- 1West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Chongqing Center for Disease Control and Prevention, Chongqing, China
- 3Department of Preventive Health Care, Sichuan University Hospital, Chengdu, Sichuan, China
- 4Food Safety Monitoring and Risk Assessment Key Laboratory of Sichuan Province, Chengdu, Sichuan, China
Introduction: Aflatoxins (AFT) identified as a Group 1 human carcinogen naturally contaminate various types of food and could increase the risk of hepatocellular carcinoma (HCC) through dietary intake. Chongqing municipality is located in Southwest China with subtropical monsoon climate which is conducive to AFT contamination in crops. However, the burden of HCC caused by the dietary exposure of the population in Chongqing to AFT has not been quantified.
Methods: The burden of HCC was estimated in terms of Disability Adjusted Life Year (DALY) using FDA-iRISK software. Dietary exposure to AFT in three food categories including grain and its products, nuts and seeds, and spices was assessed.
Results: The lifetime average daily dose (LADD) of AFT exposure for the population ranged from 2.40 to 8.25 ng/kg bw/day and 9.51 to 15.10 ng/kg bw/day at the mean and heavy (P95) AFT contamination levels, respectively. Among the three food categories, grain and its products contributed most to AFT exposure of the population. The estimated DALYs related to HCC induced by AFT were 162,000–556,000 and 641,000-1,020,000; the DALY rates were 6.47–22.20 and 25.59–40.72 per 100,000 persons per year; and the population attribution fractions (PAF) were 1.68–5.78% and 6.66–10.60%.
Discussion: Although the burden of HCC caused by dietary AFT was estimated to be relatively low among the population, the overall health burden might be underestimated owing to the uncertainties of this dataset. Thus, the overall health burden associated with AFT intake should still be of concern in further studies.
1. Introduction
Aflatoxins (AFT) are a group of toxic secondary metabolites primarily produced by Aspergillus flavus and Aspergillus parasiticus (Moss, 2002). AFT contaminating can occur in a wide variety of food crops including cereals, legumes, oilseeds, nuts and spices at any stage of food production (pre-harvest, post-harvest, drying and storage) (Winter and Pereg, 2019; Gichohi-Wainaina et al., 2021). Both epidemiological surveys and animal experiments evidenced that AFT can damage human and animal liver tissues to cause hepatocellular carcinoma (HCC) and even death (Wu et al., 2014). The International Agency for Research on Cancer (IARC) evaluated the carcinogenicity of naturally occurring AFT (AFB1, AFB2, AFG1 and AFG2) for humans as Group 1 human carcinogen (IARC, 2012). In nations worldwide with high risk of HCC, there are significantly synergistic hepato-carcinogenic effects caused by AFT and hepatitis B virus (HBV) infection (Shimakawa et al., 2016; Yang et al., 2019). A meta-analysis estimated the summary odds ratio (OR) of HCC risk from both AFT exposure and HBV was 54.1, while the summary ORs on HCC risk from AFT exposure alone and chronic HBV infection alone were 5.91 and 11.2, respectively, (Liu et al., 2012). In view of the carcinogenicity and widespread contamination of AFT, and it can be believed that almost all AFT exposure results from human diet, the risk assessment and risk management of AFT intake have been gaining global visibility. Joint Food and Agricultural Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) has evaluated AFT for several times at the thirty-first, forty-sixth, forty-ninth, fifty-sixth, sixty-eighth and eighty-third meetings (JECFA, 1987, 1997, 1998, 2002, 2007, 2017). JECFA pointed out that dietary exposure to AFT should be reduced to the lowest feasible level in order to minimize potential HCC risk in humans. For the individuals with high prevalence of HBV surface antigen-positive (HBsAg+) and high intake of AFT, the reduction of AFT intake would benefit the human health.
The burden of disease is an indicator of the health and economic impact of illness, injury and early death on societies and countries (Devleesschauwer et al., 2014). The Disability Adjusted Life Year (DALY) is a standard metric to express the burden of disease measuring the healthy life years lost due to a disease or injury. It can be used to predict the health burden of foodborne disease (Devleesschauwer et al., 2015). The WHO has estimated the global burden associated with exposure to AFT to be 21,757 (8,967-56,776) HCC cases, resulting in 19,455 (7,954-51,324) deaths and 636,869 (267,142-1,617,081) DALYs (Gibb et al., 2015). Liu and Wu calculated the global AFT-related HCC cases accounted for 4.6–28.2% of the total HCC cases each year. The most heavily afflicted parts of the world were sub-Saharan Africa, Southeast Asia and China, among which China accounted for 12.1–28.9% (Liu and Wu, 2010). However, little is known regarding the disease burden of HCC caused by foodborne AFT exposure in China. Most studies on AFT risk assessment mainly focused on dietary exposure to AFT in local population without exploring the associated disease burden. Chen et al. investigated the disease burden of HCC caused by AFT intake through DALY calculation in different areas of China; however, they just assessed two kinds of food (peanuts, corn and their products) which might underestimate the burden of disease (Chen et al., 2022).
Environmental factors such as temperature, humidity, soil and storage conditions affect fungal growth and, consequently, the occurrence of AFT in foods (Jallow et al., 2021). The aflatoxin-producing molds are isolated from a wide range of climate zones, but are more frequently found between latitudes 16° and 35° in warm climate zones (i.e., tropical and subtropical zones) (Torres et al., 2014; Mao et al., 2016). Wang et al. pointed out that high-incidence areas of HCC in China generally had a warm and humid climate (Wang et al., 1983). The southwestern region of China includes Sichuan Province, Chongqing Municipality, Yunnan Province, Guizhou Province, and the Tibet Autonomous Region. Yunnan and Guizhou provinces are located in the Yungui Plateau, while the Tibet Autonomous Region is situated in the Qinghai-Tibet Plateau, both of which experience relatively lower temperatures and low humidity, and are considered low-incidence areas for HCC in China (Chen et al., 2008). In contrast, the incidence rate of HCC is relatively higher in the Chongqing-Sichuan region in Southwest China. Because of climatic diversity in China whose latitude and longitude span more than 20°, there are five different climate regions in China. The subtropical monsoon climate in Chongqing municipality (28°-32° north latitude) located in Southwest China favors the reproduction and virulence of aflatoxin-producing fungi (Wu et al., 2016; Sun et al., 2017). No study regarding the association between AFT exposure and HCC in the local population has been found in other southwestern regions of China. However, a case–control study conducted in Chongqing revealed that AFT is an independent risk factor for HCC in the population of Chongqing (Zheng et al., 2017). In addition, consumption of grain and its products, nuts and seeds, and spices is common in the dietary pattern of the population in Chongqing. Therefore, to explore the dietary AFT intake and associated health risk of the population, this study evaluated the lifetime dietary exposure to AFT and burden of HCC based on consumption data and AFT contamination data of three food categories (grain and its products, nuts and seeds, and spices) in Chongqing municipality of China.
2. Materials and methods
2.1. Contamination data
According to the sampling guideline outlined in the China Food Safety Risk Monitoring Program, a range of factors is taken into account when determining the sampling sites and sample numbers. These factors include food production, financial situation, monitoring capacity, dietary characteristics, degree of economic development, population density, geographical distribution, and other relevant considerations specific to each region. The sampling time and monitoring frequency of various monitoring samples were determined based on the characteristics of monitoring substances and their relationship with seasons, the requirements of national food safety risk assessment. A total of 694 samples of grain and its products, nuts and seeds, and spices were collected from various retail outlets (supermarkets, shops and market stalls) in different areas of Chongqing in 2012–2021. Grain and its products include rice, corn, wheat and their products; nuts and seeds include peanuts, sunflower seeds, pine nuts and their products; and spices are mainly pepper and its products.
All samples were delivered to the laboratories during 1 week and stored under ventilated and dry conditions until analysis. The levels of total aflatoxin content in samples including AFB1, AFB2, AFG1 and AFG2 were analyzed by using high-performance liquid chromatography (HPLC) or high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) following the China National Food Safety Standard GB/T 18979–2003, GB/T 5009.23–2006 and GB 5009.22–2016 (GAQSIQ, 2003; MOHC, 2006; NHFPC, 2016). All laboratories have passed the provincial laboratory quality certification and were requested to validate the test methodology for quality control prior to formal sample analysis. The limits of detection (LODs) among laboratories ranged from 0.006 to 0.5 μg/kg which met the requirements in the above-mentioned China National Food Safety Standards. According to the processing principle proposed by the Global Environment Monitoring System-Food Contamination Monitoring and Assessment Program (GEMS/Food), all non-detected results were replaced with 0 and LOD to produce the lower bound estimate (LB) and the upper bound estimate (UB) respectively (WHO, 1995).
2.2. Consumption data
The dietary consumption data was obtained from the survey data of Chongqing municipality in China Health and Nutrition Survey of 2018. A total of 979 subjects were obtained from 6 survey sites in Chongqing including Shapingba, Nanan, Dazu, Fengjie, Jiangjin and Qijiang districts and counties. The consumption data (including all foods eaten at and out home) was collected using three consecutive 24-h dietary recall by well-trained dietary staff via face-to-face interviews. For subjects under 7 years old and over 75 years old, dietary information came from adult family members. The food consumption within each food category was summed up for each investigated person to match with the occurrence data of each food category.
2.3. Risk-assessment methods
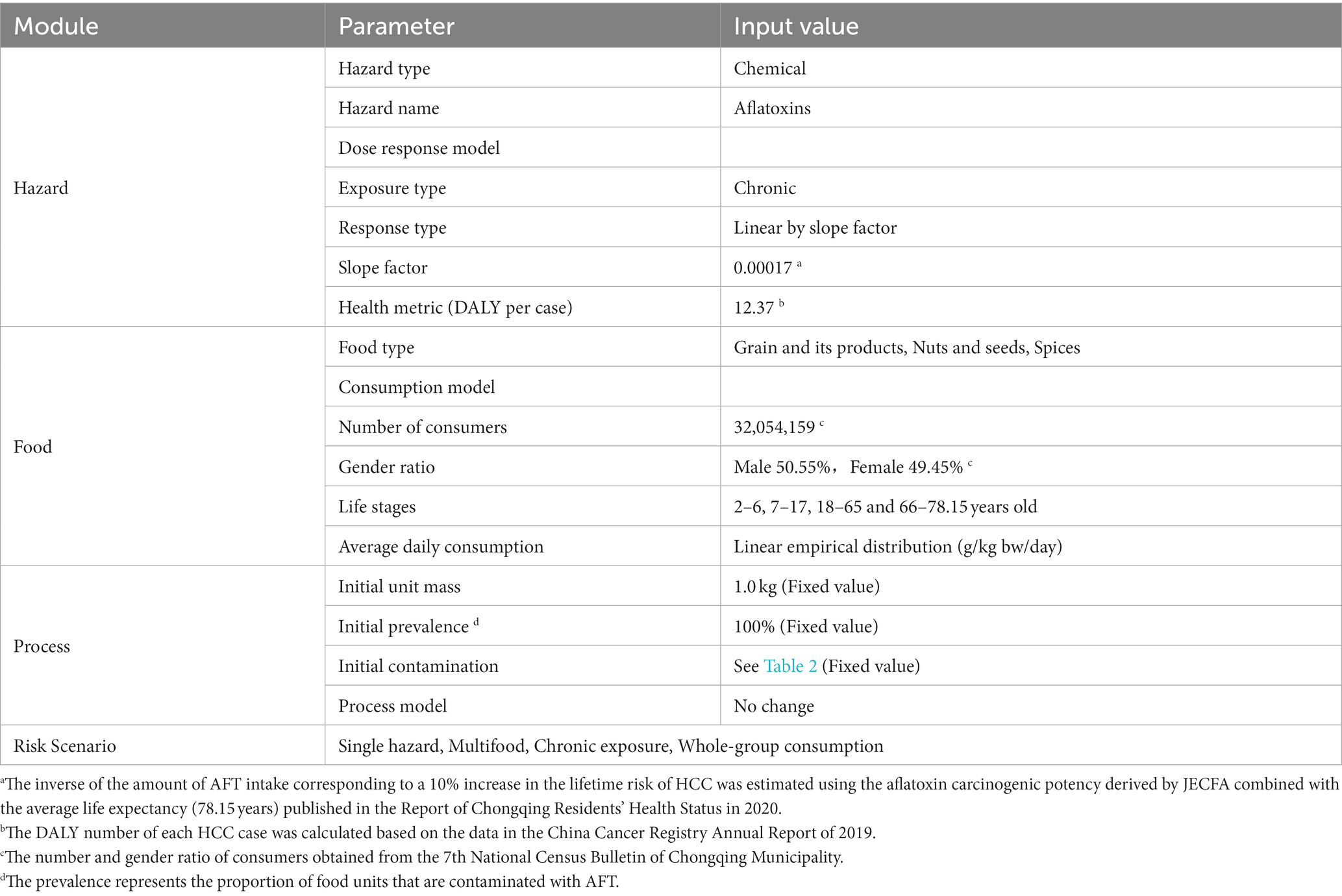
FDA-iRISK (iRISK) is a web-based risk-assessment system developed by the U.S. Food and Drug Administration (FDA) that intended for relatively rapid assessment of the risks and public health burden associated with hazards in food (Chen et al., 2013; FDA/CFSAN, 2021). This tool mainly consists of four modules: Hazard, Food, Process, and Risk Scenario. Firstly, the Hazard module describes the relationship between AFT and HCC with a dose–response model and health metric. For the Food module, food type and consumption model were both required to estimate the average daily consumption of aflatoxin-contaminated food in any life stages. In the Process module, the processes through which the prevalence and concentration of AFT in food units change at various steps in the food chain would affect the final AFT contamination level in food. Finally, the Risk Scenario module combines three above-defined modules to simulate different risk scenarios to compute and estimate the disease burden of dietary exposure to AFT. The computed risk scenario in the present study is defined as the chronic exposure for single hazard with multiple foods scenario. The schematic of the technical route is shown in Figure 1, and the key input parameters are listed in Table 1.

Figure 1. Schematic of the technical route of the chronic exposure for aflatoxin-Multifood scenario used in FDA-iRISK 4.2. LADD refers to lifetime average daily dose. HCC refers to hepatocellular carcinoma. DALY refers to Disability Adjusted Life Year.
2.3.1. Hazard module
2.3.1.1. Dose–response model
The cancer slope factor (SF) of “Liner by Slope Factor” model describes the dose–response relationship between AFT exposure and HCC incidence (Wang et al., 2019; FDA/CFSAN, 2021). The SF was derived from cancer potency factor (PF) proposed by JECFA. In the case of the dietary exposure level of AFB1 as 1 ng/kg bw/day, JECFA estimated AFB1 carcinogenic potencies which corresponded to 0.3 cancer cases/year per 100,000 subjects in HBsAg-positive individuals. For HBsAg-negative individuals, the potency estimate was 0.01 cancer cases/year per 100,000 subjects (JECFA, 1998). In our present study, the carcinogenic potency of AFT (the sum of AFB1, AFB2, AFG1 and AFG2) was conservatively estimated to equal to AFB1. Considering the HBsAg+ prevalence rate in the population of Chongqing, the PF and SF were calculated as follow:
where PHBsAg+ was the HBsAg-positive rate as 4.18% among the population of Chongqing according to the Chongqing Sero-epidemiological Survey of 2015 (Kuang et al., 2018); 10% referred to a 10% increase in the lifetime risk of HCC; L was the lifespan of the targeted population.
The PF was calculated as 0.022 cases/100,000 persons/year in the context of 1 ng/kg bw/day AFT intake. It is equivalent to a 10% increase in the lifetime risk of HCC of the general population in Chongqing ingesting 5,816 ng AFT/kg bw/day, which means the SF is 0.00017 (EFSA, 2007). The lifespan of the population was calculated as 78.15 years of the average life expectancy published in the Report of Chongqing Residents’ Health Status in 2020.
2.3.1.2. Health metric
The health burden of foodborne disease was measured using DALY (Havelaar et al., 2015). DALYs express the sum of years of life lost (YLLs) due to premature mortality and years of healthy life lost due to disability (YLDs) adjusted for the severity of a disease or health condition (Murray and Lopez, 1997). Chen et al. has calculated that the DALY number of each HCC case in China was 12.37 based on the data obtained from the China Cancer Registry Annual Report of 2019 (NCCR, 2020; Chen et al., 2022). And finally, the amount of aflatoxin-associated HCC cases multiplied by 12.37 equals the total DALYs.
2.3.2. Food module
2.3.2.1. Food type
Three food categories easily contaminated with AFT including grain and its products, nuts and seeds, and spices were assessed in our present study.
2.3.2.2. Consumption model
As different foods will be consumed by different fractions of the population in each life stage, the distribution describing the consumption level will necessarily include a proportion of consumers with zero consumption (FDA/CFSAN, 2021). As such, the cumulative empirical distribution is available in iRISK to describe consumption patterns for multi-food scenarios. Since there were only 5 subjects under 2 years old in our consumption survey, and the average life expectancy in Chongqing municipality was 78.15 years, the targeted age groups (life stages) focused on the population described and computed in iRISK were 2–6 years old, 7–17 years old, 18–65 years old and 66–78.15 years old. The values of daily consumption in grams per kilogram of body weight for the life stages were required to input in the form of liner empirical distribution. iRISK modeled chronic exposure by drawing a single value randomly from the consumption distribution of each age group to simulate a large number of individual lifetime exposure patterns that were possible within the population of consumers. In this way, the distinct variants of food exposure over the course of the lifespan were condensed into a single value representing the lifetime average daily dose (LADD). The calculation and examples of LADD are presented in Supplementary Tables S1, S2.
2.3.3. Process module
The process module combined the hazard module with the food module to calculate the AFT intake of the target population. It could estimate the impact of various interventions applied at specific steps in the farm-to-table continuum on AFT contamination. In this study, AFT content was uniformly distributed in the food, and both the mass of food and AFT content were assumed to be unchanged throughout the whole process (Wang et al., 2019). That is, the proportion of food contaminated with AFT per unit mass was 100% (initial and final prevalence) either before or after the process module running. Therefore, the setting value of unit mass had no effect on AFT contamination level in food and was defined as a fixed value of 1 kg in this study.
2.3.4. Risk scenario module
The risk scenario module combines different scenarios from the hazard, food and process modules to estimate the burden of foodborne disease. The burden of HCC caused by dietary exposure to AFT was calculated as follows:
where DALYtotal referred to the total DALY number in lifetime; DALYHCC was the DALY number per HCC case; Case was the total amount of HCC cases; N was the number of consumers; P (LADD|SF) was the mean lifetime risk probability of HCC per consumer (%); SF was cancer slope factor; LADD was lifetime average daily dose for AFT intake (ng/kg bw/day); LADCi was lifetime average daily consumption amount of food category i (g/kg bw/day); LADDi was lifetime average daily dose amount of AFT intake from food category i (ng/kg bw/day); Ci was the final AFT concentration of food category i (μg/kg); n was the number of food categories; Pi was the final AFT prevalence of food category i (%); Aj was the daily consumption per kilogram of body weight of life stage j (g/kg bw/day); Yj was the time span of life stage j (years); m was the number of life stages.
2.3.5. DALY rate and population attributable fraction
The DALY rate and population attributable fraction (PAF) were also calculated for estimating the health burden and incidence of aflatoxin-related HCC. Taking into account the number of consumers, lifetime and overall annual HCC incidence, the DALY rate and PAF were calculated as follows:
where DALY rate referred to the annual DALY number per 100,000 persons (DALY/100,000 persons/year); Rtotal was the annual all-cause HCC incidence (cases/100,000 persons/year).
2.4. Statistical analysis
SPSS 25.0 (IBM Corporation, Armonk, NY, United States) and R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis. Differences in AFT contamination among food categories were compared using the Kruskal-Wallis H-test at the 0.05 level (2-tailed). Findings were considered significant at p value <0.05. The dietary exposure and health burden of disease were estimated by using FDA-iRISK 4.2 (FDA/CFSAN, College Park, Maryland, United States) software.
3. Results
3.1. Contamination levels of AFT in three food categories
Table 2 shows the AFT contamination levels in grain and its products, nuts and seeds, and spices in Chongqing municipality of China. The total AFT detection rate of all the three food types was 17.4%, among which the highest AFT detection rate of 20.4% was found in nuts and seeds, followed by grain and its products (18.0%). In the case of the mean, median, P95 and maximum AFT contamination levels, grain and its products were more seriously contaminated with AFT than the other two food categories. The mean AFT concentration in grain and its products ranged from 0.61 to 2.08 μg/kg (LB-UB) which was 4–15 times higher than that in the other food categories, and the maximum concentration of AFT (37.10–38.30 μg/kg) was approximately 15-fold of that in the others.
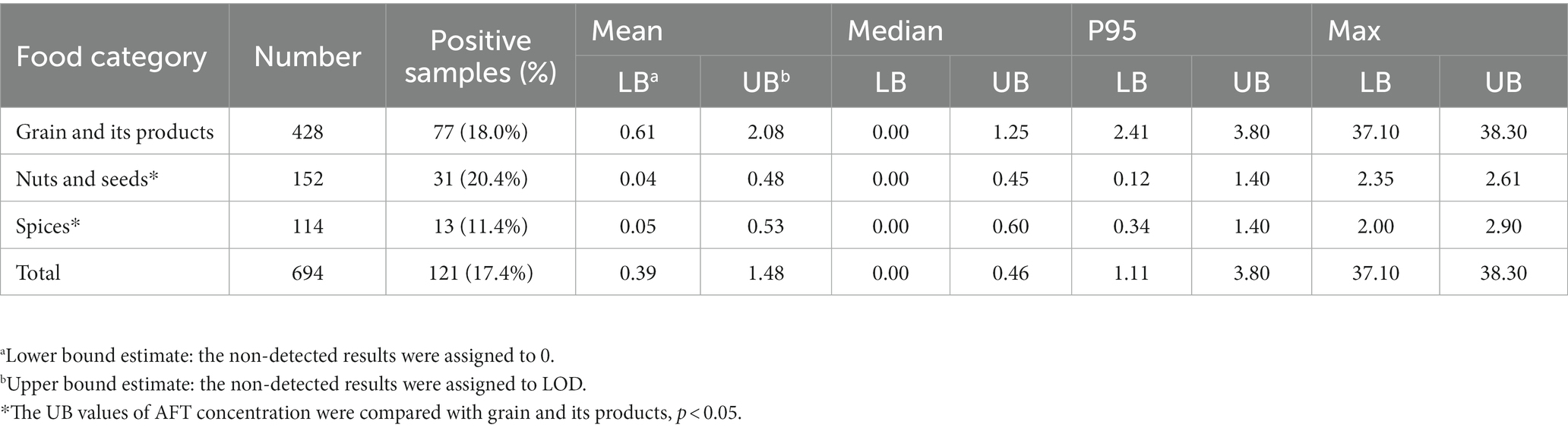
Table 2. AFT contamination levels in three food categories in Chongqing municipality of China (μg/kg).
3.2. Consumption data of three food categories
As presented in Table 3, all subjects ingested grain and its products, while a certain proportion of consumers in each age group had zero intake of spices, among which about 50% consumers aged 2–6 years consumed 0 g spices/kg bw/day. It is worth noting that the percentage of consumers ingested nuts and seeds was significantly low, with over 80% in all age groups had no consumption of nuts and seeds. The daily consumption level for grain and its products was the highest in each age group ranged from 0.43 to 24.53 g/kg bw/day, then followed by nuts and seeds (0.00–3.72 g/kg bw/day). The intake of spices was much lower at 0.00–0.49 g/kg bw/day.
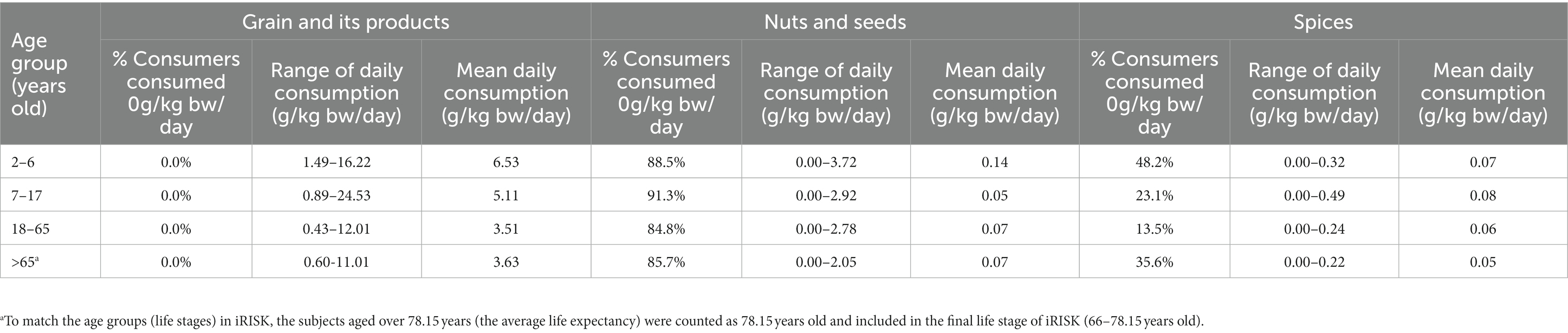
Table 3. Consumption levels of three food categories for different age groups in Chongqing municipality of China.
3.3. Dietary exposure to AFT
As shown in Figure 2 and Table 4, the percentile distributions of LADD for the population of Chongqing were calculated by simulating using the iRISK tool based on consumption data and occurrence data of three food categories. At the mean AFT contamination level in three food categories, the LADD ranged from 1.11 to 5.18 ng/kg bw/day (LB) and 3.83 to17.69 ng/kg bw/day (UB). Meanwhile, the high percentile (P95) of AFT concentration caused much higher LADD of 4.41–20.48 ng/kg bw/day (LB) and 7.05–32.48 ng/kg bw/day (UB). Detailed exposure percentile results output from iRISK are shown in Supplementary Table S3. The representative values of LADD at the mean AFT contamination level were 2.40 ng/kg bw/day (LB) and 8.25 ng/kg bw/day (UB).
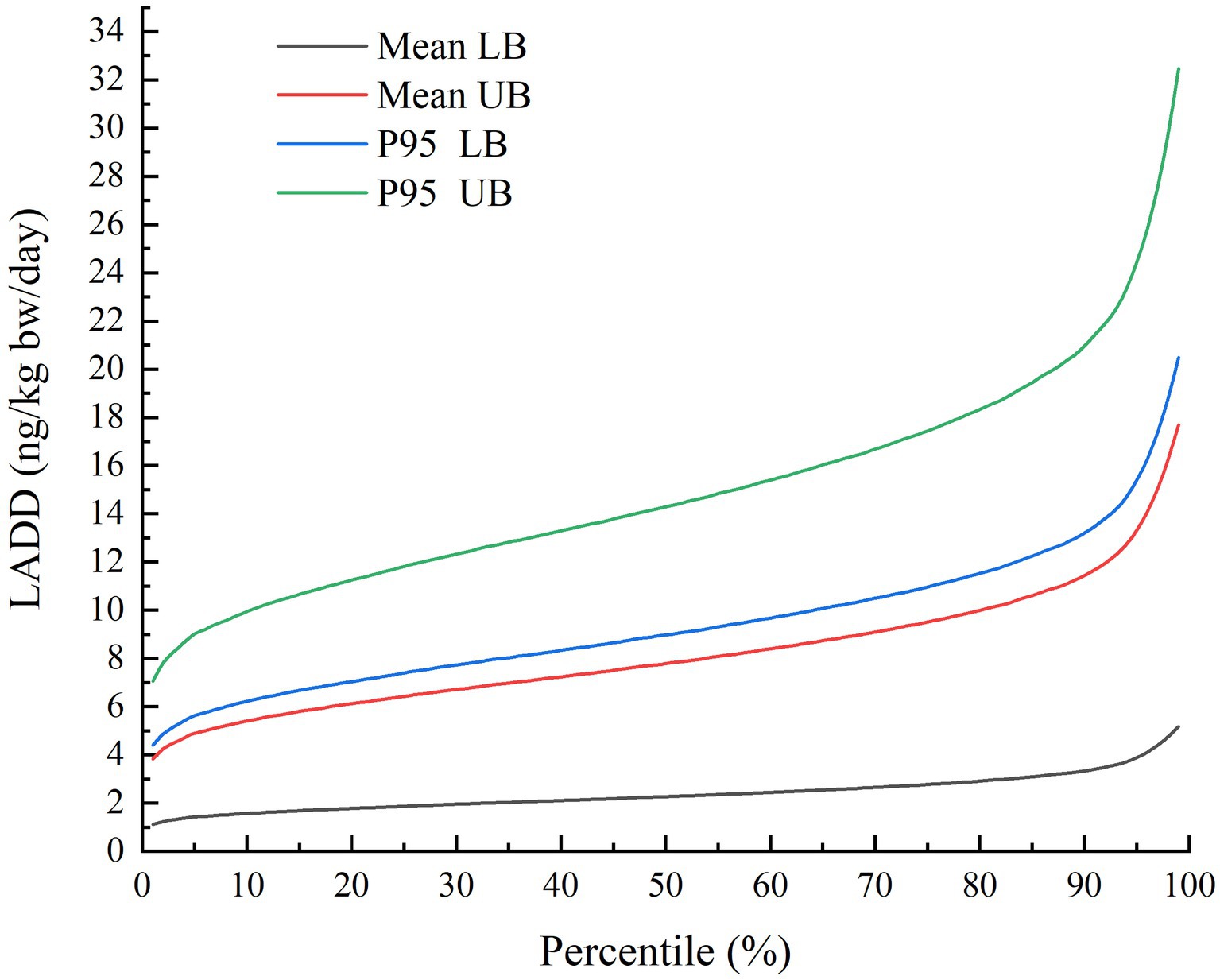
Figure 2. Percentile distributions of simulated LADD of AFT for the whole-group population in Chongqing municipality of China using FDA-iRISK 4.2.
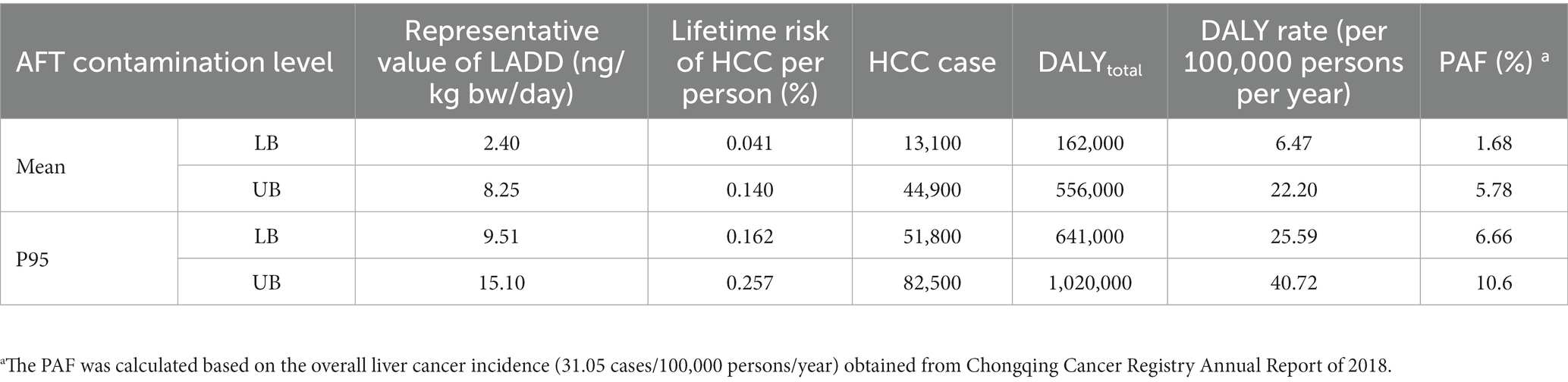
Table 4. AFT exposure levels and burden of HCC for the whole-group population in Chongqing municipality of China.
3.4. Disease burden of AFT exposure
In Table 4, the representative values of LADD at the mean AFT contamination level (LB and UB) resulted in 13,100 and 44,900 HCC cases, respectively. In addition, the total corresponding DALY numbers were 162,000 and 556,000; and the DALY rates were 6.47 and 22.20 per 100,000 persons per year. Given all the risk factors of liver cancer, the foodborne AFT intake from the food categories discussed in our present study accounted for 1.68 and 5.78% (PAF) of the overall annual liver cancer incidence. The AFT intake and associated disease burden resulted from AFT contamination at the high percentile (P95) level were approximately 2–4 times higher compared with those induced by the mean AFT contamination level.
4. Discussion
In this study, the LADD of AFT exposure from grain and its products, nuts and seeds, and spices was estimated in the population of Chongqing municipality in China. As stated above, at the mean and P95 contamination levels of AFT in three types of food, the LADD for the population were 2.40–8.25 ng/kg bw/day (LB-UB) and 9.51–15.10 ng/kg bw/day (LB-UB), respectively, which were lower than the estimated mean AFT intake of 8.3 ng/kg bw/day and 24.9 ng/kg bw/day for the Chinese adults and children in Yangtze Delta region of China (Li et al., 2014). The reason may lie in the average daily consumption per kg bw, which was calculated based on the daily consumption per capita of 0.402 kg divided by 60 kg bw for adults and 20 kg bw for children, was higher than that in our study. As for other countries of Asia, a calculated daily intake of AFB1 for the Korean population fell into the range of 0.0640–0.3612 ng/kg bw/day (Lee et al., 2009). The dietary exposure to AFB1 from all foods contaminated was only 0.003–0.004 ng/kg bw/day even though at the 95th percentile level of consumer population in Japan (Sugita-Konishi et al., 2010). Nevertheless, Malaysia estimated the dietary exposure to AFB1 to range from 24.3 to 34.0 ng/kg bw/day in the adult Malaysian diet (Chin et al., 2012). Additionally, the exposure to AFT shown in our study was higher compared with that in EFSA’s report of 2020. In that report, the dietary exposure to AFB1 from multiple foods in European population was estimated to be 0.08–6.95 ng/kg bw/day and 0.35–14.01 ng/kg bw/day for all age groups at mean and P95 exposure levels, respectively (EFSA Panel on Contaminants in the Food Chain (CONTAM) et al., 2020). Such differences in AFT intake levels could be concerned with the temperate climate of most of the EU regions and the subtropical climate of Chongqing. In contrast, dietary AFT exposure estimates in Africa (1.4–850 ng/kg bw/day) (Andrade and Caldas, 2015) were significantly higher than our findings. A survey conducted in sub-Saharan Africa revealed that the human dietary exposure to AFT, ranging between 4 and 526 ng/kg bw/day, also highlighted this discrepancy (Ingenbleek et al., 2020). The aforesaid findings agreed with the findings that the higher HCC risk occurred in the populations of sub-Saharan Africa, Southeast Asia and China, which were located in tropical and subtropical regions exposed to climate conditions favorable for AFT contamination (McGlynn et al., 2015; Sayiner et al., 2019).
Although it was not appropriate to establish a tolerable daily intake in view of the genotoxic properties of AFT, JECFA indicated that the risk of one extra cancer case per million people per year was acceptable (JECFA, 1998). The risk of extra HCC cases obtained for the target population evaluated in this study exceeded the above recognized unacceptable cancer risk. Our assessment revealed that the attributable risk of HCC caused by AFT intake in Chongqing municipality of China was estimated to be 1.68–5.78% at the mean contamination level of AFT in food. It is slightly below the global attributable risk of AFT-associated HCC (4.6–28.2%) reported by Liu and Wu (2010). Also, this is the first study to estimate the burden of disease associated with the current AFT exposure for the population in Chongqing in terms of DALY. The mean DALY rate of dietary AFT exposure for the population ranged from 6.47 to 22.20 per 100,000 persons per year (LB-UB), and increased to 25.59–40.72 per 100,000 persons per year at the 95th percentile of AFT contamination level. In 2022, Chen et al. evaluated the disease burden induced by dietary exposure to AFT in different areas of China. The national-level estimates of average DALY rate (1.53 per 100,000 persons per year) and PAF (0.69%) were both lower comparing with our results. In particular, the DALY rate of Chongqing municipality (0.01 per 100,000 persons per year) was far below our estimate of a mean of 6.47–22.20 per 100,000 persons per year. The discrepancy may be owing to the fact that only peanuts and peanut oil among oil in Chongqing municipality were included in that study, and the attributable exposure was merely 0.025 ng/kg bw/day (Chen et al., 2022). An evaluation conducted in Taiwan of China indicated the total annual DALYs caused by AFT contamination was 4,110, equivalent to 24.63 DALYs per 100,000 persons per year, which was higher than our results (Wang et al., 2019). The remarkable thing was that the HBsAg-positive rate of Taiwanese (17.3%) far above that of the population in Chongqing (4.18%). A WHO report by the Foodborne Disease Burden Epidemiology Reference Group (FERG) estimated the burden of disease related to AFT intake for each of 14 subregions (Havelaar et al., 2015; WHO, 2015). Among them, African Region D (AFR D) revealed the highest median DALY rate of 28 per 100,000 persons per year, followed by Western Pacific Region B (WPB R, including China) with a median DALY rate of 17 per 100,000 persons per year, which was close to our estimates. The two subregions with the lowest median DALY rate were Region A of the Americas (AMR A) and European Region A (EUR A), with only 0.04 and 0.3 DALYs per 100,000 persons per year, respectively. Comparisons with national studies showed the similar findings. Ricardo Assuncao et al. estimated the burden of disease associated to the current AFT exposure for Portuguese population to be 0.08–0.30 DALYs per 100,000 persons per year (Assuncao et al., 2018). Nevertheless, there was a strikingly high risk of AFT contamination in food ingested by Nigerian consumers, resulting in a national burden of 126.85–38,682.29 DALYs per 100,000 persons per year (Adetuniji et al., 2014). Furthermore, given the all-cause HCC incidence of the population of Chongqing reported to be 31.05 cases/100,000 persons/year (Rong et al., 2021), the overall HCC-burden was calculated to be 394.3 DALYs per 100,000 persons per year. In general, our findings suggest the current occurrence of AFT appears to be not a serious problem in Chongqing municipality of China, but it still causes certain risks concerning public health.
As is well-known, the environmental conditions of warm and high humidity are conducive to AFT-related fungal infection and toxin production (Do et al., 2020; Kerry et al., 2021). In this study, the AFT contamination in food of Chongqing was more severe than China’s national level, especially than that in northern China (Zhao et al., 2020; Yan et al., 2022). The subtropical monsoon climate of Chongqing municipality in Southwest China was reported to be more conducive to fungal reproduction and virulence versus the temperate continental climate and temperate monsoon climate in the northern China (Qin et al., 2021). Through the surveillance in our study, grain and its products in Chongqing were founded to be contaminated more seriously with AFT than nuts, seeds and spices. In addition, grain and its products account for a much larger proportion in the dietary consumption of the population in Chongqing than the other two types of food, so grain and its products can be considered to be the main food contributing to AFT exposure in the population’s diets. Chongqing municipality and Chengdu of Sichuan province, also located in Southwest China, once carried out AFT contamination surveys on unpackaged spices, and the AFT contamination levels were both higher than the values among this dataset, with median AFT content of 3.014–12.849 μg/kg and 1.1–57.1 μg/kg, respectively (Fu et al., 2015; Huang et al., 2020). This difference suggested that prolonged exposure of unpackaged food to external environment could increase the chance of AFT contamination as the lack of well-controlled storage conditions (Set and Erkmen, 2014; Naz et al., 2016). Various methods have been applied to degrade AFT, such as good agricultural practice (GAP), biocontrol, improving crop storage packaging, ozonolysis, alkali refining, ultraviolet (UV) irradiation and so on (Sudini et al., 2014; Mao et al., 2016; Parimi et al., 2018; Shabeer et al., 2022). However, it is expected that in the future the HCC-burden contributed by AFT exposure will increase due to climate change (Cotty and Jaime-Garcia, 2007; Assuncao et al., 2018). Reducing dietary exposures to the carcinogen AFT is likely to significantly reduce HCC risk, even in those already infected with HBV (Chen et al., 2013). Hence, consecutive monitoring and controlling AFT contamination in food to avoid future exposure of vast human populations to unacceptable AFT levels are essential ways to promote the food safety and human health.
Our study evaluated the lifetime exposure patterns of the population in Chongqing to AFT and firstly quantified the health burden of AFT-induced HCC. However, several uncertainty factors and limitations in the present study need to be addressed. Firstly, cancer potency for AFB1 was applied to ‘aflatoxin total’ because the available data could not make it possible to identify potency factors of all types of AFT. The uncertainty arising from this conservative approach should be noted, while EFSA reported that the influence of the assumption on the conclusion regarding the risk resulted from the presence of AFT in food was small (EFSA Panel on Contaminants in the Food Chain (CONTAM) et al., 2020). In the dose–response model, the derivation of the cancer potency factor (PF) only adjusted for the prevalence of hepatitis B infection in the local population and did not consider other confounding factors such as alcohol, hepatitis C infection, and consumption of raw meat. It should be noted that the population of Chongqing does not have a dietary habit of consuming raw meat, and the prevalence of hepatitis C infection is significantly lower compared to hepatitis B (Wang et al., 2007). Therefore, these two confounding factors was considered to pose little effect on the PF. However, alcohol intake is a well-recognized independent risk factor for HCC, and the lack of adjustment for it in this study might introduce some uncertainty. This would be one of the key points to address in future research. In the process module, fixed values were used for the calculations, so uncertainties ranges were not quantified due to the lack of raw data. Also, we assumed that the AFT content in food did not change throughout the whole process, but it is unclear what combined effect food processing and cooking might have had on AFT levels in consumed foods. Full studies with the farm-to-table continuum on AFT contamination are recommended in the future. Additionally, we collected samples in markets rather than in homes of residents and farmers, where storage conditions may affect aflatoxins contaminations. Total dietary studies may be conducted thereafter to reduce this uncertainty. Finally, the absent food categories in this study such as vegetable oil and beans have been the main sources of dietary exposure to AFT (Pickova et al., 2021). A full-scale estimation for the overall dietary AFT exposure of population needs to be conducted. In summary, we consider the risk assessment in our study is likely to be conservative.
5. Conclusion
This study presents the first disease burden estimates of HCC induced by the dietary exposure to AFT in three food categories including grain and its products, nuts and seeds, and spices, among population in a mountainous city (Chongqing municipality), in Southwest China. Comparing with nuts and seeds, and spices, grain and its products contributed most to dietary AFT exposure of the population. In terms of lifetime chronic exposure levels, the burden of HCC induced by AFT exposure among the population was relatively low in the context of all-cause liver cancer incidence. However, the health risk caused by AFT contamination should still be of concern. Given the future climate change, continuous monitoring and controlling of AFT contamination to restrict dietary exposure are recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MQ, JC, YL, and LZ conceived the concept. MQ, LC, XT, and YG carried out the project and collected the data. MQ analysed the data, wrote the initial draft, and revised the manuscript. JC, JH, YL, and LZ critically revised the manuscript and finalized the manuscript. JC and JH significantly contributed to review the manuscript in reply to reviewers. JZ, SL, and HZ supervised and administrated the project. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the project of Science and Technology Department of Sichuan Province in China (no 2019YJ0020), the China Postdoctoral Science Foundation (no 2022 M710549), the Natural Science Foundation of Chongqing (no cstc2021jcyj-msxmX0479), and the First batch of Key Disciplines on Public Health in Chongqing and the Scientific and Technological Innovation Center in Chongqing.
Acknowledgments
The authors would like to thank Centers for Disease Control and Prevention in all districts and counties of Chongqing municipality in China for supporting the experimental work in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1215428/full#supplementary-material
References
Adetuniji, M. C., Atanda, O. O., Ezekiel, C. N., Dipeolu, A. O., Uzochukwu, S. V. A., Oyedepo, J., et al. (2014). Distribution of mycotoxins and risk assessment of maize consumers in five agro-ecological zones of Nigeria. Eur. Food Res. Technol. 239, 287–296. doi: 10.1007/s00217-014-2221-0
Andrade, P. D., and Caldas, E. D. (2015). Aflatoxins in cereals: worldwide occurrence and dietary risk assessment. World Mycotoxin J. 8, 415–431. doi: 10.3920/wmj2014.1847
Assuncao, R., Martins, C., Viegas, S., Viegas, C., Jakobsen, L. S., Pires, S., et al. (2018). Climate change and the health impact of aflatoxins exposure in Portugal - an overview. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 35, 1610–1621. doi: 10.1080/19440049.2018.1447691
Chen, Y., Dennis, S. B., Hartnett, E., Paoli, G., Pouillot, R., Ruthman, T., et al. (2013). FDA-iRISK--a comparative risk assessment system for evaluating and ranking food-hazard pairs: case studies on microbial hazards. J. Food Prot. 76, 376–385. doi: 10.4315/0362-028X.JFP-12-372
Chen, J., Egner, P. A., Ng, D., Jacobson, L. P., Muñoz, A., Zhu, Y., et al. (2013). Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. (Phila.) 6, 1038–1045. doi: 10.1158/1940-6207.CAPR-13-0168
Chen, T., Liu, J., Li, Y., and Wei, S. (2022). Burden of disease associated with dietary exposure to aflatoxins in China in 2020. Nutrients 14:1027. doi: 10.3390/nu14051027
Chen, W., Zou, X., and Zhang, S. (2008). Analysis of liver cancer geographical characteristics in China. J. Pract. Oncol. 22, 201–203.
Chin, C. K., Abdullahb, A., and Sugita-Konishi, Y. (2012). Dietary intake of aflatoxins in the adult Malaysian population - an assessment of risk. Food Addit. Contam. Part B. Surveill. 5, 286–294. doi: 10.1080/19393210.2012.713028
Cotty, P. J., and Jaime-Garcia, R. (2007). Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 119, 109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060
Devleesschauwer, B., Haagsma, J. A., Angulo, F. J., Bellinger, D. C., Cole, D., Dopfer, D., et al. (2015). Methodological framework for World Health Organization estimates of the global burden of foodborne disease. PLoS One 10:e0142498. doi: 10.1371/journal.pone.0142498
Devleesschauwer, B., Havelaar, A. H., Maertens de Noordhout, C., Haagsma, J. A., Praet, N., Dorny, P., et al. (2014). DALY calculation in practice: a stepwise approach. Int. J. Public Health 59, 571–574. doi: 10.1007/s00038-014-0553-y
Do, T. H., Tran, S. C., Le, C. D., Nguyen, H.-B. T., Le, P.-T. T., Le, H.-H. T., et al. (2020). Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin a, fumonisin B1, and zearalenone in food from different provinces in northern Vietnam. Food Control 112:107108. doi: 10.1016/j.foodcont.2020.107108
EFSA Panel on Contaminants in the Food Chain (CONTAM)Schrenk, D., Bignami, M., Bodin, L., Chipman, J. K., del Mazo, J., et al. (2020). Risk assessment of aflatoxins in food. EFSA J. 18:e06040. doi: 10.2903/j.efsa.2020.6040
EFSA (European food safety authority) (2007). Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential in-crease of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 5, 1–127. doi: 10.2903/j.efsa.2007.446
FDA/CFSAN (Food and Drug Administration Center for Food Safety and Applied Nutrition).; JIFSAN (joint Institute for Food Safety and Applied Nutrition).; RSI (risk sciences international). (2021). FDA-iRISK® version 4.2. FDA CFSAN. College Park, Maryland.
Fu, G., Deng, X., Li, B., Wang, L., Yang, L., and Zhang, W. (2015). Investigation on contamination of cayenne powder aflatoxin B1 in Xindu District, Chengdu. J. Prev. Med. Public Health 26, 101–102.
GAQSIQ (General Administration of Quality Supervision Inspection and Quarantine of the People‘s Republic of China). De-termination aflatoxins content in food. Cleanup by immunoaffinity chromatography and determination by high-performance liquid chromatography and fluorometer. Beijing, China: China Standard Press;(2003).
Gibb, H., Devleesschauwer, B., Bolger, P. M., Wu, F., Ezendam, J., Cliff, J., et al. (2015). World health organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: a data synthesis. F1000Res 4:1393. doi: 10.12688/f1000research.7340.1
Gichohi-Wainaina, W. N., Kumwenda, N., Zulu, R., Munthali, J., and Okori, P. (2021). Aflatoxin contamination: knowledge disparities among agriculture extension officers, frontline health workers and small holder farming households in Malawi. Food Control 121:107672. doi: 10.1016/j.foodcont.2020.107672
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12:e1001923. doi: 10.1371/journal.pmed.1001923
Huang, S., Dong, X., Deng, Y., Li, H., Shi, F., Gu, S., et al. (2020). Survey on mycotoxin contamination situation in pepper, prickly ash and star anise in Chongqing. J. Food Saf. Qual. 11, 8119–8124. doi: 10.19812/j.cnki.jfsq11-5956/ts.2020.21.076
IARC (International Agency for Research on Cancer). A review of human carcinogens: Chemical agents and related occupations. Lyon, France: World Health Organization (WHO) Press (2012).
Ingenbleek, L., Verger, P., Gimou, M. M., Adegboye, A., Adebayo, S. B., Hossou, S. E., et al. (2020). Human dietary exposure to chemicals in sub-Saharan Africa: safety assessment through a total diet study. Lancet Planet Health. 4, e292–e300. doi: 10.1016/S2542-5196(20)30104-2
Jallow, A., Xie, H., Tang, X., Qi, Z., and Li, P. (2021). Worldwide aflatoxin contamination of agricultural products and foods: from occurrence to control. Compre. Rev. Food Sci. Food Saf. 20, 2332–2381. doi: 10.1111/1541-4337.12734
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain food additives and contaminants: Thirty-first report of the joint FAO/WHO expert committee on food additives. (1987). World Health Organization. Switzerland
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain food additives and contaminants: Forty-sixth report of the joint FAO/WHO expert committee on food additives. (1997). World Health Organization. Switzerland
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain food additives and contaminants: Forty-ninth report of the joint FAO/WHO expert committee on food additives. (1998). World Health Organization. Switzerland
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain mycotoxins in food: Fifty-sixth report of the joint FAO/WHO expert committee on food additives. (2002). World Health Organization. Switzerland
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain food additives: Sixty-ninth report of the joint FAO/WHO expert committee on food additives. (2007). World Health Organization. Switzerland
JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of certain contaminants in food: Eighty-third report of the joint FAOWHO expert committee on food additives. (2017). World Health Organization. Switzerland
Kerry, R., Ingram, B., Garcia-Cela, E., Magan, N., Ortiz, B. V., and Scully, B. (2021). Determining future aflatoxin contamination risk scenarios for corn in southern Georgia, USA using spatio-temporal modelling and future climate simulations. Sci. Rep. 11:13522. doi: 10.1038/s41598-021-92557-6
Kuang, S., Wang, D., Wang, Q., and Xu, J. (2018). Serological epidemiology analysis of the hepatitis B virus infection among the population aged 1 to 29 in Chongqing. J. Prev. Med. Inf. 34, 923–926.
Lee, H. M., Hwang, J. H., Ryuem, T. K., Jang, D. D., and Yang, J.-H. (2009). Risk assessment of aflatoxin B1from food consumption in the Korean general population. Hum. Ecol. Risk. Assess. 15, 1273–1285. doi: 10.1080/10807030903306760
Li, R., Wang, X., Zhou, T., Yang, D., Wang, Q., and Zhou, Y. (2014). Occurrence of four mycotoxins in cereal and oil products in Yangtze Delta region of China and their food safety risks. Food Control 35, 117–122. doi: 10.1016/j.foodcont.2013.06.042
Liu, Y., Chang, C.-C. H., Marsh, G. M., and Wu, F. (2012). Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur. J. Cancer 48, 2125–2136. doi: 10.1016/j.ejca.2012.02.009
Liu, Y., and Wu, F. (2010). Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 118, 818–824. doi: 10.1289/ehp.0901388
Mao, J., He, B., Zhang, L., Li, P., Zhang, Q., Ding, X., et al. (2016). A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in Peanut oil under UV irradiation. Toxins (Basel) 8:332. doi: 10.3390/toxins8110332
McGlynn, K. A., Petrick, J. L., and London, W. T. (2015). Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin. Liver Dis. 32, 454–464. doi: 10.1016/j.cld.2015.01.001
MOHC (Minister of Health of the People’s Republic of China).; SMC (China National Standardization Management com-mittee). Determination of aflatoxins B1, B2, G1, G2 in foods. Beijing, China: China Standard Press; (2006).
Moss, M. O. (2002). Risk assessment for aflatoxins in foodstuffs. Int. Biodeterior. Biodegradation 50, 137–142. doi: 10.1016/S0964-8305(02)00078-1
Murray, C. J., and Lopez, A. D. (1997). Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 349, 1436–1442. doi: 10.1016/S0140-6736(96)07495-8
Naz, N., Kashif, A., Kanwal, K., Khan, A. M., and Abbas, M. (2016). Quantitative Scrutinization of aflatoxins in different spices from Pakistan. Int J Anal Chem. 2016, 4907425–4907427. doi: 10.1155/2016/4907425
NCCR. China Cancer registry annual report 2019. Beijing, China: Tsinghua University Press (China); (2020).
NHFPC (National Health and Family Planning Commission of the People's Republic of China).; NHFPC (National Health and Family Planning Commission of the People's Republic of China). Determination of aflatoxins groups B and G in food. Beijing, China: China Standard Press; (2016).
Parimi, V., Kotamraju, V., and Sudini, H. (2018). On-farm demonstrations with a Set of good agricultural practices (GAPs) proved cost-effective in reducing pre-harvest aflatoxin contamination in groundnut. Agronomy 8:10. doi: 10.3390/agronomy8020010
Pickova, D., Ostry, V., and Malir, F. (2021). A recent overview of producers and important dietary sources of aflatoxins. Toxins (Basel). 13:186. doi: 10.3390/toxins13030186
Qin, M., Liang, J., Yang, D., Yang, X., Cao, P., Wang, X., et al. (2021). Spatial analysis of dietary exposure of aflatoxins in peanuts and peanut oil in different areas of China. Food Res. Int. 140:109899. doi: 10.1016/j.foodres.2020.109899
Rong, R, Tang, W, Ding, X, Lv, X, and Zhang, G. Chongqing annual report of tumor registration (2018). Chengdu, China: Sichuan Science and Technology Press; (2021).
Sayiner, M., Golabi, P., and Younossi, Z. M. (2019). Disease burden of hepatocellular carcinoma: a global perspective. Dig. Dis. Sci. 64, 910–917. doi: 10.1007/s10620-019-05537-2
Set, E., and Erkmen, O. (2014). Occurrence of aflatoxins in ground red chili pepper and pistachio nut. Int. J. Food Prop. 17, 2322–2331. doi: 10.1080/10942912.2013.800985
Shabeer, S., Asad, S., Jamal, A., and Ali, A. (2022). Aflatoxin contamination, its impact and management strategies: an updated review. Toxins (Basel) 14:307. doi: 10.3390/toxins14050307
Shimakawa, Y., Lemoine, M., Njai, H. F., Bottomley, C., Ndow, G., Goldin, R. D., et al. (2016). Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from the Gambia. Gut 65, 2007–2016. doi: 10.1136/gutjnl-2015-309892
Sudini, H., Ranga Rao, G. V., Gowda, C. L. L., Chandrika, R., Margam, V., Rathore, A., et al. (2014). Purdue improved crop storage (PICS) bags for safe storage of groundnuts. J. Stored Prod. Res. 64, 133–138. doi: 10.1016/j.jspr.2014.09.002
Sugita-Konishi, Y., Sato, T., Saito, S., Nakajima, M., Tabata, S., Tanaka, T., et al. (2010). Exposure to aflatoxins in Japan: risk assessment for aflatoxin B1. Food Addit Contam., Part A 27, 365–372. doi: 10.1080/19440040903317497
Sun, X. D., Su, P., and Shan, H. (2017). Mycotoxin contamination of Rice in China. J. Food Sci. 82, 573–584. doi: 10.1111/1750-3841.13631
Torres, A. M., Barros, G. G., Palacios, S. A., Chulze, S. N., and Battilani, P. (2014). Review on pre- and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 62, 11–19. doi: 10.1016/j.foodres.2014.02.023
Wang, Q., Chen, Y., Wang, X., Wang, W., Wang, Y., and Liang, X. (2007). Investigation and study of the prevalence of chronic hepatitis B and C in Chongqing. Mod Prev Med. 34:11.
Wang, Y., Lan, L., Ye, B., Xu, Y., Liu, Y., and Li, W. (1983). Relationship between geographical distribution of liver cancer and climate and aflatoxin B1 in China. Zhongguo Kexue. 13, 431–437.
Wang, X., You, S. H., Lien, K. W., and Ling, M. P. (2019). Using disease-burden method to evaluate the strategies for reduction of aflatoxin exposure in peanuts. Toxicol. Lett. 314, 75–81. doi: 10.1016/j.toxlet.2019.07.006
WHO (World Health Organization) (1995). Second workshop on reliable evaluation of low-level contamination of food-report on a workshop in the frame of GEMS/food-EURO. Kulmbach Germany: WHO Regional Office for Europe.
WHO (World Health Organization). WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007–2015. Geneva, Switzerland: World Health Organization; (2015).
Winter, G., and Pereg, L. (2019). A review on the relation between soil and mycotoxins: effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 70, 882–897. doi: 10.1111/ejss.12813
Wu, L. X., Ding, X. X., Li, P. W., Du, X. H., Zhou, H. Y., Bai, Y. Z., et al. (2016). Aflatoxin contamination of peanuts at harvest in China from 2010 to 2013 and its relationship with climatic conditions. Food Control 60, 117–123. doi: 10.1016/j.foodcont.2015.06.029
Wu, F., Groopman, J. D., and Pestka, J. J. (2014). Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 5, 351–372. doi: 10.1146/annurev-food-030713-092431
Yan, Z., Huang, C., and Yang, X. (2022). Status of mycotoxin contamination in staple foods in China. J. Hyg. Res. 51, 685–690. doi: 10.19813/j.cnki.weishengyanjiu.2022.04.031
Yang, J. D., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., and Roberts, L. R. (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604. doi: 10.1038/s41575-019-0186-y
Zhao, Y., Zeng, R., Chen, P., Wang, Q., Wang, X., Liu, X., et al. (2020). Investigation on the contamination of sterigmatocystin and aflatoxin B1 in food from mainland China. J. Food Saf. Qual. 11, 9109–9114. doi: 10.19812/j.cnki.jfsq11-5956/ts.2020.24.005
Keywords: aflatoxin, dietary exposure, disease burden, disability adjusted life year, hepatocellular carcinoma
Citation: Qin M, Cheng L, Li Y, Tang X, Gan Y, Zhao J, Luo S, Zhang H, Zhang L, Chen J and Huo J (2023) Disease burden contributed by dietary exposure to aflatoxins in a mountainous city in Southwest China. Front. Microbiol. 14:1215428. doi: 10.3389/fmicb.2023.1215428
Edited by:
Xiao Li Shen, Zunyi Medical University, ChinaReviewed by:
Haixia Sui, China National Center for Food Safety Risk Assessment, ChinaPhitsanu Tulayakul, Kasetsart University, Thailand
Copyright © 2023 Qin, Cheng, Li, Tang, Gan, Zhao, Luo, Zhang, Zhang, Chen and Huo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinyao Chen, dW1icmVsbGF5eUAxNjMuY29t; Jiao Huo, bGFtYXJoakAxMjYuY29t
 Mei Qin1,2
Mei Qin1,2 Yan Li
Yan Li Lishi Zhang
Lishi Zhang Jinyao Chen
Jinyao Chen