Abstract
Carbapenemase-producing Enterobacterales are rapidly spreading and adapting to different environments beyond hospital settings. During COVID-19 lockdown, a carbapenem-resistant NDM-1-positive Escherichia coli isolate (BA01 strain) was recovered from a pygmy sperm whale (Kogia breviceps), which was found stranded on the southern coast of Brazil. BA01 strain belonged to the global sequence type (ST) 162 and carried the blaNDM–1, besides other medically important antimicrobial resistance genes. Additionally, genes associated with resistance to heavy metals, biocides, and glyphosate were also detected. Halophilic behavior (tolerance to > 10% NaCl) of BA01 strain was confirmed by tolerance tests of NaCl minimal inhibitory concentration, whereas halotolerance associated genes katE and nhaA, which encodes for catalase and Na+/H+ antiporter cytoplasmic membrane, respectively, were in silico confirmed. Phylogenomics clustered BA01 with poultry- and human-associated ST162 lineages circulating in European and Asian countries. Important virulence genes, including the astA (a gene encoding an enterotoxin associated with human and animal infections) were detected, whereas in vivo experiments using the Galleria mellonella infection model confirmed the virulent behavior of the BA01 strain. WHO critical priority carbapenemase-producing pathogens in coastal water are an emerging threat that deserves the urgent need to assess the role of the aquatic environment in its global epidemiology.
Introduction
The rapid and global spread of carbapenemase-producing Enterobacterales has triggered an unprecedented public health crisis due to the lack of novel clinically effective antibiotics (Queenan and Bush, 2007; Nordmann et al., 2011a; Lee et al., 2022). Carbapenems are broad-spectrum β-lactam antibiotics that have been administered as a last-line resort, being generally reserved to treat life-threatening infections caused by multidrug-resistant (MDR) Gram-negative bacterial infections (Papp-Wallace et al., 2011; Lee et al., 2022). The production of carbapenemases by certain Enterobacterales and non-fermentative bacteria, can threaten the efficacy of these antimicrobials making them useless (Queenan and Bush, 2007; Nordmann et al., 2011a; Lee et al., 2022).
Due to their clinical impacts in human medicine, carbapenemase-producing bacteria were recent classified as critical priority pathogens by the World Health Organization (WHO) (Tacconelli et al., 2018). Particularly, the emergence of metallo-β-lactamase NDM-1-producing bacteria has been a phenomenon of global interest (Nordmann et al., 2011a,b; Dortet et al., 2014). Of epidemiological concern, although the successful spread of plasmids encoding blaNDM-type genes has been primarily related to nosocomial settings (Wu et al., 2019), there is growing evidence of their occurrence beyond the human medicine context (Mills and Lee, 2019; Ranjan and Thatikonda, 2021). Indeed, NDM-1-producing bacteria have been increasingly reported in environmental samples (mostly in anthropogenic-impacted aquatic environments) (Ranjan and Thatikonda, 2021). More critically, NDM-1-positive bacteria have begun to be documented in wild animals (Fischer et al., 2013; Liao et al., 2019; Mairi et al., 2020), which could indicate, in part, that these critical-priority bacteria can spill over into natural ecosystems, and then further spread in wildlife (Dolejska and Literak, 2019; Mills and Lee, 2019; Cohen et al., 2020; Mairi et al., 2020).
In this study, we report the identification of an NDM-1-positive Escherichia coli strain belonging to the international clone sequence type (ST) 162 in a pygmy sperm whale (Kogia breviceps), highlighting negative clinical and ecological implications related to the dissemination of WHO critical priority pathogens in the marine environment.
Materials and Methods
Bacterial Isolation, Identification, and Antimicrobial Susceptibility Testing
During a surveillance study conducted to investigate the occurrence and genomic features of critical priority Gram-negative pathogens circulating at the marine ecosystems in Brazil, part of the Grand Challenges Explorations—New Approaches to Characterize the Global Burden of Antimicrobial Resistance program, we characterized a multidrug-resistant E. coli recovered from a pygmy sperm whale (Kogia breviceps), during the COVID-19 lockdown.
The animal (Supplementary Figure 1) was received at CEPRAM/R3 Animal (Florianópolis, Santa Catarina state, Southern Brazil), as part of the Santos Basin Beach Monitoring Project, licensed by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) of the Brazilian Ministry of Environment under ABIO N° 755/2016. The whale was found stranded alive on October 19th, 2020, in a beach (−28.1663385: −48.6577602), located on the city of Imbituba, in Santa Catarina state, Southern Brazil. The animal was immediately monitored in the water by the Santos Basin Beach Monitoring Project team. External examination revealed multiple signs of interspecific interaction in the ventral and lateral regions of the body and a deep circular lesion consistent with bite marks from cookiecutter sharks (Isistius spp.). The whale was carefully monitored during transport, receiving benzodiazepines (Diazepam, 0.1 mg/kg, IM) and bronchodilators (Aminophylline, 4 mg/kg, IM, SID) to dilate the lungs’ airways. Upon arrival at Associação R3 Animal, in Florianópolis, the whale was transferred to a 60,000 L tank under continuous supervision. To replace the animal’s hydration, 1.5 L of water were initially administered through a 17 mm equine nasogastric tube and later the volume was increased to 3 L every 3 h and antibiotic therapy was started (Enrofloxacin, 5 mg/kg, IM, BID). On October 21, the animal begun to show signs of agitation, swimming erratically, jumping, and bending its body, even after being sedated. The veterinary staff observed prolonged periods of apnea and bradycardia. Cardiac massage and administration of emergency drugs were attempted, but the animal succumbed to death.
At necropsy, besides other gross alterations, the prescapular lymph nodes were clearly swollen. A yellowish purulent material was collected from the lymph nodes and placed in Stuart transport medium, and immediately sent to a microbiology laboratory for bacterial culture and antimicrobial susceptibility testing. The E. coli strain (BA01) was recovered, being identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Antimicrobial susceptibility was performed and interpreted according to the Clinical and Laboratory Standards Institute recommendations (CLSI, 2020). In this respect, human and veterinary antibiotics were tested, including amoxicillin-clavulanic acid, ceftazidime, cefotaxime, ceftriaxone, ceftiofur, cefepime, cefoxitin, aztreonam, ertapenem, meropenem, imipenem, ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole, fosfomycin, gentamicin, tetracycline, and amikacin. A drug-susceptible E. coli (ATCC 25922) was included as a control strain. Moreover, to determinate whether BA01 strain could survive in the marine environment, tolerance test of NaCl minimal inhibitory concentration (MIC) was performed using 0.1–15% NaCl solutions (Fernandes et al., 2020a).
Whole Genome Sequence Analysis
The whole genomic DNA of E. coli BA01 was extracted (PureLink™; Invitrogen) and used to prepare a library that was sequenced using the NextSeq550 platform (2 × 75-bp paired-end) (Illumina). Raw sequencing data were quality filtered to remove low-quality bases (Phred20 quality score) using Trimmomatic v0.32.1 The sequence reads were assembled De novo using default parameters of Unicycler v0.4.8.2 Draft genome sequence was automatically annotated using the NCBI Prokaryotic Genome Annotation Pipeline v.3.2.3 BA01 circular genome map (Figure 1) was performed using the Proksee platform4 and BLASTN.
FIGURE 1
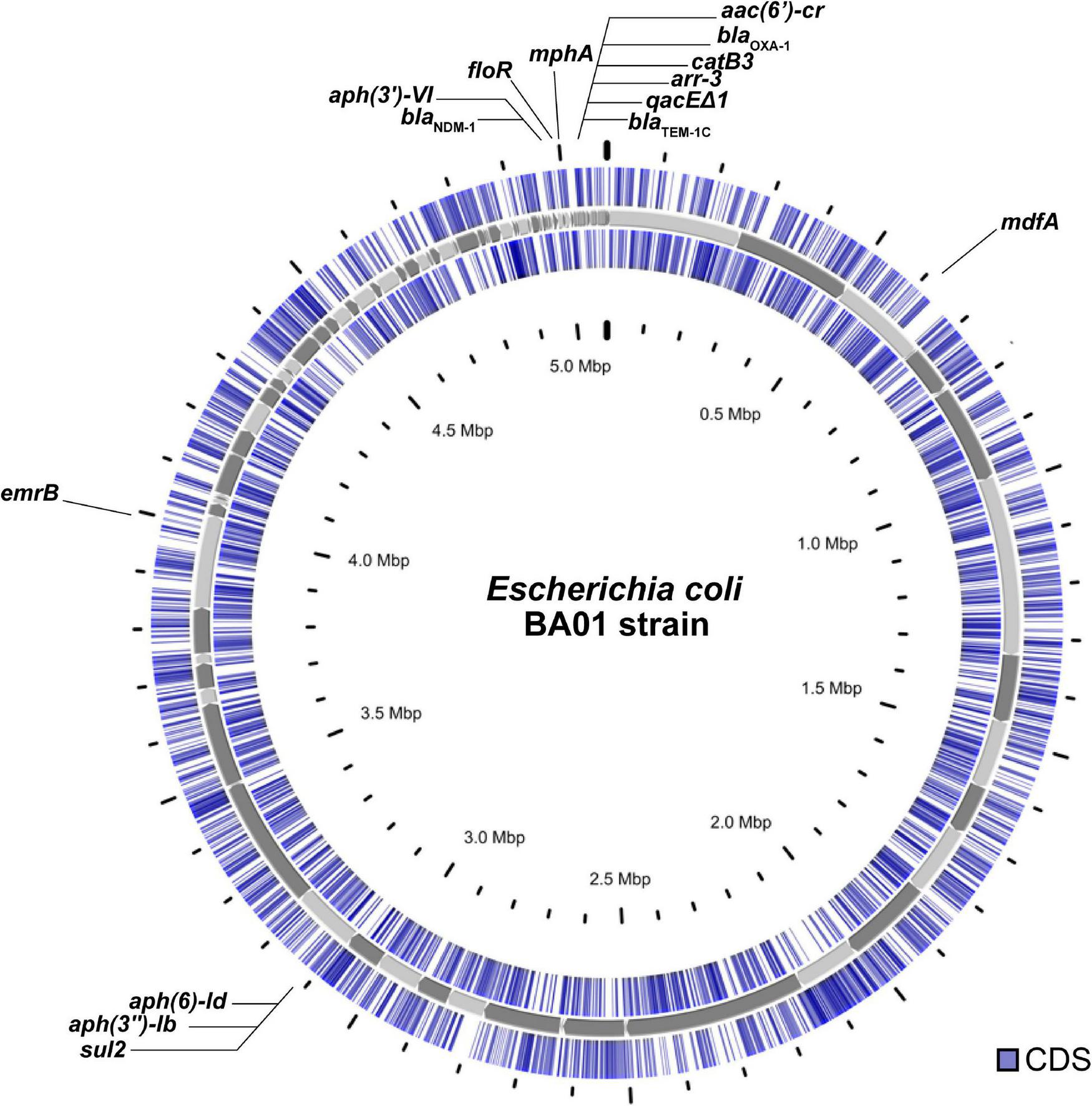
Circular genome view of the Escherichia coli BA01 strain.
Multilocus sequence type (MLST), plasmid replicons, resistome, virulome, type fimbrial, and serotype were performed in silico using MLST v2.0, PlasmidFinder v2.0, ResFinder 4.0, VirulenceFinder 2.0, FimTyper v1.0, and SerotypeFinder v2.0, respectively; available from the centre for Genomic Epidemiology.5 In addition, ABRicate v0.9.86 was used to screen putative virulence factors through VFDB database.7
The presence of heavy metal (HM) genes was predicted by comparison with the BacMet—Antibacterial Biocide and Metal Resistance Genes Database,8 whereas for detection of mercury, arsenic, and disinfectant resistance genes DRG (quaternary ammonium compounds), we performed alignment of sequenced reads against our in-house database. Moreover, the presence of halotolerance-associated genes (katE and nhaA) was in silico investigated using BLASTN. A ≥ 90% identity threshold was used as a filter for all accessed databases.
Phylogenetic Analysis
In order to compare BA01 with other E. coli strains, we performed a search for E. coli ST162 on Escherichia/Shigella database in Enterobase.9 For phylogenetic analysis purpose, FastANI v1.3210 was used to select the 30 genomes with highest average nucleotide identity (ANI) to BA01 among 542 genome assemblies of strains with data for country, year, and source of isolation downloaded from Enterobase. CSI phylogeny v1.411 was used with default settings to generate a maximum-likelihood phylogenetic tree with BA01 and the 30 selected genomes. E. coli ST162 strain W2-5 chromosome sequence (RefSeq accession number NZ_CP032989.1) was used as reference. ABRicate v1.0.1 (see text footnote 6) was used with ResFinder and PlasmidFinder databases to screen the genomes for antimicrobial resistance and plasmid replicons. Identity and coverage were set to 98 and 100%, respectively. Mutations in quinolone resistance-determining regions were assessed using CGE PointFinder pipeline.12 iTOL v613 was used to root the tree at midpoint and to annotate the tree with Enterobase and ABRicate data.
In vivo Virulence Assays in the Galleria mellonella Infection Model
To evaluate the virulence potential of strains, an in vivo experiment was carried out with the Galleria mellonella infection model (Tsai et al., 2016; Moura et al., 2018). G. mellonella larvae, of nearly 250–350 mg, were inoculated with 105 CFU of each strain and survival analysis was evaluated each hour, for 96 h. For each strain, groups of G. mellonella containing five larvae were evaluated. E. coli strain ATCC 25922 was used as non-virulent control, whereas hypervirulent meningitis/sepsis-associated K1 E. coli MNEC RS218 strain was used as hypervirulent control samples (Fuentes-Castillo et al., 2021a). Data were analyzed by the log rank test, with P of 0.05 indicating statistical significance (Graph Pad Software, San Diego, CA, United States).
Plasmid Conjugation
To evaluate the transferability of the blaNDM–1 gene, conjugation experiments were carried out. Plasmid conjugation was assessed by mating-out assay using E. coli BA01 and sodium azide-resistant E. coli C600 (lactose-negative) as donor and recipient strains, respectively. Transconjugants were obtained from MacConkey agar plates supplemented with ertapenem (4 μg/mL) and sodium azide (100 μg/mL).
Results and Discussion
The E. coli strain BA01 strain displayed a MDR profile to amoxicillin/clavulanic acid, cefotaxime, ceftriaxone, cefepime, cefoxitin, ceftiofur, ertapenem (MIC = 16 mg/L), imipenem (MIC = 16 mg/L), meropenem (MIC = 16 mg/L), amikacin, ciprofloxacin, enrofloxacin, levofloxacin, chloramphenicol, and tetracycline (Magiorakos et al., 2012), remaining susceptible to aztreonam, gentamicin, sulfamethoxazole/trimethoprim, and fosfomycin. Additionally, BA01 strain displayed NaCl tolerance (>10%), confirming its ability to survive in the marine environment.
Genomic analysis revealed a broad resistome, with genes conferring resistance to β-lactams (blaNDM–1, blaTEM–1C, and blaOXA–1), aminoglycosides [aph(6)-Id, and aph(3″)-Ib, aph(3′)-VI], macrolide, (ermB, mdfA, and mphA), rifamycin (arr-3), quinolones [aac(6′)-Ib-cr, and qnrB6], phenicols (catB3 and floR), sulfonamide (sul1 and sul2), and tetracycline (tetA) (Table 1). Additionally, chromosomal point mutations in ParC (S80I) and GyrA (S83L and D87N) were detected, which may justify the fluoroquinolone-resistant profile. Furthermore, plasmid replicons IncFIB and IncA/C2 were also detected (Table 1).
TABLE 1
| Strain | BA01 |
| Genome size (Mbp) | 5.7 |
| No. of CDSa | 4,744 |
| tRNA (n) | 56 |
| rRNA (n) | 71 |
| Non-coding RNA (n) | 11 |
| Pseudogenes | 136 |
| CRISPR | 2 |
| MLST (ST)b | 162 |
| Resistome | |
| β-lactams | blaNDM–1, blaTEM–1C, blaOXA–1 |
| Aminoglycosides | aph(6)-Id, aph(3″)-Ib, aph(3′)-VI |
| Fluoroquinolones | aac(6′)-Ib-cr, qnrB6, gyrA (S83F, D87A), parC (S80I) |
| Tetracyclines | tet(A) |
| Rifamycins | arr-3 |
| Phenicols | catB3, floR |
| Sulphonamides | sul1, sul2 |
| Macrolides | ermB, mdf, mphA |
| Heavy metal and Biocides | acrEF, arsBCR, emrDK, mdtEFKN, mvrC, phnCDGHIJKLMNOP, tehAB, tolC, yjiO |
| Halotolerance genes | katE, nhaA |
| Virulome | astA, entA, entC, entE, entB, entD, entF, entS, csgB, csgD, csgF, csgG, espX4, espX5, fdeC, fepA, fepB, fepC, fepD, fepG, fes, espL1, espR1, fimA, fimB, fimC, fimD, fimE, fimF, fimG, fimH, fimI, gspC, gspD, gspE, gspF, gspG, gspH, gspI, gspJ, gspK, gspL, gspM, espX1, iroB, iroC, iroD, iroE, iroN, iucA, iucB, iucC, iucD, iutA, ompA, ykgK/ecpR, yagZ/ecpA, yagY/ecpB, yagX/ecpC, yagW/ecpD, yagV/ecpE |
| Plasmidome | IncC-ST3, IncFIB [F18:A-:B1] |
| GenBank accession number | JAENJJ000000000 |
| OneBR ID | ONE128 |
Genomic and epidemiological data of E. coli strain BA01 isolated from a pygmy sperm whale (Kogia breviceps) in Brazil.
aCDSs, coding sequences.
bMLST, Multilocus sequence type. ST, sequence type.
Halotolerance associated genes katE and nhaA, which encodes for catalase and Na + /H + antiporter cytoplasmic membrane, respectively, were in silico predicted (Rimon et al., 2007; Prodhan et al., 2008). Furthermore, genes conferring resistance to heavy metals [i.e., arsenic resistance (arsBCR), tellurite (tehAB)] and biocides [i.e., quaternary ammonium compounds (acrEF, emrK, mdtEFKN, mvrC, tolC, yjiO) and glyphosate (phnCDEFGHIJKLMNOP)] were also detected (Table 1).
The blaNDM–1 gene was located on the IncC plasmid and was successfully transferred to the E. coli C600 strain, being confirmed by PCR-based replicon typing of transconjugant (Carattoli et al., 2005). The transconjugant E. coli displayed resistance to amoxicillin/clavulanic acid, cefotaxime, ceftriaxone, cefepime, cefoxitin, ceftiofur, ertapenem, imipenem, meropenem, amikacin, ciprofloxacin, enrofloxacin, levofloxacin, chloramphenicol, and tetracycline, remaining susceptible to aztreonam, gentamicin, sulfamethoxazole/trimethoprim, and fosfomycin. However, due to limitations of short-read sequencing technology, it was not possible to obtain complete nucleotide sequences of this plasmid. Further analysis revealed that the aminoglycoside 3′-phosphotransferase [aph(3′)-VI] and the carbapenemase-encoding blaNDM–1 genes were located downstream of ISAba125 and ISAba14 mobile genetic elements, respectively, along with the bleomycin resistance protein (bleMBL), N-(5′-phosphoribosyl)anthranilate isomerase (iso) and twin-arginine translocation pathway signal protein (tat) being harbored by a Tn125-like transposon (Figure 2A) identified in a 8,630-bp contig highly similar (100% nucleotide identity; 100% query coverage) to that found on Klebsiella pneumoniae plasmids (Genbank accession number: LR697132.1; LR697099.1; CP021961.1) and close related (100% nucleotide identity; 70% query coverage) to pAB17 plasmid (Genbank accession number: MT002974.1) identified in a nosocomial lineage of Acinetobacter baumannii in Brazil (Rossi et al., 2021). In addition, we also identified a class 1 integron carrying an integron-integrase gene (intI1) along with other genes encoding antimicrobial resistance, including aminoglycoside-6′-N-acetyltransferase-Ib [aac(6′)Ib-cr], class D beta-lactamase OXA-1 (blaOXA–1), chloramphenicol O-acetyltransferase (catB3), rifampin ADP-ribosyl transferase (arr-3), and quaternary ammonium compound (qacEΔ1) (Figure 2B). In this respect, there is a growing concern about the spread of biocides contaminating aquatic environments, especially QACs, since these compounds are widely used in domiciliary and hospital setting, including disinfectants formulations (Zubris et al., 2017). As a consequence, ecosystems impacted by heavy metal and biocides could favor the selection and persistence of MDR bacteria harboring broad resistomes (Baker-Austin et al., 2006; Kim et al., 2018).
FIGURE 2
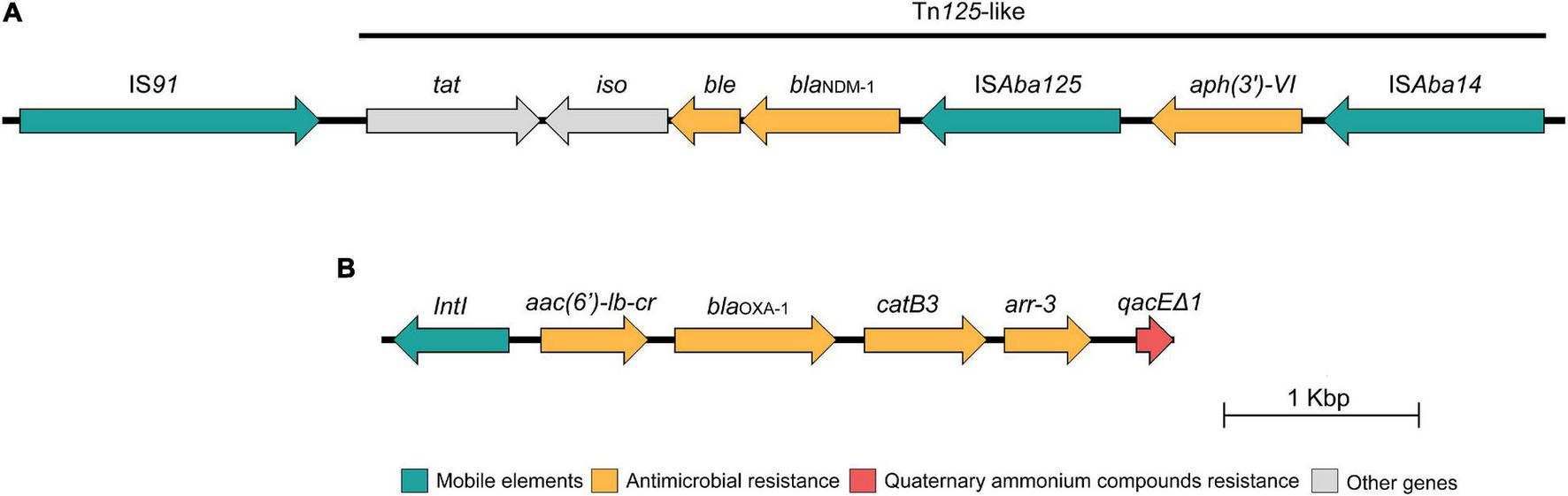
Schematic presentation of the genetic environment context of the blaNDM–1 gene (A) and the class I integron (B) identified in the Escherichia coli BA01 strain. Arrows indicate protein-coding sequences and are colored by function.
E. coli ST162 is a pandemic lineage that has been isolated from multiple sources including clinical, environmental, and domestic and wild animal samples (Fuentes-Castillo et al., 2020). When compared with BA01, the 30 selected E. coli ST162 genomes for the phylogenetic tree had ANI ranging between 99.7994 and 99.8948%. Among the 31 genomes analyzed, SNP counts variated between 0 and 1,343 (Supplementary Table 1).
Phylogenetic analysis revealed that BA01 is closely related to two strains isolated in 2018 from poultry in Hungary, differing from both strains by 59 SNPs (Figure 3 and Supplementary Table 1). While these two strains from Hungary share the same resistome, BA01 has several resistance genes that are absent in these strains [blaNDM–1, blaOXA–1, blaTEM–1C, aac(6′)-lb-cr, aph(3′)-VI, sul1, catB3, erm(B), mph(A), and qnrB6], as well as an IncC-type plasmid.
FIGURE 3
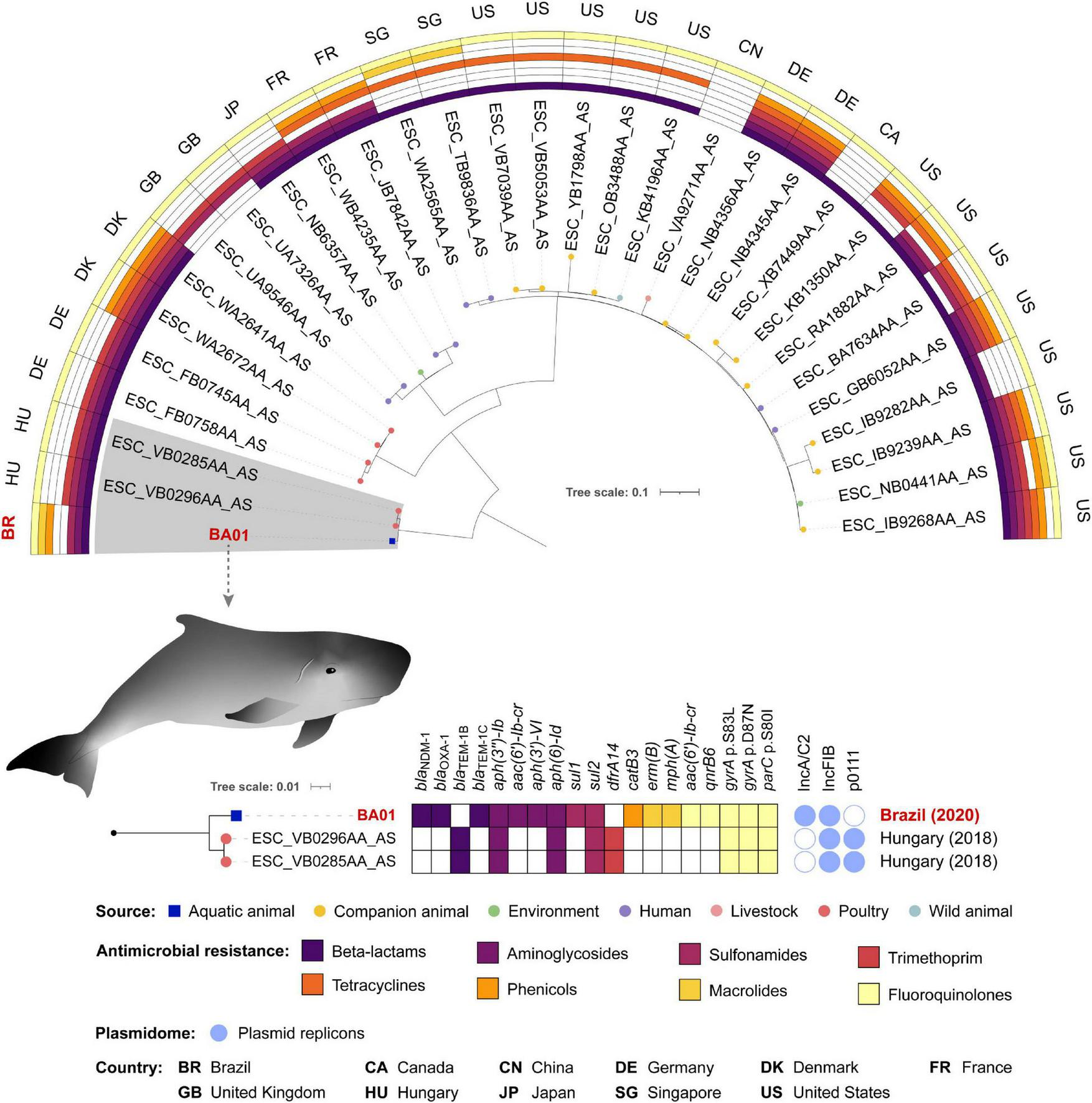
Phylogenetic tree of 31 Escherichia coli ST162 strains, plotted in a 180° arc, as well as their predicted phenotype for antimicrobial resistance, source and country of isolation. The highlighted clade with 3 strains is shown in a subtree with resistome, plasmidome, country and year of collection of each isolate.
Virulome of BA01 strain, included genes/operons that encodes to enteroaggregative EAST-1 heat-stable toxin (astA), iron acquisition systems (entACEDFS, fepABCDG, fes, iroBCDEN, iucABCD, and iutA), adherence factors (fdeC, ecpRABCDE, csgBDFG, and fimABCDEFGHI), secretion systems components (espL1, espR1, espX1, espX4, espX5, and gspCDEFGHIJKLM) and outer membrane protein A (ompA). Of note, the astA virulence factor has been commonly found in E. coli strains associated with extra-intestinal disease in animals and outbreaks of diarrhea in humans and animals worldwide (Zajacova et al., 2012; Silva et al., 2014; Maluta et al., 2016; Ochi et al., 2017; Dubreuil, 2019). Indeed, the presence of astA gene along with other virulent associated genes detected in the BA01 genome (i.e., genes encoding for adherence factors and iron acquisition systems) could favor the virulent behavior of this strain (Fuentes-Castillo et al., 2020, Fuentes-Castillo et al., 2021a), which was supported by in vivo experiments using G. mellonella larvae. In this respect, the E. coli BA01 strain and the hypervirulent meningitis/sepsis-associated K1 E. coli MNEC RS218 strain killed 70 and 100% of the G. mellonella larvae within 80 h post-infection, respectively, presenting higher mortality rates than the non-virulent E. coli ATCC 25922 strain (P < 0.05) (Figure 4).
FIGURE 4
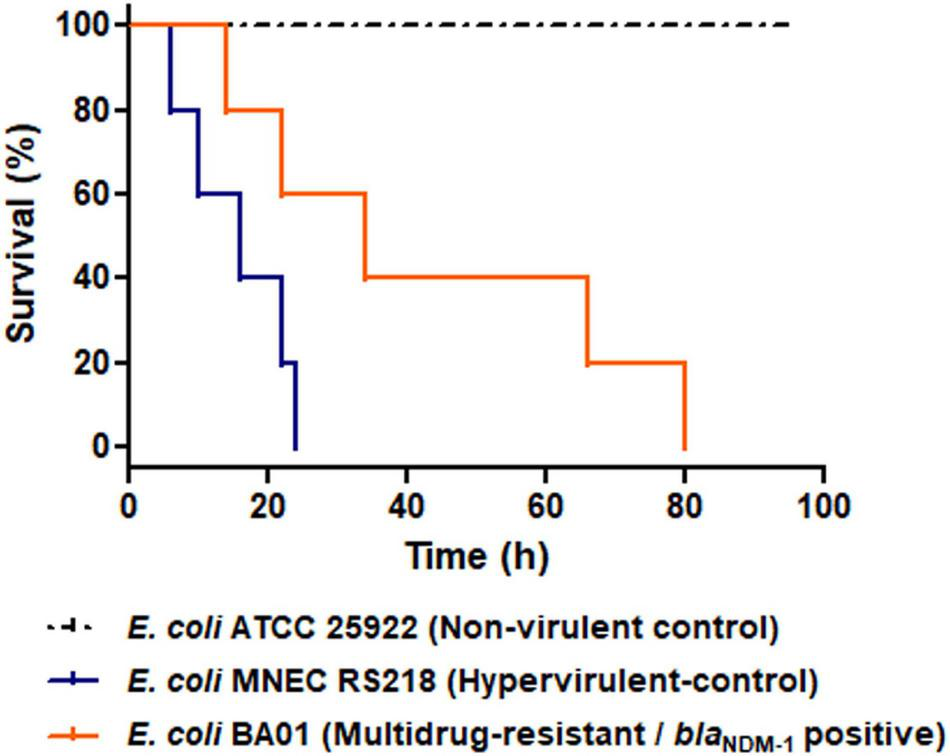
Virulent behavior of NDM-1-positive Escherichia coli isolated from a pygmy sperm whale (Kogia breviceps), in Brazil. Kaplan-Meier survival curves of G. mellonella infected with 105 CFU/larvae of the NDM-1-positive E. coli BA01 strain (orange line), the non-virulent E. coli ATCC 25922 strain (dashed black line) and the hypervirulent meningitis/sepsis associated E. coli strain MNEC RS218 strain (blue line). The E. coli BA01 strain and the hypervirulent meningitis/sepsis-associated K1 E. coli MNEC RS218 strain killed 70 and 100% of the G. mellonella larvae within 80 h post-infection, respectively, leading to higher mortality rates than the non-virulent E. coli ATCC 25922 strain (P < 0.05). For each strain, groups of G. mellonella containing five larvae were evaluated in three separate experiments.
The blaNDM–1 gene was firstly reported in Klebsiella pneumoniae and E. coli recovered from a patient in Sweden that was transferred from a New Delhi hospital in 2008 (Yong et al., 2009). Since then, NDM-type carbapenemases have triggered global attention due to their rapid epidemiologic expansion among Enterobacterales and Acinetobacter spp., and more rarely, in Pseudomonas aeruginosa (Dortet et al., 2014). Of note, recent reports have documented the spreading of NDM producers beyond the boundary of human healthcare settings where they were originally related (Ranjan and Thatikonda, 2021). The environmental spread of NDM-producing bacteria has been associated to several human activities that result in chemical and microbial pollution mostly in aquatic environments (Ranjan and Thatikonda, 2021).
Particularly for marine environments, it has been demonstrated that anthropogenic pollution by improper discharge of effluents from hospitals, domestic sewage, and industrial, urban and/or agricultural wastewaters can runoff to ocean carrying MDR bacteria, antibiotic-resistant genes (ARGs), and heavy metals (Hatosy and Martiny, 2015; Li et al., 2020; Zhang et al., 2022). While it has been suggested that beaches and coastal waters from urbanized and densely populated coastlines are more prone to be contaminated by WHO critical priority bacteria, ocean currents and migratory animals can also favor the spread of these pathogens through long distances, sometimes reaching remote geographical areas with limited human footprints such Polar regions (Hernández and González-Acuña, 2016; Akhil Prakash et al., 2021) and inhospitable oceanic islands (Ewbank et al., 2022).
In this investigation, we report the occurrence of a carbapenem-resistant NDM-1- producing E. coli isolated from a pygmy sperm whale. In this regard, the pygmy sperm whale is a small cetacean from the Kogiidae family that is found in mesopelagic regions near the continental shelves (between 600 and 1,200 m depth) of the tropical and temperate Atlantic, Indian, and Pacific Oceans (Moura et al., 2016; Brentano and Petry, 2020; Kiszka and Braulik, 2020). Although cetacean research in oceanic waters has significantly progressed over the last decades, there is scarce information on the population, distribution, and behavior of pygmy sperm whales (Kiszka and Braulik, 2020). This could be explained by their short surfacing interval, cryptic surface behavior, and long deep dives, which make challenging to see these whales in the ocean (Kiszka and Braulik, 2020). Indeed, most data come from stranded animals, being generally affected by anthropogenic material, including accidental ingestion of plastic debris (Brentano and Petry, 2020). Alarmingly, increasing reports of WHO critical priority Gram-negative pathogens (MCR-type, carbapenemase- and/or ESBL-producing bacteria) on the Brazilian coast have been occurred in the last decade, which may indicate, in part, the adaptation of such pathogens in the sea. In this regard, the occurrence of such bacteria was documented in coastal waters from in densely coastal areas (Montezzi et al., 2015; Campana et al., 2017; Fernandes et al., 2017, 2020a; Paschoal et al., 2017; Sellera et al., 2017a; Corrêa et al., 2021; Furlan et al., 2021; Cordeiro-Moura et al., 2022), in marine fishes (Sellera et al., 2018a) and benthic invertebrates (Sellera et al., 2018b; Monte et al., 2019; Fernandes et al., 2020b), and also infecting penguins (Sellera et al., 2017b; Wink et al., 2021), a sea turtle (Goldberg et al., 2019), and a dolphin (Fuentes-Castillo et al., 2021b). More specifically, the presence of NDM-1-producing bacteria have been so far identified in K. pneumoniae and Acinetobacter chengduensis from coastal waters of Rio de Janeiro (Campana et al., 2017; Paschoal et al., 2017; Corrêa et al., 2021), whereas a single case of E. coli carrying blaNDM–1 infecting a penguin was also documented in the South coast of Brazil (Wink et al., 2021).
Conclusion
In summary, we report for the first time the occurrence of the NDM-1-producing E. coli ST162 clone in a marine cetacean. Our findings are worrisome because may indicate that NDM-producing E. coli can spill over from the human clinical context to the aquatic environment reaching marine animals with serious clinical implications in wildlife with a further threat to marine ecosystem maintenance. Indeed, recent studies have already demonstrated that WHO critical priority E. coli may display halotolerant behavior (Fernandes et al., 2020a), which could favor their spread and persistence in the marine environment. Considering that marine cetaceans are usually found in nearshore waters, exposure to critical priority carbapenemase-producing bacteria could emerge as a new challenge for the conservation of these threatened species. Last but not least, strengthening the epidemiological surveillance of antimicrobial resistance in the ocean is crucial to understanding the ecological implications of these bacteria on marine populations.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was reviewed and approved by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) of the Brazilian Ministry of Environment under ABIO N°755/2016; all animal handling procedures and protocols followed the required ethics and welfare practices.
Author contributions
FS, BC, DF-C, BF, ES, and FE performed data analysis. FS, BC, DF-C, BF, ES, FE, and HF conducted the experiments. FS, BC, DF-C, BF, ES, FE, DG, and NL wrote the manuscript. NL designed, coordinated the project, and supervised. MA, LS, SS, CK, and NL reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Bill and Melinda Gates Foundation (Grand Challenges Explorations Brazil OPP1193112). Under the grant conditions of the Foundation, a CC BY or equivalent license is applied to the author accepted manuscript version arising from this submission. Additionally, this study was supported by the Fundação de Amparo ã Pesquisa do Estado de São Paulo (2020/08224-9 and 2019/15778-4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (AMR 443819/2018-1, 312249/2017-9, 422984/2021-3, and 314336/2021-4), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (88882.333054/2019-01). NL was a research fellow of CNPq (314336/2021-4). BC and HF were research fellows of CAPES (88882.333054/2019-01 and 88887.506496/2020-00). FE was a research fellow of FAPESP (2019/15578-4). BF was a research fellow of PNPD/CAPES (88887.358057/2019-00).
Acknowledgments
We are grateful to FAPESP, CAPES, and CNPq. We also thank Cefar Diagnóstica Ltda. (São Paulo, Brazil) for kindly supplying the antibiotic disks for susceptibility testing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.915375/full#supplementary-material
Footnotes
1.^https://github.com/timflutre/trimmomatic
2.^https://github.com/rrwick/Unicycler
3.^www.github.com/tseemann/prokka
5.^http://genomicepidemiology.org/
6.^https://github.com/tseemann/abricate
7.^https://github.com/haruosuz/vfdb
8.^http://bacmet.biomedicine.gu.se
9.^https://enterobase.warwick.ac.uk
10.^https://github.com/ParBLiSS/FastANI
11.^https://cge.cbs.dtu.dk/services/CSIPhylogeny/
References
1
Akhil PrakashE.HromádkováT.JabirT.VipindasP. V.KrishnanK. P.Mohamed HathaA. A.et al (2021). Dissemination of multidrug resistant bacteria to the polar environment - Role of the longest migratory bird Arctic tern.Sci. Total Environ.815:152727. 10.1016/j.scitotenv.2021.152727
2
Baker-AustinC.WrightM. S.StepanauskasR.McArthurJ. V. (2006). Co-selection of antibiotic and metal resistance.Trends Microbiol.14176–182. 10.1016/j.tim.2006.02.006
3
BrentanoR.PetryM. V. (2020). Marine debris ingestion and human impacts on the Pygmy sperm whale (Kogia breviceps) in southern Brazil.Mar. Pollut. Bull.150:110595. 10.1016/j.marpolbul.2019.110595
4
CampanaE. H.MontezziL. F.PaschoalR. P.PicãoR. C. (2017). NDM-producing Klebsiella pneumoniae ST11 goes to the beach.Int. J. Antimicrob. Agents.49119–121. 10.1016/j.ijantimicag.2016.10.006
5
CarattoliA.BertiniA.VillaL.FalboV.HopkinsK. L.ThrelfallE. F. (2005). Identification of plasmids by PCR-based replicon typing.J. Microbiol. Methods.148219–228. 10.1016/j.mimet.2005.03.018
6
CohenR.PaikinS.RokneyA.Rubin-BlumM.AstrahanP. (2020). Multidrug-resistant Enterobacteriaceae in coastal water: an emerging threat.Antimicrob. Resist. Infect. Control.9169. 10.1186/s13756-020-00826-2
7
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing —Twenty-Ninth. Edition: M100-S30.European: CLSI.
8
Cordeiro-MouraJ. R.KraycheteG. B.LongoL.CorrêaL. L.da SilvaN.CampanaE. H.et al (2022). Description and comparative genomic analysis of a Mcr-1-carrying Escherichia coli ST683/CC155 recovered from touristic coastal water in Northeastern Brazil.Infect. Genet. Evol.97:105196. 10.1016/j.meegid.2021.105196
9
CorrêaL. L.KraycheteG. B.RezendeA. M.CampanaE. H.Lima-MoralesD.WinkP. L.et al (2021). NDM-1-encoding plasmid in Acinetobacter chengduensis isolated from coastal water.Infect. Genet. Evol.93:104926. 10.1016/j.meegid.2021.104926
10
DolejskaM.LiterakI. (2019). Wildlife Is Overlooked in the Epidemiology of Medically Important Antibiotic-Resistant Bacteria.Antimicrob. Agents Chemother.63e1167–e1119. 10.1128/AAC.01167-19
11
DortetL.PoirelL.NordmannP. (2014). Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria.Biomed. Res. Int.2014:249856. 10.1155/2014/249856
12
DubreuilJ. D. (2019). EAST1 toxin: an enigmatic molecule associated with sporadic episodes of diarrhea in humans and animals.J. Microbiol.57541–549. 10.1007/s12275-019-8651-4
13
EwbankA. C.Fuentes-CastilloD.SacristánC.CardosoB.EspositoF.FugaB.et al (2022). Extended-spectrum β-lactamase (ESBL)-producing Escherichia coli survey in wild seabirds at a pristine atoll in the southern Atlantic Ocean, Brazil: first report of the O25b-ST131 clone harboring BlaCTX–M–8.Sci. Total Environ.806:150539. 10.1016/j.scitotenv.2021.150539
14
FernandesM. R.SelleraF. P.EspositoF.SabinoC. P.CerdeiraL.LincopanN. (2017). Colistin-Resistant Mcr-1-Positive Escherichia coli on Public Beaches, an Infectious Threat Emerging in Recreational Waters.Antimicrob. Agents Chemother.61e234–e217. 10.1128/AAC.00234-17
15
FernandesM. R.SelleraF. P.MouraQ.EspositoF.SabinoC. P.LincopanN. (2020a). Identification and genomic features of halotolerant extended spectrum- β-lactamase (CTX-M)-producing Escherichia coli in urban-impacted coastal waters, Southeast Brazil. Mar. Pollut. Bull.150:110689. 10.1016/j.marpolbul.2019.110689
16
FernandesM. R.SelleraF. P.CunhaM. P. V.LopesR.CerdeiraL.LincopanN. (2020b). Emergence of CTX-M-27-producing Escherichia coli of ST131 and clade C1-M27 in an impacted ecosystem with international maritime traffic in South America. J. Antimicrob. Chemother.75, 1647–1649. 10.1093/jac/dkaa069
17
FischerJ.SchmogerS.JahnS.HelmuthR.GuerraB. (2013). NDM-1 carbapenemase-producing Salmonella enterica subsp. Enterica serovar Corvallis isolated from a wild bird in Germany.J. Antimicrob. Chemother.682954–2956. 10.1093/jac/dkt260
18
Fuentes-CastilloD.EspositoF.CardosoB.DalazenG.MouraQ.FugaB.et al (2020). Genomic data reveal international lineages of critical priority Escherichia coli harbouring wide resistome in Andean condors (Vultur gryphus Linnaeus, 1758).Mol. Ecol.291919–1935. 10.1111/mec.15455
19
Fuentes-CastilloD.Navas-SuárezP. E.GondimM. F.EspositoF.SacristánC.FontanaH.et al (2021a). Genomic characterization of multidrug-resistant ESBL-producing Escherichia coli ST58 causing fatal colibacillosis in critically endangered Brazilian merganser (Mergus octosetaceus). Transbound. Emerg. Dis.68, 258–266. 10.1111/tbed.13686
20
Fuentes-CastilloD.SelleraF. P.GoldbergD. W.FontanaH.EspositoF.CardosoB.et al (2021b). Colistin-resistant Enterobacter kobei carrying mcr-9.1 and bla CTX-M-15 infecting a critically endangered franciscana dolphin (Pontoporia blainvillei), Brazil.Transbound. Emerg. Dis.68, 3048–3054. 10.1111/tbed.13980
21
FurlanJ. P. R.RamosM. S.Dos SantosL. D. R.GalloI. F. L.LopesR.StehlingE. G. (2021). Appearance of Mcr-9, BlaKPC, cfr and other clinically relevsant antimicrobial resistance genes in recreation waters and sands from urban beaches.Brazil. Mar. Pollut. Bull.167:112334. 10.1016/j.marpolbul.2021.112334
22
GoldbergD. W.FernandesM. R.SelleraF. P.CostaD. G. C.Loureiro BracarenseA. P.LincopanN. (2019). Genetic background of CTX-M-15-producing Enterobacter hormaechei ST114 and Citrobacter freundii ST265 co-infecting a free-living green turtle (Chelonia mydas).Zoonoses Public Health66540–545. 10.1111/zph.12572
23
HatosyS. M.MartinyA. C. (2015). The ocean as a global reservoir of antibiotic resistance genes.Appl. Environ. Microbiol.817593–7599. 10.1128/AEM.00736-15
24
HernándezJ.González-AcuñaD. (2016). Anthropogenic antibiotic resistance genes mobilization to the polar regions.Infect. Ecol. Epidemiol.6:32112. 10.3402/iee.v6.32112
25
KimM.WeigandM. R.OhS.HattJ. K.KrishnanR.TezelU.et al (2018). Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance.Appl. Environ. Microbiol.84e1201–e1218. 10.1128/AEM.01201-18
26
KiszkaJ.BraulikG. (2020). Kogia breviceps. The IUCN Red List of Threatened Species 2020. e.T11047A50358334. Available online at: https://www.iucnredlist.org/species/11047/50358334[accessed on Feb 3, 2022].
27
LeeY. L.ChenH. M.HiiI. M.HsuehP. R. (2022). Carbapenemase-producing Enterobacterales infections: recent advances in diagnosis and treatment.Int. J. Antimicrob. Agents.59:106528. 10.1016/j.ijantimicag.2022.106528
28
LiW.SuH.CaoY.WangL.HuX.XuW.et al (2020). Antibiotic resistance genes and bacterial community dynamics in the seawater environment of Dapeng Cove, South China.Sci. Total Environ.723:138027. 10.1016/j.scitotenv.2020.138027
29
LiaoX.YangR. S.XiaJ.ChenL.ZhangR.FangL. X.et al (2019). High colonization rate of a novel carbapenem-resistant Klebsiella lineage among migratory birds at Qinghai Lake.China. J. Antimicrob. Chemother.742895–2903. 10.1093/jac/dkz268
30
MagiorakosA.-P.SrinivasanA.CareyR. B.CarmeliY.FalagasM. E.GiskeC. G.et al (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.18, 268–281. 10.1111/j.1469-0691.2011.03570.x
31
MairiA.BarraudO.MuggeoA.de ChampsC.TouatiA. (2020). Genomic analysis of one Multidrug-resistant Klebsiella pneumoniae ST11 strain recovered from Barbary Deer (Cervus elaphus barbarus) in the Akfadou forest, Algeria.Algeria. J. Glob. Antimicrob. Resist.22515–518. 10.1016/j.jgar.2020.04.027
32
MalutaR. P.LeiteJ. L.RojasT.ScaletskyI.GuastalliE.RamosM. C.et al (2016). Variants of AstA gene among extra-intestinal Escherichia coli of human and avian origin.FEMS Microbiol. Lett.364:fnw285. 10.1093/femsle/fnw285
33
MillsM. C.LeeJ. (2019). The threat of carbapenem-resistant bacteria in the environment: evidence of widespread contamination of reservoirs at a global scale.Environ. Pollut.255:113143. 10.1016/j.envpol.2019.113143
34
MonteD. F.SelleraF. P.FernandesM. R.MouraQ.LandgrafM.LincopanN. (2019). Genome Sequencing of an Escherichia coli Sequence Type 617 Strain Isolated from Beach Ghost Shrimp (Callichirus major) from a Heavily Polluted Ecosystem Reveals a Wider Resistome against Heavy Metals and Antibiotics.Microbiol. Resour. Announc.8:e1471–e1418. 10.1128/MRA.01471-18
35
MontezziL. F.CampanaE. H.CorrêaL. L.JustoL. H.PaschoalR. P.da SilvaI. L.et al (2015). Occurrence of carbapenemase-producing bacteria in coastal recreational waters.Int. J. Antimicrob. Agents.45174–177. 10.1016/j.ijantimicag.2014.10.016
36
MouraJ. F.Acevedo-TrejosE.TavaresD. C.MeirellesA. C.SilvaC. P.OliveiraL. R.et al (2016). Stranding Events of Kogia Whales along the Brazilian Coast.PLoS One11:e0146108. 10.1371/journal.pone.0146108
37
MouraQ.FernandesM. R.SilvaK. C.MonteD. F.EspositoF.DropaM.et al (2018). Virulent nontyphoidal Salmonella producing CTX-M and CMY-2 β-lactamases from livestock, food and human infection, Brazil. Virulence9, 281–286. 10.1080/21505594.2017.1279779
38
NordmannP.NaasT.PoirelL. (2011a). Global spread of Carbapenemase-producing Enterobacteriaceae.Emerg. Infect. Dis.171791–1798. 10.3201/eid1710.110655
39
NordmannP.PoirelL.WalshT. R.LivermoreD. M. (2011b). The emerging NDM carbapenemases.Trends Microbiol.19588–595. 10.1016/j.tim.2011.09.005
40
OchiS.ShahM.OdoyoE.BundiM.MiringuG.GuyoS.et al (2017). An Outbreak of Diarrhea in Mandera, Kenya, Due to Escherichia coli Serogroup O-Nontypable Strain That Had a Coding Gene for Enteroaggregative E. coli Heat-Stable Enterotoxin 1.Am. J. Trop. Med. Hyg.96457–464. 10.4269/ajtmh.16-0310
41
Papp-WallaceK. M.EndimianiA.TaracilaM. A.BonomoR. A. (2011). Carbapenems: past, present, and future.Antimicrob. Agents Chemother.554943–4960. 10.1128/AAC.00296-11
42
PaschoalR. P.CampanaE. H.CorrêaL. L.MontezziL. F.BarruetoL. R. L.da SilvaI. R.et al (2017). Concentration and Variety of Carbapenemase Producers in Recreational Coastal Waters Showing Distinct Levels of Pollution.Antimicrob. Agents Chemother.61e1963–e1917. 10.1128/AAC.01963-17
43
ProdhanS. H.HossainA.NagamiyaK.KomamineA.MorishimaH. (2008). Improved salt tolerance and morphological variation in indica rice (Oryza sativa L.) transformed with a catalase gene from E. coli.Plant Tissue Cult. Biotechnol.1857–63. 10.3329/ptcb.v18i1.3266
44
QueenanA. M.BushK. (2007). Carbapenemases: the versatile beta-lactamases.Clin. Microbiol. Rev.20440–458. 10.1128/CMR.00001-07
45
RanjanR.ThatikondaS. (2021). β-Lactam Resistance Gene NDM-1 in the Aquatic Environment: a Review.Curr. Microbiol.783634–3643. 10.1007/s00284-021-02630-6
46
RimonA.TzuberyT.PadanE. (2007). Monomers of the NhaA Na+/H+ antiporter of Escherichia coli are fully functional yet dimers are beneficial under extreme stress conditions at alkaline pH in the presence of Na+ or Li+.J. Biol. Chem.1426810–26821. 10.1074/jbc.M704469200
47
RossiI.RoyerS.FerreiraM.BragaI. A.CamposP.BatistãoD.et al (2021). Novel ST1465/CC216 Nosocomial Lineage of Carbapenem-Resistant Acinetobacter baumannii Harboring an Unusual Plasmid Carrying BlaNDM–1 Gene.Microb. Drug. Resist.27471–475. 10.1089/mdr.2020.0219
48
SelleraF. P.FernandesM. R.MouraQ.CarvalhoM. P. N.LincopanN. (2018a). Extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in wild fishes from a polluted area in the Atlantic Coast of South America.Mar. Pollut. Bull.135183–186. 10.1016/j.marpolbul.2018.07.012
49
SelleraF. P.FernandesM. R.MouraQ.LopesR. B.SouzaT. A.CerdeiraL.et al (2018b). Draft genome sequence of a BlaCMY–2/IncI1-harbouring Escherichia coli D:ST457 isolated from coastal benthic organisms.J. Glob. Antimicrob. Resist.1483–84. 10.1016/j.jgar.2018.06.010
50
SelleraF. P.FernandesM. R.MouraQ.SouzaT. A.CerdeiraL.LincopanN. (2017a). Draft genome sequence of Enterobacter cloacae ST520 harbouring BlaKPC–2, blaCTX–M–15 and BlaOXA–17 isolated from coastal waters of the South Atlantic Ocean.J. Glob. Antimicrob. Resist.10279–280. 10.1016/j.jgar.2017.07.017
51
SelleraF. P.FernandesM. R.SartoriL.CarvalhoM. P.EspositoF.NascimentoC. L.et al (2017b). Escherichia coli carrying IncX4 plasmid-mediated Mcr-1 and BlaCTX–M genes in infected migratory Magellanic penguins (Spheniscus magellanicus).J. Antimicrob. Chemother.721255–1256. 10.1093/jac/dkw543
52
SilvaL. E.SouzaT. B.SilvaN. P.ScaletskyI. C. (2014). Detection and genetic analysis of the enteroaggregative Escherichia coli heat-stable enterotoxin (EAST1) gene in clinical isolates of enteropathogenic Escherichia coli (EPEC) strains.BMC Microbiol.14:135. 10.1186/1471-2180-14-135
53
TacconelliE.CarraraE.SavoldiA.HarbarthS.MendelsonM.MonnetD. L. (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis.Lancet Infect. Dis.18318–327. 10.1016/S1473-3099(17)30753-3
54
TsaiC. J.LohJ. M.ProftT. (2016). Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing.Virulence7, 214–229. 10.1080/21505594.2015.1135289
55
WinkP. L.Lima-MoralesD.MeurerR.BarthA. L. (2021). Escherichia coli carrying BlaNDM–1 obtained from a migratory penguin (Spheniscus magellanicus) in the Brazilian seacoast.Braz. J. Microbiol.53499–502. 10.1007/s42770-021-00652-7
56
WuW.FengY.TangG.QiaoF.McNallyA.ZongZ. (2019). NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings.Clin. Microbiol. Rev.32e115–e118. 10.1128/CMR.00115-18
57
YongD.TolemanM. A.GiskeC. G.ChoH. S.SundmanK.LeeK.et al (2009). Characterization of a new metallo-beta-lactamase gene, Bla(NDM–1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India.Antimicrob. Agents Chemother.535046–5054. 10.1128/AAC.00774-09
58
ZajacovaZ. S.KonstantinovaL.AlexaP. (2012). Detection of virulence factors of Escherichia coli focused on prevalence of EAST1 toxin in stool of diarrheic and non-diarrheic piglets and presence of adhesion involving virulence factors in AstA positive strains.Vet. Microbiol.154369–375. 10.1016/J.VETMIC.2011.07.029
59
ZhangH.WangY.LiuP.SunY.DongX.HuX. (2022). Unveiling the occurrence, hosts and mobility potential of antibiotic resistance genes in the deep ocean.Sci. Total Environ.816:151539. 10.1016/j.scitotenv.2021.151539
60
ZubrisD.MinbioleK.WuestW. (2017). Polymeric quaternary ammonium compounds: versatile antimicrobial materials.Curr. Top. Med. Chem.17305–318. 10.2174/1568026616666160829155805
Summary
Keywords
carbapenems, NDM carbapenemases, nosocomial bacteria, one health, wildlife, aquatic environment
Citation
Sellera FP, Cardoso B, Fuentes-Castillo D, Esposito F, Sano E, Fontana H, Fuga B, Goldberg DW, Seabra LAV, Antonelli M, Sandri S, Kolesnikovas CKM and Lincopan N (2022) Genomic Analysis of a Highly Virulent NDM-1-Producing Escherichia coli ST162 Infecting a Pygmy Sperm Whale (Kogia breviceps) in South America. Front. Microbiol. 13:915375. doi: 10.3389/fmicb.2022.915375
Received
07 April 2022
Accepted
18 May 2022
Published
10 June 2022
Volume
13 - 2022
Edited by
Ziad Daoud, Central Michigan University, United States
Reviewed by
Ahmed Mahrous Soliman, Kafrelsheikh University, Egypt; Andres Felipe Opazo-Capurro, University of Concepcion, Chile
Updates
Copyright
© 2022 Sellera, Cardoso, Fuentes-Castillo, Esposito, Sano, Fontana, Fuga, Goldberg, Seabra, Antonelli, Sandri, Kolesnikovas and Lincopan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fábio P. Sellera, fsellera@usp.brNilton Lincopan, lincopan@usp.br
†These authors have contributed equally to this work
This article was submitted to Antimicrobials, Resistance and Chemotherapy, a section of the journal Frontiers in Microbiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.