Abstract
Phytopathogens represent a persistent threat to global agricultural productivity, precipitating yield losses and destabilizing food security. Conventional reliance on synthetic agrochemicals, while effective in phytopathogen suppression, incurs significant economic burdens, drives environmental toxicity, and accelerates the evolution of resistant microbial strains, with collateral risks to ecosystem integrity and public health. This review synthesizes current advancements in harnessing plant- and microorganism-derived extracts, bioactivity-guided fractions, and purified phytochemicals as eco-compatible antimicrobial agents against phytopathogenic bacteria and fungi. Furthermore, we propose a novel framework for standardized prioritization of natural products, integrating efficacy thresholds, phytochemical complexity, and mechanistic specificity to guide scalable antimicrobial discovery. Meta-analysis of published studies reveals a predominant focus on Fusarium spp. as model phytopathogens, with dilution in broth and agar diffusion as the predominant in vitro assays. Quantitative benchmarks for antimicrobial potential were established: bacterial Minimum Inhibitory Concentrations (MICs) ≤ 2.5 mg/mL (crude extracts), ≤0.6 mg/mL (fractions), and ≤64 μg/mL (purified compounds), alongside fungal growth inhibition thresholds <52% (agar dilution assays). These criteria highlight the differential bioactivity of natural product tiers, emphasizing the role of compound purification in potency enhancement. By bridging phytochemical innovation with agronomic applicability, this work positions plant-derived antimicrobials as pivotal tools for sustainable disease management, circumventing agrochemical limitations while advancing One Health-aligned agricultural practices.
1 Introduction
As the global population nears >9 billion by 2050, humanity faces a critical paradox: persistent hunger and caloric insufficiency coexist with rising overnutrition and obesity (Guldan, 2020). This dual burden of malnutrition strains health systems, exacerbates socioeconomic inequalities, and necessitates innovative, equitable solutions to achieve sustainable food security through strategic agricultural investment (Ulian et al., 2020). Plant cultivation has played a foundational role in agricultural systems since the advent of human civilization, serving as a critical driver of food security and socioeconomic development with substantial economic value. Nevertheless, crop productivity and quality remain persistently threatened by microbial pathogens including viruses, bacteria, fungi and oomycetes that compromise plant health and yield. Notable phytopathogens include bacteria such as Pseudomonas viridiflava, Escherichia coli, Xanthomonas campestris, Bacillus megaterium, and Clavibacter michiganensis; fungi such as Aspergillus micheli, Alternaria alternata, Fusarium oxysporum, Penicillium digitatum, and Botrytis cinerea; oomycetes such as Phytophthora cinnamomi, Pythium aphanidermatum, and Phytophthora infestans; and plant viruses such as Dasheen mosaic virus (DMV), Sour cherry green ring mottle virus (CGRMV), and Potato leafroll virus (PLRV). These diverse pathogens are responsible for significant agricultural losses (Elshafie et al., 2021; Giachero et al., 2022; Hartman, 1974; Hernández-Díaz et al., 2021; Martin and Loper, 1999; Taliansky et al., 2003; Zhang et al., 1998). For instance, the grey mould fungus, Botrytis cinerea, is a significant contributor to pre- and post-harvest losses in fruit and vegetable production. Recently classified as a ‘high-risk’ necrotrophic pathogen, it exhibits a remarkable capacity to rapidly develop resistance to fungicides, primarily through drug efflux transport mechanisms (Weber and Petridis, 2023; Shao et al., 2021). Of particular concern, intensive and excessive fungicide applications have led to the emergence of multiresistant strains in several countries, posing a serious challenge to disease management strategies (Hahn, 2014).
Contemporary agricultural practices for pathogen management remain heavily reliant on synthetic agrochemicals. However, conventional pest control strategies frequently fail to account for their broader ecological and economic ramifications. Prolonged agrochemical use has been associated with adverse environmental impacts, including bioaccumulation within trophic networks, resulting in biomagnification of toxic compounds across food chains (Dhananjayan et al., 2020). Human health risks, such as acute intoxication and chronic poisoning, further underscore the limitations of these chemical agents (Devi et al., 2022; Lekei et al., 2014). Moreover, the indiscriminate application of agrochemicals accelerates the evolution of antimicrobial resistance, wherein phytopathogenic strains acquire adaptive mechanisms to circumvent chemical control measures. This necessitates the deployment of increasingly potent compounds, perpetuating a cycle of environmental degradation and ecological imbalance (Lekei et al., 2014). In response to these challenges, the scientific community has intensified efforts to identify sustainable alternatives that harmonize economic viability with ecological safety. Natural products, encompassing bioactive compounds, phytochemical fractions, and plant-derived nanoparticles, have emerged as promising candidates due to their biodegradability, low environmental persistence, and reduced propensity for resistance development (Chin et al., 2006). By leveraging these resources, researchers aim to mitigate the unintended consequences of industrial agrochemicals while maintaining robust antimicrobial efficacy.
However, significant research gaps hinder their translation from lab to field. First, while in vitro studies demonstrate efficacy, mechanistic insights into how plant-based compounds such as Olive mill wastewater (rich in phenolics) interact with Phytopathogenic bacteria like Pseudomonas savastanoi pv. savastanoi, Clavibacter michiganensis, and Xanthomonas campestris remain limited, impeding optimization (Košćak et al., 2023). Second, the yield of bioactive compounds extracted from a given plant is highly variable due to the influence of climatic conditions and many other factors. This inconsistency is further compounded by the absence of standardized extraction protocols for isolating phytochemicals with potential activity against phytopathogenic microorganisms, posing a significant challenge to their reliable application in plant disease management (Bitwell et al., 2023; Kumar et al., 2017; Moomin et al., 2023). Third, the potential of synergistic combinations between plant-derived compounds and biocontrol organisms or integrated phytopathogen management strategies remains largely unexplored. Despite their promise in enhancing the effectiveness and sustainability of phytopathogen control, such approaches have received limited research attention and, when applied, are largely restricted to greenhouse crops (Pandit et al., 2022). Fourth, long-term ecological impacts—such as effects on non-target species or soil microbiomes—are poorly documented, raising questions about holistic sustainability (Nadeu et al., 2023). Finally, economic barriers, including cost–benefit analyses and adoption incentives, are overlooked in favor of purely technical research, and limiting real-world uptake. This review critically examines recent advances in the application of natural products derived from plants as antiphytopathogenic agents, with a focus on their mechanistic action and efficacy. We further evaluate standardized methodologies for antimicrobial assessment and propose a unified criterion for interpreting antibacterial and antifungal activity data, aiming to establish a framework for identifying high-potency, environmentally sustainable phytopathogen control strategies.
2 Major phytopathogens
The agroecosystem plays a pivotal role in shaping local and global economies. However, agricultural productivity is frequently compromised by phytopathogens, leading to significant yield reductions and economic losses. Among viral pathogens, mosaic viruses (e.g., Tobacco mosaic virus) are particularly impactful (Palukaitis et al., 1992). Predominant fungal phytopathogens include genera such as Aspergillus, Fusarium, Penicillium, Alternaria, and Botrytis, which infect diverse plant tissues, including leaves, stems, roots, and fruits (Nakajima and Akutsu, 2014; Okungbowa and Shittu, 2012; Peever et al., 2002). Bacterial genera such as Ralstonia, Pseudomonas, Xanthomonas, Pectobacterium, and Dickeya, similarly can colonize and damage plants through either systemic or localized infections (Álvarez et al., 2010; Bashan and De-Bashan, 2002; Ge et al., 2021; Rudolph, 1993). Table 1 summarizes the most prevalent fungal and bacterial phytopathogenic genera and their associated host crops.
Table 1
| Biological group | Phytopathogens | Crops affected | Reference |
|---|---|---|---|
| Bacteria | Clavibacter spp. | Tomato, alfalfa, wheat, potato, bean | Nandi et al. (2018) and Peritore-Galve et al. (2021) |
| Dickeya spp. | Potato | Toth et al. (2011) | |
| Erwinia spp. | Ornamental plants, apples, pear and potatoes | Reiter et al. (2002) and Vrancken et al. (2013) | |
| Pectobacterium spp. | Potato, tomato, maize, cabbage, and ornamental plants, rice, maize, potato | Ma et al. (2007), Toth et al. (2021), and Werra et al. (2021) | |
| Pseudomonas spp. | Arabidopsis sp., sweet basil | Mansfield et al. (2012) and Walker et al. (2004) | |
| Ralstonia spp. | Potato, tomato, and other solanaceous plant species | Pedro-Jove et al. (2021) | |
| Xanthomonas spp. | Arabidopsis thaliana, broccoli, cabbage, cauliflower, citrus fruit | Papaianni et al. (2020) and Reynol (2017) | |
| Xylella spp. | Grape, olive, almond, citrus fruit, coffee | Bruening et al. (2014) and Landa et al. (2022) | |
| Fungi | Alternaria spp. | Tomato, olive, carrots, citrus fruits, cereals, apples | Logrieco et al. (2009) and Palou et al. (2012) |
| Aspergillus spp. | Maize, cottonseed, peanuts, tree nuts | Diener et al. (1987) and Robens and Cardwell (2003) | |
| Botrytis spp. | Strawberry, citrus fruits, grapes, chickpea, and other beans | Boddy (2016) and Pande et al. (2006) | |
| Colletotrichum spp. | Woody ornamentals and tropical foliage plants, papaya, mango, guava, avocado | Azevedo Dos Santos et al. (2020) | |
| Fusarium spp. | Wheat, soft red winter wheat, durum wheat, barley, bananas | Dita et al. (2018), Nelson (1992), and Nganje et al. (2004) | |
| Glomerella sp. | Apple | Wang et al. (2015) | |
| Penicillium spp. | Apple, garlic | Wang et al. (2023) | |
| Rhizoctonia spp. | Soybean, corn, alfalfa, winter wheat, spring barley | Malvick (2018) and Smiley (2021) | |
| Rhizopus spp. | Sunflower, strawberry, passion fruit | Schipper and Stalpers (1984) and Thompson et al. (1980) | |
| Oomycetes | Aphanomyces spp. | Pea, sugar beet, spinach alfalfa, lentils, green beans |
Wikström et al. (2025) |
| Peronospora sp. | Alfalfa | Yu et al. (2023) | |
| Phytophthora spp. | Pepper, cucumber, pumpkin, tomatoes, snapbeans | Granke et al. (2012) | |
| Pythium spp. | Avocado, pineapple, peach, chestnut, macadamia, camellia, oak, pine and eucalyptus | Hardham (2005) and Jung et al. (2013) | |
| Virus | Nepovirus | Cucumber, Blueberries | Caglayan et al. (2025) and James et al. (2024) |
| Polerovirus | Tobacco, wild-rice | Yan et al. (2025) | |
| Potyvirus | Potato | Mäkinen et al. (2023) |
Major genera of bacterial, fungal, oomycetes and viruses phytopathogens and their affected plants and fruits.
Phytopathogens encompasses a diverse spectrum of pathogens, including viroids, viruses, prokaryotic bacteria, and eukaryotic organisms such as fungi, oomycetes, and nematodes (Pandit et al., 2022). The following sections provide an overview of the major phytopathogenic fungi, bacteria, and selected viral pathogens, highlighting the diseases they cause and their economic impact on agricultural production systems. Particular attention is given to viral diseases in a dedicated section.
2.1 Fungi phytopathogens
Fungal phytopathogens play an important role in global food and health security issues, causing various diseases in many crops and fruits (Luck et al., 2011). Fungi such as Aspergillus are mainly found in soil, fruits, plants and have more than two hundred species such as A. terreus, A. fumigatus and A. oryzae. This genus produces conidiophores, a structure similar to the cnidarian “hydra,” which allows it to reproduce and spread (Jayaprakash et al., 2019). The disease caused by these fungi is mainly due to toxins such as aflatoxin (Amaike and Keller, 2011), a type of mycotoxin, which affects various stages of plant life, such as seed germination and other physiological processes, causing harm to humans and animals when ingested in contaminated foods (Abdelaziz et al., 2022). According to Eskola et al. (2020), between 60 and 80% of all crops in the world are damaged by mycotoxins such as aflatoxicins.
In the United States, maize cultivation suffers losses of approximately US$160 million per year due to aflatoxin contamination. In developing countries, the impact is even more severe, reaching around US$450 million, which accounts for 38% of global losses caused by the presence of toxins in agricultural production (Jallow et al., 2021).
Another extremely important genus is Fusarium, which mainly infects the banana plant and fruit, and includes species such as: F. oxysporum, F. sporotrichoides, F. graminearum (Ploetz, 2015). Banana cultivation has a global market turnover of $25 billion dollars (Voora et al., 2020). India is the country with the largest banana production in the world, growing more than 31 million tons annually, surpassing China with an annual production of approximately 11 million tons and Indonesia with 8 million tons (Statista, 2024). Fusarium infection of the plant mainly affects the vascular system in the stem and root (Ploetz, 2015) and may reach the xylem (Gordon, 2017). It also has the potential to infect animals and humans, with a high mortality rate (Hof, 2020). Fusarium oxysporum, the primary agent of vascular wilt, stands out as the most widespread pathogen, with global crop losses estimated at 10–50%, and even higher in India, where losses can reach up to 80% (Bai et al., 2018).
The genus Alternaria includes the species: A. alternata, A. arbusti, A. blumeae, A. brassicae, A. brassicicola, A. carotiincultae, A. conjuncta, A. dauci, A. euphorbiicola, A. infectoria, A. molesta, A. panax, A. petroselini, A. selini, A. solani, A. smyrnii, among others, are known to attack pre- and post-harvest fruit, especially tomatoes (Palou et al., 2012). Infection by this fungus consists of the appearance of black spots on the skin of the fruit due to the production of toxins and diseases such as black mould rot (Yan et al., 2015). This genus can affect numerous plant species such as citrus fruits, pears, carrots, barley, oats, olives, melons, peppers, apples, raspberries, cranberries, grapes, sunflower seeds, melons, lentils, wheat and other grains (Crous et al., 2015; Logrieco et al., 2009; Patriarca et al., 2007). As recent research has shown (Shabeer et al., 2022), this type of genus can also infect humans and cause disease (Azcarate et al., 2008).
The Botrytis genus consists of 22 species such as B. cinerea, B. convoluta, B. fabae, among others that can cause the rust disease, characterized by leaf loss, black spot and decay in the stem (Bika et al., 2021). The most commonly affected plants are strawberries, citrus fruits, grapes, chickpeas and lettuce (Pande et al., 2006).
The Glomerella and Colletotrichum genera mainly attack apples, but they can also infect strawberries, causing leaf spots of Glomerella (Gonzalez et al., 2006) or even bitter rot, appearing as black or brown spots that grow and cause necrosis in the area (Moreira et al., 2019). They can also cause the disease anthracnose, causing rot on plants and dark lesions on fruit, affecting strawberry, mango, citrus fruit, avocado, banana, coffee and some cereal crops (Cannon et al., 2012). Both genera are related to each other, as the genus Glomerella represents the teleomorphic (sexual) phase of the fungus while the asexual (anamorphic) phase is represented by the genus Colletotrichum (Guerber and Correll, 2001), like the life cycle of cnidarians, alternating between polyp and medusa. A good example of a species that symbolizes this relationship is G. cingulata, which is the teleomorph of C. cingulata. G. cingulata in the epidemic regions of China caused severe damage to susceptible apples, including 90% defoliation and diseased fruit before harvest, which resulted in reduced tree vigor, lower yields and poor fruit quality (Liu et al., 2023).
The genus Rhizoctonia is made up of numerous species such as R. fumigata, R. ferruginea, R. oryzae-sativae and R. rubi, which reduce the yield of a large number of plants, from aquatic to aerial, such as soybeans, corn, alfalfa, winter wheat and spring barley (Malvick, 2018). It is most commonly found in damp soil, initiating the infection in the roots of the plant (Baker, 1970). Rhizoctonia bare spot affected almost 20% of the plantation (approximately 2 fields of 50 hectares), and the impact of the disease caused yield reductions of up to 73% on a winter wheat and spring barley farm (Smiley, 2021). Symptoms observed on a variety of hosts include seed decay, root decay, hypocotyl decay, crown decay, stem decay, limb and pod decay, stem canker, black scab and seedling blight (Ajayi-Oyetunde and Bradley, 2018).
The Rhizopus genus includes species such as R. oryzae, R. nigricans and R. sexualis, which infect sunflowers, strawberries, passion fruit and cause flower rot (SCHIPPER & STALPERS Schipper and Stalpers, 1984). The disease can be on the flower head or on the peduncle. On the flower, the disease manifests itself as a brown spot, causing some seeds to taste bitter and fall off. On the peduncle the whole flower falls off and rots (Yildirim et al., 2010).
Oomycetes are microorganisms that produce hyphae just like fungi. Their cell walls are composed of cellulose, and they produce coenocytic hyphae, which are not composed of divided cells but rather a single elongated cell (Hardham, 2007). In literature studies have been carried out on numerous oomycetes, such as the genera Phytophthora and Pythium. One of the most studied oomycetes in the literature is Phytophthora, especially Phytophthora infestans, which causes late blight in potatoes (Kamoun et al., 2015). The Pythium genera consist of P. ultimum, P. aphanidermatum and P. irregulare. These microorganisms cause diseases such as Pythium damping off, root rot (Mavrodi et al., 2012).
Chitosan, saponins, and induced systemic resistance play a crucial role in managing fungal and oomycete pathogens such as Fusarium oxysporum, Phytophthora infestans, and Colletotrichum gloeosporioides. It was reported that, chitosan interacts with the negatively charged components of the fungal cell surface, altering membrane permeability and leading to the leakage of intracellular electrolytes and protein-rich contents (Hernández-Lauzardo et al., 2011). Saponin-rich plant extracts from Balanites aegyptiaca fruit mesocarp, Quillaja saponaria bark, and Yucca schidigera have demonstrated effective inhibitory activity against phytopathogens such as Fusarium oxysporum, Colletotrichum coccodes, and Verticillium dahliae, with varying degrees of efficacy (Chapagain et al., 2007). Interestingly, systemic acquired resistance and induced systemic resistance are plant defense mechanisms triggered by prior infection or treatment, enhancing resistance to future pathogen attacks. Instead of directly targeting pathogens, they strengthen the plant’s physical and chemical barriers through signaling pathways, particularly salicylic acid-dependent cascades, that induce broad-spectrum, long-lasting resistance (Kamle et al., 2020).
2.2 Bacterial phytopathogens
Bacterial pathogens affect the global market for various fruits and vegetables commonly used in everyday cooking by the world’s population. A very relevant genus of phytopathogenic bacteria is Ralstonia, which usually infects the host through the roots, mainly affecting tomato plantations, responsible for bacterial wilt, a disease characterized mainly by the crumpling of leaves, eventually collapsing the plant (Tahat and Sijam, 2010). This genus includes species such as R. pseudosolanacearum, R. solenacearum and R. syzygii. To start the invasion, R. solanacearum initially releases enzymes that break down the cell wall of the host cell (Coll and Valls, 2013). The current widely accepted route of colonization by R. solanacearum involves the bacterium entering the root cortex of the host plant and subsequently progressing through the intercellular space to reach the xylem. Once in the xylem, the bacterium multiplies and spreads to the above-ground parts of the host plant (Bae et al., 2015).
Another extremely important disease in crops is soft rot, which affects the roots of plants such as potatoes, causing the tissue to decompose. The pathogens responsible include Pectobacterium and Pseudomonas spp. The genus Pectobacterium sp. is made up of species such as P. carotovorum, P. atrosepticum, P. wasabiae; and the genus Pseudomonas sp. includes P. aeruginosa, P. putida, P. chlororaphis. Pectobacterium sp., which can cause soft rot on various hosts, such as cabbage, carrots, celery, cotton, cucumbers, cyclamen, hyacinth, corn, potatoes, sugar cane and tobacco (Dye, 1968). Another disease is blackleg, which damages profits and agriculture in general. Symptoms include blackening of the stem and rotting (Van der Merwe et al., 2010). Infection begins when the bacterium comes into contact with the plant’s tissues through a wound, subsequently the tissue becomes soft due to the action of pectinolytic enzymes excreted by the pathogen, presenting a fetid secretion (Perombelon, 1988).
Erwinia includes species such as Erwinia amylovora and Erwinia carotovora. It is responsible for causing diseases such as blackleg of potatoes and fire blight (Reiter et al., 2002; Zeng et al., 2021). A 2024 study estimated the economic impact of fire blight on the orchard’s long-term economic performance, and, with the worst-case scenario, there was a reduction of up to 70% in the field’s profitability (Nieto et al., 2024).
Xanthomonas sp. is another genus of great importance in agriculture and includes species such as X. citri, X. alfalfae, X. oryzae, X. vasicola and X. perforans. These pathogens can cause circular, oily spots on leaves, stems, thorns and fruit and develop into white or yellow pustules that darken and thicken, forming a brown canker and deep craters in the fruit, which can lead to defoliation and premature fruit drop (Brunings and Gabriel, 2003). It is a genus of Gram-negative bacteria, which mainly affects citrus fruits such as lemons, oranges and tangerines (Starr, 1981). In 2019, according to the Fund for Citrus Protection (Fundecitrus 2019), in one region of Brazil, the incidence of symptomatic trees reached 15%, which is significant given the severity of the disease.
The genus Dickeya comprises bacteria that infect potato, tomato, chicory, artichoke, dahlia, kalanchoe, pineapple, sweet potato, banana, corn and others, including species such as D. zeae, D. solani and D. aquatica. The main symptom of infection caused by these bacteria is soft rot, which manifests itself as internal stem necrosis or rot extending from the base of the stem. Externally, however, the base of the stem appears healthy (Toth et al., 2011).
The Clavibacter genus includes Gram-positive bacteria such as C. michiganensis, C. capsici, and C. sepedonicus. During infection they can cause ratoon dwarfism, which is a term used to describe a problem prevalent in perennial crops such as sugar cane, sweet potato and certain varieties of banana. The term “ratoon” refers to the secondary plant growth that appears after the primary crop has been harvested, originating from the remaining shoots and buds of the original plants (Davis et al., 1984). One of the main species that causes major economic losses is C. michiganensis, as there have been reports of tomato fields losing between 50 and 100% of total production, and its symptoms are wide-ranging, such as cankers on the stem, dehydration of the leaf margins, chlorotic spots on the leaves, among others (Rossi, 2014).
The Xylella genus can cause various plant diseases, such as citrus variegated chlorosis, plum leaf scald, false peach disease, olive rapid decline syndrome, Pierce’s grapevine disease, alfalfa dwarfing, margin necrosis and leaf scorch (Trkulja et al., 2022). The only member of its genus is X. fastidiosa, which is usually characterized by its subspecies as: X. fastidiosa subsp. fastidiosa, X. fastidiosa subsp. pauca, X. fastidiosa subsp. multiplex, X. fastidiosa subsp. sandyi, X. fastidiosa subsp. morus. Generally, these bacteria obstruct the transport of water and soluble minerals through the xylem, leading to various manifestations in infected plants, such as necrosis of leaf margins, wilting and subsequent drying of leaves and branches, along with stunted growth and wilting of specific parts of the plant. According to Schneider et al. (2020), the economic impact of ineffective control against Xylella subspecies would cause a batch in Italy to lose between US$2 billion and US$5.6 billion dollars (converted on 31/07/2024). Brazil, one of the higher production of citrus fruit, in 2015, had an orange production volume of 16.7 million tons (Pereira et al., 2022), and the country has been dealing with measures to protect orange and citrus production from citrus canker since 1957 (Behlau, 2021). Figure 1 illustrates the primary plant structures susceptible to infection by common bacterial and fungal phytopathogens in agricultural plantations. It highlights the specific sites of pathogen attack, providing insights into disease progression and potential impact on plant health.
Figure 1

Key plant structures affected by common bacterial and fungal phytopathogens in agricultural plantations.
Plant-derived antibacterial agents and microbiome manipulation represent sustainable strategies to combat bacterial phytopathogens. A variety of plant-derived phytochemicals (including extracts and essential oils) show potential as novel antibacterial agents for suppressing bacterial phytopathogens, offering sustainable alternatives to synthetic bactericides in agriculture (Abdullahi et al., 2020). A current trend in sustainable agriculture involves activating the soil microbiome by enhancing indigenous microbial communities, which has been shown to reduce diseases cause by phytopathogens such as bacterial wilt, brown blotch, fire blight, and crown gall. This approach offers effective plant protection while maintaining soil health, with no reported adverse effects on soil properties (Haq et al., 2024).
2.3 Viral phytopathogens
Plant viral diseases pose a significant challenge to global agriculture, reducing crop yield and quality. Besides, the increasing globalization of agriculture and international trade is contributing to the spread of viruses and their vectors into new regions, leading to unpredictable impacts on food production and natural ecosystems (Jones and Naidu, 2019). Unlike bacterial or fungal pathogens, plant viruses lack independent metabolic machinery and must rely on host cellular mechanisms for replication and systemic spread.
Plant viruses belong to various families with distinct transmission mechanisms, host ranges, and disease symptoms. The Potyviridae family includes Potato Virus Y (PVY), which affects potatoes, tomatoes, and peppers, causing mosaic symptoms, leaf necrosis, and reduced yield (Scholthof et al., 2011). Another example is Papaya Ringspot Virus (PRSV), which infects papaya and cucurbits, leading to severe leaf distortion, mosaic patterns, and ring spots on fruit (Tripathi et al., 2008). The Tombusviridae family includes Tomato Bushy Stunt Virus (TBSV), which infects tomatoes and causes stunting, yellowing, and fruit malformations (Scholthof et al., 2011). Cucumber Necrosis Virus (CNV) is another member that affects cucumbers, causing necrotic lesions and stunted growth (Dijkstra and Khan, 2024). The Geminiviridae family includes Tomato Yellow Leaf Curl Virus (TYLCV), a devastating virus in tomatoes, transmitted by whiteflies (Bemisia tabaci), causing yellowing, curling of leaves, and severe yield loss (Navas-Castillo et al., 2011). Another example is Maize Streak Virus (MSV), which affects maize crops, causing chlorotic streaking and stunted growth (Bosque-Pérez, 2000).
Host resistance is a fundamental natural control mechanism. Some plants have evolved resistance genes (R genes) that recognize specific viral proteins and activate defense responses. RNA interference (RNAi) is another natural defense in which plants degrade viral RNA to prevent replication and movement (Baulcombe, 2004). Additionally, systemic acquired resistance (SAR) primes plants against secondary infections by inducing defense-related proteins and secondary metabolites (Durrant and Dong, 2004). Beneficial microorganisms, including endophytic fungi, rhizobacteria, and mycorrhizal fungi, enhance plant immune responses against viral infections. Certain strains of Bacillus and Pseudomonas produce antimicrobial compounds and elicit induced systemic resistance (ISR), reducing viral replication and symptom severity (Kloepper et al., 2004). Some fungal biocontrol agents, such as Trichoderma spp., secrete enzymes that degrade viral coat proteins, interfering with infection (Shoresh et al., 2010).
Many plant viruses are transmitted by insect vectors such as aphids, whiteflies, and thrips. Natural enemies like ladybugs (Coccinellidae), lacewings (Chrysopidae), and parasitoid wasps (Encarsia formosa) regulate vector populations, reducing virus transmission rates. Additionally, entomopathogenic fungi such as Beauveria bassiana and Metarhizium anisopliae target insect vectors and minimize virus spread (Baverstock et al., 2010). Certain plant extracts and essential oils exhibit antiviral activity by interfering with viral replication and cell-to-cell movement. Phytochemicals such as flavonoids, alkaloids, and terpenoids have shown inhibitory effects against plant viruses (Ma and Yao, 2020). Extracts from neem (Azadirachta indica) and garlic (Allium sativum) reduce infection severity by disrupting viral coat proteins and inhibiting vector feeding behavior (Thomas et al., 2021). Integrating natural control measures into agricultural systems requires a holistic approach. Crop rotation and intercropping disrupt virus life cycles and limit vector establishment. Companion planting with repellent species, such as marigold (Tagetes spp.) and basil (Ocimum basilicum), deters insect vectors and reduces virus incidence (Hooks and Fereres, 2006). Additionally, breeding programs focusing on durable resistance genes can provide long-term solutions against viral diseases (Garcia-Ruiz, 2018).
3 Plant-based alternatives for phytopathogen control: from extraction to evaluation
3.1 Extraction of bioactive plant compounds for phytopathogen control
It is evident that phytopathogens can simultaneously affect multiple plant structures. Consequently, advancements in microbial and pest control strategies continue to evolve annually, including the development of agrochemicals with enhanced antimicrobial efficacy, the utilization of natural biopesticides, and the induction of plant resistance to specific pests (Douglas, 2018; Perdikis et al., 2011; Vurro et al., 2019). The use of herbal medicines with various biological activities, such as antimicrobial, anti-inflammatory and anticarcinogenic, among others, is very wide and variable. There are different ways of extracting natural plant extracts, such as maceration, infusion, digestion, microwave-assisted extraction, percolation and steam distillation (Zhang et al., 2018). Maceration consists of crushing the leaves and can be done in a mortar and pestle (Ishii and Yokotsuka, 2014). Infusion is an extraction method that basically consists of pouring hot water on the leaves or part of the plant. Digestion is the decomposition of plant material with acids and high temperatures (Parr et al., 2001). Microwave-assisted extraction, as the name suggests, uses microwaves to heat the base material of the plant to obtain the extract (Kaufmann and Christen, 2002). The percolation method of solvent extraction consists of extracting the plant material by continuously flowing solvent through plant material to extract soluble components (Kaufmann and Christen, 2002; Singh, 2008), and steam distillation consists of applying steam directly to the plant material followed by condensation to obtain the extract (Cassel et al., 2009). In addition to the previously mentioned extraction techniques, advanced analytical methods such as High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) are widely utilized for the precise identification and quantification of bioactive compounds in plant-derived extracts.
Moussi et al. (2015) used the chromatography technique to separate the phenolic part of the Rhamnus alaternus L. extract, where due to interactions with the molecules of both the mobile part (solvent) and the stationary part (material filling the column), only the molecule or class of molecules of interest were separated. In the case of gas chromatography, separation depends on volatility and interaction with the stationary part (Bartle and Myers, 2002). The difference between liquid and gas is what carries the sample, which can be gas (helium, nitrogen or hydrogen) or liquid (Giddings, 1965). Moussi et al. (2015) employed High-Performance Liquid Chromatography (HPLC) to isolate phenolic compounds from Rhamnus alaternus L. extracts. In HPLC, separation is governed by differential interactions between analytes and two distinct phases: a liquid mobile phase (e.g., water, methanol, or acetonitrile) that transports the sample through the system, and a solid stationary phase (e.g., silica-based or polymer-packed columns) that selectively retains molecules based on polarity, size, or affinity (Snyder et al., 2011). For phenolic compounds, which are polar, non-volatile, and thermally labile molecules, HPLC is particularly advantageous, as it preserves their structural integrity while effectively resolving complex mixtures into individual components, such as flavonoids and phenolic acids, using gradient elution or isocratic modes (Moussi et al., 2015).
In contrast, Gas Chromatography (GC) utilizes a gaseous mobile phase (e.g., helium, nitrogen, or hydrogen) to transport volatilized analytes through a temperature-controlled column coated with a stationary phase (e.g., polysiloxane). Separation in GC depends primarily on volatility and secondarily on interactions with the stationary phase (Bartle and Myers, 2002). Compounds with lower boiling points elute faster, whereas polar or less volatile molecules often require derivatization, a chemical modification that enhances thermal stability and volatility, prior to analysis (Grob and Barry, 2004). While GC is ideal for volatile organic compounds (VOCs) such as terpenes and fatty acids, it is unsuitable for non-volatile phenolics, as these compounds would degrade under the high-temperature conditions of GC (Snyder et al., 2011). Thus, the choice between HPLC and GC hinges on the physicochemical properties of the analytes. HPLC excels in separating non-volatile, thermally sensitive molecules, including polyphenols and proteins, due to its gentle operating conditions and compatibility with polar solvents. Conversely, GC is reserved for volatile, thermally stable compounds such as hydrocarbons and essential oils, leveraging its ability to resolve low-molecular-weight species under elevated temperatures (Bartle and Myers, 2002; Grob and Barry, 2004).
A natural product extract is a concentrated preparation of active substances extracted from plants, vegetables, animals, bacteria and fungi derivatives. In several studies using natural extracts with antimicrobial activity, such as Pacheco-Cano et al. (2020), which used the crude extract of Brassica oleracea var. italica against Bacillus cereus, Listeria monocytogenes, P. aeruginosa, Salmonella typhimurium and Vibrio parahaemolyticus, and Fontana et al. (2022) witch evaluated the antimicrobial activity of the methanolic and hydroalcoholic extracts of Moringa oleifera against Erwinia amylovora. Compounds, on the other hand, consist of the material or chemical that has been separated from a mixture or source and obtained in its pure form, such as the isolation of a class of molecules or a specific molecule. Some studies have used compounds as Azevedo Dos Santos et al. (2020), who evaluated the peptide portion of Capsicum chinense fruit extract against F. oxysporum, F. solani, Colletotrichum lindemuthianum and C. gloeosporioides, Kim et al. (2018) who evaluated five antifungal molecules from the crude methanolic extract of Trevesia palmat a against Alternaria porri, B. cinerea, Colletotrichum coccodes, F. oxysporum, Magnaporthe oryzae and Phytophthora infestans showing promising results.
The literature contains articles describing the antimicrobial evaluation of natural extracts coated with nanoparticles, materials with dimensions in the nanometric range, usually between 1 and 100 nanometers (nm) (El-Naggar et al., 2024; Ndolomingo et al., 2020). Due to their very small size and high surface-to-volume ratio, nanoparticles exhibit distinct and unique properties Danish et al. (2022), with green synthesis for the production of silver nanoparticles based on the natural extract of Cassia fistula (L.), evaluated against Pseudomonas syringae, Fusarium oxysporum, Rhizoctonia solani and Sarocladium sp.
3.2 The search for evidence: microbiological evaluation of plant-based antimicrobials
To evaluate the biological potential of extracts, fractions and compounds from extracts or plant products, whether coated with nanoparticles, some techniques can be used, such as agar dilution, agar diffusion, micro and macro dilution in broth, bioautography, among others. When the method for antimicrobial evaluation is carried out on agar, techniques such as agar dilution or agar diffusion can be used. In literature, it is common to use different terms to describe the same methodological approach to assessing antifungal activity in filamentous fungi. Terms such as “poisoned food technique”(Pinto et al., 2022), “poisoned food medium assay”(Kim et al., 2019), “diffusion technique on PDA growth medium”(Vuerich et al., 2023), and “mycelial growth inhibition by an agar-dilution method”(Reyes et al., 2022), to describe methods in which the tested substance is incorporated into the agar before solidification. and then inoculating the hyphae-producing microorganisms, i.e., filamentous fungi on the agar plate, and the result is normally presented as the percentage of inhibition of the growth of the hyphae of the microorganism compared to a control without the sample (Perrucci et al., 1994; Balouiri et al., 2016).
Diffusion on agar, on the other hand, can be carried out in two main ways: disk or well on agar. In the case of agar well diffusion, as carried out by Bhatti et al. (2024), the microorganisms are inoculated onto the agar. Subsequently, a well is made in the agar to place the extracts or compounds (Magaldi et al., 2004). In the case of the paper disk, as performed by Klančnik et al. (2009), subsequently the agar plates are produced normally, sequentially the microorganism will be inoculated into the agar, and finally, paper discs containing the tested substance will be placed on the previously inoculated agar. In this way the tested samples will spread in part of the agar through diffusion (Magaldi et al., 2004). The test is interpreted by measuring the growth inhibition halo after the respective incubation of the microorganism used, thus making it possible to measure and account for the antimicrobial activity of the extracts and compounds (Devillers et al., 1989).
There are other methods such as: microdilution and macrodilution, which are characterized by serial dilution, and can be performed in tubes, in the case of macrodilution (Nikitin et al., 2023), or in wells in 96-well plates in the case of microdilution (Chalo et al., 2023). After diluting the sample, microorganisms are added at specific concentrations to determine the Minimum Inhibitory Concentration (MIC), defined as the concentration of the extract, or nanoparticle capable of compounds inhibiting the growth of the microorganism or eliminating it completely (Pa, 2002). The MIC can be interpreted by adding an oxidoreductive salt, such as rezasurin, which changes color from blue to pink when in contact with living metabolites (Sarker et al., 2007). Another way of determining the MIC is by reading the optical density in a spectrophotometer, resulting in the absorbance of each well of the microplate.
Such tests can branch out into other different testing possibilities, such as the evaluation of extracts and compounds from natural products together with other antimicrobials against microorganisms, with the aim of verifying whether there is synergism or antagonism, as well as the inhibition of biofilm formation, among others. Table 2 summarizes the evaluated plant species, their antimicrobial activities against phytopathogens, the techniques applied, and the main findings. The selected studies, published between 2020 and 2024, are open-access articles from peer-reviewed journals with an impact factor above 3. These studies focus on plant-derived bioactive compounds tested against bacterial and fungal phytopathogens. The articles were retrieved from the Scopus database using various keyword combinations, including “phytopathogen and antimicrobial and plant and extract,” “plant and extract and fraction and phytopathogens,” “plant and extract and molecule and phytopathogen,” “plant and extract and isolated and compounds and phytopathogen,” and “phytopathogen and nanoparticles and antimicrobial.”
Table 2
| Plant | Phytopathogens evaluated | Extract or compounds | Techniques | Results | Reference |
|---|---|---|---|---|---|
| Achillea millefolium L., Mentha piperita L., Salvia officinalis L., Equisetum arvense L., Urtica dioica L., Taraxacum officinale (L.) Weber ex F. H. Wigg., Elymus repens (L.) Gould, Hypericum perforatum L., Rosmarinus officinalis Spenn., Humulus lupulus L., Satureja hortensis L., Carum carvi L., Nigella sativa L., Thymus vulgaris L., Lavandula angustifolia Mill., Armoracia rusticana G. Gaertn., B. Mey. and Scherb., Allium sativum L., Syzygium aromaticum (L.) Merr. and Perry, Allium cepa L., Curcuma longa L., Polygonum bistorta L. and Polygonum aviculare | Colletotrichum coccodes, Phoma exigua, Fusarium sambucinum, Rhizoctonia solani, Alternaria tenuissima, Streptomyces scabiei, Pectobacterium carotovorum, Alternaria alternata, Alternaria solani, Fusarium oxysporum | Water extracts, water-glycol extract, subcritical carbon dioxide extracts | Agar-well diffusion and agar-disc diffusion method and macro-broth dilution method | Inhibition zones between 1 and 59 mm for diffusion and with minimum inhibitory concentrations (MIC) ranging from approximately 0.9 to 25 mg/mL | Steglińska et al. (2022) |
| 33 Brassica oleracea varieties | Xanthomonas campestris, Agrobacterium rhizogenes, Fusarium oxysporum, Gibberella zeae, Phytophthora infestans | Dichloromethane extract | Kirby–Bauer disk diffusion method and two-fold serial dilution method | The extract showed zones of inhibition of 15.90 and 28.60 mm and showed overall MICs in the range of 7.81 to 31.25 μg/mL | He et al. (2024) |
| Gnaphalium uliginosum L. | Clavibacter michiganensis Erwinia carotovora spp. carotovora, Alternaria solani, Rhizoctonia solani | Ethanolic extract | Agar diffusion and serial dilutions | MIC range of 2,500 to 78 μg/mL and inhibition halos of 24 to 13 mm | Davydova et al. (2024) |
| Hibiscus rosa-sinensis | Xanthomonas oryzae pv. oryzae | Cobalt oxide nanoparticles | Micro-dilution, Lesion length in rice plants after treatment and Biofilm inhibition | Biofilm inhibition up to 79,65% and inhibition zones ranging from 2,40 cm to 2.90 cm | Ogunyemi et al. (2023) |
| Centaurea calcitrapa | Agrobacterium tumefaciens, Erwinia amylovora, Pseudomonas syringae pv. Aptata, Pseudomonas syringae pv. syringa, Xanthomonas campestris pv. campestris, Xanthomonas arboricola pv. juglandis | Methanolic extract | Well diffusion test, Two-fold serial dilutions | Inhibition of 9.83 up to 30.5 mm, and MIC values ranging of 25–750 μg/mL | Dimkić et al. (2020) |
| Camellia sinensis | Fusarium equiseti | Zinc oxide nanoparticles | Inhibitory activity on agar | Growth Inhibition up to 84.8% | Subba et al. (2024) |
| Atriplex glaucum and Calendula officinalis | Fusarium oxysporum, Colletotrichum gloeosporioides and Cladosporium cladosporioides, | Methanolic extracts | Kirby–Bauer method (Disk diffusion) |
Inhibitions halos up to 9.32 mm | Lazcano-Ramírez et al. (2023) |
| Dunaliella salina | Pseudomonas syringae pv. tomato, Bacillus subtilis and Pectobacterium. carotovorum subsp. carotovorum | Chloroform: Methanol extract, Ethanol extract and Hexane extract | Agar Disc Diffusion Method, broth dilution method and in vivo treatment evaluation in tomatoes | Inhibitions zone up to 20.0 mm and MICs of 0.3 mg/mL | Ambrico et al. (2020) |
| Hedera helix | Saccharomyces cerevisiae and Diplodia corticola | Aqueous Extract | Growth inhibition and Yeast Viability with the percentage of colony-forming units | Growth Inhibition up to 70% and percentage of colony-forming units of 0% in certain conditions | Crisóstomo et al. (2024) |
| Erismadelphus exsul | Phytophthora infestans and Zymoseptoria tritici | Ethanolic extract and fractions | Microplate serial dilution and mycelial growth inhibition test in microplates | Growth Inhibition up to 100% | Essono Mintsa et al. (2022) |
| Moringa oleifera | Erwinia amylovora | Methanolic, hydroalcoholic and hydroalcoholic with maltodextrins | Microdilution method and analysis of disease symptoms in vivo | Mic of >2 to 1 mg/mL, 80% reduction in biofilm formation, and reducing the wilting area by up to 80% | Fontana et al. (2022) |
| Sambucus nigra | Diaporthe amygdali, Phytophthora megasperm and Verticillium dahliae | Ammoniacal aqueous extract | Agar dilution method and Ex Situ protection assays on excised stems | Growth Inhibition up to 100% with a effective concentration of 50% at 193.9 μg/mL, and full protection of the stem at the concentration of 1875 μg/mL | Sánchez-Hernández et al. (2023) |
| Artemisia annua L., Artemisia dracunculus L., Artemisia santonica L., Artemisia abrotanum L. and Artemisia scoparia Waldst. and Kit | Rathayibacter iranicus, Bacillus subtilis, Xanthomonas arboricola, Agrobacterium tumefacien, Alternaria solani, Fusarium graminearum and Rhizoctonia solani | Ethanol extracts | Two-fold serial dilution and serial dilution method | MIC ranging of 310 to >5,000 μg/mL | Nikitin et al. (2023) |
| Pavlova lutheri, Chaetoceros muelleri, Chlorella sp., Dunaliella tertiolecta, Haematococcus pluvialis, Isochrysis galbana, Nannochloropsis sp., Scenedesmus sp., Tetraselmis astigmatica, Tetraselmis chuii, Tetraselmis suecica, Limnothrix sp. and Spirulina sp. | Clavibacter michiganensis and Pseudomonas syringae | Hexane, dichloromethane and methanol fractions | Disc-diffusion method, spot-on-lawn method and serial dilutions | MIC of 500 μg/mL to 1 mg/mL and inhibition halos up to 18.67 mm | Alsenani et al. (2020) |
| Larrea nitida Cav. | Fusarium oxysporum, Fusarium verticillioides and Trichoderma harzianum | Nanodispersions produced from the methanolic extract | Agar diffusion | Growth inhibition up to 48% | Rocha et al. (2023) |
| Baccharis trinervis Pers., Baccharis prunifolia Steyerm, Baccharis zumbadorensis Badillo | Botrytis cinerea | Methanol and dichloromethane extracts | Broth Microdilution Method and poisoned food technique | MIC ranging of 125 to 250 μg/mL, lowest IC50 of 3.1 μg/mL, an growth inhibition up to 72,05% | Pinto et al. (2022) |
| Quercus ilex subsp. ballota (Desf.) Samp. | Fusarium circinatum, Cryphonectria parasitica, Phytophthora cinnamomic, | Aqueous ammonia extract | Agar dilution method and Protection Tests on Artificially Inoculated Excised Stems | EC90 values of 322, 295, and 75 μg/mL and achieving protection at of 782 μg/mL | Sánchez-Hernández et al. (2022) |
| Capsicum annuum, Capsicum baccatum | Alternaria sp., Aspergillus niger, Aspergillus dimorphicus, Fusarium oxysporum, Fusarium verticillioides, Penicillium citrinum and Rhizopus arrhizus | Aqueous extracts | Well diffusion method, broth liquid dilution | Inhibition ratio up to 18% | Sepúlveda et al. (2024) |
| Retama raetam | Stemphylium vesicarium | Six compounds of the plant extract | Spot-inoculation and Inhibition of the fungal growth | Growth inhibition up to 55% | Soriano et al. (2022) |
| Silybum marianum (L.) Gaertn. | Fusarium graminearum | Peptides of the plant extract | Hyphal growth inhibition assays in microplate | Three peptides almost completely inhibited the hyphal growth | Fernández et al. (2021) |
| Cassia fistula (L.) | Pseudomonas syringae, Fusarium oxysporum, Rhizoctonia solani, Sarocladium sp. | Silver nanoparticles | Microplate antibiofilm assay, growth inhibition | Inhibitory zones of 12.2 mm and biofilm and fungal inhibition up to 78% | Danish et al. (2022) |
| Ceropegia fusca, Argyranthemum broussonetii, Artemisia thuscula, Gymnosporia cassinoides, Cistus symphytifolius, Lavandula canariensis, Salvia canariensis, Apollonias barbujana subsp. barbujana, Laurus novocanariensis, Ruta pinnata, Ruta chalepensis, Datura innoxia, Datura stramonium, Nicotiana glauca, Salpichroa origanifolia and Withania aristata. | Alternaria alternata, Botrytis cinerea, and Fusarium oxysporum | Ethanolic plant extract | Mycelial growth inhibition by an agar-dilution method | Inhibition up to 72.22% | Reyes et al. (2022) |
| Olive pomace | Xylella fastidiosa subsp. Pauca, Pseudomonas syringae pv. tomato and Pectobacterium carotovorum subsp. carotovorum | Phenolic extract | Disk diffusion assay, time-kill contact assay and Broth dilution assay | MIC ranging from 1.6 to 0.4 mg/mL | Greco et al. (2024) |
| Alseis yucatanensis, Alvaradoa amorphoides, Annona primigenia, Bakeridesia notolophium, Bravaisia berlandieriana, Byrsonima bucidifolia, Calea jamaicensis, Cameraria latifolia, Chrysophyllum mexicanum, Coccoloba sp., Croton arboreus, Croton itzaeus, Croton sp., Cupania sp., Diospyros sp., Erythroxylum confusum, Erythroxylum rotundifolium, Erythroxylum sp., Eugenia sp., Euphorbia armourii, Guettarda combsii, Helicteres baruensis, Heteropterys laurifolia, Hybanthus yucatanensis, Ipomoea clavata, Karwinskia humboldtiana, Licaria sp., Macroscepis diademata, Malpighia glabra, Morella cerifera, Mosannona depressa, Parathesis cubana, Paullinia sp., Piper neesianum, Psychotria sp., Randia aculeata, Serjania caracasana, Simarouba glauca, Stemmadenia donnell-smithii, Turnera aromatica | Fusarium equiseti and Fusarium oxysporum | Aqueous Extracts, ethanolic Extracts and fractions | Broth microdilution | Lowest MIC of 1,000 μg/mL and growth inhibition up to 100% | Cruz-Cerino et al. (2020) |
| Sideroxylon obtusifolium and Annona acutiflora | Thielaviopsis ethacetica | Ethyl acetate and butanol extract | Hole diffusion test, Mycelial inhibition test and growth curve, Microdilution test | Inhibition halos up to 35.3 mm,and MIC of 25 to 6 mg/mL | Duarte et al. (2022) |
| Monodora kerstingii | Fusarium oxysporum | Crude extracts and fractions | Serial dilutions | MIC from >1,000 to 23 μg/mL | Fotso et al. (2020) |
| Ptaeroxylon obliquum (Thunb.) Radlk | Aspergillus niger, Aspergillus parasiticus, Colletotrichum gloeosporioides, Fusarium oxysporum, Penicillium digitatum, Penicillium expansum, Penicillium italicum, Penicillium janthinellum, Rhizoctonia solani | Acetone crude extracts, fractions, and isolated compounds | Serial microdilution assay | MIC from 1,250 to 32 μg/mL | Ramadwa et al. (2024) |
| Capsicum chinense Jacq., var. ‘Habanero Mustard’, ‘Habanero Pastel’, ‘Trinidad Moruga Scorpion Red’, ‘Trinidad Moruga Scorpion Choco’, ‘Carolina Reaper’, ‘White Naga’, ‘Naga Morich Chocolate’ | Botrytis cinerea, Guignardia bidwellii, Plasmopara viticola | Oleoresin | Diffusion technique, radial growth inhibition and Inhibition sporulation on leaf discs | Inhibition activity ranging from 0.001 to 12.5 mg/mL, with the complete inhibition in certain concentration | Vuerich et al. (2023) |
| Cestrum nocturnum | Fusarium kuroshium, Fusarium solani | Methanolic Crude Extract and fractions | Mycelial growth inhibition by microplate | Some fractions achieve 100% of inhibition | Valencia-Mejía et al. (2022) |
| Zuccagnia punctata | Monilinia fructicola | Ethanolic extract | Broth microdilution technique, ex vivo Antifungal Assay on Wounded Fruits and Cell Viability Assay with MTT | MIC from 250 to 62.5 μg/mL, and the treatments reduced the brown rot sporulation index compared to the control | Di Liberto et al. (2023) |
Main techniques and methods for the antimicrobial evaluation of natural products against phytopathogens.
According to Table 2, and according to database limitations, the most studied type of microorganism was fungi of the genus Fusarium sp. (16,8%). The most evaluated natural products regarding their antimicrobial activity were aqueous extracts (18.42%) and ethanolic extracts (15.79%).
Due to the lack of articles that establish clear benchmarks for promising antimicrobial activity against phytopathogens, this study proposes tentative threshold values to guide future evaluations. Values higher than 20.90 mm of inhibition halo are suggested as a indicative of promising activity. MIC values for bacteria of less than 2.5 mg/mL for the crude extract, 0.6 mg/mL for the fractions and 64 μg/mL for the compounds are proposed as benchmarks. In the case of the percentage inhibition of the growth of filamentous fungi, values of less than 52% inhibition may be considered promising.
Figure 2 illustrates the most commonly employed techniques and methods in the literature for assessing the activity of natural products against phytopathogenic microorganisms, along with their respective usage percentages.
Figure 2
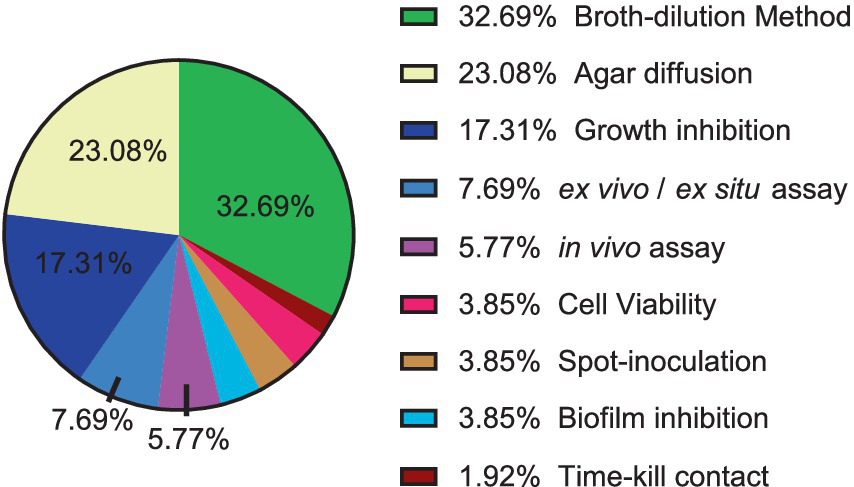
Distribution of techniques and methodologies for antibacterial and antifungal evaluation of natural products against phytopathogens.
The most used techniques in the articles for antimicrobial evaluation were dilution in broth and agar diffusion. The widespread of these techniques is due to their applicability, such as their use for solid or with difficult solubility substances, which allows the analysis of the growth of the microorganism in direct contact with the substance or compound, but there is less standardization and consequently variation between the results, in addition to the fact that the results can be difficult to interpret due to the interaction of the substance or compound with the agar. In the case of microdilution and macro-dilution in broth, the advantage is that it is well suited to samples that do not diffuse well in agar, such as for certain antibiotics (Satlin, 2019).
4 Recommended strategies, challenges and future directions
Effective phytopathogen control requires a multi-pronged strategy integrating phytochemical innovation, nanotechnology, host genetics, and ecological stewardship. Prioritizing standardized protocols, field validation, and socioeconomic feasibility will bridge the lab-to-field gap, advancing One Health-aligned agriculture (Figure 3). Plant-derived antimicrobials, including phytoalexins and phytoanticipins, represent a cornerstone of sustainable phytopathogen control. These compounds inhibit pathogens through mechanisms such as membrane disruption, enzyme inhibition, and interference with microbial signaling (González-Lamothe et al., 2009; Košćak et al., 2023). For instance, saponins like avenacin in oats and α-tomatine in tomatoes form complexes with fungal sterols, destabilizing membranes and suppressing infections (González-Lamothe et al., 2009). In addition to antagonistic bacteria and fungi, plant-derived bioactive compounds have emerged as promising biocontrol agents, demonstrating considerable efficacy in suppressing plant pathogen proliferation. These phytochemicals contribute to pathogen inhibition through direct antimicrobial activity, interference with virulence mechanisms, and the enhancement of host plant defense responses, thereby complementing microbial biocontrol strategies (da Silva et al., 2016; Najdabbasi et al., 2020). Moreover, the use of nanotechnology in plant disease diagnostics has transformational potential, enabling the creation of sophisticated instruments for the rapid and early identification of plant infections. Nanomaterials (1–100 nm) are well suited for this application owing to their superior surface-to-volume ratio and distinctive chemical, photonic, and electrical characteristics that fundamentally differ from those of bulk materials; the ability to execute precise molecular alterations, together with the unique optical properties of nanomaterials, enables ultrasensitive and effective pathogen detection systems (Ray et al., 2023). Genetic engineering and CRISPR-Cas9 genome editing provide precise, targeted alterations of plant genomes to improve resistance to phytopathogens. This method facilitates the insertion or deletion of certain genes, including pathogen-responsive R genes or susceptibility factors, while minimizing off-target effects, so expediting the creation of disease-resistant cultivars. The transfer of resistance-conferring genes across species expands the genetic repertoire beyond taxonomic boundaries, while robust integration of transgenes guarantees heritable resistance in vegetatively propagated crops, surpassing the constraints of traditional breeding methods. These solutions jointly enhance crop resilience by customized genetic modifications, reducing dependence on chemical interventions (Dong and Ronald, 2019; Gohil et al., 2024). Finally, the paradigm of plant disease management should shift from a narrow focus on crop yield to a comprehensive approach that integrates ecological sustainability, social acceptability, and economic feasibility. To achieve this, priority should be given to developing environmentally sustainable biocontrol agents, applied synergistically with other control measures under an integrated disease management system (He et al., 2021).
Figure 3
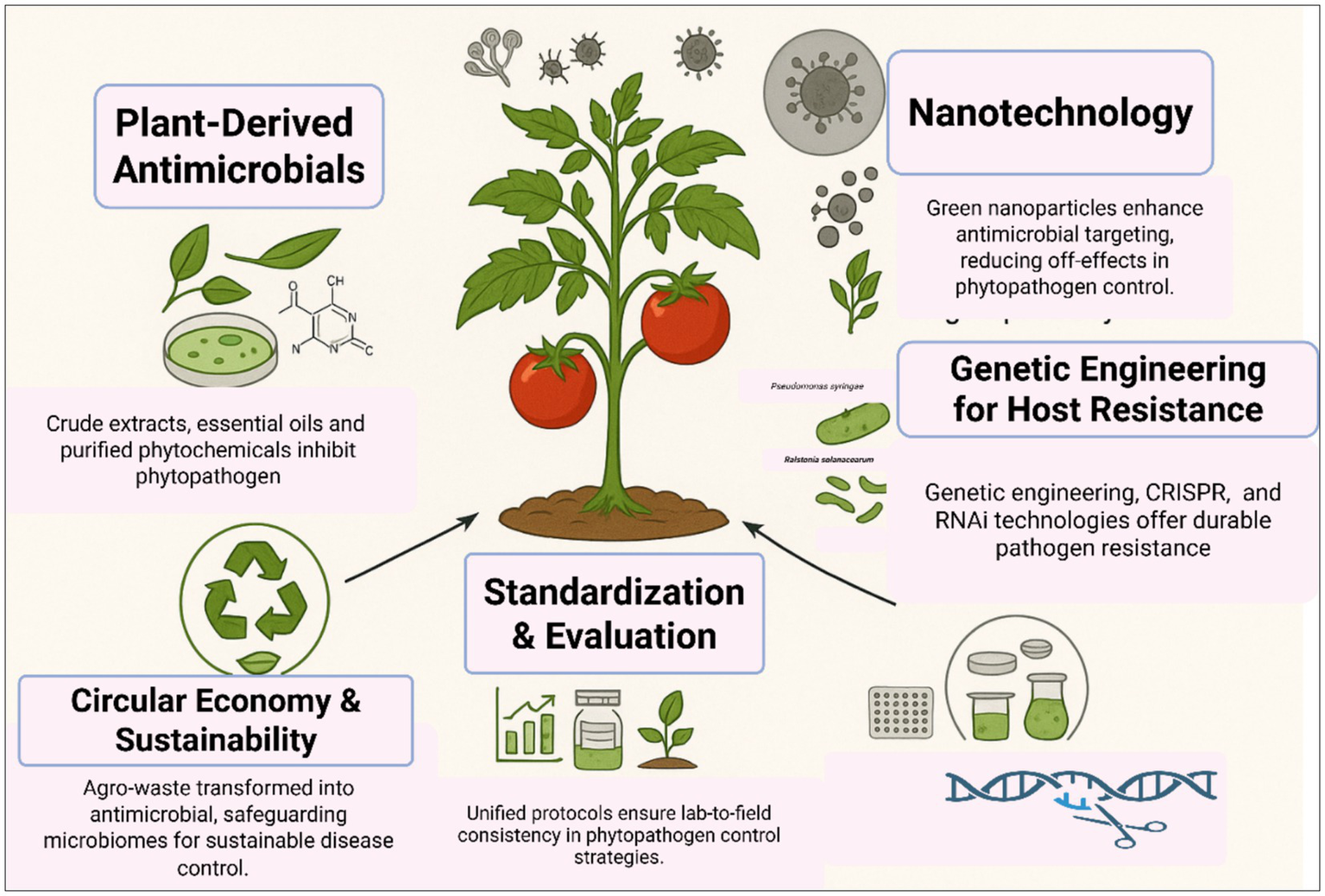
Multifaceted interventions for effective control of plant pathogens.
The control of plant viral diseases traditionally relies on chemical pesticides targeting insect vectors, cultural practices, and genetic resistance. However, natural control strategies offer an eco-friendly, sustainable alternative that minimizes environmental risks and enhances crop resilience (Figure 4). The central concept of natural control is connected to four main strategies: host resistance, biological control, vector management, and plant-derived antiviral compounds. Host resistance includes RNA interference and systemic acquired resistance. Vector management relies on natural predators and entomopathogenic fungi to limit insect-mediated virus transmission. Plant-derived antiviral compounds include flavonoids, terpenoids, and plant extracts such as neem and garlic, which interfere with viral replication and vector feeding behavior (Hudson, 2018; Jones, 2006).
Figure 4
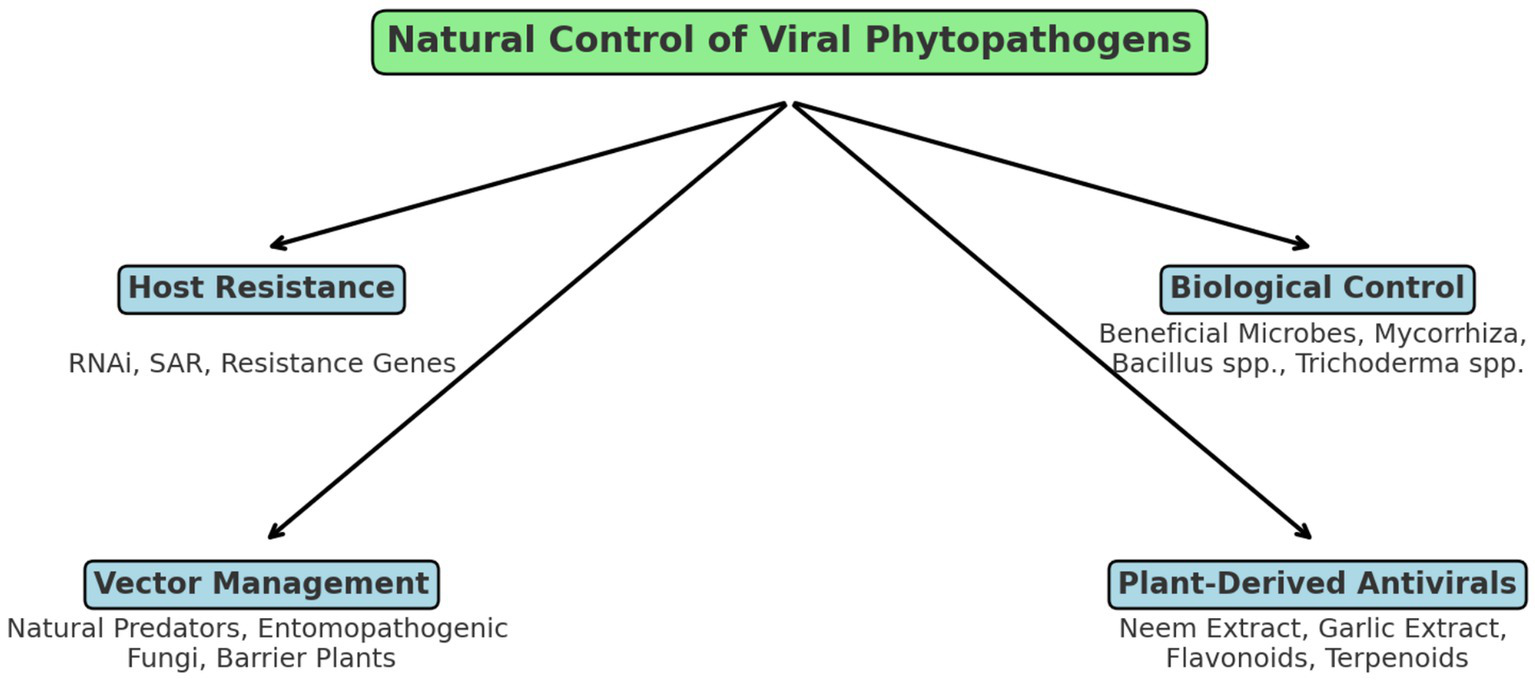
Schematic representation of natural control mechanisms against plant viral pathogens.
The different methods used in the literature to evaluate the antimicrobial activity of natural products against phytopathogens make it difficult to compare results. An article may use the agar disk diffusion method and have a 20–28 mm growth inhibition halo as Mohamed et al. (2023) and another article a MIC result of 19.5 to 117 μg/mL as Makhubu et al. (2023). These two results cannot be compared due to the use of different principles, making it necessary to develop standard or common techniques, thus being able to compare extracts or compounds or nanoparticles in an easier, safer and standardized way.
Furthermore, there are few articles that address in vivo tests such as infection of the microorganism in the plant or fruit itself. Additionally, information on the epidemiology of diseases and global economic losses is scarce and what is available is outdated. There are few articles that explore the formation of biofilms of phytopathogens and the use of extracts and compounds with antibiofilm properties. This assessment is extremely important, since most microorganisms available in the environment are in the form of biofilms, distancing themselves from the real condition of infection (Ramey et al., 2004).
Key research gaps include elucidating the mechanisms of action of bioactive compounds, which could facilitate the design of more targeted and specific molecules. Additionally, evaluating these natural products directly on plants under varying soil types and climatic conditions, while considering their interactions with the soil microbiome, remains largely unexplored. There is also a pressing need for studies integrating antimicrobial assessments with emerging agricultural technologies, as well as comprehensive investigations into the toxicity and long-term environmental impact of these natural extracts on a broader scale.
5 Conclusion
The current review demonstrated that various natural products exhibit significant antimicrobial activity against phytopathogens, yielding promising and encouraging results. According to the literature, the most extensively evaluated natural products in terms of antiphytopathogenic activity were aqueous extracts and ethanolic extracts. The most frequently studied phytopathogens were Fusarium spp., while the predominant methodologies for antimicrobial assessment were dilution in broth and agar diffusion. Moreover, this review provides a comprehensive synthesis of existing studies to establish benchmarks for evaluating plant-derived antimicrobials against phytopathogens, offering practical criteria for prioritizing natural products in sustainable agriculture. Investigating the antimicrobial potential of natural extracts, fractions, and compounds against phytopathogens represents a promising avenue for their future application. A deeper understanding of their mechanisms of action may provide valuable insights into effective strategies for crop protection. Given the devastating impact of phytopathogens on agricultural productivity, advancing research in this field is crucial for the development of sustainable and efficient plant protection solutions.
Statements
Author contributions
GC: Writing – original draft, Writing – review & editing. NS: Conceptualization, Methodology, Project administration, Writing – review & editing. BA: Funding acquisition, Writing – review & editing. EA: Writing – review & editing. CM: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025). National Council for Scientific and Technological Development (CNPq) n° 307974/2019–7, 311568/2023–8 (Grants) and 146353/2024–2 (Scholarship).
Acknowledgments
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025). National Council for Scientific and Technological Development (CNPq) n° 307974/2019–7, 311568/2023-8 (Grants) and 146353/2024-2 (Scholarship).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdelaziz A. M. El-Wakil D. A. Attia M. S. Ali O. M. AbdElgawad H. Hashem A. H. (2022). Inhibition of Aspergillus flavus growth and aflatoxin production in Zea mays L. using endophytic Aspergillus fumigatus. J. Fungi8:482. doi: 10.3390/jof8050482
2
Abdullahi A. Khairulmazmi A. Yasmeen S. Ismail I. S. Norhayu A. Sulaiman M. R. et al . (2020). Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem.13, 8012–8025. doi: 10.1016/j.arabjc.2020.09.031
3
Ajayi-Oyetunde O. Bradley C. (2018). Rhizoctonia solani: taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol.67, 3–17. doi: 10.1111/ppa.12733
4
Alsenani F. Tupally K. R. Chua E. T. Eltanahy E. Alsufyani H. Parekh H. S. et al . (2020). Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J.28, 1834–1841. doi: 10.1016/j.jsps.2020.11.010
5
Álvarez B. Biosca E. G. López M. M. (2010). “On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen” in Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microbiol. Biotechnol, vol. 1, 267–279.
6
Amaike S. Keller N. P. (2011). Aspergillus flavus. Annu. Rev. Phytopathol.49, 107–133. doi: 10.1146/annurev-phyto-072910-095221
7
Ambrico A. Trupo M. Magarelli R. Balducchi R. Ferraro A. Hristoforou E. et al . (2020). Effectiveness of Dunaliella salina extracts against Bacillus subtilis and bacterial plant pathogens. Pathogens9:613. doi: 10.3390/pathogens9080613
8
Azcarate M. P. Patriarca A. Terminiello L. Pinto V. F. (2008). Alternaria toxins in wheat during the 2004 to 2005 Argentinean harvest. J. Food Prot.71, 1262–1265. doi: 10.4315/0362-028X-71.6.1262
9
Azevedo Dos Santos L. Taveira G. B. Silva M. S. Silva Gebara R. Silva Pereira L. Perales J. et al . (2020). Antimicrobial peptides from Capsicum chinense fruits: agronomic alternatives against phytopathogenic fungi. Biosci. Rep.40:BSR20200950. doi: 10.1042/BSR20200950
10
Bae C. Han S. W. Song Y.-R. Kim B.-Y. Lee H.-J. Lee J.-M. et al . (2015). Infection processes of xylem-colonizing pathogenic bacteria: possible explanations for the scarcity of qualitative disease resistance genes against them in crops. Theor. Appl. Genet.128, 1219–1229. doi: 10.1007/s00122-015-2521-1
11
Bai A. T. Ruth C. Gopal K. Arunodhayam K. Priya B. T. Ramakrishna M. (2018). Survey and identification of Fusarium wilt disease in chilli (Capsicum annuum L.). Int. J. Curr. Microbiol.7, 1073–1078. doi: 10.20546/ijcmas.2018.706.127
12
Baker K. F. (1970). Types of Rhizoctonia diseases and their occurrence. Rhizoctonia solani, 125–148. doi: 10.1525/9780520318243-011
13
Balouiri M. Sadiki M. Ibnsouda S. K. (2016). Methods for in vitro evaluating antimicrobial activity: a review. J. Pharmaceutical Analysis6, 71–79. doi: 10.1016/j.jpha.2015.11.005
14
Bartle K. D. Myers P. (2002). History of gas chromatography. Trends Anal. Chem.21, 547–557. doi: 10.1016/S0165-9936(02)00806-3
15
Bashan Y. De-Bashan L. E. (2002). Protection of tomato seedlings against infection by Pseudomonas syringae pv. Tomato by using the plant growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol.68, 2637–2643. doi: 10.1128/AEM.68.6.2637-2643.2002
16
Baulcombe D. (2004). RNA silencing in plants. Nature431, 356–363. doi: 10.1038/nature02874
17
Baverstock J. Roy H. E. Pell J. K. (2010). Entomopathogenic fungi and insect behaviour: from unsuspecting hosts to targeted vectors. BioControl55, 89–102. doi: 10.1007/s10526-009-9238-5
18
Behlau F. (2021). An overview of citrus canker in Brazil. Trop. Plant Pathol.46, 1–12. doi: 10.1007/s40858-020-00377-2
19
Bhatti Q. A. Fakhar-e-Alam M. Adnan M. Atif M. Shah W. A. Aseer M. et al . (2024). Inshights on antifungal potential of Bryum argenteum in association with therapeutical clay smectite and silver nanoparticles. J. King Saud Univ.36:103225. doi: 10.1016/j.jksus.2024.103225
20
Bika R. Baysal-Gurel F. Jennings C. (2021). Botrytis cinerea management in ornamental production: a continuous battle. Can. J. Plant Pathol.43, 345–365. doi: 10.1080/07060661.2020.1807409
21
Bitwell C. Indra S. S. Luke C. Kakoma M. K. (2023). A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr.19:e01585. doi: 10.1016/j.sciaf.2023.e01585
22
Boddy L. (2016). “Pathogens of autotrophs” in The fungi (Third Edition). eds. WatkinsonS. C.BoddyL.MoneyN. P. (Academic Press), 245–292.
23
Bosque-Pérez N. A. (2000). Eight decades of maize streak virus research. Virus Res.71, 107–121. doi: 10.1016/S0168-1702(00)00192-1
24
Bruening G. Kirkpatrick B. Esser T. Webster R. (2014). Managing newly established pests: cooperative efforts contained spread of Pierce's disease and found genetic resistance. Calif. Agric.68, 134–141. doi: 10.3733/ca.v068n04p134
25
Brunings A. M. Gabriel D. W. (2003). Xanthomonas citri: breaking the surface. Mol. Plant Pathol.4, 141–157. doi: 10.1046/j.1364-3703.2003.00163.x
26
Caglayan K. Tunc B. Akkan R. Taş V. Roumi V. (2025). Occurrence of blueberry leaf mottle virus (Nepovirus myrtilli) in Türkiye. J. Plant Dis. Prot.132:79. doi: 10.1007/s41348-025-01071-8
27
Cannon P. F. Damm U. Johnston P. R. Weir B. S. (2012). Colletotrichum - current status and future directions. Stud. Mycol.73, 181–213. doi: 10.3114/sim0014
28
Cassel E. Vargas R. Martinez N. Lorenzo D. Dellacassa E. (2009). Steam distillation modeling for essential oil extraction process. Ind. Crop. Prod.29, 171–176. doi: 10.1016/j.indcrop.2008.04.017
29
Chalo D. M. Franke K. Nchiozem-Ngnitedem V. A. Kakudidi E. Origa-Oryem H. Namukobe J. et al . (2023). Prenylated isoflavanones with antimicrobial potential from the root bark of Dalbergia melanoxylon. Meta13:678. doi: 10.3390/metabo13060678
30
Chapagain B. P. Wiesman Z. Tsror L. (2007). In vitro study of the antifungal activity of saponin-rich extracts against prevalent phytopathogenic fungi. Ind. Crop. Prod.26, 109–115. doi: 10.1016/j.indcrop.2007.02.005
31
Chin Y.-W. Balunas M. J. Chai H. B. Kinghorn A. D. (2006). Drug discovery from natural sources. AAPS J.8, E239–E253. doi: 10.1007/BF02854894
32
Coll N. S. Valls M. (2013). Current knowledge on the Ralstonia solanacearum type III secretion system. Microb. Biotechnol.6, 614–620. doi: 10.1111/1751-7915.12056
33
Crisóstomo C. Simões L. Barros L. Finimundy T. C. Cunha A. Oliveira R. (2024). Cell wall-mediated antifungal activity of the aqueous extract of Hedera helix L. leaves against Diplodia corticola. Antibiotics13:1116. doi: 10.3390/antibiotics13121116
34
Crous P. W. Hawksworth D. L. Wingfield M. J. (2015). Identifying and naming plant-pathogenic fungi: past, present, and future. Annu. Rev. Phytopathol.53, 247–267. doi: 10.1146/annurev-phyto-080614-120245
35
Cruz-Cerino P. Cristóbal-Alejo J. Ruiz-Carrera V. Carnevali G. Vera-Ku M. Martín J. et al . (2020). Extracts from six native plants of the Yucatán peninsula hinder mycelial growth of Fusarium equiseti and F. Oxysporum, pathogens of Capsicum chinense. Pathogens9:827. doi: 10.3390/pathogens9100827
36
da Silva C. M. A. da Silva Costa B. M. da Silva A. G. de Souza E. B. dos Santos Correia M. T. de Menezes L. V. L. (2016). Antimicrobial activity of several Brazilian medicinal plants against phytopathogenic bacteria. Afr. J. Microbiol. Res.10, 578–583. doi: 10.5897/AJMR2014.6999
37
Danish M. Shahid M. Ahamad L. Raees K. Atef Hatamleh A. Al-Dosary M. A. et al . (2022). Nano-pesticidal potential of Cassia fistula (L.) leaf synthesized silver nanoparticles (ag@ Cf L-NPs): deciphering the phytopathogenic inhibition and growth augmentation in Solanum lycopersicum (L.). Front. Microbiol.13:985852. doi: 10.3389/fmicb.2022.985852
38
Davis M. J. Gillaspie A. G. Jr. Vidaver A. K. Harris R. W. (1984). Clavibacter: a new genus containing some phytopathogenic coryneform bacteria, including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and bermudagrass stunting disease. Int. J. Syst. Evol. Microbiol.34, 107–117. doi: 10.1099/00207713-34-2-107
39
Davydova L. Menshova A. Shumatbaev G. Babaev V. Nikitin E. (2024). Phytochemical study of ethanol extract of Gnaphalium uliginosum L. and evaluation of its antimicrobial activity. Antibiotics13:785. doi: 10.3390/antibiotics13080785
40
Devi P. I. Manjula M. Bhavani R. (2022). Agrochemicals, environment, and human health. Annu. Rev. Environ. Resour.47, 399–421. doi: 10.1146/annurev-environ-120920-111015
41
Devillers J. Steiman R. Seigle-Murandi F. (1989). The usefulness of the agar-well diffusion method for assessing chemical toxicity to bacteria and fungi. Chemosphere19, 1693–1700. doi: 10.1016/0045-6535(89)90512-2
42
Dhananjayan V. Jayanthi P. Jayakumar S. Ravichandran B. (2020). “Agrochemicals impact on ecosystem and bio-monitoring” in Resources use efficiency in agriculture, 349–388.
43
Di Liberto M. G. Stegmayer M. I. Fernández L. N. Quiroga A. D. Svetaz L. A. Derita M. G. (2023). Control of brown rot produced by Monilinia fructicola in peaches using a full-spectrum extract of Zuccagnia punctata Cav. Horticulturae9:1141. doi: 10.3390/horticulturae9101141
44
Diener U. L. Cole R. J. Sanders T. H. Payne G. A. Lee L. S. Klich M. A. (1987). Epidemiology of aflatoxin formation by aspergillus flavus. Annu. Rev. Phytopathol.25, 249–270. doi: 10.1146/annurev.py.25.090187.001341
45
Dijkstra J. Khan J. A. (2024). “Plant virus transmission: fungi, nematodes, and seeds” in Handbook of plant virology (Boca Raton, Florida, USA: CRC Press), 127–136.
46
Dimkić I. Petrović M. Gavrilović M. Gašić U. Ristivojević P. Stanković S. et al . (2020). New perspectives of purple starthistle (Centaurea calcitrapa) leaf extracts: phytochemical analysis, cytotoxicity and antimicrobial activity. AMB Express10, 183–121. doi: 10.1186/s13568-020-01120-5
47
Dita M. Barquero M. Heck D. Mizubuti E. S. Staver C. P. (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci.9:1468. doi: 10.3389/fpls.2018.01468
48
Dong O. X. Ronald P. C. (2019). Genetic engineering for disease resistance in plants: recent progress and future perspectives. Plant Physiol.180, 26–38. doi: 10.1104/pp.18.01224
49
Douglas A. E. (2018). Strategies for enhanced crop resistance to insect pests. Annu. Rev. Plant Biol.69, 637–660. doi: 10.1146/annurev-arplant-042817-040248
50
Duarte J. A. Fiaux S. B. Barbosa E. Toledo P. F. Silva A. C. Oliveira E. E. et al . (2022). Antifungal potential and biosafety of native plants from the Brazilian Restinga ecosystem. Cleaner Engineering and Technology8:100493. doi: 10.1016/j.clet.2022.100493
51
Durrant W. E. Dong X. (2004). Systemic acquired resistance. Annu. Rev. Phytopathol.42, 185–209. doi: 10.1146/annurev.phyto.42.040803.140421
52
Dye D. (1968). A taxonomic study of the genus Erwinia. Part II. The'carotovora'group. N. Z. J. Sci.12, 81–97.
53
El-Naggar N. E. Shiha A. M. Mahrous H. Mohammed A. B. A. (2024). A sustainable green-731 approach for biofabrication of chitosan nanoparticles, optimization, characterization, its 732 in review 30 this is a provisional file, not the final typeset article antifungal activity against phytopathogenic Fusarium culmorum and antitumor activity. Sci. 733 Rep.14:11336. doi: 10.1038/s41598-024-59702-3
54
Elshafie H. S. Caputo L. De Martino L. Sakr S. H. De Feo V. Camele I. (2021). Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) leathery exocarp extract. Plan. Theory10:153. doi: 10.3390/plants10010153
55
Eskola M. Kos G. Elliott C. T. Hajšlová J. Mayar S. Krska R. (2020). Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr.60, 2773–2789. doi: 10.1080/10408398.2019.1658570
56
Essono Mintsa M. Otogo N’nang E. Choque É. Siah A. Jacquin J. Muchembled J. et al . (2022). Combined LC-MS/MS and molecular networking approach reveals antioxidant and antimicrobial compounds from Erismadelphus exsul bark. Plan. Theory11:1505. doi: 10.3390/plants11111505
57
Fernández A. Colombo M. L. Curto L. M. Gómez G. E. Delfino J. M. Guzmán F. et al . (2021). Peptides derived from the α-core and γ-core regions of a putative Silybum marianum flower defensin show antifungal activity against Fusarium graminearum. Front. Microbiol.12:632008. doi: 10.3389/fmicb.2021.632008
58
Fontana R. Macchi G. Caproni A. Sicurella M. Buratto M. Salvatori F. et al . (2022). Control of Erwinia amylovora growth by Moringa oleifera leaf extracts: in vitro and in planta effects. Plan. Theory11:957. doi: 10.3390/plants11070957
59
Fotso G. W. Ngameni B. Storr T. E. Ngadjui B. T. Mafu S. Stephenson G. R. (2020). Synthesis of novel stilbene–coumarin derivatives and antifungal screening of Monotes kerstingii-specialized metabolites against Fusarium oxysporum. Antibiotics9:537. doi: 10.3390/antibiotics9090537
60
Fundecitrus . (2019). Citrus canker. Available online at: https://www.fundecitrus.com.br/levantamentos/cancro-citrico (Accessed July 6, 2024).
61
Garcia-Ruiz H. (2018). Susceptibility genes to plant viruses. Viruses10:484. doi: 10.3390/v10090484
62
Ge T. Ekbataniamiri F. Johnson S. B. Larkin R. P. Hao J. (2021). Interaction between Dickeya dianthicola and Pectobacterium parmentieri in potato infection under field conditions. Microorganisms9:316. doi: 10.3390/microorganisms9020316
63
Giachero M. L. Declerck S. Marquez N. (2022). Phytophthora root rot: importance of the disease, current and novel methods of control. Agronomy12:610. doi: 10.3390/agronomy12030610
64
Giddings J. C. (1965). Comparison of theoretical limit of separating speed in gas and liquid chromatography. Anal. Chem.37, 60–63. doi: 10.1021/ac60220a012
65
Gohil S. Kumari A. Prakash A. Shah N. Bhutani S. Singh M. (2024). “CRISPER-based industrial crop improvements” in Industrial crop plants (Singapore: Springer Nature Singapore), 123–162.
66
Gonzalez E. Sutton T. B. Correll J. C. (2006). Clarification of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology96, 982–992. doi: 10.1094/PHYTO-96-0982
67
González-Lamothe R. Mitchell G. Gattuso M. Diarra M. S. Malouin F. Bouarab K. (2009). Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci.10, 3400–3419. doi: 10.3390/ijms10083400
68
Gordon T. R. (2017). Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol.55, 23–39. doi: 10.1146/annurev-phyto-080615-095919
69
Granke L. L. Quesada-Ocampo L. Lamour K. Hausbeck M. K. (2012). Advances in research on Phytophthora capsici on vegetable crops in The United States. Plant Dis.96, 1588–1600. doi: 10.1094/PDIS-02-12-0211-FE
70
Greco M. Fuertes-Rabanal M. Frey C. Del Grosso C. Coculo D. Moretti P. et al . (2024). Phenolic compounds-enriched extract recovered from two-phase olive pomace serves as plant immunostimulants and broad-spectrum antimicrobials against phytopathogens including Xylella fastidiosa. Plant Stress14:100655. doi: 10.1016/j.stress.2024.100655
71
Grob R. L. Barry E. F. (Eds.) (2004). Modern practice of gas chromatography. Hoboken, New Jersey, EU: John Wiley & Sons.
72
Guerber J. C. Correll J. C. (2001). Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia93, 216–229. doi: 10.1080/00275514.2001.12063151
73
Guldan G. S. (2020). Undernutrition and overnutrition: the challenging double burden of malnutrition. Good Health and Well-Being747–759. doi: 10.1007/978-3-319-95681-7_50
74
Hahn M. (2014). The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol.7, 133–141. doi: 10.1007/s12154-014-0113-1
75
Haq I. U. Rahim K. Yahya G. Ijaz B. Maryam S. Paker N. P. (2024). Eco-smart biocontrol strategies utilizing potent microbes for sustainable management of phytopathogenic diseases. Biotechnology Reports44:e00859. doi: 10.1016/j.btre.2024.e00859
76
Hardham A. R. (2005). Phytophthora cinnamomi. Mol. Plant Pathol.6, 589–604. doi: 10.1111/j.1364-3703.2005.00308.x
77
Hardham A. R. (2007). Cell biology of plant–oomycete interactions. Cell. Microbiol.9, 31–39. doi: 10.1111/j.1462-5822.2006.00833.x
78
Hartman R. (1974). Dasheen mosaic virus and other phytopathogens eliminated from caladium, taro, and cocoyam by culture of shoot tips. Phytopathology64, 237–240. doi: 10.1094/Phyto-64-237
79
He D. C. He M. H. Amalin D. M. Liu W. Alvindia D. G. Zhan J. (2021). Biological control of plant diseases: an evolutionary and eco-economic consideration. Pathogens10:1311. doi: 10.3390/pathogens10101311
80
He L. Jiang H. Li Y. Zhang X. Sun W. Liu C. et al . (2024). Sulforaphane-enriched extracts from broccoli exhibit antimicrobial activity against plant pathogens, promising a natural antimicrobial agent for crop protection. Biomol. Ther.14:352. doi: 10.3390/biom14030352
81
Hernández-Díaz J. A. Garza-García J. J. Zamudio-Ojeda A. León-Morales J. M. López-Velázquez J. C. García-Morales S. (2021). Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric.101, 1270–1287. doi: 10.1002/jsfa.10767
82
Hernández-Lauzardo A. N. del Velázquez- Valle M. G. Guerra-Sanchez M. G. (2011). Current status of action mode and effect of chitosan against phytopathogens fungi. Afr. J. Microbiol. Res.5, 4243–4247. doi: 10.5897/AJMR11.104
83
Hof H. (2020). The medical relevance of Fusarium spp. J. Fungi (Basel)6:117. doi: 10.3390/jof6030117
84
Hooks C. R. Fereres A. (2006). Protecting crops from non-persistently aphid-transmitted viruses: a review on the use of barrier plants as a management tool. Virus Res.120, 1–16. doi: 10.1016/j.virusres.2006.02.006
85
Hudson J. B. (2018). Antiviral compounds from plants. Boca Raton, Florida, USA: CRC press.
86
Ishii S. Yokotsuka T. (2014). Maceration of plant tissues by pectintrans-eliminase. Agric. Biol. Chem.35, 1157–1159. doi: 10.1080/00021369.1971.10860051
87
Jallow A. Xie H. Tang X. Qi Z. Li P. (2021). Worldwide aflatoxin contamination of agricultural products and foods: from occurrence to control. Compr. Rev. Food Sci. Food Saf.20, 2332–2381. doi: 10.1111/1541-4337.12734
88
James A. Kryovrysanaki N. Andronis C. Pappi P. G. Kalantidis K. Katsarou K. (2024). Identification and characterisation of zucchini yellow fleck virus and a novel Nepovirus from next-generation sequencing of mixed virus infections in cucumbers (Cucumis sativus) from Crete. Ann. Appl. Biol.186, 248–261. doi: 10.1111/aab.12962
89
Jayaprakash A. Thanmalagan R. R. Roy A. Arunachalam A. Lakshmi P. (2019). Strategies to understand aspergillus flavus resistance mechanism in Arachis hypogaea L. Curr. Plant Biol.20:100123. doi: 10.1016/j.cpb.2019.100123
90
Jones R. A. (2006). Control of plant virus diseases. Adv. Vir. Res.67, 205–244. doi: 10.1016/S0065-3527(06)67006-1
91
Jones R. A. Naidu R. A. (2019). Global dimensions of plant virus diseases: current status and future perspectives. Annu. Rev. Virol.6, 387–409. doi: 10.1146/annurev-virology-092818-015606
92
Jung T. Colquhoun I. Hardy G. S. J. (2013). New insights into the survival strategy of the invasive soilborne pathogen P hytophthora cinnamomi in different natural ecosystems in W estern a ustralia. For. Pathol.43, 266–288. doi: 10.1111/efp.12025
93
Kamle M. Borah R. Bora H. Jaiswal A. K. Singh R. K. Kumar P. (2020). Systemic acquired resistance (SAR) and induced systemic resistance (ISR): role and mechanism of action against phytopathogens. Fungal Biol. Biotech.457–470. doi: 10.1007/978-3-030-41870-0_20
94
Kamoun S. Furzer O. Jones J. D. Judelson H. S. Ali G. S. Dalio R. J. et al . (2015). The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol.16, 413–434. doi: 10.1111/mpp.12190
95
Kaufmann B. Christen P. (2002). Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal.13, 105–113. doi: 10.1002/pca.631
96
Kim B. Han J. W. Thi Ngo M. Le Dang Q. Kim J.-C. Kim H. et al . (2018). Identification of novel compounds, oleanane-and ursane-type triterpene glycosides, from Trevesia palmata: their biocontrol activity against phytopathogenic fungi. Sci. Rep.8:14522. doi: 10.1038/s41598-018-32956-4
97
Kim D. S. Kwack Y. Lee J. H. Chun C. (2019). Antimicrobial activity of various parts of tomato plants varied with different solvent extracts. Plant Pathol. J.35, 149–155. doi: 10.5423/PPJ.OA.07.2018.0132
98
Klančnik A. Guzej B. Kolar M. H. Abramovič H. Možina S. S. (2009). In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot.72, 1744–1752. doi: 10.4315/0362-028X-72.8.1744
99
Kloepper J. W. Ryu C.-M. Zhang S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology94, 1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259
100
Košćak L. Lamovšek J. Đermić E. Prgomet I. Godena S. (2023). Microbial and plant-based compounds as alternatives for the control of phytopathogenic bacteria. Horticulturae9:1124. doi: 10.3390/horticulturae9101124
101
Kumar S. Yadav A. Yadav M. Yadav J. P. (2017). Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. F. BMC. Res. Notes10, 1–12. doi: 10.1186/s13104-017-2385-3
102
Landa B. B. Saponari M. Feitosa-Junior O. R. Giampetruzzi A. Vieira F. J. D. Mor E. et al . (2022). Xylella fastidiosa's relationships: the bacterium, the host plants, and the plant microbiome. New Phytol.234, 1598–1605. doi: 10.1111/nph.18089
103
Lazcano-Ramírez H. G. Garza-García J. J. Hernández-Díaz J. A. León-Morales J. M. Macías-Sandoval A. S. García-Morales S. (2023). Antifungal activity of selenium nanoparticles obtained by plant-mediated synthesis. Antibiotics12:115. doi: 10.3390/antibiotics12010115
104
Lekei E. E. Ngowi A. V. London L. (2014). Farmers' knowledge, practices and injuries associated with pesticide exposure in rural farming villages in Tanzania. BMC Public Health14, 1–13. doi: 10.1186/1471-2458-14-389
105
Liu Y. Xu R. Tian Y. Wang H. Ma F. Liu C. et al . (2023). Exogenous chitosan enhances the resistance of apple to Glomerella leaf spot. Sci. Hortic.309:111611. doi: 10.1016/j.scienta.2022.111611
106
Logrieco A. Moretti A. Solfrizzo M. (2009). Alternaria toxins and plant diseases: an overview of origin, occurrence and risks. World Mycotoxin J.2, 129–140. doi: 10.3920/wmj2009.1145
107
Luck J. Spackman M. Freeman A. Tre˛bicki P. Griffiths W. Finlay K. et al . (2011). Climate change and diseases of food crops. Plant Pathol.60, 113–121. doi: 10.1111/j.1365-3059.2010.02414.x
108
Ma B. Hibbing M. E. Kim H. S. Reedy R. M. Yedidia I. Breuer J. et al . (2007). Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology97, 1150–1163. doi: 10.1094/PHYTO-97-9-1150
109
Ma L. Yao L. (2020). Antiviral effects of plant-derived essential oils and their components: an updated review. Molecules25:2627. doi: 10.3390/molecules25112627
110
Magaldi S. Mata-Essayag S. Hartung de Capriles C. Perez C. Colella M. T. Olaizola C. et al . (2004). Well diffusion for antifungal susceptibility testing. Intl. J. Infect. Dis.8, 39–45. doi: 10.1016/j.ijid.2003.03.002
111
Makhubu F. Khosa M. McGaw L. J. (2023). Chemical profiling and inhibitory effects of selected south African plants against phytopathogenic bacteria and fungi of tomato. S. Afr. J. Bot.163, 729–735. doi: 10.1016/j.sajb.2023.11.028
112
Mäkinen K. Aspelin W. Pollari M. Wang L. (2023). How do they do it? The infection biology of potyviruses. Adv. Virus Res.117, 1–79. doi: 10.1016/bs.aivir.2023.07.001
113
Malvick D . (2018). Rhizoctonia root and stem rot on soybean. University of Minnesota Extension. Available online at: https://extension.umn.edu/pest-management/rhizoctonia-root-and-stem-rot-soybean (Accessed October 12, 2024).
114
Mansfield J. Genin S. Magori S. Citovsky V. Sriariyanum M. Ronald P. et al . (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol.13, 614–629. doi: 10.1111/j.1364-3703.2012.00804.x
115
Martin F. N. Loper J. E. (1999). Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci.18, 111–181. doi: 10.1080/07352689991309216
116
Mavrodi O. V. Walter N. Elateek S. Taylor C. G. Okubara P. A. (2012). Suppression of Rhizoctonia and Pythium root rot of wheat by new strains of Pseudomonas. Biol. Control62, 93–102. doi: 10.1016/j.biocontrol.2012.03.013
117
Mohamed N. Z. Shaaban L. Safan S. El-Sayed A. S. A. (2023). Phytochemical and metabolic profiling of the different Podocarpus species in Egypt: potential antimicrobial and antiproliferative activities. Heliyon9:e20034. doi: 10.1016/j.heliyon.2023.e20034
118
Moomin A. Russell W. R. Knott R. M. Scobbie L. Mensah K. B. Adu-Gyamfi P. K. T. et al . (2023). Season, storage and extraction method impact on the phytochemical profile of Terminalia ivorensis. BMC Plant Biol.23:162. doi: 10.1186/s12870-023-04144-8
119
Moreira R. R. Peres N. A. May De Mio L. L. (2019). Colletotrichum acutatum and C. Gloeosporioides species complexes associated with apple in Brazil. Plant Dis.103, 268–275. doi: 10.1094/PDIS-07-18-1187-RE
120
Moussi K. Nayak B. Perkins L. B. Dahmoune F. Madani K. Chibane M. (2015). HPLC-DAD profile of phenolic compounds and antioxidant activity of leaves extract of Rhamnus alaternus L. Ind. Crop. Prod.74, 858–866. doi: 10.1016/j.indcrop.2015.06.015
121
Nadeu E. Van Dijk R. Hiller N . (2023). The soil microbiome: its contribution to soil health and one health. Institute for European Environmental Policy. Available online at: https://ieep.eu/wp-content/uploads/2023/12/The-Soil-Microbiome-ESAD-IEEP-2023.pdf (Accessed December 8, 2024).
122
Najdabbasi N. Mirmajlessi S. M. Dewitte K. Landschoot S. Mänd M. Audenaert K. et al . (2020). Biocidal activity of plant-derived compounds against Phytophthora infestans: an alternative approach to late blight management. Crop Prot.138:105315. doi: 10.1016/j.cropro.2020.105315
123
Nakajima M. Akutsu K. (2014). Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol.80, 15–23. doi: 10.1007/s10327-013-0492-0
124
Nandi M. Macdonald J. Liu P. Weselowski B. Yuan Z. C. (2018). Clavibacter michiganensis ssp. michiganensis: bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol.19, 2036–2050. doi: 10.1111/mpp.12678
125
Navas-Castillo J. Fiallo-Olivé E. Sánchez-Campos S. (2011). Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol.49, 219–248. doi: 10.1146/annurev-phyto-072910-095235
126
Ndolomingo M. J. Bingwa N. Meijboom R. (2020). Review of supported metal nanoparticles: synthesis methodologies, advantages and application as catalysts. J. Mater. Sci.55, 6195–6241. doi: 10.1007/s10853-020-04415-x
127
Nelson P. E. (1992). Taxonomy and biology of Fusarium moniliforme. Mycopathologia117, 29–36. doi: 10.1007/BF00497276
128
Nganje W. E. Bangsund D. A. Leistritz F. L. Wilson W. W. Tiapo N. M. (2004). Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ.26, 332–347. doi: 10.1111/j.1467-9353.2004.00183.x
129
Nieto L. G. Ho S.-T. Rickard B. J. Reig G. Lordan J. Fazio G. et al . (2024). Estimated economic impact of fire blight on long-term orchard economic performance with susceptible and resistant rootstocks. Sci. Hortic.337:113478. doi: 10.1016/j.scienta.2024.113478
130
Nikitin E. Fitsev I. Egorova A. Logvinenko L. Terenzhev D. Bekmuratova F. et al . (2023). Five different Artemisia L. species ethanol extracts’ phytochemical composition and their antimicrobial and nematocide activity. Int. J. Mol. Sci.24:14372. doi: 10.3390/ijms241814372
131
Ogunyemi S. O. Xu X. Xu L. Abdallah Y. Rizwan M. Lv L. et al . (2023). Cobalt oxide nanoparticles: an effective growth promoter of Arabidopsis plants and nano-pesticide against bacterial leaf blight pathogen in rice. Ecotoxicol. Environ. Saf.257:114935. doi: 10.1016/j.ecoenv.2023.114935
132
Okungbowa F. I. Shittu H. O. (2012). Fusarium wilts: an overview. Environ. Res. J.6, 83–102.
133
Pa W. (2002). Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A2
134
Pacheco-Cano R. D. Salcedo-Hernández R. Casados-Vázquez L. E. Wrobel K. Bideshi D. K. Barboza-Corona J. E. (2020). Class i defensins (BraDef) from broccoli (Brassica oleracea var. italica) seeds and their antimicrobial activity. World J. Microbiol. Biotechnol.36, 1–12. doi: 10.1007/s11274-020-2807-6
135
Palou L. Taberner V. Guardado A. Montesinos-Herrero C. (2012). First report of Alternaria alternata causing postharvest black spot of persimmon in Spain. Aust Plant Dis Notes7, 41–42. doi: 10.1007/s13314-012-0043-0
136
Palukaitis P. Roossinck M. J. Dietzgen R. G. Francki R. I. (1992). Cucumber mosaic virus. Adv. Virus Res.41, 281–348. doi: 10.1016/S0065-3527(08)60039-1
137
Pande S. Galloway J. Gaur P. M. Siddique K. H. M. Tripathi H. S. Taylor P. et al . (2006). Botrytis grey mould of chickpea: a review of biology, epidemiology, and disease management. Aust. J. Agric. Res.57, 1137–1150. doi: 10.1071/ar06120
138
Pandit M. A. Kumar J. Gulati S. Bhandari N. Mehta P. Katyal R. et al . (2022). Major biological control strategies for plant pathogens. Pathogens11:273. doi: 10.3390/pathogens11020273
139
Papaianni M. Paris D. Woo S. L. Fulgione A. Rigano M. M. Parrilli E. et al . (2020). Plant dynamic metabolic response to bacteriophage treatment after Xanthomonas campestris pv. Campestris infection. Front. Microbiol.11:732. doi: 10.3389/fmicb.2020.00732
140
Parr J. F. Dolic V. Lancaster G. Boyd W. E. (2001). A microwave digestion method for the extraction of phytoliths from herbarium specimens. Rev. Palaeobot. Palynol.116, 203–212. doi: 10.1016/S0034-6667(01)00089-6
141
Patriarca A. Azcarate M. P. Terminiello L. Fernandez Pinto V. (2007). Mycotoxin production by Alternaria strains isolated from Argentinean wheat. Int. J. Food Microbiol.119, 219–222. doi: 10.1016/j.ijfoodmicro.2007.07.055
142
Pedro-Jove R. Puigvert M. Sebastia P. Macho A. P. Monteiro J. S. Coll N. S. et al . (2021). Dynamic expression of Ralstonia solanacearum virulence factors and metabolism-controlling genes during plant infection. BMC Genomics22:170. doi: 10.1186/s12864-021-07457-w
143
Peever T. Ibanez A. Akimitsu K. Timmer L. J. P. (2002). Worldwide phylogeography of the citrus brown spot pathogen, Alternaria alternata. Phytopathology92, 794–802. doi: 10.1094/PHYTO.2002.92.7.794
144
Perdikis D. Fantinou A. Lykouressis D. (2011). Enhancing pest control in annual crops by conservation of predatory Heteroptera. Biol. Control59, 13–21. doi: 10.1016/j.biocontrol.2011.03.014
145
Pereira B. S. De Freitas C. Vieira R. M. Brienzo M. (2022). Brazilian banana, guava, and orange fruit and waste production as a potential biorefinery feedstock. J. Mater. Cycles Waste Manag.24, 2126–2140. doi: 10.1007/s10163-022-01495-6
146
Peritore-Galve F. C. Tancos M. A. Smart C. D. (2021). Bacterial canker of tomato: revisiting a global and economically damaging Seedborne pathogen. Plant Dis.105, 1581–1595. doi: 10.1094/PDIS-08-20-1732-FE
147
Perombelon M. (1988). Ecology of erwinias causing stem and tuber diseases. In C. International Potato (Ed.), Bacterial diseases of the potato: report of the planning conference on bacterial diseases of the potato, Lima, Peru, 16–20 Mar. 1987 (pp. 143–177). Lima: CIP.
148
Perrucci S. Mancianti F. Cioni P. Flamini G. Morelli I. Macchioni G. (1994). In vitro antifungal activity of essential oils against some isolates of Microsporum canis and Microsporum gypseum. Planta Med.60, 184–186. doi: 10.1055/s-2006-959448
149
Pinto A. A. Ruano-González A. Ezzanad A. Pinedo-Rivilla C. Sánchez-Maestre R. Amaro-Luis J. M. (2022). Bio-guided isolation of new compounds from Baccharis spp. as antifungal against Botrytis cinerea. Meta12:1292. doi: 10.3390/metabo12121292
150
Ploetz R. C. (2015). Fusarium wilt of Banana. Phytopathology105, 1512–1521. doi: 10.1094/PHYTO-04-15-0101-RVW
151
Ramadwa T. E. Makhubu F. N. Eloff J. N. (2024). The activity of leaf extracts, fractions, and isolated compounds from Ptaeroxylon obliquum against nine phytopathogenic fungi and the nematode Meloidogyne incognita. Heliyon10. doi: 10.1016/j.heliyon.2024.e28920
152
Ramey B. E. Koutsoudis M. von Bodman S. B. Fuqua C. (2004). Biofilm formation in plant–microbe associations. Curr. Opin. Microbiol.7, 602–609. doi: 10.1016/j.mib.2004.10.014
153
Ray M. K. Mishra A. K. Mohanta Y. K. Mahanta S. Chakrabartty I. Kungwani N. A. et al . (2023). Nanotechnology as a promising tool against phytopathogens: a futuristic approach to agriculture. Agriculture13:1856. doi: 10.3390/agriculture13091856
154
Reiter B. Pfeifer U. Schwab H. Sessitsch A. (2002). Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Appl. Environ. Microbiol.68, 2261–2268. doi: 10.1128/AEM.68.5.2261-2268.2002
155
Reyes C. P. Sabina S. R. López-Cabeza R. Montelongo C. G. Giménez C. Jiménez I. A. et al . (2022). Antifungal potential of Canarian plant extracts against high-risk phytopathogens. Plan. Theory11:2988. doi: 10.3390/plants11212988
156
Reynol F . (2017). Brasil se prepara para uma das mais temidas doenças do arroz. Available online at: https://www.embrapa.br/xxi-ciencia-para-a-vida/busca-de-noticias?p_p_id=buscanoticia_WAR_pcebusca6_1portlet&p_p_lifecycle=0&p_p_state=pop_up&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_buscanoticia_WAR_pcebusca6_1portlet_groupId=10180&_buscanoticia_WAR_pcebusca6_1portlet_articleId=24103446&_buscanoticia_WAR_pcebusca6_1portlet_viewMode=print (Accessed January 13, 2025).
157
Robens J. Cardwell K. (2003). The costs of mycotoxin management to the USA: management of aflatoxins in the United States. J. Toxicol. Toxin Rev.22, 139–152. doi: 10.1081/txr-120024089
158
Rocha F. Nunes Calumby R. J. Svetaz L. Sortino M. Teixeira Ribeiro Vidigal M. C. Campos-Bermudez V. A. et al . (2023). Effects of Larrea nitida nanodispersions on the growth inhibition of phytopathogens. AMB Express13:98. doi: 10.1186/s13568-023-01605-z
159
Rossi V. (2014). Scientific opinion on the pest categorisation of Clavibacter michiganensis subsp. michiganensis (smith) Davis et al. EFSA J.12:3721. doi: 10.2903/j.efsa.2014.3721
160
Rudolph K. (1993). Infection of the plant by Xanthomonas. In Xanthomonas. eds. SwingsJ. G.CiveroloE. L. (London: Chapman and Hall), pp. 193–264.
161
Sánchez-Hernández E. Balduque-Gil J. Barriuso-Vargas J. J. Casanova-Gascón J. González-García V. Cuchí-Oterino J. A. et al . (2022). Holm oak (Quercus ilex subsp. ballota (Desf.) Samp.) bark aqueous ammonia extract for the control of invasive forest pathogens. Int. J. Mol. Sci.23:11882. doi: 10.3390/ijms231911882
162
Sánchez-Hernández E. Balduque-Gil J. González-García V. Barriuso-Vargas J. J. Casanova-Gascón J. Martín-Gil J. et al . (2023). Phytochemical profiling of Sambucus nigra L. flower and leaf extracts and their antimicrobial potential against almond tree pathogens. Int. J. Mol. Sci.24:1154. doi: 10.3390/ijms24021154
163
Sarker S. D. Nahar L. Kumarasamy Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods42, 321–324. doi: 10.1016/j.ymeth.2007.01.006
164
Satlin M. J. (2019). The search for a practical method for colistin susceptibility testing: have we found it by going back to the future?J. Clin. Microbiol.57:e01608-01618. doi: 10.1128/jcm.01608-18
165
Schipper M. A. A. Stalpers J. A. (1984). “A revision of the genus Rhizopus” in Stud. Mycol, vol. 25 (Baarn: Centraalbureau voor Schimmelcultures), 19.
166
Schneider K. van der Werf W. Cendoya M. Mourits M. Navas-Cortes J. A. Vicent A. et al . (2020). Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA117, 9250–9259. doi: 10.1073/pnas.1912206117
167
Scholthof K. B. G. Adkins S. Czosnek H. Palukaitis P. Jacquot E. Hohn T. et al . (2011). Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol.12, 938–954. doi: 10.1111/j.1364-3703.2011.00752.x
168
Sepúlveda M. Costa J. Cayún Y. Gallardo V. Barría E. Rigotto Caruso G. et al . (2024). Chemical composition and antifungal activity of Capsicum pepper aqueous extracts against plant pathogens and food spoilage fungi. Front. Cell. Infect. Microbiol.14:1451287. doi: 10.3389/fcimb.2024.1451287
169
Shabeer S. Asad S. Jamal A. Ali A. (2022). Aflatoxin contamination, its impact and management strategies: an updated review. Toxins14:307. doi: 10.3390/toxins14050307
170
Shao W. Zhao Y. Ma Z. (2021). Advances in understanding fungicide resistance in Botrytis cinerea in China. Phytopathology111, 455–463. doi: 10.1094/PHYTO-07-20-0313-IA
171
Shoresh M. Harman G. E. Mastouri F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol.48, 21–43. doi: 10.1146/annurev-phyto-073009-114450
172
Singh J. (2008). “Maceration, percolation and infusion techniques for the extraction of medicinal and aromatic plants” in Extraction technologies for medicinal aromatic plants, vol. 67, 32–35.
173
Smiley R. W. (2021). Economic loss from Rhizoctonia bare patch in rainfed winter wheat and spring barley in Oregon. Plant Dis.105, 3803–3808. doi: 10.1094/PDIS-01-21-0026-SC
174
Snyder L. R. Kirkland J. J. Dolan J. W. (2011). Introduction to modern liquid chromatography. Hoboken, New Jersey, USA: John Wiley 1076 & Sons.
175
Soriano G. Petrillo C. Masi M. Bouafiane M. Khelil A. Tuzi A. et al . (2022). Specialized metabolites from the allelopathic plant Retama raetam as potential biopesticides. Toxins14:311. doi: 10.3390/toxins14050311
176
Starr M. P. (1981). “The genus Xanthomonas” in The prokaryotes: A handbook on habitats, isolation, and identification of bacteria (Berlin, Heidelberg: Springer), 742–763.
177
Statista . (2024). Leading producers of bananas worldwide in 2022, by country (in thousand metric tons). Available online at: https://www.statista.com/statistics/811243/leading-banana-producing-countries/ (Accessed December 10, 2024).
178
Steglińska A. Bekhter A. Wawrzyniak P. Kunicka-Styczyńska A. Jastrząbek K. Fidler M. et al . (2022). Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules27:1579. doi: 10.3390/molecules27051579
179
Subba B. Rai G. B. Bhandary R. Parajuli P. Thapa N. Kandel D. R. et al . (2024). Antifungal activity of zinc oxide nanoparticles (ZnO NPs) on Fusarium equiseti phytopathogen isolated from tomato plant in Nepal. Heliyon10:e40198. doi: 10.1016/j.heliyon.2024.e40198
180
Tahat M. M. Sijam K. (2010). Ralstoina solanacearum: the bacterial wilt causal agent. Asian J. Plant Sci.9, 385–393. doi: 10.3923/ajps.2010.385.393
181
Taliansky M. Mayo M. A. Barker H. (2003). Potato leafroll virus: a classic pathogen shows some new tricks. Mol. Plant Pathol.4, 81–89. doi: 10.1046/j.1364-3703.2003.00153.x
182
Thomas E. Stewart L. E. Darley B. A. Pham A. M. Esteban I. Panda S. S. (2021). Plant-based natural products and extracts: potential source to develop new antiviral drug candidates. Molecules26:6197. doi: 10.3390/molecules26206197
183
Thompson T. Rogers C. Zimmerman D. (1980). Sunflower oil quality and quantity as affected by Rhizopus head rot. J. Am. Oil Chem. Soc.57, 106–108. doi: 10.1007/BF02678814
184
Toth I. K. Barny M.-a. Brurberg M. B. Condemine G. Czajkowski R. Elphinstone J. G. et al . (2021). Pectobacterium and Dickeya: environment to disease development. Plant diseases caused by Dickeya Pectobacterium species. Cham: Springer, 39–84.
185
Toth I. Van Der Wolf J. Saddler G. LOjkowska E. Hélias V. Pirhonen M. et al . (2011). Dickeya species: an emerging problem for potato production in Europe. Plant Pathol.60, 385–399. doi: 10.1111/j.1365-3059.2011.02427.x
186
Tripathi S. Suzuki J. Y. Ferreira S. A. Gonsalves D. (2008). Papaya ringspot virus-P: characteristics, pathogenicity, sequence variability and control. Mol. Plant Pathol.9, 269–280. doi: 10.1111/j.1364-3703.2008.00467.x
187
Trkulja V. Tomic A. Ilicic R. Nozinic M. Milovanovic T. P. (2022). Xylella fastidiosa in Europe: from the introduction to the current status. Plant Pathol. J.38, 551–571. doi: 10.5423/PPJ.RW.09.2022.0127
188
Ulian T. Diazgranados M. Pironon S. Padulosi S. Liu U. Davies L. et al . (2020). Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet2, 421–445. doi: 10.1002/ppp3.10145
189
Valencia-Mejía E. León-Wilchez Y. Y. Monribot-Villanueva J. L. Ramírez-Vázquez M. Bonilla-Landa I. Guerrero-Analco J. A. (2022). Isolation and identification of Pennogenin Tetraglycoside from Cestrum nocturnum (Solanaceae) and its antifungal activity against Fusarium kuroshium, causal agent of Fusarium dieback. Molecules27:1860. doi: 10.3390/molecules27061860
190
Van der Merwe J. J. Coutinho T. A. Korsten L. van der Waals J. E. (2010). Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. Eur. J. Plant Pathol.126, 175–185. doi: 10.1007/s10658-009-9531-2
191
Voora V. Larrea C. Bermudez S . (2020). Global market report: bananas. Retrieved from Winnipeg: https://www.evidensia.eco/resources/917/global-market-report-bananas/
192
Vrancken K. Holtappels M. Schoofs H. Deckers T. Valcke R. (2013). Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: state of the art. Microbiology159, 823–832. doi: 10.1099/mic.0.064881-0
193
Vuerich M. Petrussa E. Filippi A. Cluzet S. Fonayet J. V. Sepulcri A. et al . (2023). Antifungal activity of chili pepper extract with potential for the control of some major pathogens in grapevine. Pest Manag. Sci.79, 2503–2516. doi: 10.1002/ps.7435
194
Vurro M. Miguel-Rojas C. Perez-de-Luque A. (2019). Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci.75, 2403–2412. doi: 10.1002/ps.5348
195
Walker T. S. Bais H. P. Deziel E. Schweizer H. P. Rahme L. G. Fall R. et al . (2004). Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol.134, 320–331. doi: 10.1104/pp.103.027888
196
Wang B. Li B. H. Dong X. L. Wang C. X. Zhang Z. F. (2015). Effects of temperature, wetness duration, and moisture on the conidial germination, infection, and disease incubation period of Glomerella cingulata. Plant Dis.99, 249–256. doi: 10.1094/PDIS-04-14-0361-RE
197
Wang K. Ngea G. L. N. Godana E. A. Shi Y. Lanhuang B. Zhang X. et al . (2023). Recent advances in Penicillium expansum infection mechanisms and current methods in controlling P. expansum in postharvest apples. Crit. Rev. Food Sci. Nutr.63, 2598–2611. doi: 10.1080/10408398.2021.1978384
198
Weber R. W. Petridis A. (2023). Fungicide resistance in Botrytis spp. and regional strategies for its management in northern European strawberry production. Biotech12:64. doi: 10.3390/biotech12040064
199
Werra P. Debonneville C. Kellenberger I. Dupuis B. (2021). Pathogenicity and relative abundance of Dickeya and Pectobacterium species in Switzerland: an epidemiological dichotomy. Microorganisms9:2270. doi: 10.3390/microorganisms9112270
200
Wikström J. Chaudhary S. Persson L. Wikström M. Fatehi J. Karlsson M. (2025). Distribution of plant pathogenic Aphanomyces species in Sweden, Denmark and Lithuania and its relationship with soil factors. J. Plant Pathol.1–11. doi: 10.1007/s42161-025-01835-z
201
Yan J. Yuan S. Wang C. Ding X. Cao J. Jiang W. (2015). Enhanced resistance of jujube (Zizyphus jujuba mill. cv. Dongzao) fruit against postharvest Alternaria rot by β-aminobutyric acid dipping. Sci. Hortic.186, 108–114. doi: 10.1016/j.scienta.2015.02.018
202
Yan W. Zhu Y. Zou C. Liu W. Jia B. Niu J. et al . (2025). Virome characterization of native wild-rice plants discovers a novel pathogenic rice polerovirus with world-wide circulation. Plant Cell Environ.48, 1005–1020. doi: 10.1111/pce.15204
203
Yildirim I. Turhan H. Özgen B. (2010). The effects of head rot disease (Rhizopus stolonifer) on sunflower genotypes at two different growth stages. Turk. J. Field Crops.15, 94–98.
204
Yu L. Lin K. Xu L. Cui J. Zhang Y. Zhang Q. et al . (2023). Downy mildew (Peronospora aestivalis) infection of alfalfa alters the feeding behavior of the aphid (Therioaphis trifolii) and the chemical characteristics of alfalfa. J. Pest. Sci.96, 989–1001. doi: 10.1007/s10340-022-01571-8
205
Zeng Q. Puławska J. Schachterle J. (2021). Early events in fire blight infection and pathogenesis of Erwinia amylovora. J. Plant Pathol.103, 13–24. doi: 10.1007/s42161-020-00675-3
206
Zhang Q. W. Lin L. G. Ye W. C. (2018). Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med.13:20. doi: 10.1186/s13020-018-0177-x
207
Zhang Y.-p. Uyemoto J. Kirkpatrick B. (1998). A small-scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. J. Virol. Methods71, 45–50. doi: 10.1016/S0166-0934(97)00190-0
Summary
Keywords
plant extract, phytopathogens, economic impact, chemical composition, antimicrobial activity
Citation
Calefi GG, Silva NBS, Alhatlani BY, Abdallah EM and Martins CHG (2025) Harnessing nature’s arsenal: sustainable plant-based strategies for phytopathogen control. Front. Microbiol. 16:1588462. doi: 10.3389/fmicb.2025.1588462
Received
05 March 2025
Accepted
28 May 2025
Published
18 June 2025
Volume
16 - 2025
Edited by
Javier Pascual, Darwin Bioprospecting Excellence, Spain
Reviewed by
Gordon Webster, Cardiff University, United Kingdom
Shraddha Maitra, University of Illinois at Urbana-Champaign, United States
Emmanuel Oliver Fenibo, Hensard University, Nigeria
Updates
Copyright
© 2025 Calefi, Silva, Alhatlani, Abdallah and Martins.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bader Y. Alhatlani, balhatlani@qu.edu.sa; Emad M. Abdallah, 140208@qu.edu.sa
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.