- 1National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Shaanxi Provincial Center for Disease Control and Prevention, Xi'an, China
- 3Long County Center for Disease Control and Prevention, Baoji, China
- 4Mei County Center for Disease Control and Prevention, Baoji, China
- 5HanZhong Center for Disease Control and Prevention, Hanzhong, China
- 6Zhenba County Center for Disease Control and Prevention, Hanzhong, China
Important tick-borne diseases include spotted fever group Rickettsia (SFGR), Anaplasma, and Ehrlichia, which cause harm to animal and human health. Ixodidae are the primary vectors of these pathogens. We aimed to analyze the prevalence and genetic diversity of SFGR, Anaplasma, and Ehrlichia species in the Ixodidae in Shaanxi Province, China. Herein, 1,113 adult Ixodidae ticks were collected from domestic cattle and goats, and detected using nested PCR. A total of four Ixodidae species were collected and Ca. R. jingxinensis (20.58%, 229/1113), A. bovis (3.05%, 34/1113), A. capra (3.32%, 37/1113), A. marginale (0.18%, 2/1113), E. sp. Yonaguni138 (0.18%, 2/1113), and a potent novel Ehrlichia species named E. sp. Baoji96 (0.09%, 1/1113) were detected. A. marginale was detected for the first time in Rhipicephalus microplus. E. sp. Baoji96 was closely related to E. chaffeensis and was first identified in Haemaphysalis longicornis. In addition, co-infection with two Rickettsiales pathogens within an individual tick was detected in 10 (1.54%) ticks. This study provides a reference for the formulation of biological control strategies for ticks and tick-borne diseases in Shaanxi Province, and could lead to an improved control effect.
1 Introduction
Rickettsiales are a class of bacteria that are gram-negative obligate intracellular bacteria that can infect humans and different vertebrates via the bite of an arthropod vector. Recognized important Rickettsiales pathogens include spotted fever group Rickettsia (SFGR), Anaplasma, and Ehrlichia, and Ixodidae are their primary vectors (Qin et al., 2019). The initial symptoms are similar to those of a cold, such as high fever, weakness, pain, chills, etc., and there are also signature clinical features, rash and eschar. SFGR consists of over 30 species distributed worldwide, and at least 18 species have been identified as human pathogens in China (Robinson et al., 2019; Teng et al., 2023b). For example, Rickettsia rickettsii is the most pathogenicSFGR, which causes Rocky Mountain spotted fever (RMSF) in North America. Rickettsia conorii causes Mediterranean spotted fever (MSF) in some regions of Europe, Africa, and Asia (Robinson et al., 2019). In addition, disease names associated with pathogens often reflect the region where they are found; however, the actual endemic region is often much larger (Labruna et al., 2014). Rickettsia japonica causes Japanese spotted fever (JSF). It was first described in Japan in 1984 in three patients with high fever and rash (Mahara, 1997). But outside Japan, cases have been reported in China, South Korea, the Philippines, and Thailand (Teng et al., 2023a). At present, the genera Anaplasma and Ehrlichia contain eight known bacterial species that infect humans: A. phagocytophilum, A. platys, A. ovis, A. bovis, A. capra, E. chaffeensis, E. ewingii, and E. muris (Bakken et al., 1994; Paddock and Childs, 2003; Thomas et al., 2009; Chochlakis et al., 2010; Johnson et al., 2015; Li et al., 2015; Lu et al., 2022). Furthermore, E. chaffeensis causes human monocytotropic ehrlichiosis (HME), and A. phagocytophilum is responsible for human granulocytotropic anaplasmosis (HGA). Both HME and HGA have fairly high infection rates in the United States, and cases have also been reported in China (Ismail et al., 2010). Rickettsiales are potential pathogenic factors for numerous diseases, which can lead to rash, post-infectious arthritides, interstitial pneumonia, meningoencephalitis, acute kidney injury, multiple organ failure, and even death after human infection (Pasquale Mansueto et al., 2012; Zeidler and Hudson, 2021; Sebastian et al., 2022). Moreover, in recent years, a number of emerging and re-emerging Rickettsiales species have been discovered to infect humans (Qin et al., 2019). Thus, Rickettsiales diseases will continue to be a threat to human health.
The epidemiology of tick-borne Rickettsial disease reflects the geographic and seasonal distribution of the pathogen and transmission is mainly related to the following: the tick vector and its host, and the human behavior of people bitten after their skin is exposed to the tick (Beati et al., 1997). The distribution of tick-borne Rickettsial disease is geographically similar to that of ticks and their hosts, which tells us that understanding the distribution and changes of vectors is very important for the prevention and control of tick-borne rickettsial disease. Ticks are found in a wide range of areas, ranging from forests to roadside bushes, and even in areas without vegetation (Piotrowski and Rymaszewska, 2020). This makes us aware of the potential dangers of tick bites when we travel.
Ixodidae, the primary vectors of pathogens belonging to the Rickettsiales, comprise 111 species from 7 genera in China (Zhang et al., 2019). Ticks are found in all regions of China and infect every class of terrestrial vertebrates, including mammals, birds, reptiles, and even amphibians (Lu et al., 2019). With more and more attention being paid to the construction of urban landscaping, the contact area between man and nature has been greatly increased. This overlaps human, animal and tick habitats, greatly increasing the risk of human tick bites. Despite their ubiquity in nature, these organisms are often overlooked as an important cause of disease around the world. Treatment is easily delayed because of a lack of awareness of tick-borne diseases. Due to misdiagnosis, the best antibiotic treatment time is missed, which may lead to serious complications and even death. Hence, investigation of local tick-borne Rickettsiales pathogen prevalence is helpful for the early diagnosis and treatment of related diseases.
The terrain of Shaanxi province consists of mountains, plains, and basins, spanning three climatic zones. Southern Shaanxi is humid, central Guanzhong is semi-humid, and northern Shaanxi is semi-arid; therefore, it is rich in species and diverse in vegetation types. The Qinling Mountains are even known as a “biological gene bank.” Different ticks have different habitats. Ixodes prefer cool, moist environments, but Rhipicephalus sanguineus are adapted to high temperatures and dry environments. And, Different ticks prefer different hosts (Piotrowski and Rymaszewska, 2020). Here, ticks always find the right living conditions and plenty of hosts. In the past, Rickettsiales studies in Shaanxi Province mainly focused on Anaplasma, especially A. capra (Guo et al., 2019b). In this study, we aimed to analyze the prevalence and genetic diversity of SFGR, Anaplasma, and Ehrlichia species in Ixodidae collected from Shaanxi Province, China.
2 Materials and methods
2.1 Sample collection
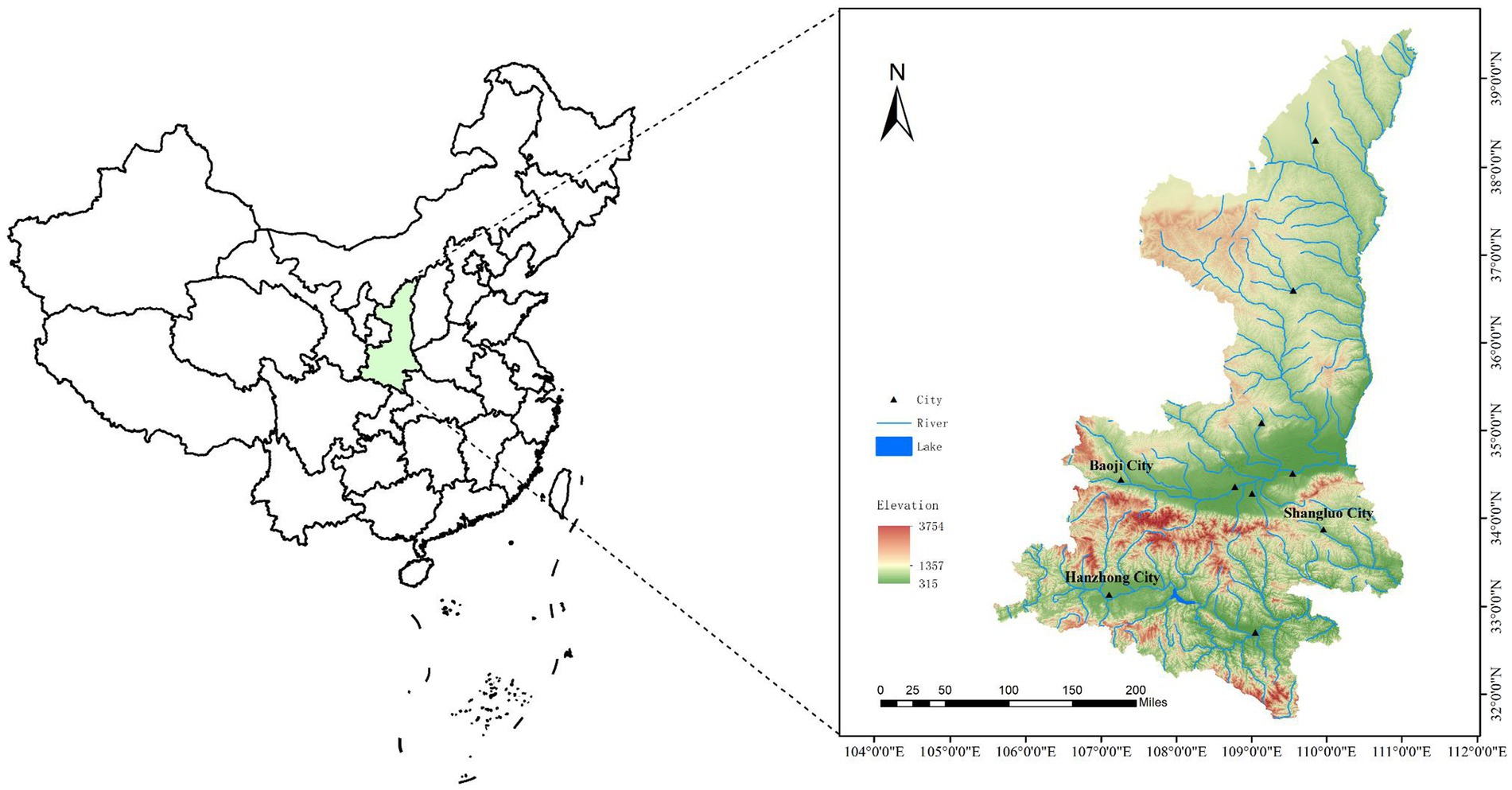
From 2022 to 2023, adult ticks were collected from locations in Shaanxi Province, China: Zhenba County of Hanzhong city (32°08′ ~ 32°50′N, 107°25′ ~ 108°16′E), Baoji City (33°35′ ~ 35°06′N, 106°18′ ~ 108°03′E), and Shangluo District of Shangluo City (33°06′ ~ 33°44′N, 10°24′ ~ 111°01′E) (Figure 1). Two methods of collecting ticks were used: the tweezers were used to pick up ticks on the body surface of domestic animals (cattle and goats), and the cloth flag method was used to collect ticks in grassland. All ticks were identified by observing their structure under a light microscope and referring to the tick classification search table (Teng and Jiang, 1991).
2.2 DNA extraction
The ticks were removed from −80°C storage, and washed successively using 0.1% Bromo-Germaine, 75% alcohol, and phosphate-buffered saline (PBS) for 10–15 min. This step is to remove impurities from the surface of ticks. The ticks were individually homogenized in PBS, and then centrifuged for 3 min at 2,500 × g. Total nucleic acids from the tick homogenates were extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and diluted to 100 μL. All the DNA extracts were stored at −20°C until use.
2.3 PCR amplification and sequencing
All ticks were further confirmed by PCR amplification and DNA sequencing of the mitochondrial cytochrome oxidase I (CO I) gene (Peng-Fei et al., 2018). Rickettsia in the ticks were simultaneously detected using nested or semi-nested PCR targeting a 440 bp region of the 17-kDa antigen-encoding (17kD) gene, a 1,100 bp region of the citrate synthase (gltA) gene, and a 1,200 bp region of the 16S rRNA (rrs) gene. A 500 bp sequence generated using universal primers of the rrs gene was used to detect Anaplasma and Ehrlichia. The clones positive for Anaplasma were further amplified using primers amplifying groEL (encoding the 60 kDa heat shock protein), gltA, and a longer fragment (1,200 bp) of the rrs gene. The clones positive for Ehrlichia were further amplified with primers for groEL, gltA, dsb (encoding the disulfide bond formation protein), ftsZ (encoding a cell division protein), and a longer fragment of the rrs gene. For the potential novel agents, positive specimens a 1,200 bp fragment of the rrs gene was amplified, and two nested PCR assays were used to amplify the 5′-end and 3′-end fragments of the rrs gene to assemble a complete gene (Wen et al., 2002). All the primers used for PCR are shown in Supplementary Table S1. All primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. And PCR was performed using Premix Taq Version 2.0 plus dye (Takara, Dalian, China). These sequences were amplified by nested or semi-nested PCR according to Lu et al. (2022). Tm is 50°C.
The PCR products were electrophoresed through 1.0% agarose gels. The target amplicons were purified using a QIAquick PCR Purification Kit (Qiagen). The purified PCR products were sent to Beijing De’aoping Biotechnology Co., Ltd. (Beijing, China) for bi-directional sequencing.
2.4 Genetic and phylogenetic analysis
The sequences obtained from the target genes were modified and assembled using the EditSeq and SeqMan programs (in DNAStar, Ver. 7.0, DNASTAR Inc., Madison, WI, United States) to make them accurate and complete. For confirmation, these sequences were compared with those uploaded to GenBank1 using the Basic Local Alignment Search Tool (BLASTn). Multiple sequence alignments of these sequences were performed using the Clustal W method (with default parameters) as implemented in the MegAlign program (DNAStar, Ver. 7.0). The evolutionary history of these sequences was inferred using the maximum likelihood method in MEGA version 7 (Bakken et al., 1994; Kumar et al., 2016). The robustness of the resultant phylogenetic trees was assessed based on bootstrap support values obtained from 1,000 replicates; values more than 70% were considered to indicate significant differences.
2.5 Nucleotide sequence accession number
The GenBank accession numbers of the nucleotide sequences obtained in this study are presented in Supplementary Table S2.
3 Results
3.1 Tick collection and identification
A total of 1,113 adult ticks were collected from three different locations in Shaanxi Province (365 from Zhenba County, 691 from Baoji City, 57 from Shangluo District) (Figure 1). Based on the tick classification characteristics and further confirmed by amplification of the COI gene, three tick species were identified: Haemaphysalis longicornis (87.42%, 973/1113), Rhipicephalus microplus (11.77%, 131/1113), Haemaphysalis flava (0.81%, 9/1113) (Table 1). The COI gene sequences of all the ticks obtained in this study showed 99–100% identities with those of the above three ticks in GenBank (OR574171–OR574179).

Table 1. Detection of Rickettsia, Anaplasma, and Ehrlichia in ticks from different areas in Shaanxi Province, China.
3.2 Detection and phylogenic analysis of Rickettsia
PCR amplification results showed that 130 ticks were positive for rrs, gltA, and 17kD, and the prevalence of Rickettsia in the ticks was 20.58% (229/1113). Rickettsia was detected in Haemaphysalis longicornis (20.04%, 195/973), Rhipicephalus microplus (23.66%, 31/131), and Haemaphysalis flava (33.33%, 3/9). Table 1 shows the situation of ticks and Rickettsia (SFGR), Anaplasma, and Ehrlichia carried by ticks in different areas of Shaanxi Province. Based on these three genes, only one spotted fever group Rickettsia was detected. Among all the amplified Rickettsia strains, there were three representative strains (rrs: OR513096–OR513098; 17KD: OR526945–OR526947; gltA: OR526950–OR526952), and other Rickettsia strains were 100% identical with these three strains. As shown in the phylogenetic tree in Figure 2, and nucleotide alignment showed that their rrs gene, gltA gene, and 17kD gene were 100, 99.89%, 99.54–100% similar to “Candidatus Rickettsia jingxinensis” (Ca. R. jingxinensis) strains (rrs: MH500204, MH923226; gltA: MH500217, OQ702260; 17kD: MH932037-MH932038, OQ702257), respectively. Figures 2–4 shows phylogenetic trees constructed with different genes, which can be used to observe the evolutionary relationships between species and to better understand the diversity of species.
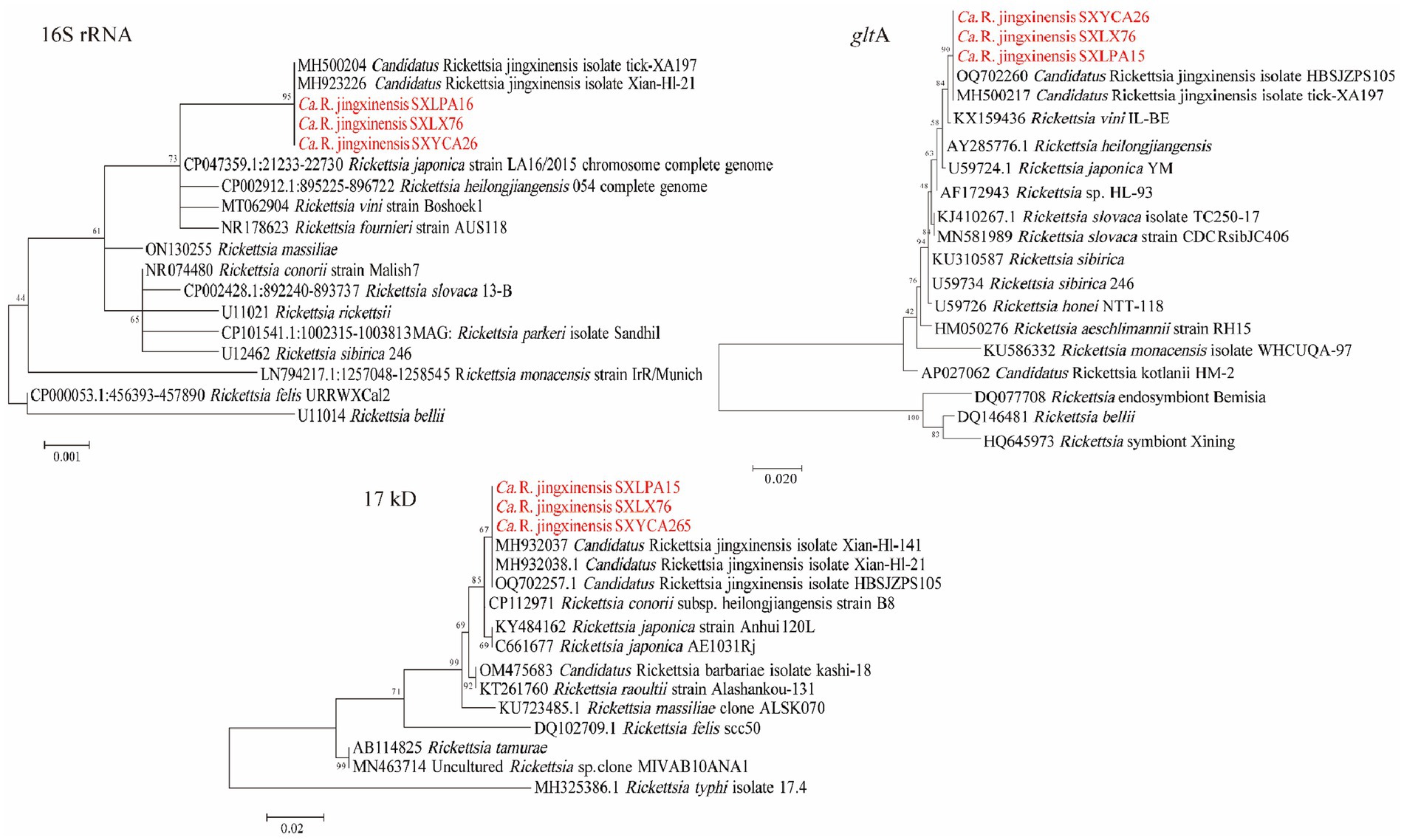
Figure 2. Phylogenetic trees of Rickettsia strains. The tree was constructed using MEGA 7.0 based on the nucleotide sequences of the 16S rRNA (1,188 bp), gltA (1,000 bp; encoding citrate synthase), and 17kD (440 bp, encoding the 17-kDa antigen) genes. Sequences obtained in this study are marked in red.
3.3 Detection and phylogenic analysis of Anaplasma
Three Anaplasma species (A. bovis, A. capra, A. marginale) were detected in H. longicornis and Rh. microplus. A. bovis detected in this study was found in H. longicornis from Zhenba County and Baoji City with a prevalence of (3.05%, 34/1113). As shown in Figure 3, in the phylogenetic analysis based on the rrs gene, the 14 sequences were clustered together with those of A. bovis strains obtained from cattle (MH255914) and goats (MH594292, MH255920) found in other provinces of China. The topologies of groEL and gltA gene-based phylogenetic trees were similar to that of the rrs gene-based phylogenetic tree. The rrs, gltA, and groEL gene sequences from the strains amplified in this study shared 99.92–100%, 99.35–100%, and 99.87–100% identity with previously reported A. bovis sequences, respectively. A. capra was detected in H. longicornis in Baoji City and Shangluo District, with a positive rate of (3.32%, 37/1113). In the rrs, gltA, groEL phylogenetic tree, the sequences obtained in this study showed a close relationship with A. capra isolate JZT12 (OQ185246) detected in Anhui province in China. DNA sequencing of the partial rrs and gltA gene showed 100% identity to A. capra isolate JZT12, while the groEL gene showed 99.88–100% similarity. The positive rate of A. marginale was (0.18%, 2/1113), which was amplified from H. longicornis from Shangluo District. Sequence analysis showed that the sequences of the two samples were consistent, and were closely related to that of the known A. marginale found in Rh. microplus from wild goats and cattle (Lu et al., 2017). The sequences of their partial rrs gene showed 100% identity to that of the A. marginale strain WHBMXZ-130 (KX987367), and their gltA and groEL gene showed 99.70 and 99.88% identity to A. marginale strain WHBMXZ-130, respectively.
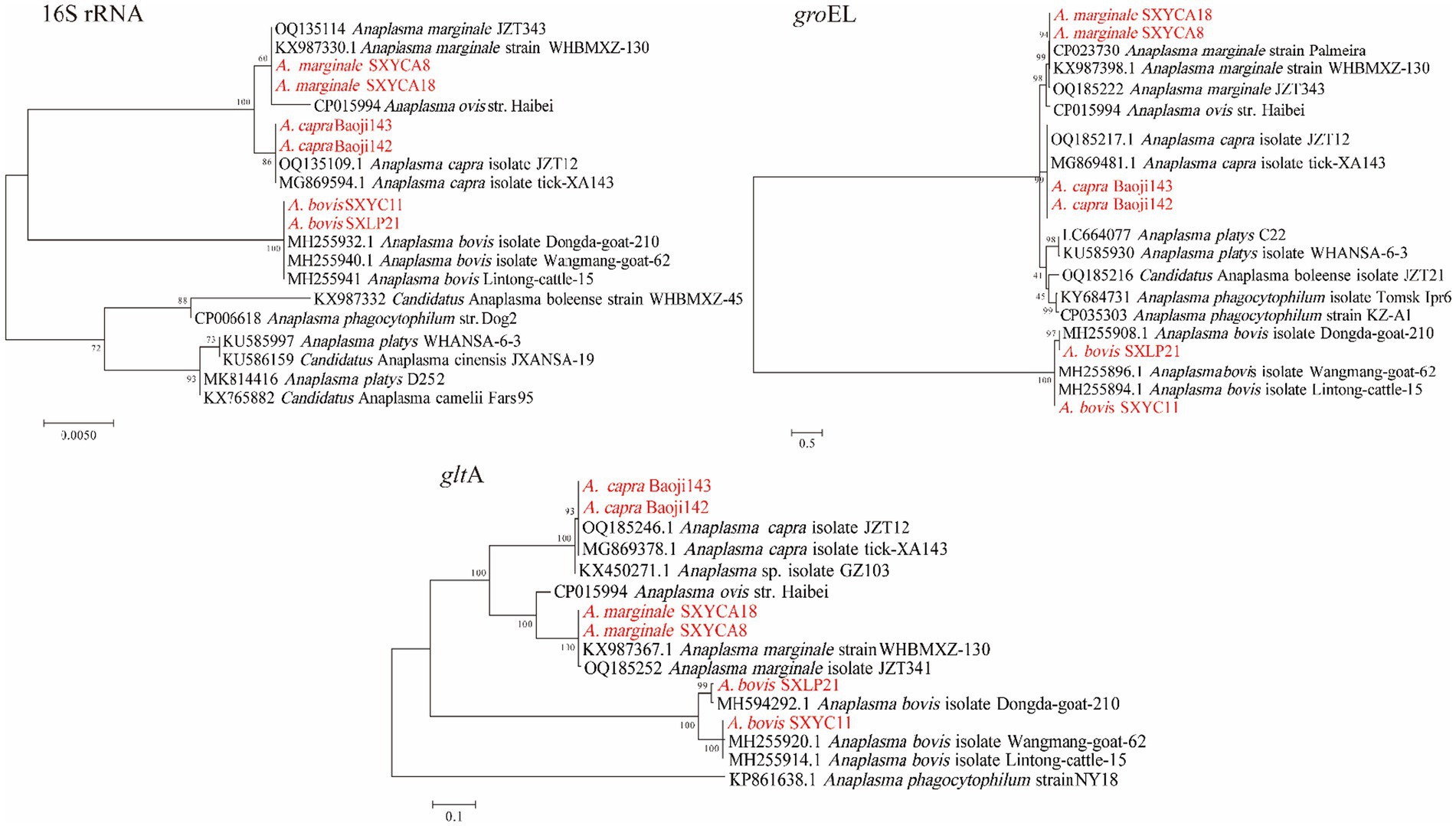
Figure 3. Phylogenetic trees of Anaplasma strains. The tree was constructed using MEGA 7.0 based on the nucleotide sequences of the 16S rRNA (1,200 bp), gltA (1,000 bp), and groEL (800 bp, encoding the 60 kDa heat shock protein) genes. Sequences obtained in this study are marked in red.
3.4 Detection and phylogenic analysis of Ehrlichia
Two Ehrlichia species were detected in H. longicornis (0.27%, 3/1113). As shown in Figure 4, phylogenetic analysis of the rrs and groEL gene sequences of one detected from Zhenba revealed that it was most closely related to E. sp. Yonaguni138 (HQ697588) found in Japan (Matsumoto et al., 2011). Its rrs and groEL genes were 100% identical to that of Ehrlichia sp. Yonaguni138. However, its dsb gene showed the highest identity (90.28%) to Ca. E. pampeana isolate S12HM25, and the ftsZ gene showed the highest identity (90.28%) to Ehrlichia sp. MieHfN113. This was probably because of the absence of the counterparts of dsb and ftsZ gene sequences from Ehrlichia sp. Yonaguni138 in the GenBank database.
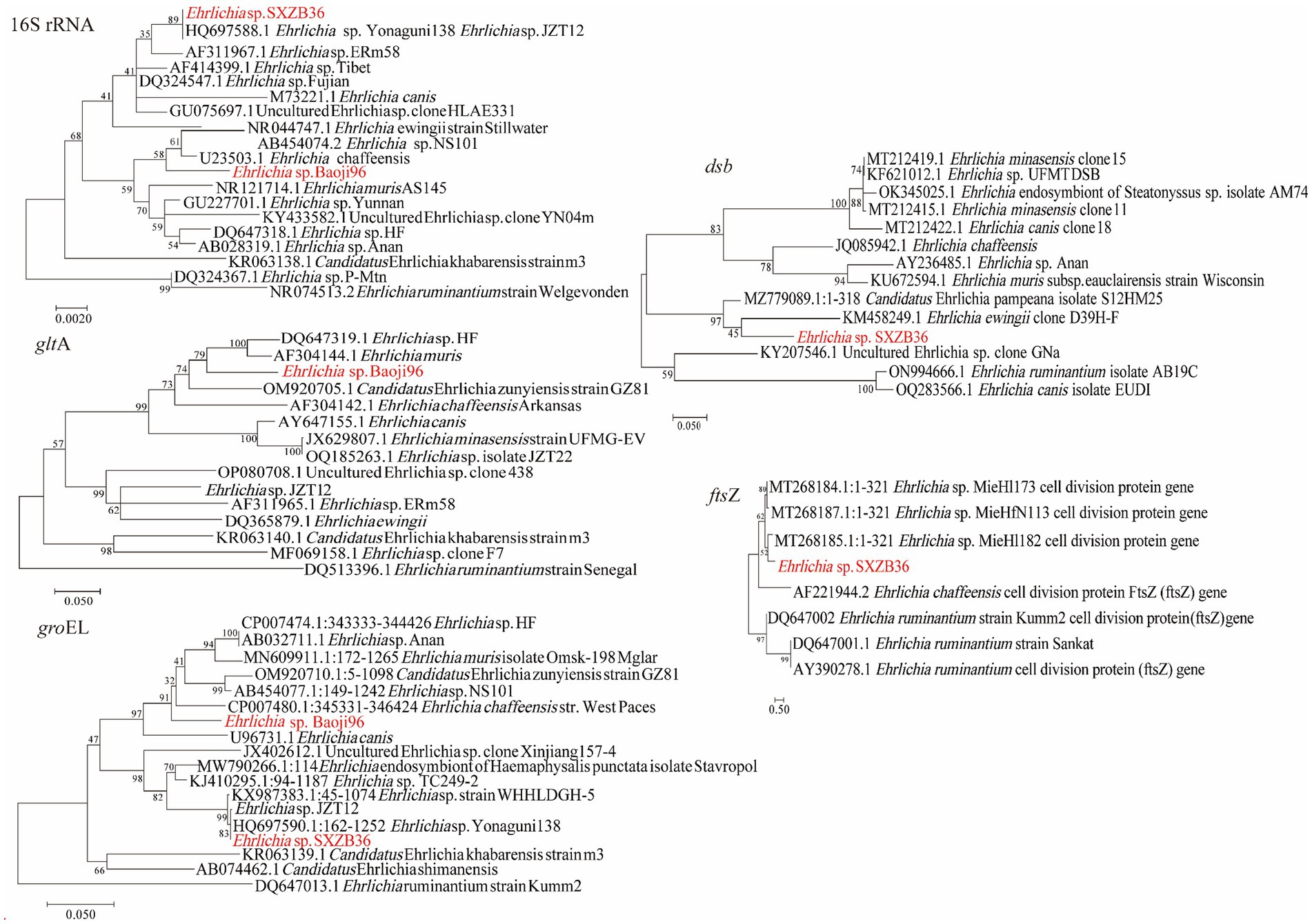
Figure 4. Phylogenetic trees of Ehrlichia strains. The tree was constructed using MEGA 7.0 based on the nucleotide sequences of the 16S rRNA (1,110 bp), gltA (804 bp), groEL (1,030 bp), dsb (400 bp; encoding the disulfide bond formation protein) and ftsZ (343 bp; encoding a cell division protein) genes. Sequences obtained in this study are marked in red.
Nucleotide alignment showed that the other Ehrlichia species from Baoji City was 99.20% identical to E. chaffeensis strain Arkansas (NR_074500) based on the rrs gene, 89.69% to Ehrlichia muris AS145 (CP006917) based on the gltA gene, and 94.91% to Ehrlichia sp. NS101 (AB454077) based on the groEL gene. Thus, we believed it represented a putative novel species, which we named as “Ehrlichia sp. Baoji96” according to the site where it was detected. To identify Ehrlichial agents at the species level, the 5′-end and 3′-end fragments of the rrs gene of Ehrlichia sp. Baoji96 was amplified and assembled with the parts already acquired into a complete gene of 1,504 bp. The whole 16S rRNA gene sequence of this novel species was compared with those of other species of Anaplasma family using the Clustal W method in the multiple sequence alignment program of DNAStar. It showed that there were interspecific differences between Ehrlichia sp. Baoji96 and other Ehrlichia species in the hypervariable region at the first 200 bp of the 5′-end of the 16S rRNA gene (Figure 5). The levels of sequence divergences and similarities between the novel species and the strains of Anaplastidae are shown in Figure 6. As can be seen from the table, the entire sequence was most similar to the 16S rRNA gene sequences of E. chaffeensis. The similarity is 99.2%, which is less than 99.7%, and is thus in the range for a new species (Qin et al., 2019).
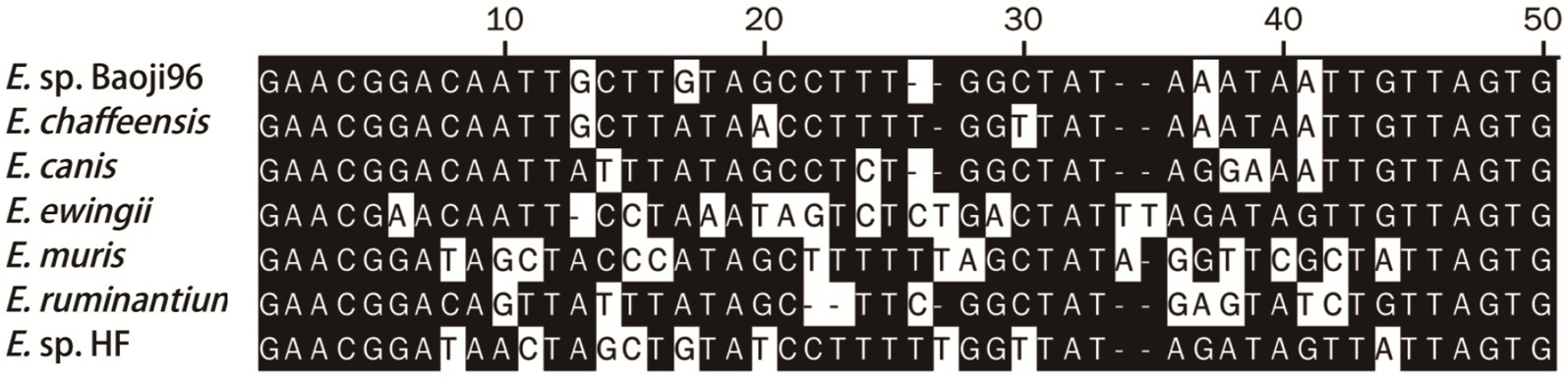
Figure 5. The differences in the 16S rRNA gene sequences of E. sp. Baoji96 and representative strains of Ehrlichia in a 50 bp hypervariable region located at the 5′-end of the 16S rRNA gene after multiple sequence alignment.
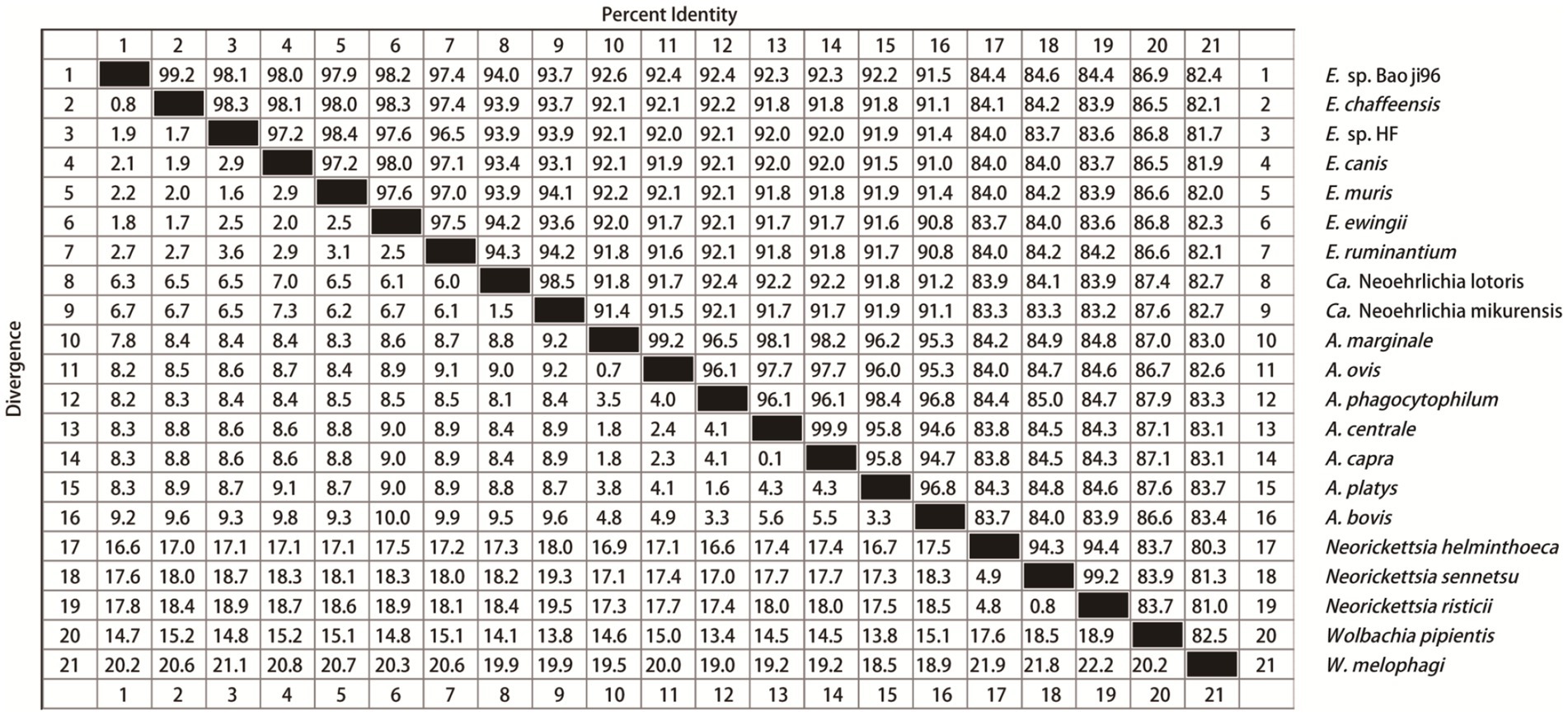
Figure 6. Levels of genetic identity and divergence between E. sp. Baoji96 and Anaplasmataceae species in the 16S rRNA gene. Twenty one sequences of representative strains of all genera of Anaplasmataceae were selected. The Clustal W algorithm was used to compare these 21 sequences in pairs and to calculate the genetic distance between them. The values on the upper right are the corrected levels of nucleotide identities for 1,390 bases.
3.5 Co-infection of Rickettsiales in ticks
As is shown in Table 2, co-infection with two Rickettsiales pathogens within an individual tick was detected in 10 (0.90%, 10/1113) ticks. Five (0.45%, 5/1113) ticks were co-infected with Ca. R. jingxinensis and A. bovis, two (0.18%, 2/1113) ticks were co-infected with Ca. R. jingxinensis and A. capra, two (0.18%, 2/1113) ticks were co-infected with Ca. R. jingxinensis and A. marginale, and only one tick was co-infected with Ca. R. jingxinensis and Ehrlichia spp.
4 Discussion
In the present study, the prevalence and genetic diversity of SFGR, Anaplasma, and Ehrlichia species in Ticks (Ixodidae) in Shaanxi Province was analyzed using molecular methods. A total of 1,113 ticks, including H. longicornis, Rh. microplus and H. flava, were collected in Shaanxi Province from 2022 to 2023. Among these ticks, one species of Rickettsia, two species of Ehrlichia, and three species of Anaplasma were identified.
The Rickettsia species detected in this study was Ca. R. jingxinensis, which was first found in Japan (Ishikura et al., 2003) and named in 2016 in Jingxin city, Jilin Province, China (Liu et al., 2016). In recent years, it has been found in both H. longicornis and Rh. microplus from Liaoning, Hebei, Shaanxi, Anhui, Hubei, and Yunnan provinces of China (Dong et al., 2014; Liu et al., 2016; Wang et al., 2021b; Jin et al., 2023). In addition to H. longicornis and Rh. microplus, it was also detected in H. flava in Shaanxi Province in the present study. The prevalence of Ca. R. jingxinensis in the ticks was as high as 20.58%. And our study showed that the gltA genes of Ca. R. jingxinensis in H. longicornis, Rh. microplus, and H. flava were 100% identical with the gltA gene of R. sp. strain WHBMXZ-80 and Ca. R. longicornii, suggesting an identification of the two organisms as one species, which is consistent with previous reports (Jiang et al., 2018; Jiao et al., 2021). This suggests that Ca. R. jingxinensis has a much wider distribution than previously realized and might be emerging as a dominant SFG species in the epidemic distribution of the vector species. Moreover, the gltA gene sequence of Ca. R. jingxinensis was found in a patient (KU853023) (Guo et al., 2018; Guo et al., 2019a). Furthermore, we found that it can coexist with pathogenic A. bovis and A. capra in ticks. Hence, we should pay continuous attention to the agent and raise awareness of its potential pathogenicity.
A. bovis, A. capra, and A. marginale discovered in this study have been identified as human or animal pathogens. A. bovis and A. capra have emerged as known zoonoses in recent years, which cause considerable harm to both humans and animals (Li et al., 2015; Lu et al., 2019). In 2017, A. bovis was reported to be capable of infecting cattle as well as humans (Lu et al., 2019). The emergence of A. capra as a human pathogen was observed in Heilongjiang Province in 2015 (Li et al., 2015). Patients infected with these two pathogens have similar clinical symptoms, such as fever, chills, headache, dizziness, myalgia, rash, eschar, and lymphadenopathy (Li et al., 2015; Lu et al., 2019). A previous study showed that infections with A. bovis and A. capra in goats of Shaanxi Province were frequent in summer, perhaps because the vector ticks were more active in summer (Wang et al., 2021a). In this study, the prevalence of A. bovis and A. capra were 3.05 and 3.32%, respectively. Although there were not many A. bovis and A. capra found in this study, combined with previous studies in Shaanxi Province, A. bovis and A. capra have always existed in Shaanxi Province, suggesting that we should strengthen the investment in vector monitoring and control. Additionally, in Asia, the most important rickettsial disease for cattle is bovine anaplasmosis caused by A. marginale (Rodríguez et al., 2009). A. marginale is a pathogen belonging to the Rickettsiales, which can cause progressive anemia in ruminants, resulting in huge economic losses (Kumar et al., 2015). A study showed the presence of the disease in more than 50% of cattle sampled in tropical and subtropical regions of Mexico (Rodríguez et al., 2009). The main vectors of A. marginale were Dermacentor and Rhipicephalus ticks (Rodríguez et al., 2009). In this study, the DNA of A. marginale was detected for the first time in two Rh. Microplus (0.18%) collected in Zhenba County, Shaanxi Province, which proved the prevalence of A. marginale in Shaanxi Province. Zhenba County has both a subtropical climate and shows the presence of A. marginale, which reminds us that the health of cattle in this area might be facing the problem of A. marginale infection.
In recent decades, with the widespread use of laboratory diagnostic methods, the number of new Rickettsiales and their associated diseases has increased, and many bacteria that were previously considered non-pathogenic are now associated with human disease (Lu et al., 2017). For example, the first infection caused by Rickettsia parkeri was reported 70 years after it was first identified in Amblyomma maculatum ticks (Piotrowski and Rymaszewska, 2020). Rickettsia slovaca was first isolated from Dermacentor marginatus ticks in Czechoslovakia, several years before the first human cases were reported (Piotrowski and Rymaszewska, 2020). And, in 2021, our research group found an emerging tick-borne pathogen, named as Ca. Ehrlichia erythraense, which is associated with human febrile illness discovered in the Dabieshan mountain area of China. The bacteria obtained from the ticks was described as Ehrlichia sp. JZT12 (Lu et al., 2023). In the present study, a putative novel Ehrlichia species closely related to E. chaffeensis was first identified by gene analysis of 16S rRNA in Ixodidae from Shaanxi Province. It was named Ehrlichia sp. Baoji96, which showed genetic similarities for the rrs, gltA, and groEL genes of 99.20% with E. chaffeensis strain Arkansas, 89.69% with Ehrlichia muris AS145, and 94.91% with Ehrlichia sp. NS101, respectively. In addition, the similarities of the rrs, gltA and groEL genes of Ehrlichia sp. Baoji96 and Ehrlichia sp. JZT12 were 98.23, 84.24 and 91.39%, respectively. Further research is needed to determine whether this bacteria can cause disease in animals or humans.
5 Conclusion
In this study, we detected Ixodidae parasitized on cattle and goats in warm temperate and subtropical areas of Shaanxi Province, and analyzed the prevalence and genetic diversity of SFGR, Anaplasma, and Ehrlichia species in Ixodidae in these regions. Shaanxi Province has a diverse terrain and climate, and we have made some new discoveries: A. marginale was detected for the first time in Rh. microplus collected in Zhenba County, and a novel Ehrlichia species closely related to E. chaffeensis was first identified in H. longicornis. For tick-borne diseases, tick prevention is the key to avoiding infection. Hence, continuous surveillance of Rickettsiales pathogens in Chinese ticks should be conducted to assess the potential risk of transmission to animals and humans by colonizing species in disease-causing pathogens or vectors. This study provides a reference for the formulation of biological control strategies for ticks and tick-borne diseases in this area, and could improve the control effect.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found at: https://www.ncbi.nlm.nih.gov/; OR513096-OR513098, OR520945- OR520951, OR526930- OR526952.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
XZ: Methodology, Writing – original draft, Writing – review & editing. WL: Methodology, Writing – original draft, Writing – review & editing. ZT: Investigation, Methodology, Writing – review & editing. NZ: Investigation, Methodology, Writing – review & editing. YZ: Investigation, Methodology, Writing – review & editing. DM: Investigation, Methodology, Writing – review & editing. LM: Investigation, Methodology, Writing – review & editing. YC: Data curation, Software, Writing – review & editing. JW: Data curation, Software, Writing – review & editing. JH: Conceptualization, Data curation, Software, Writing – review & editing. WM: Writing – review & editing. DL: Writing – original draft, Writing – review & editing. TQ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant number 81671985), the Science Foundation for the State Key Laboratory for Infectious Disease Prevention and Control of China (grant numbers 2022SKLID209 and 2019SKLID403), and the Public Health Service Capability Improvement Project of the National Health Commission of the People’s Republic of China (grant number 2100409002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1331434/full#supplementary-material
Footnotes
References
Bakken, J. S., Dumler, J. S., Chen, S. M., Eckman, M. R., Van Etta, L. L., and Walker, D. H. (1994). Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272, 212–218. doi: 10.1001/jama.1994.03520030054028
Beati, L., Meskini, M., Thiers, B., and Raoult, D. (1997). Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 47, 548–554. doi: 10.1099/00207713-47-2-548
Chochlakis, D., Ioannou, I., Tselentis, Y., and Psaroulaki, A. (2010). Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 16, 1031–1032. doi: 10.3201/eid1606.090175
Dong, X., Chen, X.-P., Liu, N., Dumler, S. J., and Zhang, Y.-Z. (2014). Co-circulation of multiple species of Rickettsiales bacteria in one single species of hard ticks in Shenyang, China. Ticks Tick Borne Dis. 5, 727–733. doi: 10.1016/j.ttbdis.2014.05.011
Guo, W.-P., Huang, B., Zhao, Q., Xu, G., Liu, B., Wang, Y.-H., et al. (2018). Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Negl. Trop. Dis. 12:e0006916. doi: 10.1371/journal.pntd.0006916
Guo, W.-P., Wang, Y.-H., Lu, Q., Xu, G., Luo, Y., Ni, X., et al. (2019a). Molecular detection of spotted fever group rickettsiae in hard ticks, northern China. Transbound. Emerg. Dis. 66, 1587–1596. doi: 10.1111/tbed.13184
Guo, W.-P., Zhang, B., Wang, Y.-H., Xu, G., Wang, X., Ni, X., et al. (2019b). Molecular identification and characterization of Anaplasma capra and Anaplasma platys-like in Rhipicephalus microplus in Ankang, Northwest China. BMC Infect. Dis. 19:434. doi: 10.1186/s12879-019-4075-3
Ishikura, M., Ando, S., Shinagawa, Y., Matsuura, K., Hasegawa, S., Nakayama, T., et al. (2003). Phylogenetic analysis of spotted fever group rickettsiae based on gltA, 17-kDa, and rOmpA genes amplified by nested PCR from ticks in Japan. Microbiol. Immunol. 47, 823–832. doi: 10.1111/j.1348-0421.2003.tb03448.x
Ismail, N., Bloch, K. C., and McBride, J. W. (2010). Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 30, 261–292. doi: 10.1016/j.cll.2009.10.004
Jiang, J., An, H., Lee, J. S., O'Guinn, M. L., Kim, H.-C., Chong, S.-T., et al. (2018). Molecular characterization of Haemaphysalis longicornis-borne rickettsiae, Republic of Korea and China. Ticks Tick Borne Dis. 9, 1606–1613. doi: 10.1016/j.ttbdis.2018.07.013
Jiao, J., Zhang, J., He, P., OuYang, X., Yu, Y., Wen, B., et al. (2021). Identification of tick-borne pathogens and genotyping of Coxiella burnetii in Rhipicephalus microplus in Yunnan Province, China. Front. Microbiol. 12:736484. doi: 10.3389/fmicb.2021.736484
Jin, X., Liao, J., Chen, Q., Ding, J., Chang, H., Lyu, Y., et al. (2023). Diversity of Rickettsiales bacteria in five species of ticks collected from Jinzhai County, Anhui Province, China in 2021-2022. Front. Microbiol. 14:1141217. doi: 10.3389/fmicb.2023.1141217
Johnson, D. K. H., Schiffman, E. K., Davis, J. P., Neitzel, D. F., Sloan, L. M., Nicholson, W. L., et al. (2015). Human infection with Ehrlichia muris-like pathogen, United States, 2007-2013(1). Emerg. Infect. Dis. 21, 1794–1799. doi: 10.3201/eid2110.150143
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Kumar, T., Sindhu, N., Charaya, G., Kumar, A., Kumar, P., Chandratere, G., et al. (2015). Emerging status of anaplasmosis in cattle in Hisar. Vet. World 8, 768–771. doi: 10.14202/vetworld.2015.768-771
Labruna, M. B., Santos, F. C. P., Ogrzewalska, M., Nascimento, E. M. M., Colombo, S., Marcili, A., et al. (2014). Genetic identification of rickettsial isolates from fatal cases of Brazilian spotted fever and comparison with Rickettsia rickettsii isolates from the American continents. J. Clin. Microbiol. 52, 3788–3791. doi: 10.1128/JCM.01914-14
Li, H., Zheng, Y.-C., Ma, L., Jia, N., Jiang, B.-G., Jiang, R.-R., et al. (2015). Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect. Dis. 15, 663–670. doi: 10.1016/S1473-3099(15)70051-4
Liu, H., Li, Q., Zhang, X., Li, Z., Wang, Z., Song, M., et al. (2016). Characterization of rickettsiae in ticks in northeastern China. Parasit. Vectors 9:498. doi: 10.1186/s13071-016-1764-2
Lu, M., Chen, Q., Qin, X., Lyu, Y., Teng, Z., Li, K., et al. (2022). Anaplasma bovis infection in fever and thrombocytopenia patients - Anhui Province, China, 2021. China CDC Week. 4, 249–253. doi: 10.46234/ccdcw2022.053
Lu, M., Li, F., Liao, Y., Shen, J.-J., Xu, J.-M., Chen, Y.-Z., et al. (2019). Epidemiology and diversity of Rickettsiales Bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 9:13176. doi: 10.1038/s41598-019-49059-3
Lu, M., Qin, X.-C., Jiang, Y.-Z., Guo, Q., Jin, X.-J., Teng, Z.-Q., et al. (2023). Emergence of ehrlichiosis by a new tick-borne Ehrlichia species in China. Int. J. Infect. Dis. 131, 32–39. doi: 10.1016/j.ijid.2023.03.038
Lu, M., Tian, J.-H., Yu, B., Guo, W.-P., Holmes, E. C., and Zhang, Y.-Z. (2017). Extensive diversity of rickettsiales bacteria in ticks from Wuhan, China. Ticks Tick Borne Dis. 8, 574–580. doi: 10.1016/j.ttbdis.2017.03.006
Mahara, F. (1997). Japanese spotted fever: report of 31 cases and review of the literature. Emerg. Infect. Dis. 3, 105–111. doi: 10.3201/eid0302.970203
Matsumoto, K., Takeuchi, T., Yokoyama, N., Katagiri, Y., Ooshiro, M., Zakimi, S., et al. (2011). Detection of the new Ehrlichia species closely related to Ehrlichia ewingii from Haemaphysalis longicornis in Yonaguni Island, Okinawa, Japan. J. Vet. Med. Sci. 73, 1485–1488. doi: 10.1292/jvms.11-0007
Paddock, C. D., and Childs, J. E. (2003). Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16, 37–64. doi: 10.1128/CMR.16.1.37-64.2003
Pasquale Mansueto, G. V., Cascio, A., Seidita, A., Pepe, I., Carroccio, A., di Rosa, S., et al. (2012). New insight into immunity and immunopathology of Rickettsial diseases. Clin. Dev. Immunol. 2012, 1–26. doi: 10.1155/2012/967852
Peng-Fei, Y., Qing-Li, Y., Qi, S. U., Fang, H. E., Li, T., Ya-Dong, X., et al. (2018). Application of DNA barcoding technolodge based on the mitochondrial gene COI to identify Haemaphysalis longicornis along the Yangtse River in Jiangsu province. Jiangsu J. Prevent. Med. doi: 10.3724/sp.j.1035.2009.00271
Piotrowski, M., and Rymaszewska, A. (2020). Expansion of tick-borne rickettsioses in the world. Microorganisms 8. doi: 10.3390/microorganisms8121906
Qin, X. R., Han, H. J., Han, F. J., Zhao, F. M., Zhang, Z. T., Xue, Z. F., et al. (2019). Rickettsia japonica and novel Rickettsia species in ticks, China. Emerg. Infect. Dis. 25, 992–995. doi: 10.3201/eid2505.171745
Robinson, M. T., Satjanadumrong, J., Hughes, T., Stenos, J., and Blacksell, S. D. (2019). Diagnosis of spotted fever group Rickettsia infections: the Asian perspective. Epidemiol. Infect. 147:e286. doi: 10.1017/S0950268819001390
Rodríguez, S. D., Ortiz, M. Á. G., Ocampo, R. J., and Murguía, C. A. V. Y. (2009). Molecular epidemiology of bovine anaplasmosis with a particular focus in Mexico. Infect. Genet. Evol. 9, 1092–1101. doi: 10.1016/j.meegid.2009.09.007
Sebastian, S. A., Co, E. L., Mehendale, M., Sudan, S., Manchanda, K., and Khan, S. (2022). Challenges and updates in the diagnosis and treatment of infective endocarditis. Curr. Probl. Cardiol. 47:101267. doi: 10.1016/j.cpcardiol.2022.101267
Teng, K.F., and Jiang, Z.J. (1991). Economic insect fauna of China, Fasc 39 Acari: Ixodidae (in Chinese).
Teng, Z., Gong, P., Wang, W., Zhao, N., Jin, X., Sun, X., et al. (2023a). Clinical forms of Japanese spotted fever from case-series study, Zigui County, Hubei Province, China, 2021. Emerg. Infect. Dis. 29, 202–206. doi: 10.3201/eid2901.220639
Teng, Z., Shi, Y., Zhao, N., Zhang, X., Jin, X., He, J., et al. (2023b). Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) Ticks from free-ranging domestic sheep in Hebei Province, China. Pathogens (Basel, Switzerland) 12. doi: 10.3390/pathogens12060763
Thomas, R. J., Dumler, J. S., and Carlyon, J. A. (2009). Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev. Anti-Infect. Ther. 7, 709–722. doi: 10.1586/eri.09.44
Wang, K., Yan, Y., Zhou, Y., Zhao, S., Jian, F., Wang, R., et al. (2021a). Seasonal dynamics of Anaplasma spp. in goats in warm-temperate zone of China. Ticks Tick Borne Dis. 12:101673. doi: 10.1016/j.ttbdis.2021.101673
Wang, Q., Guo, W.-B., Pan, Y.-S., Jiang, B.-G., Du, C.-H., Que, T.-C., et al. (2021b). Detection of novel spotted fever group Rickettsiae (Rickettsiales: Rickettsiaceae) in ticks (Acari: Ixodidae) in southwestern China. J. Med. Entomol. 58, 1363–1369. doi: 10.1093/jme/tjaa294
Wen, B., Jian, R., Zhang, Y., and Chen, R. (2002). Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J. Clin. Microbiol. 40, 3286–3290. doi: 10.1128/JCM.40.9.3286-3290.2002
Zeidler, H., and Hudson, A. P. (2021). Reactive arthritis update: spotlight on new and rare infectious agents implicated as pathogens. Curr. Rheumatol. Rep. 23:53. doi: 10.1007/s11926-021-01018-6
Keywords: SFGR, Anaplasma, Ehrlichia, Ixodidae, Shaanxi Province
Citation: Zhang X, Lv W, Teng Z, Zhao N, Zhou Y, Ma D, Ma L, Cheng Y, Wei J, He J, Ma W, Liu D and Qin T (2024) Molecular detection of Rickettsiales and a potential novel Ehrlichia species closely related to Ehrlichia chaffeensis in ticks (Acari: Ixodidae) from Shaanxi Province, China, in 2022 to 2023. Front. Microbiol. 14:1331434. doi: 10.3389/fmicb.2023.1331434
Edited by:
Hong Yin, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Tian Luo, University of Texas Medical Branch at Galveston, United StatesThankGod Emmanuel Onyiche, University of Maiduguri, Nigeria
David H. Walker, University of Texas Medical Branch at Galveston, United States
Copyright © 2024 Zhang, Lv, Teng, Zhao, Zhou, Ma, Ma, Cheng, Wei, He, Ma, Liu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Qin, cWludGlhbkBpY2RjLmNu; Dongli Liu, bGRsMDI5QGZveG1haWwuY29t
 Xue Zhang
Xue Zhang Wen Lv2
Wen Lv2 Na Zhao
Na Zhao Tian Qin
Tian Qin