- 1Yanchi Research Station, School of Soil and Water Conservation, Beijing Forestry University, Beijing, China
- 2School of Soil and Water Conservation, Beijing Forestry University, Beijing, China
- 3Key Laboratory of State Forestry Administration on Soil and Water Conservation, Beijing Forestry University, Beijing, China
- 4State Key Laboratory of Efficient Production of Forest Resource, Beijing Forestry University, Beijing, China
- 5Engineering Research Center of Forestry Ecological Engineering, Ministry of Education, Beijing Forestry University, Beijing, China
Introduction: Biocrusts are critical components of desert ecosystems, performing vital functions including soil stabilization, nutrient enrichment, and regulation of carbon (C) and nitrogen (N) cycles. This study investigated the microbial mechanisms underlying biocrust-mediated soil multifunctionality (SMF) in the Mu Us Desert by comparing algal-, lichen-, and moss-dominated crusts.
Methods: We systematically sampled biocrust layers and underlying subcrust soils (0–5 cm depth), employing metagenomic sequencing and co-occurrence network analysis to characterize microbial community structures and functional properties. SMF was quantified using an integrative index based on ten parameters associated with C, N, and phosphorus (P) cycling processes.
Results: Results revealed significant variation in SMF among biocrust types, with moss crusts exhibiting the highest level, followed by lichen and algal crusts. Microbial community characteristics indicated that although lichen crusts showed the highest taxonomic diversity and network complexity, moss crusts showed a significant positive correlation with SMF. Network topological parameters, particularly network density within the biocrust layers, correlated significantly positively with SMF (p < 0.05), contrasting with the non-significant relationship observed for taxonomic diversity. Functional gene analysis revealed that the diversity of C degradation and N cycling genes exhibited a significant positive correlation with SMF.
Discussion: Our findings demonstrate that biocrusts enhance SMF primarily by mediating both direct and indirect effects on N cycling functional gene diversity and microbial network complexity. This study underscores the critical role of functional gene diversity in driving biocrust-mediated ecological functions in desert ecosystems and provides a theoretical framework for developing sustainable land management and ecological restoration strategies in drylands.
1 Introduction
Biocrusts represent vital components of desert ecosystems, dominating vegetation-free surfaces and occupying > 40% of ground cover in these regions, equivalent to approximately 12% of the Earth’s continental land area (Rodriguez-Caballero et al., 2018; Li Y. H. et al., 2021). Biocrusts comprise photoautotrophic communities (cyanobacteria, algae, lichens and bryophytes) that co-occur with heterotrophic bacteria, archaea and fungi, forming cohesive surface layers on arid soils (Rodriguez-Caballero et al., 2018). Based on biological composition, biocrusts are categorized into algal-, lichen-, and moss-dominated types, each exhibiting unique ecological functions due to divergent physicochemical properties (Li X. R. et al., 2021; Li Y. H. et al., 2021). Crucially, biocrusts enhance soil physical structure, promote nutrient accumulation, and elevate carbon (C) and nitrogen (N) stocks in desert ecosystems. Through these synergistic effects, they enhance soil multifunctionality (SMF) (Billings et al., 2003; Housman et al., 2006; Bu et al., 2013). Biocrusts do not function as isolated ecosystem units; their formation, stability, and functionality are tightly coupled with and interact dynamically with the underlying subcrust soil (Dou et al., 2022; Liu Q. et al., 2024). Biocrust development critically depends on physical support, water storage, and nutrient supply provided by the subcrust soils. Simultaneously, biocrusts profoundly modify subcrust soils physicochemical and biological properties through microclimate alteration, enhanced organic matter input, and regulation of water infiltration and nutrient cycling (Eldridge et al., 2020; Mackelprang et al., 2022; Li et al., 2023).
SMF defined as the capability of soils to simultaneously sustain multiple ecological processes such as nutrient cycling, organic carbon transformation, and fertility maintenance – is fundamental to ecosystem stability (Deng et al., 2024; Hu X. G. et al., 2024). Although considerable advances have elucidated SMF in forest, agricultural, and grassland ecosystems, understanding of biocrusts – mediated SMF in deserts remains limited. Current knowledge faces two principal constraints: First, while microorganisms are primary drivers of biocrust development and function, the specific mechanisms through which microbial communities regulate SMF remain unresolved (Zhai et al., 2024). Second, existing studies predominantly focus on microbial taxonomic diversity, neglecting the governing roles of microbial interaction networks and functional gene composition in determining SMF (Xiao et al., 2023). These critical gaps hinder efforts to enhance the functional stability of desert ecosystems.
As core components of biocrusts, microorganisms directly regulate soil biogeochemical processes through their diversity and functional traits (Maier et al., 2018). Enhanced microbial diversity typically increases SMF via functional redundancy and complementarity (Wagg et al., 2019). However, studies in agricultural ecosystems demonstrate that microbial interactions are stronger predictors of SMF than taxonomic diversity alone (Jiao et al., 2022). Furthermore, functional genes (e.g., those involved in C, N, and P cycling) drive the synergistic enhancement of SMF by underpinning multiple individual processes, such as nutrient cycling and organic matter decomposition (Liu L. et al., 2024; Liu Q. et al., 2024). Divergent effects on SMF are observed across biocrust types: algal crusts primarily facilitate carbon and nitrogen accumulation, whereas moss crusts enhance phosphorus mineralization; collectively, these contribute to sustaining overall SMF (Mager and Thomas, 2011; Xu et al., 2023). Nevertheless, the drivers of SMF exhibit substantial variation across different ecosystems, and a generalizable framework for its comprehensive assessment remains elusive (Byrnes et al., 2013). Notably, within desert ecosystems, the mechanisms by which biocrusts influence SMF are poorly understood. In particular, the relative contributions of taxonomic diversity, microbial network interactions, and functional gene assemblages require quantitative assessment.
This investigation focuses on algal-, lichen-, and moss-dominated biocrusts in the Mu Us Desert. Utilizing a stratified sampling design (biocrust layer and the underlying subcrust layers) combined with metagenomic sequencing, we examine microbial community structure and functional gene profiles across biocrust types. Specifically, the study aims to address the following: (1) Assess differences in SMF among biocrust types and between biocrusts layer and their underlying soils; (2) Determine how microbial taxonomic diversity and co-occurrence network topology within biocrusts relate to SMF; (3) Evaluate the influence of functional gene composition and diversity in biocrusts on SMF. By integrating these perspectives, this research advances our understanding of the mechanisms by which biocrusts modulate ecosystem functioning in the Mu Us Desert. The findings not only address critical knowledge gaps regarding microbial drivers of SMF but also provide a scientific basis for managing and accessing soil ecosystem health in dryland regions.
2 Materials and methods
2.1 Study site
This research was conducted at the Yanchi Research Station (37 °40′–38 °10′N, 106 °30′–107 °41′E), located on the southwestern fringe of the Mu Us Desert in northern China at 1,530 m elevation (Zhu et al., 2024). The site experiences a semi-arid mid-temperate continental monsoon climate. Meteorological records (1954–2022) from Yanchi Meteorological Station (~20 km northeast) indicate a mean annual temperature of 8.4 °C and precipitation of 292 mm, with ~80% occurring during the growing season (May–September). Annual potential evaporation averages 2024 mm. Soils are sandy-textured, with bulk density of 1.54 ± 0.08 g cm−3 and field capacity of 20.31 ± 3.33% (0–20 cm depth) (Jia et al., 2018; Hao et al., 2023). Dominant vegetation includes Artemisia ordosica, Hedysarum mongolicum, Salix psammophila, and Caragana korshinskii, with biocrusts colonizing plant interspaces across xeric-to-mesic microhabitats. Biocrust communities comprise algae (Oscillatoria chlorina, Microcoleus vaginatus), lichens (Collema tenax), and mosses (Bryum argenteum) (She et al., 2024).
2.2 Experiment design and sampling
Three biocrust types (algal, lichen, moss) were sampled at Yanchi Research Station in June 2017. Within three spatially segregated 1-km2 plots (one per biocrust type), we established three 50 × 50 m subplots (n = 3 per biocrust type; minimum 50 m spacing). Biocrust samples (1–2 cm natural thickness) and underlying subcrust soils (5 cm below crust interface) were collected using a diagonal intersection strategy (5 points: center + corners). At each subplot, 12 biocrust layer samples were randomly obtained using sterilized cutting rings (inner diameter: 9 cm; depth: 1 cm), which were homogenized into one composite sample per subplot (yielding 9 composite biocrust samples). Subcrust soils were similarly collected and composited using the same method, yielding 9 composite soil samples. This resulted in a total of 18 composite soil samples. Plant litter was removed from biocrust surfaces prior to sampling, and all equipment was sterilized with 75% ethanol between samples. Samples were immediately stored in cooling boxes (4 °C) and transported to the laboratory within 4 h. Each composite sample was then divided into three aliquots: (1) fresh subsamples for determining physicochemical properties and enzyme activities; (2) Flash-frozen (−80 °C) for DNA sequencing; (3) Refrigerated (4 °C) for microbial biomass.
2.3 Soil physicochemical properties determination
The fresh subsamples were used for physicochemical properties. Soil organic carbon (SOC) was quantified by K₂SO₄ extraction. Total nitrogen (TN) and phosphorus (TP) were determined using Kjeldahl micro-analysis (FOSS 2200) and molybdenum-blue spectrophotometry, respectively. Inorganic nitrogen fractions were measured as: NO₃−-N by UV spectrophotometry and NH₄+-N by continuous flow analysis after CaCl₂ extraction. Enzyme activities were assessed calorimetrically: invertase via 3,5-DNS method, urease by phenol-hypochlorite, and alkaline phosphatase using phenyl phosphate disodium substrate. The soil samples stored at 4 °C used to determine microbial biomass carbon (MBC) and nitrogen (MBN) via chloroform fumigation-K₂SO₄ extraction.
2.4 Shotgun metagenome sequencing
Soil samples stored at −20 °C were subjected to metagenomic sequencing on an Illumina HiSeq platform (Novogene Co., Beijing). Raw sequences underwent adapter trimming and quality filtering (discarding reads <40 bp, average quality score ≤38, or exceeding thresholds for N-base content/adapter overlap). High-quality reads were assembled into contigs using Megahit v1.2.9, retaining contigs ≥500 bp for downstream analysis. Open reading frames (ORFs) were predicted from contigs using Metagene Mark v3.38. For taxonomic annotation, ORFs were aligned against the NCBI NR database using BLASTP (e-value ≤ 1e−5). The lowest common ancestor (LCA) algorithm assigned classifications at kingdom to species levels, with gene abundance calculated per taxon. Functional annotation was performed via DIAMOND v2.0.15 against KEGG (2023 release) using best-hit criteria (e-value ≤ 1e−5). KEGG Orthology (KO), pathway, and module abundances were quantified based on aligned gene counts following Liu et al. (2023).
2.5 Soil multifunctionality calculations
For this study, ten key indicators of ecosystem functioning were selected to characterize soil multifunctionality. These indicators include SOC, TN, TP, MBC, MBN, NO₃−-N, NH₄+-N, urease activity, alkaline phosphatase activity, and invertase activity. Closely linked to the storage and cycling of C, N, and P, these metrics collectively provide a robust representation of multiple ecosystem functions, including nutrient sequestration and utilization, soil fertility, and biogeochemical cycles (Hu W. et al., 2024; Hu X. G. et al., 2024). Each of these ten indicators was standardized using the Z-score method (Equation 1), followed by the calculation of soil multifunctionality. The formula for Z-score standardization is as follows:
2.6 Soil microbial community and functional characteristics
Microbial taxonomic α-diversity (Shannon, species richness, Chao1) and functional gene α-diversity (Shannon, Chao1, Simpson for C/N/P cycling genes) were calculated using vegan v2.6-4 package in R v.4.4.1. Co-occurrence networks were constructed from species-level abundance data (relative abundance > 0.002%; occurrence frequency >1/6) via the WGCNA v1.72 package in R v.4.4.1 (Langfelder and Horvath, 2008). Spearman’s rank correlation coefficients (r) were computed using the psych v2.5.6 package in R v.4.4.1 Nodes and edges were retained only when meeting the threshold criteria of |r| > 0.55 with statistical significance of p < 0.01 (FDR-corrected) (Benjamini and Hochberg, 1995). Weighted undirected networks were constructed from the correlation matrices using the igraph v.2.2.4 package in R v.4.4.1. Edge weights were assigned based on correlation coefficients, and categorical attributes were added to nodes. Common topological metrics were subsequently calculated. Network visualization was implemented in Gephi 0.9.2. For each sampling site, sub-networks were extracted from the integrated network using the subgraph function in igraph v.2.2.4, from which node counts, edge counts, and network density were derived (Zhang B. et al., 2018; Wagg et al., 2019; Qin et al., 2021).
2.7 Statistical analysis
Differences in soil parameters and microbial diversity across biocrust types/layers were assessed using ANOVA with LSD post-hoc tests (α = 0.05). Parametric assumptions (normality: Shapiro–Wilk; homogeneity: Levene’s test) were verified, with log-transformation applied to non-normal variables. Pearson correlations quantified relationships between microbial characteristics and SMF. To clarify the contributions of microbial communities versus functional traits to ecosystem multifunctionality, we conducted variance partitioning analysis (VPA) using the varpart function in the vegan v2.6-4 package in R v.4.4.1, followed by rigorous validation of unique components through partial redundancy analysis (pRDA) combined with 999 permutations. Structural equation modeling (SEM) elucidated direct/indirect pathways linking microbial parameters to ecosystem multifunctionality. The SEM was deemed acceptable if it satisfied all the following goodness-of-fit criteria: 0 ≤ Chisq/df ≤ 2 with 0.05 < p ≤ 1.00; 0.90 ≤ Goodness of Fit Index (GFI) ≤ 1.00; 0.90 ≤ Comparative Fit Index (CFI) ≤ 1.00; and 0.90 ≤ Normed Fit Index (NFI) ≤ 1.00.
3 Results
3.1 Differences in soil properties and multifunctionality of biocrusts
Among all measured parameters, NO₃−-N and urease concentrations were lower in the moss crust layer compared to its subcrust layer (Supplementary Figure S1). In contrast, all other indicators were significantly higher in the crust layers than in the subcrust layers. Within the biocrust layers, SOC, TN, and MBC contents differed significantly among the three biocrust types (p < 0.01, Supplementary Figure S1). However, the subcrust layers, exhibited no significant differences in TN, TP, MBC, MBN, NH₄+-N, urease, or invertase across the biocrust types.
SMF was significantly higher in biocrust layers than in subcrust layers (p < 0.05) and differed significantly among biocrust types (p < 0.05, Figure 1). Moss crusts had the highest SMF, followed by lichen and algal crusts, in both biocrust and subcrust layers. Positive SMF values (>0) were only observed in the lichen and moss crusts layers. Quantitative values for biocrust layers SMF were −0.25 ± 0.02 (algae), 0.49 ± 0.32 (lichen), and 1.31 ± 0.21 (moss). Corresponding values for subcrust layers were: −0.91 ± 0.06, −0.49 ± 0.23, and −0.15 ± 0.19.

Figure 1. Soil multifunctionality of different biocrusts. Different lowercase letters indicate significant differences (p < 0.05), data are shown as mean ± SE.
3.2 Difference in microbial taxonomic and functional properties
Species richness and the Chao1 index were significantly higher in both lichen crust layers and their subcrust layers compared to the corresponding layers in algal crusts and moss crusts (Figure 2). Conversely, Shannon diversity indices showed an inverse pattern: algal crust layers had the highest values, followed by moss crust layers, while lichen crust layers had significantly lower values. Across the three biocrust types, there were generally no significant differences in these taxonomic diversity indices between biocrust layers and subcrust layers. However, an exception was observed in algal crusts, where the biocrust layer had a significantly higher Shannon index than its subcrust layer.
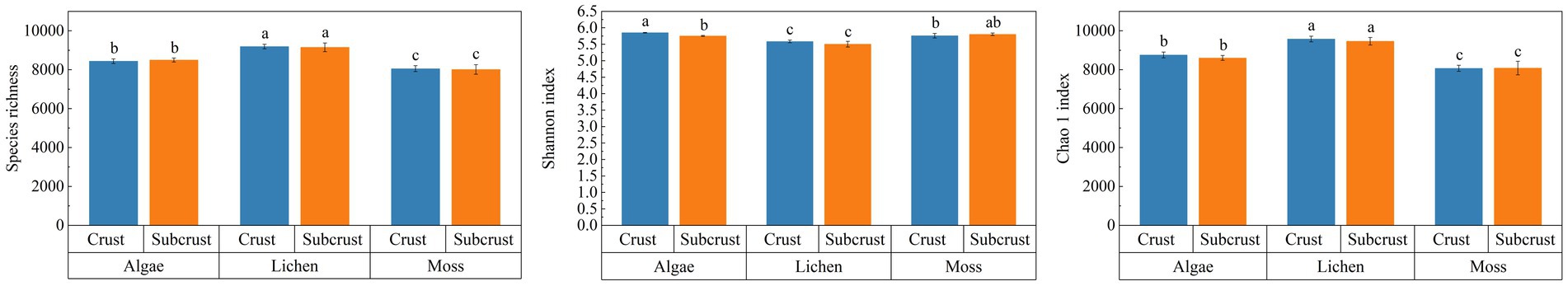
Figure 2. Soil microbial taxonomic diversity in different biocrusts. Different lowercase letters indicate significant differences (p < 0.05), data are shown as mean ± SE.
Co-occurrence network complexity, as indicated by network density, was significantly higher in lichen crusts (0.2043) than in algal crusts (0.0178) or moss crusts (0.0138) (Figure 3). Furthermore, the biocrust layer (0.1174) exhibited greater network complexity than its respective subcrust layer (0.0220). Across all networks, positive interactions between nodes consistently exceeded 50%, indicating predominantly cooperative relationships among soil microbial species. The phyla Proteobacteria, Actinobacteria, and Firmicutes (each with relative abundance >10%) were key components contributing to network stability.
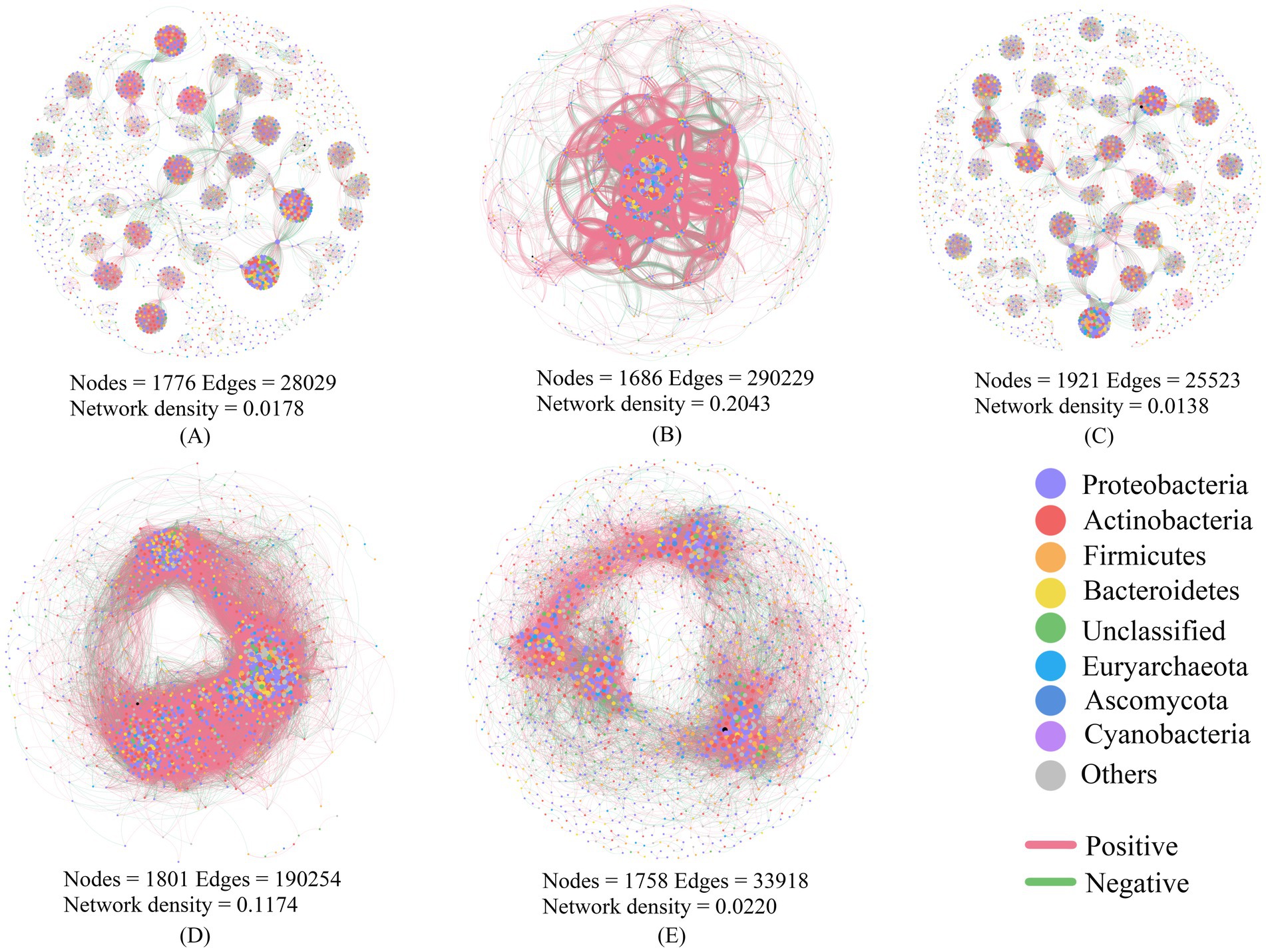
Figure 3. Microbial co-occurrence networks of soil microorganisms with different biocrusts. (A) algal crusts, (B) lichen crusts, (C) moss crusts, (D) biocrust layer, (E): subcrust layer.
Comparative analysis revealed significant differences (p < 0.05) between biocrust layers and subcrust layers in the Shannon and Simpson indices of microbial functional genes associated with C fixation, C degradation, and P cycling (Supplementary Figure S2). Notably, the subcrust layer exhibited greater diversity in C fixation genes than the biocrust layer. Among the three biocrust types, significant differences in the Shannon and Simpson indices of functional genes were only observed for N cycling genes (p < 0.05).
3.3 Influence of microbial taxonomic and functional properties on soil multifunctionality
Pearson correlation analysis revealed no significant correlation between SMF and taxonomic α-diversity of biocrusts (Supplementary Figure S3). However, in biocrust layers, network density showed a significant positive correlation with SMF (p < 0.05), contrasting with a significant negative correlation (p < 0.05) in subcrust soils. In biocrust layers, α-diversity of C fixation gene (Simpson index), C degradation (Chao 1), and N cycling (Shannon and Simpson index) exhibited a significant positive association with SMF (Figure 4). Conversely, the α-diversity (Simpson index) of P cycling genes showed a significant negative correlation with SMF (p < 0.05). In subcrust layers, only the α-diversity of P cycling genes exhibited a significant association (negative) with SMF; α-diversity indices for C- and N-related genes showed no significant relationships.
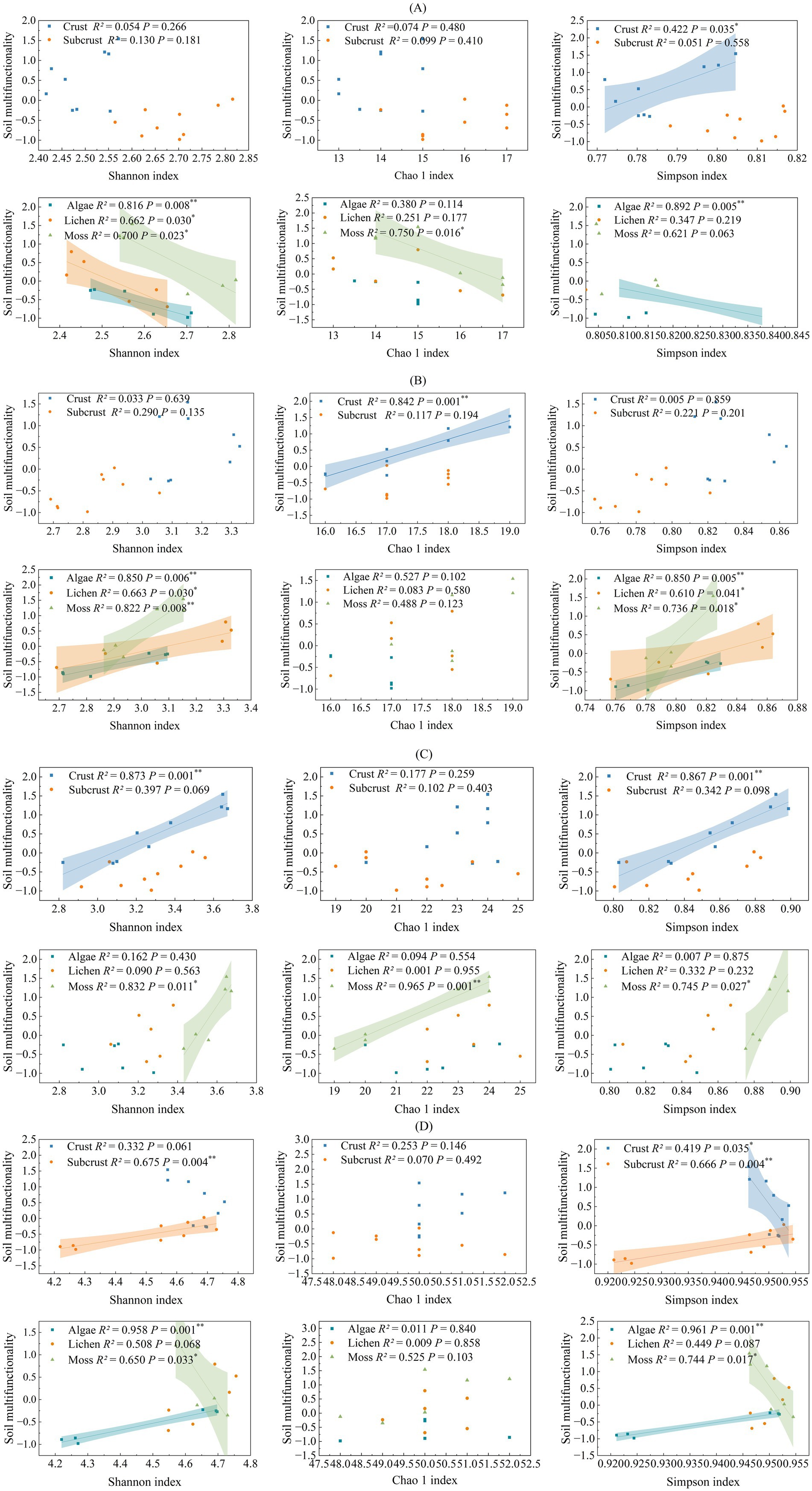
Figure 4. Relationship between soil multifunctionality (Z-score) and soil microbial functional diversity. Shadow regions indicate 95% confidence intervals around the regressions. R2, Coefficient of determination; * p < 0.05; ** p < 0.01. (A) carbon fixation, (B) carbon degradation. (C) nitrogen cycle, (D) phosphorus cycle.
Examining correlations by biocrust types revealed consistent negative associations between C fixation gene α-diversity and SMF across all three types. C-degrading functional genes α-diversity showed consistent positive associations (Shannon and Simpson indices). For N cycling genes, significant positive correlations were observed only in moss crusts. P cycling gene α-diversity exhibited divergent correlations: positive in algal crusts but strongly negative in moss crusts.
Variance partitioning analysis (VPA, Figures 5A,B) revealed that functional genes diversity (C degradation, C fixation, N cycling, and P cycling) collectively accounted for 86% of the variance in SMF. Among them, C degradation gene diversity contributed the most to SMF, explaining up to 74% of the variation, followed by N cycling gene diversity at 60%. VPA also indicated that microbial taxonomic diversity and network topology together explained 26% of the variance in SMF; this explained variance was attributable solely to network topology.
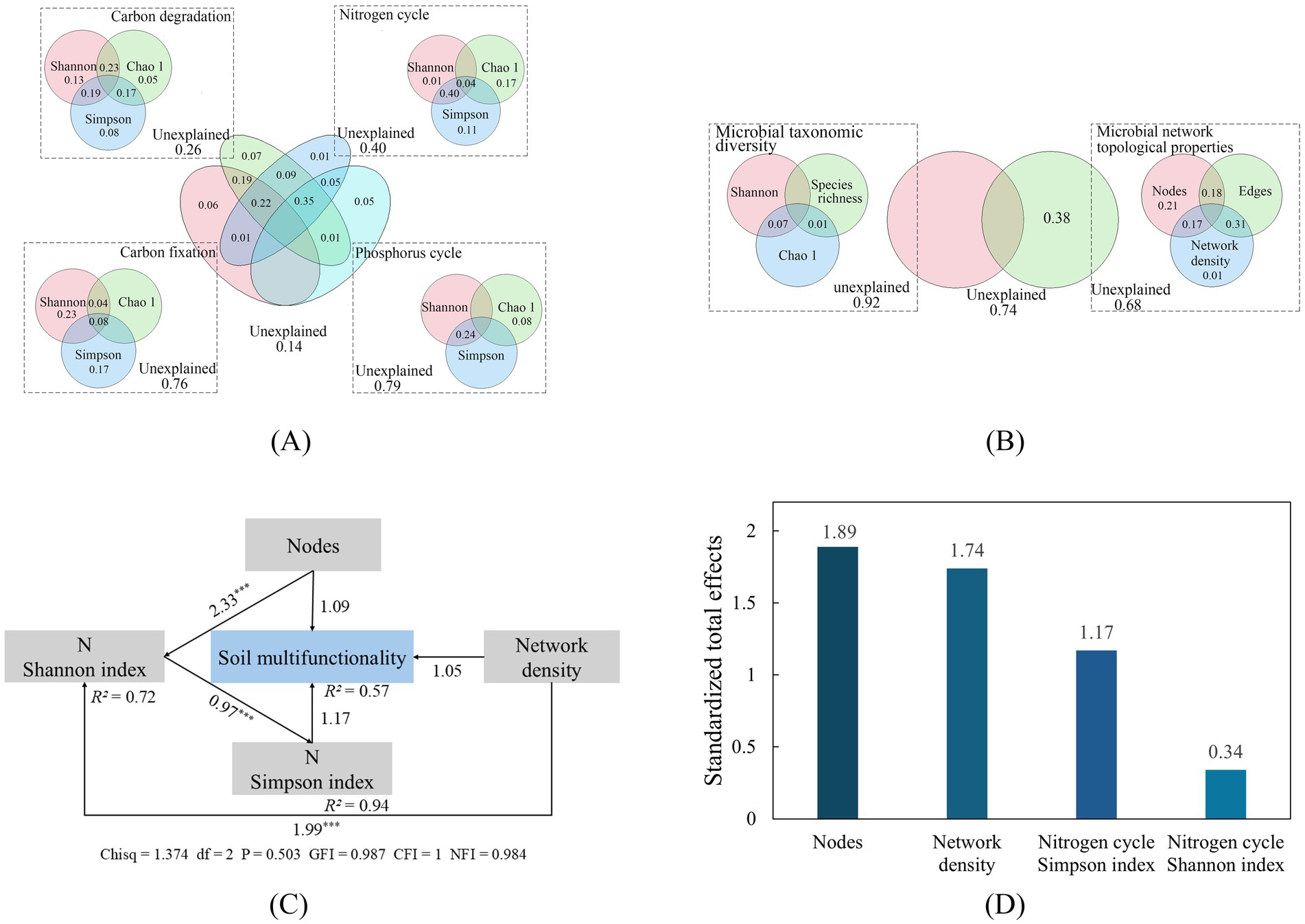
Figure 5. Percentage explanation of functional properties (A) and microbial taxonomic (B) on SMF (variance partition analysis); structural equation modeling (C) and standardized total effect (D) of microbial parameters on SMF. Arrows represent positive correlations, the value on the arrow is the standardized path coefficient and the degree of correlation, and R2 represents the degree of explanation of the model for the variable; GFI, Goodness fit index; CFI, Comparative fit index; NFI, Normed fit index; * p < 0.05; ** p < 0.01; *** p < 0.001.
Structural equation modeling (SEM, Figure 5C) explained 57% of the variance in SMF (R2 = 0.57). Key drivers identified by the SEM included microbial network node number, network density, and the α-diversity (Shannon and Simpson indices) of N cycling functional genes. The model indicated significant paths where microbial network topology positively influenced N cycling gene α-diversity, which in turn was positively associated with SMF. The SEM exhibited excellent fit: Chisq = 1.374 (df = 2, p = 0.503), indicating a nonsignificant discrepancy with observed data. This was corroborated by a GFI of 0.987, along with other fit indices confirming strong model performance, demonstrating robust explanatory power and reliability.
4 Discussion
4.1 Soil multifunctionality dynamics: vertical stratification and biocrust type differentiations
4.1.1 Biocrusts enhance soil multifunctionality across stratified soil layers
This study demonstrated that SMF increased significantly in both biocrust layers and subcrust soils during biocrust development and succession (Figure 1). This pattern indicates a vertical facilitation effect of biocrusts. This result aligns with observations from the Gurbantunggut Desert (Huang et al., 2023). The elevated SMF in biocrust layers primarily arises from two key mechanisms: (1) atmospheric deposition and capture of dust, nutrient-rich litter, and moisture facilitated by biocrust structures (Drahorad et al., 2022); and (2) synergistic enhancements in carbon and nitrogen sequestration, phosphorus mineralization rates, enzymatic activities, soil structure, and microbial diversity and activity (Sun et al., 2020).
4.1.2 Differences in soil multifunctionality among different biocrusts
Our findings demonstrate that SMF follows the order moss crusts > lichen crusts > algal crusts. The superior multifunctional performance of moss crusts stems from four reasons: (1) Carbon-driven nutrient accumulation, previous research reveals that moss crusts exhibit higher photosynthetic carbon fixation rates (51.57 g C m−2 yr.−1) compared to algal (30.64 g C m−2 yr.−1) and lichen crusts (32.71 g C m−2 yr.−1) (Feng, 2014). SOC content beneath moss crusts was 5.9-fold and 2.0-fold greater than under algal and lichen crusts, respectively (Figure 1). High carbon fixation rates significantly increase soil total carbon content while simultaneously elevating nutrient levels such as total nitrogen and available phosphorus, thereby improving the soil environment and enhancing multifunctionality (Yan et al., 2025). (2) The rhizoid network of moss plants enhances erosion resistance through microaggregate formation. These microaggregates significantly elevate soil organic carbon, total nitrogen, and total phosphorus content (Zhao et al., 2006). This process not only mitigates erosion but also enhances soil multifunctionality by modulating microbial community composition and activity (Cheng et al., 2020). (3) Moss crusts exhibited substantially higher mean soil water content (9.33%) compared to lichen (2.35%) and algal crusts (0.85%). This hydrological advantage stems from their greater specific surface area and elevated fine-particle content, which collectively enhance capillary forces (Cheng et al., 2018). The resultant improvement in water adsorption capacity promotes microbial activity and accelerates carbon-nitrogen cycling processes, thereby directly amplifying SMF (Lei et al., 2024). (4) The rapidly decomposing litter of moss crusts facilitates humus formation. This humus provides bioavailable carbon substrates that stimulate microbial metabolism, elevating soil carbon, nitrogen, and phosphorus concentrations. Consequently, accelerated nutrient cycling enhances soil multifunctionality through synergistic biogeochemical feedback (Cheng et al., 2019; Lei et al., 2024).
4.2 Relationship between soil microbial community characteristics and soil multifunctionality
Our results indicated that among biocrust types, only algal crusts showed a significant positive correlation between microbial taxonomic diversity and SMF, whereas no such association occurred in lichen or moss crusts. In contrast, network complexity exerted a significantly stronger influence on SMF across all biocrust types. This pattern likely arises because microbially driven processes depend less on the additive effects of microbial abundance than on complex interspecies interactions that generate integrated metabolic processes (Morrison et al., 2020). Such interactions enhance cross-functional resource utilization and metabolic coordination, thereby supporting ecosystem multifunctionality. Collectively, these findings suggest that tightly connected microbial networks underpin higher ecosystem functioning (Morrien et al., 2017).
The lack of correlation between taxonomic diversity and SMF in lichen and moss biocrusts may stem from three factors: (1) Functional antagonism among microbial taxa, where archaeal richness negatively correlates with SMF, counterbalancing positive bacterial/fungal contributions (Wang et al., 2024); (2) Aridity-driven function decoupling, as regional aridity index (0.5–0.8) weakens microbial diversity-function linkages while amplifying plant diversity dominance (Hu et al., 2021); (3) Environmental context dependency, where SMF-diversity correlations turn positive when environmental factors jointly enhance (or suppress) both properties, but become negative/non-significant under opposing environmental pressures (Rillig et al., 2019). Conversely, algal crusts maintained a positive diversity-SMF relationship, potentially attributable to their significantly higher Shannon diversity (Figure 2). This elevated richness may enable synergistic functional complementarity (Bowker et al., 2010).
Among biocrust types, lichen crusts demonstrated the highest species richness and network complexity, consistent with observations from the Gurbantunggut Desert (Zhang B. C. et al., 2018; Zhang B. et al., 2018). Three key mechanisms collectively drive this pattern: (1) Lichen acid secretion promotes soil particle cementation, while fungal hyphae bind soil particles and encapsulates microorganisms. This dual mechanism not only enhances soil aggregate stability and erosion resistance but also reduces the loss of keystone microbial taxa, thereby strengthening the resilience of microbial networks to environmental disturbances. Consequently, it ensures dual structural stability in both soil physical architecture and microbial community composition (Tumur et al., 2006; Zhang and Wang, 2010). (2) Symbiosis-mediated functional complementarity, where fungal-photobiont consortia develop complex networks via resource partitioning (hyphal protection/photosynthetic C fixation/secondary metabolite exchange) (Spribille et al., 2016). (3) Succession-dependent complexity optimization, peaking in mid-late stages through synergistic functional redundancy and keystone species coexistence, then declining due to keystone taxa loss (Wang et al., 2021; Jiao, 2023).
The biocrust layer demonstrates significantly greater network complexity than subcrust soil, confirming its critical role in augmenting soil stability. This emergent complexity is associated with the co-occurrence and potential interplay of two key factors: (1) Nutrient-enzyme co-drivers: Elevated nutrient availability coupled with enhanced enzymatic activity in the biocrust layer supplies sustained metabolic energy, facilitating coordinated microbial network assembly; (2) Heterogeneity-mediated adaptive selection: Exposure to steep spatiotemporal gradients of temperature, moisture and nutrients drives the evolution of diversified microbial survival strategies, amplifying interaction complexity through niche partitioning (Ding et al., 2023; Yang et al., 2025).
4.3 Relationship between soil microbial functional properties and soil multifunctionality
Research revealed a significant negative correlation between C-fixing functional gene diversity and SMF (Figure 4A). This pattern is primarily attributable to chemoautotrophic C-fixing microorganisms, which dominate subcrust microenvironments through high abundance and diversity, thereby suppressing heterotrophic microbial activity and disrupting functional equilibrium (Lynn et al., 2017). Furthermore, under environmental stress, competitively dominant C-fixing bacteria suppress other functional groups, driving increased gene diversity but decreased SMF (Chen et al., 2022). Conversely, a strong positive correlation exists between C-degrading functional gene diversity and SMF (Figure 4B). Biocrust types exhibit differential C utilization: algal and lichen crusts primarily assimilate microbially derived carbon, while moss crusts rely more heavily on autochthonous litter and microbial necromass carbon. The microbial growth-death-decomposition cycle sustains community activity via C degradation metabolism (He et al., 2024). Critically, during biocrust succession, accumulating necromass and litter drives the enrichment of C-degrading functional microorganisms, facilitating organic matter mineralization, nutrient liberation, and soil fertility enhancement (Li et al., 2024).
During biocrusts development, N cycling functional gene diversity increased significantly and exhibited a strong positive correlation with SMF (Figure 4C), primarily mediated through N-driven regulation of N transformation, organic matter turnover, and nutrient provisioning. Specifically, enriched nitrate assimilation, nitrification, and dissimilatory nitrate reduction genes synergistically enhanced N cycle efficiency (Shu et al., 2024), while elevated functional gene diversity sustained nitrogen metabolism under carbon-limited conditions via functional complementarity-driven energy allocation strategies (Zhu et al., 2023; Ma et al., 2024). Concurrently, functional redundancy conferred resilience against disturbances, when environmental stressors (e.g., pH decline) suppress specific denitrification genes, compensatory mechanisms maintained transformation efficiency (Hao et al., 2022). Furthermore, multifunctional keystone genes (e.g., phoD) critically amplified multifunctionality through coordinated N-P co-activation (Jia et al., 2025), collectively demonstrating how N cycling infrastructure orchestrates enhanced SMF. A context-dependent relationship was observed between the diversity P cycling functional genes and SMF across biocrust types and soil layer. Specifically, algal crusts exhibited a positive correlation, whereas moss crusts showed a negative correlation. Spatially, a negative correlation dominated in the biocrust layer, shifting to positive correlation in the subcrust layer (Figure 4D). These patterns arise from distinct mechanistic driver: (1) The higher P availability in moss crusts (compared to algal crusts) potentially suppresses the expression of some P cycling functional genes via negative feedback, inducing microbial functional trade-offs (Liu et al., 2020). Under these conditions, microbes enhance P acquisition concurrently impairing other processes, ultimately leading to a decline in multifunctionality (Delgado-Baquerizo et al., 2016). (2) Low pH in the moss crust layer reducing the abundance and diversity (Shannon index) of genes involved in inorganic P solubilization (e.g., gcd), further strengthening the negative correlation within this layer (Liao et al., 2023). In contrast, algal crusts, as an earlier stage of biocrust succession, exhibit lower functional gene diversity compared to moss crusts (Supplementary Figure S3). Consequently, an increase in P cycling functional gene diversity in these biocrusts tends to directly enhance nutrient supply and promote multifunctionality. Furthermore, P availability per se, rather than the diversity of functional genes, appears to be the key factor in alleviating microbial P limitation and promoting soil functions (Peng et al., 2023).
4.4 Microbial network complexity outweighs taxonomic diversity in driving soil multifunctionality in arid ecosystems
Our integrated analysis reveals a fundamental principle governing SMF in arid ecosystems: the synergistic interactions among microbial network topological properties and functionality supersedes taxonomic diversity as the primary driver. SEM (Figures 5C,D) further elucidated the mechanistic pathways: network topology (specifically node and network density) not only directly enhances SMF but also exerts significant indirect effects by boosting the diversity of N cycling functional genes. Critically, high network density enhances microbial synergies, significantly amplifying N-transformation efficiency. This empirically confirms that microbial network complexity underpins the sustainability of SMF (Faust and Raes, 2012).
This work provides quantitative evidence for the superior contribution of network topological properties over taxonomic diversity, identifies N cycling as a key functional module amplified by network complexity, furnishes direct mechanistic evidence linking network density to enhanced N-transformation efficiency, and underscores the pivotal role of microbial network topological properties. Given the inherent structural complexity of arid ecosystems, land management strategies should prioritize fostering conditions that build and stabilize dense, cooperative microbial functional networks, moving beyond the conventional paradigm focused solely on maximizing taxonomic diversity. Management practices should specifically target the coordinated regulation of the N cycle, recognizing this functional module as central to maintaining soil health and multifunctionality. Future research should validate the generality of the ‘network complexity-N cycling-SMF’ framework across diverse biomes and employ manipulative experiments to directly test the causal relationships between network structure, N cycling processes, and SMF.
5 Conclusion
This study investigated algal, lichen, and moss crusts in the Mu Us Desert to elucidate how microbial community composition and functional traits govern SMF. Moss crusts exhibited the highest level of SMF, followed by lichen and algal crusts. Compared to microbial taxonomic diversity, network topological properties exerted a stronger influence on SMF, particularly through the indirect effects mediated by network complexity (node and edge numbers). Critically, functional gene diversity metrics accounted for up to 96% of the variation in SMF, primarily driven by the diversity of genes involved in carbon degradation and nitrogen cycling. Overall, biocrusts enhanced SMF by mediating the formation of high-density microbial networks that synergistically enhanced nitrogen cycling gene diversity. These findings highlight the crucial roles of microbial network architecture and functional gene diversity in driving SMF within biocrust systems, providing important insights for managing dryland ecosystem functions and services, evaluating soil resources, and developing sustainable land-use strategies.
Data availability statement
The research data is available on Mendeley Data, V2, doi: 10.17632/cs37fxxptr.2 (Link: https://data.mendeley.com/drafts/cs37fxxptr).
Author contributions
YJ: Writing – original draft, Visualization, Software, Data curation, Writing – review & editing, Investigation. XZ: Resources, Funding acquisition, Writing – review & editing, Validation, Investigation. ML: Methodology, Investigation, Writing – review & editing, Resources. JY: Investigation, Methodology, Writing – review & editing, Resources. YZ: Resources, Funding acquisition, Writing – review & editing, Supervision, Investigation. SQ: Supervision, Validation, Investigation, Writing – review & editing, Funding acquisition, Resources. WF: Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project ‘Integrated Technology Demonstration for the Comprehensive Management of Mountains, Waters, Forests, Farmlands, Lakes, Grasslands, and Deserts’ (Grant No. 2024YFF1306305).
Acknowledgments
The authors gratefully acknowledge the invaluable technical assistance provided by the research station staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1656706/full#supplementary-material
References
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Billings, S. A., Schaeffer, S. M., and Evans, R. D. (2003). Nitrogen fixation by biological soil crusts and heterotrophic bacteria in an intact Mojave desert ecosystem with elevated CO2 and added soil carbon. Soil Biol. Biochem. 35, 643–649. doi: 10.1016/S0038-0717(03)00011-7
Bowker, M. A., Maestre, F. T., and Escolar, C. (2010). Biological crusts as a model system for examining the biodiversity-ecosystem function relationship in soils. Soil Biol. Biochem. 42, 405–417. doi: 10.1016/j.soilbio.2009.10.025
Bu, C. F., Wu, S. F., Xie, Y. S., and Zhang, X. C. (2013). The study of biological soil crusts: hotspots and prospects. Clean Soil Air Water 41, 899–906. doi: 10.1002/clen.201100675
Byrnes, J. E. K., Gamfeldt, L., Isbell, F., Lefcheck, J. S., Griffin, J. N., Hector, A., et al. (2013). Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods Ecol. Evol. 5, 111–124. doi: 10.1111/2041-210x.12143
Chen, G. X., Wu, C. F., Ge, T. D., Chen, J. P., and Deng, Y. W. (2022). Response of soil multifunctionality to reduced microbial diversity. Environ. Sci. 43, 5274–5285. doi: 10.13227/j.hjkx.202201095
Cheng, L., Jia, X. H., Wu, B., Li, Y. S., Zhao, X. B., and Zhou, H. (2018). Effects of biological sol crusts on the characteristics of hygroscopic and condensate water deposition in albinesandy lands. Acta Ecol. Sin. 38, 5037–5046. doi: 10.5846/stxb201704070595
Cheng, C., Li, Y. J., Long, M. Z., and Li, X. N. (2019). Application potential of bryophyte soil crust on the control of karst rocky desertification. Chin. J. Appl. Ecol. 30, 2501–2510. doi: 10.13287/j.1001-9332.201907.008
Cheng, C., Li, Y. J., Zhang, Y. D., Gao, M., and Li, X. N. (2020). Effects of moss crusts on soil nutrients and ecological stoichiometry characteristics in karst rocky desertification region. Acta Ecol. Sin. 40, 9234–9244. doi: 10.5846/stxb202002110228
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., et al. (2016). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 7:8. doi: 10.1038/ncomms10541
Deng, H. Y., Ma, X. Z., Liu, Z. K., Hu, H. W., Di, H. J., Liu, Y. J., et al. (2024). Soil ecosystem multifunctionality is strongly linked with crop yield after four decades chemical fertilization in black soil. Agric. Ecosyst. Environ. 368:109007. doi: 10.1016/j.agee.2024.109007
Ding, Y., Geng, Y. C., Zhou, W. C., and Li, D. H. (2023). Habitat-specific environmental factors regulate the spatial variability of biological soil crust microbial communities on the Qinghai-Tibet plateau. Sci. Total Environ. 901:165937. doi: 10.1016/j.scitotenv.2023.165937
Dou, W. Q., Xiao, B., Wang, Y. F., and Kidron, G. J. (2022). Contributions of three types of biocrusts to soil carbon stock and annual efflux in a small watershed of northern Chinese loess plateau. Appl. Soil Ecol. 179:104596. doi: 10.1016/j.apsoil.2022.104596
Drahorad, S., Felix-Henningsen, P., Siemens, J., Marschner, B., and Heinze, S. (2022). Patterns of enzyme activities and nutrient availability within biocrusts under increasing aridity in Negev desert. Ecosphere 13:4051. doi: 10.1002/ecs2.4051
Eldridge, D. J., Reed, S., Travers, S. K., Bowker, M. A., Maestre, F. T., Ding, J., et al. (2020). The pervasive and multifaceted influence of biocrusts on water in the world's drylands. Glob. Change Biol. 26, 6003–6014. doi: 10.1111/gcb.15232
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Feng, W. (2014). Photosynthetic carbon fixation process of biological soil crusts and its impact on soil carbon emission in Mu us Sandland. [doctoral dissertation]. Beijing: Beijing Forestry University.
Hao, J. Q., Feng, Y. Z., Wang, X., Yu, Q., Zhang, F., Yang, G. H., et al. (2022). Soil microbial nitrogen cycling gene abundances in response to crop diversification: a meta-analysis. Sci. Total Environ. 838:156621. doi: 10.1016/j.scitotenv.2022.156621
Hao, S. R., Jia, X., Mu, Y. M., Zha, T. S., Qin, S. G., Liu, P., et al. (2023). Canopy greenness, atmospheric aridity, and large rain events jointly regulate evapotranspiration partitioning in a temperate semiarid shrubland. Agric. For. Meteorol. 333:109425. doi: 10.1016/j.agrformet.2023.109425
He, H. Y., Liu, W., Chang, Z. Q., Hou, C. M., Sun, L. W., and Chi, X. L. (2024). Effects of revegetation on soil organic carbon composition and stability in the southern edge of the Tenggel desert. J. Desert Res. 44, 307–317. doi: 10.7522/j.issn.1000-694X.2024.00148
Housman, D. C., Powers, H. H., Collins, A. D., and Belnap, J. (2006). Carbon and nitrogen fixation differ between concessional stages of biological soil crusts in the Colorado plateau and Chihuahuan Desert. J. Arid Environ. 66, 620–634. doi: 10.1016/j.jaridenv.2005.11.014
Hu, W. G., Ran, J. Z., Dong, L. W., Du, Q. J., Ji, M. F., Yao, S. R., et al. (2021). Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 12:5350. doi: 10.1038/s41467-021-25641-0
Hu, X. G., Xie, Y. Y., Zhou, Q. X., and Mu, L. (2024). Machine learning approach for studying the multifunctionality of soil against global climate changes. Ecol. Indicat. 169:112772. doi: 10.1016/j.ecolind.2024.112772
Hu, W., Zhang, Y. P., Rong, X. M., Zhou, X., Fei, J. C., Peng, J. W., et al. (2024). Biochar and organic fertilizer applications enhance soil functional microbia abundance and agroecosystem multifunctionality. Biochar 6:3. doi: 10.1007/s42773-023-00296-w
Huang, Y. J., Li, Y. G., Zhou, X. B., Yin, B. F., Tao, Y., and Zhang, Y. M. (2023). Moss patch size as a factor profoundly influencing soil nutrient characteristics and multifunctionality of temperate desert in Central Asia. Ecol. Indic. 155:110975. doi: 10.1016/j.ecolind.2023.110975
Jia, J. Y., de Goede, R., Li, Y. Z., Zhang, J. Z., Wang, G. Z., Zhang, J. L., et al. (2025). Unlocking soil health: are microbial functional genes effective indicators? Soil Biol. Biochem. 204:109768. doi: 10.1016/j.soilbio.2025.109768
Jia, X. S., Zha, T. S., Gong, J. N., Zhang, Y. Q., Wu, B., Qin, S. G., et al. (2018). Multi-scale dynamics and environmental controls on net ecosystem CO2 exchange over a temperate semiarid shrubland. Agric. For. Meteorol. 259, 250–259. doi: 10.1016/j.agrformet.2018.05.009
Jiao, B. J. (2023). Nitrogen fixation potential and microbiological mechanisms of biological soil crusts in the water-wind erosion crisscross region of the loess plateau (master's thesis). Taiyuan: Shanxi Normal University.
Jiao, S., Lu, Y. H., and Wei, G. H. (2022). Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Change Biol. 28, 140–153. doi: 10.1111/gcb.15917
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics, 9, 559. doi: 10.1186/1471-2105-9-559
Li, Y. H., Bu, C. F., Guo, Q., and Wei, Y. X. (2021). Ecological funcions compaison of moss crust and algae crust in the Mu us sand land. J. Desert Res. 41, 138–144. doi: 10.7522/j.issn.1000-694X.2020.00101
Li, X. R., Hui, R., Tan, H. J., Zhao, Y., Liu, R. T., and Song, N. P. (2021). Biocrust research in China: recent progress and application in land degradation control. Front. Plant Sci. 12:751521. doi: 10.3389/fpls.2021.751521
Li, X. R., Sun, H. X., Zhang, H. X., Tan, H. J., Hui, R., Qi, J. H., et al. (2023). Warming decreases desert ecosystem functioning by altering biocrusts in drylands. J. Appl. Ecol. 60, 2676–2687. doi: 10.1111/1365-2664.14528
Li, C. H., Zhang, L., Liu, S. Y., Han, L. L., Yao, B. M., Shen, C. C., et al. (2024). Distribution characteristics of carbon and nitrogen contents and related functional ge-nes in shrub soils of the Tibetan plateau. Acta Ecol. Sin. 44, 392–404. doi: 10.20103/j.stxb.202301310161
Liao, X. H., Zhao, J., Yi, Q., Li, J. N., Li, Z. L., Wu, S. S., et al. (2023). Metagenomic insights into the effects of organic and inorganic agricultural managements on soil phosphorus cycling. Agric. Ecosyst. Environ. 343:108281. doi: 10.1016/j.agee.2022.108281
Lei, Y. F., Li, X. S., Tao, Y., Yin, B. F., Rong, X. Y., Zhang, J., et al. (2024). Characterization of soil multifunctionality and its determining factors under moss crust cover in the arid regions of Northwest China. Arid Zone Res. 41, 812–820. doi: 10.13866/j.azr.2024.05.09
Liu, L., Gao, Z., Liu, W. Y., Li, H. F., Wang, Z. H., and Liu, J. S. (2024). Phosphorus fertilizer input threshold shifts bacterial community structure and soil multifunctionality to maintain dryland wheat production. Soil Tillage Res. 243:101174. doi: 10.1016/j.still.2024.106174
Liu, J. J., Guo, Y. P., Gu, H. D., Liu, Z. X., Hu, X. J., Yu, Z. H., et al. (2023). Conversion of steppe to cropland increases spatial heterogeneity of soil functional genes. ISME J. 17, 1872–1883. doi: 10.1038/s41396-023-01496-9
Liu, J. S., Ma, Q., Hui, X. L., Ran, J. Y., Ma, Q. X., Wang, X. S., et al. (2020). Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol. Biochem. 149:107918. doi: 10.1016/j.soilbio.2020.107918
Liu, Q., Zhou, S., Zhang, B., Zhao, K., Wang, F., Li, K., et al. (2024). The development of the biological soil crust regulates the fungal distribution and the stability of fungal networks. Front. Microbiol. 15:1347704. doi: 10.3389/fmicb.2024.1347704
Lynn, T. M., Ge, T., Yuan, H. M., Wei, X. M., Wu, X. H., Xiao, K. Q., et al. (2017). Soil carbon-fixation rates and associated bacterial diversity and abundance in three natural ecosystems. Microb. Ecol. 73, 645–657. doi: 10.1007/s00248-016-0890-x
Ma, Q. H., Zhu, Y., Wang, Y., Liu, T., Qing, X., Liu, J. S., et al. (2024). Livestock grazing modifies soil nematode body size structure in mosaic grassland habitats. J. Environ. Manag. 351:119600. doi: 10.1016/j.jenvman.2023.119600
Mackelprang, R., Vaishampayan, P., and Fisher, K. (2022). Adaptation to environmental extremes structures functional traits in biological soil crust and hypolithic microbial communities. mSystems 7:e0141921. doi: 10.1128/msystems.01419-21
Mager, D. M., and Thomas, A. D. (2011). Extracellular polysaccharides from cyanobacterial soil crusts: a review of their role in dryland soil processes. J. Arid Environ. 75, 91–97. doi: 10.1016/j.jaridenv.2010.10.001
Maier, S., Tamm, A., Wu, D. M., Caesar, J., Grube, M., and Weber, B. (2018). Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J. 12, 1032–1046. doi: 10.1038/s41396-018-0062-8
Morrien, E., Hannula, S. E., Snoek, L. B., Helmsing, N. R., Zweers, H., de Hollander, M., et al. (2017). Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 8:14349. doi: 10.1038/ncomms14349
Morrison, B. M. L., Brosi, B. J., and Dirzo, R. (2020). Agricultural intensification drives changes in hybrid network robustness by modifying network structure. Ecol. Lett. 23, 359–369. doi: 10.1111/ele.13440
Peng, Z. Y., Wu, Y. T., Guo, L. L., Yang, L., Wang, B., Wang, X., et al. (2023). Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 238, 1033–1044. doi: 10.1111/nph.18797
Qin, L. P., Zhang, Q., Zhu, H. S., Reich, P. B., Banerjee, S., van der Heijden, M. G. A., et al. (2021). Erosion reduction soil microbial diversity, network complexity and multifunctionality. ISME J. 15, 2474–2489. doi: 10.1038/s41396-021-00913-1
Rillig, M. C., Ryo, M., Lehmann, A., Aguilar-Trigueros, C. A., Buchert, S., Wulf, A., et al. (2019). The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366, 886–890. doi: 10.1126/science.aay2832
Rodriguez-Caballero, E., Belnap, J., Büdel, B., Crutzen, P. J., Andreae, M. O., Pöschl, U., et al. (2018). Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 11, 185–189. doi: 10.1038/s41561-018-0072-1
She, W. W., Zhou, Y., Luo, W. T., Bai, Y. X., Feng, W., Lai, Z. R., et al. (2024). Precipitation and plant functional composition mediate desert canopy nutrient responses to water and nitrogen addition. Plant Soil 496, 609–621. doi: 10.1007/s11104-023-06384-x
Shu, D. T., Banerjee, S., Mao, X. Y., Zhang, J. Q., Cui, W. L., Zhang, W., et al. (2024). Conversion of monocropping to intercropping promotes rhizosphere microbiome functionality and soil nitrogen cycling. Sci. Total Environ. 949:174953. doi: 10.1016/j.scitotenv.2024.174953
Spribille, T., Tuovinen, V., Resl, P., Vanderpool, D., Wolinski, H., Aime, M. C., et al. (2016). Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 353, 488–492. doi: 10.1126/science.aaf8287
Sun, Y. Q., Feng, W., Zhang, Y. Q., Qin, S. G., and Mao, H. N. (2020). Effects of biological soil crusts on soil enzyme activities of Artemisia ordosica community in the Mu Us Desert of northwestern China. J. Beijing For. Univ. 42, 82–90. doi: 10.12171/j.1000-1522.20190082
Sun, J., Wang, Y., and Liu, G. H. (2021). Linkages of aboveground plant carbon accumulation rate with ecosystem multifunctionality in alpine grassland, Qingzang plateau. Chin. J. Plant Ecol. 45, 496–506. doi: 10.17521/cjpe.2020.0180
Tumur, A., Wang, Y. L., Abbas, A., Wang, Y. L., and Abbas, A. (2006). Species composition and distribution of lichens in soil crust of southern fringe of Zhungeer basin in Xinjiang. J. Plant Resour. Environ. 15, 35–38.
Wagg, C., Schlaeppi, K., Banerjee, S., Kuramae, E. E., and van der Heijden, M. G. A. (2019). Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 10:4841. doi: 10.1038/s41467-019-12798-y
Wang, N., Cheng, J. H., Liu, Y. H., Xu, Q. C., Zhu, C., Ling, N., et al. (2024). Relative importance of altitude shifts with plant and microbial diversity to soil multifunctionality in grasslands of North-Western China. Plant Soil 504, 545–560. doi: 10.1007/s11104-024-06641-7
Wang, Q., Han, Y. C., Lan, S. B., and Hu, C. X. (2021). Metagenomic insight into patterns and mechanism of nitrogen cycle during biocrust succession. Front. Microbiol. 12:633428. doi: 10.3389/fmicb.2021.633428
Xiao, Y. Y., Feng, W., Qiao, Y. G., Zhang, Y. Q., and Qin, S. G. (2023). Effects of soil microbial community characteristics on soil multifunctionality in sand-fixation shrublands. Biodivers. Sci. 31, 128–141. doi: 10.17520/biods.2022585
Xu, L., Li, X. Z., Li, C. N., Kou, Y. P., Li, J. B., Yao, M. J., et al. (2023). Disentangling the relative importance of precipitation, biocrust succession, and shrub cover in mediating soil phoD-harbouring communities and organic phosphorus mineralisation. Soil Biol. Biochem. 186:109165. doi: 10.1016/j.soilbio.2023.109165
Yan, Z. Y., Qu, M. M., and Ma, C. (2025). Study on bryophyte crust and its ecological function in Ordos surface coal mine. Acta Agrestia Sin. 9, 241–249. doi: 10.11733/j.issn.1007-0435.2025.01.027
Yang, K. W., Zhang, L., Ruiz-Valencia, A., Song, X., Vogel, T. M., and Zhang, X. J. (2025). Heterogeneity in the composition and catabolism of indigenous microbiomes in subsurface soils cocontaminated with BTEX and chlorinated aliphatic hydrocarbons. Environ. Sci. Technol. 59, 4540–4550. doi: 10.1021/acs.est.4c10071
Zhai, C. C., Han, L. L., Xiong, C., Ge, A. H., Yue, X. J., Li, Y., et al. (2024). Soil microbial diversity and network complexity drive the ecosystem multifunctionality of temperate grasslands under changing precipitation. Sci. Total Environ. 906:167217. doi: 10.1016/j.scitotenv.2023.167217
Zhang, Y. M., and Wang, X. Q. (2010). Summary on formation and developmental characteristics of biological soil crusts in desert areas. Acta Ecol. Sin. 30, 4484–4492. doi: 10.20103/j.stxb.2010.16.026
Zhang, B. C., Zhang, Y. Q., Li, X. Z., and Zhang, Y. M. (2018). Successional changes of fungal communities along the biocrust development stages. Biol. Fertil. Soils 54, 285–294. doi: 10.1007/s00374-017-1259-0
Zhang, B., Zhang, J., Liu, Y., Shi, P., and Wei, G. (2018). Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 118, 178–186. doi: 10.1016/j.soilbio.2017.12.011
Zhao, Y. G., Xu, M. X., Wang, Q. J., and Shao, M. A. (2006). Impact of biological soil crust on soil physical and chemical properties of rehabilitated grassland in hilly loess plateau, China. J. Nat. Resour. 21, 441–448. doi: 10.11849/zrzyxb.2006.03.014
Zhu, G. N., Nong, H. J., Fang, S. Y., Qin, S. G., and Zhang, Y. Q. (2024). Arbuscular mycorrhizal symbiosis reshapes the drought adaptation strategies of a dominant sand-fixation shrub species in northern China. Sci. Total Environ. 955:177135. doi: 10.1016/j.scitotenv.2024.177135
Keywords: biocrusts, soil multifunctionality, microbial co-occurrence networks, microbial taxonomic diversity, microbial functional gene diversity
Citation: Jiang Y, Zhang X, Li M, Yang J, Zhang Y, Qin S and Feng W (2025) Microbial network properties and functional gene diversity drive soil multifunctionality during biocrust succession. Front. Microbiol. 16:1656706. doi: 10.3389/fmicb.2025.1656706
Edited by:
Samina Mehnaz, Forman Christian College, PakistanCopyright © 2025 Jiang, Zhang, Li, Yang, Zhang, Qin and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Feng, d2VpZmVuZ0BiamZ1LmVkdS5jbg==
 Yawen Jiang
Yawen Jiang Xuexia Zhang1,2
Xuexia Zhang1,2 Shugao Qin
Shugao Qin