- 1Department of Veterinary Medical Science, Alma Mater Studiorum – University of Bologna, Bologna, Italy
- 2Department of Agricultural and Food Sciences, Alma Mater Studiorum – University of Bologna, Bologna, Italy
- 3Department of Industrial Engineering, University of Trento, Trento, Italy
- 4Centre Agriculture Food Environment, University of Trento, Trento, Italy
Non-thermal atmospheric plasma (NTAP) has gained attention as a decontamination and shelf-life extension technology. In this study its effect on psychrotrophic histamine-producing bacteria (HPB) and histamine formation in fish stored at 0–5°C was evaluated. Mackerel filets were artificially inoculated with Morganella psychrotolerans and Photobacterium phosphoreum and exposed to NTAP to evaluate its effect on their viability and the histidine decarboxylase (HDC) activity in broth cultures and the accumulation of histamine in fish samples, stored on melting ice or at fridge temperature (5°C). NTAP treatment was made under wet conditions for 30 min, using a dielectric barrier discharge (DBD) reactor. The voltage output was characterized by a peak-to-peak value of 13.8 kV (fundamental frequency around 12.7 KHz). This treatment resulted in a significant reduction of the number of M. psychrotolerans and P. phosphoreum (≈3 log cfu/cm2) on skin samples that have been prewashed with surfactant (SDS) or SDS and lactic acid. A marked reduction of their histamine-producing potential was also observed in HDC broth incubated at either 20 or 5°C. Lower accumulation of histamine was observed in NTAP-treated mackerel filets that have been inoculated with M. psychrotolerans or P. phosphoreum and pre-washed with either normal saline or SDS solution (0.05% w/v) and stored at 5°C for 10 days. Mean histamine level in treated and control groups for the samples inoculated with either M. psychrotolerans or P. phosphoreum (≈5 log cfu/g) varied from 7 to 32 and from 49 to 66 μg/g, respectively. No synergistic effect of SDS was observed in the challenge test on meat samples. Any detectable amount of histamine was produced in the meat samples held at melting ice temperature (0–2°C) for 7 days. The effects of NTAP on the quality properties of mackerel’s filets were negligible, whereas its effect on the psychrotrophic HPB might be useful when time and environmental conditions are challenging for the cool-keeping capacity throughout the transport/storage period.
Introduction
Histamine is a biogenic amine and can be produced during processing and/or storage in fish, usually by the action of spoilage bacteria. The intoxication by histamine occurs after the consumption of fish and fish products containing histamine at concentrations higher than 500 ppm (Gonzaga et al., 2009; Tao et al., 2011). Hazard characterization studies identified 50 mg of histamine per meal as the dose where either adverse effects were not noted (NOAEL) or the estimate of additional risk (lower confidence level) is low, but this dosage level will not apply to individuals with a specific sensitivity to histamine and would not apply to children (FAO/WHO, 2013).
Histamine poisoning is the most common cause of human foodborne illness due to the consumption of fish products. According to the European Food Safety Authority and the European Centre for Disease Prevention and Control, 599 histamine food poisoning outbreaks were notified in the European Union (EU) during the period 2010–17 and levels of more than 3,000 ppm have been recorded in fish products implicated in outbreaks of histamine poisoning (European Food Safety Authority (EFSA), 2017; European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC), 2018). Histamine accumulates in the tissues due to the enzymatic activity of bacteria that have contaminated the fish meat and reaches the highest concentrations in fish containing high levels of free histidine. Therefore, the intoxication is most often associated with the consumption of fish belonging to the Scombridae and Scomberesocidae families (tuna, mackerel, bonito, and jack) and some non-scombroid fish, including mahi-mahi, sardines, pilchards, anchovies, herring, marlin, and blue fin. The number and activity of Histamine-Producing Bacteria (HPB) is directly related to the probability of histamine level exceeding the microbiological criteria for the products placed on the market (Hungerford, 2010; Tao et al., 2011). The majority of HPB that have been isolated from implicated fish are Gram-negative. The psychrotrophic species that are able to produce toxic concentrations of histamine at 0–5°C, such as Morganella psychrotolerans (Emborg et al., 2006) and Photobacterium phosphoreum (Kanki et al., 2004), appear to be important in fish stored under refrigeration and mild temperature abuse (FAO/WHO, 2013). Cooling and then keeping the chill temperature of the fish is considered to be the main factor impacting the growth and/or survival of histidine-decarboxylase bacteria and consequently of histamine accumulation during storage and transport (United States Department of Health and Human Services (HHS), Food and Drug Administration (FDA), and Applied Nutrition Center for Food Safety (CFSAN), 2020). Once histidine decarboxylase (HDC) is produced, it can continue to produce histamine, even though bacterial growth has been prevented by cooling to 4°C (Kanki et al., 2007). The HDC activity is correlated with the levels of contamination and the HDC potential of bacteria. Small fishes such as mackerels are stored on-board without bleeding or gutting and are chilled to achieve a temperature close to that of melting ice. The storage can be in fish boxes or tubs with ice, also in mixture with seawater or freshwater. In order to maintain the cold chain re-icing and removal of melted ice through drainage hole of the containers are periodically made, but the cooling-keeping process could be challenged during transport and storage (EFSA Biohaz Panel et al., 2020).
Non-thermal plasma is one of the most promising minimal processing methods for the food industry (Bourke et al., 2017; López et al., 2019; Sonawane and Sonar Patil, 2020). However, up until now there are limited studies that report the application and effect of non-thermal plasma on processed seafood (Kulawik and Kumar Tiwari, 2018; Zhao et al., 2019; Dityanawarman et al., 2020; Andoni et al., 2021; Ekonomou and Boziaris, 2021). Non-thermal Atmospheric Plasma (NTAP), also called Cold Plasma, is generated under atmospheric conditions at temperatures of 30–60°C requiring low energy (Misra et al., 2011). Although the exact mechanism of bactericidal action is yet to be elucidated, it is known that NTAP is a source of multiple chemically reactive species, including reactive oxygen (ROS) and reactive nitrogen species (RNS), with a high bactericidal potency (Ziuzina et al., 2015b; Liao et al., 2016; Mai-Prochnow et al., 2016; López et al., 2019). Previous studies have indicated that cells in planktonic state show a higher sensitiveness toward NTAP than cells within biofilms (Jahid et al., 2014; Ziuzina et al., 2015a; López et al., 2019). In this respect, the use of a surfactant like sodium dodecyl sulfate (SDS) might increase the sanitation efficacy of NTAP. A synergistic effect of low pH and NTAP-derived reactive species on microbial inactivation has been also proposed (López et al., 2019) and a strong bactericidal action was demonstrated on Listeria monocytogenes and verotoxin-producing Escherichia coli when NTAP was applied in combination with SDS and lactic acid to decontaminate leafy vegetables (Berardinelli et al., 2016; Trevisani et al., 2017).
The objective of this work was to investigate the effect of NTAP and the synergic activity of SDS and lactic acid against the potential of histamine production of M. psychrotolerans and P. phosphoreum in order to explore its possible use in the processing of fresh fish.
Materials and Methods
Samples
Fresh mackerels (Scomber scombrus) were purchased in two supermarkets at different times (six different batches). The fishes (n = 12) were carried in insulated boxes and frozen within 1 h. Fishes were kept at −18°C for 7 days to reduce P. phosphoreum that can be part of the microbial background flora (Guldager et al., 1998; Emborg et al., 2002). After 7 days they were de-frozen in a cool room at 5 ± 1°C for 24 h. Surface disinfection by exposure to germicidal UV-light (254 nm, 25 w) for 30 min was also used to reduce the presence of background bacteria.
Strains Used for the Inoculum
Five strains of M. psychrotolerans isolated from different samples of Clupeids pilchardus, Engraulis encrasicolus, Clupea harengus and Thunnus albacares (Costanza et al., 2013) and four strains of P. phosphoreum isolated from different samples of Thunnus albacares (FAO area 57) were used. Their ability to produce histamine was determined by inoculating the isolates in HDC broth, which was formulated with Tryptone Soy Broth (Oxoid, Basingstoke, United Kingdom), 1% l-histidine, 2% NaCl and 0.0005% pyridoxal 5-phosphate (Sigma-Aldrich, Milano, Italy) (TSBH+) and measuring with a biosensor the amount of histamine after incubation at 20°C (M. psychrotolerans) and 5°C (P. phosphoreum) for 2 and 4 days, respectively (Kanki et al., 2004). The strains have also been tested by PCR for the reference gene vasD (M. psychrotolerans) (Podeur et al., 2015) and gyrB (P. phosphoreum) (Macé et al., 2013) and for the HDC–encoding genes (De Las Rivas et al., 2005).
Growth of Bacteria and Inoculum
Bacteria were grown in TSBH+ at 20°C (M. psychrotolerans) and 15°C (P. phosphoreum) for 2 and 4 days, respectively. Subcultures of each strain at a cell density of ≈108 cfu/ml (40% transmittance at 540 nm) were mixed and used for the inoculum, which was made either on the skin or on meat samples. Spot-inoculation of the skin of entire fishes was aimed at simulating natural skin contamination and the effect of NTAP, SDS and lactic acid on the viability and histamine-producing ability that was assessed using in vitro test (cultures in HDC broth). With this aim entire mackerels were laid on trays and the suspensions of bacteria (either M. psychrotolerans or P. phosphoreum) (20 μl, ≈6 log cfu) were pipetted on nine different spots at a minimum distance from each other of 2.5 cm. The fish skin black lines just below the lateral line were used to mark them. All operations were done in a laminar flow hood. The contaminated fishes were then covered with an aluminum foil. The raised edges of the tray prevented it from touching the samples. In order to evaluate histamine production on fish meat stored at chilling temperature (on melting ice or at 5°C) a subsequent challenge test was made, using 5-g meat samples (1 cm thick) including the skin that were cut with sterile blades from mackerels, put in sterile weighting vessels and contaminated with ≈5.5 log cfu/g. The inoculated samples, either entire fish or meat, were held at refrigeration temperature (5 ± 1°C) overnight to allow the bacterial attachment.
Challenge Tests
After overnight storage the mackerel’s skin samples (≈5 cm2) were excised with a sterile scalpel and put into weighing vessels. Not-inoculated skin samples were analyzed to detect the background HPB bacteria. The inoculated meat and skin samples were submerged in water solutions containing SDS (0.05% w/v) or in normal saline (NaCl 0.85% w/v). The effect of lactic acid (LA, 2% w/v) in combination with SDS (0.05% w/v) was also assessed on the skin samples. All reagents were purchased from Sigma-Aldrich (Milano, Italy). The preliminary washing was performed at room temperature (20–25°C) for 5 min. During this period the containers were tilted repeatedly for 5 s to increase the washing effect. After this step samples were rinsed with 18 ml of sterile deionized water to remove the residue of SDS and LA, as well as the not attached bacteria. NTAP treatment was carried out under wet conditions and at temperature of 33 ± 1°C, as previously described by Berardinelli et al. (2016). In brief, the weighting vessels with the inoculated samples were put in a plastic hermetic chamber (135 mm × 220 mm × 178 mm) housing a Dielectric Barrier Discharge (DBD) plasma source; a fan mounted above the couple of parallel electrodes was used to direct the plasma species against the sample. The vessels were filled with deionized water to a height of 0.6 cm. The distance between the fluid and the electrodes was about 2 cm. Voltage at the electrodes was produced by high voltage (HV) transformers and power switching transistors supplied by a stabilized DC power supply (Elektro-Automatik GmbH & Co., KG, EA-PS 2042-06B). Treatment parameters were fixed at 19.15 V and 3.15 ± 0.5 A for 30 min and air was used as working gas. Voltage output had a like-shaped sinusoidal waveform; during measurement its peak-to-peak value was around 13.8 kV with a fundamental frequency of oscillation around 12.7 KHz. Control samples, inoculated and washed with normal saline, were also rinsed and left for 30 min immersed in water. To ensure result reproducibility, each experiment had three replicates (skin/meat samples, 5 cm2/g) and was repeated two times (meat samples) or three times (skin samples) in different days using samples from different fish batches. pH of the water solutions (SDS, SDS+LA and normal saline) was measured before and immediately after the NTAP treatment. The meat samples were stored after the treatments at melting ice temperature (0–2°C) for 7 days (n = 6, two lots, three repetitions) and at fridge temperature (5 ± 1°C) for 10 days (n = 6, two lots, three repetitions). The effect of NTAP on planktonic (freely suspended) bacteria diluted in normal saline, with or without SDS, was also evaluated (n = 3 repetitions). Microbial suspensions (10 ml) of either M. psychrotolerans and P. phosphoreum were put in small weighing vessels and treated with NTAP as described before. The numbers of the target bacteria were counted before and after the treatment.
Microbiological Assessments
Skin samples (5 cm2; ≈5 g) were homogenized in TSBH+ (1:10 dilution). Psychotropic Aerobic Viable Count (PAVC) and luminous colonies (LC) were determined by spread plating of appropriate dilutions on pre-chilled plates of Marine Agar (MA, 0.5% peptone, 0.3% yeast extract, 0.3% glycerol, agar 1.5% in micro filtrated aged sea water). Plates were incubated at 5°C and PAVC were counted after 5 days. LC were also counted in a dark room. Two colonies from MA plates of the highest dilution showing growth were collected and purified on Trypticase Soy Agar (Oxoid) supplemented with 2.0% NaCl. Then isolates were analyzed by PCR for the reference gene gyrB (Macé et al., 2013) to determine whether they belonged to the P. phosphoreum species. M. psychrotolerans was counted instead by spread plating the sample homogenates on MacConkey agar. Plates were incubated at 25°C for 2 days and the lactose negative colonies were counted. Five colonies picked from plates containing 10–50 cfu were transferred on Niven agar plates (0.5% Tryptone (Oxoid), 0.5% of Yeast extract (Oxoid), 0.5% NaCl (Sigma), 2% L-histidine (Sigma), 0.1% CaCO3 (Sigma), 0.006% bromocresol purple (Sigma), and 2% agar (Oxoid) (pH 5.3) (Mavromatis and Quantick, 2002). Two light purple colonies corresponding to the highest dilution showing growth were picked, streaked on trypticase soy agar (Oxoid) to obtain pure cultures and tested with the PCR assay for the reference gene vasD of M. psychrotolerans (Podeur et al., 2015). Incubation at ≈2°C (in insulated boxes with melting ice) for 7 days was used for assessing viability after NTAP treatment of freely suspended bacteria.
Histamine Measurement in Cultures and in Meat
Skin samples (5 cm2) inoculated with either M. psychrotolerans or P. phosphoreum were homogenized in TSBH+ (dilution 1:10) and incubated at 20 ± 1°C and 5 ± 1°C for 1 and 5 days, respectively. The low temperature was used to inhibit the growth of mesophilic microorganisms that could prevail (Dalgaard et al., 1997). The same procedure was used for the cultures of the strains in order to assess their histamine producing potential. Meat samples (5 g) were homogenized (dilution 1:10 in phosphate buffer saline (PBS). Cultures and fish sample homogenates were sterilized at 121°C for 15 min. Then filtration by filter paper (Whatman 1004 110, Fisher Scientific, Italy) was used to remove debris. The histamine was measured with a histamine biosensor as described by Trevisani et al. (2019). Briefly, the reduction current mediated by ferrocenium ions (HCFe) was measured with an amperometer connected to a personal computer (Autolab PGSTAT 10, Methrom EcoChemie, NL) and a carbon screen-printed carbon electrode (SPCE) modified with diamine oxidase (DAO) and peroxidase (HRP). The applied potential was −0.025 mV (vs. Ag/AgCl) and measurements (mA) were taken after 120 s.
Effect of NTAP on Quality Properties of Fish
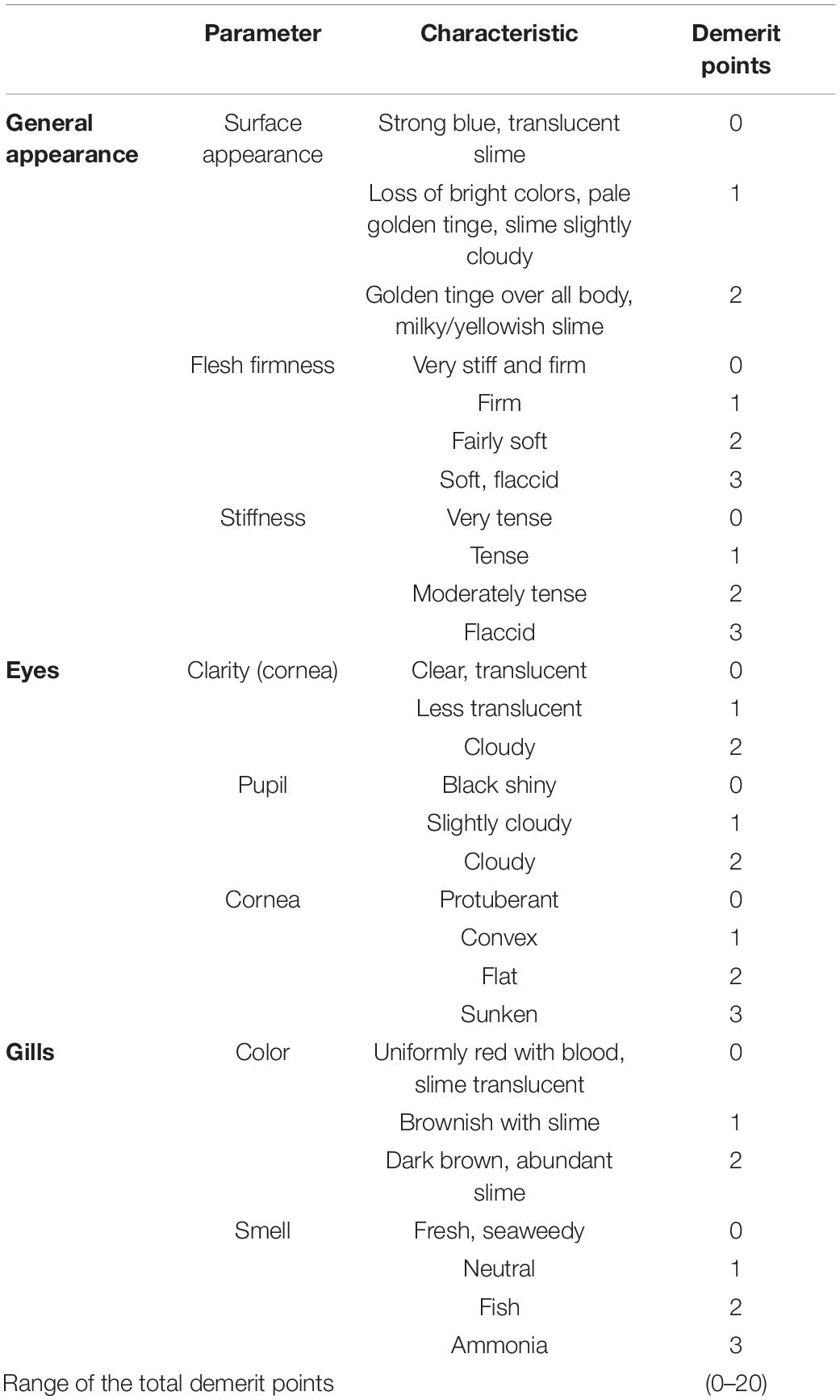
Quality properties were evaluated by a trained sensory panel on 12 fishes before the treatment, immediately after the treatment (SDS+NTP, SDS+LA+NTP and NaCl) and at one, 2 and 5 days of storage. The samples were placed in a polystyrene box covered with ice flakes and stored at 4°C, up to 5 days. For the sensory evaluation, a panel of five members evaluated the fishes according to the Quality Index Method (QIM) (Özyurt et al., 2009). This sensory scale is based on the freshness quality grading system for Atlantic mackerel (Scomber scombrus) developed by Andrade et al. (1997). The QIM is based on the evaluation of appropriate sensory attributes of the raw fish. Demerit score ranging from 0 to 1, 2 or 3 depending on the different sensory attributes are assigned (Table 1). To obtain the quality index, the scores for all characteristics are then summed to give an overall sensory score (Botta, 1995). The scale gives zero score for absolutely fresh fish and increasing total demerit points during fish deterioration. The basic parameters of this scheme are: surface appearance; stiffness and flesh firmness; clarity of cornea, clouding of the pupil; color and smell of the gills. Color measurements of the skin were conducted by means of a Minolta ChromaMeter CR-400 reflectance colorimeter (Minolta, Milan, Italy). The acquisitions were performed on two different fish zones: the abdomen characterized by a white color and the dorsal area characterized by a dark color (Figure 1). For each acquisition, an average value of three measurements was calculated. The CIELab system L∗, a∗ and b∗ was considered (CIE, 1976) and the Chroma values were calculated as C∗=.
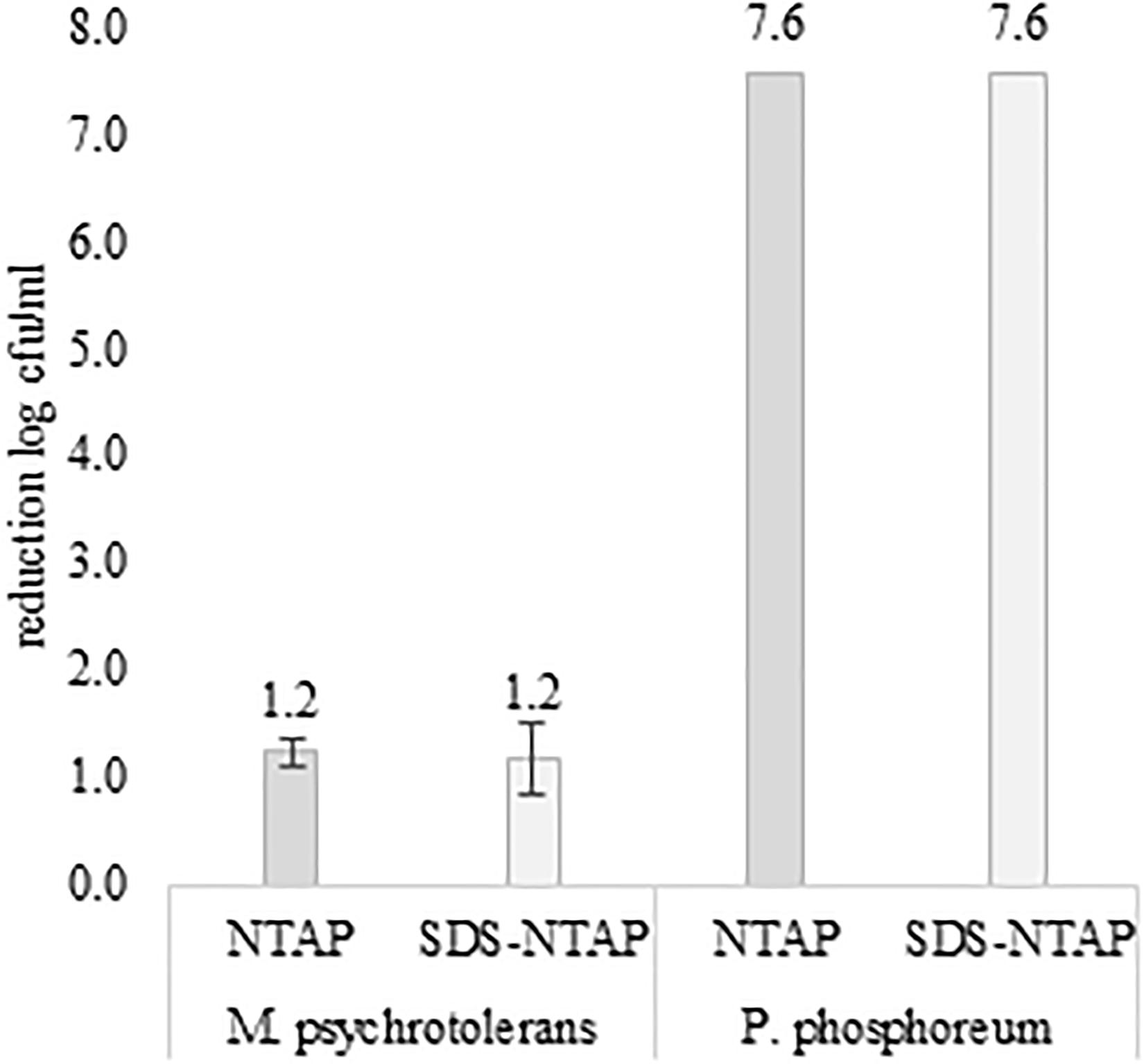
Figure 1. Effect of Not-Thermal Atmospheric Plasma and SDS (0.05% w/v) on M. psychrotolerans and P. phosphoreum. Counts after incubation at 2–4°C for 7 days.
Statistical Methods
Significant differences between the means of the quality parameters were evaluated by the analysis of variance (ANOVA with Post hoc Tukey HSD). Two-way ANOVA with replication was performed to assess the effectiveness of NTAP and its synergy with SDS/LA on the different batches of mackerels. Welch T test was used to analyze differences between groups where the homogeneity of variance assumption was not met. Statistical elaboration was made by Matlab R2018a.
Results
Effect of NTAP on Histamine-Producing Bacteria
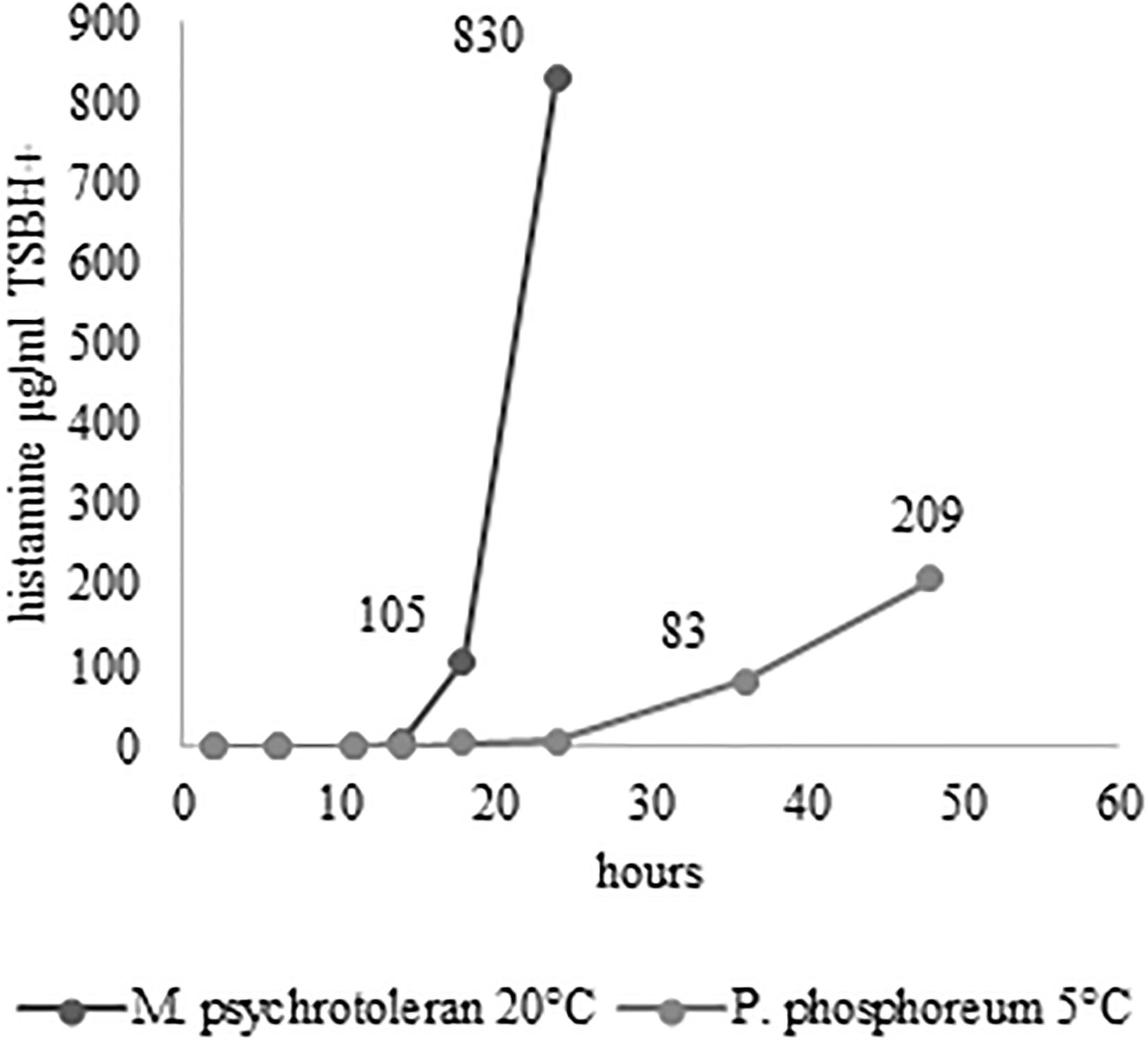
After 30 min of plasma exposure, the number of P. phosphoreum in normal saline, with or without SDS, decreased form 7.56 log cfu/ml to zero. The results were reproduced three times. A much lower antimicrobial efficacy of plasma exposure was observed with the M. psychrotolerans suspensions (Figure 1). No difference was observed when comparing microbial suspensions in normal saline and in SDS solutions. Temperature and availability of high concentration of free histidine (1% in TSBH+) influenced the level of histamine accumulated in the test in-vitro (Figure 2).
The challenge tests carried out with the skin samples demonstrated that NTAP can reduce the numbers and the histamine-producing potential of M. psychrotolerans and P. phosphoreum in association with an SDS pre-washing step (Table 2 and Figure 3), while lactic acid had no synergic effect. The inhibitory effect of NTAP and SDS on the viability of the target bacteria was particularly relevant with the samples incubated at 20°C, which is close to the optimum temperature for the growth of M. psychrotolerans, but it was significant even with the samples incubated at 5°C (p < 0.001). It has to be underlined that M. psychrotolerans and P. phosphoreum were detected in almost all samples independently from the inoculum and therefore, the measurements made in the challenge tests were relative to the sum of the inoculated and indigenous HPB. The concentrations of histamine produced in HDC broth at different times in two samples inoculated with similar concentration of either M. psychrotolerans or P. phosphoreum is shown in Figure 3. It is indicative of the histamine-producing potential of these bacteria in the in-vitro test by using similar inoculum (6 log cfu). Approximately 100 μg/ml of histamine was produced by M. psychrotolerans after 18 h at 20°C and similar amount (≈82 μg/ml) was produced after 36 h at 5°C by the P. phosphoreum.
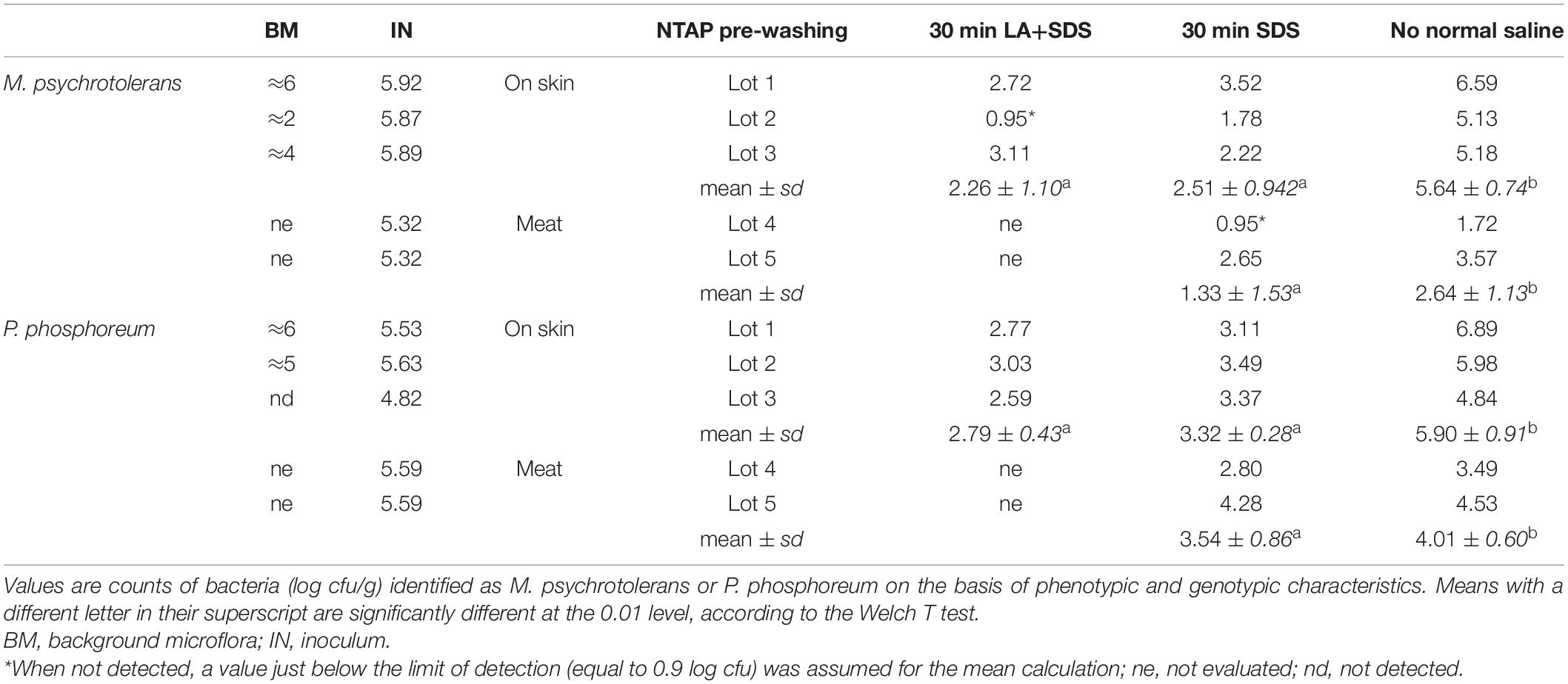
Table 2. Inactivation of M. psychrotolerans and P. phosphoreum by non-thermal plasma associated with SDS and Lactic acid.
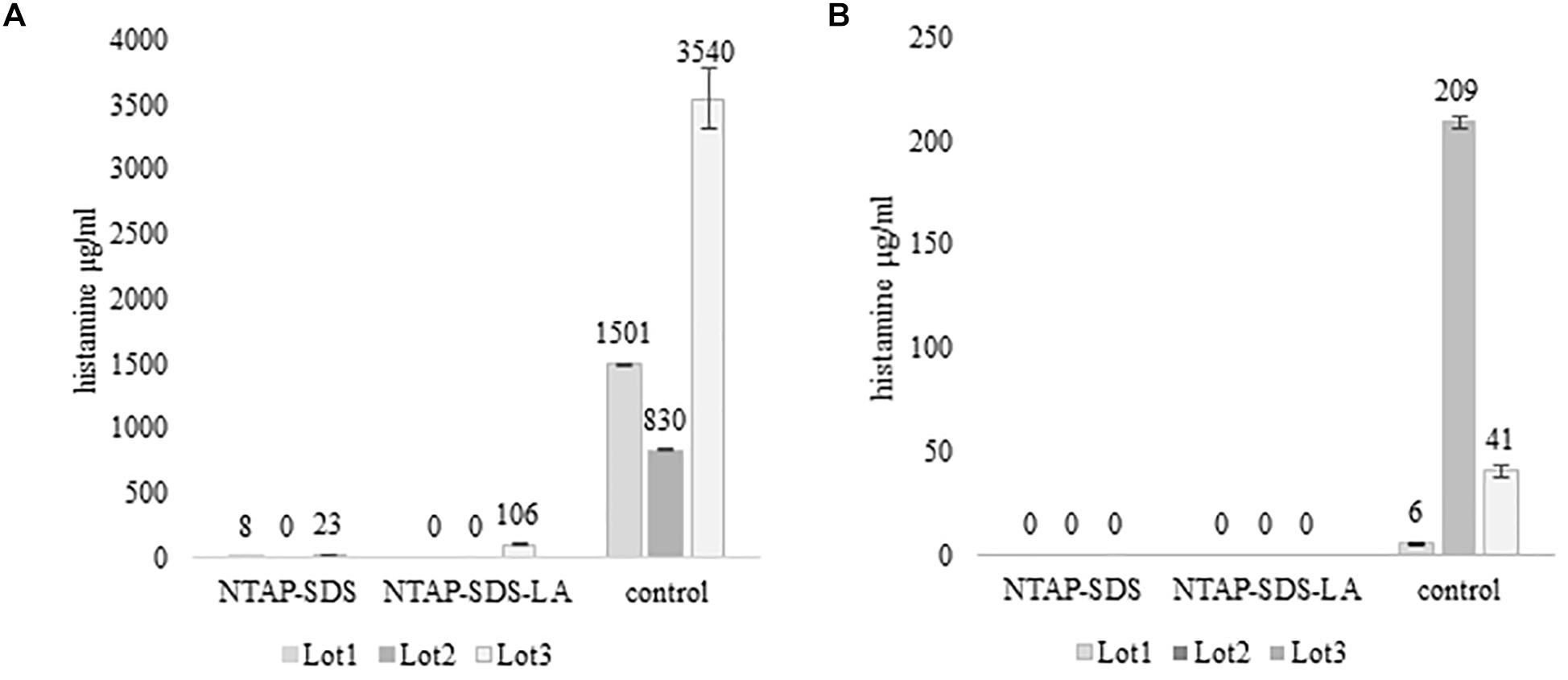
Figure 3. Activity of HPB at different temperature and time. Histamine produced in TSBH+. Samples not treated with NTAP and inoculated with either M. psychrotolerans (Mp) or P. phosphoreum (Pp) and incubated at 20°C and 5°C, respectively.
Histamine was not detected in fish samples incubated at melting ice temperature for 1 week, either NTAP treated and control samples, while it was detected in those stored in fridge at 5°C for 10 days (Figure 4). The effect of NTAP on histamine production was more relevant in the samples inoculated with M. psychrotolerans (p < 0.001) leading to histamine concentrations that were approximately five times lower than in controls (i.e., 7 vs. 33 mean log cfu/g). No differences were observed in the NTAP treated samples prewashed or not with SDS. In the samples inoculated with P. phosphoreum the concentration of histamine was higher than in previous group and the mean difference between NTAP treated and controls was 22 μg/g (p < 0.01), precisely it was 28 and 17 μg/g in the samples prewashed or not with SDS. The differences observed as a consequence of pre-washing with SDS were not significant (p = 0.13). Inhibition of the microbial viability was also observed (Table 2), although differences between NTAP treated and controls were small (0.5–1.3 log cfu/g).
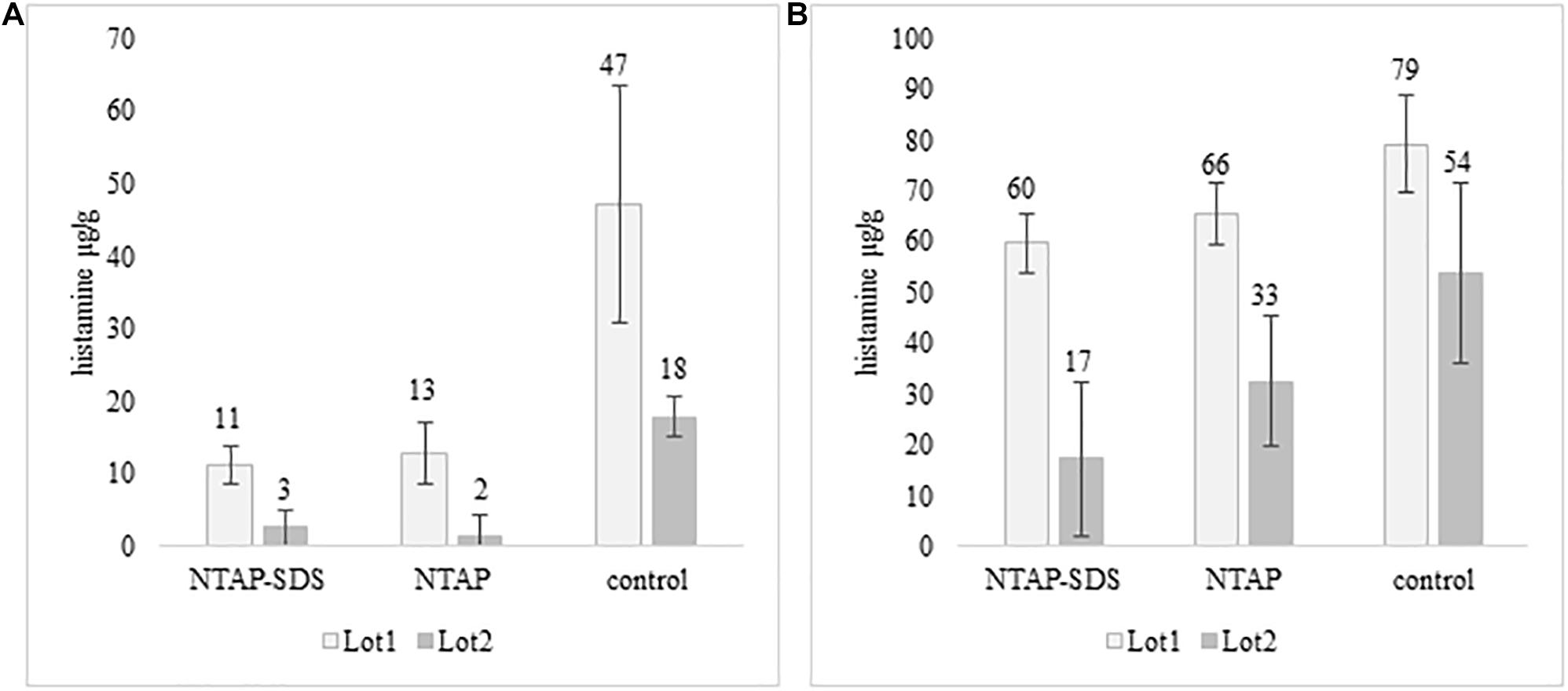
Figure 4. Histamine concentration (μg/g) in fish meat inoculated with M. psychrotolerans (A) and P. phosphoreum (B) after 10 days at 5°C.
Effect of NTAP on Quality Properties of Fish
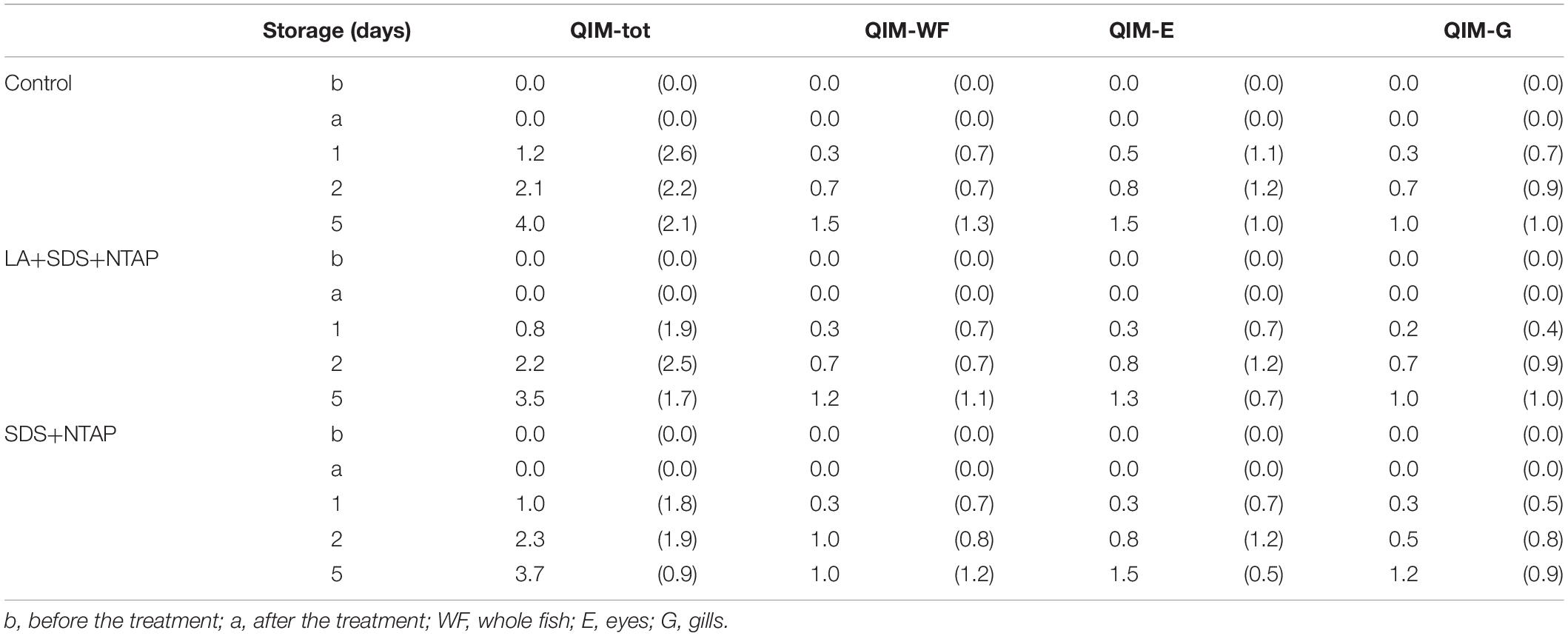
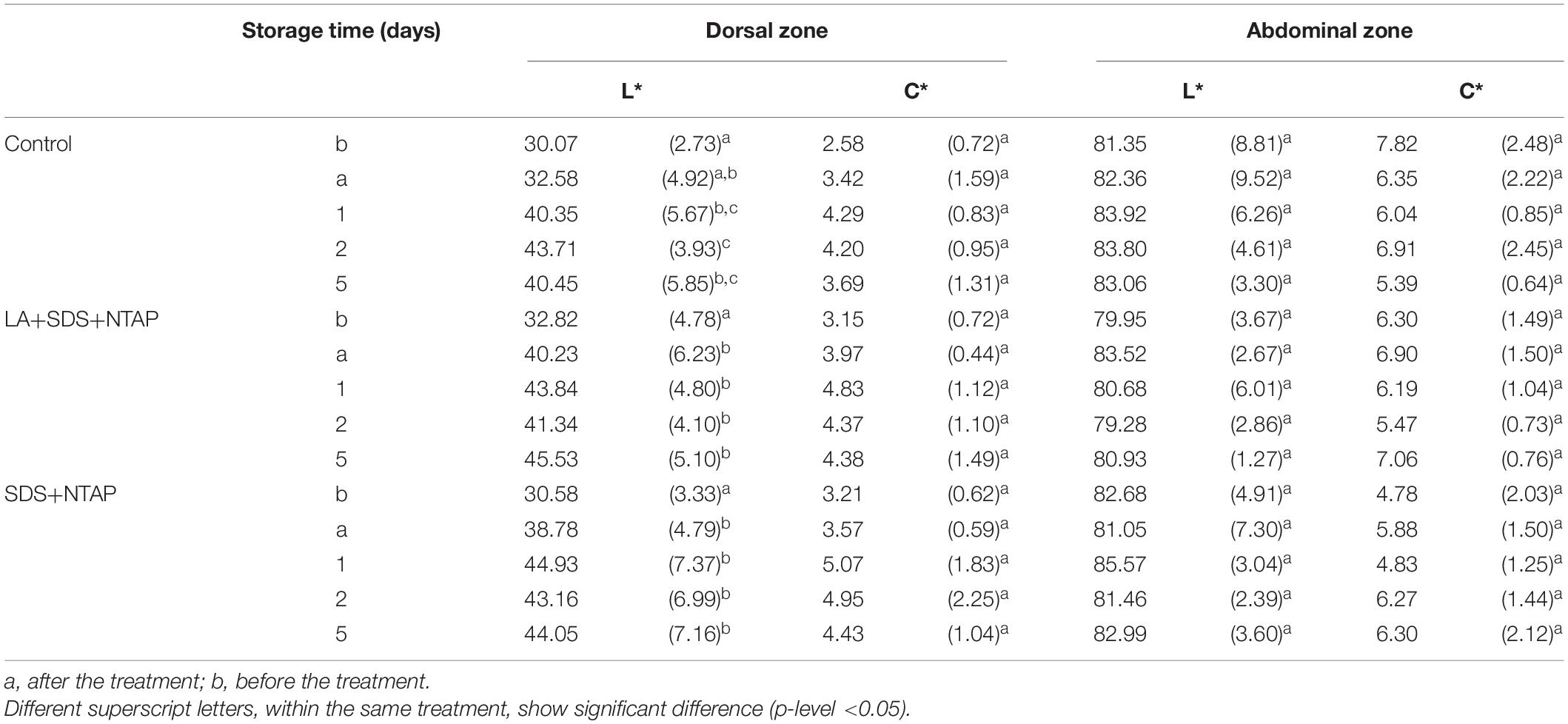
Results of sensory evaluation, in terms of means of QIM demerit points, are shown in Table 3. Quality descriptors were related to the visual appearance of whole fish (QIM-WF), eyes (QIM-E), gills (QIM-G) and to the sum of total demerit points (QIM-tot). Demerit points (QIM-tot) increased (passing from 0 to 3.72 ± 0.21) as function of storage time, following a linear trend (R2 from 0.948 to 0.984), as reported for horse and Atlantic mackerel stored in ice, by Andrade et al. (1997). No differences were reported between treated and control samples, within the same storage time, confirming that the NTP does not induce sensory modifications. Concerning the color evaluation, L∗ and C∗ values are reported in Table 4. For both fish zones, significant differences were not observed during the storage for the C∗ parameter, while a slight significant increase in lightness was reported, for the dorsal zone, between the NTAP treated samples before and after the treatment.
Effect of NTAP and Residues of Sanitizers on pH
The pH of the distilled water used for NTAP treatments changed from 6.7 to 6.0 when the fish samples have been pre-washed with SDS, while remained at pH 6.1 when they have been pre-washed with LA and SDS. The pH of water used for the inoculated control samples pre-washed with normal saline was 6.7.
Discussion
A major goal of this study was to evaluate the effect of NTAP on psychrotrophic bacteria that were associated with many incidents caused by histamine in fish and the suitability of NTAP treatment as a decontamination technique for mackerels. The experimental data demonstrated that NTAP can significantly reduce the microbial load of M. psychrotolerans and P. phosphoreum on mackerels’ skin and affect significantly their histamine-producing potential. The effect of NTAP on muscle can be affected by the buffer capability and microstructure of the proteins (Abe, 2000) that counteract the oxidative species and protect bacteria. The greater effect demonstrated using the in-vitro tests was nevertheless most probably related to the temperature and growth rate of the target bacteria, which have their optimum in cultures at 26–27°C (M. psychrotolerans) and 15–20°C (P. phosphoreum) (Budsberg et al., 2003; Emborg and Dalgaard, 2008) as well as to the concentration of free histidine and the decarboxylase activity of bacteria. With regard to the free histidine content of mackerels it has seasonal variations and average values between 0.20 and 0.45 g/100 g (Osako et al., 2004; FAO/WHO, 2013), while it was 1% in TSBH+. The in vitro model can be useful for the evaluation of NTAP activity, but the tests made using refrigerated mackerel meat have demonstrated that the treatment can be useful only for fish that are exposed to a temperature abuse and if manipulations facilitate the contamination of meat. It was found that mackerel meat samples stored at 5°C did not accumulate histamine to levels greater than 50–70 mg/kg during up to 10 days of storage when the initial level of P. phosphoreum was ≈3.5 – 4.5 log cfu/g, but the time to accumulate ≈82 μg/ml in TSBH+ was 36 h when the initial level was ≈ 6.0 log cfu/g. Even if enzymatic methods are specific, other biogenic amines, such as cadaverine and tyramine and especially putrescine, can interfere with histamine biosensors that are based on the DAO activity (Trevisani et al., 2017). The measured levels of histamine in fish meat could be overestimated for the presence of these diamines. Nevertheless, these values are correlated with the reduction in the numbers of M. psychrotolerans (1.3 log cfu/g) and P. phosphoreum (0.5 log cfu/g) and therefore indicative of the antimicrobial activity of NTAP treatment.
Previous studies have indicated that the presence of a critical pH of the solution, i.e., pH ≈4.7 influences the bactericidal effect by low-temperature plasma when bacteria suspensions are diluted in distilled water (Ikawa et al., 2010). They reported that acidification by plasma exposure is caused by the dissolution of nitrogen oxides generated from ambient air by the plasma and that the pH values evaluated from the NO concentration, is in good agreement with those directly measured by a pH meter. The acidity without plasma exposure, however, was ineffective and the bacterial inactivation of the target Gram- bacteria in their study arises only from the combined effect of acidity and plasma exposure. Their hypothesis was that at low pH superoxide anion radicals generated by the plasma source superoxide anion radicals are converted to hydroperoxy radicals (HOO•), which can penetrate the cell membrane and damage intercellular components.
The inhibitory effects of atmospheric CP in addition to the use of a surfactant and lactic acid on Listeria monocytogenes, and E. coli O157:H7 in red chicory was studied by Trevisani et al. (2017). They found that pre-washing red chicory with SDS before NTAP treatment reduced E. coli O157:H7 counts by 2.89 log CFU/g and pre-washing with lactic acid + SDS gave a much higher VTEC counts reduction of 4.78 log CFU/g. They also observed that after the treatments with (LA + SDS) 15 min + ACP 15 min and SDS 10 min + ACP 15 min, the pH values of water solutions used to immerge the leaves were 3.23 ± 0.12 and 3.32 ± 0.17, respectively. In the present study, SDS did not appear to have an additional effect on the viability of M. psychrotolerans that are suspended in saline solution and could not be observed in the test with P. phosphoreum, where NTAP alone reduced their number from 7.56 to below the detection limit. When bacteria are on food surface the activity of SDS could be most probably related to the detachment of bacteria and this effect could be different on rough or smooth hydrophobic surfaces. The plasma generated reactive oxygen and nitrogen species (RONS) can directly affect microbial cells or interact with the medium (buffer, growth media, or water) and eventually destroy microbial cells rather than damage food tissues (i.e., through oxidative stress) when the threshold level of the oxidative stress harmful to the tissues is higher than that harmful to microbial cells (Bhawana et al., 2020). Surfactants may promote the detachment and dispersion of bacteria form the surface of vegetables when they are not internalized, thus enhancing NTAP effect on microorganisms, but this effect might be easier on the surface of intact leaves (Ziuzina et al., 2015b) or the smooth surface of mackerels’ skin than on muscles.
In the present study different factors influenced the results of the challenge tests. The pH of water remained above 6.0 after the NTAP treatments even in the samples pre-washed with LA most probably as a consequence of the buffering capacity of the proteins of muscle tissue. This occurred also with the “skin samples” because a thin layer of muscle remained attached to the skin when it was excised from the fishes and it could have LA ineffective.
Differences in the temperature and in the substrate are other factors that influenced the rate of histamine production in the test made to evaluate the effect of treatments on the HPB.
Even the surfactant activity of SDS can produce different results when the washing solution are in contact with smooth surfaces like mackerel skin or on the fibrillar structure of the meat.
Ziuzina et al. (2015b) observed that the presence of organic matter in the bacterial suspension might present a protective effect against the action of RONS on bacterial cells and hypothesized that NTAP generated reactive species could not penetrate through complex biofilm matrices. The lower prevalence of bacteria that are exposed to the action of the oxidative species generated by plasma, as well as the low temperature, might explain the weak differences observed between the fish meat treated and control samples. At the melting ice temperature histamine was not produced in either control and treated samples.
The preliminary results reported in the present study would need further trials with cold plasma discharge systems capable of treating entire fishes, because in real conditions it would be the bacteria surviving to the eventual NTAP treatment made using bigger cold plasma discharge systems that produce histamine after they penetrate the natural barriers (skin, gut) in consequence of manipulations and post-mortem degradation of the tissues.
The effects of NTAP on the qualitative aspects of fresh fish were analyzed in some recent reviews (Kulawik and Kumar Tiwari, 2018; Olatunde and Benjakul, 2018). They reported that NTAP treatment induces oxidative changes of treated fish and seafood, but this does not necessary lead to decrease in sensory quality or shelf-life of the final products. In a study on mackerel filets Albertos et al. (2017) observed that NTAP (generated using a DBD plasma system operating at 50 Hz, 70–80 kV, 1–5 min) can reduce the numbers of psychrotrophic lactic acid bacteria and Pseudomonas up to 2 log cfu/g and that the treatment did not affect the proximate composition and color, but it induced an increase in lipid oxidation parameters, such as peroxide value and conjugated dienes, while TBARS analysis did not show significant differences. Chiper et al. (2011) reported that cold plasma generated in Ar/CO2 gas at 15 Hz, 11 kV in gas plasma can reduce the number of Photobacterium phosphoreum in cold smoked salmon of less than 3 log cfu/g in 2 min.
In the present study the sensory evaluation results obtained after treatment of whole mackerels suggest that the NTAP does not induce appreciable modifications after storage at 4°C for 5 days. Only a slight increase in lightness was observed, as reported also by Albertos et al. (2017) on mackerel filets treated by a DBD plasma system. This change was probably due to oxidation of hemoproteins (Kulawik and Kumar Tiwari, 2018).
In conclusion, NTAP shows potential for use as a decontamination technology in the processing of mackerels. The treatment of fishes before long distance transportations may contribute to safety and extend their shelf life. NTAP treatment might be especially useful when time and environmental conditions are challenging for the cool-keeping capacity throughout the transport/storage period.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MT, AB, and CC conceived the study. MT and MC made the bacteriological and chemical analyses. AB and CC made the quality and sensorial tests. MT wrote the manuscript and all authors contributed with their review.
Funding
This work was supported by the University of Bologna (Oriented Fundamental Research fund, RFO).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.653597/full#supplementary-material
References
Abe, H. (2000). Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc.) 65, 891–900.
Albertos, I., Martín-Diana, A. B., Cullen, P. J., Tiwari, B. K., Ojha, S. K., Bourke, P., et al. (2017). Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 44, 117–122. doi: 10.1016/j.ifset.2017.07.006
Andoni, E., Ozuni, E., Bijo, B., Shehu, F., Branciari, R., Miraglia, D., et al. (2021). Efficacy of non-thermal processing methods to prevent fish spoilage. J. Aquat. Food Prod. Technol. 30, 228–245, doi: 10.1080/10498850.2020.1866131
Andrade, A., Nunes, M., and Batista, I. (1997). “Freshness quality grading of small pelagic species by sensory analysis,” in Proceedings of the Final Meeting of the Concerted Action “Evaluation of Fish Freshness”, AIR3CT94 2283, Nantes Conference, November 12–14 1997: Methods to Determine the Freshness of Fish in Research and Industry, eds G. Ólafsdóttir, J. Luten, P. Dalgaard, M. Careche, V. Verrez-Bagnis, E. Martinsdóttir, et al. (Paris: International Institute of Refrigeration), 333–338.
Berardinelli, A., Pasquali, F., Cevoli, C., Trevisani, M., Ragni, L., Mancusi, R., et al. (2016). Sanitisation of fresh-cut celery and radicchio by gas plasma treatments in water medium. Postharvest Biol. Technol. 111, 297–304. doi: 10.1016/j.postharvbio.2015.09.026
Bhawana, A., Kamonporn, P., Mayura, V., Sarmistha, M., and Gyungsoon, P. (2020). Plant disease control by non-thermal atmospheric-pressure plasma. Front. Plant Sci. 14:2. doi: 10.3389/fpls.2020.00077
Bourke, P., Ziuzina, D., Han, L., Cullen, P. J., and Gilmore, B. F. (2017). Microbiological interactions with cold plasma. J. Appl. Microbiol. 123, 308–324. doi: 10.1111/jam.13429
Budsberg, K. J., Wimpee, C. F., and Braddock, J. F. (2003). Isolation and Identification of Photobacterium phosphoreum from an unexpected niche: migrating salmon. Appl. Environ. Microbiol. 69, 6938–6942. doi: 10.1128/aem.69.11.6938-6942.2003
Chiper, A. S., Chen, W., Mejlholm, O., Dalgaard, P., and Stamate, E. (2011). Atmospheric pressure plasma produced inside a closed package by a dielectric barrier discharge in Ar/CO2 for bacterial inactivation of biological samples. Plasma Sources Sci. Technol. 20:2. doi: 10.1088/0963-0252/20/2/025008
Costanza, C., Cecchini, M., Mancusi, R., Mosso, A., Giani, G., Rosmini, R.,, et al. (2013). Evaluation and identification of histamine-forming bacteria on fish products of middle Adriatic Sea. Ital. J. Food Saf. 2:e7 doi: 10.4081/ijfs.2013.944
Dalgaard, P., Mejlholm, O., Christiansen, T. J., and Huss, H. H. (1997). Importance of Photobacterium phosphoreum in relation to spoilage of modified atmosphere-packed fish products. Lett. Appl. Microbiol. 24, 373–378. doi: 10.1046/j.1472-765x.1997.00152.x
De Las Rivas, B., Marcobal, Á, and Muñoz, R. (2005). Improved multiplex-PCR method for the simultaneous detection of food bacteria producing biogenic amines. FEMS Microbiol. Lett. 244, 367–372. doi: 10.1016/j.femsle.2005.02.012
Dityanawarman, A., Puspita Dewi, I., Endah Ratnawati, S., Ekantari, N., and Tamplin, M. (2020). Growth rate and histamine production of Klebsiella sp. CK02 Isolated from skipjack tuna compared with Morganella morganii ATCC 25830 at various incubation temperatures. Squalen 15:1. doi: 10.15578/squalen.v15i1.441
EFSA Biohaz Panel, Koutsomanis, K., Allende, A., Alvarez-Ordonez, A., Bolton, D., Chemaly, M., et al. (2020). Scientific opinion on the use of the so-called ‘tubs’ for transporting and storing fresh fishery products. EFSA J. 18:6091. doi: 10.2903/j.efsa.2020.6091
Ekonomou, S. I., and Boziaris, I. S. (2021). Non-thermal methods for ensuring the microbiological quality and safety of seafood. Appl. Sci. 11:833. doi: 10.3390/app11020833
Emborg, J., and Dalgaard, P. (2008). Growth, inactivation and histamine formation of Morganella psychrotolerans and Morganella morganii—development and evaluation of predictive models. IJFM 128, 234–243. doi: 10.1016/j.ijfoodmicro.2008.08.015
Emborg, J., Dalgaard, P., and Ahrens, P. (2006). Morganella psychrotolerans sp. nov., a histamine-producing bacterium isolated from various seafoods. Int. J. Syst. Evol. Microbiol. 56, 2473–2479. doi: 10.1099/ijs.0.64357-0
Emborg, J., Laursen, B. G., Rathjen, T., and Dalgaard, P. (2002). Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2°C. J. Appl. Microbiol. 92, 790–799. doi: 10.1046/j.1365-2672.2002.01588.x
European Food Safety Authority (EFSA) (2017). Assessment of the incidents of histamine intoxication in some EU countries. EFSA Support. Publ. 14:1301E. doi: 10.2903/sp.efsa.2017.EN-1301
European Food Safety Authority (EFSA), and European Centre for Disease Prevention and Control (ECDC) (2018). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. J. EFSA 16:5500. doi: 10.2903/j.efsa.2018.5500
FAO/WHO (2013). Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Joint FAO/WHO Expert Meeting Report. Available online at: https://www.who.int/foodsafety/publications/histamine_risk/en/ (accessed December 14, 2020).
Gonzaga, V. E., Lescano, A. G., Huamán, A. A., Salmón- Mulanovich, G., and Blazes, D. L. (2009). NIH Public access. J. Food Protect. 72, 1112–1115.
Guldager, H. S., Bøknæs, N., Østerberg, C., Nielsen, J., and Dalgaard, P. (1998). Thawed cod fillets spoil less rapidly than unfrozen fillets when stored under modified atmosphere at 2°C. J. Food Protect. 61, 1129–1136. doi: 10.4315/0362-028x-61.9.1129
Hungerford, J. M. (2010). Scombroid poisoning: a review. Toxicon 56, 231–243. doi: 10.1016/j.toxicon.2010.02.006
Ikawa, S., Kitano, K., and Hamaguchi, S. (2010). Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Processes Polym. 7, 33–42. doi: 10.1002/ppap.200900090
Jahid, I. K., Han, N., and Ha, S. (2014). Inactivation kinetics of cold oxygen plasma depend on incubation conditions of Aeromonas hydrophila biofilm on lettuce. Int. Food Res. J. 55, 181–189. doi: 10.1016/j.foodres.2013.11.005
Kanki, M., Yoda, T., Ishibashi, M., and Tsukamoto, T. (2004). Photobacterium phosphoreum caused a histamine fish poisoning incident. Int. J. Food Microbiol. 92, 79–87. doi: 10.1016/j.ijfoodmicro.2003.08.019
Kanki, M., Yoda, T., Tsukamoto, T., and Baba, E. (2007). Histidine decarboxylases and their role in accumulation of histamine in tuna and dried saury. Appl. Environ. Microbiol. 73, 1467–1473. doi: 10.1128/aem.01907-06
Kulawik, P., and Kumar Tiwari, B. (2018). Recent advancements in the application of non-thermal plasma technology for the seafood industry. Criti. Rev. Food Sci. Nutr. 59, 3199–3210. doi: 10.1080/10408398.2018.1510827
Liao, X., Liu, D., Xiang, Q., Ahn, J., Chen, S., Ye, X., et al. (2016). Inactivation mechanisms of non-thermal plasma on microbes: a review. Food Control 75, 83–91. doi: 10.1016/j.foodcont.2016.12.021
López, M., Calvo, T., Prieto, M., Múgica-Vidal, R., Muro-Fraguas, I., Alba-Elías, F., et al. (2019). A review on non-thermal atmospheric plasma for food preservation: mode of action, determinants of effectiveness, and applications. Front. Microbiol. 10:4. doi: 10.3389/fmicb.2019.00622
Macé, S., Mamlouk, K., Chipchakova, S., Prévost, H., Joffraud, J. J., Dalgaard, P., et al. (2013). Development of a rapid real-time PCR method as a tool to quantify viable Photobacterium phosphoreum bacteria in salmon (Salmo salar) steaks. Appl. Environ. Microbiol. 79, 2612–2619. doi: 10.1128/aem.03677-12
Mai-Prochnow, A., Clauson, M., Hong, J., and Murphy, A. B. (2016). Gram positive and gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 6:38610. doi: 10.1038/srep38610
Mavromatis, P., and Quantick, P. C. (2002). Modification of Niven’s medium for the enumeration of histamine-forming bacteria and discussion of the parameters associated with its use. J. Food Protect. 65, 546–551. doi: 10.4315/0362-028x-65.3.546
Misra, N., Tiwari, B., Rahavarao, K., and Cullen, P. (2011). Nonthermal plasma inactivation of food borne pathogens. Food Eng. Rev. 3, 159–170. doi: 10.1007/s12393-011-9041-9
Olatunde, O. O., and Benjakul, S. (2018). Nonthermal processes for shelf-life extension of seafoods: a revisit. Compr. Rev. Food Sci. Food Safety 17, 892–904. doi: 10.1111/1541-4337.12354
Osako, K., Kurokawa, T., Kuwahara, K., and Nozaki, Y. (2004). Seasonal variations in taurine and histidine levels of horse mackerel caught in the East China Sea. Fish. Sci. 70, 1180–1182. doi: 10.1111/j.1444-2906.2004.00922.x
Özyurt, G., Kuley, E., Özkütük, S., and Özogul, F. (2009). Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem. 114, 505–510. doi: 10.1016/j.foodchem.2008.09.078
Podeur, G., Dalgaard, P., Leroi, F., Prévost, H., Emborg, J., Martinussen, J., et al. (2015). Development of a real-time PCR method coupled with a selective pre-enrichment step for quantification of Morganella morganii and Morganella psychrotolerans in fish products. Int. J. Food Microbiol. 203, 55–62. doi: 10.1016/j.ijfoodmicro.2015.03.005
Sonawane, S. K., and Sonar Patil, M. T. (2020). Non-thermal plasma: an advanced technology for food industry. Food Sci. Technol. Int. 26, 727–740. doi: 10.1177/1082013220929474
Tao, Z., Sato, M., Zhang, H., Yamaguchi, T., and Nakano, T. (2011). A survey of histamine content in seafood sold in markets of nine countries. Food Control 22, 430–432. doi: 10.1016/j.foodcont.2010.09.018
Trevisani, M., Berardinelli, A., Cevoli, C., Cecchini, M., Ragni, L., and Pasquali, F. (2017). Effects of sanitizing treatments with atmospheric cold plasma, SDS and lactic acid on verotoxin-producing Escherichia coli and Listeria monocytogenes in red chicory (radicchio). Food Control 78, 138–143. doi: 10.1016/j.foodcont.2017.02.056
Trevisani, M., Cecchini, M., Fedrizzi, G., Corradini, A., Mancusi, R., and Tothill, I. E. (2019). Biosensing the histamine producing potential of bacteria in Tuna. Front. Microbiol. 10:8. doi: 10.3389/fmicb.2019.01844
United States Department of Health and Human Services (HHS), Food and Drug Administration (FDA), and Applied Nutrition Center for Food Safety (CFSAN) (2020). Fish and Fishery Products Hazards and Controls Guidance. Fish and Fishery Products Hazard and Control Guidance Fourth Edition (March). Available online at: https://www.fda.gov/media/80637/download (accessed December 14, 2020).
Zhao, Y. M., de Alba, M., Sun, D. W., and Tiwari, B. (2019). Principles and recent applications of novel non-thermal processing technologies for the fish industry—a review. Crit. Rev. Food Sci. Nutr. 59, 728–742. doi: 10.1080/10408398.2018.1495613
Ziuzina, D., Boehm, D., Patil, S., Cullen, P. J., and Bourke, P. (2015a). Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One 10:9. doi: 10.1371/journal.pone.0138209
Keywords: non-thermal atmospheric plasma, sodium dodecyl sulfate, histamine, Morganella psychrotolerans, Photobacterium phosphoreum, mackerel (Scomber scombrus), food safety
Citation: Trevisani M, Cevoli C, Ragni L, Cecchini M and Berardinelli A (2021) Effect of Non-thermal Atmospheric Plasma on Viability and Histamine-Producing Activity of Psychotrophic Bacteria in Mackerel Fillets. Front. Microbiol. 12:653597. doi: 10.3389/fmicb.2021.653597
Received: 14 January 2021; Accepted: 01 July 2021;
Published: 27 July 2021.
Edited by:
David Rodriguez-Lazaro, University of Burgos, SpainReviewed by:
Avelino Alvarez-Ordóñez, Universidad de León, SpainCengiz Gokbulut, Balıkesir University, Turkey
Copyright © 2021 Trevisani, Cevoli, Ragni, Cecchini and Berardinelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcello Trevisani, bWFyY2VsbG8udHJldmlzYW5pQHVuaWJvLml0
 Marcello Trevisani
Marcello Trevisani Chiara Cevoli
Chiara Cevoli Luigi Ragni
Luigi Ragni Matilde Cecchini
Matilde Cecchini Annachiara Berardinelli
Annachiara Berardinelli