- 1Infectious Disease Department, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China
- 2Department of Laboratory, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China
- 3Department of Infectious Diseases, Shanghai Xuhui Central Hospital/Zhongshan-Xuhui Hospital, Fudan University, Shanghai, China
- 4Department of Urology, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China
Objective: To investigate the distribution characteristics of methicillin-resistant Staphylococcus aureus (MRSA) infection in people living with HIV (PLWH), to analyse the risk factors of MRSA colonisation in the nasopharynx of PLWH, and to provide a scientific basis for the prevention of hospital-acquired MRSA infection in PLWH.
Methods: This study used a cross-sectional research design to analyse 1,100 PLWH attending the AIDS outpatient clinic of the People’s Hospital of Dongyang City, Zhejiang Province, from January 2022 to December 2024. Nasal swabs were collected with informed consent, and epidemiological information was collected via questionnaire. Standard microbiological methods were used for isolation and identification of strains, with drug susceptibility testing performed using the K-B paper diffusion method. PCR was used to detect virulence genes pvl and tst. Statistical analysis was conducted using Stata13.0 software.
Results: Of the 1,100 PLWH enrolled, 275 (25%) were colonized with S. aureus, of which 98 (35.63%) carried MRSA and 177 (64.37%) carried MSSA. The mean age of MRSA carriers (51.32 ± 15.87 years) was significantly higher than that of MSSA carriers (44.26 ± 18.93 years) (p < 0.001). MRSA had a high prevalence of resistance to a wide range of antibiotics, including penicillin, oxacillin, amoxicillin and amoxicillin/clavulanic acid. The virulence gene pvl was detected more frequently in MSSA than in MRSA. In PLWH, respiratory infections in the previous 12 months, lack of antiretroviral therapy and heterosexual transmission were associated with a higher risk of nasal carriage of MRSA.
Conclusion: The study provides valuable insights into the characteristics and distribution of MRSA and MSSA cases, as well as the factors influencing the nasal carriage of Staphylococcus aureus, particularly MRSA, in PLWH. These findings can guide clinical practice and infection control measures to reduce the incidence and spread of MRSA infections in high-risk populations.
1 Introduction
Methicillin-Resistant Staphylococcus aureus (MRSA) is a global public health problem that can cause multi-organ, skin and soft tissue infections and increase the risk of death in hospital settings (Chalmers and Wylam, 2020). The colonisation and infection rates of Staphylococcus aureus (S. aureus) and MRSA in people living with HIV (PLWH) are significantly higher than those in non-HIV-infected individuals (Sabbagh et al., 2019; Hsu et al., 2020), making this population particularly vulnerable to complications from these infections (Reid et al., 2017b).
The rate of nasal MRSA colonisation in PLWH differs between countries and regions, with CC30-ST36 being common in the United States and the United Kingdom, CC45 predominant in Europe, and CC5-ST5 and CC22-ST22 most common in Asian countries (Bhattacharya and Horswill, 2024). Studies of MRSA susceptibility in nasopharyngeal screening of PLWH from Taiwan and Brazil showed significant differences in drug resistance to sulfamethoxazole-trimethoprim, suggesting regional differences in MRSA strains carried by PLWH (Popovich et al., 2013).
Studies show that MRSA is predominantly nasal in PLWH. In North America, the prevalence of nasal carriage was 8.8%, with the risk of MRSA in PLWH with a history of hospitalisation in the past 12 months being 3.1 times higher than in those without (Donkor et al., 2019). Results from Atlanta showed an overall nasal and axillary carriage rate of 13% (Kotpal et al., 2016), while a Johns Hopkins University study showed a 15.4% prevalence of MRSA in PLWH, with 59.7% from the nasal cavity (Zervou et al., 2014).
The prevalence of S. aureus or MRSA varies regionally, possibly related to differences in strain virulence, colonisation properties, antimicrobial drug utilization, and infection control methods. Risk factors for MRSA colonization in PLWH have been reported to include CD4 cell counts, history of infections, hospitalization, antibiotic use, and demographic factors, though findings are inconsistent across studies (Peters et al., 2011; Popovich et al., 2013; Farley et al., 2015).
Currently, the prevalence of MRSA in nasopharyngeal colonisation of PLWH in Zhejiang Province has not been reported, and there is a lack of drug susceptibility data for this regional population. Therefore, it is necessary to investigate the prevalence of MRSA in nasopharyngeal colonisation of PLWH in this region, analyze its drug resistance characteristics, and identify risk factors associated with MRSA colonization in this specific population.
The primary objective of this study is to determine the prevalence of nasal MRSA colonization among PLWH in Zhejiang Province and to identify associated risk factors. The secondary objectives are to characterize the antimicrobial resistance patterns and virulence factors of MRSA isolates from this population.
2 Methods
2.1 Ethics statement
The study was approved by the Ethics and Programme Review Committee of the People’s Hospital of Dongyang City, Zhejiang Province, under the ethical approval number “DYRM2022-135.”
2.2 Research design
In this study, a cross-sectional research design was used to collect nasal swab specimens from PLWH and a questionnaire was administered using a combination of face-to-face and self-administered methods. The study was conducted from January 2022 to December 2024.
2.3 Inclusion and exclusion criteria
2.3.1 Inclusion criteria
(1) Patients aged ≥18 years who are HIV-positive in the initial screening and confirmatory test who voluntarily submit to the investigation; (2) Staphylococcus aureus detected as MRSA by oxacillin disk assay and mecA gene PCR.
2.3.2 Exclusion criteria
(1) Patients with MRSA infection detected by admission screening; (2) Patients not screened for MRSA on admission or within 48 h; (3) Patients with MRSA infection detected within 48 h of admission; (4) Patients who died, were discharged without healing, or discharged voluntarily; (5) Patients with hospital stay less than 48 h; (6) Patients without weekly MRSA screening; (7) Patients transferred after treatment at other hospitals; (8) Patients with incomplete clinical information.
2.4 Sample collection and processing
A total of 1,100 PLWH were enrolled in the study, and one nasal swab specimen was collected from each participant. Nasal swabs were collected by trained personnel following standard protocols. Briefly, sterile cotton swabs moistened with sterile saline were used to sample the bilateral nasal vestibules of subjects. Samples were placed in sterile tubes containing 7.5% NaCl broth and transported to the laboratory within 6 h for processing.
2.5 Laboratory methods
Staphylococcus aureus identification was performed using mannitol salt agar (MSA, BD Difco™, USA) as a selective medium to differentiate S. aureus from coagulase-negative staphylococci such as S. epidermidis. S. aureus identification and drug susceptibility testing were performed according to standard clinical microbiological procedures. The Kirby-Bauer (K-B) disk diffusion method was used for antimicrobial susceptibility testing according to Clinical and Laboratory Standards Institute (CLSI) 2023 guidelines (Clinical and Laboratory Standards Institute, 2023). MRSA identification was performed using both phenotypic and genotypic methods. For phenotypic identification, cefoxitin (30 μg) disk diffusion was used, with an inhibition zone diameter ≤21 mm indicating MRSA. For genotypic confirmation, PCR detection of the mecA gene was performed using the following primers: mecA-F: 5’-GTAGAAATGACTGAACGTCCGATAA-3′ and mecA-R: 5’-CCAATTCCACATTGTTTCGGTCTAA-3′, with an expected product size of 310 bp.
2.6 Statistical analysis
Categorical data were expressed as frequencies and percentages; normally distributed data were expressed as mean and standard deviation; and non-normally distributed data were expressed as median and interquartile range. For the comparison of characteristics between MRSA and MSSA carriers, chi-square tests were used for categorical variables and t-tests or Wilcoxon rank-sum tests were used for continuous variables, as appropriate. All percentages were calculated based on the actual number of subjects in each group (MRSA n = 98, MSSA n = 177).
Unconditional logistic regression models were used to investigate risk factors for MRSA colonization among S. aureus carriers. The odds ratio (OR) and 95% confidence interval (95%CI) were calculated for each factor, with factors showing p < 0.20 in univariate analysis included in the multivariate model.
3 Results
3.1 Demographic characteristics of MRSA and MSSA patients
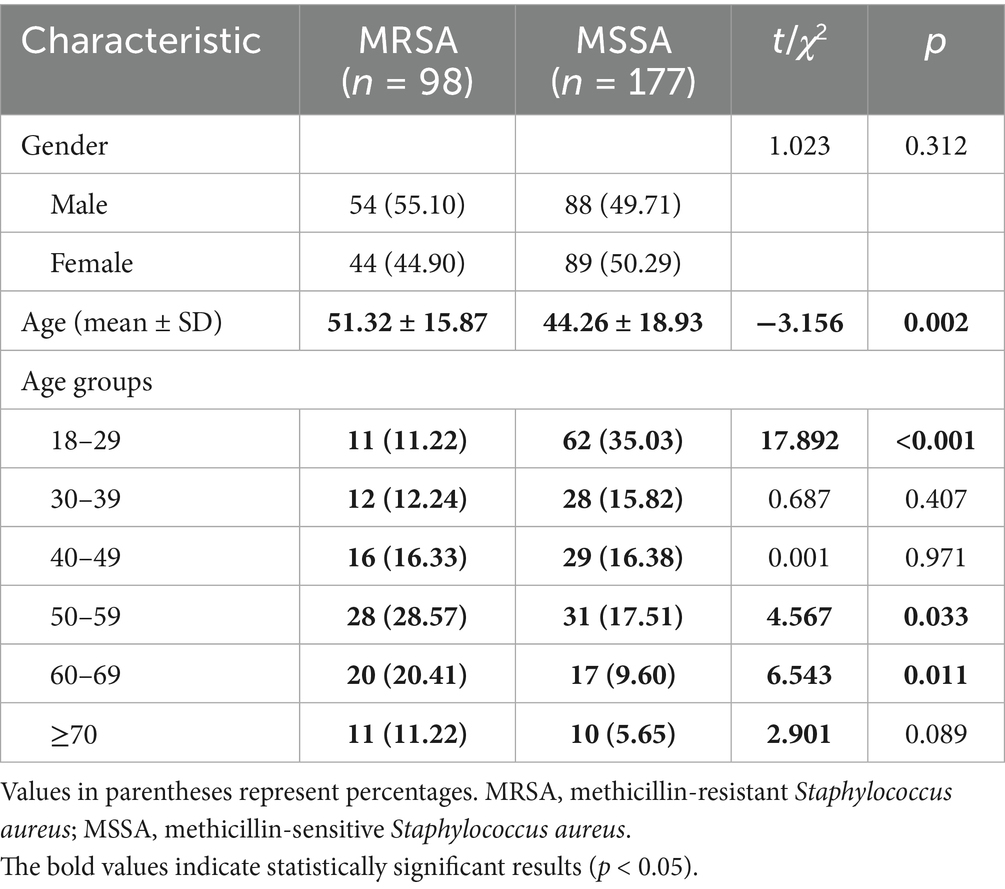
Of the 1,100 PLWH enrolled, 275 (25%) were colonized with S. aureus, of which 98 (35.63%) carried MRSA and 177 (64.37%) carried MSSA (see Table 1).
The gender distribution between MRSA and MSSA groups showed no significant difference (p = 0.312). However, the mean age of MRSA carriers (51.32 ± 15.87 years) was significantly higher than that of MSSA carriers (44.26 ± 18.93 years) (p = 0.002). Age group analysis showed that MSSA was significantly more common in younger age groups (18–29 years), while MRSA was more prevalent in older age groups (50–59 and 60–69 years) (see Table 2).
3.2 Distribution of MRSA resistance to antibiotics
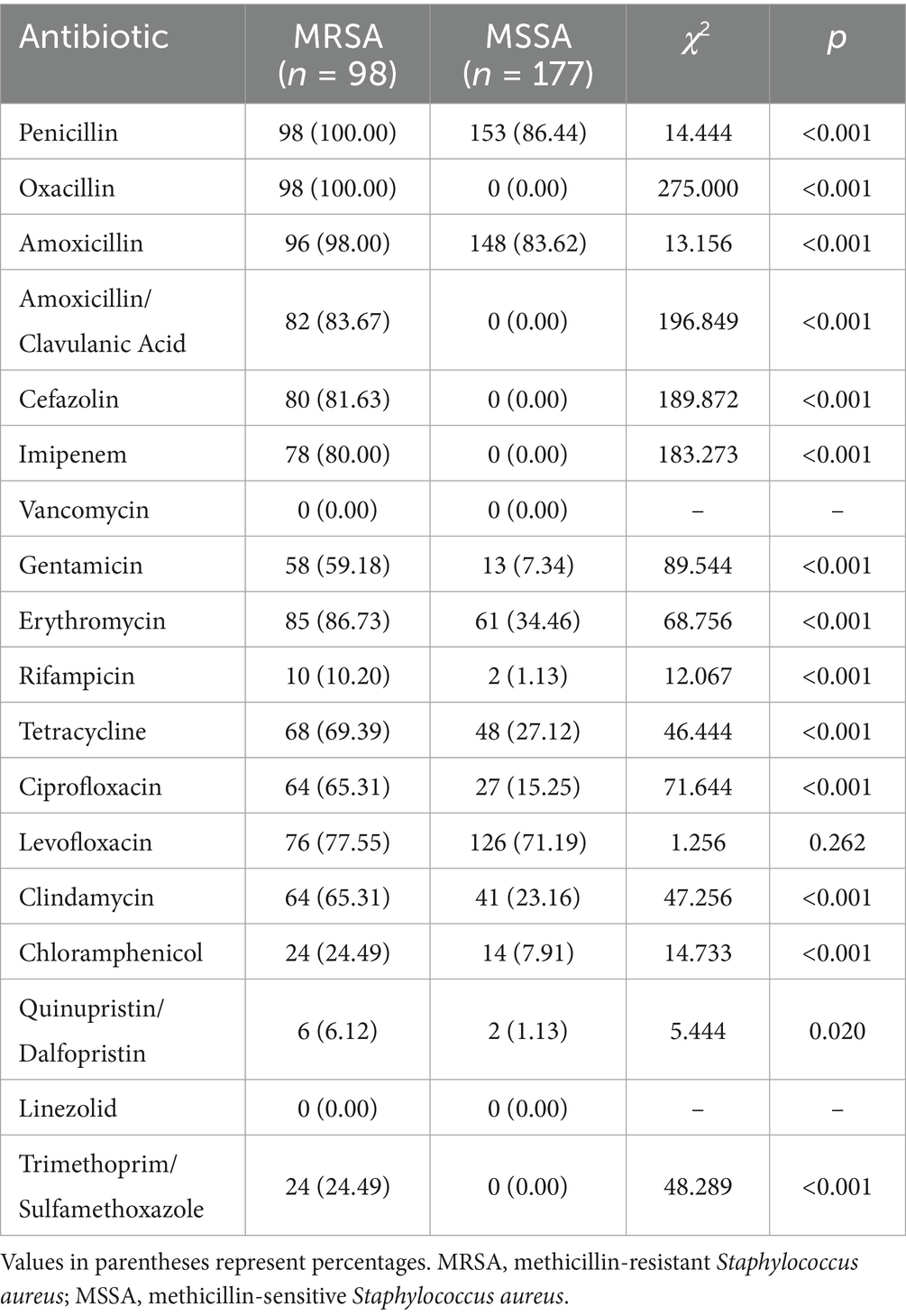
MRSA isolates showed 100% resistance to penicillin and oxacillin, with high resistance rates to amoxicillin (98%), amoxicillin/clavulanic acid (83.67%), cefazolin (81.63%), and imipenem (80%). MRSA exhibited significantly higher resistance rates than MSSA to most antibiotics tested (p < 0.001), with the exception of levofloxacin (p = 0.262). Notably, no resistance to vancomycin and linezolid was detected in either MRSA or MSSA isolates (see Table 3).
3.3 Detection of virulence genes
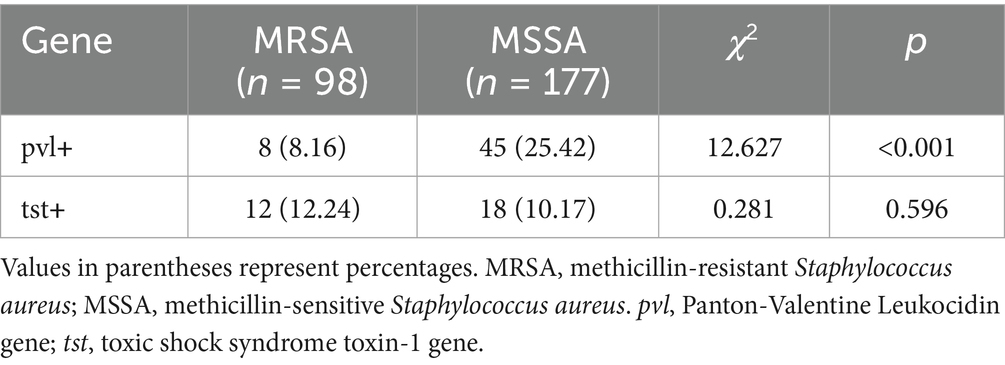
The pvl gene was detected at a significantly lower rate in MRSA isolates (8.16%) compared to MSSA isolates (25.42%) (p < 0.001). In contrast, the prevalence of the tst gene showed no significant difference between MRSA (12.24%) and MSSA (10.17%) isolates (p = 0.596) (see Table 4).
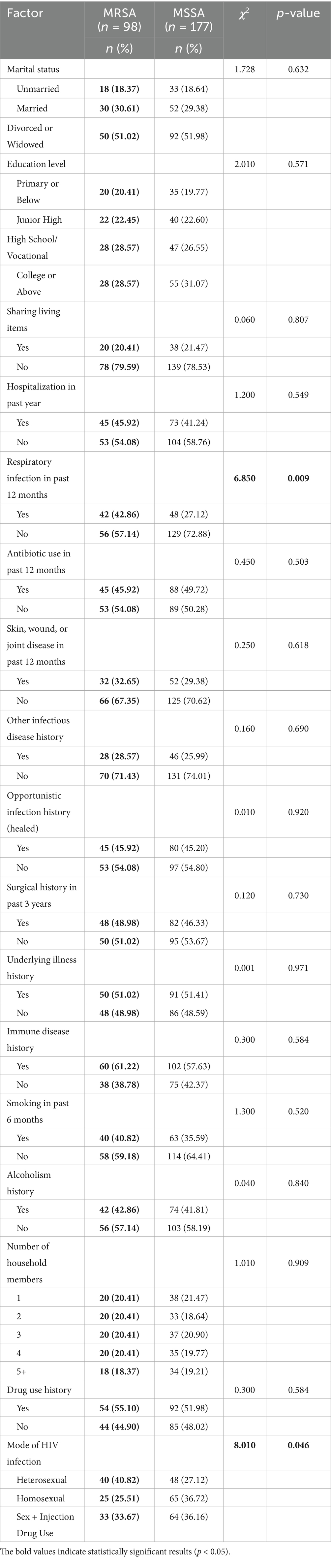
Table 4. Univariate analysis of factors associated with MRSA nasal colonization among S. aureus carriers.
3.4 Risk factors for MRSA colonization in PLWH
In the univariate analysis comparing characteristics between MRSA carriers (n = 98) and MSSA carriers (n = 177), respiratory infection in the past 12 months (42.86% vs. 27.12%, p = 0.009) and mode of HIV infection (p = 0.046) showed statistically significant associations with MRSA colonization. Specifically, MRSA carriers had a higher proportion of respiratory infections and were more likely to have acquired HIV through heterosexual transmission (40.82% vs. 27.12%).
3.5 Multifactorial analysis of risk factors
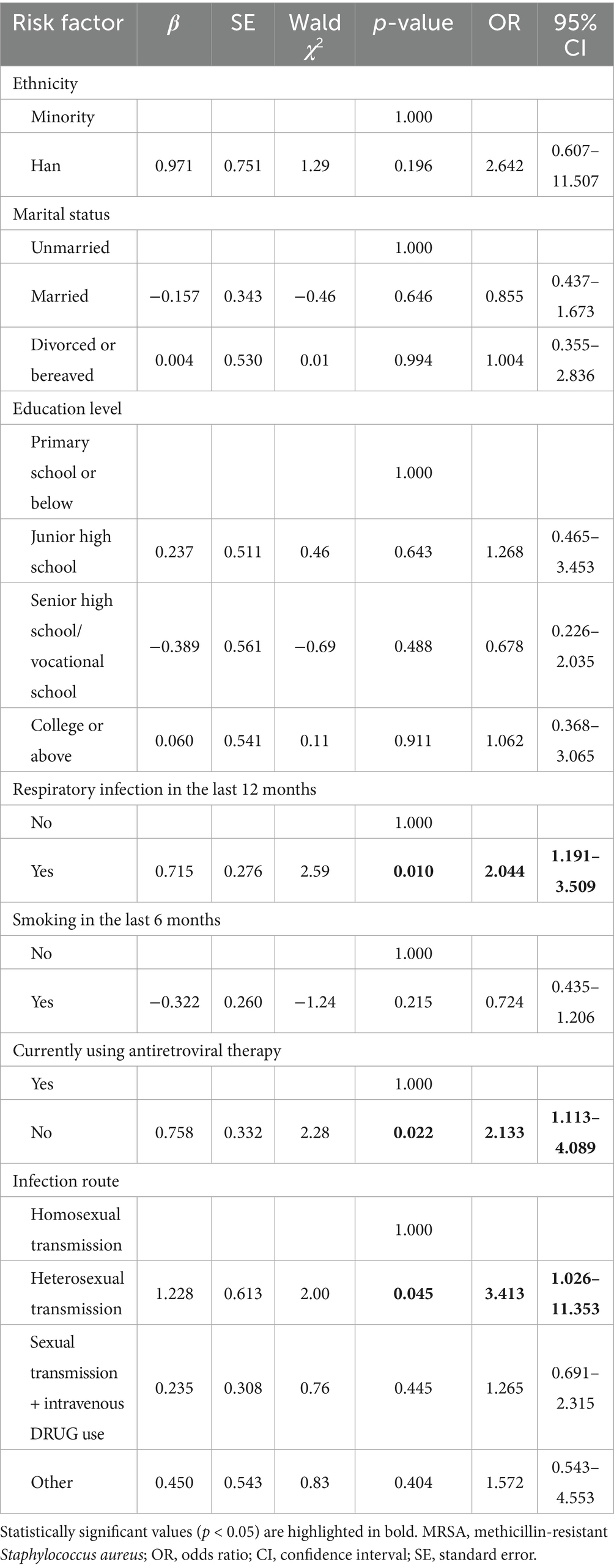
Based on the corrected univariate analysis, variables with p < 0.20 were included in the multifactorial logistic regression model to identify independent risk factors for MRSA colonization among S. aureus carriers (see Table 5).
Multifactorial logistic regression analysis identified three independent risk factors for MRSA nasal carriage in PLWH: respiratory infection in the previous 12 months (OR = 2.044, 95% CI: 1.191–3.509, p = 0.010), not receiving antiretroviral therapy (OR = 2.133, 95% CI: 1.113–4.089, p = 0.022), and heterosexual transmission of HIV (OR = 3.413, 95% CI: 1.026–11.353, p = 0.045).
4 Discussion
Staphylococcus aureus is an important pathogen causing infections in PLWH and AIDS patients. PLWH are a group with a high prevalence of S. aureus and MRSA carriage and infection (Vyas et al., 2011). Our study found a 25% nasal carrier rate of S. aureus among PLWH, with 35.63% of these being MRSA carriers, which is comparable to some international studies but with a notably higher MRSA proportion.
Previous studies have shown varying carriage rates of S. aureus and MRSA among different geographical areas and populations. In Texas, USA, the prevalence of S. aureus among PLWH was 29.7%, with MRSA accounting for 3% (Hidron et al., 2011). In Northern Ethiopia, S. aureus was isolated from 32.5% of respondents and MRSA from 2.4% (Gebremedhn et al., 2016). African regions generally show higher rates, with a study in Botswana reporting 55% prevalence of S. aureus in PLWH (Reid et al., 2017a, b). Studies from the United States show S. aureus detection rates of 36–39% among PLWH, with MRSA rates of 3.2–6% (Sayana et al., 2010; Lee et al., 2013). In Brazil, nasal S. aureus carriage was reported at 34.2% with MRSA colonization at 10.4% (Vieira et al., 2016), while a study in Iran found MRSA nasal carriage at 12.8% (Hassanzadeh et al., 2015). A study from Taiwan reported that the nasal carriage rate of S. aureus in PLWH was 21.5%, with an MRSA carriage rate of 3.4% (Hsu et al., 2020).
Our study revealed several important findings. First, the age distribution differed significantly between MRSA and MSSA carriers, with MRSA more common in older patients. This age-related difference may reflect different exposure risks or immune status changes with aging in PLWH. Second, MRSA isolates showed high resistance rates to multiple antibiotics, particularly beta-lactams, aminoglycosides, macrolides, and fluoroquinolones, limiting treatment options. However, all isolates remained susceptible to vancomycin and linezolid, which is encouraging for treatment of severe infections.
The higher prevalence of the pvl gene in MSSA compared to MRSA isolates is noteworthy, as this virulence factor is associated with increased pathogenicity, particularly in skin and soft tissue infections (Sullivan et al., 2016). This finding suggests that MSSA infections might potentially cause severe clinical manifestations despite their generally better antibiotic susceptibility profile.
Our multifactorial analysis identified three independent risk factors for MRSA nasal carriage: recent respiratory infections, lack of antiretroviral therapy, and heterosexual transmission route. Respiratory infections may provide opportunities for MRSA acquisition during healthcare encounters or may reflect compromised respiratory immunity. The association with lack of antiretroviral therapy suggests that improved immune function through effective HIV treatment may protect against MRSA colonization. The association with heterosexual transmission differs from some studies that found higher risks with homosexual transmission or injection drug use (Leung et al., 2015; Leung et al., 2017; Wu et al., 2017), and may reflect regional differences in HIV transmission patterns and associated behaviors.
5 Limitations
There are some limitations to this study. First, the data were derived from PLWH at a specific time and place, so the representativeness of the sample may be limited. The results may not be fully generalisable to other populations or wider geographical areas. Second, the study only reflects the factors associated with nasal carriage of S. aureus and its drug resistance in PLWH, but does not delve into the mechanisms of interaction between these factors. Third, the age distribution in our study population may not fully represent all PLWH in the region, as older patients may be overrepresented due to higher healthcare utilization.
6 Conclusion
In conclusion, our study provides important regional data on MRSA nasal colonization in PLWH in Zhejiang Province, China. The identified risk factors and antibiotic resistance patterns can guide targeted screening, infection control measures, and empirical antibiotic therapy for this vulnerable population. Future studies should explore the molecular epidemiology of these isolates and investigate intervention strategies to reduce MRSA colonization and subsequent infection risks in PLWH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by This study was approved by the Ethics Committee of the Affiliated Dongyang Hospital of Wenzhou Medical University, Zhejiang, People’s Republic of China (Approval No. DYRM2022-135). Written informed consent was obtained from all participants, and all procedures were conducted in accordance with the ethical standards set by the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DH: Funding acquisition, Project administration, Formal analysis, Supervision, Writing – review & editing, Software, Writing – original draft. LH: Writing – review & editing, Writing – original draft, Investigation. TY: Conceptualization, Writing – review & editing, Writing – original draft. BY: Writing – original draft, Formal analysis, Writing – review & editing. LW: Formal analysis, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jinhua public welfare project in 2023, Project No.: 2023-4-212.
Acknowledgments
The authors would like to thank the AIDS outpatient clinic staff at the People’s Hospital of Dongyang City for their collaboration in patient recruitment and sample collection. We sincerely thank Reviewer 1 for the careful review and identification of calculation errors in Table 4. The reviewer’s attention to detail has significantly improved the accuracy of our data presentation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1634460/full#supplementary-material
References
Bhattacharya, M., and Horswill, A. R. (2024). The role of human extracellular matrix proteins in defining Staphylococcus aureus biofilm infections. FEMS Microbiol. Rev. 48:fuae002. doi: 10.1093/femsre/fuae002
Chalmers, S. J., and Wylam, M. E. (2020). Methicillin-resistant Staphylococcus aureus infection and treatment options. Methods Mol. Biol. 2069, 229–251. doi: 10.1007/978-1-4939-9849-4_16
Clinical and Laboratory Standards Institute (2023). Performance standards for antimicrobial susceptibility testing, CLSI supplement M100. 33rd Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Donkor, E. S., Kotey, F. C. N., Dayie, N. T. K. D., Duodu, S., Tetteh-Quarcoo, P. B., Osei, M. M., et al. (2019). Colonization of HIV-infected children with methicillin-resistant Staphylococcus aureus. Pathogens 8:35. doi: 10.3390/pathogens8010035
Farley, J. E., Hayat, M. J., Sacamano, P. L., Ross, T., and Carroll, K. (2015). Prevalence and risk factors for methicillin-resistant Staphylococcus aureus in an HIV-positive cohort. Am. J. Infect. Control 43, 329–335. doi: 10.1016/j.ajic.2014.12.024
Gebremedhn, G., Gebremariam, T. T., Wasihun, A. G., Dejene, T. A., and Saravanan, M. (2016). Prevalence and risk factors of methicillin-resistant Staphylococcus aureus colonization among HIV patients in Mekelle, northern Ethiopia. Springerplus 5:877. doi: 10.1186/s40064-016-2613-7
Hassanzadeh, P., Hassanzadeh, Y., Mardaneh, J., Rezai, E., and Motamedifar, M. (2015). Isolation of methicillin-resistant Staphylococcus aureus (MRSA) from HIV patients referring to HIV referral center, shiraz, Iran, 2011-2012. Iran. J. Med. Sci. 40, 526–530.
Hidron, A. I., Moanna, A., and Rimland, D. (2011). The rise and fall of methicillin-resistant Staphylococcus aureus infections in HIV patients. AIDS 25, 1001–1003. doi: 10.1097/QAD.0b013e328343c595
Hsu, Y. Y., Wu, D., Hung, C. C., Huang, S. S., Yuan, F. H., Lee, M. H., et al. (2020). Methicillin-resistant Staphylococcus aureus nasal colonization among HIV-infected patients in Taiwan: prevalence, molecular characteristics and associated factors with nasal carriage. BMC Infect. Dis. 20:254. doi: 10.1186/s12879-020-04979-8
Kotpal, R., S, K. P., Bhalla, P., Dewan, R., and Kaur, R. (2016). Incidence and risk factors of nasal carriage of Staphylococcus aureus in HIV-infected individuals in comparison to HIV-uninfected individuals: a case-control study. J. Int. Assoc. Provid. AIDS Care 15, 141–147. doi: 10.1177/2325957414554005
Lee, L. K., Win, M. K., Veeraraghavan, M. A., Wong, C. S., Chow, A. L., and Leo, Y. S. (2013). Short communication: risk factors for methicillin-resistant Staphylococcus aureus colonization among HIV patients at hospital admission. AIDS Res. Hum. Retrovir. 29, 796–798. doi: 10.1089/aid.2012.0074
Leung, N. S., Padgett, P., Robinson, D. A., and Brown, E. L. (2015). Prevalence and behavioural risk factors of Staphylococcus aureus nasal colonization in community-based injection drug users. Epidemiol. Infect. 143, 2430–2439. doi: 10.1017/S0950268814003227
Leung, N. S., Vidoni, M. L., Robinson, D. A., Padgett, P., and Brown, E. L. (2017). A community-based study of Staphylococcus aureus nasal colonization and molecular characterization among men who have sex with men. LGBT Health 4, 345–351. doi: 10.1089/lgbt.2017.0016
Peters, P. J., Brooks, J. T., Limbago, B., Lowery, H. K., McAllister, S. K., Mindley, R., et al. (2011). Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiol. Infect. 139, 998–1008. doi: 10.1017/S0950268810002013
Popovich, K. J., Hota, B., Aroutcheva, A., Kurien, L., Patel, J., Lyles-Banks, R., et al. (2013). Community-associated methicillin-resistant Staphylococcus aureus colonization burden in HIV-infected patients. Clin. Infect. Dis. 56, 1067–1074. doi: 10.1093/cid/cit010
Reid, M. J. A., Fischer, R. S. B., Mannathoko, N., Muthoga, C., McHugh, E., Essigmann, H., et al. (2017a). Prevalence of Staphylococcus aureus nasal carriage in human immunodeficiency virus-infected and uninfected children in Botswana: prevalence and risk factors. Am. J. Trop. Med. Hyg. 96, 795–801. doi: 10.4269/ajtmh.16-0650
Reid, M. J. A., Steenhoff, A. P., Mannathoko, N., Muthoga, C., McHugh, E., Brown, E. L., et al. (2017b). Staphylococcus aureus nasal colonization among HIV-infected adults in Botswana: prevalence and risk factors. AIDS Care 29, 961–965. doi: 10.1080/09540121.2017.1282600
Sabbagh, P., Riahi, S. M., Gamble, H. R., and Rostami, A. (2019). The global and regional prevalence, burden, and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV-infected people: a systematic review and meta-analysis. Am. J. Infect. Control 47, 323–333. doi: 10.1016/j.ajic.2018.06.023
Sayana, S., Ricaurte, J. C., and Khanlou, H. (2010). An unusual pathogen for a liver abscess in a human immunodeficiency virus-infected individual. Am J Med Sci 339, 290–291. doi: 10.1097/MAJ.0b013e3181c6ef05
Sullivan, S. B., Kamath, S., McConville, T. H., Gray, B. T., Lowy, F. D., Gordon, P. G., et al. (2016). Staphylococcus epidermidis protection against Staphylococcus aureus colonization in people living with human immunodeficiency virus in an inner-city outpatient population: a cross-sectional study. Open Forum Infect. Dis. 3:ofw234. doi: 10.1093/ofid/ofw234
Vieira, M. T., Marlow, M. A., Aguiar-Alves, F., Pinheiro, M. G., Freitas Alves, M. F., Santos Cruz, M. L., et al. (2016). Living conditions as a driving factor in persistent methicillin-resistant Staphylococcus aureus colonization among HIV-infected youth. Pediatr. Infect. Dis. J. 35, 1126–1131. doi: 10.1097/INF.0000000000001246
Vyas, K., Hospenthal, D. R., Mende, K., and Crum-Cianflone, N. F. (2011). Recurrent community-acquired methicillin-resistant Staphylococcus aureus infections in an HIV-infected person. J. Clin. Microbiol. 49, 2047–2053. doi: 10.1128/JCM.02423-10
Wu, C. J., Ko, W. C., Ho, M. W., Lin, H. H., Yang, Y. L., Lin, J. N., et al. (2017). Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization among human immunodeficient virus-infected outpatients in Taiwan: oral Candida colonization as a comparator. J. Oral Microbiol. 9:1322446. doi: 10.1080/20002297.2017.1322446
Keywords: MRSA, people living with HIV, risk factors, nasal carriage, drug resistance
Citation: Hu D, Hong L, Ye T, Yang B and Wang L (2025) Risk factors of methicillin-resistant Staphylococcus aureus colonization in the nasal cavity of people living with HIV: a cross-sectional study from Dongyang hospital, Zhejiang Province. Front. Microbiol. 16:1634460. doi: 10.3389/fmicb.2025.1634460
Edited by:
Swayam Prakash, University of California, Irvine, United StatesReviewed by:
Suleiman Adeiza Shuaibu, Ahmadu Bello University, NigeriaArwa Othman, Sana’a University, Yemen
Copyright © 2025 Hu, Hong, Ye, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixia Wang, MTg3NTc5MzI0NDlAMTYzLmNvbQ==
 Dongping Hu
Dongping Hu Li Hong1
Li Hong1 Bin Yang
Bin Yang