- 1Key Laboratory of Protection and Utilization of Subtropic Plant Resources of Jiangxi Province, College of Life Sciences, Jiangxi Normal University, Nanchang, China
- 2Key Laboratory of Bioprocess Engineering of Jiangxi Province, College of Life Sciences, Jiangxi Science and Technology Normal University, Nanchang, China
- 3College of Life Sciences, Gannan Normal University, Ganzhou, China
Understanding the evolution of microorganisms and metabolites during wine fermentation is essential for controlling its production. The structural composition and functional capacity of the core microbiota determine the quality and quantity of fruit wine. Nanfeng tangerine wine fermentation involves a complex of various microorganisms and a wide variety of metabolites. However, the microbial succession and functional shift of the core microbiota in this product fermentation remain unclear. Therefore, high-throughput sequencing (HTS) and headspace-gas chromatography-mass spectrometry (HS/GC-MS) were employed to reveal the core functional microbiota for the production of volatile flavors during spontaneous fermentation (SF) and inoculated fermentation (IF) with Saccharomyces cerevisiae of Nanfeng tangerine wine. A total of 13 bacterial and 8 fungal genera were identified as the core microbiota; Lactobacillus and Acetobacter were the dominant bacteria in SF and IF, respectively. The main fungal genera in SF and IF were Hanseniaspora, Pichia, and Saccharomyces with a clear succession. In addition, the potential correlations analysis between microbiota succession and volatile flavor dynamics revealed that Lactobacillus, Acetobacter, Hanseniaspora, and Saccharomyces were the major contributors to the production of the volatile flavor of Nanfeng tangerine wine. The results of the present study provide insight into the effects of the core functional microbiota in Nanfeng tangerine wine and can be used to develop effective strategies for improving the quality of fruit wines.
Introduction
Fruit wines are fermented alcoholic beverages made of fruits other than grapes, and they may also have additional flavors taken from other fruits, flowers, and herbs. The high demand for fruit wine, a derived product, has provided an opportunity for the development of value-added products. The fruit wine market is now progressing toward diversification by developing original, novel, and enriched products through innovative formulas, technologies, and alternative raw materials (Joshi et al., 2017). For a long time, citrus has been one of the most widely cultivated fruits worldwide, and abundant citrus varieties and resources are available in China (Liu R. et al., 2015). The flavor of citrus wine varies depending on the variety and processing method. Nanfeng tangerine, a unique citrus cultivar and a royal tribute in ancient China, has been cultivated in Nanfeng district (Jiangxi Province, China) for over 1,300 years. In recent decades, the yield and area cultivated for Nanfeng tangerine have increased to meet the strong market demand for this product, which contains various nutrients, such as amino acids, glucose, fructose, citric acid, vitamin C, vitamin B1, vitamin B2, calcium, phosphorus, iron, and other inorganic salts (Zhang et al., 2011). However, the characteristics of high sugar content and thin peel make it difficult to preserve at room temperature, and large amounts of rotten Nanfeng tangerine are inevitably wasted and disposed of annually, resulting in serious environmental pollution and huge economic losses to farmers (Lai et al., 2017; Kahramanolu et al., 2020). Processing berries into wine and other fermented beverages may provide some solutions to circumvent these problems. Brewing wine with Nanfeng tangerines may not only reduce yield loss due to rotting but also increase farmers’ income by promoting value-added products. This strategy can meet the need for high-quality fruit wine and prevent environmental pollution. Fermentation of berries other than grapes, such as cherry, blueberry, blackberry, and raspberry, has recently gained wide interest in response to the growing need of consumers for diversified berry wines (Liu et al., 2018). However, tangerine wine is still not widely produced. Only few studies focused on fermentation optimization (Liao and Wang, 2014; Chen et al., 2015) and Non-Saccharomyces isolation (Yu et al., 2019) for high-quality tangerine wine production. Further research is warranted to support the industrial manufacturing of this product.
The microbiota in natural environments is complex and often includes thousands of genera from a diverse range of species (Astudillo-García et al., 2017). In natural environments or under artificially controlled conditions, fermentation microbiota can potentially utilize different raw materials to produce various metabolites (Lu et al., 2016). These metabolites are commonly characterized by the presence of specific microorganisms, orderly microbial succession, and an unusual functional shift (Cagno et al., 2014; Kong et al., 2014). However, the core microbiota that influence the quantity and quality of tangerine wine are poorly understood (Cocolin et al., 2013). Traditionally, species were isolated and identified from food fermentation processes via culture-dependent techniques. However, the knowledge gained by these previous studies about the core structural microbiota in fermented foods remains limited (Wu et al., 2013). With the development of high-throughput sequencing (HTS) techniques, complex microbial communities can now be identified (Sinclair et al., 2015; Wei et al., 2018).
The community structure of the core microbiota in fermented foods and the correlation between microbiota and metabolites during different fermentation processes are important (Papagianni, 2014). Humans have been making wine for thousands of years, and microorganisms play an integral part in this process as they not only drive fermentation but also significantly influence the flavor, aroma, and quality of finished wines (Morrison-Whittle and Goddard, 2018). The diversity and evolution of microorganisms during fermentation of fruit wine, such as grape wine (Portillo and Mas, 2016; Tapsoba et al., 2016) and Palm Wine (Djeni et al., 2020), had attracted more scientists’ attention. Many different species of fungi and bacteria are found naturally associated with fruits and their fermentation (Chanprasartsuk et al., 2010; Nicholas et al., 2014; Liu et al., 2016).
Tangerine wine is a beverage with low alcohol content and variety of flavor components through a series of biochemical reactions. The fermentation mechanism required for tangerine wine therefore involves complex microbiota and metabolites. Moreover, the fermentation microbiotas use tangerine juice as raw materials to form a variety of flavor components through a series of biochemical reactions. However, the functional correlation between the core microbiota and important metabolites remains to be established in tangerine wine. More specifically, microbial succession and the functional shift in the core microbiota tangerine wine have not been clarified to date. Spontaneous fermentation (SF) and inoculated fermentation (IF) are the two main fermentation methods used in producing tangerine wine. Therefore, in this study, we employed several HTS technologies (16S rRNA gene amplicon sequencing, ITS amplicon sequencing, and metatranscriptomics sequencing) to explore the structure and function of core microorganisms in fermentation microbiota. Moreover, we combined ultraperformance liquid chromatography (UPLC) and headspace-gas chromatography-mass spectrometry (HS/GC-MS) to explore the fluctuations in major flavor components. On the basis of this information, we explored the correlations between the core microbiota and important metabolites of Nanfeng tangerine wine.
Materials and Methods
Sample Collection
Tangerines (Nanfeng tangerine) were harvested at optimal maturity from Nanfeng County, Jiangxi Province, China, in 2016. The fresh tangerine fruits were washed, squeezed to obtain the juice, with 97 g/L total sugar, 12 oBrix soluble solids, and 8.3 g/L citric acid, and no sulfur dioxide was added into the juice. SF was induced spontaneously by the indigenous microbes in tangerine in a 500 m3 fermenter, and IF was carried out in a 500 m3 fermenter with 5 kg Angel® commercial Saccharomyces cerevisiae (for fruit wine, Angel yeast Co., Ltd., China) as the population of cell in the fermentation broth was about 1 × 106 cfu⋅mL–1. The loading volume was both approximately 80% of the tank capacity. SF and IF were both conducted at a controlled temperature of 25°C for 10 days. Samples were collected every 2 days for genomic DNA extraction, main products, and flavor component analysis after fermentation. Each sample was analyzed in triplicates.
Analysis of Chemical and Physical Properties
For the detection of chemical concentration, the samples were centrifuged at 4°C at 8,000 rpm for 5 min. Total sugar content was determined using the concentrated sulfuric acid-phenol method, and ethanol content was measured by gas chromatography (GC). For the detection of organic acids, the samples were centrifuged and filtered through a 0.22 μm MCE filter, and metabolite contents were quantified via UPLC. An Ultimate AQ-C18 column measuring 4.6 mm × 250 mm was used. Refractive index was monitored using a refractive index detector (RID). As the mobile phase, 0.025% trifluoroacetic acid solution and methanol isocratic elution at a ratio of 95:5 were added at a rate of 0.8 mL/min. Column temperature was set to 25°C. Absorbance was monitored at 210 nm by using a Ultraviolet–visible spectroscopy (UV/VIS) detector. For the detection of volatile compounds, the samples were quantified via HS-GC-MS by using an Agilent GC (7,000C) equipped with an Agilent mass selective detector (GC-MSD). The target analytes were separated using a DB-FFAP column (30 m length × 0.25 mm ID × 0.32 μm film thickness; Agilent, CA, United States). The MS source temperature was 230°C, and the mass (MS) quad temperature was 180°C. All mass spectra were acquired in electron impact mode at 70 eV via full scan within the scanning range of 30–500 amu.
DNA Extraction, PCR Amplification, and MiSeq Sequencing
Genomic DNA was extracted from 33 samples taken from five fermentation stages of SF and IF by using E.Z.N.A. soil DNA Kit (OMEGA, United States). For prokaryotes, the V3 hypervariable region of 16S rRNA genes was amplified using the universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GACTACHVGGGTWT CTAAT-3′). For eukaryotes, the ITS1 region of fungal rRNA gene was amplified using the universal primers ITS3F (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4R (5′-TCCTCCGCTTATTGATATGC-3′). Primers 338F and ITS1 were added with barcodes. PCR was conducted in triplicate in a 25 μL reaction mixture containing 1 μL of each primer, 2.5 μL of 10 × Pyrobest reaction buffer, 2 μL of dNTPs (2.5 mmol/L), 0.4 μL of Pyrobest DNA Polymerase (Takara), and 15 ng of template DNA. The amplification program consisted of an initial denaturation step at 95°C for 2 min, 35 amplification cycles (each cycle consisted of 95°C for 30 s, 55°C for 1 min, and 72°C for 30 s), and a final incubation of 72°C for 10 min. The amplicons were confirmed by electrophoresis agarose gels (2% agarose in TAE buffer) and purified with AxyPrepDNA gel extraction kit. The amplified products were sent to the Illumina Miseq sequencing platform for sequencing. The raw data for both experiments have been deposited at the China National GeneBank DataBase (CNGBdb) with accession number CNP0001643.
Sequencing Data Analysis
Raw sequences were sorted with their unique barcodes. Sequences with low quality, read length below 300 bp, and average base quality score of less than 20 were filtered out. Chimera sequences were removed using the Uchime algorithm. The sequences were clustered into operational taxonomic units (OTUs) at 97% identity threshold. The most frequently occurring sequence was extracted as the representative sequence for each OTU and was screened for further annotation using the bacterial SILVA database (Release132, for 16S) (Quast et al., 2012) and the UNITE database (Release 7.2, for ITS) (Kõljalg et al., 2013) with the confidence threshold set to default to ≥0.5. Each sample was rarefied to the same number of reads for both alpha-diversity and beta-diversity (PCoA, UniFrac) analyses.
Statistical Analysis
Data of volatile compounds were processed using the SPSS version 20.0 statistical package. Principal Component Analysis (PCA) was performed using MATLAB software (version 7.0; Mathworks Inc., Natick, MA, United States) to recognize the wine with different volatile flavors. Heatmap analyses were performed using the OmicShare tools1. Calculate the Spearman correlation between microorganisms and metabolites (ρ), find the connection with ρ > 0.5 and P < 0.05 as visible object. For co-occurrence between microorganisms analysis, calculate the Spearman coefficient between the microorganisms correlation, and then select | ρ| > 0.5 and P < 0.05 as the interaction object. To characterize the contribution of microorganisms to metabolites and co-occurrence between microbial genera and major metabolites, the correlation networks between the selected flavor metabolites and microbial community were visualized via the Cytoscape software (version 3.7.2).
Results
Microbial Community Structure and Diversity
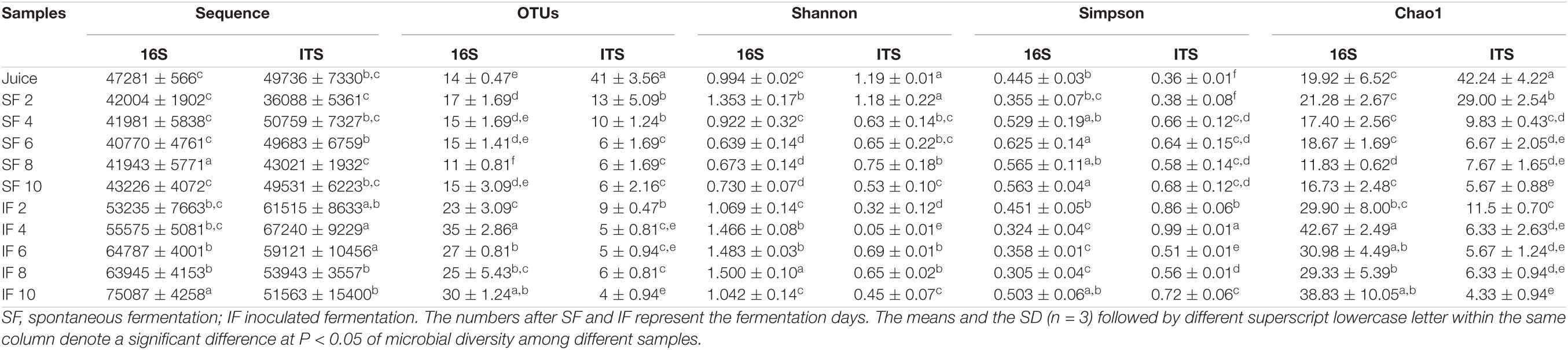
A total of 569,834 and 572,200 sequence reads were obtained from the bacteria and fungi samples, respectively. A total of 57 bacterial OTUs were obtained, and 52 fungal OTUs were observed from all samples on the basis of 97% similarity. The rarefaction curves and Simpson diversity indices for bacteria and fungi are shown in Supplementary Figure 1. Although the rarefaction curves were not parallel to the x-axis, the Simpson diversity indices reached saturation, suggesting that additional phenotypes could be added with additional sequencing. Nevertheless, the great majority of microbial diversity was captured. The various diversity indices, such as Shannon, Simpson, and Chao1, of bacteria and fungi at different fermentation stages are shown in Table 1. The Shannon diversity index of bacteria in SF initially increased and then decreased, whereas that of fungi decreased during SF. In IF, the Shannon diversity index of bacteria increased and then decreased at the end of the process, whereas that of fungi slightly decreased and then increased. The indices revealed that the microbial community structure during tangerine wine fermentation was continuously adjusted, balanced, and stable.
Microbial Community Dynamics During Wine Fermentation
The microorganisms in fermentation broth were found to belong to five bacterial phyla and two identified fungal phyla (Supplementary Figure 2). At the genus level, 13 bacterial genera were identified in tangerine wine fermentation (Figure 1A). In SF, Cyanobacteria, Tatumella, Acetobacter, and Gluconobacter were abundant in the early stage of fermentation, although their abundance gradually decreased as fermentation progressed and Acetobacter became predominant during the middle up to the last stages of fermentation. Lactobacillus was detected on the 4th day of fermentation and became one of the most dominant genera in the latter stage of fermentation. In IF, Cyanobacteria and Tatumella were consistently detected with relatively higher abundance throughout the entire IF than that in SF. Interestingly, the majority of OTUs in the last stage of IF belonged to Lactobacillus, whereas Acetobacter dominated in SF. This result arose likely because of certain wine characteristics, such as the contents of sugar, ethanol, and organic acids. Analyses of both clusters and significant differences revealed a sharp distinction between SF and IF in terms of bacterial communities (Supplementary Figures 3A, 4A). Partial least squares discriminant analysis (PLS-DA) also showed significant differences among the original juice, SF, and IF (Supplementary Figure 5A).
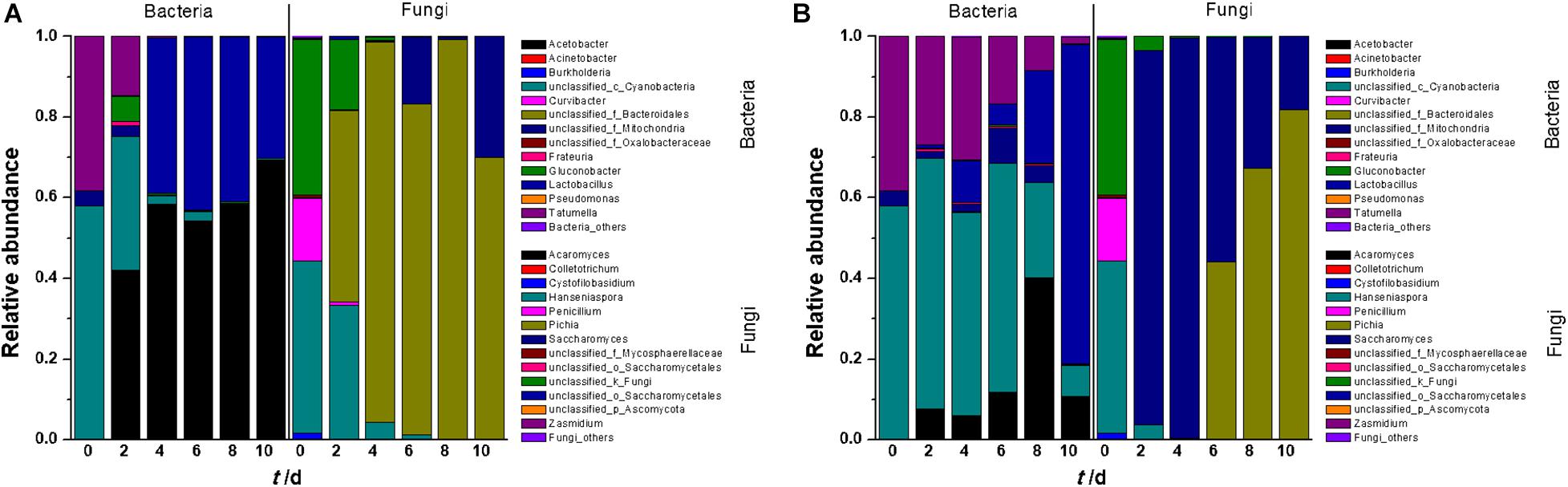
Figure 1. Relative abundance plots of microbial community composition during the entire fermentation period (A: SF; B: IF). Each value was the mean of triplicate samples.
Compared with bacterial diversity, only eight fungal genera were found in tangerine juice (Figure 1B). In SF, Hanseniaspora was the dominant genus in the early stage of fermentation, but its proportion sharply decreased from 43 to 5% during the subsequent stages. Pichia existed almost throughout the entire fermentation process and became the dominant fungus in the broth. Saccharomyces, the inoculant of commercial yeast, dominated the fungal community in the early and middle stages of IF, whereas Hanseniaspora, Pichia, and Penicillium were inhibited during these stages. However, Saccharomyces rapidly declined on day six when Pichia greatly increased and became the dominant genus in the subsequent IF stages. Cluster analysis revealed an overlapping but clear and remarkable difference in fungal communities between SF and IF (Supplementary Figure 3B). Analysis of significant differences showed that SF and IF were obviously different in terms of the fungal communities of Saccharomyces and Hanseniaspora (Supplementary Figure 4B). PLS-DA analysis also demonstrated sharp distinctions in fungal microbiota in juice, SF, and IF (Supplementary Figure 5B). The diversity of fungal communities in juice was greater than that in fermentation broth, indicating that the fungi were sensitive to alcohol fermentation.
General Features of Nanfeng Tangerine Wine Fermentation
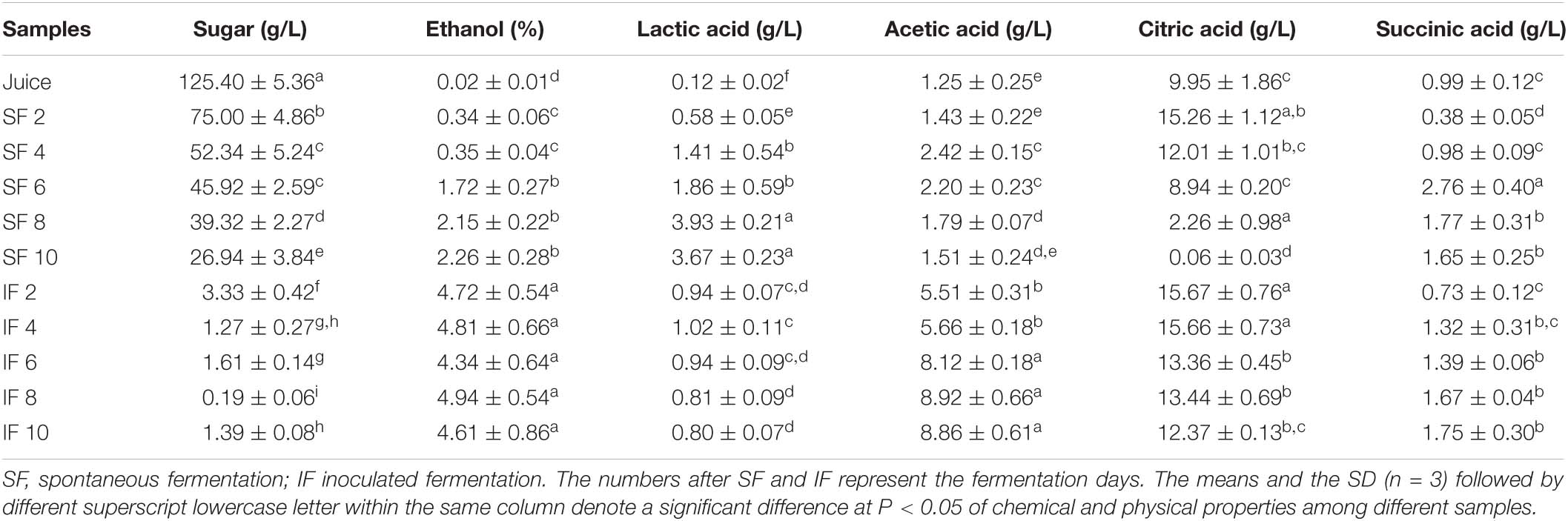
The reducing sugar, alcohol content, pH, and organic acids in the fermentation of Nanfeng tangerine wine were investigated to determine its fermentation characteristics (Table 2). As a sweet fruit variety, the sugar content of tangerine juice can reach up to 125.40 g/L. With the juice as the medium, sugar consumption rate was smaller in SF than that in IF, and the concentration of reducing sugars in the broth were 26.94 and 1.39 g/L, respectively, at the end of fermentation. Consequently, ethanol production in IF (4.61%) was higher than that in SF (2.2%), suggesting that the microbial community in IF which dominated by S. cerevisiae, customarily used for alcoholic wine fermentation, has excellent performance on sugar utilization and alcohol production.
The main organic acids detected in tangerine juice were lactic acid, acetic acid, citric acid, and succinic acid, with a concentration of 0.12, 1.25, 9.95, and 0.99 g/L, respectively (Table 2). In SF, lactic acid concentration gradually increased during fermentation and was higher than that in IF. By contrast, acetic acid production in SF was considerably lower than that in IF during fermentation. The citric acid in both processes accumulated in the early stage of fermentation and then was gradually consumed in the middle and late stages. In particular, the citric acid in SF was almost depleted by the end of fermentation. Succinic acid was continuously consumed and synthesized during fermentation. In IF broth, lactic acid concentration slightly increased in the early stage of fermentation (0–4 days) and then slightly decreased in subsequent stages, possibly because of the esterification of some of the lactic acid during fermentation. Citric acid and succinic acid were maintained at relatively stable concentrations in IF because they are normal fermentation products of alcohol fermentation. In general, IF wine had higher organic acid content than SF probably because of the presence of commercial yeast in IF, which has a high rate of glucose metabolism and leads to accumulation of organic acids.
Flavor Evolution in Fermentation
Tangerine wine fermentation can produce large amounts of volatile aromatic compounds, such as alkanes, alkenes, esters, alcohols, and acids. The main aromatic components of Nanfeng tangerine juice were alkenes and alkanes, including 3-decene, α-phellandrene, terpinene, 1,3-cyclohexadiene, d-limonene, cyclotetrasiloxane, and small amounts of esters and alcohols, such as α-ethyl aspartate, methyl nitrate, and α-methyl-benzeneethanol (Figure 2).
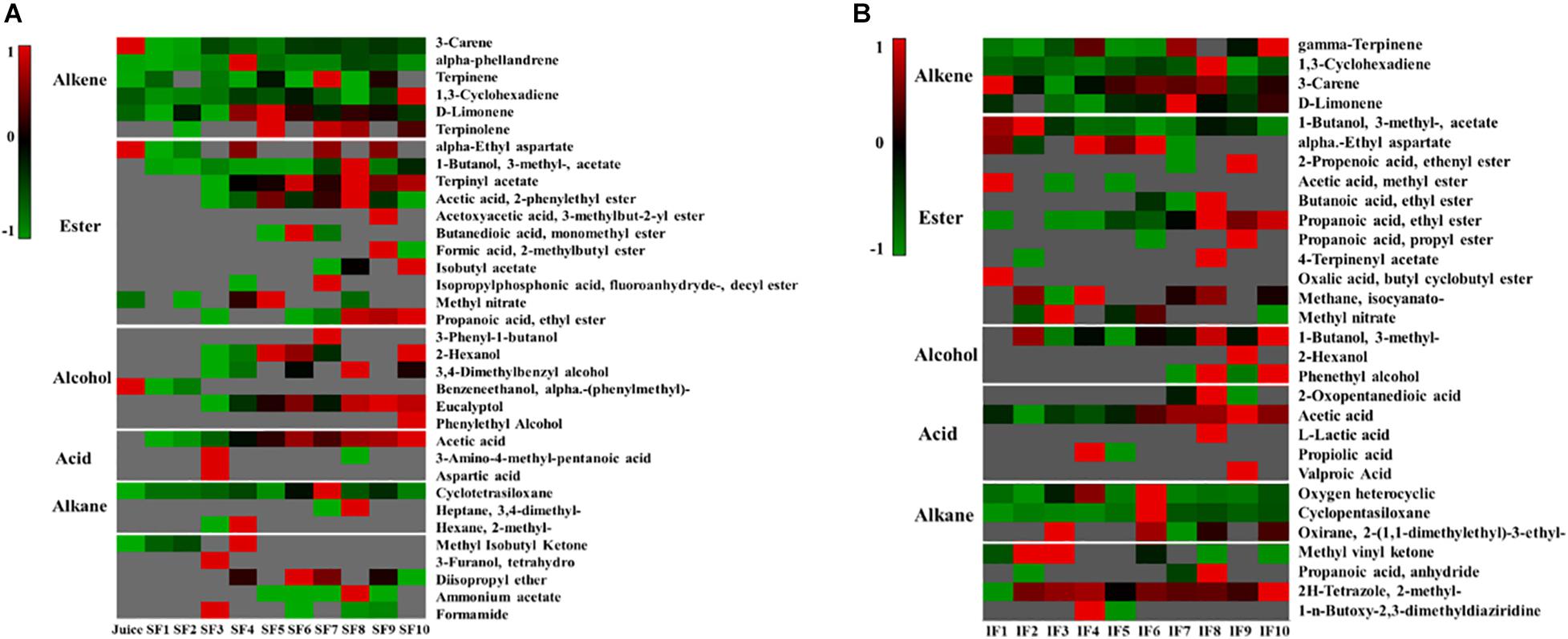
Figure 2. Heatmap of change in metabolic profiling during the fermentation process (A: SF; B: IF). Clustering analysis was performed using Pearson correlation and Euclidean distance based on the relative content of metabolites during the fermentation process. Data were processed by z-score transformation.
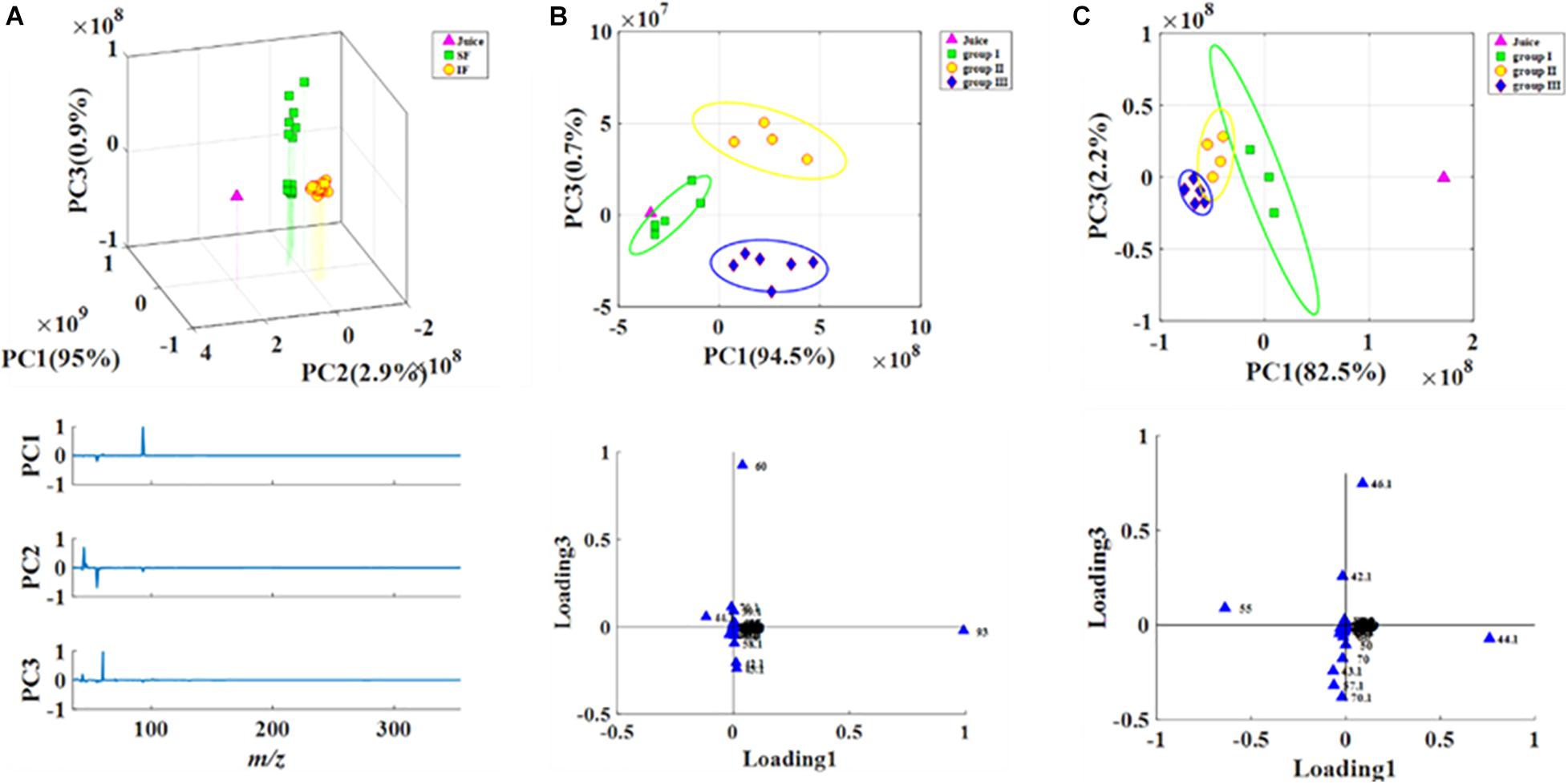
Headspace-gas chromatography-mass spectrometry was performed to evaluate the metabolic profiles of the volatile compounds of SF and IF. PCA illustrated that the samples collected from SF and IF formed a group in the negative quadrants of PC1, PC2, and PC3. Three PCs (PC1 eigenvalue, 95%; PC2 eigenvalue, 2.9%; PC3 eigenvalue, 0.9%) representing about 98.8% of the total variance (Figure 3A) were selected for modeling. SF samples were more scattered than IF samples, indicating that the volatile compounds of SF were more variable than those of IF. The primary characteristic substances in both SF and IF were acetic acid, isoamyl acetate, and terpinyl acetate. In SF, these samples could be divided into three groups (group I: SF 1–3 days; group II: SF 4–7 days; group III: SF 8–10 days) according to their compounds’ loadings on the axes (Figure 3B). Each group was completely separated. In the early stage of fermentation (group I), small amounts of acids and esters, such as acetic acid, 3-amino-4-methyl-pentanoic acid, aspartic acid, 1-butanol 3-methyl-acetate, and terpinyl acetate, were formed. As the fermentation progressed, several alcohols, including 2-hexanol, eucalyptol, and 3,4-dimethylbenzyl alcohol, were synthesized. The content of acetic acid and terpinyl acetate gradually increased, and certain esters, such as acetic acid 2-phenylethyl ester, butanedioic acid monomethyl ester, and propanoic acid ethyl ester, were formed. The contents of aromatic compounds 1-butanol 3-methyl-acetate, acetic acid, eucalyptol, and terpinyl acetate gradually increased in the late stage of SF, whereas certain esters, such as formic 2-methylbutyl ester, isobutyl acetate, and propanoic acid ethyl ester, were produced. According to the corresponding loading plots, the primary substances that substantially influenced the changes in groups were terpinyl acetate, acetic acid, 1-butanol, and 3-methyl-acetate (Figure 3B). Results showed that the content of acetic acid in SF wine was comparatively high and thus negatively affected the quality of wine.
IF was also divided into three groups (group I: IF 1–2 days; group II: IF 3–6 days; group III: IF 7–10 days) (Figure 3C). In the early stage of IF, various alcohols and esters, such as 1-butanol 3-methyl-acetate, acetic acid methyl ester, propanoic acid ethyl ester, isocyanato-methane, and ethyl nitrate, were synthesized. In the middle stage, the content of acetic acid increased, whereas that of acetic acid and methyl ester decreased. Moreover, the traditional aromatic components of IF in the middle and late stages of fermentation were quite similar according to principal component plots. Small amounts of phenethyl alcohol formed in the late stage of fermentation.
Correlation Between Microbial Communities and Metabolites
Mantel test was performed to assess the significance of Spearman’s correlation on the basis of bacterial and fungal communities and metabolites and thus elucidate the relationships between these elements. The bacteria in wine fermentation were significantly positively correlated with ethanol, acetic acid, and citric acid but negatively correlated with sugar content (Figure 4A). Acetobacter was negatively associated with citric acid but positively correlated with lactic acid. Lactobacillus was positively correlated to acetic acid and lactic acid. Except for Saccharomyces and Pichia, the fungi had a significantly positive correlation with sugar. Furthermore, most of the fungi were negatively correlated with ethanol, lactic acid, and acetic acid (Figure 4B). However, Saccharomyces was significantly positively correlated with ethanol. Hanseniaspora was significantly negatively correlated with ethanol, lactic acid, acetic acid, and succinic acid but significantly positively correlated with sugar. Penicillium was significantly negatively correlated with ethanol and acetic acid but positively correlated with sugar; however, this correlation gradually diminished as fermentation progressed. Saccharomyces was significantly positively correlated with ethanol, acetic acid, and citric acid. Pichia was significantly positively correlated with lactic acid but significantly negatively correlated with citric acid.
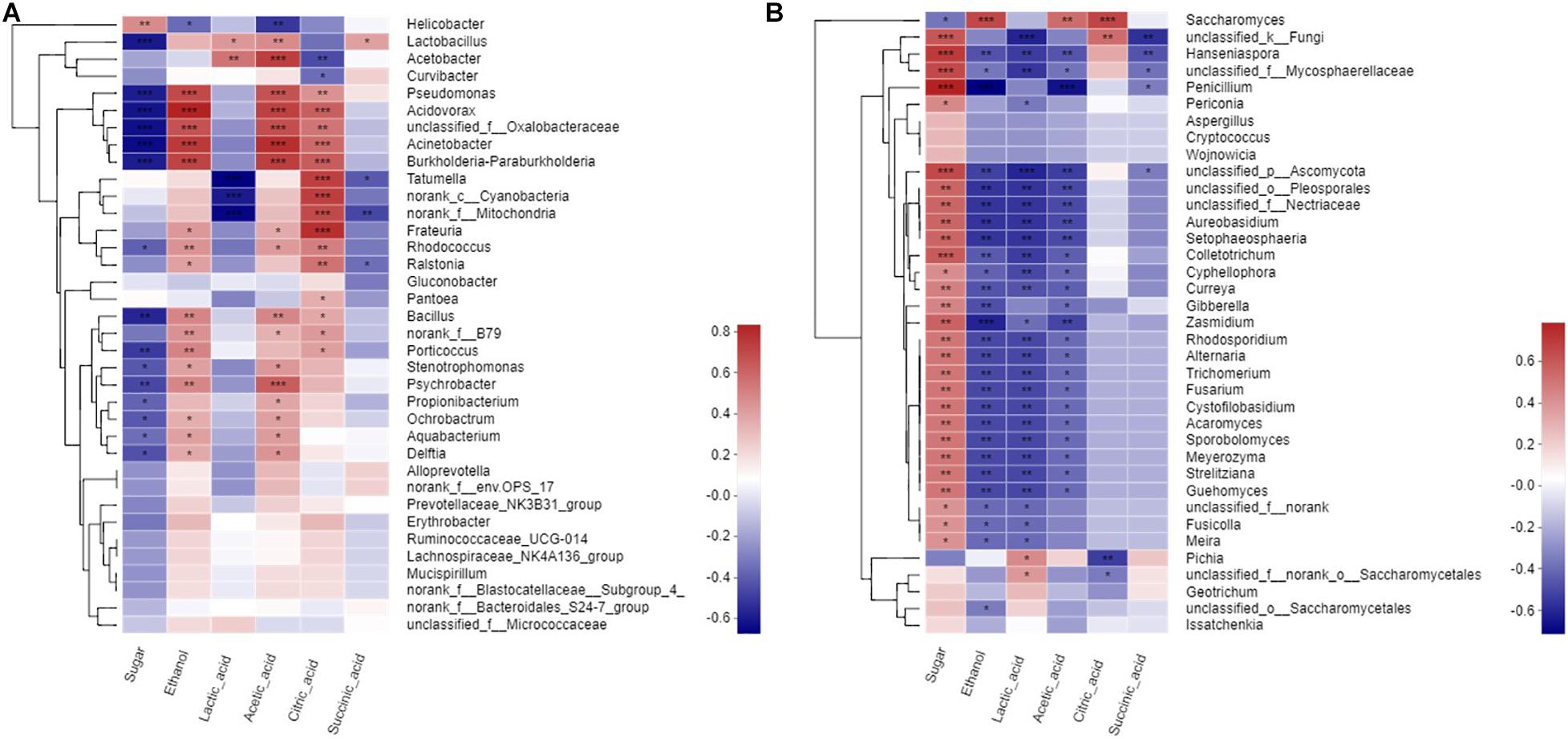
Figure 4. Spearman corelation heatmap of metabolites to microbial. (A) a prokaryotic community, (B) eukaryotic community at genus level.
Spearman correlation and diversity analyses were combined to explore the positive and negative correlations between the core microbiota and volatile compounds (Figure 5); | ρ| > 0.5 was selected as the interaction object. In general, a significant and robust relationship (edge) was established between 18 genera (nodes) and 34 metabolites (P < 0.05). Lactobacillus, Acetobacter, Hanseniaspora, and Saccharomyces were the largest contributors to the production of the volatile flavor of Nanfeng tangerine wine. At the genus level, the bacteria Lactobacillus, Acetobacter, and Tatumella and the fungi Hanseniaspora, Saccharomyces, and Pichia were identified as the core functional microbiota for the production of the volatile flavor of Nanfeng tangerine wine.
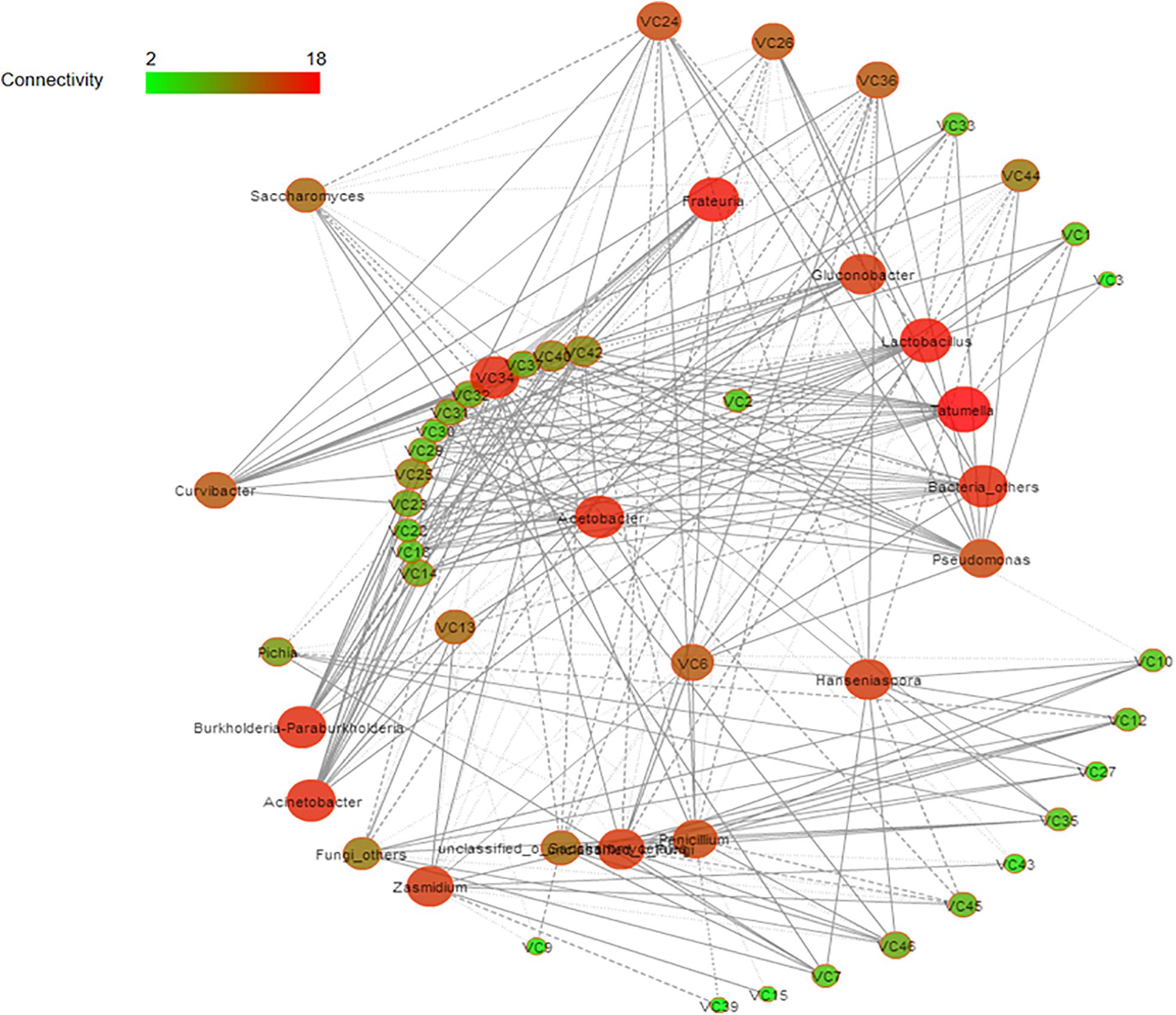
Figure 5. Correlation network of co-occurring microbial genera in fermented grains. Statistically significant (P < 0.05) Spearman correlation coefficient (| ρ| > 0.5) indicates the robust correlations. The size of nodes indicates the degree of connections. The same color of nodes indicates the same order. Edge thickness represents the proportional to the value of Spearman’s correlation.
Spearman analysis identified Lactobacillus as the core microbe that enhances the production of volatile flavor. Lactobacillus was strongly positively associated with esters, including terpinyl acetate, methyl nitrate, and acetic acid 2-phenylethyl ester. Acetobacter was significantly positively correlated with isobutyl acetate, 1-butanol 3-methyl-acetate, propanoic acid ethyl ester, as well as with acids and alcohols, such as acetic acid, eucalyptol, 3,4-dimethylbenzyl alcohol, and 2-hexanol. Hanseniaspora was correlated with esters, such as methyl nitrate, butanoic acid ethyl ester, terpinenyl acetate, and propanoic acid ethyl ester. Furthermore, Saccharomyces was positively correlated with terpinyl acetate, 1-butanol, 3- methyl-, and acetic acid. However, Pichia had partially contributed to the volatile flavor of Nanfeng tangerine wine and was mainly related to butanoic acid ethyl ester, acetic acid, and terpinyl acetate.
Discussion
Fruits wines are characterized by peculiar aromatic notes, antioxidant potential, phenolic composition, alcohol content, and other parameters. Using tangerine juice as a raw material to brew a unique flavor of fruit wine could help to reduce postharvest and production losses of tangerine. Some efforts have been made to optimize the fermentation process and use Non-Saccharomyces to improve the quality of tangerine wine (Liao and Wang, 2014; Chen et al., 2015; Yu et al., 2019). As the basic processes of fruit wines are similar to grape juice production and fermentation, both endogenous and exogenous microorganisms are involved in the process of fruit wine fermentation. Understanding the diversity and evolution of microorganisms during fruit wine fermentation is essential for controlling its production. Recent studies focused on the microbial composition and diversity during the wine fermentation based on culture-dependent methods (Ghosh et al., 2015; Liu R. et al., 2015) or culture-independent methods (Portillo and Mas, 2016; Tapsoba et al., 2016; Wei et al., 2018). These diverse microbial populations and their potential interplay in the different stages of alcoholic fermentation constitute a complex biological process that contributes largely to the final chemical composition (Gammacurta et al., 2017) of wine and, therefore, to its sensory properties (Hu et al., 2020). SF and IF are the main fermentation methods employed in producing wine. Analysis obtained from spontaneous and IFs has shown significant differences in the chemical composition and sensory properties of wine (Blanco et al., 2008). Exploring the microbial diversity and dynamics in different fermentation methods of wine is essential both to understand the role of core functional microbiota in the process, and to select autochthonous starter strains that are potentially able to contribute wines with unique regional characteristics, avoiding the risks associated with SFs (Knight et al., 2015; Capozzi et al., 2020). In this study, we systematically investigated the dynamics of microbial communities and metabolites during SF and IF. To enhance our understanding of the microbial dynamics during tangerine fermentation, we employed 16S rRNA gene and ITS amplicon sequencing (Benjamin et al., 2014) and determined the changes in bacterial and fungal communities during fermentation. The ITS sequence is more suitable for the analysis of fungal communities because its highly variable regions are more taxonomically informative than 18S rDNA variable regions.
Diversity indices indicated that the diversity of bacterial communities in IF was more abundant than that in SF. In SF, the diversity of bacterial communities initially increased and then decreased presumably because the high content of nutrients in the juice was suitable for bacterial growth. By contrast, the environmental conditions during the late fermentation stage and yeast growth had a certain inhibitory effect on bacterial growth. In IF, bacterial diversity remained stable, suggesting that the bacteria were able to tolerate the highly acidic and highly ethanolic fermentation environment. The diversity of bacteria in IF was more abundant than that in SF. In terms of bacterial communities, 13 genera were identified during tangerine wine fermentation. Acetobacter, Lactobacillus Gluconobacter, and Cyanobacteria are frequently detected in wine (Liu S. et al., 2015; Piao et al., 2015; Chen et al., 2017). The high abundance of Lactobacillus in IF might be related to the fact that, in wine, S. cerevisiae inoculation can modulate the bacterial consortium, improving the concentration of protechnological bacteria (Berbegal et al., 2019). Acetobacter is moderately resistant to alcohol; thus, this bacterium was detected in the late stages of fermentation. Although Acetobacter has been identified as a wine spoilage bacterium and is rarely observed in wine, several studies also detected Acetobacter during wine fermentation, suggesting that the population of acetic acid bacteria might have often been underestimated via culture-dependent methods because of the lack of appropriate cultivation techniques (Millet and Lonvaud-Funel, 2010). Lactobacillus, which can convert malic acid to lactic acid and enhance the taste and sensory quality of wine (Gammacurta et al., 2018), was the dominant bacterium in SF wine. Lactic acid has a buffering effect on blending wine taste, and it can be synthesized as a series of lactate esters with alcohol to enrich the esters in wine (Liang et al., 2015). The observed increase in Lactobacillus abundance at the late stage of IF could be expected to generate the appropriate conditions for subsequent malolactic fermentation (Portillo and Mas, 2016). Gluconobacter, as a wine spoilage bacterium, is often found in grapes and fruit surfaces. Gluconobacter preferentially utilizes sugar as a carbon source, whereas Acetobacter prioritizes the use of alcohol; hence, the latter is more resistant to alcohol than the former, an observation reported in previous studies (Piao et al., 2015). This fact may explain why the abundance of Gluconobacter detected in SF wine was higher than that in IF wine. A comparison of the dynamics of bacterial communities in SF and IF that the abundance of Cyanobacteria and Tatumella was significantly higher in the middle stage of IF than that at the same stage in SF probably because these bacterial genera were inhibited by Acetobacter.
The diversity of fungal communities in both SF and IF decreased with the progress of fermentation suggesting that the fungi involved in brewing might not be adaptable to the highly acidic and highly ethanolic fermentation environment. Harsh conditions reportedly inhibit fungal communities until they become adapted to, or even tolerant of, the harsh fermentation environment (Wang and Liu, 2013). The most abundant fungal genera during SF were Hanseniaspora, Pichia, and Saccharomyces with a clear succession during SF, whereas those during IF were Saccharomyces, Hanseniaspora, and Pichia. These fungal genera have been frequently isolated in wine fermentation (Liu R. et al., 2015). The abundance of Hanseniaspora gradually decreased during fermentation because it is sensitive to ethanol, as was observed in previous studies (Moreira et al., 2011; Raymond et al., 2017). The major difference between SF and IF of Nanfeng tangerine wine employed in this study was the addition of commercial yeast; thus, Saccharomyces dominated at the pre-fermentation stage of IF. Interestingly, Pichia began to appear on the 6th day of IF and gradually dominated in the late fermentation stage. This result was somewhat unexpected because Pichia is moderately resistant to alcohol and usually detected in the middle stage of fermentation (Portillo and Mas, 2016). Some Pichia species in Nanfeng tangerine wine probably had a better ethanol tolerance than Saccharomyces or they were more adaptable to the environment of the late fermentation period. This finding was similar to that of several studies that reported that some strains of P. pastoris have better ethanol tolerance than Saccharomyces (Benito et al., 2011; Portillo and Mas, 2016). Saccharomyces became predominant at the middle and late stages of fermentation because of its efficient fermentation catabolism and ethanol tolerance and highly competitive ability (Wang and Liu, 2013; Huang et al., 2018). In this study, Saccharomyces was detected in the late stage of SF unlike that in previous studies. Previous studies argued that Saccharomyces rarely appears on the surface of berries and hardly exists in citrus wine SF (Barata et al., 2012; Liu R. et al., 2015). As important generators of various secondary metabolites, the other fungal genera were dominant in the pre-fermentation stage of Nanfeng tangerine wine, implying that these genera can be used to produce large amounts of flavor substances and thus improve wine quality.
Wines produced via IF and SF had a final ethanol concentration of 2.2 and 4.61%, respectively. The content of residual sugar in IF (1.39 g/L) was considerably lower than that in SF (26.94 g/L). These results suggested that IF can generate Nanfeng tangerine wines with a higher alcohol level and a lower residual sugar level than SF. The total acid content was higher in IF than in SF; the contents of acetic acid and citric acid were substantially higher in IF than those in SF, whereas that of lactic acid was more abundant in SF than that in IF likely because of the complex microbial activities involved in fermentation (Cai et al., 2018). In this work, Mantel test was performed to assess the significance of Spearman’s correlation on the basis of bacterial and fungal communities and metabolites. Results showed that the bacteria were significantly positively correlated with citric acid, ethanol, and acetic acid but negatively correlated with sugar content. In addition, acetic acid and lactic acid production was correlated with Lactobacillus, and the increase in acidity was apparently correlated with the abundance of Acetobacter, a result that was consistent with that of previous studies (Papalexandratou et al., 2013; Tapsoba et al., 2016; Gammacurta et al., 2018). Correlation analysis revealed that Acetobacter was strongly correlated with acetic acid possibly because the pathway of acetic acid production was enhanced by Acetobacter in Nanfeng tangerine wine fermentation. Except for Saccharomyces, almost all of the fungi were negatively correlated with ethanol. This observation can explain the fact that yeast succession in wine concerning other fungal genera is followed by S. cerevisiae after the middle stage of fermentation (Zhang et al., 2017; Cai et al., 2018). Saccharomyces was significantly positively correlated with ethanol, acetic acid, and citric acid, a finding that explained why the contents of ethanol, acetic acid, and citric acid were substantially higher in IF than in SF. Pichia was the main contributor to lactic acid and significantly negatively correlated with citric acid. Previous studies reported that some Pichia species, such as P. kudriavzevii, can degrade L-malic acid, increase pH, and produce low levels of ethanol and acceptable amounts of acetic acid (Mónaco et al., 2014).
In the present study, 34 and 36 volatile compounds were detected in SF and IF, respectively. PCA showed significant differences between SF and IF. The wine produced via SF had distinct aromatic profiles unlike that produced via IF. SF wine was characterized by abundant esters and high amounts of volatile acids, whereas IF wine contained high amounts of alcohols, such as isoamyl alcohol. The content of acetic acid in SF wine was relatively higher than that in IF wine that had adverse effect on quality. In addition, SF wine contained high amounts of esters, including terpinyl acetate, 1-butanol 3-methyl-acetate, acetic acid 2-phenylethyl ester, butanedioic acid monomethyl ester, propanoic acid ethyl ester, formic acid 2-methylbutyl ester, and isobutyl acetate. These esters impart a unique fruit aroma to wines, and they play an important role in the formation of the overall aromatic profile of tangerine wine. Small amounts of isoamyl alcohol, which is an important flavor in wine, can impart an elegant aroma to wines. However, amounts of this alcohol beyond the sensory threshold adversely affect the flavor of wines and may even cause poisoning. Deficiency in volatile aroma is one of the limiting factors of wine production. The volatile aromatic profiles of fruit wines can be improved by inoculating the fermentation with Non-Saccharomyces yeast.
The relationship between microbiota succession and volatile flavor dynamics during SF and IF of Nanfeng tangerine wine remains unclear. The core functional microbiota responsible for the production of volatile flavor can be explored by considering both dominance and functionality. In this study, a significant and robust relationship (edge) was established between 18 genera (nodes) and 34 metabolites (P < 0.05). Lactobacillus, Acetobacter, Hanseniaspora, and Saccharomyces were the largest contributors to the production of the volatile flavor of Nanfeng tangerine wine. Lactobacillus became the predominant bacterium in wine and it was the major producer of lactic acid and acetic acid under strict anaerobic conditions during wine fermentation (Song et al., 2017). Strict anaerobic conditions ensure the stability of wines and improve their aromas and flavors by enhancing ester production (Wang et al., 2014; Song et al., 2017; Gammacurta et al., 2018; Xu et al., 2018). Hanseniaspora was the main contributor to some esters (Tufariello et al., 2021), a finding that explained why the esters in IF wine were poor; the wild yeasts in Nanfeng tangerine likely had difficulty competing against the commercial yeast. Aside from Saccharomyces, the other indigenous fungal genera could adapt well to the local fermentation conditions and substantially contribute to the characters of specific regional aroma. Nonetheless, the actual contribution of yeasts other than Saccharomyces to the final volatile flavor of Nanfeng tangerine wine requires further study. Moreover, the mixed culture of Saccharomyces and other fungal genera is a valuable tool for modulating the volatile profiles and improving the aromatic complexity of wine (Liu et al., 2016). Therefore, further research is warranted to highlight the beneficial enological traits of non-Saccharomyces yeasts. This end may be achieved via sequential co-fermentation with S. cerevisiae or by changing the inoculation time and ratio of non-Saccharomyces yeasts.
Conclusion
Spontaneous fermentation could improve the aromatic profiles of Nanfeng tangerine wine. Non-Saccharomyces yeasts and Lactobacillus played an important role in determining the aromatic and flavor profile of wine produced via SF by producing abundant esters and high amounts of volatile acids. The results of the present study can be used to develop effective strategies for selecting beneficial microorganism to improve the quality of Nanfeng tangerine wine.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: China National GeneBank DataBase (CNGBdb) (https://db.cngb.org/), accession number CNP0001643.
Author Contributions
XQ: conceptualization, methodology, validation, investigation, and writing – original draft. LY: investigation, methodology, validation, and writing – review and editing. WW: data curation and software. RY: conceptualization, writing – review and editing, visualization, project administration, and funding acquisition. ZZ: investigation and methodology. HY: investigation. DZ: conceptualization, methodology, project administration, and funding acquisition. BZ: resources. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31760021) and the Key Research Plan of the Jiangxi Province Science Foundation (Grant No. 20171ACF60007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.649978/full#supplementary-material
Footnotes
References
Astudillo-García, C., Bell, J. J., Webster, N. S., Glasl, B., Jompa, J., Montoya, J. M., et al. (2017). Evaluating the core microbiota in complex communities: a systematic investigation. Environ. Microbiol. 19, 1450–1462. doi: 10.1111/1462-2920.13647
Barata, A., Malfeito-Ferreira, M., and Loureiro, V. (2012). The microbial ecology of wine grape berries. Int. J. Food Microbiol. 153, 243–259. doi: 10.1016/j.ijfoodmicro.2011.11.025
Benito, S., Morata, A., Palomero, F., González, M. C., and Suárez-Lepe, J. A. (2011). Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chem. 124, 15–23. doi: 10.1016/j.foodchem.2010.05.096
Benjamin, E. W., Julie, E. B., Marcela, S., and Rachel, J. D. (2014). Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 158, 422–433. doi: 10.1016/j.cell.2014.05.041
Berbegal, C., Borruso, L., Fragasso, M., Tufariello, M., Russo, P., Brusetti, L., et al. (2019). A metagenomic-based approach for the characterization of bacterial diversity associated with spontaneous malolactic fermentations in wine. Int. J. Mol. Sci. 20:3980. doi: 10.3390/ijms20163980
Blanco, P., Vázquez-Alén, M., and Losada, A. (2008). Influence of yeast population on characteristics of the wine obtained in spontaneous and inoculated fermentations of must from Vitis vinifera Lado. J. Industr. Microbiol. Biotechnol. 35, 183–188. doi: 10.1007/s10295-007-0282-9
Cagno, R. D., Pontonio, E., Buchin, S., Angelis, M. D., Lattanzi, A., Valerio, F., et al. (2014). Diversity of the lactic acid bacterium and yeast microbiota in the switch from firm- to liquid-sourdough fermentation. Appl. Environ. Microbiol. 80, 3161–3172. doi: 10.1128/AEM.00309-14
Cai, H., Zhang, T., Zhang, Q., Luo, J., Cai, C., and Mao, J. (2018). Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol. 73, 319–326. doi: 10.1016/j.fm.2018.02.002
Capozzi, V., Fragasso, M., and Russo, P. (2020). Microbiological safety and the management of microbial resources in artisanal foods and beverages: the need for a transdisciplinary assessment to conciliate actual trends and risks avoidance. Microorganisms 8:306. doi: 10.3390/microorganisms8020306
Chanprasartsuk, O.-o., Prakitchaiwattana, C., Sanguandeekul, R., and Fleet, G. H. (2010). Autochthonous yeasts associated with mature pineapple fruits, freshly crushed juice and their ferments; and the chemical changes during natural fermentation. Bioresour. Technol. 101, 7500–7509. doi: 10.1016/j.biortech.2010.04.047
Chen, G., Chen, C., and Lei, Z. (2017). Meta-omics insights in the microbial community profiling and functional characterization of fermented foods. Trends Food Sci. Technol. 65, 23–31. doi: 10.1016/j.tifs.2017.05.002
Chen, Q., Jian, Q., Wang, J., and Sun, A. (2015). Fermentation technology of tangerine fruit wine. China Brew. 34, 168–171. doi: 10.11882/j.issn.0254-5071.2015.01.039
Cocolin, L., Alessandria, V., Dolci, P., Gorra, R., and Rantsiou, K. (2013). Culture independent methods to assess the diversity and dynamics of microbiota during food fermentation. Int. J. Food Microbiol. 167, 29–43. doi: 10.1016/j.ijfoodmicro.2013.05.008
Djeni, T. N., Kouame, K. H., Ake, F. D. M., Amoikon, L. S. T., Dje, M. K., and Jeyaram, K. (2020). Microbial diversity and metabolite profiles of palm wine produced from three different palm tree species in Côte d’ivoire. Sci. Rep. 10:1715. doi: 10.1038/s41598-020-58587-2
Gammacurta, M., Lytra, G., Marchal, A., Marchand, S., Barbe, J. C., Moine, V., et al. (2018). Influence of lactic acid bacteria strains on ester concentrations in red wines: specific impact on branched hydroxylated compounds. Food Chem. 239, 252–259. doi: 10.1016/j.foodchem.2017.06.123
Gammacurta, M., Marchand, S., Moine, V., and de Revel, G. (2017). Influence of different yeast/lactic acid bacteria combinations on the aromatic profile of red Bordeaux wine. J. Sci. Food Agricult. 97, 4046–4057. doi: 10.1002/jsfa.8272
Ghosh, S., Bagheri, B., Morgan, H. H., Divol, B., and Setati, M. E. (2015). Assessment of wine microbial diversity using ARISA and cultivation-based methods. Annal. Microbiol. 65, 1833–1840. doi: 10.1007/s13213-014-1021-x
Hu, L., Liu, R., Wang, X., and Zhang, X. (2020). The sensory quality improvement of citrus wine through co-fermentations with selected Non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 8:323. doi: 10.3390/microorganisms8030323
Huang, Z., Hong, J., Xu, J., Li, L., Guo, W., Pan, Y., et al. (2018). Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 76, 487–496. doi: 10.1016/j.fm.2018.07.014
Joshi, V. K., Panesar, P. S., Rana, V. S., and Kaur, S. (2017). “Chapter 1 – Science and technology of fruit wines: an overview,” in Science and Technology of Fruit Wine Production, eds M. R. Kosseva, V. K. Joshi, and P. S. Panesar, (San Diego, CA: Academic Press), 1–72.
Kahramanolu, B., Chen, C., Chen, Y., Chen, J., and Wan, C. (2020). Improving storability of “Nanfeng” mandarins by treating with postharvest hot water dipping. J. Food Qual. 2020, 1–12. doi: 10.1155/2020/8524952
Knight, S., Klaere, S., Fedrizzi, B., and Goddard, M. R. (2015). Regional microbial signatures positively correlate with differential wine phenotypes: evidence for a microbial aspect to terroir. Sci. Rep. 5:14233. doi: 10.1038/srep14233
Kõljalg, U., Nilsson, R. H., Abarenkov, K., Tedersoo, L., Taylor, A. F. S., Bahram, M., et al. (2013). Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277. doi: 10.1111/mec.12481
Kong, Y., Wu, Q., Zhang, Y., and Xu, Y. (2014). In situ analysis of metabolic characteristics reveals the key yeast in the spontaneous and solid-state fermentation process of Chinese light-style liquor. Appl. Environ. Microbiol. 80, 3667–3676. doi: 10.1128/AEM.04219-13
Lai, Q., Wang, X., Wang, C., and Zheng, S. (2017). Current processing and utilization situation of fruit peels and research progress. J. Food Saf. Qual. 8, 876–881. doi: 10.19812/j.cnki.jfsq11-5956/ts.2017.03.027
Liang, H., Li, W., Luo, Q., Liu, C., Wu, Z., and Zhang, W. (2015). Analysis of the bacterial community in aged and aging pit mud of Chinese Luzhou-flavour liquor by combined PCR-DGGE and quantitative PCR assay. J. Sci. Food Agric. 95, 2729–2735. doi: 10.1002/jsfa.7013
Liao, L., and Wang, S. (2014). Brewing technology of mixed fruit wine. China Brew. 33, 157–160. doi: 10.11882/j.issn.0254-5071.2014.07.036
Liu, P., Lu, L., Duan, C., and Yan, G. (2016). The contribution of indigenous non- Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT Food Sci. Technol. 71, 356–363. doi: 10.1016/j.lwt.2016.04.031
Liu, R., Zhang, Q., Chen, F., and Zhang, X. (2015). Analysis of culturable yeast diversity in spontaneously fermented orange wine, orange peel and orangery soil of a Ponkan plantation in China. Annal. Microbiol. 65, 2387–2391. doi: 10.1007/s13213-015-1081-6
Liu, S., Laaksonen, O., Kortesniemi, M., Kalpio, M., and Yang, B. (2018). Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and SchizoSaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 266, 262–274. doi: 10.1016/j.foodchem.2018.06.003
Liu, S., Mao, J., Liu, Y., Meng, X., Ji, Z., Zhou, Z., et al. (2015). Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World J. Microbiol. Biotechnol. 31, 1907–1921. doi: 10.1007/s11274-015-1931-1
Lu, Z. M., Liu, N., Wang, L. J., Wu, L. H., Gong, J. S., Yu, Y. J., et al. (2016). Elucidating and regulating the acetoin-producing role of microbial functional group in multispecies acetic acid fermentation. Appl. Environ. Microbiol. 82, 5860–5868. doi: 10.1128/AEM.01331-16
Millet, V., and Lonvaud-Funel, A. (2010). The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 30, 136–141. doi: 10.1046/j.1472-765x.2000.00684.x
Mónaco, S. M., Del Barda, N. B., Rubio, N. C., and Caballero, A. C. (2014). Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 117, 451–464. doi: 10.1111/jam.12547
Moreira, N., Pina, C., Mendes, F., Couto, J. A., Hogg, T., and Vasconcelos, I. (2011). Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control 22, 662–667. doi: 10.1016/j.foodcont.2010.07.025
Morrison-Whittle, P., and Goddard, M. (2018). From vineyard to winery: a source map of microbial diversity driving wine fermentation. Environ. Microbiol. 20, 75–84. doi: 10.1111/1462-2920.13960
Nicholas, B., Thorngate, J., Richardson, P., and Mills, D. A. (2014). Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. U.S.A. 111, E139–E148. doi: 10.1073/pnas.1317377110
Papagianni, M. (2014). Recent advances in solid-state fermentation applications for the food industry. Curr. Biochem. Eng. 1, 2–8. doi: 10.1002/chin.201442298
Papalexandratou, Z., Lefeber, T., Bahrim, B., Lee, O. S., Daniel, H. M., and Vuyst, L. D. (2013). Hanseniaspora opuntiae, Saccharomyces cerevisiae, Lactobacillus fermentum, and Acetobacter pasteurianus predominate during well-performed Malaysian cocoa bean box fermentations, underlining the importance of these microbial species for a successful cocoa bean fermentation process. Food Microbiol. 35, 73–85. doi: 10.1016/j.fm.2013.02.015
Piao, H., Hawley, E., Kopf, S., Descenzo, R., Sealock, S., Henick-Kling, T., et al. (2015). Insights into the bacterial community and its temporal succession during the fermentation of wine grapes. Front. Microbiol. 6:809. doi: 10.3389/fmicb.2015.00809
Portillo, M. D. C., and Mas, A. (2016). Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high-throughput barcoding sequencing. LWT Food Sci. Technol. 72, 317–321. doi: 10.1016/j.lwt.2016.05.009
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., and Glckner, F. O. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596. doi: 10.1093/nar/gks1219
Raymond, E. M. L., Reynoso, C., Lauret, S. C., and Rosa, A. L. (2017). Isolation and Identification of the indigenous yeast population during spontaneous fermentation of isabella (Vitis labrusca L.) grape must. Front. Microbiol. 8:532. doi: 10.3389/fmicb.2017.00532
Sinclair, L., Osman, O. A., Bertilsson, S., and Eiler, A. (2015). Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the illumina platform. PLoS One 10:e0116955. doi: 10.1371/journal.pone.0116955
Song, Z., Du, H., Zhang, Y., and Xu, Y. (2017). Unraveling core functional microbiota in traditional solid-state fermentation by high-throughput amplicons and Metatranscriptomics sequencing. Front. Microbiol. 8:1294. doi: 10.3389/fmicb.2017.01294
Tapsoba, F., Savadogo, A., Legras, J. L., Zongo, C., and Traore, A. S. (2016). Microbial diversity and biochemical characteristics of Borassus akeassii wine. Lett. Appl. Microbiol. 63, 297–306. doi: 10.1111/lam.12619
Tufariello, M., Fragasso, M., Pico, J., Panighel, A., Castellarin, S. D., Flamini, R., et al. (2021). Influence of Non-Saccharomyces on wine chemistry: a focus on aroma-related compounds. Molecules 26:644. doi: 10.3390/molecules26030644
Wang, C., and Liu, Y. (2013). Dynamic study of yeast species and Saccharomyces cerevisiae strains during the spontaneous fermentations of Muscat blanc in Jingyang, China. Food Microbiol. 33, 172–177. doi: 10.1016/j.fm.2012.09.014
Wang, P., Mao, J., Meng, X., Li, X., Liu, Y., and Feng, H. (2014). Changes in flavour characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control 44, 58–63. doi: 10.1016/j.foodcont.2014.03.018
Wei, Y. J., Wu, Y., Yan, Y. Z., Zou, W., Xue, J., Ma, W. R., et al. (2018). High-throughput sequencing of microbial community diversity in soil, grapes, leaves, grape juice and wine of grapevine from China. PLoS One 13:e0193097. doi: 10.1371/journal.pone.0193097
Wu, Q., Chen, L. Q., and Xu, Y. (2013). Yeast community associated with the solid state fermentation of traditional Chinese Maotai-flavor liquor. Int. J. Food Microbiol. 166, 323–330. doi: 10.1016/j.ijfoodmicro.2013.07.003
Xu, J., Wu, H., Wang, Z., Zheng, F., Lu, X., Li, Z., et al. (2018). Microbial dynamics and metabolite changes in Chinese Rice Wine fermentation from sorghum with different tannin content. Sci. Rep. 8:4639. doi: 10.1038/s41598-018-23013-1
Yu, L., Xiong, J., Zhang, Z., Wang, Y., Yang, H., Yan, R., et al. (2019). Isolation, identification, fermentation characteristics and volatile aroma components analysis of non-Saccharomyces for tangerine wine. Sci. Technol. Food Industry 40:22. doi: 10.13386/j.issn1002-0306.2019.07.022
Zhang, F., Du, L., and Wang, M. (2011). Research on the fermentation technology of low-alcohol nanfeng mandarin orange wine. Liquor Mak. Sci. Technol. 2, 93–95. doi: 10.13746/j.njkj.2011.02.021
Keywords: tangerine wine, high-throughput sequencing, fermentation microbiota, metabolites, Non-Saccharomyces cerevisiae
Citation: Qiu X, Yu L, Wang W, Yan R, Zhang Z, Yang H, Zhu D and Zhu B (2021) Comparative Evaluation of Microbiota Dynamics and Metabolites Correlation Between Spontaneous and Inoculated Fermentations of Nanfeng Tangerine Wine. Front. Microbiol. 12:649978. doi: 10.3389/fmicb.2021.649978
Received: 06 January 2021; Accepted: 31 March 2021;
Published: 11 May 2021.
Edited by:
Vittorio Capozzi, Institute of Sciences of Food Production, Italian National Research Council, ItalyReviewed by:
Yu Zheng, Tianjin University of Science and Technology, ChinaXiao-Na Pang, Beijing University of Agriculture, China
Copyright © 2021 Qiu, Yu, Wang, Yan, Zhang, Yang, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riming Yan, eWFucmltaW5nQGp4bnUuZWR1LmNu; Du Zhu, emh1ZHUxMkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xiangyu Qiu
Xiangyu Qiu Linlin Yu1†
Linlin Yu1† Riming Yan
Riming Yan Du Zhu
Du Zhu