904000-00018
Desai, N. (2013). Stable Pexiganan Formulation. Google Patents No, WO/2013/188286. International Research Report (Art. 21 (3)).
Di Nardo, A., Braff, M. H., Taylor, K. R., Na, C., Granstein, R. D., McInturff, J. E., et al. (2007). Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 178, 1829–1834. doi: 10.4049/jimmunol.178.3.1829
Dobias, J., Poirel, L., and Nordmann, P. (2017). Cross-resistance to human cationic antimicrobial peptides and to polymyxins mediated by the plasmid-encoded MCR-1? Clin. Microbiol. Infect. 23, 676.e1–676.e5. doi: 10.1016/j.cmi.2017.03.015
Doi, K., Hu, X., Yuen, P. S. T., Leelahavanichkul, A., Yasuda, H., Kim, S. M., et al. (2008). AP214, an analogue of α-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 73, 1266–1274. doi: 10.1038/ki.2008.97
Dosler, S., Karaaslan, E., and Alev Gerceker, A. (2016). Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against gram-negative bacteria. J. Chemother. 28, 95–103. doi: 10.1179/1973947815Y.0000000004
Dvorchik, B. H., Brazier, D., DeBruin, M. F., and Arbeit, R. D. (2003). Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47, 1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003
Easton, D. M., Nijnik, A., Mayer, M. L., and Hancock, R. E. W. (2009). Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27, 582–590. doi: 10.1016/j.tibtech.2009.07.004
Ebenhan, T., Gheysens, O., Kruger, H. G., Zeevaart, J. R., and Sathekge, M. M. (2014). Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. Biomed Res. Int. 2014:867381. doi: 10.1155/2014/867381
Eckert, R., Brady, K. M., Greenberg, E. P., Qi, F., Yarbrough, D. K., He, J., et al. (2006). Enhancement of antimicrobial activity against Pseudomonas aeruginosa by coadministration of G10KHc and tobramycin. Antimicrob. Agents Chemother. 50, 3833–3838. doi: 10.1128/AAC.00509-06
Eckert, R., Sullivan, R., and Shi, W. (2012). Targeted antimicrobial treatment to re-establish a healthy microbial flora for long-term protection. Adv. Dent. Res. 24, 94–97. doi: 10.1177/0022034512453725
Edgerton, M., Koshlukova, S. E., Araujo, M. W. B., Patel, R. C., Dong, J., and Bruenn, J. A. (2000). Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob. Agents Chemother. 44, 3310–3316. doi: 10.1128/AAC.44.12.3310-3316.2000
Edsfeldt, S., Holm, B., Mahlapuu, M., Reno, C., Hart, D. A., and Wiig, M. (2017). PXL01 in sodium hyaluronate results in increased PRG4 expression: a potential mechanism for anti-adhesion. Ups. J. Med. Sci. 122, 28–34. doi: 10.1080/03009734.2016.1230157
Endermann, R., Vente, A., and Labischinski, H. (2007). “Friulimicin B, a cyclic lipopeptide, exhibits potent efficacy in a murine pneumococcal pneumonia model,” in Poster Presentation at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago.
Falagas, M. E., and Kasiakou, S. K. (2006). Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care 10:R27.
Falagas, M. E., Kasiakou, S. K., Tsiodras, S., and Michalopoulos, A. (2006). The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin. Med. Res. 4, 138–146. doi: 10.3121/cmr.4.2.138
Falanga, A., Lombardi, L., Franci, G., Vitiello, M., Iovene, M. R., Morelli, G., et al. (2016). Marine antimicrobial peptides: nature provides templates for the design of novel compounds against pathogenic bacteria. Int. J. Mol. Sci. 17:785. doi: 10.3390/ijms17050785
Fang, Z., Wusgal, L., Cheng, H., and Liang, L. (2017). “Natural biodegradable medical polymers: therapeutic peptides and proteins,” in Science and Principles of Biodegradable and Bioresorbable Medical Polymers: Materials and Properties, ed. X. C. Zhang (Amsterdam: Elsevier Inc), 321–350. doi: 10.1016/B978-0-08-100372-5.00011-8
Fulco, P., and Wenzel, R. P. (2006). Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent. Expert Rev. Anti. Infect. Ther. 4, 939–945. doi: 10.1586/14787210.4.6.939
Gajski, G., and Garaj-Vrhovac, V. (2013). Melittin: a lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 36, 697–705. doi: 10.1016/j.etap.2013.06.009
Galar, A., Muñoz, P., Valerio, M., Cercenado, E., García-González, X., Burillo, A., et al. (2019). Current use of daptomycin and systematic therapeutic drug monitoring: clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 53, 40–48. doi: 10.1016/j.ijantimicag.2018.09.015
Gaussier, H., Morency, H., Lavoie, M. C., and Subirade, M. (2002). Replacement of trifluoroacetic acid with HCl in the hydrophobic purification steps of pediocin PA-1: a structural effect. Appl. Environ. Microbiol. 68, 4803–4808. doi: 10.1128/aem.68.10.4803-4808.2002
Genetic Engineering, and Biotechnology News (2016). Dipexium’s Diabet. Foot Ulcer Candidate Fail. Phase III Trials. Available online at: https://www.genengnews.com/news/dipexiums-diabetic-foot-ulcer-candidate-fails-phase-iii-trials/ (accessed September 30, 2020).
Gentilucci, L., De Marco, R., and Cerisoli, L. (2010). Chemical modifications designed to improve peptide stability: incorporation of non-natural amino Acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 16, 3185–3203. doi: 10.2174/138161210793292555
Giroir, B. P., Scannon, P. J., and Levin, M. (2001). Bactericidal/permeability-increasing protein—Lessons learned from the phase III, randomized, clinical trial of rBPI21 for adjunctive treatment of children with severe meningococcemia. Crit. Care Med. 29, S130–S135. doi: 10.1097/00003246-200107001-00039
Gordon, Y. J., Romanowski, E. G., and McDermott, A. M. (2005). A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 30, 505–515. doi: 10.1080/02713680590968637
Gottler, L. M., and Ramamoorthy, A. (2009). Structure, membrane orientation, mechanism, and function of pexiganan—a highly potent antimicrobial peptide designed from magainin. Biochim. Biophys. Acta Biomembr. 1788, 1680–1686. doi: 10.1016/j.bbamem.2008.10.009
Gualillo, O., Lago, F., Gómez-Reino, J., Casanueva, F. F., and Dieguez, C. (2003). Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 552, 105–109. doi: 10.1016/s0014-5793(03)00965-7
Guinan, E. C., Barbon, C. M., Kalish, L. A., Parmar, K., Kutok, J., Mancuso, C. J., et al. (2011). Bactericidal/permeability-increasing protein (rBPI21) and fluoroquinolone mitigate radiation-induced bone marrow aplasia and death. Sci. Transl. Med. 3:110ra118. doi: 10.1126/scitranslmed.3003126
Guo, L., Mclean, J. S., Yang, Y., Eckert, R., Kaplan, C. W., Kyme, P., et al. (2015). Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 112, 7569–7574. doi: 10.1073/pnas.1506207112
Håkansson, J., Ringstad, L., Umerska, A., Johansson, J., Andersson, T., Boge, L., et al. (2019). Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide DPK-060 used for topical treatment. Front. Cell. Infect. Microbiol. 9:174. doi: 10.3389/fcimb.2019.00174
Hale, J. D. F., and Hancock, R. E. W. (2007). Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti. Infect. Ther. 5, 951–959. doi: 10.1586/14787210.5.6.951
He, J., Yarbrough, D. K., Kreth, J., Anderson, M. H., Shi, W., and Eckert, R. (2010). Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob. Agents Chemother. 54, 2143–2151. doi: 10.1128/AAC.01391-09
Heinemann, B., and Williams, R. (1966). Inactivation of nisin by pancreatin. J. Dairy Sci. 49, 312–314. doi: 10.3168/jds.S0022-0302(66)87854-2
Hirunwiwatkul, P., and Wachirasereechai, K. (2005). Effectiveness of combined antibiotic ophthalmic solution in the treatment of hordeolum after incision and curettage: a randomized, placebo-controlled trial: a pilot study. J. Med. Assoc. Thail. 88, 647–650.
Hou, M., Zhang, N., Yang, J., Meng, X., Yang, R., Li, J., et al. (2013). Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell. Physiol. Biochem. 32, 614–623. doi: 10.1159/000354465
Ingham, A. B., and Moore, R. J. (2007). Recombinant production of antimicrobial peptides in heterologous microbial systems. Biotechnol. Appl. Biochem. 47:207. doi: 10.1042/ba20060207
Isaksson, J., Brandsdal, B. O., Engqvist, M., Flaten, G. E., Svendsen, J. S. M., and Stensen, W. (2011). A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J. Med. Chem. 54, 5786–5795. doi: 10.1021/jm200450h
Ishida, H., Nguyen, L. T., Gopal, R., Aizawa, T., and Vogel, H. J. (2016). Overexpression of antimicrobial, anticancer, and transmembrane peptides in Escherichia coli through a calmodulin-peptide fusion system. J. Am. Chem. Soc. 138, 11318–11326. doi: 10.1021/jacs.6b06781
Itoh, H., Tokumoto, K., Kaji, T., Paudel, A., Panthee, S., Hamamoto, H., et al. (2017). Total synthesis and biological mode of action of WAP-8294A2: a menaquinone-targeting antibiotic. J. Org. Chem. 83, 6924–6935. doi: 10.1021/acs.joc.7b02318
Jiang, Z., Vasil, A. I., Hale, J. D., Hancock, R. E. W., Vasil, M. L., and Hodges, R. S. (2008). Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Biopolym. Pept. Sci. Sect. 90, 369–383. doi: 10.1002/bip.20911
Johansson, J., Gudmundsson, G. H., Rottenberg, M. E., Berndt, K. D., and Agerberth, B. (1998). Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273, 3718–3724. doi: 10.1074/jbc.273.6.3718
Jorgensen, J. H., Crawford, S. A., Kelly, C. C., and Patterson, J. E. (2003). In vitro activity of daptomycin against vancomycin-resistant Enterococci of various van types and comparison of susceptibility testing methods. Antimicrob. Agents Chemother. 47, 3760–3763. doi: 10.1128/AAC.47.12.3760-3763.2003
Kampshoff, F., Willcox, M. D. P., and Dutta, D. (2019). A pilot study of the synergy between two antimicrobial peptides and two common antibiotics. Antibiotics 8:60. doi: 10.3390/antibiotics8020060
Kaplan, C. W., Sim, J. H., Shah, K. R., Kolesnikova-Kaplan, A., Shi, W., and Eckert, R. (2011). Selective membrane disruption: mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob. Agents Chemother. 55, 3446–3452. doi: 10.1128/AAC.00342-11
Khan, I., and Oh, D. H. (2016). Integration of nisin into nanoparticles for application in foods. Innov. Food Sci. Emerg. Technol. 34, 376–384. doi: 10.1016/j.ifset.2015.12.013
Kim, S. H., Nguyen, T. H., and Maynard, H. D. (2017). Polymeric drug conjugates by controlled radical polymerization. Comprehens. Biomater. II 4, 493–505. doi: 10.1016/B978-0-08-100691-7.00020-3
Kramer, N. E., Hasper, H. E., van den Bogaard, P. T. C., Morath, S., de Kruijff, B., Hartung, T., et al. (2008). Increased D-alanylation of lipoteichoic and a thickened septum are main determinants in the nisin resistance mechanism of Lactococcus lactis. Microbiology 154, 1755–1762. doi: 10.1099/mic.0.2007/015412-0
Kudrimoti, M., Curtis, A., Azawi, S., Worden, F., Katz, S., Adkins, D., et al. (2016). Dusquetide: a novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled Phase 2a clinical study. J. Biotechnol. 239, 115–125. doi: 10.1016/j.jbiotec.2016.10.010
Kumarasamy, K. K., Toleman, M. A., Walsh, T. R., Bagaria, J., Butt, F., Balakrishnan, R., et al. (2010). Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10, 597–602. doi: 10.1016/S1473-3099(10)70143-2
Kwa, A., Kasiakou, S. K., Tam, V. H., and Falagas, M. E. (2007). Polymyxin B: similarities to and differences from colistin (polymyxin E). Expert Rev. Anti. Infect. Ther. 5, 811–821. doi: 10.1586/14787210.5.5.811
Lacey, K. A., Geoghegan, J. A., and McLoughlin, R. M. (2016). The role of Staphylococcus aureus virulence factors in skin infection and their potential as vaccine antigens. Pathogens 5:22. doi: 10.3390/pathogens5010022
Larrick, J. W., Hirata, M., Balint, R. F., Lee, J., Zhong, J., and Wright, S. C. (1995). Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63, 1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995
Laverty, G. (2014). Cationic antimicrobial peptide cytotoxicity. SOJ Microbiol. Infect. Dis. 2:112. doi: 10.15226/sojmid.2013.00112
Le, C. F., Fang, C. M., and Sekaran, S. D. (2017). Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 61:e02340-16. doi: 10.1128/AAC.02340-16
Le Lay, C., Dridi, L., Bergeron, M. G., Ouellette, M., and Fliss, I. I. (2016). Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J Med Microbiol 65, 169–175. doi: 10.1099/jmm.0.000202
Lee, G., and Bae, H. (2016). Anti-inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules 21:616. doi: 10.3390/molecules21050616
Leeds, J. A., Sachdeva, M., Mullin, S., Dzink-Fox, J., and LaMarche, M. J. (2012). Mechanism of action of and mechanism of reduced susceptibility to the novel anti-Clostridium difficile compound LFF571. Antimicrob. Agents Chemother. 56, 4463–4465. doi: 10.1128/aac.06354-11
Lenhard, J. R., Nation, R. L., and Tsuji, B. T. (2016). Synergistic combinations of polymyxins. Int. J. Antimicrob. Agents 48, 607–613. doi: 10.1016/j.ijantimicag.2016.09.014
Li, J., Koh, J.-J., Liu, S., Lakshminarayanan, R., Verma, C. S., and Beuerman, R. W. (2017). Membrane active antimicrobial peptides: translating mechanistic insights to design. Front. Neurosci. 11:73. doi: 10.3389/fnins.2017.00073
Li, J., and Nation, R. L. (2006). Old polymyxins are back: is resistance close? Clin. Infect. Dis. 43, 663–664. doi: 10.1086/506571
Li, J., Nation, R. L., Milne, R. W., Turnidge, J. D., and Coulthard, K. (2005). Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int. J. Antimicrob. Agents 25, 11–25. doi: 10.1016/j.ijantimicag.2004.10.001
Li, M., Cha, D. J., Lai, Y., Villaruz, A. E., Sturdevant, D. E., and Otto, M. (2007). The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66, 1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x
Liang, S. C., Tan, X. Y., Luxenberg, D. P., Karim, R., Dunussi-Joannopoulos, K., Collins, M., et al. (2006). Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279. doi: 10.1084/jem.20061308
Lipsky, B. A., Holroyd, K. J., and Zasloff, M. (2008). Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin. Infect. Dis. 47, 1537–1545. doi: 10.1086/593185
Liu, Y.-Y., Wang, Y., Walsh, T. R., Yi, L.-X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/s1473-3099(15)00424-7
Luo, X.-L., Li, J.-X., Huang, H.-R., Duan, J.-L., Dai, R.-X., Tao, R.-J., et al. (2019). LL37 inhibits Aspergillus fumigatus infection via directly binding to the fungus and preventing excessive inflammation. Front. Immunol. 10:283. doi: 10.3389/fimmu.2019.00283
Mahlapuu, M., Håkansson, J., Ringstad, L., and Björn, C. (2016). Antimicrobial peptides: an emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 6:194. doi: 10.3389/fcimb.2016.00194
Malanovic, N., Leber, R., Schmuck, M., Kriechbaum, M., Cordfunke, R. A., Drijfhout, J. W., et al. (2015). Phospholipid-driven differences determine the action of the synthetic antimicrobial peptide OP-145 on Gram-positive bacterial and mammalian membrane model systems. Biochim. Biophys. Acta Biomembr. 1848, 2437–2447. doi: 10.1016/j.bbamem.2015.07.010
Marr, A. K., Gooderham, W. J., and Hancock, R. E. (2006). Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 6, 468–472. doi: 10.1016/j.coph.2006.04.006
Martin-Loeches, I., Dale, G. E., and Torres, A. (2018). Murepavadin: a new antibiotic class in the pipeline. Expert Rev. Anti. Infect. Ther. 16, 259–268. doi: 10.1080/14787210.2018.1441024
Matsuzaki, K. (1999). Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta Biomembr. 1462, 1–10. doi: 10.1016/S0005-2736(99)00197-2
McCrudden, M. T. C., McLean, D. T. F., Zhou, M., Shaw, J., Linden, G. J., Irwin, C. R., et al. (2014). The host defence peptide LL-37 is susceptible to proteolytic degradation by wound fluid isolated from foot ulcers of diabetic patients. Int. J. Pept. Res. Ther. 20, 457–464. doi: 10.1007/s10989-014-9410-3
Meikle, T. G., Conn, C. E., Separovic, F., and Drummond, C. J. (2016). Exploring the structural relationship between encapsulated antimicrobial peptides and the bilayer membrane mimetic lipidic cubic phase: studies with gramicidin A′. RSC Adv. 6, 68685–68694. doi: 10.1039/c6ra13658c
Memariani, H., Memariani, M., Moravvej, H., and Shahidi-Dadras, M. (2020). Melittin: a venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 39, 5–17. doi: 10.1007/s10096-019-03674-0
Mensa, B., Howell, G., Scott, R., and DeGrado, W. (2014). Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob. Agents Chemother. 58, 5136–5145. doi: 10.1128/AAC.02955-14
Mercer, D. K., Robertson, J. C., Miller, L., Stewart, C. S., and O’Neil, D. A. (2020). NP213 (Novexatin®): a unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 58, 1064–1072. doi: 10.1093/mmy/myaa015
Midura-Nowaczek, K., and Markowska, A. (2014). Antimicrobial peptides and their analogs: searching for new potential therapeutics. Perspect. Med. Chem. 6:PMC.S13215. doi: 10.4137/PMC.S13215
Miller, L. S., Sørensen, O. E., Liu, P. T., Jalian, H. R., Eshtiaghpour, D., Behmanesh, B. E., et al. (2005). TGF-α regulates TLR expression and function on epidermal keratinocytes. J. Immunol. 174, 6137–6143. doi: 10.4049/jimmunol.174.10.6137
Mishra, B., Reiling, S., Zarena, D., and Wang, G. (2017). Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr. Opin. Chem. Biol. 38, 87–96. doi: 10.1016/j.cbpa.2017.03.014
Mitra, D., Yadav, A., Prithyani, S., John, L. E., Rodrigues, S., and Shah, R. (2019). The antiplaque efficacy of lantibiotic Nisin extract mouthrinse. J. Indian Soc. Periodontol. 23, 31–34. doi: 10.4103/jisp.jisp_326_18
Miyake, O., Ochiai, A., Hashimoto, W., and Murata, K. (2004). Origin and diversity of alginate lyases of families PL-5 and -7 in Sphingomonas sp. strain A1. J. Bacteriol. 186, 2891–2896. doi: 10.1128/JB.186.9.2891-2896.2004
Mohamed, M. F., Abdelkhalek, A., and Seleem, M. N. (2016). Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 6, 1–14. doi: 10.1038/srep29707
Montesinos, E., and Bardají, E. (2008). Synthetic antimicrobial peptides as agricultural pesticides for plant-disease control. Chem. Biodivers. 5, 1225–1237. doi: 10.1002/cbdv.200890111
Morrison, D. C., and Jacobs, D. M. (1976). Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13, 813–818. doi: 10.1016/0019-2791(76)90181-6
Muchintala, D., Suresh, V., Raju, D., and Sashidhar, R. B. (2020). Synthesis and characterization of cecropin peptide-based silver nanocomposites: its antibacterial activity and mode of action. Mater. Sci. Eng. C 110:110712. doi: 10.1016/j.msec.2020.110712
Nagaoka, I., Suzuki, K., Niyonsaba, F., Tamura, H., and Hirata, M. (2012). Modulation of neutrophil apoptosis by antimicrobial peptides. ISRN Microbiol. 2012:345791. doi: 10.5402/2012/345791
Nagy, I., Pivarcsi, A., Kis, K., Koreck, A., Bodai, L., McDowell, A., et al. (2006). Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microb. Infect. 8, 2195–2205. doi: 10.1016/j.micinf.2006.04.001
Nell, M. J., Tjabringa, G. S., Wafelman, A. R., Verrijk, R., Hiemstra, P. S., Drijfhout, J. W., et al. (2006). Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 27, 649–660. doi: 10.1016/j.peptides.2005.09.016
Neu, T., and Henrich, B. (2003). New thermosensitive delivery vector and its use to enable nisin-controlled gene expression in Lactobacillus gasseri. Appl. Environ. Microbiol. 69, 1377–1382. doi: 10.1128/AEM.69.3.1377-1382.2003
Nibbering, P. H., Ravensbergen, E., Welling, M. M., van Berkel, L. A., van Berkel, P. H. C., Pauwels, E. K. J., et al. (2001). Human lactoferrin and peptides derived from Its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 69, 1469–1476. doi: 10.1128/IAI.69.3.1469-1476.2001
Nordström, R., and Malmsten, M. (2017). Delivery systems for antimicrobial peptides. Adv. Colloid Interf. Sci. 242, 17–34. doi: 10.1016/j.cis.2017.01.005
Oo, T. Z., Cole, N., Garthwaite, L., Willcox, M. D. P., and Zhu, H. (2010). Evaluation of synergistic activity of bovine lactoferricin with antibiotics in corneal infection. J. Antimicrob. Chemother. 65, 1243–1251. doi: 10.1093/jac/dkq106
Ooi, N., Miller, K., Hobbs, J., Rhys-Williams, W., Love, W., and Chopra, I. (2009). XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J. Antimicrob. Chemother. 64, 735–740. doi: 10.1093/jac/dkp299
Orlov, D., Hong, T., Menzel, L. P., Azimov, R., Falla, T. J., Waring, A. J., et al. (1805). “Bactericidal mechanism of iseganan (IB-367), a rapidly acting antimicrobial protegrin peptide,” in Proceedings of the 41st Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago.
Park, S.-C., Kim, J.-Y., Shin, S.-O., Jeong, C.-Y., Kim, M.-H., Shin, S. Y., et al. (2006). Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem. Biophys. Res. Commun. 343, 222–228. doi: 10.1016/j.bbrc.2006.02.090
Perona, J. J., and Craik, C. S. (1997). Evolutionary divergence of substrate specificity within the chymotrypsin-like serine protease fold. J. Biol. Chem. 272, 29987–29990. doi: 10.1074/jbc.272.48.29987
Petrof, E. O., Dhaliwal, R., Manzanares, W., Johnstone, J., Cook, D., and Heyland, D. K. (2012). Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit. Care Med. 40, 3290–3302. doi: 10.1097/CCM.0b013e318260cc33
Peyrusson, F., Butler, D., Tulkens, P. M., and Van Bambeke, F. (2015). Cellular pharmacokinetics and intracellular activity of the novel peptide deformylase inhibitor GSK1322322 against Staphylococcus aureus laboratory and clinical strains with various resistance phenotypes: studies with human THP-1 monocytes and J774 murine macrophages. Antimicrob. Agents Chemother. 59, 5747–5760. doi: 10.1128/AAC.00827-15
Pfalzgraff, A., Brandenburg, K., and Weindl, G. (2018). Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 9:281. doi: 10.3389/fphar.2018.00281
Phee, L. M., Betts, J. W., Bharathan, B., and Wareham, D. W. (2015). Colistin and fusidic acid, a novel potent synergistic combination for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 59, 4544–4550. doi: 10.1128/aac.00753-15
Piper, C., Hill, C., Cotter, P. D., and Ross, R. P. (2011). Bioengineering of a nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb. Biotechnol. 4, 375–382. doi: 10.1111/j.1751-7915.2010.00207.x
Prajna, N. V., George, C., Selvaraj, S., Lu, K. L., McDonnell, P. J., and Srinivasan, M. (2001). Bacteriologic and clinical efficacy of ofloxacin 0.3% versus ciprofloxacin 0.3% ophthalmic solutions in the treatment of patients with culture-positive bacterial keratitis. Cornea 20, 175–178. doi: 10.1097/00003226-200103000-00013
Prince, A., Sandhu, P., Kumar, P., Dash, E., Sharma, S., Arakha, M., et al. (2016). Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci. Rep. 6, 1–15. doi: 10.1038/srep37908
Rand, K. H., and Houck, H. J. (2004). Synergy of daptomycin with oxacillin and other β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 48, 2871–2875. doi: 10.1128/AAC.48.8.2871-2875.2004
Rivas-Santiago, B., Trujillo, V., Montoya, A., Gonzalez-Curiel, I., Castañeda-Delgado, J., Cardenas, A., et al. (2012). Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 65, 19–26. doi: 10.1016/j.jdermsci.2011.09.013
Rodriguez, C. H., De Ambrosio, A., Bajuk, M., Spinozzi, M., Nastro, M., Bombicino, K., et al. (2010). In vitro antimicrobials activity against endemic Acinetobacter baumannii multiresistant clones. J. Infect. Dev. Ctries. 4, 164–167. doi: 10.3855/jidc.604
Rogers, J. M., and Suga, H. (2015). Discovering functional, non-proteinogenic amino acid containing, peptides using genetic code reprogramming. Org. Biomol. Chem. 13, 9353–9363. doi: 10.1039/c5ob01336d
Rosenfeld, Y., Papo, N., and Shai, Y. (2006). Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides: peptide properties and plausible modes of action. J. Biol. Chem. 281, 1636–1643. doi: 10.1074/jbc.M504327200
Rubinchik, E., Dugourd, D., Algara, T., Pasetka, C., and Friedland, H. D. (2009). Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int. J. Antimicrob. Agents 34, 457–461. doi: 10.1016/j.ijantimicag.2009.05.003
Ryu, S., Song, P., Seo, C., Cheong, H., and Park, Y. (2014). Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 15, 8753–8772. doi: 10.3390/ijms15058753
Sakamoto, I., Igarashi, M., Kimura, K., Takagi, A., Miwa, T., and Koga, Y. (2001). Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J. Antimicrob. Chemother. 47, 709–710. doi: 10.1093/jac/47.5.709
Salmon, J. K., Armstrong, C. A., and Ansel, J. C. (1994). The skin as an immune organ. West. J. Med. 160, 146–152.
Sarma, P., Mahendiratta, S., Prakash, A., and Medhi, B. (2018). Specifically targeted antimicrobial peptides: a new and promising avenue in selective antimicrobial therapy. Indian J. Pharmacol. 50, 1–3. doi: 10.4103/ijp.IJP_218_18
Schmidtchen, A., Pasupuleti, M., and Malmsten, M. (2014). Effect of hydrophobic modifications in antimicrobial peptides. Adv. Colloid Interf. Sci. 205, 265–274. doi: 10.1016/j.cis.2013.06.009
Schneider, T., Gries, K., Josten, M., Wiedemann, I., Pelzer, S., Labischinski, H., et al. (2009). The lipopeptide antibiotic friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 53, 1610–1618. doi: 10.1128/AAC.01040-08
Schroder, G., Brandenburg, K., and Seydel, U. (1992). Polymyxin B induces transient permeability fluctuations in asymmetric planar lipopolysaccharide/phospholipid bilayers. Biochemistry 31, 631–638. doi: 10.1021/bi00118a001
Schultz, H., and Weiss, J. P. (2007). The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease. Clin. Chim. Acta 384, 12–23. doi: 10.1016/j.cca.2007.07.005
Segev-Zarko, L., Saar-Dover, R., Brumfeld, V., Mangoni, M. L., and Shai, Y. (2015). Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 468, 259–270. doi: 10.1042/BJ20141251
Severina, E., Severin, A., and Tomasz, A. (1998). Antibacterial efficacy of nisin against multidrug-resistant grampositive pathogens. J. Antimicrob. Chemother. 41, 341–347. doi: 10.1093/jac/41.3.341
Shin, J. M., Ateia, I., Paulus, J. R., Liu, H., Fenno, J. C., Rickard, A. H., et al. (2015). Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 6:617. doi: 10.3389/fmicb.2015.00617
Shurko, J. F., Galega, R. S., Li, C., and Lee, G. C. (2018). Evaluation of LL-37 antimicrobial peptide derivatives alone and in combination with vancomycin against S. aureus. J. Antibiot. 71, 971–974. doi: 10.1038/s41429-018-0090-7
Sikora, K., Jaśkiewicz, M., Neubauer, D., Bauer, M., Bartoszewska, S., Barańska-Rybak, W., et al. (2018). Counter-ion effect on antistaphylococcal activity and cytotoxicity of selected antimicrobial peptides. Amino Acids 50, 609–619. doi: 10.1007/s00726-017-2536-9
Sivertsen, A., Isaksson, J., Leiros, H.-K. S., Svenson, J., Svendsen, J.-S., and Brandsdal, B. O. (2014). Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 14:4. doi: 10.1186/1472-6807-14-4
Son, D. J., Lee, J. W., Lee, Y. H., Song, H. S., Lee, C. K., and Hong, J. T. (2007). Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 115, 246–270. doi: 10.1016/j.pharmthera.2007.04.004
Sørensen, O. E., Thapa, D. R., Roupé, K. M., Valore, E. V., Sjöbring, U., Roberts, A. A., et al. (2006). Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J. Clin. Invest. 116, 1878–1885. doi: 10.1172/JCI28422
Srinivas, N., Jetter, P., Ueberbacher, B. J., Werneburg, M., Zerbe, K., Steinmann, J., et al. (2010). Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327, 1010–1013. doi: 10.1126/science.1182749
Starr, C. G., He, J., and Wimley, W. C. (2016). Host cell interactions are a significant barrier to the clinical utility of peptide antibiotics. ACS Chem. Biol. 11, 3391–3399. doi: 10.1021/acschembio.6b00843
Steenbergen, J. N., Mohr, J. F., and Thorne, G. M. (2009). Effects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studies. J. Antimicrob. Chemother. 64, 1130–1138. doi: 10.1093/jac/dkp346
Stevens, K. A., Sheldon, B. W., Klapes, N. A., and Klaenhammer, T. R. (1991). Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl. Environ. Microbiol. 57, 3613–3615. doi: 10.1128/aem.57.12.3613-3615.1991
Strömstedt, A. A., Pasupuleti, M., Schmidtchen, A., and Malmsten, M. (2009). Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob. Agents Chemother. 53, 593–602. doi: 10.1128/AAC.00477-08
Taylor, S. D., and Palmer, M. (2016). The action mechanism of daptomycin. Bioorganic Med. Chem. 24, 6253–6268. doi: 10.1016/j.bmc.2016.05.052
Taylor, T. M., Gaysinsky, S., Davidson, P. M., Bruce, B. D., and Weiss, J. (2007). Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2, 1–9. doi: 10.1007/s11483-007-9023-x
Tempera, G., Mangiafico, A., Genovese, C., Giudice, E., Mastrojeni, S., Nicolosi, D., et al. (2009). In vitro evaluation of the synergistic activity of neomycin-polymyxin b association against pathogens responsible for otitis externa. Int. J. Immunopathol. Pharmacol. 22, 299–302. doi: 10.1177/039463200902200206
Terwilligert, T. C., and Eisenbergg, D. (1982). The structure of Melittin. J. Biol. Chem. 257, 6016–6022.
Thwaite, J. E., Hibbs, S., Titball, R. W., and Atkins, T. P. (2006). Proteolytic degradation of human antimicrobial peptide LL-37 by Bacillus anthracis may contribute to virulence. Antimicrob. Agents Chemother. 50, 2316–2322. doi: 10.1128/AAC.01488-05
Todd, P., and Pierre, K. (2015). GlobeNewswire. C3 Jian Complet. Second Phase 2 Clin. Trial Anti-Cavity Drug. Available online at: https://www.globenewswire.com/news-release/2015/07/13/751454/0/en/C3-Jian-Completes-Second-Phase-2-Clinical-Trial-of-Anti-Cavity-Drug.html (accessed September 18, 2020).
Travis, S., Yap, L. M., Hawkey, C., Warren, B., Lazarov, M., Fong, T., et al. (2005). RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 11, 713–719. doi: 10.1097/01.mib.0000172807.26748.16
Ugurlu, T., Turkoglu, M., Gurer, U. S., and Akarsu, B. G. (2007). Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur. J. Pharm. Biopharm. 67, 202–210. doi: 10.1016/j.ejpb.2007.01.016
Valencia, R., Arroyo, L. A., Conde, M., Aldana, J. M., Torres, M.-J., Fernández-Cuenca, F., et al. (2009). Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect. Control Hosp. Epidemiol. 30, 257–263. doi: 10.1086/595977
Van Den Bogaart, G., Guzmán, J. V., Mika, J. T., and Poolman, B. (2008). On the mechanism of pore formation by melittin. J. Biol. Chem. 283, 33854–33857. doi: 10.1074/jbc.M805171200
Van Epps, H. L. (2006). René dubos: unearthing antibiotics. J. Exp. Med. 203:259. doi: 10.1084/jem.2032fta
Van Groenendael, R., Kox, M., Van Eijk, L. T., and Pickkers, P. (2018). Immunomodulatory and kidney-protective effects of the human chorionic gonadotropin derivate EA-230. Nephron 140, 148–151. doi: 10.1159/000490772
Wecke, T., Zühlke, D., Mäder, U., Jordan, S., Voigt, B., Pelzer, S., et al. (2009). Daptomycin versus friulimicin B: in-depth profiling of Bacillus subtilis cell envelope stress responses. Antimicrob. Agents Chemother. 53, 1619–1623. doi: 10.1128/AAC.01046-08
Welling, M., Brouwer, C., Roscini, L., Cardinali, G., Corte, L., Casagrande Pierantoni, D., et al. (2018). Structure-activity relationship study of synthetic variants derived from the highly potent human antimicrobial peptide hLF(1-11). Cohesive J. Microbiol. Infect. Dis. doi: 10.31031/CJMI.2018.01.000512
Wimley, W. C., and Hristova, K. (2011). Antimicrobial peptides: successes, challenges and unanswered questions. J. Membr. Biol. 239, 27–34. doi: 10.1007/s00232-011-9343-0
Woong, S. J., Xuewei, S. L., Sun, J. N., and Edgerton, M. (2008). The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 52, 497–504. doi: 10.1128/AAC.01199-07
Wu, C.-L., Hsueh, J.-Y., Yip, B.-S., Chih, Y.-H., Peng, K.-L., and Cheng, J.-W. (2020). Antimicrobial peptides display strong synergy with vancomycin against vancomycin-resistant E. faecium, S. aureus, and wild-type E. coli. Int. J. Mol. Sci. 21:4578. doi: 10.3390/ijms21134578
Yang, C. H., Chen, Y. C., Peng, S. Y., Tsai, A. P. Y., Lee, T. J. F., Yen, J. H., et al. (2018). An engineered arginine-rich α-helical antimicrobial peptide exhibits broad-spectrum bactericidal activity against pathogenic bacteria and reduces bacterial infections in mice. Sci. Rep. 8, 1–14. doi: 10.1038/s41598-018-32981-3
Yasir, M., Dutta, D., Hossain, K. R., Chen, R., Ho, K. K. K., Kuppusamy, R., et al. (2020). Mechanism of action of surface immobilized antimicrobial peptides against Pseudomonas aeruginosa. Front. Microbiol. 10:3053. doi: 10.3389/fmicb.2019.03053
Yasir, M., Dutta, D., and Willcox, M. D. P. (2019). Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS One 14:e0215703. doi: 10.1371/journal.pone.0215703
Yu, H. B., Kielczewska, A., Rozek, A., Takenaka, S., Li, Y., Thorson, L., et al. (2009). Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 284, 36007–36011. doi: 10.1074/jbc.c109.073627
Yu, K., Lo, J. C. Y., Yan, M., Yang, X., Brooks, D. E., Hancock, R. E. W., et al. (2017). Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116, 69–81. doi: 10.1016/j.biomaterials.2016.11.047
Yu, Z., Qin, W., Lin, J., Fang, S., and Qiu, J. (2015). Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed. Res. Int. 2015:679109. doi: 10.1155/2015/679109
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature 415, 389–395. doi: 10.1038/415389a
Zavascki, A. P., Goldani, L. Z., Li, J., and Nation, R. L. (2007). Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60, 1206–1215. doi: 10.1093/jac/dkm357
Zhang, J., and Zhong, J. (2015). The journey of nisin development in China, a natural-green food preservative. Protein Cell 6, 709–711. doi: 10.1007/s13238-015-0214-9
Keywords: antimicrobial peptide, clinical trial, resistance, infection, cytotoxicity, mechanism of action, improvement strategies, peptide modifications
Citation: Dijksteel GS, Ulrich MMW, Middelkoop E and Boekema BKHL (2021) Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 12:616979. doi: 10.3389/fmicb.2021.616979
Received: 13 October 2020; Accepted: 29 January 2021;
Published: 22 February 2021.
Edited by:
Mariano Martinez-Vazquez, National Autonomous University of Mexico, MexicoReviewed by:
Sónia Gonçalves, University of Lisbon, PortugalPeter Bergman, Karolinska Institutet (KI), Sweden
Copyright © 2021 Dijksteel, Ulrich, Middelkoop and Boekema. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bouke K. H. L. Boekema, YmJvZWtlbWFAYnVybnMubmw=
 Gabrielle S. Dijksteel
Gabrielle S. Dijksteel Magda M. W. Ulrich
Magda M. W. Ulrich Esther Middelkoop1,2
Esther Middelkoop1,2 Bouke K. H. L. Boekema
Bouke K. H. L. Boekema