Abstract
The gastrointestinal (GI) microbiota is essential in maintaining human health. Alteration of the GI microbiota or gut microbiota (GM) from homeostasis (i.e., dysbiosis) is associated with several communicable and non-communicable diseases. Thus, it is crucial to constantly monitor the GM composition and host–microbe interactions in the GI tract since they could provide vital health information and indicate possible predispositions to various diseases. Pathogens in the GI tract must be detected early to prevent dysbiosis and related diseases. Similarly, the consumed beneficial microbial strains (i.e., probiotics) also require real-time monitoring to quantify the actual number of their colony-forming units within the GI tract. Unfortunately, due to the inherent limitations associated with the conventional methods, routine monitoring of one’s GM health is not attainable till date. In this context, miniaturized diagnostic devices such as biosensors could provide alternative and rapid detection methods by offering robust, affordable, portable, convenient, and reliable technology. Though biosensors for GM are still at a relatively preliminary stage, they can potentially transform clinical diagnosis in the near future. In this mini-review, we have discussed the significance and recent advancements of biosensors in monitoring GM. Finally, the progresses on future biosensing techniques such as lab-on-chip, smart materials, ingestible capsules, wearable devices, and fusion of machine learning/artificial intelligence (ML/AI) have also been highlighted.
1. Introduction
Trillions of microorganisms (bacteria, archaea, viruses, fungi, and protozoa) colonize in human gastrointestinal tract forming GM, which has a significant role in maintaining gut homeostasis (Schmidt et al., 2018). Within GM, these microbes co-evolve with the host and maintain a mutually beneficial relationship (Shreiner et al., 2015; Cani, 2018; Durack and Lynch, 2019). However, such symbiotic relationships are at constant threat of disruption, leading to the overgrowth of a particular pathobiont. The resulting gut dysbiosis is known to be linked to several communicable and non-communicable diseases (Kho and Lal, 2018; Daliri et al., 2020). Besides, the human gut may also get infected by enteric pathogens leading to serious health concerns, including mortality. The resident microbiota and the invading pathogens generate many metabolic products, which positively or negatively impact human health (Federici, 2019; de Vos et al., 2022).
Since the resident GM influences many physiological activities of the host, like immune response, nutrition, and metabolism, it is crucial to assess and monitor the composition of GM in real-time. In order to identify and determine the GI microflora, different approaches such as biochemical (culture-dependent) and molecular (culture-independent) techniques have been explored (Jian et al., 2020). Furthermore, many advanced high-throughput bio-analytical equipment is available to detect various microbes and their metabolites. Though such equipment offers highly sensitive, selective, and high throughput results, they are expensive, time-consuming, and require technical expertise to operate and interpret the results. Therefore, such instruments cannot be used by everyone and anywhere (Montes-Cebrián et al., 2018).
Since the inception of glucose biosensors such as glucometer, different biosensors have been developed for various applications. With advanced biomedical engineering and biotechnology, nanotechnology, and microtechnology, modern biosensors are extremely useful in healthcare. Recently, several biosensors have been developed to detect pathogens in environmental samples (Ngashangva et al., 2022). In addition, integrating biosensors with inter-disciplinary research fields and advanced information technology, such as wireless and machine learning, is expanding rapidly (Cui et al., 2020). In this mini-review, we briefly discuss the potential of using biosensors as Point-of-Care (POC) and personalized devices for monitoring the pathogenic and beneficial microbes in GM.
1.1. GM in health and disease
Microbiota is the consortium of all microbial members colonized in a particular niche. Different human body niches carry different microbiota with distinct qualitative and quantitative compositions. In the human body, the GM is composed of the highest number of microbes of different kingdoms, including members of protozoa, archaea, eukaryotes, viruses, and bacteria (Barko et al., 2018). It is interesting to note that the composition and physiology of the GM that influence human health are also highly dependent on human lifestyle (e.g., diet, alcohol consumption, smoking, medication, stress, and sleep) and the surrounding environment. GM contributes to maintaining human health by preventing pathogenic infections (by occupying the gut space and modulating immunity) and by contributing to the nutrition and metabolism of the host (Thursby and Juge, 2017; Schmidt et al., 2018). However, the homeostasis in the GM may get disrupted due to alterations in either lifestyle or environment, leading to dysbiosis and associated complications (Bajaj et al., 2014). Dysbiosis in GM, inadequate nutrition, exposure to enteric pathogens due to improper hygiene early in life may result in environmental enteropathy, which impairs the immune, metabolic, and neuroendocrine physiology that may lead to the long-term cognitive deficit and poor vaccine efficacy (Watanabe and Petri, 2016; Hajela et al., 2020; Singhvi et al., 2020). In addition, dysbiosis may produce microbe-derived metabolites that are detrimental to the host and may result in diseases like non-alcoholic fatty liver disease (NAFLD) (Yuan et al., 2019). Maintaining a high diversity in GM is essential for gut homeostasis. The beneficial microbes like Lactobacillus plantarum and the bacteria-derived short-chain fatty acids such as butyrate are crucial in maintaining epithelial integrity (Karczewski et al., 2010).
Moreover, recent studies found that the abundance of two bacterial species, Faecalibacterium prausnitzii and Roseburia hominis, in the colon is significantly lower for the patients with ulcerative colitis than for the controls (Machiels et al., 2014). Likewise, a lower abundance of Bifidobacterium in the gut is related to peptic ulcer and gastric cancer (Devi et al., 2021). In contrast, Fusobacterium nucleatum colonization is positively associated with colon cancer (Kostic et al., 2012; Repass et al., 2018). Besides, by incorporating metagenomic sequencing technologies, large data comparison and analysis of the microbial communities have yielded the link between microbiome alteration with human diseases such as cancer (Yu et al., 2017; Thomas et al., 2019; Wirbel et al., 2019), type II diabetes (Wang et al., 2012), cirrhosis (Oh et al., 2020). For detailed perspectives on the inter-relationship between gut microbiota and human health, the authors refer to Zheng et al. (2018), Durack and Lynch (2019), and Gibbons et al. (2022).
Understanding the interplay among GM, gut microbial metabolites, and the host in homeostasis and disease is one of the top challenges in modern science. Modern research on GM is now rapidly moving from relative to quantitative approaches, potentially revealing more information relevant to human health.
2. Biosensors as point-of-care diagnostics for GM
Microbial colonization in the human body can be identified using high-throughput diagnostic equipment that detects microbial nucleic acids, microbial proteins, and human antibody titers against specific antigens. Molecular techniques such as Enzyme-Linked Immuno-Sorbent Assay (ELISA) (Yilmaz et al., 2006), polymerase chain reaction (PCR) (Kim et al., 2020), fluorescent in situ hybridization (FISH) (Frickmann et al., 2017), etc., have been utilized to analyze human GI microflora. Additionally, microarray techniques such as DNA (Rivas et al., 2018), oligonucleotide (Wang et al., 2002), phylogenetic-microarray (Rigsbee et al., 2011), etc., are explored to meet the demand of simultaneous detection and quantifications of thousands of genes or target sequences within shorter period.
However, such classical instruments have several limitations, like high expense, less portability, the requirement of highly trained personnel, and lengthier procedure. In contrast, miniaturized diagnostic devices such as biosensors are now extensively explored in healthcare monitoring due to their easy operation and portability (Kim et al., 2019).
A biosensor is a device that yields a quantifiable and processable signal corresponding to the concentration of the target analyte. It usually integrates biological sensing elements or bioreceptors or biorecognition element (such as antibodies/enzymes/cell/nucleic acid/aptamer, etc.), transducers of the physicochemical signals (semi-conducting materials/nanomaterials, etc.), and digital displays (along with signal amplifier; Perumal and Hashim, 2014). Compared to classical bioanalytical instruments, biosensors may be more affordable, portable, user-friendly, rapid, among others. Additionally, patients can easily use them for routine health monitoring at the POC—diagnostic testing at or near-the-patient—or point-of-need (PON)—broader spectrum including on-site testing of environment, food samples, etc. Personalized healthcare may be achieved as individuals/patients can monitor their health or the efficacy of the treatment by biosensors.
Biosensors as POC testing for pathogenic, beneficial gut microbes, and gut microbial metabolites are rapidly emerging Some of the recently developed biosensors and bioelectronics for GI microbes, and gut microbial metabolites are summarized in Table 1, and a schematic representation of the biosensors for GM is shown in Figure 1.
Table 1
| Biosensors | Biorecognition element | Target analyte | LOD (Sample) | Range | Reference |
|---|---|---|---|---|---|
| Optical | Oligonucleotide – AuNPs | Helicobacter pylori | 25 cfu/mL (Feces) | 100–1,000 cfu/mL | Fei et al. (2022) |
| Optical | Cu2+ Nanoflowers | Helicobacter pylori | 50 cfu/mL (Artificial saliva) | 0–105 cfu/mL | Wang T. et al. (2021) |
| Optical | Aptamer-Fe3O4 super-paramagnetic NPs | Helicobacter pylori | 1 cfu/mL (Human feces) | 10–107 cfu/mL | Wang Z. et al. (2021) |
| Electrochemical | Nucleic acid (DNA) | Helicobacter pylori | 12 fM (Dental plague) | 6.55 pM–32.8 fM | Chen et al. (2018) |
| Electrochemical | Nucleic acid (DNA) | Helicobacter pylori DNA | ~6 pmol | 5–20 pmol | Del Pozo et al. (2005) |
| Electrochemical | Bismuth-immobilized carbon nanotube | Helicobacter pylori DNA | 0.6 μg/mL (patient’s gastric tissue) | 0.72–7.92 μg/mL | Ly et al. (2011) |
| Optical (Colorimetric) | AuNPs | Shigella | 10 fg (culture), 5.86 cfu/mL (human fecal) | 10 ng–10 fg | Wang et al. (2016) |
| Optical (Plasmonic) | E. faecalis imprinted nanoparticles | Enterococcus faecalis | ~100 bac/mL (Sea water) | 2 × 104–1 × 108 cfu/mL | Erdem et al. (2019) |
| Piezoelectric | Sulfo-LC-SPDP & MHDA | Bifidobacterium bifidum 01356 & Lactobacillus acidophilus 01132 | 103 cfu/mL (Milk) | 103–5 × 105 cfu/ml | Szalontai et al. (2012) |
| Optical (Fluorescence) | DNAzyme-copper nanoclusters | E. coli 0157:H7 | 1.57 cfu/mL (Drinking water, apple juice) | 10–1,000 cfu/mL | Zhou et al. (2020) |
| Electrochemical (Impedance) | OCMCS-Fe3O4 NPs | Campylobacter jejuni | 1 × 103 cfu/mL (Stool) | 103–107 cfu/mL | Huang et al. (2010) |
| Electrochemical | Molecularly imprinted polymer | TMAO | 1 ppm/mL (Urine) | 1–15 ppm | Lakshmi et al. (2021) |
| Optical (Colorimetric) | PAH@MnO2 nanozyme | TMAO | 6.7 μM [Blood (rats)] | 15.6–500 μM | Chang Y. C. et al. (2021) |
| Optical (Colorimetric) | Antibody on lateral flow platform | Salmonella Typhi | 10 cfu/mL (Fecal) | 101–107 cfu/mL | Amalina et al. (2021) |
| Salmonella Paratyphi A | 102 cfu/mL (Fecal) | ||||
| Optical (Fluorescence) | Engineered E. coli Nissle 1917 | Nitrate | 39 μM [Fecal, colon (mice)] | 0–10 mM | Woo et al. (2020) |
| Piezoelectric | Antibody-AuNPs | Bifidobacterium bifidum | 2.1 × 102 cfu/ml [Fecal, food (milk)] | 103–105 cfu/mL | Hou et al. (2020) |
| Optical (Fluorescence) | DNA-mediated Au@Ag@silica nanopopcorn | Lactobacillus Plantarum | 15 cfu/mL (Mice) | 105–109 cfu/mL | Gao et al. (2022) |
| Optical | Aptamer-decorated porous Si NS | Lactobacillus acidophilus | 106 cells/mL (selection buffer) | 106–107 cfu/mL | Urmann et al. (2016) |
| Electrochemical (Amperometric) | Lyophilized bacterial cell | Lactate | 0.012 mM (Milk, buttermilk, kefir) | 0.1–1.0 mM | Canbay et al. (2015) |
| Pyruvate | 0.018 mM | ||||
| Optical (Colorimetric) | Aptamer-AuNPs | Salmonella enteritidis | 101 cfu/mL (Milk) | 101–1012 cfu/mL | Fang et al. (2014) |
| Electrochemical (Impedance) | PPy-Co-CPy – aptamer | Salmonella typhimurium | 3 cfu/mL (Apple juice) | 102–108 cfu/mL | Sheikhzadeh et al. (2016) |
| Optical (FOLSPR) | Aptamer | Salmonella typhimurium | 128 cfu/mL (Chicken) | 5 × 102–1 × 108 cfu/mL | Xu et al. (2018) |
Some of the recent development of biosensors and bioelectronics for GI microbes and gut metabolites.
AuNP, Gold nanoparticle; NP, Nanoparticle; LOD, limit of detection; bac/mL, bacteria/mL; cfu, colony forming unit; TMAO, Trimethylamine N-oxide; sulfo-LC-SPDP, sulfosuccinimidyl 6-[3-(2-pyridyldithio)propionamido] hexanoate; MHDA, 16-mercapto-hexadecanoic acid; PAH@MnO2, Polyallylamine hydrochloride-capped manganese dioxide; OCMCS-Fe3O4 NP, O-carboxymethyl chitosan surface modified Fe3O4 nanoparticle; PPy-Co-CPy, poly[pyrrole-co-3-carboxyl-pyrrole]copolymer; FOLSPR, Fiber-optic localized surface plasmon resonance; Si NS, Silicon nanostructure.
Figure 1
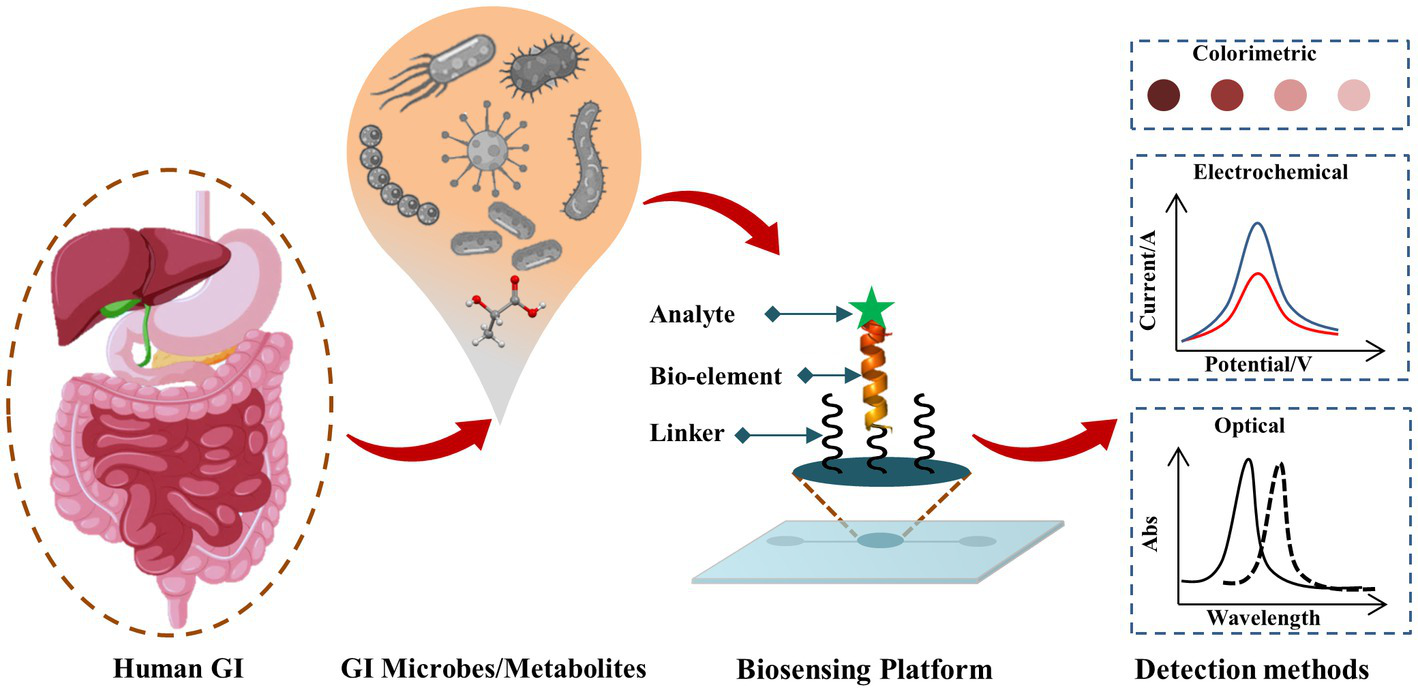
Schematic representation of biosensing techniques for GI microbes and gut metabolites. The target analyte (such as GI microbe or gut metabolite of interest) reacts with the corresponding bio-recognition element (bioelement or bioprobe) of the biosensor and such analyte-bioprobe interaction is selectively and sensitively analyzed using different detection methods such as optical, electrochemical, piezoelectric, thermoelectric, field-effect transistor (FET) approaches.
However, as compared to other areas of applications, biosensors for GM remained less progressed, which is partly due to many technical challenges such as: (i) most GI microbes cannot be cultured in vitro till date, (ii) it is challenging to isolate and perform standard assays from humans, (iii) challenges that exist to mimic the GI microbial ecosystem in the artificial model, (iv) GM gets altered by many other factors (e.g., diet).
2.1. Biosensors for the pathogenic microbes in GM
The biomarkers from GM can be used for assessing various disease progressions, the nature of severity, and the response toward medical treatments. Microbial biomarkers have been proposed for different health problems, such as lung cancer (Liu et al., 2019; Temraz et al., 2019), obesity & diabetes (Zheng et al., 2018; Singer-Englar et al., 2019), and cirrhosis (Bajaj, 2019). Despite difficulties and low success rates, many studies attempted to target gut microbes for diagnostic purposes. Most of these attempts were focused on estimating the metabolites such as glucose, volatiles, endotoxins, etc. (Kassal et al., 2018). Due to the recent attention to the relationship between GM and health, novel microbial biomarkers are now being discovered.
Due to the advancement of synthetic biology and genetic engineering, biosensors such as whole-cell biosensors (WCBs) can detect target analytes directly from complex environments like wastewater or clinical samples. Bacterial biosensors, also called bactosensors, employ whole bacterial cells to determine various pathological biomarkers. Whole-cell bacterial bioreporters are designed to detect toxic chemicals. By integrating with microengineering, genetic circuits could be developed that translate the biochemical signals into quantifiable reporter protein signals (Van Der Meer and Belkin, 2010). Rutter et al. developed an engineered bacterial biosensor using Caenorhabditis elegans as a novel model organism to determine isopropyl β-D-1-thiogalactopyranoside (IPTG) in the gut (Rutter et al., 2019). Further advancement in designing the engineered bacterial circuit may help monitor human GM and detect gut inflammation by nitrate sensing (Woo et al., 2020). Sensing the gut metabolites could also provide crucial information on the host–microbe interactions in the gut. With the help of bactosensors, metabolites such as benzoate, lactate, anhydrotetracycline, and bile acids could be detected in human fecal samples. Microbe-associated fecal metabolites has the potential to be used as diagnostics and theranostics explorations (Zúñiga et al., 2022). Another artificial receptor platform called EMeRALD (Engineered Modularized Receptors Activated via Ligand-induced Dimerization) was developed and utilized to detect bile salts (a biomarker of liver dysfunction; Chang H. J. et al., 2021). Biosensors for other essential metabolites, such as trimethylamine-N-oxide (TMAO) (Chang Y. C. et al., 2021; Lakshmi et al., 2021) and indole, have also been developed recently (Wang J. et al., 2021).
Biosensors for detecting gastric pathogen Helicobacter pylori have been developed based on different detection methods such as piezoelectric (Su and Li, 2001) and electrochemical (Del Pozo et al., 2005; Ly et al., 2011). Real-time monitoring of the interaction of H. pylori with the human gastric mucin was also studied using a resonant mirror-based biosensor (Hirmo et al., 1999). Conversely, Bifidobacterium bifidum, proposed to have protective roles against H. pylori induced gastric diseases (Alexander et al., 2021; Devi et al., 2021), can be detected using quartz crystal microbalance immunosensor (Hou et al., 2020).
There has been considerable growth in biosensor technology for gut microbiome recently. One of the reasons is the advancement of nanotechnologies/nanoengineering and their use in biosensors technologies (Yadav et al., 2022). Due to their intrinsic properties, nanoparticles are now increasingly used to detect various microbes and their activities in GM (Fuentes-Chust et al., 2021). For example, a plasmonic sensor— based on the molecularly imprinted nanoparticles— has been developed for detecting Enterococcus faecalis (Erdem et al., 2019), whereas Shigella spp. were detected using gold nanoparticles on lateral flow biosensor (Wang et al., 2016). Another emerging biorecognition element for biosensors is the aptamer biomolecule. Aptamer-based biosensors are significantly explored as they can replace conventional antibody-based diagnostic technologies due to their high specificity and selectivity to bind with the target analyte. A DNA aptamer-based biosensor has recently been developed to study and detect human gut bacterium Akkermansia muciniphila (Raber et al., 2021).
2.2. Biosensors for beneficial GM
Probiotics are specific strains of beneficial microbial species that provide health benefits to the host upon consumption in sufficient amounts. They are microorganisms with Generally Recognized As Safe (GRAS) status that are critical in preventing diseases like diarrhea, gut inflammation, viral infection, and even colorectal cancer (Uccello et al., 2012; Ghosh, 2018). The probiotics may help to increase the vaccine efficacy in developing countries (Hajela et al., 2020). Nowadays, probiotics are being used even as functional foods. However, probiotics could induce an individualized impact on the gut transcriptome, and the effect may not be universal (Zmora et al., 2018). Recently, probiotics have also initiated personalized therapies (Kort, 2014). Therefore, monitoring quantitative estimation of the probiotics within GM is very important for monitoring GI health and safety, and biosensor could become an instrumental technology.
Although studying the properties of probiotics by using biosensors is not widely used, some attempts have been made. The aptamer (Hemag1P) based biosensors were developed to detect Lactobacillus acidophilus with high selectivity and specificity (Urmann et al., 2016). This label-free, simple, and rapid method could distinguish between live and dead bacteria. An aptamer-based electronic biosensor has recently been developed for monitoring the gut bacterium Roseburia intestinalis (Xing et al., 2022). The yeasts, also being used as probiotics, have been detected by biosensors (Dacquay and McMillen, 2021). Many essential metabolites from beneficial microbes could be targeted to assess human health. Biosensors have developed for metabolites such as short chain fatty acid butyrate (Bai and Mansell, 2020; Chang Y. C. et al., 2021), lactic acid (Radoi et al., 2010) and indoxyl sulfate (Filik et al., 2016). The immunostimulatory effects usually exerted by the probiotics (e.g., some of the Lactobacillus strains) could also be traced by biosensors (Rocha-Ramírez et al., 2017). Importantly, probiotic microbes have been engineered to function as biosensors for detecting autoinducer peptide-I, a quorum-sensing molecule produced by Staphylococcus sp. (Lubkowicz et al., 2018). The engineered microbes can increase the complexity, stability, and safety during diagnosis or therapeutics, as observed with smart engineered probiotics (Rottinghaus et al., 2020).
2.3. Emerging biosensors for GM
New biosensing concepts and techniques such as in vivo wireless and ingestible capsules, microfluidic chips, and internet of things (IoT) have emerged in the recent pasts that are more robust, compact, multiplex, programmable, and reliable. These innovative technologies and smart devices would augment conventional biosensors as efficient and robust POCT devices shortly.
Smart and responsive materials are now being explored to design and develop better and more robust biosensors (Ngashangva et al., 2020). Using such advanced materials, the ingestible capsules were designed to collect microbiome samples from the GI tract. The capsule consisted of 3D-printed acrylic housing, hydrogel, and flexible PDMS membrane (Waimin et al., 2020). This non-invasive sampling technique was validated using Escherichia coli. By employing ingestible electronic capsules and a self-powered biosensing system, the crucial metabolite composition of small intestines could also be monitored (De la Paz et al., 2022). Additionally, an ingestible probiotic biosensor has been developed to diagnose GI bleeding in swine. Such ingestible micro-bio-electronic devices have the potential to transform disease management and diagnosis of GI diseases (Mimee et al., 2018). Volatile and gas molecules from a patient’s sample are used as bio-signatures. An electronic nose device based on an array of 13 commercial electro-chemical and optical sensors has been designed to study the microbial volatile metabolites in urine sample of colorectal cancer patients (Westenbrink et al., 2015).
Due to the limitation of the animal model, microfluidic chip such as organ-on-chip has become a promising tool as the physiology and function of tissues or organs can be recapitulated in microfluidic in vitro devices. Gut-on-chips are explored to enhance understanding the complex nature of gut microbiota-host interaction (Puschhof et al., 2021; Signore et al., 2021). Another possibility for exploring lab-on-chip technology is multiplex assay and detecting multiple target analytes on a single platform (Shah et al., 2016). The concept of cost-effective, instrument-less, simple, and user-friendly sensing platforms such as microfluidic paper-based analytical devices (μ-PADs) have added benefits to the existing lab-on-chip based biosensor (Takahashi et al., 2022).
As clinical governance is becoming challenging, demanding, and laborious, ML/AI and the internet of medical things must be incorporated with the biosensing concept. Additionally, medical science is transforming from traditional healthcare to digital healthcare monitoring systems that enable improved access to quality healthcare for patients, clinicians, and remote communities. Despite all the advantages, conventional biosensors have limitations such as low specificity, sensitivity, and selectivity compared to other bioanalytical techniques. The overall performance of the biosensor could be improved by integrating with ML/AI approaches. As the individual gut health involves complex interactions between diet, host, microbiota, real-time GM monitoring using ML-based big data collection and analysis with the help of a biosensor could be established (Sosnowski et al., 2020). The ML could also be used to screen the cause-effect relationship between GM dysbiosis and diseases (e.g., cardiovascular diseases; Aryal et al., 2020). ML/AI-based approaches are meta-metabolic network models that are useful to predict the pattern and acquire insights into the synergistic, dysbiotic relationships, and phenotypic outcomes (Lloyd-Price et al., 2019; Cammarota et al., 2020). By understanding such interconnections, development of robust personalized POC biosensors for gut health could be enhanced tremendously.
3. Conclusion and future perspective
Since GM is linked to maintaining human physiological processes, monitoring of GM eubiosis is likely to become fundamental in personalized healthcare. Biosensors could be a potential candidate as POC diagnostic devices for personalized monitoring of GM homeostasis. Though biosensors for diagnostics purposes are rapidly advancing, they are still in the preparatory stages for specifically monitoring GM. However, several approaches emerging from diverse fields of science and technology are promising. It is interesting to note that several groups have attempted to develop biosensors for GM by integrating advanced information technologies like ML. It is possible that precise non-invasive monitoring of GM health would be possible in the near future by employing ingestible 3D printed capsules and pairing them with wireless and self-powered wearable electronic devices or even smartphones.
Research on developing biosensors for various gut microbes other than gut bacteria and gut metabolites/biomarkers should be explored to reinforce the personalized biosensor or POCT for GM. Since the results of different studies on gut microbiota are highly variable even in the same disease, methods to develop ultra-sensitive and specificity of biosensors may be focused on in the future, thereby enhancing the early personalized diagnosis and effective medical treatment. Moreover, developing ultra-sensitive biosensors for mental and gut health is an absolute need, as there is a bidirectional relationship between gut microbiota and cognitive behaviors. Furthermore, safety and psychological challenges are associated with continuous monitoring and in vivo monitoring devices like ingestible sensors for GM. A minimally invasive approach (such as wearable devices), affordable, and user-friendly (such as μ-PADs) could be more focused in academia and the clinical industries. We believe that the rapid progress of nanotechnology and emerging multidisciplinary approaches would enable early on-site detection of gut metabolites and microbes that empower personalized POC diagnosis with efficient treatment.
Funding
This work was supported by intra-mural funding from RGCB, DBT, Govt. of India to LN and SC. It is partially supported by grants to LN (EEQ/2021/000731, SERB-DST), and to SC (MED/2017/46, DBT).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
LN designed and drafted the manuscript. SC reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Dr. Chandrabhas Narayana, Director, RGCB for encouragement and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Alexander S. M. Retnakumar R. J. Chouhan D. Devi T. N. B. Dharmaseelan S. Devadas K. et al . (2021). Helicobacter pylori in human stomach: the inconsistencies in clinical outcomes and the probable causes. Front. Microbiol.12:713955. doi: 10.3389/fmicb.2021.713955
2
Amalina Z. N. Khalid M. F. Rahman S. F. Ahmad M. N. Ahmad Najib M. Ismail A. et al . (2021). Nucleic acid-based lateral flow biosensor for salmonella typhi and salmonella paratyphi: a detection in stool samples of suspected carriers. Diagnostics11, 1–12. doi: 10.3390/diagnostics11040700
3
Aryal S. Alimadadi A. Manandhar I. Joe B. Cheng X. (2020). Machine learning strategy for gut microbiome-based diagnostic screening of cardiovascular disease. Hypertension76, 1555–1562. doi: 10.1161/HYPERTENSIONAHA.120.15885
4
Bai Y. Mansell T. J. (2020). Production and sensing of butyrate in a probiotic escherichia coli strain. Int. J. Mol. Sci.21, 1–12. doi: 10.3390/ijms21103615
5
Bajaj J. S. (2019). Gut microbiota as biosensors in patients with cirrhosis. Cell. Mol. Gastroenterol. Hepatol.8, 231–233. doi: 10.1016/j.jcmgh.2019.04.016
6
Bajaj J. S. Heuman D. M. Hylemon P. B. Sanyal A. J. White M. B. Monteith P. et al . (2014). Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol.60, 940–947. doi: 10.1016/j.jhep.2013.12.019
7
Barko P. C. McMichael M. A. Swanson K. S. Williams D. A. (2018). The gastrointestinal microbiome: a review. J. Vet. Intern. Med.32, 9–25. doi: 10.1111/jvim.14875
8
Cammarota G. Ianiro G. Ahern A. Carbone C. Temko A. Claesson M. J. et al . (2020). Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol.17, 635–648. doi: 10.1038/s41575-020-0327-3
9
Canbay E. Habip A. Kara G. Eren Z. Akyilmaz E. (2015). A microbial biosensor based on Lactobacillus delbruecki sp. bacterial cells for simultaneous determination of lactic and pyruvic acid. Food Chem.169, 197–202. doi: 10.1016/j.foodchem.2014.07.140
10
Cani P. D. (2018). Human gut microbiome: hopes, threats and promises. Gut67, 1716–1725. doi: 10.1136/gutjnl-2018-316723
11
Chang Y. C. Chu Y. H. Wang C. C. Wang C. H. Tain Y. L. Yang H. W. (2021). Rapid detection of gut microbial metabolite trimethylamine n-oxide for chronic kidney disease prevention. Biosensors11, 1–10. doi: 10.3390/bios11090339
12
Chang H. J. Zúñiga A. Conejero I. Voyvodic P. L. Gracy J. Fajardo-Ruiz E. et al . (2021). Programmable receptors enable bacterial biosensors to detect pathological biomarkers in clinical samples. Nat. Commun.12:5216. doi: 10.1038/s41467-021-25538-y
13
Chen L. L. Cui H. F. Fan S. F. Li Z. Y. Han S. Y. Ma X. et al . (2018). Detection of Helicobacter pylori in dental plaque using a DNA biosensor for noninvasive diagnosis. RSC Adv.8, 21075–21083. doi: 10.1039/c8ra03134g
14
Cui F. Yue Y. Zhang Y. Zhang Z. Susan Zhou H. (2020). Advancing biosensors with machine learning. ACS Sensors5, 3346–3364. doi: 10.1021/acssensors.0c01424
15
Dacquay L. C. McMillen D. R. (2021). Improving the design of an oxidative stress sensing biosensor in yeast. FEMS Yeast Res.21, 1–13. doi: 10.1093/femsyr/foab025
16
Daliri E. B. M. Ofosu F. K. Chelliah R. Lee B. H. Oh D. H. (2020). Health impact and therapeutic manipulation of the gut microbiome. High-Throughput9, 1–22. doi: 10.3390/ht9030017
17
De la Paz E. Maganti N. H. Trifonov A. Jeerapan I. Mahato K. Yin L. et al . (2022). A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun.13, 7405–7411. doi: 10.1038/s41467-022-35074-y
18
de Vos W. M. Tilg H. Van Hul M. Cani P. D. (2022). Gut microbiome and health: mechanistic insights. Gut71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
19
Del Pozo M. V. Alonso C. Pariente F. Lorenzo E. (2005). DNA biosensor for detection of Helicobacter pylori using phen-dione as the electrochemically active ligand in osmium complexes. Anal. Chem.77, 2550–2557. doi: 10.1021/ac0489263
20
Devi T. B. Devadas K. George M. Gandhimathi A. Chouhan D. Retnakumar R. J. et al . (2021). Low Bifidobacterium abundance in the lower gut microbiota is associated with Helicobacter pylori-related gastric ulcer and gastric Cancer. Front. Microbiol.12, 1–14. doi: 10.3389/fmicb.2021.631140
21
Durack J. Lynch S. V. (2019). The gut microbiome: relationships with disease and opportunities for therapy. J. Exp. Med.216, 20–40. doi: 10.1084/jem.20180448
22
Erdem Ö. Saylan Y. Cihangir N. Denizli A. (2019). Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron.126, 608–614. doi: 10.1016/j.bios.2018.11.030
23
Fang Z. Wu W. Lu X. Zeng L. (2014). Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement amplification. Biosens. Bioelectron.56, 192–197. doi: 10.1016/J.BIOS.2014.01.015
24
Federici M. (2019). Gut microbiome and microbial metabolites: a new system affecting metabolic disorders. J. Endocrinol. Investig.42, 1011–1018. doi: 10.1007/s40618-019-01022-9
25
Fei Y. Fang R. Xiao L. Zhang Y. Fan K. Jiang Y. et al . (2022). The development of a colorimetric biosensing assay for the detection of Helicobacter pylori in feces. Anal. Biochem.651, 114737–114736. doi: 10.1016/j.ab.2022.114737
26
Filik H. Avan A. A. Aydar S. (2016). Voltammetric sensing of uremic toxin Indoxyl sulfate using high performance disposable screen-printed Graphene electrode. Curr. Pharm. Anal.12, 36–42. doi: 10.2174/1573412911666150520000605
27
Frickmann H. Zautner A. E. Moter A. Kikhney J. Hagen R. M. Stender H. et al . (2017). Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review. Cri. Rev. Microbiol43, 263–293. doi: 10.3109/1040841X.2016.1169990
28
Fuentes-Chust C. Parolo C. Rosati G. Rivas L. Perez-Toralla K. Simon S. et al . (2021). The microbiome meets nanotechnology: opportunities and challenges in developing new diagnostic devices. Adv. Mater.33, 2006104–2006118. doi: 10.1002/adma.202006104
29
Gao F. Zhou S. Lu C. Li Y. Lin J. Zheng J. (2022). DNA-mediated au@Ag@silica nanopopcorn fluorescent probe for in vivo near-infrared imaging of probiotic Lactobacillus Plantarum. Biosens. Bioelectron.212:114421. doi: 10.1016/j.bios.2022.114421
30
Ghosh A. R. (2018). Probiotics in the Rescue of gut Inflammation. Ther. Probiotic, Unconv. Foods, 101–116. doi: 10.1016/B978-0-12-814625-5.00006-6
31
Gibbons S. M. Gurry T. Lampe J. W. Chakrabarti A. Dam V. Everard A. et al . (2022). Perspective: leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv. Nutr.13, 1450–1461. doi: 10.1093/advances/nmac075
32
Hajela N. Chattopadhyay S. Nair G. B. Ganguly N. K. (2020). Intestinal microbiota and vaccine efficacy in children from resource poor settings – potential impact for the usefulness of probiotics?Benef. Microbe11, 319–328. doi: 10.3920/BM2019.0155
33
Hirmo S. Artursson E. Puu G. Wadstrom T. Nilsson B. (1999). Helicobacter pylori interactions with human gastric mucin studied with a resonant mirror biosensor. J. Methods Microbiol. J. Microbiol. Methods37, 177–182. doi: 10.1016/s0167-7012(99)00060-3
34
Hou K. Zhao P. Chen Y. Li G. Lin Y. Chen D. et al . (2020). Rapid detection of Bifidobacterium bifidum in feces sample by highly sensitive quartz crystal microbalance Immunosensor. Front. Chem.8, 1–8. doi: 10.3389/fchem.2020.00548
35
Huang J. Yang G. Meng W. Wu L. Zhu A. Jiao X. (2010). An electrochemical impedimetric immunosensor for label-free detection of Campylobacter jejuni in diarrhea patients’ stool based on O-carboxymethylchitosan surface modified Fe3O4 nanoparticles. Biosens. Bioelectron.25, 1204–1211. doi: 10.1016/J.BIOS.2009.10.036
36
Jian C. Luukkonen P. Yki-Järvinen H. Salonen A. Korpela K. (2020). Quantitative PCR provides a simple and accessible method for quantitative microbiota profiling. PLoS One15, e0227285–e0227210. doi: 10.1371/journal.pone.0227285
37
Karczewski J. Troost F. J. Konings I. Dekker J. Kleerebezem M. Brummer R.-J. M. et al . (2010). Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol.298, 851–859. doi: 10.1152/ajpgi.00327.2009.-Lactobacillus
38
Kassal P. Steinberg M. D. Steinberg I. M. (2018). Wireless chemical sensors and biosensors: a review. Sensors Actuators B Chem.266, 228–245. doi: 10.1016/J.SNB.2018.03.074
39
Kho Z. Y. Lal S. K. (2018). The human gut microbiome – a potential controller of wellness and disease. Front. Microbiol.9, 1–23. doi: 10.3389/fmicb.2018.01835
40
Kim J. Campbell A. S. de Ávila B. E. F. Wang J. (2019). Wearable biosensors for healthcare monitoring. Nat. Biotechnol.37, 389–406. doi: 10.1038/s41587-019-0045-y
41
Kim H. B. Kim E. Yang S. M. Lee S. Kim M. J. Kim H. Y. (2020). Development of real-time PCR assay to specifically detect 22 Bifidobacterium species and subspecies using comparative genomics. Front. Microbiol.11, 1–12. doi: 10.3389/fmicb.2020.02087
42
Kort R. (2014). Personalized therapy with probiotics from the host by TripleA. Trends Biotechnol.32, 291–293. doi: 10.1016/j.tibtech.2014.04.002
43
Kostic A. D. Gevers D. Pedamallu C. S. Michaud M. Duke F. Earl A. M. et al . (2012). Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res.22, 292–298. doi: 10.1101/gr.126573.111
44
Lakshmi G. B. V. S. Yadav A. K. Mehlawat N. Jalandra R. Solanki P. R. Kumar A. (2021). Gut microbiota derived trimethylamine N-oxide (TMAO) detection through molecularly imprinted polymer based sensor. Sci. Rep.11:1338. doi: 10.1038/s41598-020-80122-6
45
Liu F. Li J. Guan Y. Lou Y. Chen H. Xu M. et al . (2019). Dysbiosis of the gut microbiome is associated with tumor biomarkers in lung cancer. Int. J. Biol. Sci.15, 2381–2392. doi: 10.7150/ijbs.35980
46
Lloyd-Price J. Arze C. Ananthakrishnan A. N. Schirmer M. Avila-Pacheco J. Poon T. W. et al . (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature569, 655–662. doi: 10.1038/s41586-019-1237-9
47
Lubkowicz D. Ho C. L. Hwang I. Y. Yew W. S. Lee Y. S. Chang M. W. (2018). Reprogramming probiotic Lactobacillus reuteri as a biosensor for Staphylococcus aureus derived AIP-I detection. ACS Synth. Biol.7, 1229–1237. doi: 10.1021/acssynbio.8b00063
48
Ly S. Y. Yoo H. S. Choa S. H. (2011). Diagnosis of Helicobacter pylori bacterial infections using a voltammetric biosensor. J. Microbiol. Methods87, 44–48. doi: 10.1016/j.mimet.2011.07.002
49
Machiels K. Joossens M. Sabino J. De Preter V. Arijs I. Eeckhaut V. et al . (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut63, 1275–1283. doi: 10.1136/GUTJNL-2013-304833
50
Mimee M. Nadeau P. Hayward A. Carim S. Flanagan S. Jerger L. et al . (2018). An ingestible bacterial-electronic system to monitor gastrointestinal health. Science360, 915–918. doi: 10.1126/science.aas9315
51
Montes-Cebrián Y. del Torno-de Román L. Álvarez-Carulla A. Colomer-Farrarons J. Minteer S. D. Sabaté N. et al . (2018). ‘Plug-and-power’ point-of-care diagnostics: a novel approach for self-powered electronic reader-based portable analytical devices. Biosens. Bioelectron.118, 88–96. doi: 10.1016/j.bios.2018.07.034
52
Ngashangva L. Goswami P. Chakma B. (2020). “Smart materials for developing sensor platforms” in Advanced materials and techniques for biosensors and bioanalytical applications. ed. GoswamiP. (Poca Raton, FL: CRC Press), 47–68.
53
Ngashangva L. Hemdan B. A. El-Liethy M. A. Bachu V. Minteer S. D. Goswami P. (2022). Emerging bioanalytical devices and platforms for rapid detection of pathogens in environmental samples. Micromachines13, 1–36. doi: 10.3390/MI13071083
54
Oh T. G. Kim S. M. Caussy C. Downes M. Evans R. M. Loomba R. et al . (2020). A universal gut-microbiome-derived signature predicts cirrhosis. Cell Metab.32, 878–888.e6. doi: 10.1016/j.cmet.2020.06.005
55
Perumal V. Hashim U. (2014). Advances in biosensors: principle, architecture and applications. J. Appl. Biomed.12, 1–15. doi: 10.1016/J.JAB.2013.02.001
56
Puschhof J. Pleguezuelos-Manzano C. Clevers H. (2021). Organoids and organs-on-chips: insights into human gut-microbe interactions. Cell Host Microbe29, 867–878. doi: 10.1016/j.chom.2021.04.002
57
Raber H. F. Kubiczek D. H. Bodenberger N. Kissmann A. K. D’souza D. Hu X. et al . (2021). Flucell-selex aptamers as specific binding molecules for diagnostics of the health relevant gut bacterium akkermansia muciniphila. Int. J. Mol. Sci.22, 1–16. doi: 10.3390/ijms221910425
58
Radoi A. Moscone D. Palleschi G. (2010). Sensing the lactic acid in probiotic yogurts using an l-lactate biosensor coupled with a microdialysis fiber inserted in a flow analysis system. Anal. Lett.43, 1301–1309. doi: 10.1080/00032710903518716
59
Repass J. Iorns E. Denis A. Williams S. R. Perfito N. Errington T. M. (2018). Replication study: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. elife7, 299–306. doi: 10.7554/eLife.25801
60
Rigsbee L. Agans R. Foy B. D. Paliy O. (2011). Optimizing the analysis of human intestinal microbiota with phylogenetic microarray. FEMS Microbiol. Ecol.75, 332–342. doi: 10.1111/j.1574-6941.2010.01009.x
61
Rivas L. Reuterswärd P. Rasti R. Herrmann B. Mårtensson A. Alfvén T. et al . (2018). A vertical flow paper-microarray assay with isothermal DNA amplification for detection of Neisseria meningitidis. Talanta183, 192–200. doi: 10.1016/j.talanta.2018.02.070
62
Rocha-Ramírez L. M. Pérez-Solano R. A. Castañón-Alonso S. L. Moreno Guerrero S. S. Ramírez Pacheco A. García Garibay M. et al . (2017). Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res2017, 1–14. doi: 10.1155/2017/4607491
63
Rottinghaus A. G. Amrofell M. B. Moon T. S. (2020). Biosensing in smart engineered probiotics. Biotechnol. J.15:1900319. doi: 10.1002/biot.201900319
64
Rutter J. W. Ozdemir T. Galimov E. R. Quintaneiro L. M. Rosa L. Thomas G. M. et al . (2019). Detecting changes in the Caenorhabditis elegans intestinal environment using an engineered bacterial biosensor. ACS Synth. Biol.8, 2620–2628. doi: 10.1021/acssynbio.9b00166
65
Schmidt T. S. B. Raes J. Bork P. (2018). The human gut microbiome: from association to modulation. Cells172, 1198–1215. doi: 10.1016/j.cell.2018.02.044
66
Shah P. Fritz J. V. Glaab E. Desai M. S. Greenhalgh K. Frachet A. et al . (2016). A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun.7:11535. doi: 10.1038/ncomms11535
67
Sheikhzadeh E. Chamsaz M. Turner A. P. F. Jager E. W. H. Beni V. (2016). Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron.80, 194–200. doi: 10.1016/J.BIOS.2016.01.057
68
Shreiner A. B. Kao J. Y. Young V. B. (2015). The gut microbiome in health and in disease. Curr. Opin. Gastroenterol.31, 69–75. doi: 10.1097/MOG.0000000000000139
69
Signore M. A. De Pascali C. Giampetruzzi L. Siciliano P. A. Francioso L. (2021). Gut-on-Chip microphysiological systems: latest advances in the integration of sensing strategies and adoption of mature detection mechanisms. Sens. Bio-Sensing Res.33, 100443–100426. doi: 10.1016/j.sbsr.2021.100443
70
Singer-Englar T. Barlow G. Mathur R. (2019). Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev. Gastroenterol. Hepatol.13, 3–15. doi: 10.1080/17474124.2019.1543023
71
Singhvi N. Gupta V. Gaur M. Sharma V. Puri A. Singh Y. et al . (2020). Interplay of human gut microbiome in health and wellness. Indian J. Microbiol.60, 26–36. doi: 10.1007/s12088-019-00825-x
72
Sosnowski K. Akarapipad P. Yoon J. (2020). The future of microbiome analysis: biosensor methods for big data collection and clinical diagnostics. Med. Devices Sensors3, 1–19. doi: 10.1002/mds3.10085
73
Su X. Li S. F. Y. (2001). Serological determination of Helicobacter pylori infection using sandwiched and enzymatically amplified piezoelectric biosensor. Anal. Chim. Acta429, 27–36. doi: 10.1016/S0003-2670(00)01262-9
74
Szalontai H. Adányi N. Kiss A. (2012). Development of piezoelectric Immunosensor for the detection of probiotic Bacteria. Anal. Lett.45, 1214–1229. doi: 10.1080/00032719.2012.673095
75
Takahashi M. K. Tan X. Dy A. J. (2022). Cell-free paper-based analysis of gut microbiota and host biomarkers. Methods Mol. Biol.2433, 351–374. doi: 10.1007/978-1-0716-1998-8_22/COVER
76
Temraz S. Nassar F. Nasr R. Charafeddine M. Mukherji D. Shamseddine A. (2019). Gut microbiome: a promising biomarker for immunotherapy in colorectal cancer. Int. J. Mol. Sci.20, 1–11. doi: 10.3390/ijms20174155
77
Thomas A. M. Manghi P. Asnicar F. Pasolli E. Armanini F. Zolfo M. et al . (2019). Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med.25, 667–678. doi: 10.1038/s41591-019-0405-7
78
Thursby E. Juge N. (2017). Introduction to the human gut microbiota. Biochem. J.474, 1823–1836. doi: 10.1042/BCJ20160510
79
Uccello M. Malaguarnera G. Basile F. Dagata V. Malaguarnera M. Bertino G. et al . (2012). Potential role of probiotics on colorectal cancer prevention. BMC Surg.12 Suppl 1, 1–8. doi: 10.1186/1471-2482-12-S1-S35
80
Urmann K. Arshavsky-Graham S. Walter J. G. Scheper T. Segal E. (2016). Whole-cell detection of live: Lactobacillus acidophilus on aptamer-decorated porous silicon biosensors. Analyst141, 5432–5440. doi: 10.1039/c6an00810k
81
Van Der Meer J. R. Belkin S. (2010). Where microbiology meets microengineering: design and applications of reporter bacteria. Nat. Rev. Microbiol.8, 511–522. doi: 10.1038/nrmicro2392
82
Waimin J. F. Nejati S. Jiang H. Qiu J. Wang J. Verma M. S. et al . (2020). Smart capsule for non-invasive sampling and studying of the gastrointestinal microbiome. RSC Adv.10, 16313–16322. doi: 10.1039/c9ra10986b
83
Wang R. F. Beggs M. L. Robertson L. H. Cerniglia C. E. (2002). Design and evaluation of oligonucleotide-microarray method for the detection of human intestinal bacteria in fecal samples. FEMS Microbiol. Lett.213, 175–182. doi: 10.1016/S0378-1097(02)00802-9
84
Wang T. Li X. Chen L. Zhang Y. Zheng Y. Yu L. et al . (2021). The preparation of bifunctional hybrid nano-flowers and their application in the enzyme-linked immunosorbent assay for Helicobacter pylori detection. Analyst146, 338–347. doi: 10.1039/d0an01533d
85
Wang J. Qin J. Li Y. Cai Z. Li S. Zhu J. et al . (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature490, 55–60. doi: 10.1038/nature11450
86
Wang Z. Wang H. Cheng X. Geng J. Wang L. Dong Q. et al . (2021). Aptamer-superparamagnetic nanoparticles capture coupling siderophore-Fe3+ scavenging actuated with carbon dots to confer an “off-on” mechanism for the ultrasensitive detection of Helicobacter pylori. Biosens. Bioelectron.193, 113551–113559. doi: 10.1016/j.bios.2021.113551
87
Wang Y. Wang Y. Xu J. Ye C. (2016). Development of multiple cross displacement amplification label-based gold nanoparticles lateral flow biosensor for detection of Shigella spp. Front. Microbiol.7, 1–13. doi: 10.3389/fmicb.2016.01834
88
Wang J. Zhang C. Childers W. S. (2021). A biosensor for detection of Indole metabolites. ACS Synth. Biol.10, 1605–1614. doi: 10.1021/acssynbio.1c00090
89
Watanabe K. Petri W. A. (2016). Environmental Enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine10, 25–32. doi: 10.1016/j.ebiom.2016.07.030
90
Westenbrink E. Arasaradnam R. P. O’Connell N. Bailey C. Nwokolo C. Bardhan K. D. et al . (2015). Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron.67, 733–738. doi: 10.1016/j.bios.2014.10.044
91
Wirbel J. Pyl P. T. Kartal E. Zych K. Kashani A. Milanese A. et al . (2019). Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med.25, 679–689. doi: 10.1038/s41591-019-0406-6
92
Woo S. G. Moon S. J. Kim S. K. Kim T. H. Lim H. S. Yeon G. H. et al . (2020). A designed whole-cell biosensor for live diagnosis of gut inflammation through nitrate sensing. Biosens. Bioelectron.168, 112523–112510. doi: 10.1016/j.bios.2020.112523
93
Xing H. Zhang Y. Krämer M. Kissmann A. K. Amann V. Raber H. F. et al . (2022). A polyclonal Aptamer library for the specific binding of the gut bacterium Roseburia intestinalis in mixtures with other gut microbiome Bacteria and human stool samples. Int. J. Mol. Sci.23, 1–13. doi: 10.3390/ijms23147744
94
Xu Y. Luo Z. Chen J. Huang Z. Wang X. An H. et al . (2018). Ω-shaped Fiber-optic probe-based localized surface Plasmon resonance biosensor for real-time detection of Salmonella Typhimurium. Anal. Chem.90, 13640–13646. doi: 10.1021/ACS.ANALCHEM.8B03905/SUPPL_FILE/AC8B03905_SI_001.PDF
95
Yadav A. K. Verma D. Sajwan R. K. Poddar M. Yadav S. K. Verma A. K. et al . (2022). Nanomaterial-based electrochemical Nanodiagnostics for human and gut metabolites diagnostics: recent advances and challenges. Biosensors12, 1–32. doi: 10.3390/bios12090733
96
Yilmaz Ö. Sen N. Küpelioǧlu A. A. Simsel I. (2006). Detection of H pylori infection by ELISA and Western blot techniques and evaluation of anti CagA seropositivity in adult Turkish dyspeptic patients. World J. Gastroenterol.12, 5375–5378. doi: 10.3748/wjg.v12.i33.5375
97
Yu J. Feng Q. Wong S. H. Zhang D. Yi Liang Q. Qin Y. et al . (2017). Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut66, 70–78. doi: 10.1136/gutjnl-2015-309800
98
Yuan J. Chen C. Cui J. Lu J. Yan C. Wei X. et al . (2019). Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab.30, 675–688.e7. doi: 10.1016/j.cmet.2019.08.018
99
Zheng P. Li Z. Zhou Z. (2018). Gut microbiome in type 1 diabetes: a comprehensive review. Diabetes Metab. Res. Rev.34, e3043–e3049. doi: 10.1002/dmrr.3043
100
Zhou Z. Zhang Y. Guo M. Huang K. Xu W. (2020). Ultrasensitive magnetic DNAzyme-copper nanoclusters fluorescent biosensor with triple amplification for the visual detection of E. coli O157:H7. Biosens. Bioelectron.167:112475. doi: 10.1016/j.bios.2020.112475
101
Zmora N. Zilberman-Schapira G. Suez J. Mor U. Dori-Bachash M. Bashiardes S. et al . (2018). Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cells174, 1388–1405.e21. doi: 10.1016/j.cell.2018.08.041
102
Zúñiga A. Muñoz-Guamuro G. Boivineau L. Mayonove P. Conejero I. Pageaux G. P. et al . (2022). A rapid and standardized workflow for functional assessment of bacterial biosensors in fecal samples. Front. Bioeng. Biotechnol.10:859600. doi: 10.3389/fbioe.2022.859600
Summary
Keywords
probiotics, biosensors, point-of-care devices, personalized healthcare, gastrointestinal microbiota
Citation
Ngashangva L and Chattopadhyay S (2023) Biosensors for point-of-care testing and personalized monitoring of gastrointestinal microbiota. Front. Microbiol. 14:1114707. doi: 10.3389/fmicb.2023.1114707
Received
02 December 2022
Accepted
19 April 2023
Published
05 May 2023
Volume
14 - 2023
Edited by
Emmanuelle Maguin, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), France
Reviewed by
Nar Singh Chauhan, Maharshi Dayanand University, India; Jesus Garcia-Gil, Department of Biology, University of Girona, Spain
Updates
Copyright
© 2023 Ngashangva and Chattopadhyay.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lightson Ngashangva, ng.lightson@rgcb.res.in
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.