- 1Department of Laboratory Medicine, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Department of Laboratory Medicine, Deyang Hospital Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Deyang, Sichuan, China
- 3Department of Clinical Laboratory, Ya’an People’s Hospital, Yaan, Sichuan, China
- 4College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Department of Nuclear Medicine, Ya’an People’s Hospital, Yaan, Sichuan, China
The increasing prevalence of antimicrobial resistance (AMR) has led to the gradual decline in the effectiveness of existing antibiotics, posing a significant threat to global health. Many phytochemicals have antimicrobial activity, but few have been developed for clinical use. Berberine, an alkaloid found in various medicinal plants, has been recognized as a promising strategy to combat AMR due to its notable antimicrobial activity and role in reversing resistance. Here, we present a systematic, comprehensive and objective overview of the antimicrobial activity, mechanism of action, and limitations of berberine. Additionally, we discuss the antimicrobial efficacy of berberine extracts and nanoformulations. Berberine demonstrates broad-spectrum antimicrobial activity by inhibiting FtsZ, disrupting cell membranes and cell walls, and interfering with DNA and RNA synthesis. However, due to its low bioavailability and lack of systematic in vivo validation, the efficacy of berberine as a standalone treatment for bacterial infections requires further investigation. Nevertheless, it can serve as an antibiotic adjuvant to enhance the efficacy of conventional antibiotics and reverse AMR. Moreover, the excellent antimicrobial effects exhibited by berberine extracts and nanoformulations may overcome these limitations, representing potential future applications of berberine. In conclusion, berberine has great potential as an antimicrobial agent and antibiotic adjuvant in combating AMR, but systematic and comprehensive in vivo and clinical trials are still needed to evaluate the therapeutic efficacy of berberine and optimize its use.
Graphical Abstract. Antibacterial mechanisms of berberine in vitro and in vivo and its future applications.
1 Introduction
In recent decades, the rise of antimicrobial resistance (AMR) has elevated bacterial infections to one of the most pressing global public health threats (Larkin, 2023). Pathogenic microorganisms have developed various resistance mechanisms through continuous adaptation and evolution, such as the production of inactivating enzymes, reduced membrane permeability, and antibiotic efflux pumps, which have reduced the available options and clinical efficacy of antibiotics, leading to alarming increases in mortality (Guedes et al., 2024). In 2021, approximately 4.71 million deaths globally were associated with AMR, with 1.14 million directly attributed to AMR. Projections suggest that by 2050, AMR could result in 8.22 million related deaths annually, including 1.91 million directly caused by resistant infections (Kariuki, 2024). This alarming trend is fueled by the overuse and misuse of antibiotics in healthcare and agriculture, a lack of new antimicrobial agents, and inadequate infection control strategies (Hays et al., 2022; Caioni et al., 2024; Lewnard et al., 2024). Importantly, the declining cost-effectiveness of developing new antibiotics, combined with the lack of direct inhibitory effects of resistance mechanism inhibitors on bacterial cells, has resulted in a severe imbalance between the urgent need for antibiotics and the current pace of their development (Seukep et al., 2020; Cook and Wright, 2022). Therefore, there is an urgent need to develop broad-spectrum antibiotics that not only exhibit direct bactericidal activity but also effectively counter AMR.
Today, pharmacologically active plants continue to serve as the primary pharmacopeia in many developing countries, with their clinical efficacy proven through centuries of traditional medicine (Porras et al., 2021). Regrettably, between 1981 and 2019, 50% of the 162 antimicrobials approved by the U.S. Food and Drug Administration were derived from microbial natural products and their derivatives, rather than from plant sources (Porras et al., 2021). However, many excellent recent reviews describe the great potential of plant natural products such as phenolic derivatives, terpenoids, and alkaloids as antimicrobial agents (Newman and Cragg, 2020; Herman and Herman, 2023; Lu et al., 2024). Among them, berberine is considered one of the most promising candidates for antimicrobial drug development. Found in medicinal plants such as Hydrastis canadensis, Berberis aristata, Coptis rhizome, Coptis japonica, and Phellodendron amurense, berberine has a long history of therapeutic use worldwide (Gasmi et al., 2024). It exhibits broad-spectrum antiviral and antifungal activity both in vitro and in vivo and has been shown to act as an antibiotic adjuvant, reversing fungal and bacterial resistance (Warowicka et al., 2020; Zhou H. et al., 2023; Ding et al., 2024). In addition, berberine exhibits a range of other pharmacological effects, including anti-tumor, anti-inflammatory, antimicrobial, and cardiovascular protective properties (Patel, 2021). These attributes enhance its economic viability and clinical application. More importantly, berberine’s low cost, availability, and accessibility offer a practical and feasible strategy for managing antibiotic resistance, particularly in developing countries. Against this backdrop, we provide a comprehensive and systematic review of berberine’s antimicrobial activity and mechanisms, as well as its limitations, with a focus on its effects on a range of pathogenic bacteria over the past two decades (Figure 1). Furthermore, we describe the antimicrobial properties of berberine-containing natural extracts and nanoformulations, exploring potential pathways for its future clinical applications. By addressing the global challenge of bacterial infections, this review aims to provide a theoretical foundation for the further development of berberine and offer practical solutions for managing global AMR.
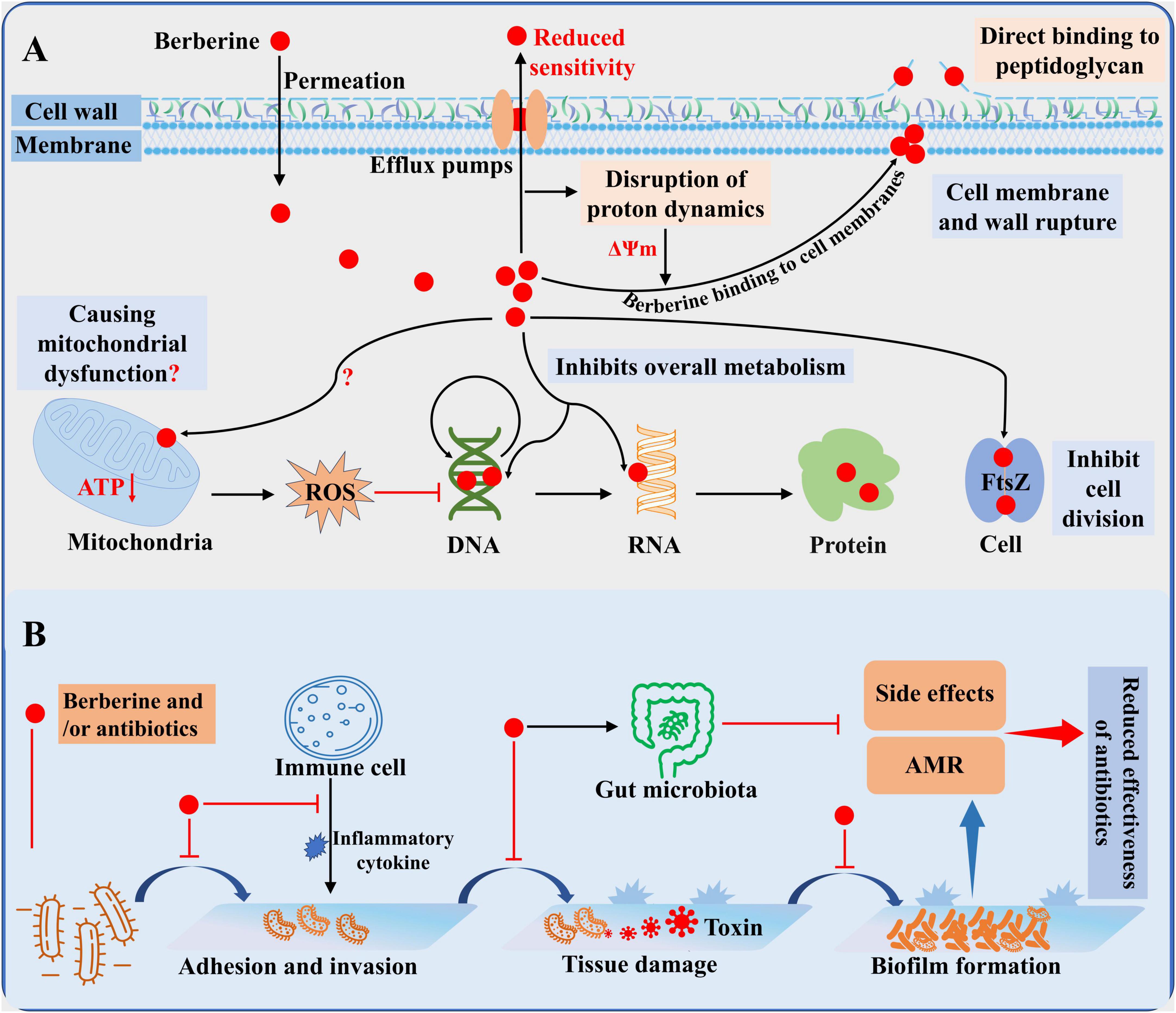
Figure 1. General overview of the antibacterial activity of berberine. (A) Antibacterial mechanism of berberine. (B) Mode of action of berberine alone or in combination in vivo.
2 Literature search strategy
A comprehensive literature search was conducted in three major databases: PubMed, Google Scholar, and Web of Science. The search was restricted to English-language publications from 2000 to 2025. Keywords used included “Berberine,” “Bacteria,” “Antibacterial activity,” “Antibacterial mechanism,” “In vivo,” “In vitro,” “Nanoparticles,” “Extracts,” and various combinations of these keywords. The initial search results were imported into EndNote software for reference management and removal of duplicates. Titles, abstracts, and full texts of the retrieved articles were carefully screened for relevance. Studies were included if they investigated the antibacterial effects and mechanisms of berberine, its nanoformulations, or natural extracts, either in vitro or in vivo, and provided the source of berberine whenever available. Studies not involving bacterial pathogens, relevant infection models, or those unrelated to berberine-based interventions were excluded from the analysis.
3 Antibacterial activities of berberine
3.1 Antibacterial properties of berberine against pathogenic bacteria in vitro
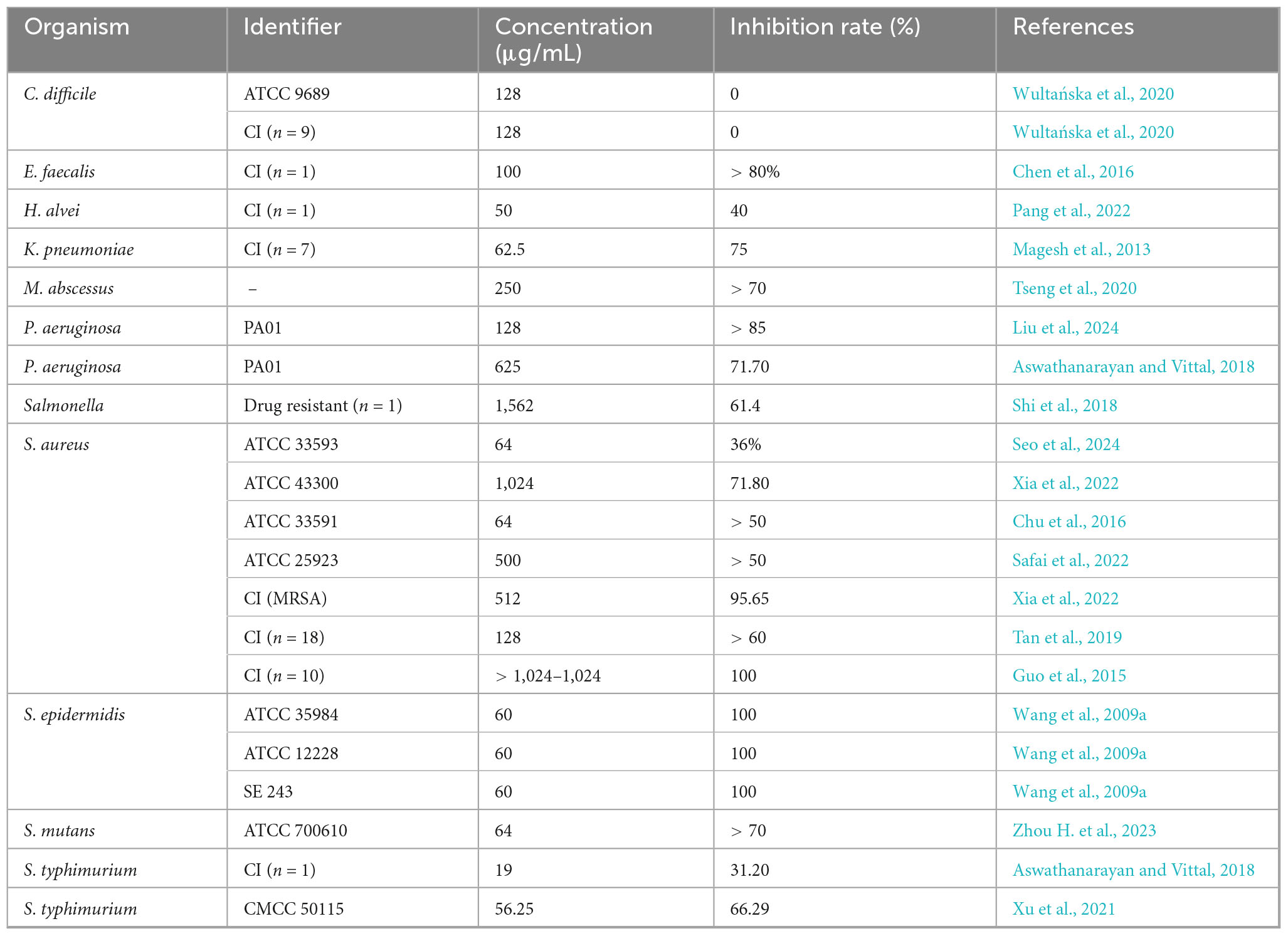
Berberine exhibits broad-spectrum antimicrobial activity (Table 1) and demonstrates moderate efficacy against various pathogens, including World Health Organization priority pathogens such as Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, and Staphylococcus aureus. It also inhibits the growth and proliferation of Prevotella bryantii, Bacteroides fragilis, Acetoanaerobium sticklandii, and Porphyromonas gingivalis (Lakes et al., 2020; Okuda et al., 2023). Meanwhile, berberine inhibits spore growth of C. difficile and Bacillus cereus, thereby reducing the potential harm caused by spore germination (Wang et al., 2016). Moreover, antimicrobial effects of berberine are dose- and time-dependent; for instance, in Escherichia coli and methicillin-resistant S. aureus (MRSA), their survival rates gradually decrease with increasing concentrations of berberine or extended incubation times (Li et al., 2018c; Zhou F. F. et al., 2023). Unlike bacteriostatic agents such as chloramphenicol and clindamycin, berberine exerts bactericidal activity against various pathogens, including MRSA, Staphylococcus epidermidis, C. difficile, and Salmonella typhimurium, though the effective concentrations are substantially higher than their minimum inhibitory concentrations (MICs) (Wang et al., 2009b; Zhang et al., 2013; Peng et al., 2015; Wultańska et al., 2020). For example, the minimum bactericidal concentration for MRSA is 2,560 μg/mL, which is 25 times its MIC (Qiu and Xu, 2024). However, the MIC range of berberine against bacteria varies widely, from 0.78 μg/mL against Streptococcus agalactiae to as high as 100,000 μg/mL against Helicobacter pylori (Huang et al., 2015; Peng et al., 2015). Even for the same pathogen, this variability can be significant. For example, Li et al. (2018a) reported the MIC of berberine against H. pylori to be 25,000–100,000 μg/mL, while Huang et al. (2015) reported it to be 50 μg/mL. These discrepancies may be attributed to differences in their antimicrobial susceptibility testing methods (Columbia blood agar with agar dilution vs. Brucella broth with broth dilution). The antifungal activity of berberine is also influenced by the culture medium (Ding et al., 2024). Indeed, prolonged exposure to berberine led to increased energy demands in E. coli, and the amino acid maintenance strategy shifted from transport to synthesis (Budeyri Gokgoz et al., 2017). Therefore, it can be inferred that berberine’s antimicrobial activity is susceptible to the influence of nutritional substrates, which is also related to its antimicrobial mechanisms.
Another notable characteristic of berberine is the low likelihood of pathogens developing resistance to it. Studies have shown that after 200 generations of exposure to berberine, the MIC of E. coli remained unchanged, while the MICs of neomycin and cefotaxime increased more than 10-fold (Jin et al., 2010). As an amphipathic cation, berberine is a natural substrate for bacterial efflux pumps, which are among the most critical mechanisms of resistance (Seukep et al., 2020). This property renders existing resistance mechanisms less likely to affect berberine’s activity. Consequently, berberine can act as an antibiotic adjuvant, competitively binding to efflux pumps and reducing drug efflux, thereby enhancing the antimicrobial activity of other antibiotics. However, it is important to note that berberine’s effects on efflux pumps vary across species. Recent studies found that low concentrations of berberine promoted the growth and resistance of Klebsiella pneumoniae by upregulating the expression of the efflux pump KmrA, while higher concentrations inhibited its growth (Li Y. et al., 2021). Similarly, overexpression of the efflux pump HmrM in Haemophilus influenzae resulted in an elevated MIC for berberine (Xu et al., 2003). In contrast, in P. aeruginosa, berberine reduced AMR by inhibiting the MexXY-OprM efflux pump (Su and Wang, 2018). This complex interaction is consistent with the varying antimicrobial activities of berberine against different strains. Additionally, low doses of berberine have been reported to have mitohormesis, offering protective benefits to neuroprotective cells (Zhu et al., 2020). Indeed, low concentration of berberine also promotes the growth of C. difficile biofilms, as well as Enterobacter cloacae and A. baumannii (Wultańska et al., 2020; Li Y. et al., 2021). Therefore, although berberine exhibits significant antimicrobial activity, the potential toxicity at low doses and the impact of drug efflux pumps on its efficacy should still be considered.
3.2 Toxicity-modulating effects of berberine
Virulence factors such as adhesion, biofilms, toxins, and quorum-sensing molecules not only help pathogenic bacteria evade host immune surveillance to promote colonization, but also synergistically invade host cells to cause damage (Lu et al., 2024). Berberine has been reported to directly inhibit the production of enterotoxin in certain Vibrio cholerae and E. coli for the treatment of bacterial diarrhea (Fu et al., 2010). In Aeromonas hydrophila, berberine similarly inhibits endotoxin and hemolysin secretion in a dose-dependent manner, reducing its hemolytic activity (Xue et al., 2015). Recent studies have also shown that berberine can inhibit the activities of pyocyanin and urease, thereby reducing the virulence and colonization of P. aeruginosa and H. pylori (Li et al., 2018a; Zhao et al., 2022). Our study also demonstrated that that subinhibitory concentrations of berberine reduce the production of C. difficile toxins TcdA and TcdB by inhibiting toxin synthesis genes, thereby decreasing its cellular invasiveness (Yang et al., 2025). In addition to directly inhibiting toxin production, berberine also exhibits significant anti-adhesion and anti-invasion properties. Berberine was found to inhibit adhesion and migration of HEp-2 cells induced by Chlamydia pneumoniae infection, thereby reducing the invasive power of HEp-2 cells (Zhang et al., 2011). In bacterial infections, berberine (20 μg/mL) reduced Salmonella Typhimurium adhesion and invasion of colon cells by 54.86% and 55.37%, respectively (Aswathanarayan and Vittal, 2018). Moreover, berberine could attenuate the adhesion and intracellular invasion of MRSA on epithelial cells and reduce its induced apoptosis in a dose-dependent manner (Yu et al., 2005; Xiong et al., 2014). Importantly, at concentrations effective against bacterial virulence, berberine does not exhibit toxicity to red blood cells, thymocytes, or splenocytes (Laudadio et al., 2019; Jhanji et al., 2021). Additionally, berberine downregulates the synthesis of staphyloxanthin by inhibiting the expression of the S. aureus Fni gene. Staphyloxanthin stabilizes the cell membrane by reducing membrane fluidity, enhancing its resistance to both host defenses and antibiotics (Qiu and Xu, 2024). N-acetyltransferase, associated with AMR in bacteria, promotes bacterial tolerance to aminoglycoside antibiotics. Berberine down-regulated N-acetyltransferase protein and gene expression in S. aureus, H. pylori, and Salmonella typhi in a dose-dependent manner (Wu et al., 2005; Wang et al., 2008; Chang et al., 2011).
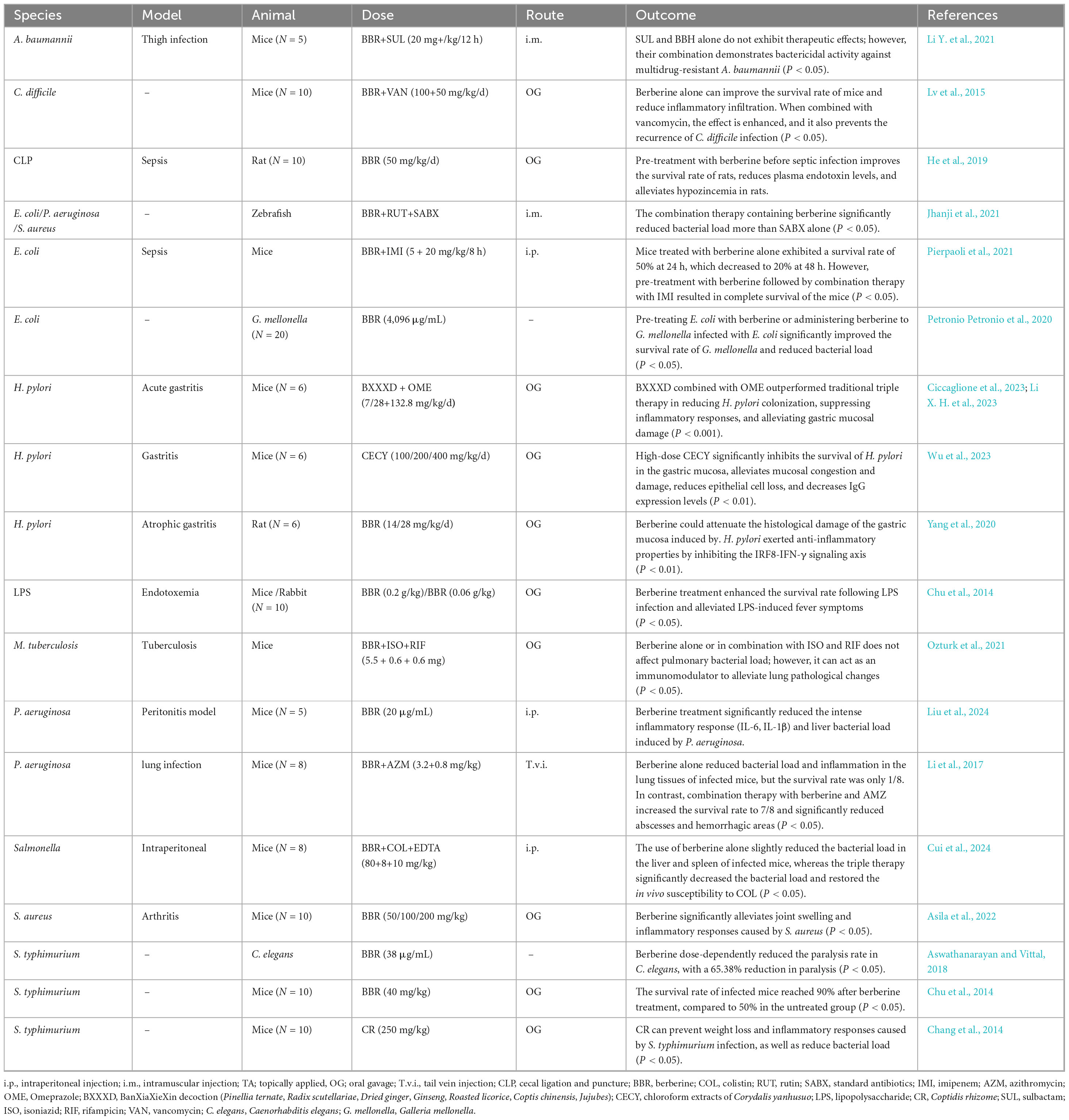
Biofilms are critical virulence factors for many pathogenic microorganisms. Bacteria commonly adhere to host tissues such as carious teeth or the lungs of cystic fibrosis patients, or the surfaces of medical devices including artificial joints, heart valves, and urinary catheters, by forming biofilms. These structured microbial communities enhance antibiotic resistance, facilitate immune evasion, promote chronic infections, and can lead to secondary infections (Bouhrour et al., 2024). As shown in Table 2, berberine at concentrations ranging from 50 to 500 μg/mL significantly inhibited biofilm formation by clinically relevant pathogens, including S. aureus, S. epidermidis, P. aeruginosa, and K. pneumoniae, with inhibition rates exceeding 50% across all strains. In S. aureus, berberine not only inhibits biofilm formation in a dose-dependent manner but also interferes with the late-stage dispersal phase of biofilm development, thereby preventing the establishment of persistent bacterial colonies and reducing the risk of recurrent infections (Chu et al., 2016; Zhang et al., 2022). More importantly, studies have shown that a berberine-loaded liposomal hydrogel can effectively disrupt S. aureus biofilms and reduce biofilm biomass in infected mouse wounds, thereby promoting wound healing (Li S. et al., 2023). Another study demonstrated that when used as a root canal irrigant, berberine reduced bacterial counts by up to 99% in a multi-species dentin biofilm model containing Fusobacterium nucleatum, E. faecalis, and Prevotella intermedia (Xie et al., 2012). Wang et al. (2009a) also reported that berberin significantly inhibited the initial adhesion of S. epidermidis to titanium alloy disks (a common orthopedic implant material) within just 2 h, thereby preventing biofilm formation. Moreover, in biofilms formed by clinical isolates of P. aeruginosa, berberine significantly enhanced the antibacterial activity of tobramycin, reducing bacterial tolerance to the antibiotic by 10- to 1,000-fold (Mangiaterra et al., 2021). Shi et al. (2018) also found that berberine, when combined with ciprofloxacin, exerts a synergistic effect against biofilms formed by multidrug-resistant Salmonella strains by inhibiting the expression of the quorum-sensing system. Indeed, berberine disrupts biofilm formation and prevents dispersal of biofilm cells by downregulating related genes, inhibiting extracellular genomic DNA release and expression of polysaccharide intercellular adhesins, and interacting with quorum-sensing receptors (Guo et al., 2015; Zhou H. et al., 2023). Therefore, berberine shows significant potential in inhibiting bacterial biofilm formation and enhancing antibiotic sensitivity, offering a promising adjunctive strategy for the prevention and treatment of biofilm-associated infections.
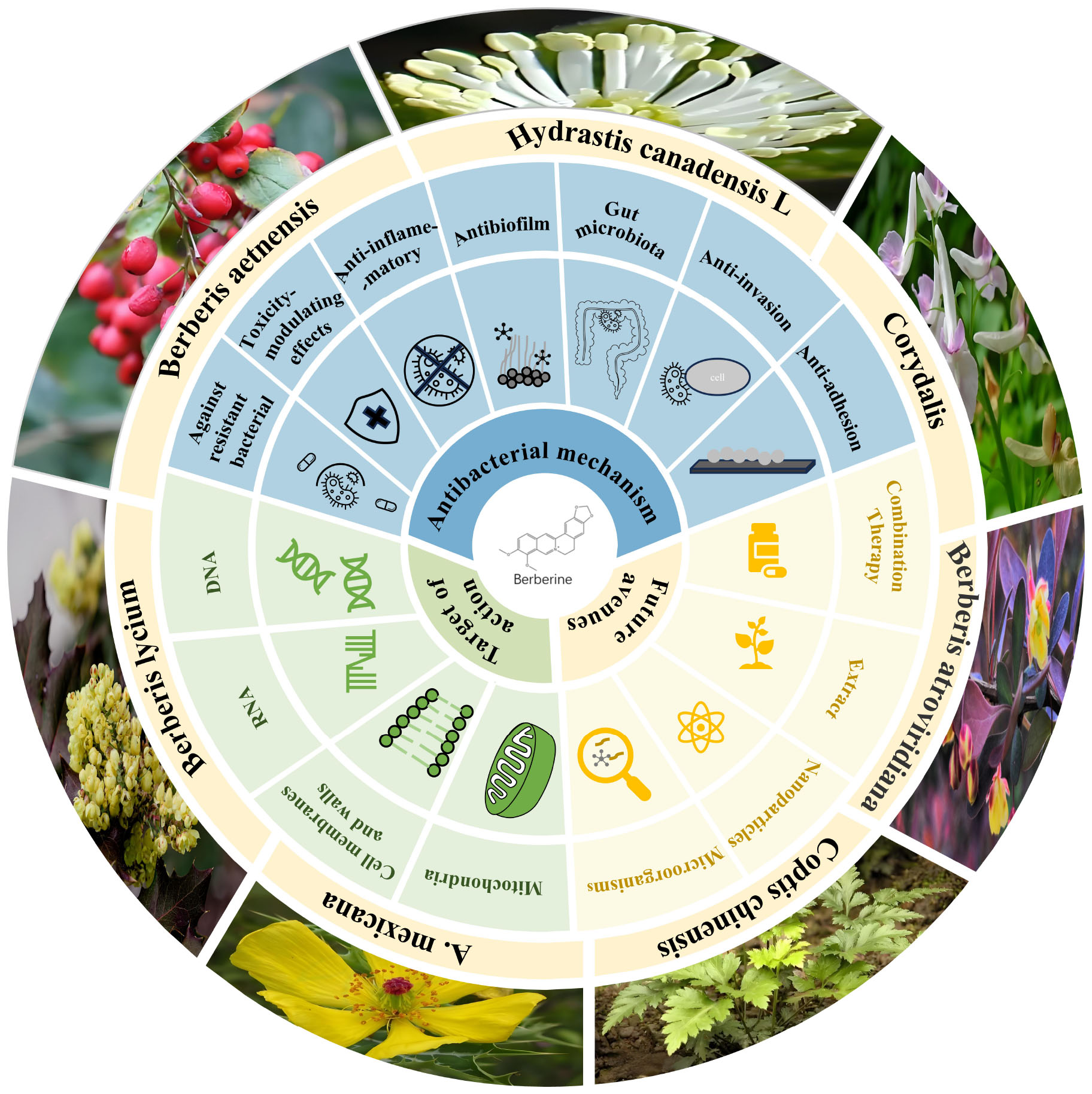
3.3 Therapeutic efficacy of berberine in treating bacterial infections in vivo
Although the antibacterial activity of berberine has been well-characterized in vitro, limited in vivo studies have not fully elucidated its therapeutic potential. Table 3 provides a detailed summary of in vivo studies on berberine. Briefly, berberine exhibited a strong therapeutic effect on inflammatory responses induced by bacterial infections in vivo, whereas its direct antibacterial activity is comparatively weaker. For example, in P. aeruginosa infections, berberine reduces the bacterial burden in infected mice, but more noteworthy is its potent anti-inflammatory activity (Liu et al., 2024). Previous studies have also reported that berberine significantly reduces osteoclast recruitment and bone resorption, demonstrating a therapeutic effect on lipopolysaccharide-induced osteolysis (Zhou et al., 2012). Indeed, berberine can attenuate inflammatory responses, coagulation activation, and organ dysfunction caused by bacterial infections through multiple mechanisms, including inhibition of the caspase-11 pathway and inhibition of COX-2 overexpression (Feng et al., 2012; Yuan et al., 2021). These aspects have been comprehensively reviewed by Izadparast et al. (2022), and the reader is referred to their work for more detailed information ( doi: 10.1080/15384101.2022.2100682). However, the antibacterial activity of berberine in vivo is relatively weaker compared to its in vitro efficacy. The probable reason for this is the low oral utilization and intestinal absorption of berberine and its very rapid blood clearance (Singh et al., 2021). After oral administration of 40 mg/kg berberine to mice, only trace amounts of berberine were detected in the plasma (Zuo et al., 2006). In human subjects, plasma berberine concentrations ranged from 1.23 to 2.10 ng/mL at 24 h after a 500 mg oral dose (Solnier et al., 2023). Although no side effects or adverse events were reported, such low plasma levels may prevent berberine from achieving effective antibacterial concentrations at infection sites, thereby limiting its clinical application. It is noteworthy that the cytotoxic threshold of berberine varies significantly across different cell lines: in L929 mouse fibroblast cells, cell viability decreases at concentrations as low as 50 μg/mL, whereas the half-maximal inhibitory concentrations in human HepG2 liver cells, NIH/3T3 fibroblasts, and 293T kidney cells are above 90, 100, and 80 μg/mL, respectively (Gu et al., 2015; Tong et al., 2021). Although in vitro results may not fully reflect in vivo conditions, its safety profile in vivo remains insufficiently characterized, particularly due to a lack of systematic evaluation of the effects of long-term or high-dose administration on major organs and tissues. Therefore, further investigation into the toxicological mechanisms of berberine in vivo is necessary to comprehensively clarify its safety. Nevertheless, as shown in Table 3, berberine exhibits significant synergistic effects with antibiotics in vivo, enhancing their antibacterial efficacy. In two randomized, open-label, non-inferiority clinical trials, a berberine-containing quadruple therapy demonstrated similar eradication rates and symptom improvement compared to conventional quadruple therapies for H. pylori infection (Zhang et al., 2017, 2020). In addition, berberine can alleviate drug-induced diarrhea and intestinal mucosal damage by modulating the intestinal microbiota, such as the anticancer agents irinotecan and 5-fluorouracil (Chen et al., 2020; Yue et al., 2021). Therefore, berberine holds promise as an antibiotic adjuvant for clinical antimicrobial therapy, helping to address the growing threat of AMR. Notably. antibiotics can negatively impact the gut microbiota, thereby reducing berberine’s bioavailability (Feng et al., 2015). Further studies on synergistic administration regimens of berberine and antibiotics are still needed in the future. Given berberine’s high tolerance and high LD50 (oral: 329 mg/kg, injection: 23 mg/kg) (Gasmi et al., 2024), along with its low propensity to induce resistance, increasing the berberine dose in combination therapy while reducing the antibiotic dose could be considered as a strategy to mitigate the occurrence of AMR.
Overall, berberine exhibits dose- and time-dependent antimicrobial effects against clinically relevant pathogens, with its anti-inflammatory properties, low potential for resistance, and ability to mitigate drug side effects highlighting its potential as both an antimicrobial agent and antibiotic adjuvant. However, its low bioavailability, potential cytotoxicity, and lack of comprehensive in vivo evaluation hinder its clinical application. Future research should focus on addressing these challenges, particularly through systematic in vivo studies. Additionally, standardized evaluation methods are needed to resolve the MIC discrepancies observed in current studies, with the approach proposed by Alharthi et al. (2021) providing a solution ( doi: 10.1016/j.bmc.2021.116527).
4 Antibacterial mechanisms of berberine
4.1 Berberine inhibits bacterial division by targeting the FtsZ protein
Filamentous temperature-sensitive mutant Z (FtsZ) is a key organizer of bacterial cell division. During the division process, FtsZ associates with membrane-associated proteins and assembles into protofilaments through GTP-dependent polymerization, forming a Z-ring that ensures the correct localization of other division proteins such as FtsA and ZipA (Cameron and Margolin, 2024). Berberine can target FtsZ to inhibit bacterial growth. In E. coli, berberine significantly reduces Z-ring formation, and silencing the FtsZ gene enhances bacterial sensitivity to berberine, reducing its MIC by 2-fold. Conversely, overexpression of FtsZ increases resistance to berberine (Boberek et al., 2010). Consistently, another study demonstrated that berberine treatment severely disrupts E. coli cell division, resulting in significantly elongated cells (Budeyri Gokgoz et al., 2017). Berberine spontaneously binds to the GTP-binding pocket of FtsZ in a dose-dependent manner and a 1:1 ratio, inhibiting FtsZ monomer interactions and disrupting the formation of FtsZ protofilaments. This results in the mislocalization and spatial disorganization of the Z-ring, thereby hindering cell division (Domadia et al., 2008). Notably, FtsZ is highly conserved and widely present across various bacterial species. In B. anthracis, MRSA, and E. faecium, berberine also exhibits significant inhibitory effects on the GTPase and polymerization activities of FtsZ (Park et al., 2014; Sun et al., 2014). Through virtual screening and computational methods, recent studies have revealed that berberine can form stable complexes with the FtsZ of Mycobacterium tuberculosis and Salmonella typhi, demonstrating high binding affinity (Akinpelu et al., 2022; Naz et al., 2022). This may explain the broad-spectrum antibacterial activity and dose-dependent inhibitory effects exhibited by berberine.
4.2 Berberine targets bacterial cell membranes and walls to disrupt cell structure
The cell membranes and cell walls are primary targets for existing antibiotics. For instance, β-lactam antibiotics prevent the cross-linking of peptidoglycan in the bacterial cell wall. Peptide antibiotics interfere with cell membrane synthesis by inhibiting lipid integration into the cell membrane (Baran et al., 2023). Berberine, however, binds to cell membranes and cell walls by a mechanism of action different from the above, thereby inhibiting bacterial growth. Due to the lack of extensive hydrogen bonding, berberine, in its positively charged form, can intercalate into lipid bilayers and penetrate the cell interior. Nevertheless, it also disrupts the phospholipid bilayer (Sokolov et al., 2023). Upon exposure to berberine, bacteria such as P. aeruginosa, S. agalactiae, and A. pleuropneumoniae exhibit features of membrane lysis and cell wall damage, including cytoplasmic shrinkage and leakage of cell contents (Kang et al., 2015; Peng et al., 2015; Liu et al., 2024). Recent studies have shown that berberine increases membrane permeability in MRSA in a dose-dependent manner and directly adheres to the bacterial cell wall, disrupting its structure and leading to cell lysis (Zhou F. F. et al., 2023). Similar alterations in cell surface structure were observed in E. coli, accompanied by the release of Ca2 + and K+ ions (Jin et al., 2010). Indeed, berberine can directly bind to cell wall components, such as lipopolysaccharides and peptidoglycans, disrupting normal cell wall physiological processes (Li et al., 2018c). Notably, in fungi, berberine also damages the cell membrane by inhibiting enzymes and downregulating genes involved in ergosterol synthesis (Ding et al., 2024). However, bacteria like E. coli upregulate genes related to cell wall and membrane transport and synthesis after berberine exposure (Zhang et al., 2009; Karaosmanoglu et al., 2014). This response may represent a stress reaction to membrane and wall damage, but also indicates that berberine does not inhibit the expression of these genes to disrupt the cell membrane. As previously mentioned, berberine is a natural substrate of efflux pumps, which increase membrane potential by exporting protons, thereby attracting positively charged molecules such as berberine. Zhao et al. (2023) demonstrated that berberine efflux via drug efflux pumps dissipates membrane potential, resulting in increased intracellular accumulation of berberine and heightened membrane instability. This may also explain the time-dependent antibacterial activity of berberine and its effects on the cell membrane. However, due to the effect of the efflux pump, this can also lead to a decrease in bacterial sensitivity to berberine, as has been demonstrated in several studies (Xu et al., 2003; Li et al., 2018b). In summary, berberine primarily exerts its effects on bacterial cell walls and membranes through its physical properties, disrupting the normal structure of the cell.
4.3 Berberine inhibits the fundamental metabolic processes of bacteria
Berberine has a high affinity for DNA and RNA. It causes DNA damage by inserting into the DNA structure and forming strong interactions through hydrogen bonding, van der Waals forces, and electrostatic forces (Budeyri Gokgoz et al., 2017). Studies have shown that berberine exerts anticancer activity by disrupting cell division and inducing apoptosis through binding to histone-DNA complexes (Sokolov et al., 2023). In bacteria, berberine not only binds to DNA and RNA, causing damage, but also inhibits essential biological processes such as DNA, RNA, and protein synthesis. Binding kinetics indicate that berberine readily binds to and remains tightly bound to DNA and RNA in E. coli, thereby inhibiting DNA replication, RNA transcription, and protein synthesis to exert antibacterial activity (Jin et al., 2010). Consistently, in A. pleuropneumoniae and S. agalactiae, berberine reduced DNA and protein levels in a time-dependent manner, probably due to its gradual accumulation within the cell (Kang et al., 2015; Peng et al., 2015). In addition, berberine disrupts the redox homeostasis of bacteria, generating reactive oxygen species (ROS) that attack key cellular components such as DNA, membranes, and mitochondria. Avci et al. (2019) reported that berberine induced oxidative stress and intracellular accumulation of reactive substances in E. coli. Similarly, elevated ROS levels were observed in P. aeruginosa and Streptococcus pyogenes after treatment with berberine, and co-culture with antioxidants partially attenuated their antimicrobial activity (Du et al., 2020; Liu et al., 2024). In fungi, mitochondrial dysfunction caused by ROS is the main antifungal mechanism of berberine (Ding et al., 2024). Although berberine has been reported to inhibit intracellular ATP production (Liu et al., 2024), its specific effect on bacterial mitochondrial function remains unclear and deserves further investigation. Nevertheless, ROS production still contributes to the antibacterial activity of berberine. However, bacterial DNA damage triggers the SOS response (a post-replicative DNA repair system), which inhibits bacterial division. In E. coli, berberine inhibits bacterial division in wild-type and SOS-negative strains, while SOS-negative strains do not respond to SOS-induced inhibition of cell division (Boberek et al., 2010). Furthermore, recent studies have found that only about 5% of the berberine accumulated in S. aureus cells binds to DNA (Zhao et al., 2023). This suggests that the primary mechanism of berberine’s inhibition of bacterial division involves targeting FtsZ, while its effects on DNA, RNA, and proteins predominantly influence bacterial metabolic processes. In various bacteria, including E. coli, Yersinia pestis, and S. flexneri, significant changes have been observed in metabolic pathways such as carbohydrate metabolism, energy production and conversion, DNA replication and repair, pyrimidine metabolism, RNA degradation, and ribosome function (Zhang et al., 2009; Fu et al., 2010; Budeyri Gokgoz et al., 2017). Notably, DNA replication, repair, and pyrimidine metabolism are significantly upregulated in response to berberine-induced DNA damage. Thus, berberine exerts its antibacterial activity synergistically by targeting key biomolecules and disrupting essential bacterial metabolic processes.
In conclusion, berberine exhibits antibacterial activity through a multifaceted mechanism, including targeting FtsZ, disrupting the cell membrane and cell wall, and interacting with DNA, RNA, proteins, and bacterial redox homeostasis. This multi-target mode of action not only disrupts fundamental bacterial processes but also hinders the development of resistance to berberine. However, the adverse effects of drug efflux pumps significantly limit the application of berberine by reducing its intracellular accumulation and thus diminishing its antibacterial efficacy. Therefore, future research aimed at overcoming efflux pump-mediated resistance holds promise for enhancing the therapeutic potential of berberine.
5 Future avenues of application for berberine
5.1 Synergistic antibacterial activity and mechanisms of berberine-containing natural extracts
Berberine is found in various medicinal plants, including Hydrastis canadensis, Berberis aristata, Coptis chinensis, and Coptis rhizome (Zhou H. et al., 2023). These plants are distributed globally and offer significant advantages such as accessibility and low cost. More importantly, the extracts from these plants also exhibit direct antimicrobial activity. Supplementary Table 1 (Antibacterial activity of berberine (BBR) extracts against bacteria) summarizes the antimicrobial activity of berberine extracts. In brief, berberine extracts display antimicrobial activity similar to or even superior to that of pure berberine, although the results are not universally consistent. For instance, in the same study, Hydrastis canadensis extract had an MIC of 15 mg/mL against P. aeruginosa, whereas the MIC of berberine was > 120 mg/mL, and the reverse was observed for S. aureus (Scazzocchio et al., 2001). This differential effect may be related to the mode of interaction between berberine and other active ingredients in the extract. Previous studies have demonstrated that 5′-methoxyhydnocarpin isolated from Berberis fremontii can inhibit drug efflux pumps, thereby increasing the intracellular accumulation of berberine and reducing its MIC against S. aureus by 8-fold (Stermitz et al., 2000). Moreover, the extracts of Lupinus argenteus and Hydrastis canadensis L. also demonstrate synergistic effects with berberine (Morel et al., 2003; Ettefagh et al., 2011). Table 4 summarizes other plant compounds that exhibit synergistic effects when combined with berberine. While no antagonistic effects have been reported with berberine, it is plausible that such compounds may exist in plant extracts. Notably, antimicrobial activity varies among different parts of the same plant. For example, the MIC of Berberis microphylla root extract against S. aureus is 2–3 times higher than that of its leaf and stem extracts (Manosalva et al., 2016). Furthermore, plant extracts also exhibit in vivo anti-inflammatory and antimicrobial activities, as outlined in Table 3. Recent studies have demonstrated that Coptis chinensis extract inhibits the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and the NF-κB signaling pathway induced by Propionibacterium acnes, showing its potential for treating acne-related inflammatory skin conditions (Lee et al., 2018). In another study, Berberis aristata extract demonstrated not only in vitro antibacterial activity against Shigella but also exhibited antidiarrheal activity in vivo, with an LD50 > 5,000 mg/kg (Joshi et al., 2011). Given that these plants have been used in traditional ethnomedicine for centuries, their extracts possess great therapeutic potential in combating antimicrobial infections, potentially offering a promising strategy to address the escalating global threat of AMR.
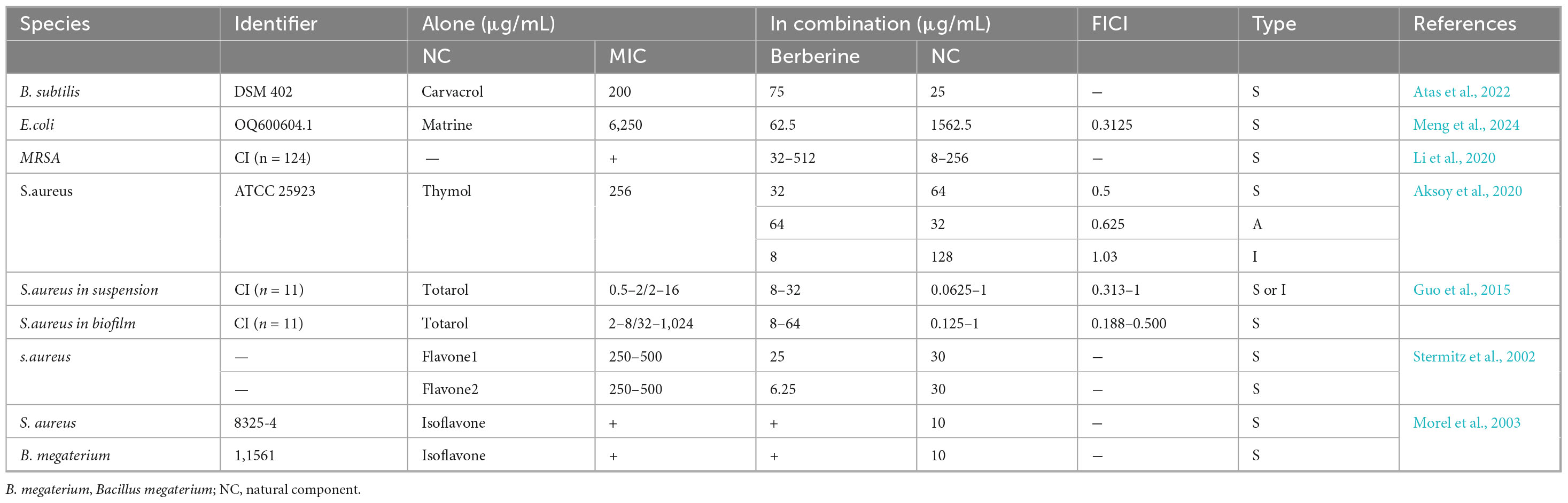
Table 4. The effect of the combination of berberine (BBR) and natural components on the minimum inhibitory concentrations (MICs) of bacterial.
5.2 Antibacterial activity and physical properties of berberine nanoparticles
Although berberine has potential cytotoxicity and poor bioavailability, its combination with nanotechnology can overcome these limitations. As summarized in Supplementary Table 2 (MICs of berberine nanoformulations against bacteria), when berberine is combined with nanocarrier systems such as liposomes, shellac, and metal ions, it exhibits improved biocompatibility, low toxicity, high bioavailability, and enhanced antimicrobial activity. Compared to free berberine, lipid-reconstituted nanoparticle-coated poly (lactic-co-glycolic acid) nanoparticles loaded with berberine have a significantly lower MIC of 5 μg/mL against Mycobacterium smegmatis, whereas the MIC of free berberine is 100 μg/mL (Pu et al., 2024). Al-Obaidy et al. (2019) also observed that dual-functionalized shellac nanocarriers can enhance the local concentration of berberine, thereby improving its biological stability and bioavailability. In another study, gold nanoparticles were shown to double the antimicrobial activity of berberine against S. aureus biofilms. In an infected skin model, berberine-loaded gold nanoparticles reduced the survival rate of MRSA to only 2.7%, with no observed toxicity in mouse fibroblast cells (Sadeghi et al., 2024). In addition, the physical and chemical properties of different nanocarriers significantly influence their performance in drug delivery systems and in vivo applications. For example, liposomes encapsulating berberine achieve an encapsulation efficiency of up to 69.8% (Pu et al., 2024). In contrast, shellac and metal-organic frameworks (MOFs) exhibit lower encapsulation efficiencies of approximately 60% and 35%, respectively. However, under near-physiological pH conditions, shellac and MOFs demonstrate higher drug release rates, reaching up to 80%, whereas liposomes release only 57.3% of the encapsulated drug (Al-Obaidy et al., 2019; Hu et al., 2023; Pu et al., 2024). Furthermore, liposomal encapsulation of berberine can improve its bioavailability by prolonging its in vivo retention time (Sun et al., 2024). According to the findings of Abo El-Enin et al. (2022), the concentration of berberine at the target site was 13.2 times higher in the liposome-treated group compared to the control group, indicating significantly enhanced targeting efficiency. The shellac-based delivery system exhibits strong adhesion to microbial cell walls, which further improves targeting and enhances antimicrobial activity (Sun et al., 2024). Remarkably, MOFs have demonstrated pronounced advantages in targeted delivery. For instance, Wang et al. (2017) developed magnetic mesoporous silica nanoparticles capable of controlled drug release under an external magnetic field. However, the elemental composition and surface charge of metallic nanoparticles may increase their toxicity (Sun et al., 2024). Despite these promising findings, many types of nanocarriers still lack comprehensive in vivo evaluations. Therefore, further preclinical and clinical investigations are warranted to substantiate their safety and therapeutic efficacy. Moreover, drug self-assembled nanoparticles, which do not require carriers, not only retain these advantages but also exhibit higher drug-loading capacity. For example, self-assembled nanoparticles of berberine and flavonoids show enhanced affinity for S. aureus, leading to bacterial collapse and reduced biofilm formation, while demonstrating good biocompatibility in zebrafish toxicity assessments (Li et al., 2019). Recent studies have also shown that gallic acid and berberine nanoparticles, formed through electrostatic interactions, π-π stacking, and hydrophobic interactions, exhibit antimicrobial and anti-biofilm activities in a S. aureus wound infection model, along with potent anti-inflammatory and pro-angiogenic effects (Chen et al., 2023). Therefore, utilizing the unique properties of nanomaterials can enhance the antimicrobial efficacy of berberine in vivo, offering an alternative strategy to combat the growing threat of AMR. However, the research on self-assembly still has problems such as preparation stability, which will be a direction for subsequent research.
In summary, berberine extracts and nanomaterial-based formulations offer distinct advantages in antibacterial therapy. Berberine extracts have a long history of use in treating inflammation and bacterial diarrhea, with promising applications in combating bacterial infections. In contrast, berberine nanomaterials exhibit enhanced bioavailability and lower toxicity, further improving both efficacy and biological safety. However, comprehensive in vivo studies are still lacking. Moreover, the significant antibacterial activity exhibited by berberine derivatives provides new avenues for its further development, as systematically summarized by Xiao et al. (2018), Jamshaid et al. (2020).
6 Conclusion and perspective
To address the growing global threat of AMR, natural bioactive compounds offer a promising therapeutic strategy. Compared to existing single-target antimicrobial drugs, the natural active compound berberine not only exhibits a multi-target mechanism of action against bacteria, but also has lower toxicity, fewer side effects, and offers beneficial effects by reducing the adverse reactions associated with antibiotics. Importantly, not only are berberine-containing medicinal plants widely distributed and traditionally used throughout the world, but the extraction of berberine from medicinal plants is also consistent with healthcare economics. These factors highlight the significant potential of berberine as an antimicrobial agent. However, the clinical application of berberine is significantly limited by factors such as potential cytotoxicity, low bioavailability, insufficient systematic in vivo evaluation of its antimicrobial activity, and the impact of drug efflux pumps. Although combining with nanotechnology may improve the above-mentioned drawbacks of berberine, systematic in vivo validation is lacking. More importantly, there is a lack of systematic research and in-depth discussion on the current status of clinical trials and the regulatory landscape of berberine in the field of antibacterial therapy, which significantly limits its clinical translation for infectious diseases. In the current context, the clinical use of berberine may be limited to its use as an antibiotic adjuvant against AMR bacterial infections or the use of berberine decoction for the treatment of mild infections such as skin and mucous membrane infections. Future research should focus on optimizing berberine-based formulations and conducting systematic in vivo and clinical studies to thoroughly evaluate its long-term safety, in vivo efficacy, and clinical applicability, thereby advancing the clinical application of berberine. To date, no plant-derived active compound has successfully passed clinical trials. Further research on berberine may pave the way for the application of plant-derived compounds in antimicrobial therapy. Achieving this milestone requires the collective effort of researchers, but it remains the ultimate goal for pharmacologists and microbiologists.
Author contributions
XY: Visualization, Project administration, Formal analysis, Methodology, Writing – review & editing, Conceptualization, Writing – original draft. YW: Conceptualization, Project administration, Visualization, Methodology, Writing – review & editing, Writing – original draft. LL: Project administration, Writing – review & editing, Visualization, Methodology, Conceptualization, Writing – original draft. DT: Investigation, Data curation, Validation, Writing – original draft. ZY: Data curation, Resources, Writing – original draft. ML: Data curation, Resources, Writing – original draft. JJ: Writing – original draft, Formal analysis, Data curation. DB: Funding acquisition, Supervision, Project administration, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Science and Technology Research Special Project of the Sichuan Provincial Administration of Traditional Chinese Medicine (2024MS577).
Acknowledgments
We thank all the editors and reviewers who participated in the review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1643409/full#supplementary-material
References
Abo El-Enin, H. A., Elkomy, M. H., Naguib, I. A., Ahmed, M. F., Alsaidan, O. A., Alsalahat, I., et al. (2022). Lipid nanocarriers overlaid with chitosan for brain delivery of berberine via the nasal route. Pharmaceuticals 15:281. doi: 10.3390/ph15030281
Aghayan, S. S., Kalalian Mogadam, H., Fazli, M., Darban-Sarokhalil, D., Khoramrooz, S. S., Jabalameli, F., et al. (2017). The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J. Med. Biotechnol. 9, 2–7.
Ahmadi, F., Khalvati, B., Eslami, S., Mirzaii, M., Roustaei, N., Mazloomirad, F., et al. (2022). The inhibitory effect of thioridazine on adeB efflux pump gene expression in multidrug-resistant Acinetobacter baumannii isolates using real time PCR. Avicenna J. Med. Biotechnol. 14, 132–136. doi: 10.18502/ajmb.v14i2.8884
Akinpelu, O. I., Kumalo, H. M., Mhlongo, S. I., and Mhlongo, N. N. (2022). Identifying the analogues of berberine as promising antitubercular drugs targeting Mtb-FtsZ polymerisation through ligand-based virtual screening and molecular dynamics simulations. J. Mol. Recognit. 35:e2940. doi: 10.1002/jmr.2940
Aksoy, C. S., Avci, F. G., Ugurel, O. M., Atas, B., Sayar, N. A., and Akbulut, B. S. (2020). Potentiating the activity of berberine for Staphylococcusaureus in a combinatorial treatment with thymol. Microb. Pathog. 149:104542. doi: 10.1016/j.micpath.2020.104542
Alharthi, S., Ziora, Z. M., and Moyle, P. M. (2021). Optimized protocols for assessing libraries of poorly soluble sortase A inhibitors for antibacterial activity against medically-relevant bacteria, toxicity and enzyme inhibition. Bioorg. Med. Chem. 52:116527. doi: 10.1016/j.bmc.2021.116527
Al-Obaidy, S. S. M., Greenway, G. M., and Paunov, V. N. (2019). Dual-functionalised shellac nanocarriers give a super-boost of the antimicrobial action of berberine. Nanoscale Adv. 1, 858–872. doi: 10.1039/c8na00121a
Asila, A., Liu, J., Liu, J., Li, L., and Liao, J. (2022). Immunomodulatory effects of berberine on Staphylococcus aureus-induced septic arthritis through down-regulation of Th17 and Treg signaling pathways. Acta Biochim. Pol. 69, 215–226. doi: 10.18388/abp.2020_5948
Aswathanarayan, J. B., and Vittal, R. R. (2018). Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 8, 36133–36141. doi: 10.1039/c8ra06413j
Atas, B., Aksoy, C. S., Avci, F. G., Sayar, N. A., Ulgen, K., Ozkirimli, E., et al. (2022). Carvacrol enhances the antimicrobial potency of berberine in Bacillus subtilis. Curr. Microbiol. 79:135. doi: 10.1007/s00284-022-02823-7
Avci, F. G., Atas, B., Aksoy, C. S., Kurpejovic, E., Gulsoy Toplan, G., Gurer, C., et al. (2019). Repurposing bioactive aporphine alkaloids as efflux pump inhibitors. Fitoterapia 139:104371. doi: 10.1016/j.fitote.2019.104371
Bandyopadhyay, S., Patra, P. H., Mahanti, A., Mondal, D. K., Dandapat, P., Bandyopadhyay, S., et al. (2013). Potential antibacterial activity of berberine against multi drug resistant enterovirulent Escherichia coli isolated from yaks (Poephagus grunniens) with haemorrhagic diarrhoea. Asian Pac. J. Trop. Med. 6, 315–319. doi: 10.1016/s1995-7645(13)60063-2
Baran, A., Kwiatkowska, A., and Potocki, L. (2023). Antibiotics and bacterial resistance-A short story of an endless arms race. Int. J. Mol. Sci. 24:57777. doi: 10.3390/ijms24065777
Boberek, J. M., Stach, J., and Good, L. (2010). Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS One 5:e13745. doi: 10.1371/journal.pone.0013745
Bouhrour, N., Nibbering, P. H., and Bendali, F. (2024). Medical device-associated biofilm infections and multidrug-resistant pathogens. Pathogens 13:393. doi: 10.3390/pathogens13050393
Budeyri Gokgoz, N., Avci, F. G., Yoneten, K. K., Alaybeyoglu, B., Ozkirimli, E., Sayar, N. A., et al. (2017). Response of Escherichia coli to prolonged berberine exposure. Microb. Drug Resist. 23, 531–544. doi: 10.1089/mdr.2016.0063
Caioni, G., Reyes, C. P., Laurenti, D., Chiaradia, C., Dainese, E., Mattioli, R., et al. (2024). Biochemistry and future perspectives of antibiotic resistance: An eye on active natural products. Antibiotics 13:1071. doi: 10.3390/antibiotics13111071
Cameron, T. A., and Margolin, W. (2024). Insights into the assembly and regulation of the bacterial divisome. Nat. Rev. Microbiol. 22, 33–45. doi: 10.1038/s41579-023-00942-x
Chang, C. H., Huang, W. Y., Lai, C. H., Hsu, Y. M., Yao, Y. H., Chen, T. Y., et al. (2011). Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 7, 593–603. doi: 10.1016/j.actbio.2010.08.028
Chang, C. H., Yu, B., Su, C. H., Chen, D. S., Hou, Y. C., Chen, Y. S., et al. (2014). Coptidis rhizome and Si Jun Zi Tang can prevent Salmonella enterica serovar Typhimurium infection in mice. PLoS One 9:e105362. doi: 10.1371/journal.pone.0105362
Chen, H., Zhang, F., Li, R., Liu, Y., Wang, X., Zhang, X., et al. (2020). Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 124:109829. doi: 10.1016/j.biopha.2020.109829
Chen, L., Bu, Q., Xu, H., Liu, Y., She, P., Tan, R., et al. (2016). The effect of berberine hydrochloride on Enterococcus faecalis biofilm formation and dispersion in vitro. Microbiol. Res. 186-187, 44–51. doi: 10.1016/j.micres.2016.03.003
Chen, Z., Soni, N., Pinero, G., Giotti, B., Eddins, D. J., Lindblad, K. E., et al. (2023). Monocyte depletion enhances neutrophil influx and proneural to mesenchymal transition in glioblastoma. Nat. Commun. 14:1839. doi: 10.1038/s41467-023-37361-8
Chu, M., Ding, R., Chu, Z. Y., Zhang, M. B., Liu, X. Y., Xie, S. H., et al. (2014). Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement. Altern. Med. 14:89. doi: 10.1186/1472-6882-14-89
Chu, M., Zhang, M. B., Liu, Y. C., Kang, J. R., Chu, Z. Y., Yin, K. L., et al. (2016). Role of berberine in the treatment of methicillin-resistant Staphylococcus aureus infections. Sci. Rep. 6:24748. doi: 10.1038/srep24748
Ciccaglione, M., McDaniel, K., Michels, A. W., Exner, A. A., and Benninger, R. K. (2023). 296-OR: Submicron ultrasound contrast agents as therapeutic delivery vehicles in type 1 diabetes. Diabetes 72, 296–OR. doi: 10.2337/db23-296-or
Cook, M. A., and Wright, G. D. (2022). The past, present, and future of antibiotics. Sci. Transl. Med. 14:eabo7793. doi: 10.1126/scitranslmed.abo7793
Cui, X. D., Liu, X. K., Ma, X. Y., Li, S. H., Zhang, J. K., Han, R. J., et al. (2024). Restoring colistin sensitivity in colistin-resistant Salmonella and Escherichia coli: Combinatorial use of berberine and EDTA with colistin. mSphere 9:e00182-24. doi: 10.1128/msphere.00182-24
Ding, J., Yan, Z., Peng, L., Li, J., Yang, F., and Zheng, D. (2024). Inhibitory effects of berberine on fungal growth, biofilm formation, virulence, and drug resistance as an antifungal drug and adjuvant with prospects for future applications. World J. Microbiol. Biotechnol. 41:5. doi: 10.1007/s11274-024-04223-4
Domadia, P. N., Bhunia, A., Sivaraman, J., Swarup, S., and Dasgupta, D. (2008). Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 47, 3225–3234. doi: 10.1021/bi7018546
Du, G. F., Le, Y. J., Sun, X., Yang, X. Y., and He, Q. Y. (2020). Proteomic investigation into the action mechanism of berberine against Streptococcus pyogenes. J. Proteomics 215:103666. doi: 10.1016/j.jprot.2020.103666
Dziedzic, A., Wojtyczka, R. D., and Kubina, R. (2015). Inhibition of oral streptococci growth induced by the complementary action of berberine chloride and antibacterial compounds. Molecules 20, 13705–13724. doi: 10.3390/molecules200813705
Ettefagh, K. A., Burns, J. T., Junio, H. A., Kaatz, G. W., and Cech, N. B. (2011). Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 77, 835–840. doi: 10.1055/s-0030-1250606
Feng, A. W., Gao, W., Zhou, G. R., Yu, R., Li, N., Huang, X. L., et al. (2012). Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARγ pathway during acute endotoxemia. Int. Immunopharmacol. 12, 182–188. doi: 10.1016/j.intimp.2011.11.009
Feng, R., Shou, J. W., Zhao, Z. X., He, C. Y., Ma, C., Huang, M., et al. (2015). Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 5:12155. doi: 10.1038/srep12155
Fu, H., Liu, L. G., Peng, J. P., Leng, W. C., Yang, J., and Jin, Q. (2010). Transcriptional profile of the Shigella flexneri response to an alkaloid: Berberine. FEMS Microbiol. Lett. 303, 169–175. doi: 10.1111/j.1574-6968.2009.01872.x
Gasmi, A., Asghar, F., Zafar, S., Oliinyk, P., Khavrona, O., Lysiuk, R., et al. (2024). Berberine: Pharmacological features in health, disease and aging. Curr. Med. Chem. 31, 1214–1234. doi: 10.2174/0929867330666230207112539
Gong, T., Cui, Y. J., Zhou, X. D., Tang, B. Y., Ren, B., and Li, Y. Q. (2020). [Synergistic Antibacterial activity of berberine in combination with amylmetacresol against Enterococcus faecalis in vitro]. Sichuan Da Xue Xue Bao Yi Xue Ban 51, 749–754. doi: 10.12182/20201160501
Gu, M., Xu, J., Han, C., Kang, Y., Liu, T., He, Y., et al. (2015). Effects of berberine on cell cycle, DNA, reactive oxygen species, and apoptosis in L929 murine fibroblast cells. Evid Based Complement. Alternat. Med. 2015:796306. doi: 10.1155/2015/796306
Guedes, B. N., Krambeck, K., Durazzo, A., Lucarini, M., Santini, A., Oliveira, M., et al. (2024). Natural antibiotics against antimicrobial resistance: Sources and bioinspired delivery systems. Braz. J. Microbiol. 55, 2753–2766. doi: 10.1007/s42770-024-01410-1
Guo, N., Zhao, X., Li, W., Shi, C., Meng, R., Liu, Z., et al. (2015). The synergy of berberine chloride and totarol against Staphylococcus aureus grown in planktonic and biofilm cultures. J. Med. Microbiol. 64, 891–900. doi: 10.1099/jmm.0.000106
Hays, J. P., Ruiz-Alvarez, M. J., Roson-Calero, N., Amin, R., Murugaiyan, J., and van Dongen, M. B. M. (2022). Perspectives on the ethics of antibiotic overuse and on the implementation of (new) antibiotics. Infect. Dis. Ther. 11, 1315–1326. doi: 10.1007/s40121-022-00656-2
He, Y., Yuan, X., Zuo, H., Li, X., Sun, Y., and Feng, A. (2019). Berberine induces ZIP14 expression and modulates zinc redistribution to protect intestinal mucosal barrier during polymicrobial sepsis. Life Sci. 233:116697. doi: 10.1016/j.lfs.2019.116697
Herman, A., and Herman, A. P. (2023). Herbal products and their active constituents used alone and in combination with antibiotics against multidrug-resistant bacteria. Planta Med. 89, 168–182. doi: 10.1055/a-1890-5559
Hu, J. J., Yu, X. Z., Zhang, S. Q., Zhang, Y. X., Chen, X. L., Long, Z. J., et al. (2023). Hydrogel with ROS scavenging effect encapsulates BR@Zn-BTB nanoparticles for accelerating diabetic mice wound healing via multimodal therapy. iScience 26:106775. doi: 10.1016/j.isci.2023.106775
Huang, Y. Q., Huang, G. R., Wu, M. H., Tang, H. Y., Huang, Z. S., Zhou, X. H., et al. (2015). Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. 21, 4225–4231. doi: 10.3748/wjg.v21.i14.4225
Iwasa, K., Nanba, H., Lee, D. U., and Kang, S. I. (1998). Structure-activity relationships of protoberberines having antimicrobial activity. Planta Med. 64, 748–751. doi: 10.1055/s-2006-957572
Izadparast, F., Riahi-Zajani, B., Yarmohammadi, F., Hayes, A. W., and Karimi, G. (2022). Protective effect of berberine against LPS-induced injury in the intestine: A review. Cell Cycle 21, 2365–2378. doi: 10.1080/15384101.2022.2100682
Jamshaid, F., Dai, J., and Yang, L. X. (2020). New development of novel berberine derivatives against bacteria. Mini Rev. Med. Chem. 20, 716–724. doi: 10.2174/1389557520666200103115124
Jhanji, R., Singh, A., and Kumar, A. (2021). Antibacterial potential of selected phytomolecules: An experimental study. Microbiol. Immunol. 65, 325–332. doi: 10.1111/1348-0421.12890
Jin, J., Hua, G., Meng, Z., and Gao, P. J. (2010). Antibacterial mechanisms of berberine and reasons for little resistance of bacteria. Chinese Herbal Med. 3, 27–35. doi: 10.3969/j.issn.1674-6384.2011.01.007
Joshi, P. V., Shirkhedkar, A. A., Prakash, K., and Maheshwari, V. L. (2011). Antidiarrheal activity, chemical and toxicity profile of Berberis aristata. Pharm. Biol. 49, 94–100. doi: 10.3109/13880209.2010.500295
Kang, S., Li, Z., Yin, Z., Jia, R., Song, X., Li, L., et al. (2015). The antibacterial mechanism of berberine against Actinobacillus pleuropneumoniae. Nat. Prod. Res. 29, 2203–2206. doi: 10.1080/14786419.2014.1001388
Karaosmanoglu, K., Sayar, N. A., Kurnaz, I. A., and Akbulut, B. S. (2014). Assessment of berberine as a multi-target antimicrobial: A multi-omics study for drug discovery and repositioning. Omics 18, 42–53. doi: 10.1089/omi.2013.0100
Kariuki, S. (2024). Global burden of antimicrobial resistance and forecasts to 2050. Lancet 404, 1172–1173. doi: 10.1016/s0140-6736(24)01885-3
Lakes, J. E., Richards, C. I., and Flythe, M. D. (2020). Inhibition of bacteroidetes and Firmicutes by select phytochemicals. Anaerobe 61:102145. doi: 10.1016/j.anaerobe.2019.102145
Larkin, H. (2023). Increasing antimicrobial resistance poses global threat. WHO says. JAMA 329:200. doi: 10.1001/jama.2022.23552
Laudadio, E., Cedraro, N., Mangiaterra, G., Citterio, B., Mobbili, G., Minnelli, C., et al. (2019). Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: In silico and in vitro studies. J. Nat. Prod. 82, 1935–1944. doi: 10.1021/acs.jnatprod.9b00317
Lee, J. W., Kang, Y. J., Choi, H. K., and Yoon, Y. G. (2018). Fractionated coptis chinensis extract and its bioactive component suppress propionibacterium acnes-stimulated inflammation in human keratinocytes. J. Microbiol. Biotechnol. 28, 839–848. doi: 10.4014/jmb.1712.12051
Lewnard, J. A., Charani, E., Gleason, A., Hsu, L. Y., Khan, W. A., Karkey, A., et al. (2024). Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: An evidence review and modelling analysis. Lancet 403, 2439–2454. doi: 10.1016/s0140-6736(24)00862-6
Li, C., Huang, P., Wong, K., Xu, Y., Tan, L., Chen, H., et al. (2018a). Coptisine-induced inhibition of Helicobacter pylori: Elucidation of specific mechanisms by probing urease active site and its maturation process. J. Enzyme Inhib. Med. Chem. 33, 1362–1375. doi: 10.1080/14756366.2018.1501044
Li, S., Wang, Y., Wang, S., Xie, J., Fu, T., and Li, S. (2023). In situ gelling hydrogel loaded with berberine liposome for the treatment of biofilm-infected wounds. Front. Bioeng. Biotechnol. 11:1189010. doi: 10.3389/fbioe.2023.1189010
Li, T., Wang, P., Guo, W., Huang, X., Tian, X., Wu, G., et al. (2019). Natural berberine-based chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 13, 6770–6781. doi: 10.1021/acsnano.9b01346
Li, X. H., Xu, J. Y., Wang, X., Liao, L. J., Huang, L., Huang, Y. Q., et al. (2023). BanXiaXieXin decoction treating gastritis mice with drug-resistant Helicobacter pylori and its mechanism. World J. Gastroenterol. 29, 2818–2835. doi: 10.3748/wjg.v29.i18.2818
Li, X., Song, Y., Wang, L., Kang, G., Wang, P., Yin, H., et al. (2021). A potential combination therapy of berberine hydrochloride with antibiotics against multidrug-resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 11:660431. doi: 10.3389/fcimb.2021.660431
Li, X., Wang, P., Hu, X., Zhang, Y., Lu, X., Li, C., et al. (2020). The combined antibacterial effects of sodium new houttuyfonate and berberine chloride against growing and persistent methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. BMC Microbiol. 20:317. doi: 10.1186/s12866-020-02003-2
Li, Y., Cao, Z. T., Wang, X. Y., and Ge, X. Z. (2018b). Expression of the TetA gene encoding TetA efflux protein in E. coli contributes to its increased bacterial resistance toward berberine. J. Asian Nat. Prod. Res. 20, 374–384. doi: 10.1080/10286020.2017.1384818
Li, Y., Huang, J., Li, L., and Liu, L. (2017). Synergistic activity of berberine with azithromycin against Pseudomonas Aeruginosa isolated from patients with cystic fibrosis of lung in vitro and in vivo. Cell. Physiol. Biochem. 42, 1657–1669. doi: 10.1159/000479411
Li, Y., Wen, H., and Ge, X. (2021). Hormesis effect of berberine against Klebsiella pneumoniae is mediated by up-regulation of the efflux pump KmrA. J. Nat. Prod. 84, 2885–2892. doi: 10.1021/acs.jnatprod.1c00642
Li, Y., Yin, Y. M., Wang, X. Y., Wu, H., and Ge, X. Z. (2018c). Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 20, 148–162. doi: 10.1080/10286020.2017.1329300
Liang, R. M., Yong, X. L., Duan, Y. Q., Tan, Y. H., Zeng, P., Zhou, Z. Y., et al. (2014). Potent in vitro synergism of fusidic acid (FA) and berberine chloride (BBR) against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). World J. Microbiol. Biotechnol. 30, 2861–2869. doi: 10.1007/s11274-014-1712-2
Liu, Q., Niu, H., Zhang, W., Mu, H., Sun, C., and Duan, J. (2015). Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 60, 421–430. doi: 10.1111/lam.12401
Liu, Q., Tang, Y., Jiang, S., Yu, X., Zhu, H., Xie, X., et al. (2024). Mechanisms of action of berberine hydrochloride in planktonic cells and biofilms of Pseudomonas aeruginosa. Microb. Pathog. 193:106774. doi: 10.1016/j.micpath.2024.106774
Lu, L., Wang, J., Wang, C., Zhu, J., Wang, H., Liao, L., et al. (2024). Plant-derived virulence arresting drugs as novel antimicrobial agents: Discovery, perspective, and challenges in clinical use. Phytother. Res. 38, 727–754. doi: 10.1002/ptr.8072
Lv, Z., Peng, G., Liu, W., Xu, H., and Su, J. (2015). Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrob. Agents Chemother. 59, 3726–3735. doi: 10.1128/aac.04794-14
Magesh, H., Kumar, A., Alam, A., Priyam, Sekar, U., Sumantran, V. N., et al. (2013). Identification of natural compounds which inhibit biofilm formation in clinical isolates of Klebsiella pneumoniae. Indian J. Exp. Biol. 51, 764–772.
Mangiaterra, G., Cedraro, N., Laudadio, E., Minnelli, C., Citterio, B., Andreoni, F., et al. (2021). The natural alkaloid berberine can reduce the number of Pseudomonas aeruginosa tolerant cells. J. Nat. Prod. 84, 993–1001. doi: 10.1021/acs.jnatprod.0c01151
Manosalva, L., Mutis, A., Urzúa, A., Fajardo, V., and Quiroz, A. (2016). Antibacterial activity of alkaloid fractions from Berberis microphylla G. forst and study of synergism with ampicillin and cephalothin. Molecules 21:76. doi: 10.3390/molecules21010076
Meng, J. W., Ding, J. X., Wang, W. R., Gu, B. L., Zhou, F. T., Wu, D. S., et al. (2024). Reversal of gentamicin sulfate resistance in avian pathogenic Escherichia coli by matrine combined with berberine hydrochloride. Arch. Microbiol. 206:292. doi: 10.1007/s00203-024-04021-4
Mohtar, M., Johari, S. A., Li, A. R., Isa, M. M., Mustafa, S., Ali, A. M., et al. (2009). Inhibitory and resistance-modifying potential of plant-based alkaloids against methicillin-resistant Staphylococcus aureus (MRSA). Curr. Microbiol. 59, 181–186. doi: 10.1007/s00284-009-9416-9
Morel, C., Stermitz, F. R., Tegos, G., and Lewis, K. (2003). Isoflavones as potentiators of antibacterial activity. J. Agric. Food Chem. 51, 5677–5679. doi: 10.1021/jf0302714
Naz, F., Kumar, M., Koley, T., Sharma, P., Haque, M. A., Kapil, A., et al. (2022). Screening of plant-based natural compounds as an inhibitor of FtsZ from Salmonella Typhi using the computational, biochemical and in vitro cell-based studies. Int. J. Biol. Macromol. 219, 428–437. doi: 10.1016/j.ijbiomac.2022.07.241
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi: 10.1021/acs.jnatprod.9b01285
Okuda, T., Jo, R., Tsutsumi, K., Watai, D., Ishihara, C., Yama, K., et al. (2023). An in vitro study of the effects of Phellodendron bark extract and berberine chloride on periodontal pathogenic bacteria in the oral microbiome. J. Oral. Biosci. 65, 72–79. doi: 10.1016/j.job.2022.11.003
Ozturk, M., Chia, J. E., Hazra, R., Saqib, M., Maine, R. A., Guler, R., et al. (2021). Evaluation of berberine as an adjunct to TB treatment. Front. Immunol. 12:656419. doi: 10.3389/fimmu.2021.656419
Pang, Y., Wang, S., Tao, J., Wang, J., Xue, Z., and Wang, R. (2022). Mechanism of berberine hydrochloride interfering with biofilm formation of Hafnia alvei. Arch. Microbiol. 204:126. doi: 10.1007/s00203-021-02617-8
Park, H. C., Gedi, V., Cho, J. H., Hyun, J. W., Lee, K. J., Kang, J., et al. (2014). Characterization and in vitro inhibition studies of Bacillus anthracis FtsZ: A potential antibacterial target. Appl. Biochem. Biotechnol. 172, 3263–3270. doi: 10.1007/s12010-014-0752-2
Patel, P. (2021). A bird’s eye view on a therapeutically ‘wonder molecule’: Berberine. Phytomed. Plus 1:100070. doi: 10.1016/j.phyplu.2021.100070
Peng, L., Kang, S., Yin, Z., Jia, R., Song, X., Li, L., et al. (2015). Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 8, 5217–5223.
Petronio Petronio, G., Cutuli, M. A., Magnifico, I., Venditti, N., Pietrangelo, L., Vergalito, F., et al. (2020). In vitro and in vivo biological activity of berberine chloride against uropathogenic E. coli strains using galleria mellonella as a host model. Molecules 25:5010. doi: 10.3390/molecules25215010
Pierpaoli, E., Cirioni, O., Simonetti, O., Orlando, F., Giacometti, A., Lombardi, P., et al. (2021). Potential application of berberine in the treatment of Escherichia coli sepsis. Nat. Prod. Res. 35, 4779–4784. doi: 10.1080/14786419.2020.1721729
Porras, G., Chassagne, F., Lyles, J. T., Marquez, L., Dettweiler, M., Salam, A. M., et al. (2021). Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 121, 3495–3560. doi: 10.1021/acs.chemrev.0c00922
Pu, X., Wang, Y., Wang, X., Sang, X., Jiang, M., Qi, D., et al. (2024). Lipids extracted from mycobacterial membrane and enveloped PLGA nanoparticles for encapsulating antibacterial drugs elicit synergistic antimicrobial response against mycobacteria. Mol. Pharm. 21, 2238–2249. doi: 10.1021/acs.molpharmaceut.3c01001
Puk, K., and Guz, L. (2022). Effect of alkaloid berberine on the susceptibility of nontuberculous mycobacteria to antibiotics. Pol. J. Vet. Sci. 25, 479–481. doi: 10.24425/pjvs.2022.142034
Qiu, M., and Xu, Z. (2024). Berberine hydrochloride reduces staphyloxanthin synthesis by inhibiting fni genes in methicillin-resistant Staphylococcus aureus. Mol. Biol. Rep. 51:761. doi: 10.1007/s11033-024-09698-w
Rouquette-Loughlin, C., Dunham, S. A., Kuhn, M., Balthazar, J. T., and Shafer, W. M. (2003). The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 185, 1101–1106. doi: 10.1128/jb.185.3.1101-1106.2003
Sadeghi, S., Agharazi, F., Hosseinzadeh, S. A., Mashayekhi, M., Saffari, Z., Shafiei, M., et al. (2024). Gold nanoparticle conjugation enhances berberine’s antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Talanta 268:125358. doi: 10.1016/j.talanta.2023.125358
Safai, S. M., Khorsandi, K., and Falsafi, S. (2022). Effect of berberine and blue LED irradiation on combating biofilm of Pseudomonas aeruginosa and Staphylococcus aureus. Curr. Microbiol. 79:366. doi: 10.1007/s00284-022-03063-5
Scazzocchio, F., Cometa, M. F., Tomassini, L., and Palmery, M. (2001). Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 67, 561–564. doi: 10.1055/s-2001-16493
Seo, Y., Kim, M., and Kim, T. J. (2024). Enhanced efficacy of ciprofloxacin and tobramycin against Staphylococcus aureus when combined with corydalis tuber and berberine through efflux pump inhibition. Antibiotics 13:469. doi: 10.3390/antibiotics13050469
Seukep, A. J., Kuete, V., Nahar, L., Sarker, S. D., and Guo, M. (2020). Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 10, 277–290. doi: 10.1016/j.jpha.2019.11.002
Shi, C., Li, M., Muhammad, I., Ma, X., Chang, Y., Li, R., et al. (2018). Combination of berberine and ciprofloxacin reduces multi-resistant Salmonella strain biofilm formation by depressing mRNA expressions of luxS, rpoE, and ompR. J. Vet. Sci. 19, 808–816. doi: 10.4142/jvs.2018.19.6.808
Singh, S., Pathak, N., Fatima, E., and Negi, A. S. (2021). Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur. J. Med. Chem. 226:113839. doi: 10.1016/j.ejmech.2021.113839
Slobodníková, L., Kost'álová, D., Labudová, D., Kotulová, D., and Kettmann, V. (2004). Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 18, 674–676. doi: 10.1002/ptr.1517
Sokolov, S., Zyrina, A., Akimov, S., Knorre, D., and Severin, F. (2023). Toxic effects of penetrating cations. Membranes 13:841. doi: 10.3390/membranes13100841
Solnier, J., Zhang, Y., Kuo, Y. C., Du, M., Roh, K., Gahler, R., et al. (2023). Characterization and pharmacokinetic assessment of a new berberine formulation with enhanced absorption in vitro and in human volunteers. Pharmaceutics 15:2561. doi: 10.3390/pharmaceutics15112567
Stermitz, F. R., Lorenz, P., Tawara, J. N., Zenewicz, L. A., and Lewis, K. (2000). Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. U S A. 97, 1433–1437. doi: 10.1073/pnas.030540597
Stermitz, F. R., Scriven, L. N., Tegos, G., and Lewis, K. (2002). Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 68, 1140–1141. doi: 10.1055/s-2002-36347
Su, F., and Wang, J. (2018). Berberine inhibits the MexXY-OprM efflux pump to reverse imipenem resistance in a clinical carbapenem-resistant Pseudomonas aeruginosa isolate in a planktonic state. Exp. Ther. Med. 15, 467–472. doi: 10.3892/etm.2017.5431
Sun, L., Lan, J., Li, Z., Zeng, R., Shen, Y., Zhang, T., et al. (2024). Transforming cancer treatment with nanotechnology: The role of berberine as a star natural compound. Int. J. Nanomed. 19, 8621–8640. doi: 10.2147/ijn.S469350
Sun, N., Chan, F. Y., Lu, Y. J., Neves, M. A., Lui, H. K., Wang, Y., et al. (2014). Rational design of berberine-based FtsZ inhibitors with broad-spectrum antibacterial activity. PLoS One 9:e97514. doi: 10.1371/journal.pone.0097514
Tan, J., Wang, J., Yang, C., Zhu, C., Guo, G., Tang, J., et al. (2019). Antimicrobial characteristics of Berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Complement. Altern. Med. 19:218. doi: 10.1186/s12906-019-2558-9
Tocci, N., Iannelli, F., Bidossi, A., Ciusa, M. L., Decorosi, F., Viti, C., et al. (2013). Functional analysis of pneumococcal drug efflux pumps associates the MATE DinF transporter with quinolone susceptibility. Antimicrob. Agents Chemother. 57, 248–253. doi: 10.1128/aac.01298-12
Tong, Y., Zhang, J., Sun, N., Wang, X. M., Wei, Q., Zhang, Y., et al. (2021). Berberine reverses multidrug resistance in Candida albicans by hijacking the drug efflux pump Mdr1p. Sci. Bull. 66, 1895–1905. doi: 10.1016/j.scib.2020.12.035
Tseng, C. Y., Sun, M. F., Li, T. C., and Lin, C. T. (2020). Effect of coptis chinensis on biofilm formation and antibiotic susceptibility in Mycobacterium abscessus. Evid. Based Complement. Alternat. Med. 2020:9754357. doi: 10.1155/2020/9754357
Wang, D., Yu, L., Xiang, H., Fan, J., He, L., Guo, N., et al. (2008). Global transcriptional profiles of Staphylococcus aureus treated with berberine chloride. FEMS Microbiol. Lett. 279, 217–225. doi: 10.1111/j.1574-6968.2007.01031.x
Wang, S., Setlow, B., Setlow, P., and Li, Y. Q. (2016). Uptake and levels of the antibiotic berberine in individual dormant and germinating Clostridium difficile and Bacillus cereus spores as measured by laser tweezers Raman spectroscopy. J. Antimicrob. Chemother. 71, 1540–1546. doi: 10.1093/jac/dkv504
Wang, X., Qiu, S., Yao, X., Tang, T., Dai, K., and Zhu, Z. (2009a). Berberine inhibits Staphylococcus epidermidis adhesion and biofilm formation on the surface of titanium alloy. J. Orthop. Res. 27, 1487–1492. doi: 10.1002/jor.20917
Wang, X., Yao, X., Zhu, Z., Tang, T., Dai, K., Sadovskaya, I., et al. (2009b). Effect of berberine on Staphylococcus epidermidis biofilm formation. Int. J. Antimicrob. Agents 34, 60–66. doi: 10.1016/j.ijantimicag.2008.10.033
Wang, Z., Wang, Y. S., Chang, Z. M., Li, L., Zhang, Y., Lu, M. M., et al. (2017). Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem. Biol. Drug Des. 89, 464–469. doi: 10.1111/cbdd.12866
Warowicka, A., Nawrot, R., and Goździcka-Józefiak, A. (2020). Antiviral activity of berberine. Arch. Virol. 165, 1935–1945. doi: 10.1007/s00705-020-04706-3
Wen, S. Q., Jeyakkumar, P., Avula, S. R., Zhang, L., and Zhou, C. H. (2016). Discovery of novel berberine imidazoles as safe antimicrobial agents by down regulating ROS generation. Bioorg. Med. Chem. Lett. 26, 2768–2773. doi: 10.1016/j.bmcl.2016.04.070
Wijaya, V., Janďourek, O., Křoustková, J., Hradiská-Breiterová, K., Korábečný, J., Sobolová, K., et al. (2022). Alkaloids of Dicranostigma franchetianum (Papaveraceae) and berberine derivatives as a new class of antimycobacterial agents. Biomolecules 12:844. doi: 10.3390/biom12060844
Wojtyczka, R. D., Dziedzic, A., Kępa, M., Kubina, R., Kabała-Dzik, A., Mularz, T., et al. (2014). Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules 19, 6583–6596. doi: 10.3390/molecules19056583
Wu, H., Sun, Q., Dong, H., Qiao, J., Lin, Y., Yu, C., et al. (2023). Gastroprotective action of the extract of Corydalis yanhusuo in Helicobacter pylori infection and its bioactive component, dehydrocorydaline. J. Ethnopharmacol. 307:116173. doi: 10.1016/j.jep.2023.116173
Wu, L. T., Tsou, M. F., Ho, C. C., Chuang, J. Y., Kuo, H. M., and Chung, J. G. (2005). Berberine inhibits arylamine N-acetyltransferase activity and gene expression in Salmonella typhi. Curr. Microbiol. 51, 255–261. doi: 10.1007/s00284-005-4569-7
Wultańska, D., Piotrowski, M., and Pituch, H. (2020). The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1391–1399. doi: 10.1007/s10096-020-03857-0
Xia, S., Ma, L., Wang, G., Yang, J., Zhang, M., Wang, X., et al. (2022). In vitro antimicrobial activity and the mechanism of berberine against methicillin-resistant Staphylococcus aureus isolated from bloodstream infection patients. Infect. Drug Resist. 15, 1933–1944. doi: 10.2147/idr.S357077
Xiao, D., Liu, Z., Zhang, S., Zhou, M., He, F., Zou, M., et al. (2018). Berberine derivatives with different pharmacological activities via structural modifications. Mini. Rev. Med. Chem. 18, 1424–1441. doi: 10.2174/1389557517666170321103139
Xie, Q., Johnson, B. R., Wenckus, C. S., Fayad, M. I., and Wu, C. D. (2012). Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J. Endod. 38, 1114–1117. doi: 10.1016/j.joen.2012.04.023
Xiong, C. Y., Fu, Y. H., Hu, H. B., Bi, A. F., and Pei, D. C. (2014). [Berberine inhibited apoptosis of human umbilical vein endothelial cells induced by Staphylocoocus aureus: An experimental research]. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 710–713.
Xu, C., Wang, F., Huang, F., Yang, M., He, D., and Deng, L. (2021). Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl. Microbiol. Biotechnol. 105, 1563–1573. doi: 10.1007/s00253-021-11116-1
Xu, X. J., Su, X. Z., Morita, Y., Kuroda, T., Mizushima, T., and Tsuchiya, T. (2003). Molecular cloning and characterization of the HmrM multidrug efflux pump from Haemophilus influenzae Rd. Microbiol. Immunol. 47, 937–943. doi: 10.1111/j.1348-0421.2003.tb03467.x
Xue, D. F., Zou, Z. Y., Chen, B., Wang, Y. Z., Wu, H., Ye, X. L., et al. (2015). Study on membrane injury mechanism of total alkaloids and berberine from Coptidis Rhizoma on Aeromonas hydrophila. Zhongguo Zhong Yao Za Zhi 40, 1787–1792.
Yang, T., Wang, R., Zhang, J., Bao, C., Zhang, J., Li, R., et al. (2020). Mechanism of berberine in treating Helicobacter pylori induced chronic atrophic gastritis through IRF8-IFN-γ signaling axis suppressing. Life Sci. 248:117456. doi: 10.1016/j.lfs.2020.117456
Yang, X., Zheng, D., Yong, J., Li, Y., Sun, Y., Zhao, F., et al. (2025). Baicalein and berberine inhibit the growth and virulence of Clostridioides difficile. Pathogens 14:662. doi: 10.3390/pathogens14070662
Yao, L., Wu, L. L., Li, Q., Hu, Q. M., Zhang, S. Y., Liu, K., et al. (2018). Novel berberine derivatives: Design, synthesis, antimicrobial effects, and molecular docking studies. Chin. J. Nat. Med. 16, 774–781. doi: 10.1016/s1875-5364(18)30117-1
Yu, H. H., Kim, K. J., Cha, J. D., Kim, H. K., Lee, Y. E., Choi, N. Y., et al. (2005). Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J. Med. Food 8, 454–461. doi: 10.1089/jmf.2005.8.454
Yuan, C., Wu, M., Xiao, Q., Zhao, W., Li, H., Zhong, Y., et al. (2021). Blocking Msr1 by berberine alkaloids inhibits caspase-11-dependent coagulation in bacterial sepsis. Signal. Transduct. Target. Ther. 6:92. doi: 10.1038/s41392-021-00483-w
Yuan, M., Shi, D. Z., Wang, T. Y., Zheng, S. Q., Liu, L. J., Sun, Z. X., et al. (2016). Transformation of trollioside and isoquercetin by human intestinal flora in vitro. Chin. J. Nat. Med. 14, 220–226. doi: 10.1016/s1875-5364(16)30019-x
Yue, B., Gao, R., Lv, C., Yu, Z., Wang, H., Geng, X., et al. (2021). Berberine improves irinotecan-induced intestinal mucositis without impairing the anti-colorectal cancer efficacy of irinotecan by inhibiting bacterial β-glucuronidase. Front. Pharmacol. 12:774560. doi: 10.3389/fphar.2021.774560
Zhang, C., Li, Z., Pan, Q., Fan, L., Pan, T., Zhu, F., et al. (2022). Berberine at sub-inhibitory concentration inhibits biofilm dispersal in Staphylococcus aureus. Microbiology 168:1243. doi: 10.1099/mic.0.001243
Zhang, D., Ke, L., Ni, Z., Chen, Y., Zhang, L. H., Zhu, S. H., et al. (2017). Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase IV trial. Medicine 96:e7697. doi: 10.1097/md.0000000000007697
Zhang, J., Han, C., Lu, W. Q., Wang, N., Wu, S. R., Wang, Y. X., et al. (2020). A randomized, multicenter and noninferiority study of amoxicillin plus berberine vs tetracycline plus furazolidone in quadruple therapy for Helicobacter pylori rescue treatment. J. Dig. Dis. 21, 256–263. doi: 10.1111/1751-2980.12870
Zhang, J., Zuo, G., Bai, Q., Wang, Y., Yang, R., and Qiu, J. (2009). Microarray expression profiling of Yersinia pestis in response to berberine. Planta Med. 75, 396–398. doi: 10.1055/s-0028-1088381
Zhang, L. J., Zhang, L. J., Quan, W., Wang, B. B., Shen, B. L., Zhang, T. T., et al. (2011). Berberine inhibits HEp-2 cell invasion induced by Chlamydophila pneumoniae infection. J. Microbiol. 49, 834–840. doi: 10.1007/s12275-011-1051-z
Zhang, S. L., Chang, J. J., Damu, G. L., Fang, B., Zhou, X. D., Geng, R. X., et al. (2013). Novel berberine triazoles: Synthesis, antimicrobial evaluation and competitive interactions with metal ions to human serum albumin. Bioorg. Med. Chem. Lett. 23, 1008–1012. doi: 10.1016/j.bmcl.2012.12.036
Zhao, N., Isguven, S., Evans, R., Schaer, T. P., and Hickok, N. J. (2023). Berberine disrupts staphylococcal proton motive force to cause potent anti-staphylococcal effects in vitro. Biofilm 5:100117. doi: 10.1016/j.bioflm.2023.100117
Zhao, Z., Guo, M., Xu, X., Hu, Y., Liu, D., Wang, C., et al. (2022). In vitro synergistic inhibitory activity of natural alkaloid berberine combined with azithromycin against alginate production by Pseudomonas aeruginosa PAO1. Oxid. Med. Cell. Longev. 2022:3858500. doi: 10.1155/2022/3858500
Zhou, F. F., Gu, X. M., Wang, L., and Lin, M. (2023). The mechanism of berberine on Methicillin resistant Staphylococcus aureus in vitro. Zhonghua Yu Fang Yi Xue Za Zhi 57, 1217–1221. doi: 10.3760/cma.j.cn112150-20230206-00081
Zhou, H., Wang, W., Cai, L., and Yang, T. (2023). Potentiation and mechanism of berberine as an antibiotic adjuvant against multidrug-resistant bacteria. Infect. Drug Resist. 16, 7313–7326. doi: 10.2147/idr.S431256
Zhou, X. Y., Ye, X. G., He, L. T., Zhang, S. R., Wang, R. L., Zhou, J., et al. (2016). In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. J. Antibiot. 69, 741–746. doi: 10.1038/ja.2016.15
Zhou, X., Zhang, C., Wang, X., An, B., Zhang, P., and Zhu, Z. (2012). Berberine inhibits lipopolysaccharide- and polyethylene particle-induced mouse calvarial osteolysis in vivo. J. Surg. Res. 173, e47–e52. doi: 10.1016/j.jss.2011.11.004
Zhou, Y., Liu, Z., Wen, J., Zhou, Y., and Lin, H. (2023). The inhibitory effect of berberine chloride hydrate on Streptococcus mutans biofilm formation at different pH values. Microbiol. Spectr. 11:e0217023. doi: 10.1128/spectrum.02170-23
Zhu, X., Wei, Y., Yang, B., Yin, X., and Guo, X. (2020). The mitohormetic response as part of the cytoprotection mechanism of berberine: Berberine induces mitohormesis and mechanisms. Mol. Med. 26:10. doi: 10.1186/s10020-020-0136-8
Keywords: berberine, bacterial, antimicrobial resistance, natural products, antimicrobial agents
Citation: Yang X, Wang Y, Li L, Tang D, Yan Z, Li M, Jiang J and Bi D (2025) Berberine and its nanoformulations and extracts: potential strategies and future perspectives against multi-drug resistant bacterial infections. Front. Microbiol. 16:1643409. doi: 10.3389/fmicb.2025.1643409
Received: 08 June 2025; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Amira Zairi, University of Sousse, TunisiaReviewed by:
Shahper Nazeer Khan, University of Manitoba, CanadaAli Parsaeimehr, South Dakota State University, United States
Neville S. Ng, University of Wollongong, Australia
Copyright © 2025 Yang, Wang, Li, Tang, Yan, Li, Jiang and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongming Bi, ZG9uZ21pbmdiaUBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
 Xue Yang1†
Xue Yang1† Dongming Bi
Dongming Bi