- 1Biotech Research Institute, Grape King Bio Ltd., Taoyuan, Taiwan
- 2Department of Animal Science and Technology, National Taiwan University, Taipei, Taiwan
- 3Department of Food Science, Nutrition, and Nutraceutical Biotechnology, Shih Chien University, Taipei, Taiwan
- 4Institute of Food Science and Technology, National Taiwan University, Taipei, Taiwan
- 5Center for Biotechnology, National Taiwan University, Taipei, Taiwan
- 6Department of Animal Science, National Chung Hsing University, Taichung, Taiwan
- 7The iEGG and Animal Biotechnology Research Center, National Chung Hsing University, Taichung, Taiwan
Background/objectives: Atopic dermatitis (AD) is a prevalent chronic skin condition, especially in young children, with rising incidence in developed countries. AD causes repeated scratching, and thus affecting quality of life. This study evaluated the effects and mechanisms of the probiotic Lactiplantibacillus plantarum GKK1 on AD symptoms in mice.
Methods: Five-week-old BALB/c mice were divided into four groups (n = 8): control, AD, low-dose GKK1 (107 CFU/day), and high-dose GKK1 (109 CFU/day). GKK1 was intragastrically administered daily for 42 days. AD symptoms, skin histology, serum antibodies, inflammatory cytokine levels, gut microbiota composition, and short-chain fatty acids (SCFAs) in the intestines were assessed.
Results: GKK1 showed improved skin appearance and reduced inflammation in AD mice, with high-dose GKK1 significantly reducing histological inflammation. The GKK1 treatment upregulated splenic interleukin (IL)-2, suppressed IL-4, IL-5 and IL-17 levels and increased intestinal Lactobacillus and Bifidobacterium spp., contributing to higher SCFAs production in intestine.
Conclusion: Oral L. plantarum GKK1 effectively ameliorated AD symptoms and reduced inflammation in mice. Therefore, L. plantarum GKK1 may serve as a potential treatment for AD.
1 Introduction
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disorder which is caused by immunological imbalance, defective skin barriers, gene mutation, and environmental risk factors (Thomsen, 2014). People typically develop the symptoms of AD in childhood, and one fourth of them continue to suffer from AD in adulthood, which means the prevalence of AD is about 20% people during their lifetime, and the prevalence is on the rise (Spergel and Paller, 2003). Besides, people with AD will have an increased risk to develop asthma and allergic rhinitis (Serrano et al., 2019). The clinical manifestation was intensely pruritic and scaly papules. Repeated scratching constitutes disruption of sleep, loss of employment, and financial burden for their family, which imposes a great impact on the patient’s quality of life (Kemp, 2003).
AD primarily results from two reasons: skin barrier defects and immunological imbalance. Firstly, people with the mutation of filaggrin (FLG) gene, which can produce epidermal proteins responsible for intact skin barriers, lead to dry skin and higher risk of allergens and bacteria penetration to skin, consequently causing AD (De Benedetto et al., 2012; Xiao et al., 2021). Emollients are applied to prevent water loss of skin and soothe itching but cannot ultimately ameliorate AD. Secondly, AD is associated with imbalance of T helper (Th) cells type 1, 2, 17, and 22 and regulatory T (Treg) cells. Th2 differentiation dominates in patients with AD, and therefore production of cytokines IL-4, IL-5, IL-13, and IL-31 were induced, which then facilitate B cells to produce immunoglobulin E (IgE), and thus Th1 differentiation was suppressed (Eyerich and Novak, 2013). Medical treatment such as corticosteroids, Janus kinase inhibitors, calcineurin inhibitors, and anti-IL-31 monoclonal antibody are used to inhibit immune responses (Liberman et al., 2018; Reynolds and Al-Daraji, 2002; McInnes et al., 2019; Silverberg et al., 2020). However, due to side effects and usage restrictions on age, we attempt to search for alternative treatment for AD.
Probiotics play a protective role on the health of gut and skin. Probiotics help alter gut microbiota composition, and because of the association of gut and skin, the symptoms of AD are thus improved. In AD patients, Staphylococcus aureus, Escherichia coli, and Clostridium difficile are enriched, whereas Lactobacillus, Bifidobacterium and Bacteroides spp. are reduced in the gut microbiota, which thus results in Th1/Th2 imbalance (Lee et al., 2018). Probiotics can help regulate immune reaction by inducing cell activation signaling and suppressing proinflammatory responses (Ueno et al., 2011; Smits et al., 2005). For example, L. plantarum LM1004 significantly improved the AD-like symptoms by reducing Th2 and Th17 cell transcription factor levels and enhancing the transcription factor levels of Treg, Th1 cells, and FLG (Kim et al., 2019). Furthermore, probiotics contribute to maintain the intestinal epithelial integrity via elevated expression of tight junction protein (Hill et al., 2014). Probiotics metabolites such as short chain fatty acids can modulate immune system by inducing Treg cells and can inhibit survival of pathogens because of low pH (Salem et al., 2018). Research revealed that the severity of AD was negatively associated with the richness of butyrate-producing bacteria (Nylund et al., 2015). The underlying mechanisms of probiotics against AD include improvement of gut microbiota, regulation of immune responses, and protection of gut and skin barriers.
Probiotic Lactiplantibacillus plantarum (formerly Lactobacillus plantarum), with a long history in food industry, demonstrates various beneficial activities to humans, such as anti-inflammatory, antioxidative, and antimicrobial activities (Garcia-Gonzalez et al., 2021). Although previous studies have shown that L. plantarum can modulate Th1 and Th2 cytokine and alleviate clinical symptoms of AD, the alteration of gut microbiota and production of short chain fatty acids (SCFAs) after administration of L. plantarum did not mention before (Prameswari et al., 2017; Prakoeswa et al., 2022). The rise in prevalence of AD is concerning. Therefore, in this study, we investigated the effects of oral administration of Lactiplantibacillus plantarum GKK1, previously identified through in-vitro screening for its significant stimulation of TNF-α and IL-10, on the treatment of AD in mice.
2 Materials and methods
2.1 Preparation of L. plantarum GKK1
L. plantarum GKK1 was isolated from pickled chili peppers in Hualien, Taiwan, in 2015, and was provided by Biotechnology Center, Grape King Bio Ltd. (Taoyuan, Taiwan). GKK1 was first cultivated in MRS broth (Difco Laboratories, Detroit, MI, USA) with 1% (v/v) stock for 24 h at 37°C anaerobically. The phosphate-buffered saline (PBS) (Uni Biotech Co., Ltd., Ye-san-Gun, South Korea) was used to wash the pellet centrifuged. The pellet was then adjusted to concentrations of 107 and 109 CFU/mL by PBS, and the samples prepared once every 2 days was stored at 4°C for use.
2.2 AD protocol
Five-week-old male BALB/c mice were acquired from National Laboratory Animal Center (Taipei City, Taiwan) and were maintained in the National Taiwan University Animal Resource Center. In this study, 32 mice were divided into 4 groups (n = 8/group): control, AD, low-dosage L. plantarum GKK1 (GKK1-L) (107 CFU/mouse/day), and high-dosage L. plantarum GKK1 (GKK1-H) (109 CFU/mouse/day). The mice were first adapted for 1 week, and GKK1-L and GKK1-H groups were fed intragastrically with low and high dosage of GKK1 respectively, while 200 μL sterile PBS was supplied for control and AD groups daily. On day 14, the hair on the back was applied with hair removal cream (Church & Dwight Co., Inc., Ewing, NJ, USA) and shaved by a hair clipper (OSCAR 2000, Frega Enterprise Co., Ltd., Conquitlam, BC, Canada). Later, 1 cm2 surgical fabric adhesive tape (Symphon Medical Technology Co., Ltd., New Taipei City, Taiwan) was used to repeatedly strip and stick the mice’s back for five times to destroy skin stratum corneum. On days 15, 22, and 36, 20 μL acetone and olive oil (Sigma-Aldrich Inc., St. Louis, MO, USA) (acetone: olive oil = 1:3) with 1% 2,4-Dinitrochlorobenzene (DNCB, Sigma-Aldrich Inc.) was applied on the mice’s back in AD, GKK1-L, and GKK1-H groups; on day 29, 20 μL acetone and olive oil (acetone: olive = 1:3) with 0.5% DNCB was applied to all mice without control group. On days 19, 26, 33, and 40, 20 μL 0.5% Tween 20 in PBS with 10 mg/mL house dust mite extract (HDM, Stallergenes Greer Inc., MA, USA) was applied to AD and probiotic groups. As for control group, 20 μL acetone and olive oil (acetone: olive = 1:3) was given at the mentioned timepoint. Mice were sacrificed humanely by cervical dislocation under gas anesthesia by using isoflurane (Abbott Laboratories, Chicago, IL, USA)-soaked cotton on the 42th day. The experimental design was presented as Figure 1A. The study was registered on Institutional Animal Care and Use Committee (IACUC) of National Taiwan University under number 00038 in 2019.
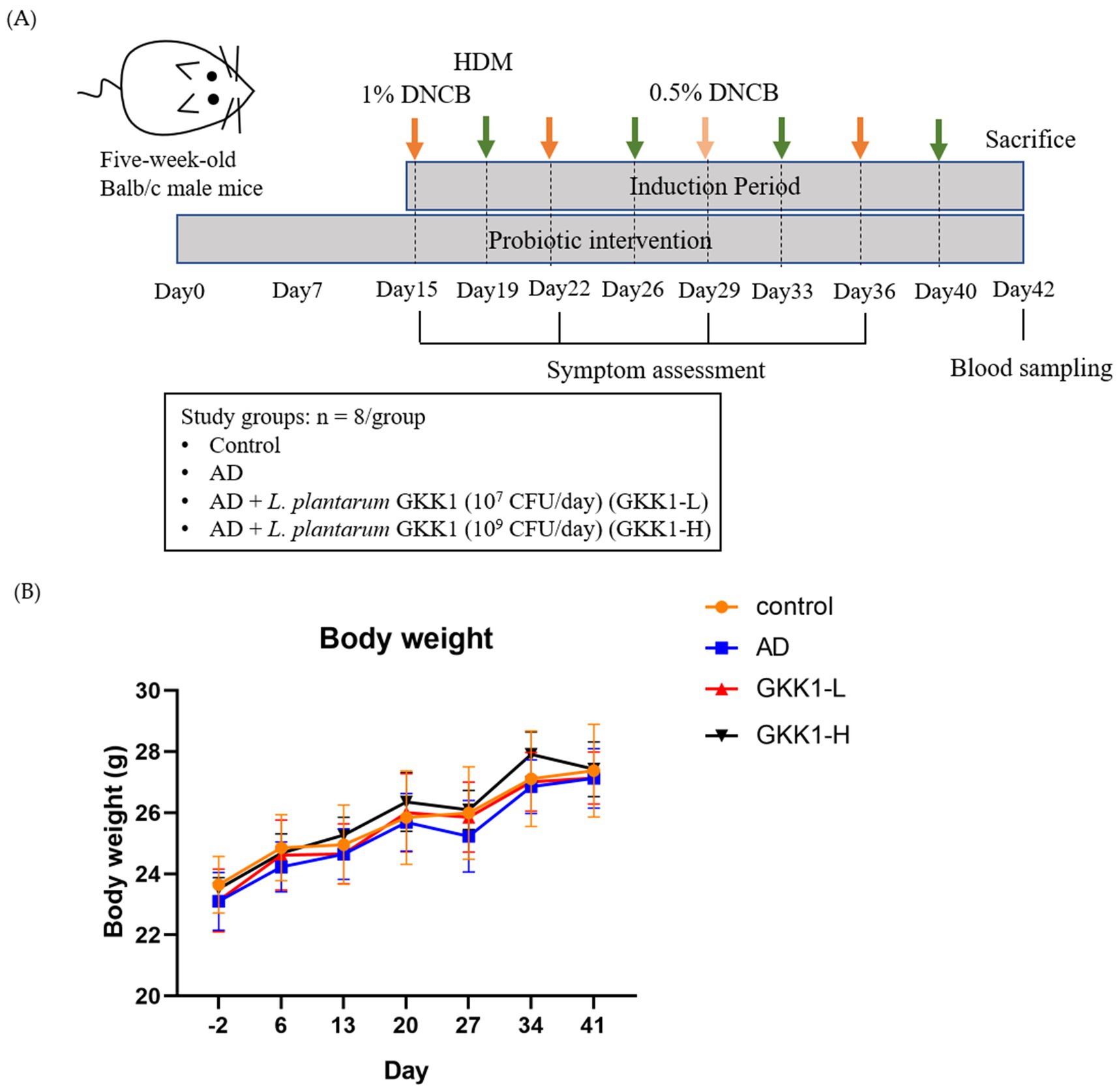
Figure 1. (A) The experimental design and (B) the body weight changes during the experiment period. Data are expressed as mean ± SD (n = 8 mice per group). Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
2.3 Evaluation of symptoms of AD
The appearance of the skin in four groups of mice were observed before and after induction of AD. At the end of the study, the symptoms of AD were evaluated by the Yu et al.’s (2018) methods. Three categories, namely erythema/hemorrhage, scarring/dryness, and excoriation/erosion, were scored from 0 (mild) to 5 (severe) according to the area and the severity of the symptoms, and the total scores were 15.
2.4 Examination of histopathology and evaluation of dermatitis severity
After sacrifice, the skin and muscle tissue of the back (length 0.5 cm x width 1 cm) were removed and perfused in 10% formaldehyde in aqueous phosphate buffer (Mallinckrodt baker, Inc., Philipsburg, USA) for 24 h to fix the tissue. National Laboratory Animal Center was authorized for tissue slicing and staining. The tissues were embedded in paraffin and then sectioned. Later, the sections were stained with hematoxylin and eosin (hematoxylin and eosin stain, H&E stain) and observed by optical microscope. Further, the histological sections were evaluated by Duke Veterinary Clinic (Taipei, Taiwan) according to the scoring system (Fick et al., 2005) including epidermis and dermis. Thickness, acanthosis (thickening of the stratum spinosum, diffuse epidermal hyperplasia), hyperkeratosis (thickening of the stratum corneum), parakeratosis (retention of the keratinocyte nuclei in the stratum corneum), inflammation (intraepithelial inflammatory cell infiltration), erosion and ulceration (complete loss of epidermal cells) were determined in epidermis, whereas thickness, inflammation, necrosis, follicular keratosis, and fibrosis were determined in dermis. Considering ratio of area of symptoms to that of the sections, every symptom was graded from 0 (within normal limits) to 5 (severe). As for thickness score, it was ranged from −4 (severely thin) to +4 (severely thick) compared with the thickness of control group.
2.5 Analysis of serum concentration of IgE and IgG1/IgG2a ratio
Before euthanasia, the blood was collected from orbital sinus to blood collection tubes (BD Biosciences, Franklin Lakes, NJ, USA). The serum was obtained after centrifugation at 4,600 rpm for 5 min at 4°C and was then stored at −80°C for antibody analysis. The serum concentration of IgE, IgG1, and IgG2a were measured by enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instruction (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.6 Splenocyte cytokine profile analysis after HDM stimulation
After sacrificing the mice, spleens were placed in sterile RPMI 1640 medium and transferred to a sterile workbench. They were ground using a sterile syringe plunger on a cell strainer and washed with 10 mL RPMI 1640 medium. The filtered solution was centrifuged at 300 × g for 5 min, and the supernatant was discarded. Red blood cell lysis buffer was added, mixed, and centrifuged again. The residue was washed with PBS, centrifuged, and resuspended in RPMI 1640 medium with 10% FBS, penicillin, and streptomycin. Cells were adjusted to 5 × 107 cells/mL, centrifuged, and resuspended in RPMI 1640 medium. The cell suspension was added to a 24-well plate with HDM and co-cultured for 48 h. The supernatant was collected and stored at −80°C for cytokine analysis. Cytokines IL-2, IL-4, IL-5, IL-10, and IL-17 in the serum samples were analyzed by ELISA. The steps could be followed according to the booklets of mouse cytokine kits (R&D Systems, Inc., Mckinley, MN, USA), and the absorbance of the samples was detected by spectrum 450 and 540 nm using spectrophotometer (Biotek Instruments Inc., Winooski, VY, USA).
2.7 Analysis of short chain fatty acids in guts
The analysis method was modified by Torii et al. (2010). After sacrifice, the samples in fresh ceca, large intestines, and feces were collected in the tubes, then 70% ethanol was added with 9 times as the weight of the samples. After homogenization, the samples were centrifuged at 13,200 rpm for 10 min at 4°C, and the supernatant was stored at −80°C for further analysis. Standards of acetic acid, propionic acid, butyric acid were obtained from Sigma-Aldrich. Working solutions of 0.0625, 0.125, 0.25, 0.5, and 1 mM were prepared by diluting standards with 50% methanol, and 2-ethylbutyric acid (2-BA, Sigma-Aldrich Inc.) was used as internal standard adjusted to concentration of 800 μM with 100% ethanol.
Each sample was undergone derivatization process according to Torii et al.’s (2010) method. The sample (150 μL) was mixed with internal standard (50 μL) and 150 μL each of Reagent 1 [3% pyridine (Aldrich Chemical Co., Inc., Milwaukee, WI, USA) ethanol (Honeywell International Inc.) solution], Reagent 2 [1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, EDC-HCl (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) ethanol solution], and Reagent 3 [2-nitrophenylhydrazine hydrochloride, 2-NPH-HCl (Tokyo Chemical Industry Co., Ltd.) ethanol solution] in a 15 mL centrifuge tube, reacted at 60°C for 20 min, and then mixed with 200 μL of Reagent 4 [15% potassium hydroxide solution (Sigma-Aldrich Inc.), methanol (Aencore Chemical Co., Ltd., Surry Hills, NSW, Australia) solution], and reacted at 60°C for 20 min. After cooling, the mixture was shaken with 1.5 mL of Reagent 5 [0.5 M phosphoric acid, H3PO4 aqueous solution (Aldrich Chemical Co., Inc.)] and 2 mL of diethyl ether (Honeywell International Inc.) and stayed still for 3 min and then centrifuged at 1,500 rpm for 3 min. The obtained ether layer was shaken with 2.0 mL of water for 3 min and centrifuged. The ether layer was transferred to a 2 mL Eppendorf tube, and ether was eliminated in a hood. The obtained fatty acid hydrazide was dissolved in 200 μL of 100% methanol and filtered through a 0.22 μm filter, and the sample was subjected to a high-performance liquid chromatography system (PU-2089 Quaternary HPLC Pump, JASCO International Co., Ltd., Tokyo, Japan). The chromatographic column used is a C18 column (ReproSil 100 C18 5 μM, 250 × 4.6 mm, Dr. Maisch GmbH, Ammerbuch, Germany). The mobile phase was comprised of acetonitrile (J.T. Baker Chemical Company, Phillipsburg, NJ, USA)–methanol–water (30:16:54), adjusted to a pH of 4.5 with 0.1% trichloroacetic acid (Sigma-Aldrich Inc.).
2.8 Microbiota analysis
The samples in fresh large intestines were first mixed with ddH2O (9 times the weight of the sample) and 1 mm grinding balls before vortexing at 1,500 rpm for 1 min. Then, an aliquot of 200 μL suspension was collected and mixed with 1 mL sterile PBS thoroughly in the homogeneous tube, followed by a centrifuge step (12,000 rpm, 5 min, 4°C). Later, the supernatant was removed, and the samples were stored at −20°C for further DNA extraction via the process (Neumann et al., 1992). The target bacteria genus Lactobacillus spp. and Bifidobacterium spp. were also undergone DNA extraction and determined the count by hemocytometer (SLGC, Tokyo, Japan). Then, real-time polymerase chain reaction [qPCR, StepOne Real-Time PCR System (Thermo Fisher Scientific Inc.)] was performed with serial 100-, 10-, and 1-fold dilutions of the target strains to obtain the standard curves of cycle threshold (Ct) value versus count (log CFU/g), and the populations of the target strains in the samples were calculated by Ct value from the standard curves. An Optical 8 Strip QPCR Tube (Bioman Scientific Co., Ltd., New Taipei City, Taiwan) contained 2 μL of DNA sample, 5 μL of KAPA SYBR FAST qPCR Master Mix (2×) (Kapa Biosystems Ltd., Salt River, Cape Town, South Africa), 0.2 μL of 10 μM forward primer, 0.2 μL of 10 μM reverse primer, and 2.6 μL of sterilized ddH2O. The annealing condition at qPCR stage was 55°C for Lactobacillus and 60°C for Bifidobacterium. The primer sets used in this study were referenced from Lubbs et al. (2009). For Lactobacillus spp., the forward primer was GGAAACAG(A/G)TGCTAATACCG (Lab-0159) and the reverse primer was ATCGTATTACCGCGGCTGCTGGCA (Univ-0515). For Bifidobacterium spp., the forward primer was CTCCTGGAAACGGGTGG (g-Bifid-F) and the reverse primer was GGTGTTCTTCCCGATATCTACA (g-Bifid-R).
2.9 Statistical analysis
The values were presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) using Duncan’s post-hoc test was conducted to evaluate the differences between every group. The value of statistical significance was set at p < 0.05. The analysis was performed using the SPSS statistical software package.
3 Results
3.1 L. plantarum GKK1 improved AD-like symptoms in mice
There were no significant differences on the body weight among experimental groups (Figure 1B), indicating that treatment of L. plantarum GKK1 did not affect the growth of mice. The clinical signs of AD in four groups were observed weekly (Figures 2A–D). After induction of AD (on day 36) by topical application of DNCB and HDM, the AD group exhibited severe erythema and hemorrhage. However, symptoms improved with oral administration of L. plantarum GKK1. Specifically, skin symptoms were scored at the end of the study (Figure 2E). The AD group had a score of 7.88 ± 1.66, significantly higher than the control group’s score of 0.19 ± 0.26 (p < 0.05), indicating successful induction of skin lesions. The scores of GKK1-L and GKK1-H groups were 5.50 ± 1.51 and 4.81 ± 1.71, respectively, both significantly lower than the AD group (p < 0.05).
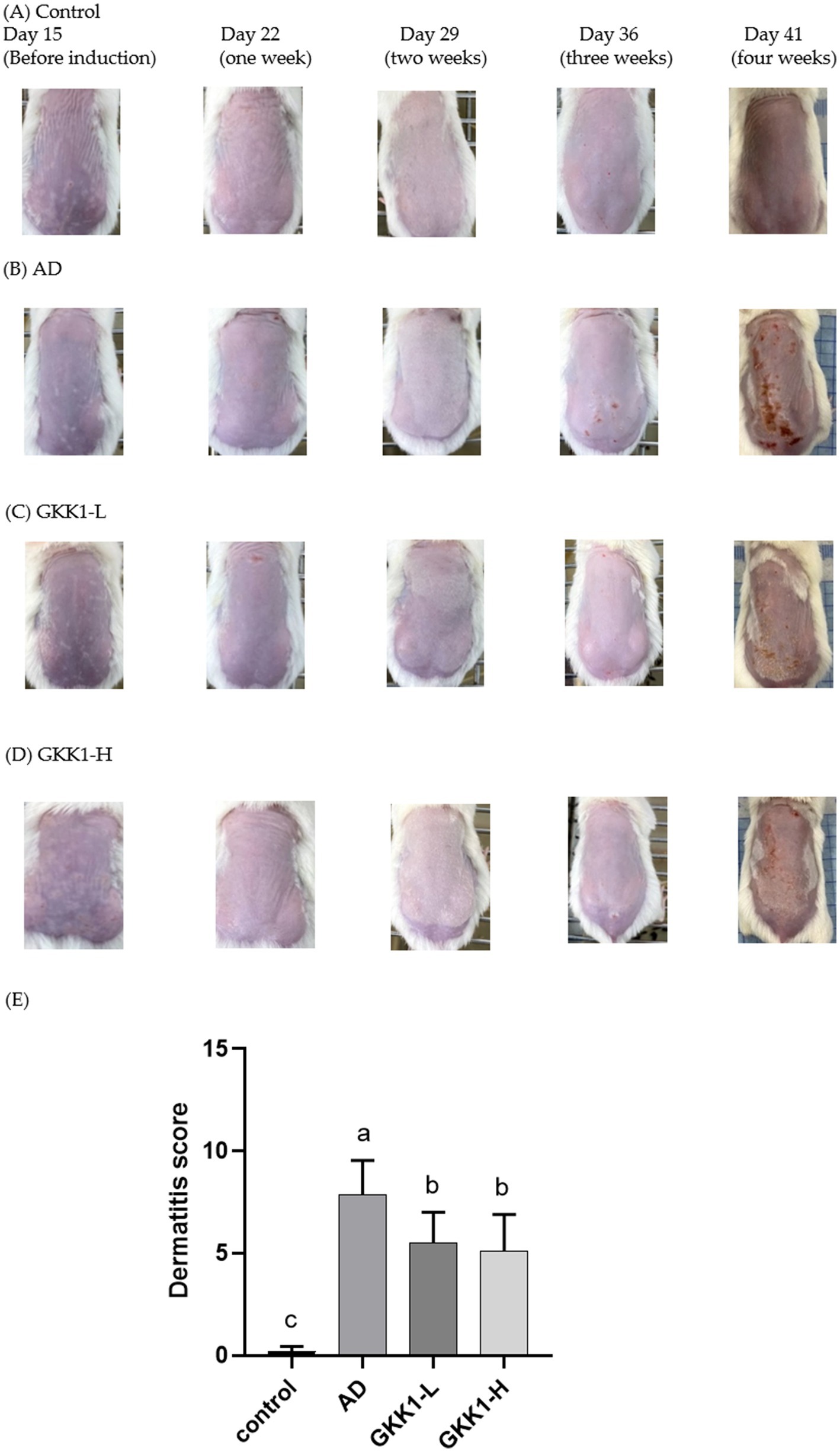
Figure 2. The appearance of skin in (A) control, (B) AD, (C) GKK1-L, (D) GKK1-H groups observed during the study, and (E) the scores of the clinical signs in four groups at the end of the study. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
3.2 L. plantarum GKK1 recovered the histology of epidermis and dermis in AD mice
The skin sections stained with hematoxylin and eosin were examined by the veterinarian. In epidermis analysis (Figure 3A), the score in the AD group was significantly higher than that of control group (p < 0.05), while the score in GKK1-L group was lower than that of AD group. The GKK1-H group had a significantly lower score than the AD group (p < 0.05). Regarding the dermis examination (Figure 3B), the AD group’s score was significantly higher than the control group’s (p < 0.05). Furthermore, there were significant differences in dermis scores between AD and two probiotics groups (p < 0.05).
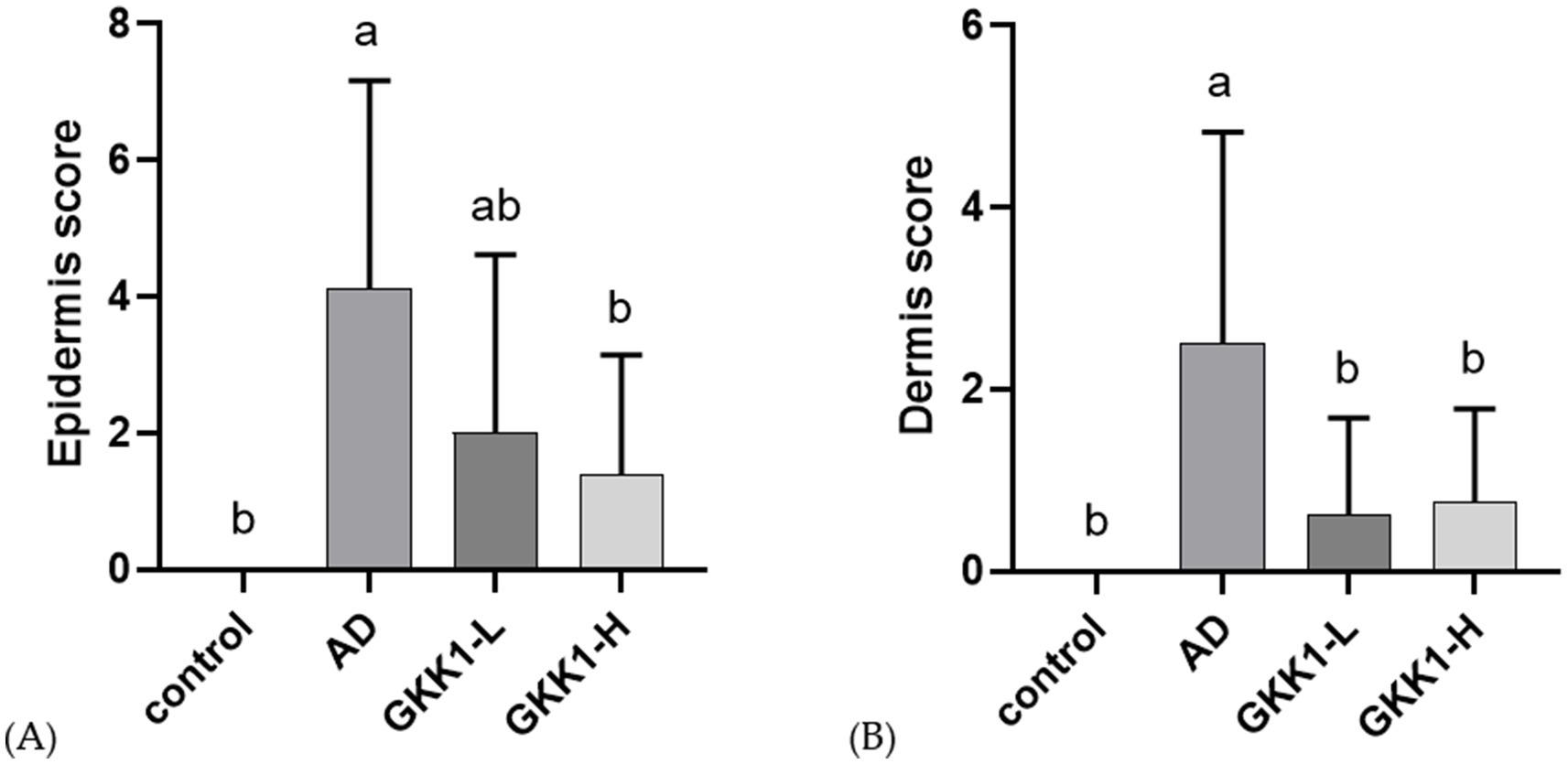
Figure 3. Quantitative evaluation of (A) epidermis, (B) dermis analysis of mouse skin tissue in different experimental groups. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
3.3 L. plantarum GKK1 downregulated serum concentration of IgE and IgG1/IgG2a ratio in AD mice
The serum collected on day 42 was analyzed for the concentration of IgE, IgG1, and IgG2a. The results showed no significant differences in IgE levels between the probiotics groups (GKK1-L and GKK1-H) and the AD group (Figure 4A). However, IgE concentration tended to decrease following the administration of L. plantarum GKK1. As shown in Figure 4B, no statistically significant differences in the IgG1/IgG2a ratio were observed between the probiotic groups (GKK1-L and GKK1-H) and the AD group, though the ratio was lower in both probiotics’ groups compared to the AD group.
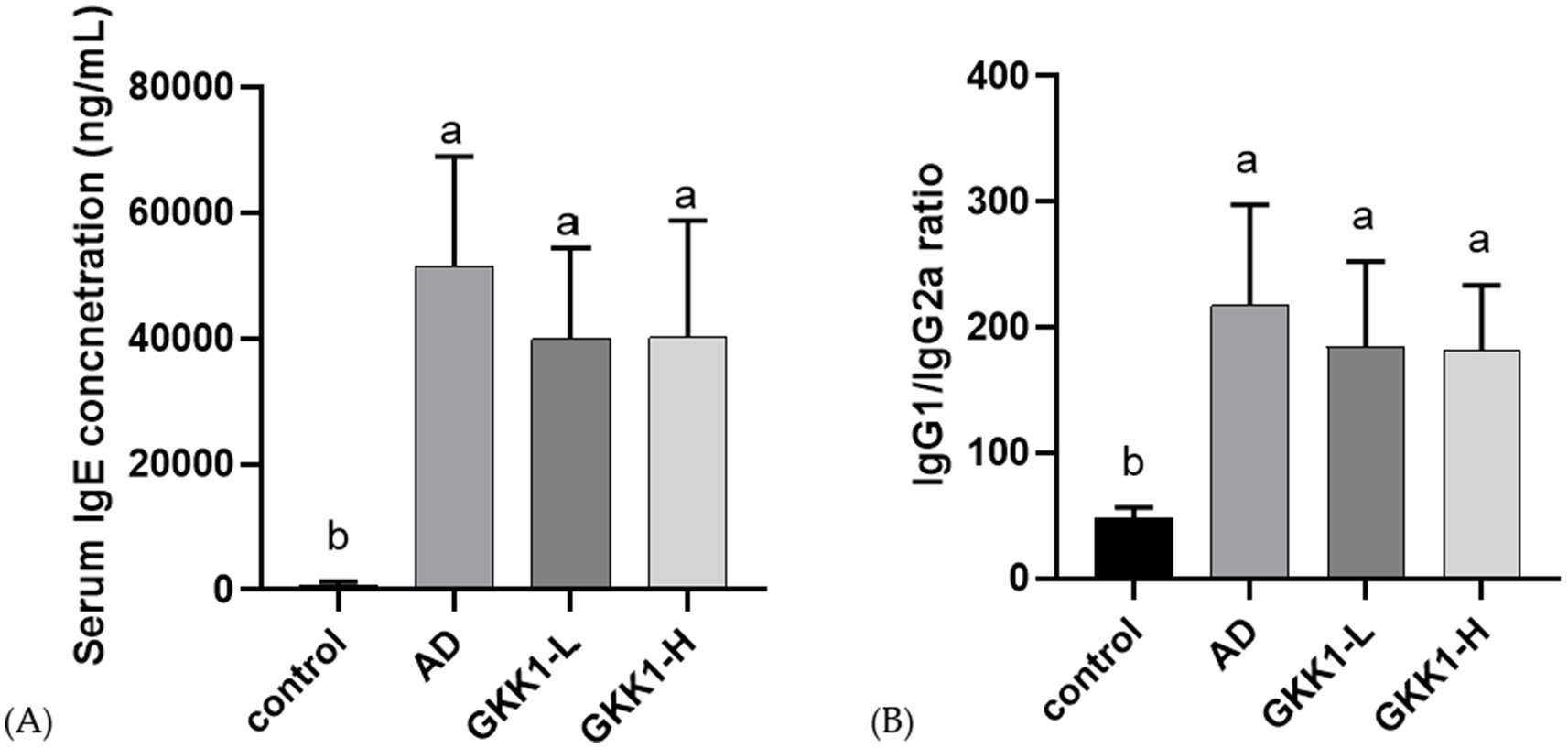
Figure 4. Serum antibody analysis in control, AD, GKK1-L, GKK1-H groups. (A) Serum IgE concentration (B) IgG1/IgG2a ratio. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
3.4 L. plantarum GKK1 raised the levels of cytokines IL-2 and lowered IL-4, IL-5 and IL-17 in spleen of AD mice
After 42 days of probiotics intervention, the concentrations of IL-4, IL-10 and IL-17 significant decreased in GKK1-H group compared to the AD group (p < 0.05), as shown in Figures 5B,D,E. Additionally, the GKK1-L group significantly increased IL-2 levels (Figure 5A), while the GKK1-L and GKK1-H groups had significantly lowered IL-5 levels compared to the AD group (Figure 5C).
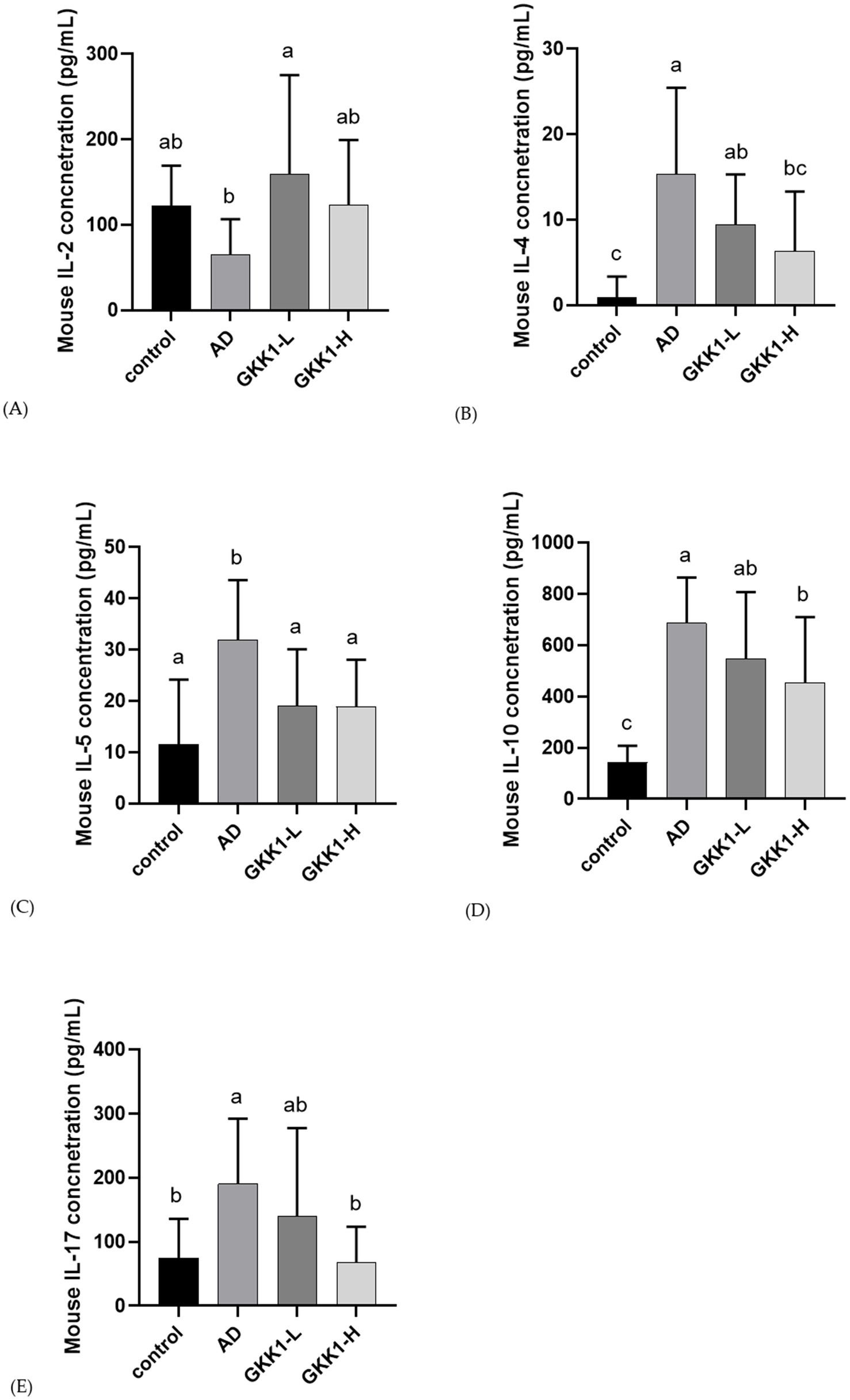
Figure 5. Splenic cytokine analysis in different experiment groups. (A) IL-2 (B) IL-4 (C) IL-5 (D) IL-10 (E) IL-17. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
3.5 Effects of L. plantarum GKK1 administration on Lactobacillus and Bifidobacterium in gut of AD mice
The results of the Lactobacillus and Bifidobacterium content in the guts were shown in Figure 6. Lactobacillus levels were significantly increased in the AD, GKK1-L, and GKK1-H groups compared to the control group (p < 0.05). Additionally, Bifidobacterium content was higher in the GKK1-L group compared to the AD group without significant difference.
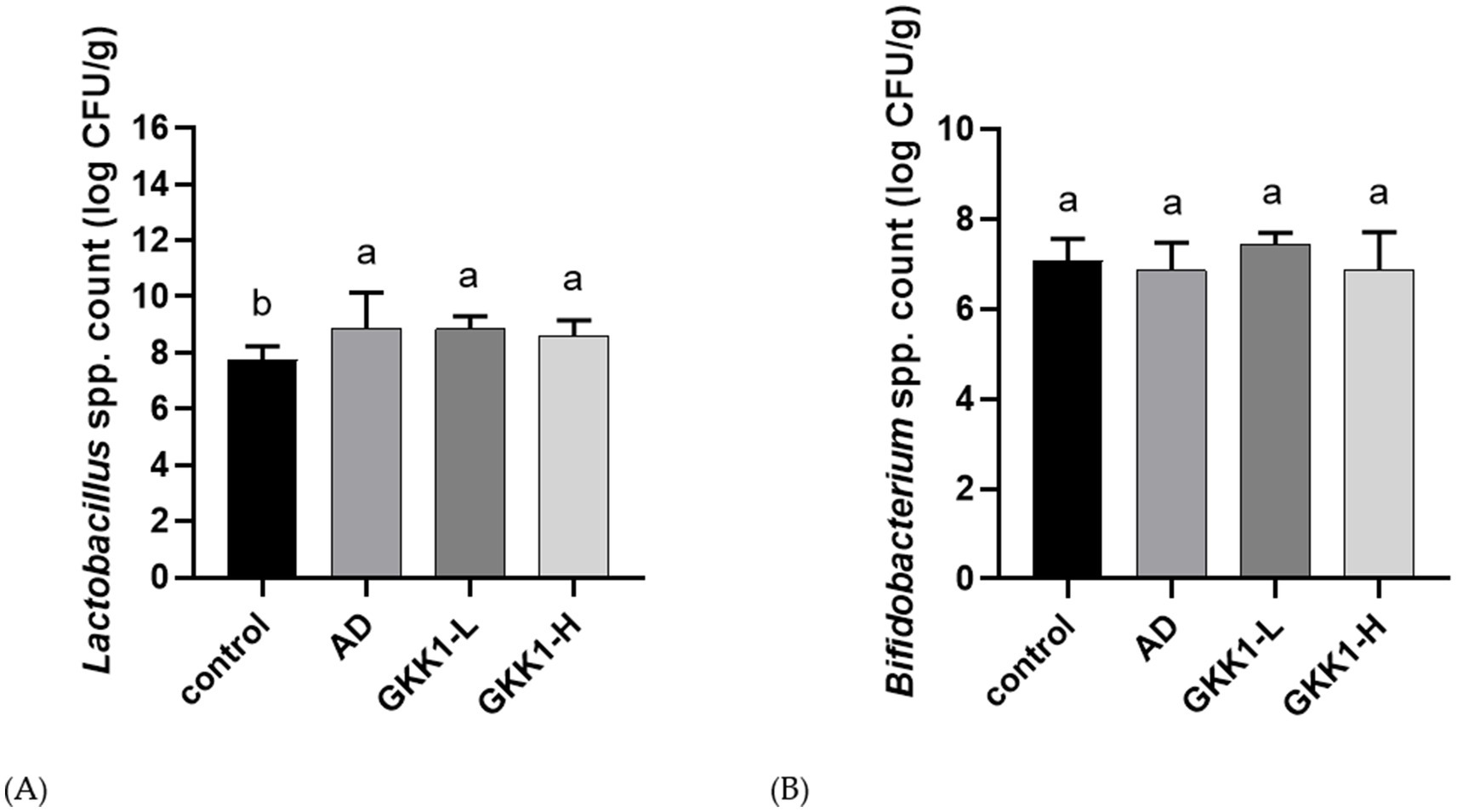
Figure 6. Quantification of intestinal (A) Lactobacillus spp. (B) Bifidobacterium spp. in different experiment groups. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1.
3.6 L. plantarum GKK1 increased acetic acid and propionic acids in gut of AD mice
Concentrations of intestinal acetic acid in the GKK1-H group was significantly higher than that in the AD group (p < 0.05) (Figure 7A). The concentration of propionic acid in GKK1-L group was significantly higher than control group (p < 0.05) (Figure 7B). The levels of butyric acid and total SCFAs did not reach statistical significances (Figures 7C,D). However, total SCFAs showed an upward trend in the GKK1-H group compared to the control group (p < 0.1).
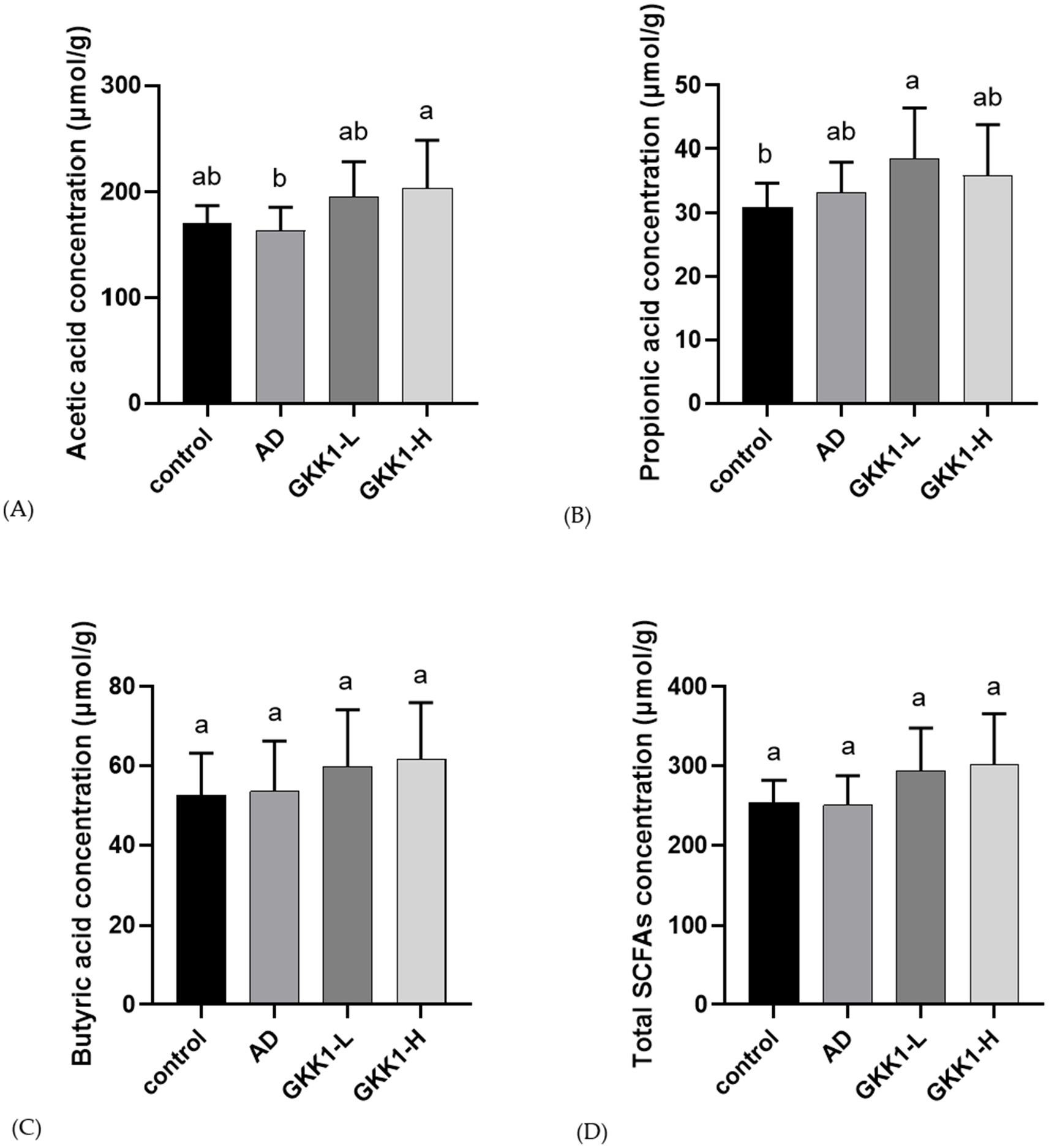
Figure 7. Quantification of short-chain fatty acids in mouse cecum in different experiment groups. (A) Acetic acid (B) Propionic acid (C) Butyric acid (D) Total SCFAs. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b) indicates significant difference at p < 0.05 as determined by one-way ANOVA. Control: normal mouse control treated with phosphate-buffered saline (PBS), AD: 2,4-dinitrochlorbenzene (DNCB)-induced atopic dermatitis (AD) mouse treated with PBS, GKK1-L: DNCB-induced AD mouse treated with low-dosage L. plantarum GKK1; GKK1-H: DNCB-induced AD mouse treated with high-dosage L. plantarum GKK1; SCFAs: short-chain fatty acids.
4 Discussion
In the present study, we demonstrated that administration of L. plantarum GKK1 showed the AD preventing effect in HDM-extract induced AD mice. The clinical symptoms of AD include erythema, edema, dryness/scarring, and excoriation/erosion (Lee et al., 2010), and the severity of AD was measured according to the symptoms. The BALB/c mice developed AD-like lesions after application of DNCB and HDM-extract, consistent with previous finding (Yoon et al., 2015). The consumption of both low and high dosages of GKK1 significantly improved the symptoms of AD. Skin biopsies from the AD group revealed skin lesions characterized by thickened epidermis and dermis, along with lymphocytic infiltrates in the dermis. Additionally, severe pyknosis of nuclei in the epidermis, indicative of cell apoptosis in AD, was observed (Hou et al., 2016). In line with previous studies, the thickness and inflammation severity of the epidermis and dermis were measured and scored in this AD mouse model. Consistent with prior findings, the AD group exhibited higher scores compared to the control group (Jang et al., 2018). In our study, the high dosage of GKK1 resulted in a significant improvement in both epidermal and dermal conditions, demonstrating a dose-effect relationship.
The mouse with AD exhibited upregulated serum IgE levels and an increase IgG1 to IgG2a ratio, which are markers of AD progression (Lee et al., 2010). Following intervention with probiotics GKK1, the elevation of serum IgE and the IgG1 to IgG2a ratio were suppressed without significant differences. During AD development, CD4+ T cells are activated and subdivided into Th1, Th2, Th17, and Treg cells (Su et al., 2017). IL-2, a cytokine produced by Th1 cells, is responsible for activating Treg cells and inhibiting Th17 cells (Hoyer et al., 2008). After administering probiotics to mice with DNCB-induced AD for 42 days, splenic IL-2 production significantly increased in GKK1-L group. Th2 cells, B cells, and mast cells are the major cell types involved in AD. Furthermore, Th2 cytokines, including IL-4 and IL-5, are associated with IgE secretion and eosinophil development, survival, and proliferation, and their up-regulation has been observed in patients with AD (Hoyer et al., 2008). In this study, IL-4 and IL-5 production in the spleen of AD mice treated with probiotics was suppressed, and high dosage of GKK1 showed better effect than low dosage of GKK1. These results indicate that probiotics GKK1 are beneficial for elevating Th1 cytokines and reducing Th2 cytokines.
IL-10 is an essential cytokine produced by Treg cells for suppression of immune inflammation (Ouyang et al., 2011). The level of IL-10 has been observed to inversely correlate with the severity of AD. Our investigation showed that the splenic IL-10 levels was higher in the AD group compared to the control group, consistent with previous studies (Laouini et al., 2003). In other studies, IL-10 production significantly increased after probiotics treatment (Lim et al., 2017; Pessi et al., 2000). However, in our investigation, IL-10 secretion was downregulated after probiotics intervention compared to the AD group. We believe that the timing of IL-10 evaluation could affect the results. It’s possible that IL-10 levels increased immediately after GKK1 treatment to control immune responses, but we only analyzed cytokines on day 42. We speculate that by day 42, the symptoms of AD had improved, leading to decreased IL-10 levels. Regarding IL-17, produced by Th17 cells, it plays a crucial role in the immune defense against allergies (Sugaya, 2020). Previous studies have shown that Th17 cell proportions and IL-17 levels are elevated in the peripheral blood of AD patients (Koga et al., 2008). With the intervention of a high dose of GKK1, IL-17 levels significantly decreased, indicating that GKK1 can modulate the Th17 immune system.
There is a cross-talk between gut microbiota and development of AD (Stefanovic et al., 2020). Hence, we further investigated whether gut microbial composition was altered after the treatment of probiotic GKK1. The results revealed that the probiotic GKK1 increased the content of Lactobacillus and Bifidobacterium in the gut. Previous studies have shown that the patients receiving both Lactobacillus and Bifidobacterium, or even either of them, experienced significant improvement in AD symptoms (Iemoli et al., 2012; Klewicka et al., 2011). These findings support our results, suggesting that an increase in Lactobacillus and Bifidobacterium in the gut can alleviate AD clinical manifestation. Treatment with the probiotic GKK1 contributed to altering the gut microbial environment, leading to increased production of SCFAs, including acetic and propionic acids, through bacterial fermentation of indigestible carbohydrates. SCFAs in the gut can inhibit the growth of other pathogens by lowering pH and can also enhance immune cell levels, such as Treg cells (Smith et al., 2013; Duncan et al., 2009).
Based on the results in this research, we speculate that the probiotic L. plantarum GKK1 may have the similar therapeutic effects on AD in humans, so the clinical trial of AD patients can be further conducted to verify in the future.
5 Conclusion
Our study highlights the therapeutic potential of L. plantarum GKK1 in alleviating symptoms of atopic dermatitis (AD) through its immunomodulatory effects, regulation of cytokine profiles, ability to modulate gut microbiota composition and SCFA productions. While further investigations are warranted to fully elucidate its mechanisms of action, L. plantarum GKK1 emerges as a compelling candidate for therapeutic intervention in AD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of National Taiwan University (IACUC of NTU). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
T-CL: Data curation, Formal analysis, Software, Visualization, Writing – original draft. Y-CW: Formal analysis, Investigation, Methodology, Software, Writing – original draft. Y-ST: Validation, Writing – review & editing. S-WL: Validation, Writing – review & editing. C-CC: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. M-JC: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. Y-PC: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
T-CL, Y-ST, S-WL, and C-CC were employed by Grape King Biotechnology Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1566594/full#supplementary-material
References
De Benedetto, A., Kubo, A., and Beck, L. A. (2012). Skin barrier disruption: a requirement for allergen sensitization? J. Invest. Dermatol. 132, 949–963. doi: 10.1038/jid.2011.435
Duncan, S. H., Louis, P., Thomson, J. M., and Flint, H. J. (2009). The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 11, 2112–2122. doi: 10.1111/j.1462-2920.2009.01931.x
Eyerich, K., and Novak, N. (2013). Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 68, 974–982. doi: 10.1111/all.12184
Fick, J. L., Novo, R. E., and Kirchhof, N. (2005). Comparison of gross and histologic tissue responses of skin incisions closed by use of absorbable subcuticular staples, cutaneous metal staples, and polyglactin 910 suture in pigs. Am. J. Vet. Res. 66, 1975–1984. doi: 10.2460/ajvr.2005.66.1975
Garcia-Gonzalez, N., Battista, N., Prete, R., and Corsetti, A. (2021). Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 9:349. doi: 10.3390/microorganisms9020349
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hou, L., Liu, K., Li, Y., Ma, S., Ji, X., and Liu, L. (2016). Necrotic pyknosis is a morphologically and biochemically distinct event from apoptotic pyknosis. J. Cell Sci. 129, 3084–3090. doi: 10.1242/jcs.184374
Hoyer, K. K., Dooms, H., Barron, L., and Abbas, A. K. (2008). Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 226, 19–28. doi: 10.1111/j.1600-065X.2008.00697.x
Iemoli, E., Trabattoni, D., Parisotto, S., Borgonovo, L., Toscano, M., Rizzardini, G., et al. (2012). Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J. Clin. Gastroenterol. 46, S33–S40. doi: 10.1097/MCG.0b013e31826a8468
Jang, H.-Y., Koo, J.-H., Lee, S.-M., and Park, B.-H. (2018). Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp. Mol. Med. 50, 1–9. doi: 10.1038/s12276-018-0104-3
Kemp, A. S. (2003). Cost of illness of atopic dermatitis in children: a societal perspective. PharmacoEconomics 21, 105–113. doi: 10.2165/00019053-200321020-00003
Kim, I. S., Lee, S. H., Kwon, Y. M., Adhikari, B., Kim, J. A., Kim, G. I., et al. (2019). Oral administration of β-glucan and Lactobacillus plantarum alleviates atopic dermatitis-like symptoms. J. Microbiol. Biotechnol. 29, 1693–1706. doi: 10.4014/jmb.1907.07011
Klewicka, E., Cukrowska, B., Libudzisz, Z., Slizewska, K., and Motyl, I. (2011). Changes in gut microbiota in children with atopic dermatitis administered the bacteria Lactobacillus casei DN–114001. Pol. J. Microbiol. 60, 329–333. doi: 10.33073/pjm-2011-047
Koga, C., Kabashima, K., Shiraishi, N., Kobayashi, M., and Tokura, Y. (2008). Possible pathogenic role of Th17 cells for atopic dermatitis. J. Invest. Dermatol. 128, 2625–2630. doi: 10.1038/jid.2008.111
Laouini, D., Alenius, H., Bryce, P., Oettgen, H., Tsitsikov, E., and Geha, R. S. (2003). IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J. Clin. Invest. 112, 1058–1066. doi: 10.1172/JCI18246
Lee, S.-H., Heo, Y., and Kim, Y.-C. (2010). Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 11, 35–41. doi: 10.4142/jvs.2010.11.1.35
Lee, E. B., Kim, K. W., Hong, J. Y., Jee, H. M., Sohn, M. H., and Kim, K. E. (2010). Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 21, e457–e460. doi: 10.1111/j.1399-3038.2009.00919.x
Lee, S.-Y., Lee, E., Park, Y. M., and Hong, S.-J. (2018). Microbiome in the gut-skin axis in atopic dermatitis. Allergy, Asthma Immunol. Res. 10, 354–362. doi: 10.4168/aair.2018.10.4.354
Liberman, A. C., Budziñski, M. L., Sokn, C., Gobbini, R. P., Steininger, A., and Arzt, E. (2018). Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front. Endocrinol. 9:235. doi: 10.3389/fendo.2018.00235
Lim, S. K., Kwon, M.-S., Lee, J., Oh, Y. J., Jang, J.-Y., Lee, J.-H., et al. (2017). Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci. Rep. 7:40040. doi: 10.1038/srep40040
Lubbs, D. C., Vester, B. M., Fastinger, N. D., and Swanson, K. S. (2009). Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J. Anim. Physiol. Anim. Nutr. 93, 113–121. doi: 10.1111/j.1439-0396.2007.00788.x
McInnes, I. B., Byers, N. L., Higgs, R. E., Lee, J., Macias, W. L., Na, S., et al. (2019). Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res. Ther. 21, 183–110. doi: 10.1186/s13075-019-1964-1
Neumann, B., Pospiech, A., and Schairer, H. U. (1992). Rapid isolation of genomic DNA from gram-negative bacteria. Trends Genet. 8, 332–333. doi: 10.1016/0168-9525(92)90269-a
Nylund, L., Nermes, M., Isolauri, E., Salminen, S., De Vos, W., and Satokari, R. (2015). Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 70, 241–244. doi: 10.1111/all.12549
Ouyang, W., Rutz, S., Crellin, N. K., Valdez, P. A., and Hymowitz, S. G. (2011). Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29, 71–109. doi: 10.1146/annurev-immunol-031210-101312
Pessi, T., Sütas, Y., Hurme, M., and Isolauri, E. (2000). Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy 30, 1804–1808. doi: 10.1046/j.1365-2222.2000.00948.x
Prakoeswa, C., Bonita, L., Karim, A., Herwanto, N., Umborowati, M., Setyaningrum, T., et al. (2022). Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: a randomized controlled trial. J. Dermatol. Treat. 33, 1491–1498. doi: 10.1080/09546634.2020.1836310
Prameswari, R., Astari, L., Hidayati, A. N., and Prakoeswa, C. R. S. (2017). Effect of Lactobacillus plantarum on total immunoglobulin E serum and scoring atopic dermatitis (SCORAD) index in children with atopic dermatitis. Berkala Ilmu Kesehatan Kulit dan Kelamin 29, 91–97. doi: 10.20473/bikk.V31.3.2019.78-84
Reynolds, N., and Al-Daraji, W. (2002). Calcineurin inhibitors and sirolimus: mechanisms of action and applications in dermatology. Clin. Exp. Dermatol. 27, 555–561. doi: 10.1046/j.1365-2230.2002.01148.x
Salem, I., Ramser, A., Isham, N., and Ghannoum, M. A. (2018). The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 9:1459. doi: 10.3389/fmicb.2018.01459
Serrano, L., Patel, K. R., and Silverberg, J. I. (2019). Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 80, 904–912. doi: 10.1016/j.jaad.2018.11.028
Silverberg, J. I., Pinter, A., Pulka, G., Poulin, Y., Bouaziz, J.-D., Wollenberg, A., et al. (2020). Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 145, 173–182. doi: 10.1016/j.jaci.2019.08.013
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly, Y. M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Smits, H. H., Engering, A., van der Kleij, D., de Jong, E. C., Schipper, K., van Capel, T. M., et al. (2005). Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 115, 1260–1267. doi: 10.1016/j.jaci.2005.03.036
Spergel, J. M., and Paller, A. S. (2003). Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 112, S118–S127. doi: 10.1016/j.jaci.2003.09.033
Stefanovic, N., Flohr, C., and Irvine, A. D. (2020). The exposome in atopic dermatitis. Allergy 75, 63–74. doi: 10.1111/all.13946
Su, C., Yang, T., Wu, Z., Zhong, J., Huang, Y., Huang, T., et al. (2017). Differentiation of T-helper cells in distinct phases of atopic dermatitis involves Th1/Th2 and Th17/Treg. Eur J. Inflamm. 15, 46–52. doi: 10.1177/1721727X17703271
Sugaya, M. (2020). The role of Th17-related cytokines in atopic dermatitis. Int. J. Mol. Sci. 21:1314. doi: 10.3390/ijms21041314
Thomsen, S. F. (2014). Atopic dermatitis: natural history, diagnosis, and treatment. Int. Sch. Res. Notices 2014:354250. doi: 10.1155/2014/354250
Torii, T., Kanemitsu, K., Wada, T., Itoh, S., Kinugawa, K., and Hagiwara, A. (2010). Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: specimen stability. Ann. Clin. Biochem. 47, 447–452. doi: 10.1258/acb.2010.010047
Ueno, N., Fujiya, M., Segawa, S., Nata, T., Moriichi, K., Tanabe, H., et al. (2011). Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm. Bowel Dis. 17, 2235–2250. doi: 10.1002/ibd.21597
Xiao, C., Sun, Z., Gao, J., Bai, Y., Zhang, C., Pang, B., et al. (2021). Enhanced phenotype of calcipotriol-induced atopic dermatitis in filaggrin-deficient mice. FASEB J. 35:e21574. doi: 10.1096/fj.202002709R
Yoon, H.-J., Jang, M.-S., Kim, H.-W., Song, D.-U., Nam, K.-I., Bae, C.-S., et al. (2015). Protective effect of diet supplemented with rice prolamin extract against DNCB-induced atopic dermatitis in BALB/c mice. BMC Complement. Altern. Med. 15, 353–357. doi: 10.1186/s12906-015-0892-0
Keywords: atopic dermatitis, Lactiplantibacillus plantarum, interleukin 2, interleukin 4, interleukin 5, interleukin 17, gut microbiota, short-chain fatty acids
Citation: Lin T-C, Wu Y-C, Tsai Y-S, Lin S-W, Chen C-C, Chen M-J and Chen Y-P (2025) Oral administration of Lactiplantibacillus plantarum GKK1 ameliorates atopic dermatitis in a mouse model. Front. Microbiol. 16:1566594. doi: 10.3389/fmicb.2025.1566594
Edited by:
Valério Monteiro-Neto, Federal University of Maranhão, BrazilReviewed by:
Tales Fernando da Silva, Institute of Biological Sciences, BrazilJianming Zhang, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2025 Lin, Wu, Tsai, Lin, Chen, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Ju Chen, Y21qQG50dS5lZHUudHc=; Yen-Po Chen, Y2hlbnlwQGRyYWdvbi5uY2h1LmVkdS50dw==
†These authors have contributed equally to this work
 Tzu-Chun Lin
Tzu-Chun Lin Yu-Chieh Wu2
Yu-Chieh Wu2 Chin-Chu Chen
Chin-Chu Chen Ming-Ju Chen
Ming-Ju Chen Yen-Po Chen
Yen-Po Chen