- 1Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, Milan, Italy
- 2Department of Physics, University of Central Florida, Orlando, FL, United States
- 3School of Environment and Safety Engineering, Jiangsu University, Zhenjiang, China
The future of Mars colonization hinges on the ability to construct durable infrastructure using locally available resources. Given the high cost and logistical complexity of transporting construction materials to Mars, the development of autonomous in situ resource utilization (ISRU) technologies is imperative. This perspective article explores the potential of biomineralization as a low-energy, sustainable alternative to conventional construction methods, such as Portland cement and thermal sintering approaches proposed for lunar applications, which are often energy-intensive and constrained by material specificity. Following an assessment of the chemical composition of Martian regolith, its suitability as a substrate for various biomineralization pathways, particularly those aligned with ISRU constraints, is evaluated. Special emphasis is placed on identifying biological pathways that are not only metabolically compatible with Martian geochemistry but can also function as a co-culture, mutually supporting each other’s survival and activity under Martian environmental stresses. The most promising microbial consortia for biocementation are proposed for future extraterrestrial construction applications. The integration of robotics and automation in biocementation-based additive manufacturing using Martian regolith as a construction feedstock is discussed. Advanced robotic systems equipped with multi-axis extrusion nozzles, sensor suites, and real-time flow control are proposed for the construction of structurally resilient geometries on Mars. As a flexible, scalable, and ISRU-compatible technology, biocementation holds promise not only for infrastructure construction but also for integrated resource cycles, producing oxygen and ammonia as byproducts. Biocementation-based ISRU construction represents a synergistic pathway toward sustainable human presence on Mars, enabling robotic fabrication of critical infrastructure from locally available materials.
1 Introduction
Human colonization of other planets has long been envisioned, but its realization relies on the ability to construct safe and sustainable infrastructure off-Earth. Mars is thought to have a relatively similar geological history to Earth and hence has long served as a target of fascination and robotic exploration missions and is a leading candidate for future human settlement. However, long-term human and robotic presence on Mars remains highly challenging due to extreme environmental conditions, such as a thin atmosphere that freezes and thaws throughout the Martian day, extreme radiation levels, and wide temperature fluctuations, as well as significant power and time constraints for mission operations. Global space agencies and organizations, such as the United States National Aeronautics and Space Administration (NASA), aim to develop human habitats on Mars (NASA, 2022). However, substantial technological and scientific advances are required to ensure safe, efficient, and cost-effective exploration and infrastructure development. A critical challenge is the sourcing of construction materials, as transporting them from Earth is prohibitively expensive and constrained by launch costs, limited payload mass, and payload volume constraints (Naser, 2019; Wang et al., 2022). Therefore, in situ resource utilization (ISRU) is critical for human and robotic missions’ sustainability beyond Earth (Sanders et al., 2022). Martian regolith is analogous to terrestrial soil and is abundant in oxygen and extractable metals (Schreiner et al., 2015), offering a viable resource for local construction, manufacturing, and propellant production (Farries et al., 2021). It can be used as a bulk material for infrastructure such as landing pads, berms, habitats, and roadways, significantly reducing reliance on Earth-based supply chains for the development of Martian infrastructure.
Given the high risk and cost associated with human spaceflight and off-Earth construction, autonomous or remotely operated robotic systems offer advantages by enhancing safety and construction consistency. Robotic systems equipped with imaging and geotechnical instruments, such as vane shears, cone penetrometers, and bearing plates, can be used to evaluate potential construction sites, additively manufactured parts, and infrastructure element mechanical properties (e.g., compressive strength and shear strength) before, during, and after construction. Such tools have been successfully deployed on lunar and Martian missions (Zent et al., 2009), demonstrating their effectiveness in assessing environmental and regolith properties. However, the mechanical behavior of planetary regolith differs significantly from terrestrial soils (Long-Fox and Britt, 2023; Long-Fox et al., 2023; Dotson et al., 2024; Lucas et al., 2024), necessitating careful adaptation and calibration of measurement techniques. Employing robotic geotechnical tools and imaging systems improves the accuracy, repeatability, and reliability of site assessments, ultimately reducing time, energy, and cost in extraterrestrial construction.
Cement is the most predominant construction material on Earth (Hosseini et al., 2024b). In recent years, biomineralization, a process in which microorganisms facilitate mineral formation under ambient conditions with low energy input (Zhu et al., 2024), has garnered considerable attention as a sustainable cement alternative. The biomineralization process typically begins when microbes adhere to a substrate containing the requisite ions for mineralization (Lowenstam et al., 1989). A key element in microbial–mineral interactions is the secretion of extracellular polymeric substances (EPS), a matrix of polysaccharides, proteins, and sometimes DNA fragments that enhances surface adhesion (Dupraz and Visscher, 2005). Within the EPS, microorganisms concentrate essential ions, creating a microenvironment that favors mineral nucleation and growth by altering local environmental parameters such as pH, redox conditions, or ion saturation (Lowenstam et al., 1989). Nucleation begins when ions aggregate to form a stable crystal “seed” on organic or inorganic surfaces. Once nucleated, the mineral crystals grow under biological control, often influenced by organic molecules that bind to specific crystal faces and regulate morphology (Mann, 2001). The final stage often involves the hardening, aging, or remodeling of the mineralized structure, including transitions from amorphous to crystalline phases (Mann, 2001).
This manuscript explores the feasibility of employing biomineralization for Martian construction. First, the chemical composition of Martian regolith is analyzed since the composition of the regolith determines the feasibility and best approach to biomineralization. Then, various biomineralization pathways are presented with an emphasis on those that can exploit native Martian minerals as potential substrates. This work then further explores how Mars’ extreme environmental conditions may constrain biomineralization processes and influence their effectiveness. The authors assess the viability of biomineralization for Martian construction, highlighting the most promising pathways, potential integration with ISRU, and the prospects for automation and remote operation. Finally, key challenges and current research gaps are discussed.
2 Martian resources and environmental challenges for biomineralization
2.1 Martian soil composition
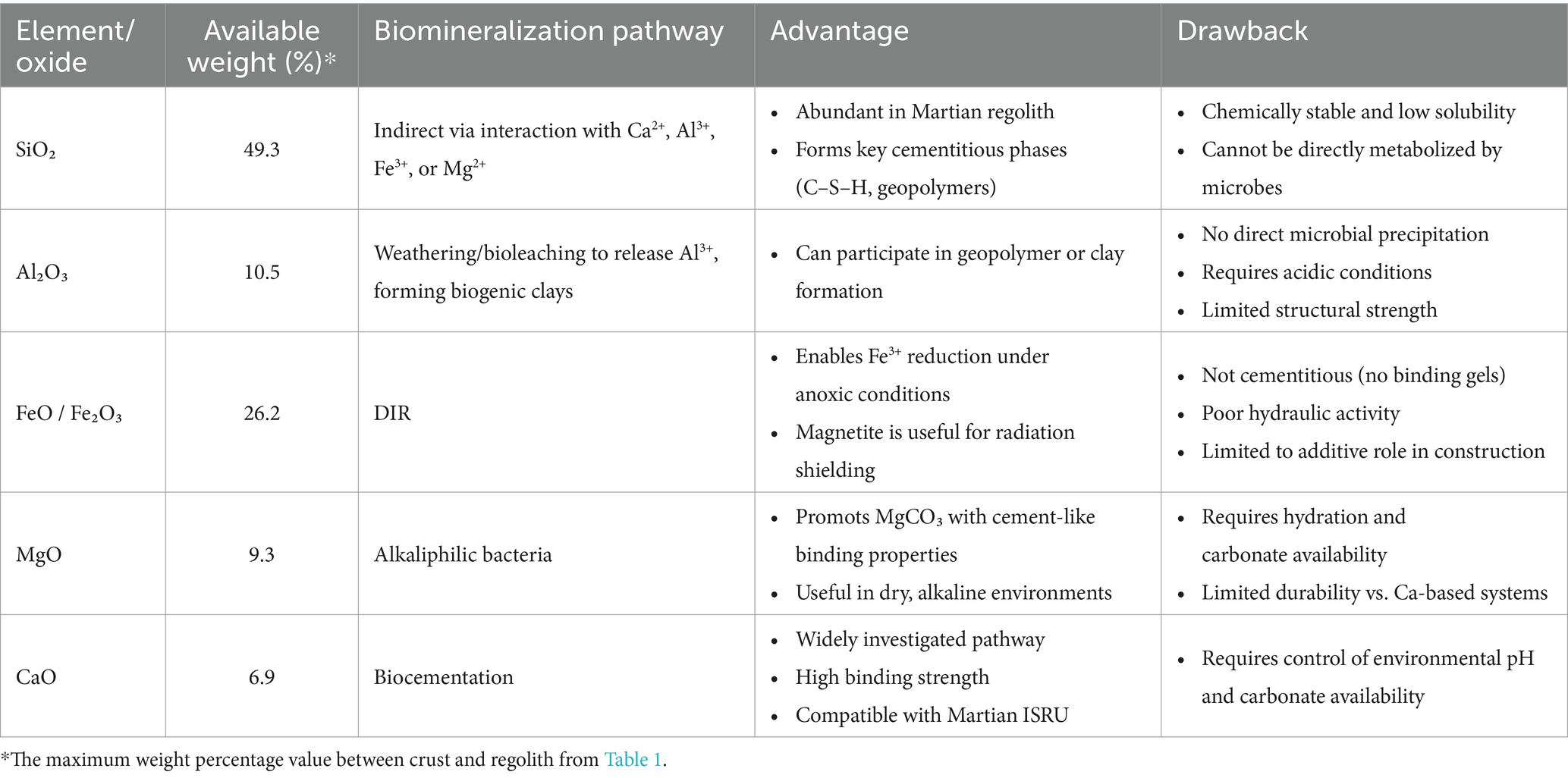
Martian regolith has only been analyzed through data collected by surface robotic or orbital missions. Despite regional variations, its chemical composition shares similarities with that of Portland cement, particularly in key oxides. As shown by the equivalent oxides given in Table 1, Martian regolith is rich in silica (SiO₂), constituting ~42%–47% of the regolith and ~49% of the crust—significantly higher than the 17%–25% typically found in Portland cement. Alumina (Al₂O₃) levels on Mars (~7%–10% in regolith, ~10.5% in crust) are also comparable to cement, where it generally accounts for <6%. Iron oxides—FeO (10%–26%) and Fe₂O₃ (4%–7%)—are present in substantial amounts, with FeO comprising ~18% of the bulk elemental composition of the Martian crust. Magnesium oxide (MgO) is similarly abundant (6%–9% in regolith, ~9% in crust). However, calcium oxide (CaO), the principal component of Portland cement (~60%–67%), is present on Mars in much lower concentrations (~5.7%–6.9%). This significant shortfall in CaO implies that in situ production of a true Portland cement analog on Mars is unlikely without external supplementation. In the near term, CaO would likely need to be imported from Earth, limiting the feasibility of producing standard Portland cement using only Martian-derived materials. It should be noted that the values presented here are equivalent oxides, representative of bulk composition rather than mineralogic species present; the oxides mentioned here generally will not be found in their oxide state on Mars.
2.2 Martian regolith as a substrate for biomineralization pathways
Given the presence of Si, Al, Fe, Mg, and Ca in Martian regolith, this section explores the potential of leveraging microbial metabolic pathways to generate biominerals from the local Martian materials, aligning with ISRU strategies. Crystalline silica (SiO₂), particularly as quartz, is prevalent in Martian regolith but is highly stable and chemically inert. Unlike redox-active elements such as Fe or Mn, Si does not participate in biological electron transfer reactions (Konhauser, 2016). Moreover, SiO2 is not metabolically useful to most microorganisms, offering neither energy nor a carbon source. Its low solubility at neutral pH (~0.1–1 ppm) further limits its bioavailability (Konhauser, 2016), making it unsuitable for direct microbial mineralization. However, microbes can indirectly contribute to the formation of cementitious materials when Si2− interacts with other cations. For example, its reaction with Ca2+ can produce calcium silicate hydrate (C–S–H), the primary binding phase in Portland cement (Konhauser, 2016). Similarly, under alkaline conditions, interactions between SiO₂ and Al3+, Fe3+, or Mg2+ can yield geopolymers or alkali-activated materials with cement-like properties.
Although microbes do not directly precipitate Al, microbial activity can facilitate its incorporation into secondary minerals through bioleaching and weathering processes (Gadd, 2007). For instance, under acidic conditions, alumina dissolves in the presence of protons, releasing Al ions (Al3+) and water (Al₂O₃ + 6H+ → 2Al3+ + 3H₂O). The released Al3+ ions can subsequently interact with dissolved silica (SiO₂) and hydroxyl ions (OH−) to form aluminosilicate hydroxide structures (Al3+ + SiO₂ + OH− → Al–Si–OH), precursors to clay minerals, such as biogenic formation of kaolinite(Gadd, 2007; Provis and Deventer, 2009).
Fe oxides can participate in biomineralization through dissimilatory iron reduction (DIR), a microbial process in which bacteria like Shewanella reduce Fe(III) to Fe(II) under anoxic conditions, leading to the precipitation of Fe minerals like magnetite (Fe₃O₄) and siderite (FeCO₃) (Lovley, 1991). However, neither magnetite nor siderite, common Fe-bearing minerals on the Martian surface, exhibit cementitious properties; they lack hydraulic activity and do not form binding hydrates like calcium silicate hydrate (C–S–H) or calcium aluminate hydrate (C–A–H), which are critical in Portland cement systems. Consequently, their binding capacity is insufficient to generate a hardened matrix capable of stabilizing mineral aggregates such as the Martian regolith. Nonetheless, due to its high density and Fe content, magnetite may serve as a functional additive for radiation-shielding concretes, relevant to systems designed to protect from cosmic and solar radiation (Hassler et al., 2014). Regarding MgO, some alkaliphilic microorganisms (e.g., cyanobacteria or sulfate-reducers) promote MgCO₃-based biomineral precipitation through alkalinization or photosynthesis (Hassler et al., 2014). MgO, upon hydration (MgO + H₂O → Mg(OH)₂), releases Mg2+ ions that can subsequently react with carbonate ions to form magnesium carbonates, such as hydromagnesite, nesquehonite, or magnesite.
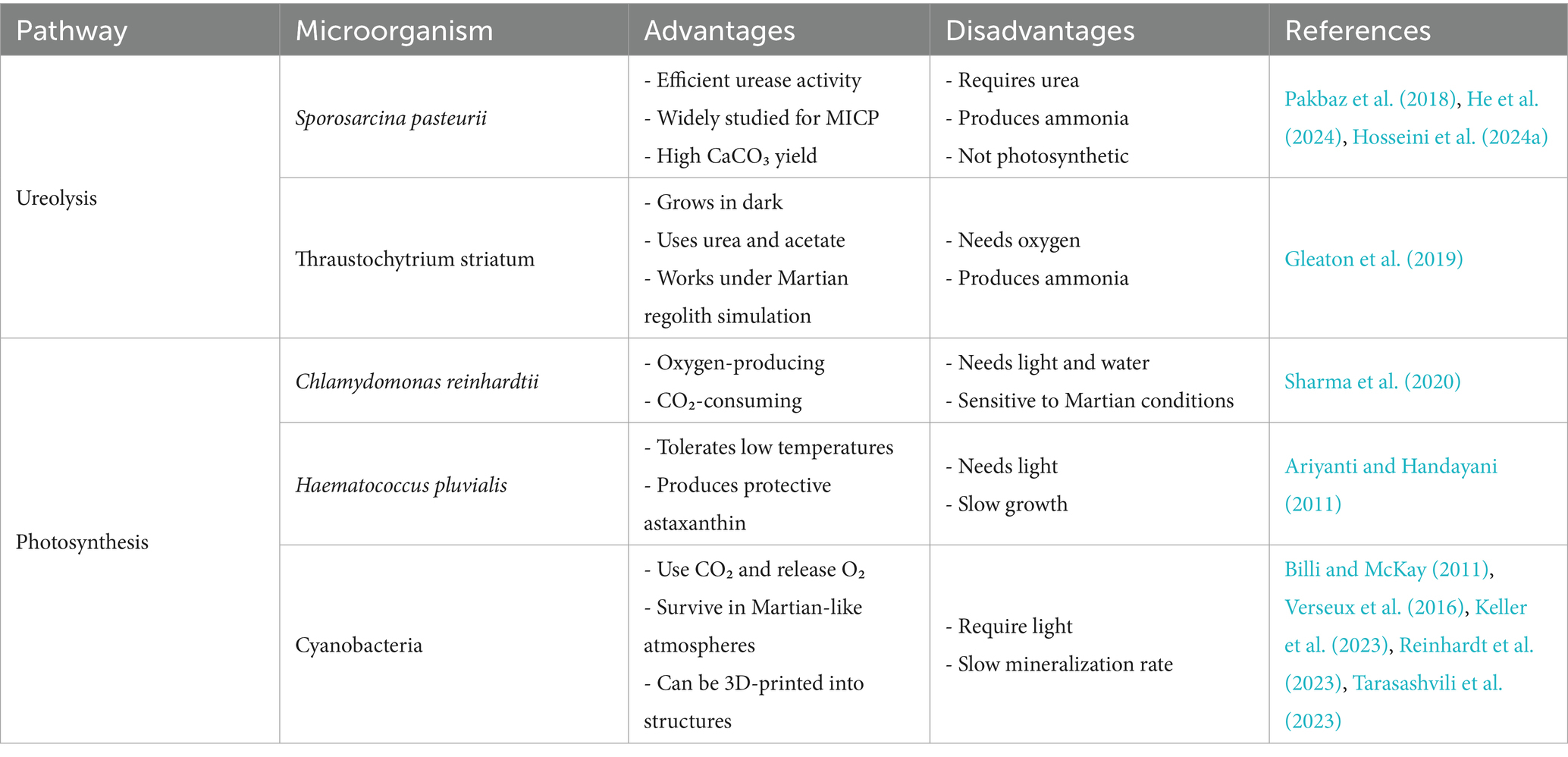
CaO, which has the potential to facilitate calcium carbonate (CaCO3) precipitation via the biocementation process, is perhaps the most promising pathway for biomineralization for Martian applications. Microbially Induced Calcium carbonate Precipitation (MICP) has been the most extensively studied biomineralization pathway on Earth over the past two decades, with applications explored in soil stabilization (Mujah et al., 2017; Sharaky et al., 2018; Omoregie et al., 2020; Yin et al., 2022), mitigation of wind-induced desertification (Zomorodian et al., 2019; Fattahi et al., 2020; Devrani et al., 2021; Dubey et al., 2021), and sustainable building construction (Castro-Alonso et al., 2019; Iqbal et al., 2021; Bagga et al., 2022; Khoshtinat, 2023a). CaCO₃ precipitation can occur via several microbial mechanisms, typically classified into six categories: ureolysis, amino acid ammonification, photosynthesis, denitrification, sulfate reduction, and methanogenesis-induced pathways (Dupraz and Visscher, 2005; DeJong et al., 2010; Van Paassen et al., 2010; Achal et al., 2015; Zhu and Dittrich, 2016; Castro-Alonso et al., 2019). For brevity, only some of these mechanisms are discussed in detail here. Among these pathways, the ureolytic pathway is one of the most extensively studied. In this process, urease-producing bacteria, notably Sporosarcina pasteurii, catalyse the hydrolysis of urea into ammonia and carbonate ions. The production of ammonia increases the pH of the environment, which, in the presence of calcium ions, leads to the precipitation of CaCO₃ (CO(NH₂)₂ + 2H₂O + Ca2+ → 2NH₄+ + CaCO₃↓)(Pakbaz et al., 2018; Hosseini et al., 2024a). The second most extensively studied biocementation pathway involves photosynthetic microorganisms. In this mechanism, organisms such as cyanobacteria facilitate CaCO₃ precipitation through their metabolic activity. By consuming CO₂ during photosynthesis, they reduce dissolved CO₂ concentrations, leading to an increase in local pH. This shift alters the carbonate equilibrium, favoring the formation of carbonate ions, which then react with calcium ions to precipitate CaCO₃ (CO₂ + H₂O + light + Ca2+ → 2H+ + CaCO₃)(Billi and McKay, 2011; Sharma et al., 2020; Reinhardt et al., 2023). Table 2 shows a selection of the latest intriguing studies on biocementation via ureolysis and photosynthesis.
2.3 Environmental constraints
Although Mars is considered to be very similar to Earth due to its rocky surface and axial tilt (~25.2°), which induces seasonal cycles, it differs markedly in environmental conditions. Mars has lower gravity (1.62 m/s2, ~1/3 that of Earth), no global magnetic field, and a thin CO₂-dominated atmosphere (~95.3%) with a relatively low surface pressure of 610 Pa (NASA, 2021). These factors expose the surface to intense cosmic and solar radiation and contribute to low average surface temperatures (−63 °C). These conditions significantly impact the viability and metabolic activity of microorganisms, as well as the hydration and curing behavior of cementitious materials. Few astrobiological studies have directly examined the effects of Martian environmental parameters on microbial activity, and none has investigated microbes with biomineralization potential. Simulated Martian conditions, low pressure, CO₂-rich atmosphere, and freeze–thaw cycles have been shown to markedly impair bacterial viability (Foster et al., 1978). However, certain psychrotrophic microbes can exhibit modest proliferation when moisture and nutrients are present, with up to three times increase in viable cells over 7–14 days. For example, Escherichia coli, Serratia liquefaciens (Berry et al., 2010), and Psychrobacter cryohalolentis (Zaccaria et al., 2024) have demonstrated moderate to high growth under specific Martian stressors. Nonetheless, overall biomass remains constrained due to desiccation and ionizing radiation.
While lower gravity can reduce structural loads, it also negatively impacts construction processes by disrupting particle settling and water distribution, which leads to non-uniform mixing, poor hydration kinetics, and increased porosity and microcracking, ultimately compromising material strength. Crystallization process experiments aboard the International Space Station revealed that microgravity suppresses sedimentation and bleeding, resulting in more uniform hydration but also in a 20% increase in porosity and larger pore sizes compared to Earth-based conditions, leading to weaker, more heterogeneous microstructures (Moraes Neves et al., 2019). In the context of biomineralization, low gravity presents additional challenges by impairing microbial adhesion and biofilm formation, thereby affecting the uniformity and efficacy of microbial-induced cementation. Mars’ extreme temperature fluctuations, from −90 °C in winter to 26.6 °C in summer, pose a significant barrier to biomineralization by disrupting microbial enzymatic activity. Even slight temperature shifts (1–2 °C) can alter enzymatic reaction rates by 10%–20% (Berry et al., 2010; Khoshtinat, 2023b). Moreover, most mesophilic bacteria become inactive near 5 °C, restricting biomineralization to a limited window of midday hours during Martian summer.
Elevated ultraviolet and ionizing radiation levels on Mars present a major challenge to microbial viability. While some Bacillus strains, such as B. pumilus SAFR-032, exhibit high resistance to Martian-like UV radiation (Newcombe et al., 2005; Osman et al., 2008), others, like B. subtilis, show reduced survival under regolith-shielded ionizing radiation (Moeller et al., 2010). In contrast, extremophiles like the cyanobacterium Chroococcidiopsis display resistance to both UV and ionizing radiation, making them more suitable candidates for Mars-based applications (Billi et al., 2000). These microorganisms have demonstrated the capacity to survive for days under simulated Martian conditions such as temperatures around −27 °C, a low-pressure CO₂-dominated atmosphere (~0.8 kPa), and direct exposure to Martian regolith simulants (Billi et al., 2019b; Keller et al., 2023). Moreover, their exceptional desiccation resistance, as demonstrated by the survival of dried biofilms for 672 days in the vacuum of space during the EXPOSE-R2 mission aboard the International Space Station, followed by successful revival upon rehydration, further underscores their potential for extraterrestrial applications (Baqué et al., 2013b; Billi et al., 2019a, 2019b).
3 Can biomineralization be adapted for Martian construction?
This section presents the authors’ perspective on the feasibility of applying biomineralization for Martian construction. It evaluates which biomineralization pathway is deemed suitable for Martian conditions and how it can be integrated with robotic systems and ISRU strategies. Additionally, it explores the potential of its incorporation into automated systems to achieve scalable, self-sustaining construction using local regolith. Finally, it outlines future research directions to address existing knowledge gaps.
3.1 Promising biomineralization pathway
Among the biomineralization pathways considered for Martian construction, biocementation appears the most promising due to its capacity to generate robust, cementitious materials suitable for extraterrestrial environments. As mentioned in Section 2.2, ureolysis and photosynthesis show particular promise for Martian construction applications. Table 3 summarizes the key concepts related to Martian regolith as a medium for biocementation discussed in Section 2.2.
In this context, co-culturing microorganisms with distinct biocementation pathways and extreme environmental condition resistance can offer synergistic advantages. The proposed system features a synthetic bioreactor containing a co-culture of photosynthetic cyanobacteria Chroococcidiopsis and ureolytic bacteria Sporosarcina pasteurii. Although photosynthetic biocementation is significantly slower than ureolysis, the demonstrated resilience of Chroococcidiopsis under Martian-like conditions supports sustained production of oxygen and EPS, creating a favorable microenvironment for S. pasteurii and enhancing calcium carbonate precipitation in extraterrestrial settings. In this scenario, the bacterial co-culture would be applied to basaltic Martian regolith. Astronaut urine would supply the key ions (Ca2+, K+), supporting microbial growth and urea source for ureolysis. As demonstrated, a nutrient-rich “Martian medium” can be created by leaching regolith with water and enriching it with urine, achieving nutrient levels comparable to BG-11 medium (Concas et al., 2023). Photosynthetic activity by Chroococcidiopsis consumes dissolved CO₂ and HCO₃−, raising the pH and secreting EPS, providing a suitable microenvironment as a nucleation site for S. pasteurii CaCO₃ precipitation.
However, certain environmental controls are essential to ensure successful biocementation, leading the process to better performance in controlled, laboratory-like conditions. To maintain water in a stable liquid phase, a pressurized enclosure approximating Earth’s atmospheric pressure is preferred. Such pressurization also supports the retention of oxygen produced by photosynthetic microorganisms, gradually shifting the reactor environment from anoxic to oxic conditions, an essential transition for the aerobic metabolism of Sporosarcina pasteurii. Although Chroococcidiopsis have demonstrated resistance to ultraviolet (UV) radiation, incorporating a transparent UV-blocking membrane that excludes UVA and UVB wavelengths, the pressurized enclosure envelope can improve environmental conditions for S. pasteurii. This protective layer complements the intrinsic UV resilience of Chroococcidiopsis and helps maintain microbial viability and metabolic functionality. The biocementation reactor would operate through cyclic wet–dry phases: moistening facilitates microbial proliferation and calcium carbonate precipitation via ureolysis, while drying enhances structural consolidation and mineralization of the formed biocement (Baqué et al., 2013a,b; Gat et al., 2014; Mosca et al., 2019; Xu et al., 2020).
3.2 Integration with ISRU
From the authors’ perspective, biocementation is considered a promising biomineralization pathway due to its strong potential for integration with ISRU strategies, both during early Mars colonization and in later phases, where its byproducts could support secondary resource cycles. Key parameters include water and calcium, with additional substrates varying by pathway (e.g., urea for ureolysis and CO₂ and light for photosynthesis). Calcium, abundant in Martian basalt (McSween et al., 2009), can be extracted via leaching processes developed for metal or oxygen production, where it may arise as a byproduct. In case of crewed mission, urea can be derived from human waste (Zuo et al., 2023), creating a closed-loop system that repurposes metabolic waste for construction. Biocementation, when automated and adapted for Martian conditions, could support Mars’ development by integrating with ISRU systems, yielding byproducts such as oxygen (via photosynthesis) and ammonia (via ureolysis). It can function both as a construction method and as auxiliary support for critical operations. CO₂, a key input, also supports oxygen production via solid oxide electrolysis (Hoffman et al., 2022) and plant growth in greenhouses (Fackrell et al., 2024). In long-term scenarios, ammonia may also serve as a nitrogen source for Martian agriculture (Wamelink et al., 2014; Fackrell et al., 2024).
Water, the most critical aspect (Cheng and Cord-Ruwisch, 2012), may be sourced from subsurface ice or hydrated minerals. For example, radar observations from the Mars Advanced Radar for Subsurface and Ionospheric Sounding (MARSIS) instrument suggest the presence of stratified subsurface structures within the Medusae Fossae Formation, consistent with ice-rich deposits buried beneath approximately 300–600 m of dry overburden (Watters et al., 2024). In addition, bright basal radar reflections detected near the Martian south pole have been interpreted as possible subglacial liquid water bodies at depths of ~1.5 km of overlying ice (Orosei et al., 2018). However, certain precautions must be considered, as most compositional data currently available have been obtained through robotic missions, and the Mars Sample Return program continues to experience delays (NASA Sets Path to Return Mars Samples, Seeks Innovative Designs - NASA, 2024). Therefore, the extent to which such ice or water is contaminated with perchlorates, highly oxidizing and potentially toxic salts that also act as strong desiccants threatening organic life (Hecht et al., 2009; Quinn et al., 2011), cannot be determined until physical samples are returned to Earth. Consequently, the potential necessity of purifying any water extracted from Martian resources must be carefully considered for every use, whether its human use, agricultural use, or for ISRU/construction.
Regarding energy consumption, unlike thermal or microwave-based sintering of regolith reliant on solar, stored electrical, or nuclear energy (Nething et al., 2020; Reinhardt et al., 2023), biocementation operates at low temperatures with low energy demands, making it suitable for Mars’ limited power systems. However, the energy demand for extraterrestrial construction depends on multiple process-specific factors, including the processing method, local resource utilization potential, and the type of instrumentation and equipment used. Since adapting biocementation for lunar construction is impractical due to extreme daytime surface temperatures of ~127 °C (Chen et al., 2024), which surpass the thermal tolerance of the microorganisms involved. Additionally, biocementation is a relatively recent concept for Martian application, there is a lack of data regarding biocementation energy consumption for extraterrestrial use. Given the limited availability of extraterrestrial construction data, only a first-order comparison of energy consumption can be attempted by assuming that the material payload delivered from Earth is constant (e.g., hardware mass, volume, storage requirements, etc.) across different construction strategies. According to available data, the production of 1 tonne of CaCO₃ by biocementation on Earth consumes approximately 29.3 MJ in total (0.029 MJ/kg) (Deng et al., 2021). In contrast, conventional thermal sintering is estimated to require about 1,372 MJ/tonne (Spedding et al., 2020), while cold microwave sintering, reported to consume ~6.66 times less energy than thermal sintering (Liu et al., 2024), requires approximately 206 MJ/tonne. Although these values should be interpreted cautiously, they suggest that biocementation has a substantial energy efficiency advantage, on the order of 1–2 magnitudes, over other proposed extraterrestrial construction techniques.
3.3 Automation and remote operability
On Mars, where payload delivery from Earth is costly, additive manufacturing (AM) using Martian regolith as feedstock can significantly lower mission costs and complexity. AM, especially 3D printing, enables precise, automated, and resource-efficient construction (Azami et al., 2024). Its modularity and scalability allow infrastructure to grow with mission needs. Remotely commanded design updates to robotic AM systems enable rapid iteration and enable damaged structures to be repaired by reprinting parts as needed, which is crucial given limited resupply opportunities on Mars. Biocementation-based AM offers flexibility in material properties and construction methods techniques (Nething et al., 2020; Reinhardt et al., 2023; Pu et al., 2024). The process can extrude a slurry of regolith and microbes (Pu et al., 2024), enabling complex, strong geometries.
Advanced nozzles and multi-axis robotic arms will facilitate varied printing orientations, supporting arches and domes that withstand pressurization and external loads like dust storms. Since biocementation begins immediately upon mixing of feedstocks, precise control of mixing and flow rates is essential to prevent nozzle clogging from premature solidification. A conceptual biocementation nozzle may use a multi-channel system, delivering bacterial solution, nutrient media, and regolith slurry through separate feed lines with controlled flow rates. The bacterial solution is introduced last, just before extrusion, minimizing residence time and reducing the risk of clogging from premature calcium carbonate precipitation. This modular design enables real-time adjustment of mix ratios, allowing tailored material properties for specific structural or environmental needs. For Martian use, the nozzle would include dust-sealing features to mitigate contamination and abrasion, along with thermal controls, such as localized heating or insulation, to maintain fluid viscosity and microbial viability. Flow dynamics must also be calibrated for Mars’ reduced gravity to ensure consistent mixing and deposition across various print orientations.
Automating biocementation on Mars requires integrating regolith treatment with robotic systems capable of precisely delivering bacteria and reagents for CaCO3 precipitation. Rovers, aerial drones/helicopters, or subsurface crawlers could inject microbial cultures and nutrient solutions directly into the regolith. These robots would feature onboard reservoirs, automated mixing units, and sensor suites to control dosage and monitor key environmental parameters, including moisture, pH, and ion concentrations that govern microbial activity and calcite formation (Yang et al., 2023). Advanced robotic systems for biocementation could employ biosensors to detect microbial byproducts and noninvasive tools such as ground-penetrating radar, cone penetrometers, or ultrasound to monitor biocement spread and buildup. Onboard navigation systems (including imaging, positioning, and inertial sensors) enable precise surface or subsurface movement along predefined treatment paths. Remote operation allows for enhanced process control, while AI or machine learning can analyze sensor data to autonomously adjust injection timing, spatial targeting, and dosing. Operators can intervene as needed to ensure safety and responsiveness. Predictive models simulating fluid flow, microbial growth (Veiskarami et al., 2023; Mahdy et al., 2024; Khoshtinat and Marano, 2025; Khoshtinat et al., 2025), and geochemical reactions can further support real-time visualization and treatment planning. However, as previously mentioned, biocementation-based construction and additive manufacturing will work better in controlled conditions. Therefore, authors recommend that additive manufacturing-based biocementation processes be conducted in laboratory settings using high-fidelity Martian regolith simulants under controlled environmental conditions. This approach allows for systematic optimization of process parameters and microbial performance, thereby ensuring that methodologies can be rapidly adapted once actual Martian samples become available and once construction efforts on Mars begin in earnest.
4 Concluding remarks, challenges, and knowledge gaps
Given the relatively early stage of research on biocementation for Martian construction, significant gaps remain to be addressed. Biocementation for Martian construction is fundamentally multidisciplinary, requiring integration of microbiology, geochemistry, materials science, robotics, and construction engineering. Yet, current research often lacks the cross-disciplinary coordination needed to address its inherent complexity. Even on Earth, managing the interdependent factors (e.g., nutrient availability, microbial behavior, precipitation kinetics, and mechanical performance) is challenging. Martian conditions further complicate this through low gravity, high radiation, perchlorate-rich regolith, and low atmospheric pressure. In the absence of a standardized, system-level methodological framework, studies remain fragmented and difficult to compare, limiting progress toward viable implementation.
Developing planetary surface technologies requires testing under conditions that closely replicate the target environment’s gravity, temperature, atmospheric chemistry, pressure, and radiation environment. For biocementation systems designed to interact with Martian regolith, using compositionally accurate Martian regolith simulants is essential during research and development, as inadequate analogs compromise experimental validity, repeatability, and reproducibility. Although several Martian regolith simulants exist (Peters et al., 2008; Cannon et al., 2019; Long-Fox and Britt, 2023), Mars’ geological diversity further demands site-specific matching of particle size and geochemistry and large-scale testing in bulk simulant testbeds increases cost and complexity. Simulating Martian surface conditions (e.g., in thermal vacuum chambers with integrated radiation sources) remains technically challenging and resource-limited. Additionally, the effects of Martian gravity on microbial growth and biofilm dynamics are virtually unexplored, as current methods (e.g., parabolic and suborbital flights) offer only short-duration reduced gravity conditions and are hence unsuitable for sustained biocementation studies.
Fundamental biological questions remain unanswered, particularly regarding the response of bacteria to Martian conditions. While Chroococcidiopsis shows tolerance to desiccation and radiation its behavior in the presence of S. pasteurii under Martian stressors remains speculative. Gene expression under such conditions is largely uncharacterized, and the combined effects of these stressors on metabolic pathways, biocementation, and stress responses are unknown. Genomic/transcriptomic analyses under simulated Martian environments are essential but currently lacking. Without empirical insight into these organisms’ adaptation or failure under relevant Martian conditions, attempts at co-culture engineering remain premature. Dual-species systems may offer synergistic benefits, but microbial interactions are highly sensitive to environmental stress. Mutualism, competition, and allelopathy must be managed carefully in closed-loop systems where resource cycling and ecological stability are critical. Scarcity of nitrogen and phosphorus on Mars adds further complexity, requiring external supplementation or engineered metabolic pathways. To date, there is no empirical evidence supporting long-term co-culture stability under Martian constraints (Mosca et al., 2019; Billi et al., 2021; Koehle et al., 2023; Di Stefano et al., 2025).
Translating laboratory findings into functional technologies introduces further challenges. Bioreactor systems must withstand extreme accelerations during takeoff and landing, thermal cycling, handle abrasive regolith, and prevent clogging from CaCO3 precipitation and biofilm detachment. Gas exchange, water management, and light delivery must be optimized under Martian gravity and atmospheric conditions. Reactor scalability, robustness, and mass efficiency are nontrivial engineering hurdles, particularly when constrained by launch payload limits and ISRU strategies. Integration into life support systems for recycling waste gases, supplying oxygen, or generating construction materials requires comprehensive safety and reliability assessments. Without integrated, long-duration testing in analog or space environments, the pathway from concept to application remains highly speculative.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SK: Investigation, Supervision, Conceptualization, Validation, Project administration, Writing – review & editing, Writing – original draft. JL-F: Writing – review & editing, Writing – original draft, Validation, Investigation. SH: Writing – original draft, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a NASA Space Technology Graduate Research Opportunity (NSTGRO) Fellowship [NASA Cooperative Agreement 80NSSC23K1173] and by the NASA Solar System Exploration Research Virtual Institute (SSERVI) Center for Lunar and Asteroid Surface Science (CLASS) [NASA Cooperative Agreement 80NSSSC19M0124] (JL-F).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. ChatGPT was utilized to enhance grammatical accuracy and text fluency. The following prompt was used: “I give you texts. Refine them from a grammatical and fluency point of view. Double-check for scientific accuracy. Keep the scientific tone and accuracy.”
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achal, V., Mukherjee, A., Kumari, D., and Zhang, Q. (2015). Biomineralization for sustainable construction – a review of processes and applications. Earth Sci. Rev. 148, 1–17. doi: 10.1016/j.earscirev.2015.05.008
Ariyanti, D., and Handayani, N. A. (2011). An overview of biocement production from microalgae, vol. 2, 30–33.
ASTM C150 (2023). Standard Specification for Portland Cement. Available online at: https://www.astm.org/c0150_c0150m-23.html (Accessed March 15, 2025).
Azami, M., Kazemi, Z., Moazen, S., Dubé, M., Potvin, M.-J., and Skonieczny, K. (2024). A comprehensive review of lunar-based manufacturing and construction. Prog. Aerosp. Sci. 150:101045. doi: 10.1016/j.paerosci.2024.101045
Bagga, M., Hamley-Bennett, C., Alex, A., Freeman, B. L., Justo-Reinoso, I., Mihai, I. C., et al. (2022). Advancements in bacteria based self-healing concrete and the promise of modelling. Constr. Build. Mater. 358:129412. doi: 10.1016/j.conbuildmat.2022.129412
Baqué, M., de Vera, J.-P., Rettberg, P., and Billi, D. (2013a). The BOSS and BIOMEX space experiments on the EXPOSE-R2 mission: endurance of the desert cyanobacterium Chroococcidiopsis under simulated space vacuum, Martian atmosphere, UVC radiation and temperature extremes. Acta Astronaut. 91, 180–186. doi: 10.1016/j.actaastro.2013.05.015
Baqué, M., Scalzi, G., Rabbow, E., Rettberg, P., and Billi, D. (2013b). Biofilm and planktonic lifestyles differently support the resistance of the desert cyanobacterium Chroococcidiopsis under space and Martian simulations. Orig. Life Evol. Biosph. 43, 377–389. doi: 10.1007/s11084-013-9341-6
Berry, B. J., Jenkins, D. G., and Schuerger, A. C. (2010). Effects of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl. Environ. Microbiol. 76, 2377–2386. doi: 10.1128/AEM.02147-09
Billi, D., Friedmann, E. I., Hofer, K. G., Caiola, M. G., and Ocampo-Friedmann, R. (2000). Ionizing-radiation resistance in the desiccation-tolerant Cyanobacterium Chroococcidiopsis. Appl. Environ. Microbiol. 66, 1489–1492. doi: 10.1128/AEM.66.4.1489-1492.2000
Billi, D., Gallego Fernandez, B., Fagliarone, C., Chiavarini, S., and Rothschild, L. J. (2021). Exploiting a perchlorate-tolerant desert cyanobacterium to support bacterial growth for in situ resource utilization on Mars. Int. J. Astrobiol. 20, 29–35. doi: 10.1017/S1473550420000300
Billi, D., Staibano, C., Verseux, C., Fagliarone, C., Mosca, C., Baqué, M., et al. (2019a). Dried biofilms of desert strains of Chroococcidiopsis survived prolonged exposure to space and Mars-like conditions in low earth orbit. Astrobiology 19, 1008–1017. doi: 10.1089/ast.2018.1900
Billi, D., Verseux, C., Fagliarone, C., Napoli, A., Baqué, M., and de Vera, J.-P. (2019b). A desert cyanobacterium under simulated Mars-like conditions in low earth orbit: implications for the habitability of Mars. Astrobiology 19, 158–169. doi: 10.1089/ast.2017.1807
Cannon, K. M., Britt, D. T., Smith, T. M., Fritsche, R. F., and Batcheldor, D. (2019). Mars global simulant MGS-1: a Rocknest-based open standard for basaltic Martian regolith simulants. Icarus 317, 470–478. doi: 10.1016/j.icarus.2018.08.019
Castro-Alonso, M. J., Montañez-Hernandez, L. E., Sanchez-Muñoz, M. A., Macias Franco, M. R., Narayanasamy, R., and Balagurusamy, N. (2019). Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front. Mater. 6:126. doi: 10.3389/fmats.2019.00126
Chen, Z., Zhang, L., Tang, Y., and Chen, B. (2024). Pioneering lunar habitats through comparative analysis of in-situ concrete technologies: a critical review. Constr. Build. Mater. 435:136833. doi: 10.1016/j.conbuildmat.2024.136833
Cheng, L., and Cord-Ruwisch, R. (2012). In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 42, 64–72. doi: 10.1016/j.ecoleng.2012.01.013
Clark, B. C., Baird, A. K., Weldon, R. J., Tsusaki, D. M., Schnabel, L., and Candelaria, M. P. (1982). Chemical composition of Martian fines. J. Geophys. Res. Solid Earth 87, 10059–10067. doi: 10.1029/JB087iB12p10059
Concas, A., Fais, G., Enna, M., Zucchelli, S., Caboni, P., Lai, N., et al. (2023). Modeling and experimental assessment of Synechococcus nidulans cultivation using simulated Martian medium and astronauts’ urine. Acta Astronaut. 205, 185–198. doi: 10.1016/j.actaastro.2023.01.027
DeJong, J. T., Mortensen, B. M., Martinez, B. C., and Nelson, D. C. (2010). Bio-mediated soil improvement. Ecol. Eng. 36, 197–210. doi: 10.1016/j.ecoleng.2008.12.029
Deng, X., Li, Y., Liu, H., Zhao, Y., Yang, Y., Xu, X., et al. (2021). Examining energy consumption and carbon emissions of microbial induced carbonate precipitation using the life cycle assessment method. Sustainability 13:4856. doi: 10.3390/su13094856
Devrani, R., Dubey, A. A., Ravi, K., and Sahoo, L. (2021). Applications of bio-cementation and bio-polymerization for aeolian erosion control. J. Arid Environ. 187:104433. doi: 10.1016/j.jaridenv.2020.104433
Di Stefano, G., Baqué, M., Garland, S., Lorek, A., de Vera, J.-P., Gangi, M. E., et al. (2025). Resilience of metabolically active biofilms of a desert cyanobacterium capable of far-red photosynthesis under Mars-like conditions. Life 15:622. doi: 10.3390/life15040622
Dotson, B., Sanchez Valencia, D., Millwater, C., Easter, P., Long-Fox, J., Britt, D., et al. (2024). Cohesion and shear strength of compacted lunar and Martian regolith simulants. Icarus 411:115943. doi: 10.1016/j.icarus.2024.115943
Dubey, A. A., Devrani, R., Ravi, K., Dhami, N. K., Mukherjee, A., and Sahoo, L. (2021). Experimental investigation to mitigate aeolian erosion via biocementation employed with a novel ureolytic soil isolate. Aeolian Res. 52:100727. doi: 10.1016/j.aeolia.2021.100727
Dupraz, C., and Visscher, P. T. (2005). Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 13, 429–438. doi: 10.1016/j.tim.2005.07.008
Fackrell, L. E., Humphrey, S., Loureiro, R., Palmer, A. G., and Long-Fox, J. (2024). Overview and recommendations for research on plants and microbes in regolith-based agriculture. npj Sustain. Agric. 2:15. doi: 10.1038/s44264-024-00013-5
Farries, K. W., Visintin, P., Smith, S. T., and Van Eyk, P. (2021). Sintered or melted regolith for lunar construction: state-of-the-art review and future research directions. Constr. Build. Mater. 296:123627. doi: 10.1016/j.conbuildmat.2021.123627
Fattahi, S. M., Soroush, A., and Huang, N. (2020). Biocementation control of sand against wind erosion. J. Geotech. Geoenviron. Eng. 146:4020045. doi: 10.1061/(ASCE)GT.1943-5606.0002268
Foster, T. L., Winans, L., Casey, R. C., and Kirschner, L. E. (1978). Response of terrestrial microorganisms to a simulated Martian environment. Appl. Environ. Microbiol. 35, 730–737. doi: 10.1128/aem.35.4.730-737.1978
Gadd, G. M. (2007). Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 111, 3–49. doi: 10.1016/j.mycres.2006.12.001
Gat, D., Tsesarsky, M., Shamir, D., and Ronen, Z. (2014). Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences 11, 2561–2569. doi: 10.5194/bg-11-2561-2014
Gellert, R., Rieder, R., Anderson, R. C., Brückner, J., Clark, B. C., Dreibus, G., et al. (2004). Chemistry of rocks and soils in Gusev crater from the alpha particle X-ray spectrometer. Science 305, 829–832. doi: 10.1126/science.1099913
Gleaton, J., Lai, Z., Xiao, R., Chen, Q., and Zheng, Y. (2019). Microalga-induced biocementation of Martian regolith simulant: effects of biogrouting methods and calcium sources. Constr. Build. Mater. 229:116885. doi: 10.1016/j.conbuildmat.2019.116885
Hassler, D. M., Zeitlin, C., Wimmer-Schweingruber, R. F., Ehresmann, B., Rafkin, S., Eigenbrode, J. L., et al. (2014). Mars’ surface radiation environment measured with the Mars science laboratory’s curiosity rover. Science 343:1244797. doi: 10.1126/science.1244797
He, S., Zheng, J., He, R., Hosseini, S. M. J., Zhu, T., Cheng, L., et al. (2024). Influence of edge scour on lateral responses of monopiles with precast microbial reinforcement. Ocean Eng. 313:119493. doi: 10.1016/j.oceaneng.2024.119493
Hecht, M. H., Kounaves, S. P., Quinn, R. C., West, S. J., Young, S. M. M., Ming, D. W., et al. (2009). Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix Lander site. Science 325, 64–67. doi: 10.1126/science.1172466
Hoffman, J. A., Hecht, M. H., Rapp, D., Hartvigsen, J. J., SooHoo, J. G., Aboobaker, A. M., et al. (2022). Mars oxygen ISRU experiment (MOXIE)—preparing for human Mars exploration. Sci. Adv. 8:eabp8636. doi: 10.1126/sciadv.abp8636
Hosseini, S. M. J., Dawei, G., and Cheng, L. (2024a). Modification of all-in-one solution for improvement of 1m sand columns. Geomicrobiol J. 41, 870–882. doi: 10.1080/01490451.2024.2396508
Hosseini, S. M. J., Guan, D., and Cheng, L. (2024b). Ground improvement with a single injection of a high-performance all-in-one MICP solution. Geomicrobiol J. 41, 636–647. doi: 10.1080/01490451.2024.2364797
Iqbal, D. M., Wong, L. S., and Kong, S. Y. (2021). Bio-cementation in construction materials: a review. Materials 14:2175. doi: 10.3390/ma14092175
Keller, R., Goli, K., Porter, W., Alrabaa, A., and Jones, J. A. (2023). Cyanobacteria and algal-based biological life support system (BLSS) and planetary surface atmospheric revitalizing bioreactor brief concept review. Life 13:816. doi: 10.3390/life13030816
Khoshtinat, S. (2023a). Advancements in exploiting Sporosarcina pasteurii as sustainable construction material: a review. Sustainability 15:13869. doi: 10.3390/su151813869
Khoshtinat, S. (2023b). State-of-the-art review of aliphatic polyesters and polyolefins biodeterioration by microorganisms: from mechanism to characterization. Corros. Mater. Degrad. 4, 542–572. doi: 10.3390/cmd4040029
Khoshtinat, S., and Marano, C. (2025) in Numerical Modeling of the pH effect on the calcium carbonate precipitation by Sporosarcina pasteurii. eds. M. Kioumarsi and B. Shafei (Springer Nature Switzerland), 141–153.
Khoshtinat, S., Marano, C., and Kioumarsi, M. (2025). Computational modeling of biocementation by S. pasteurii: effect of initial pH. Discov. Mater. 5:65. doi: 10.1007/s43939-025-00241-7
Koehle, A. P., Brumwell, S. L., Seto, E. P., Lynch, A. M., and Urbaniak, C. (2023). Microbial applications for sustainable space exploration beyond low earth orbit. npj Microgravity 9:47. doi: 10.1038/s41526-023-00285-0
Liu, Z., Li, J., Yang, C., Wang, X., Xiao, J., Wang, L., et al. (2024). Cold sintering: a promising in situ resource utilisation strategy to densify lunar regolith simulants for construction applications. Mater. Des. 238:112674. doi: 10.1016/j.matdes.2024.112674
Long-Fox, J. M., and Britt, D. T. (2023). Characterization of planetary regolith simulants for the research and development of space resource technologies. Front. Space Technol. 4:1255535. doi: 10.3389/frspt.2023.1255535
Long-Fox, J. M., Landsman, Z. A., Easter, P. B., Millwater, C. A., and Britt, D. T. (2023). Geomechanical properties of lunar regolith simulants LHS-1 and LMS-1. Adv. Space Res. 71, 5400–5412. doi: 10.1016/j.asr.2023.02.034
Lovley, D. R. (1991). Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55, 259–287. doi: 10.1128/mr.55.2.259-287.1991
Lowenstam, H. A., Weiner, S., Lowenstam, H. A., and Weiner, S. (1989). On Biomineralization. Oxford, New York: Oxford University Press.
Lucas, M. P., Neal, C. R., Long-Fox, J. M., and Britt, D. (2024). Simulating lunar highlands regolith profiles on earth to inform infrastructure development and ISRU activities on the moon. Acta Astronaut. 224, 161–171. doi: 10.1016/j.actaastro.2024.08.019
Mahdy, O. S., Ali, A. B. M., Mahdi, M. S., Jasim, D. J., Kazemi-Varnamkhasti, H., Goli, M., et al. (2024). Thermal performance of nanofluid natural convection magneto-hydrodynamics within a chamber equipped with a hot block. Int. J. Thermofluids 24:100873. doi: 10.1016/j.ijft.2024.100873
Mann, S. (2001). Biomineralization principles and concepts in bioinorganic materials chemistry. New York: Oxford University Press.
McSween, H. Y., Taylor, G. J., and Wyatt, M. B. (2009). Elemental composition of the Martian crust. Science 324, 736–739. doi: 10.1126/science.1165871
Moeller, R., Rohde, M., and Reitz, G. (2010). Effects of ionizing radiation on the survival of bacterial spores in artificial Martian regolith. Icarus 206, 783–786. doi: 10.1016/j.icarus.2009.11.014
Moraes Neves, J., Collins, P. J., Wilkerson, R. P., Grugel, R. N., and Radlińska, A. (2019). Microgravity effect on microstructural development of tri-calcium silicate (C3S) paste. Front. Mater. 6:83. doi: 10.3389/fmats.2019.00083
Morris, R. V., Klingelhöfer, G., Schröder, C., Rodionov, D. S., Yen, A., Ming, D. W., et al. (2006). Mössbauer mineralogy of rock, soil, and dust at Meridiani planum, Mars: opportunity’s journey across sulfate-rich outcrop, basaltic sand and dust, and hematite lag deposits. J. Geophys. Res. Planets 111:2006JE002791. doi: 10.1029/2006JE002791
Mosca, C., Rothschild, L. J., Napoli, A., Ferré, F., Pietrosanto, M., Fagliarone, C., et al. (2019). Over-expression of UV-damage DNA repair genes and ribonucleic acid persistence contribute to the resilience of dried biofilms of the desert Cyanobacetrium Chroococcidiopsis exposed to Mars-like UV flux and Long-term desiccation. Front. Microbiol. 10:2312. doi: 10.3389/fmicb.2019.02312
Mujah, D., Shahin, M. A., and Cheng, L. (2017). State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol J. 34, 524–537. doi: 10.1080/01490451.2016.1225866
NASA (2021). With Mars methane mystery unsolved, Curiosity Serves Scientists a New One: Oxygen. Available online at: https://www.nasa.gov/missions/with-mars-methane-mystery-unsolved-curiosity-serves-scientists-a-new-one-oxygen/ (Accessed March 15, 2025).
NASA Sets Path to Return Mars Samples, Seeks Innovative Designs - NASA (2024). NASA. Available online at: https://www.nasa.gov/news-release/nasa-sets-path-to-return-mars-samples-seeks-innovative-designs/ (Accessed August 27, 2025).
Naser, M. Z. (2019). Extraterrestrial construction materials. Prog. Mater. Sci. 105:100577. doi: 10.1016/j.pmatsci.2019.100577
Nething, C., Smirnova, M., Gröning, J. A. D., Haase, W., Stolz, A., and Sobek, W. (2020). A method for 3D printing bio-cemented spatial structures using sand and urease active calcium carbonate powder. Mater. Des. 195:109032. doi: 10.1016/j.matdes.2020.109032
Newcombe, D. A., Schuerger, A. C., Benardini, J. N., Dickinson, D., Tanner, R., and Venkateswaran, K. (2005). Survival of spacecraft-associated microorganisms under simulated Martian UV irradiation. Appl. Environ. Microbiol. 71, 8147–8156. doi: 10.1128/AEM.71.12.8147-8156.2005
Omoregie, A. I., Palombo, E. A., Ong, D. E. L., and Nissom, P. M. (2020). A feasible scale-up production of Sporosarcina pasteurii using custom-built stirred tank reactor for in-situ soil biocementation. Biocatal. Agric. Biotechnol. 24:101544. doi: 10.1016/j.bcab.2020.101544
Orosei, R., Lauro, S. E., Pettinelli, E., Cicchetti, A., Coradini, M., Cosciotti, B., et al. (2018). Radar evidence of subglacial liquid water on Mars. Science 361, 490–493. doi: 10.1126/science.aar7268
Osman, S., Peeters, Z., La Duc, M. T., Mancinelli, R., Ehrenfreund, P., and Venkateswaran, K. (2008). Effect of shadowing on survival of Bacteria under conditions simulating the Martian atmosphere and UV radiation. Appl. Environ. Microbiol. 74, 959–970. doi: 10.1128/AEM.01973-07
Pakbaz, M. S., Behzadipour, H., and Ghezelbash, G. R. (2018). Evaluation of shear strength parameters of sandy soils upon microbial treatment. Geomicrobiol J. 35, 721–726. doi: 10.1080/01490451.2018.1455766
Peters, G. H., Abbey, W., Bearman, G. H., Mungas, G. S., Smith, J. A., Anderson, R. C., et al. (2008). Mojave Mars simulant—characterization of a new geologic Mars analog. Icarus 197, 470–479. doi: 10.1016/j.icarus.2008.05.004
Provis, J. L., and Deventer, J. S. J. van (2009). Geopolymers: Structures, processing, properties and industrial applications. Cambridge; New Dehli; Boca Raton, FL: Elsevier. Available online at: https://www.google.it/books?id=NqijAgAAQBAJ (Accessed April 21, 2025).
Pu, X., Wu, Y., Liu, J., and Wu, B. (2024). 3D bioprinting of microbial-based living materials for advanced energy and environmental applications. Chem. Bio. Eng. 1, 568–592. doi: 10.1021/cbe.4c00024
Quinn, R. C., Chittenden, J. D., Kounaves, S. P., and Hecht, M. H. (2011). The oxidation-reduction potential of aqueous soil solutions at the Mars Phoenix landing site. Geophys. Res. Lett. 38. doi: 10.1029/2011GL047671
Reinhardt, O., Ihmann, S., Ahlhelm, M., and Gelinsky, M. (2023). 3D bioprinting of mineralizing cyanobacteria as novel approach for the fabrication of living building materials. Front. Bioeng. Biotechnol. 11:1145177. doi: 10.3389/fbioe.2023.1145177
Rieder, R., Gellert, R., Anderson, R. C., Brückner, J., Clark, B. C., Dreibus, G., et al. (2004). Chemistry of rocks and soils at Meridiani planum from the alpha particle X-ray spectrometer. Science 306, 1746–1749. doi: 10.1126/science.1104358
Sanders, G., Kleinhenz, J., and Linne, D. (2022). NASA plans for in situ resource utilization (ISRU) development, demonstration, and implementation.
Schreiner, S., Sibille, L., Dominguez, J., Sirk, A., Hoffman, J., and Sanders, G. (2015). “Development of a molten Regolith electrolysis reactor model for lunar in-situ resource utilization” in In 8th Symposium on Space Resource Utilization (Reston, VA: American Institute of Aeronautics and Astronautics).
Sharaky, A. M., Mohamed, N. S., Elmashad, M. E., and Shredah, N. M. (2018). Application of microbial biocementation to improve the physico-mechanical properties of sandy soil. Constr. Build. Mater. 190, 861–869. doi: 10.1016/j.conbuildmat.2018.09.159
Sharma, T., Sharma, S., Kamyab, H., and Kumar, A. (2020). Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: a review. J. Clean. Prod. 247:119138. doi: 10.1016/j.jclepro.2019.119138
Spedding, C. P., Nuttall, W. J., and Lim, S. (2020). Energy requirements of a thermally processed ISRU radiation shield for a lunar habitat. Adv. Space Res. 65, 2467–2474. doi: 10.1016/j.asr.2020.03.015
Tarasashvili, M. V., Elbakidze, K., Doborjginidze, N. D., and Gharibashvili, N. D. (2023). Carbonate precipitation and nitrogen fixation in AMG (artificial Martian ground) by cyanobacteria. Life Sci. Space Res. 37, 65–77. doi: 10.1016/j.lssr.2023.03.002
Taylor, S. R., and McLennan, S. (2008) Planetary crusts: Their composition, origin and evolution Cambridge Cambridge University Press Available online at: https://www.cambridge.org/core/product/EAAB6EB7E8C1306728F63CA3748C7626
Paassen, L. A.Van, Daza, C. M., Staal, M., Sorokin, D. Y., Van Der, Zon W., and Loosdrecht, M. C. M.Van (2010). Potential soil reinforcement by biological denitrification. 36, 168–175
Veiskarami, M., Roshanali, L., and Habibagahi, G. (2023). A theoretical study on the hydraulic conductivity of anisotropic granular materials by implementing the microstructure tensor. Granul. Matter 25:66. doi: 10.1007/s10035-023-01352-9
Verseux, C., Baqué, M., Lehto, K., de Vera, J.-P. P., Rothschild, L. J., and Billi, D. (2016). Sustainable life support on Mars – the potential roles of cyanobacteria. Int. J. Astrobiol. 15, 65–92. doi: 10.1017/S147355041500021X
Wamelink, G. W. W., Frissel, J. Y., Krijnen, W. H. J., Verwoert, M. R., and Goedhart, P. W. (2014). Can plants grow on Mars and the moon: a growth experiment on Mars and moon soil simulants. PLoS One 9:e103138. doi: 10.1371/journal.pone.0103138
Wang, Y., Hao, L., Li, Y., Sun, Q., Sun, M., Huang, Y., et al. (2022). In-situ utilization of regolith resource and future exploration of additive manufacturing for lunar/Martian habitats: a review. Appl. Clay Sci. 229:106673. doi: 10.1016/j.clay.2022.106673
Watters, T. R., Campbell, B. A., Leuschen, C. J., Morgan, G. A., Cicchetti, A., Orosei, R., et al. (2024). Evidence of ice-rich layered deposits in the Medusae fossae formation of Mars. Geophys. Res. Lett. 51:e2023GL105490. doi: 10.1029/2023GL105490
Xu, P., Fan, H., Leng, L., Fan, L., Liu, S., and Chen, P. (2020). Feasibility of microbially induced carbonate precipitation through a Chlorella-Sporosaricina co-culture system. Algal Res. 47:101831. doi: 10.1016/j.algal.2020.101831
Yang, W., Song, Y., Fang, H., Feng, Y., Zhao, C., and Song, X. (2023). Advances in microbial induced carbonate precipitation (MICP) technology for extraterrestrial construction. SSRN. doi: 10.2139/ssrn.4574872
Yin, J., Wu, J.-X., Zhang, K., Shahin, M. A., and Cheng, L. (2022). Comparison between MICP-based bio-cementation versus traditional Portland cementation for oil-contaminated soil stabilisation. Sustainability 15:434. doi: 10.3390/su15010434
Zaccaria, T., de Jonge, M. I., Domínguez-Andrés, J., Netea, M. G., Beblo-Vranesevic, K., and Rettberg, P. (2024). Survival of environment-derived opportunistic bacterial pathogens to Martian conditions: is there a concern for human missions to Mars? Astrobiology 24, 100–113. doi: 10.1089/ast.2023.0057
Zent, A. P., Hecht, M. H., Cobos, D. R., Campbell, G. S., Campbell, C. S., Cardell, G., et al. (2009). Thermal and electrical conductivity probe (TECP) for Phoenix. J. Geophys. Res. Planets 114:2007JE003052. doi: 10.1029/2007JE003052
Zhu, T., and Dittrich, M. (2016). Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front. Bioeng. Biotechnol. 4:4. doi: 10.3389/fbioe.2016.00004
Zhu, T., He, R., Hosseini, S. M. J., He, S., Cheng, L., Guo, Y., et al. (2024). Influence of precast microbial reinforcement on lateral responses of monopiles. Ocean Eng. 307:118211. doi: 10.1016/j.oceaneng.2024.118211
Zomorodian, S. M. A., Ghaffari, H., and O’Kelly, B. C. (2019). Stabilisation of crustal sand layer using biocementation technique for wind erosion control. Aeolian Res. 40, 34–41. doi: 10.1016/j.aeolia.2019.06.001
Keywords: Mars, construction, biomineralization, in situ resource utilization, robotics, additive manufacturing
Citation: Khoshtinat S, Long-Fox J and Hosseini SMJ (2025) From Earth to Mars: a perspective on exploiting biomineralization for Martian construction. Front. Microbiol. 16:1645014. doi: 10.3389/fmicb.2025.1645014
Edited by:
Yong Fu, Southern University of Science and Technology, ChinaReviewed by:
Shrihari Sankarasubramanian, University of Texas at San Antonio, United StatesCopyright © 2025 Khoshtinat, Long-Fox and Hosseini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiva Khoshtinat, c2hpdmEua2hvc2h0aW5hdEBwb2xpbWkuaXQ=
 Shiva Khoshtinat
Shiva Khoshtinat Jared Long-Fox
Jared Long-Fox Seyed Mohammad Javad Hosseini
Seyed Mohammad Javad Hosseini