Abstract
New Delhi metallo-β-lactamase 1 (NDM-1), a member of the B-type metallo-β-lactamase (MBL) family, emerged as a major focus in resistance research, raising serious concerns about the treatment of bacterial infections over the past decade. Kluyvera ascorbata, generally considered a bacterium of low pathogenicity and rarely associated with severe infections, has nonetheless demonstrated significant resistance to a broad spectrum of antibiotics in recent studies. In this study, we successfully isolated a K. ascorbata strain harboring the antibiotic-resistant genes blaNDM-1 and blaCTX-M-77 from a fecal sample of a patient with diarrhea in China. The strain was accurately identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Additionally, the minimum inhibitory concentration (MIC) of the strain against various antimicrobial agents was determined using agar dilution and microdilution method. The results indicated that the strain exhibited resistance to all tested antimicrobial agents. The blaNDM-1 resistance gene was located on an IncFIA (HI1), IncR plasmid, as revealed by whole-genome sequencing. A detailed analysis of the plasmid’s size, number, and location was conducted using S1-nuclease pulsed-field gel electrophoresis (S1-PFGE), Southern blotting, and conjugation experiments. These experiments successfully demonstrated the transfer of the plasmid carrying blaNDM-1 into the recipient bacterium Escherichia coli EC600. These findings underscore the urgent need for continuous surveillance of the blaNDM-1-carrying plasmid in clinical isolate of K. ascorbata to prevent and contain its further dissemination in China.
1 Introduction
Kluyvera ascorbata, a Gram-negative bacillus, has been increasingly recognized in recent years as an opportunistic pathogen of potential clinical relevance, particularly among immunocompromised individuals. The genus Kluyvera was initially identified as a novel taxonomic group corresponding to the previously termed “Enteric Group 8” (also known as API Group 1) (Farmer et al., 1981). Similar to most members of the family Enterobacteriaceae, Kluyvera species exhibit typical phenotypic characteristics: they are Gram-negative, motile via peritrichous flagella, catalase-positive, oxidase-negative, capable of growing on MacConkey agar, and ferment d-glucose with the production of acid and gas. These organisms were historically considered susceptible to a wide range of antibiotics (Ulloa-Clavijo et al., 2023; Ochi et al., 2017; Lee et al., 2019; Akiki et al., 2023). In recent years, with the development of molecular phylogenetics, researchers have employed methods such as 16S rRNA sequencing, multilocus sequence analysis (MLSA), and whole-genome sequencing (WGS) to further elucidate the phylogenetic relationship between the genus Kluyvera and Enterobacter. These studies have shown that the two genera share a close evolutionary relationship on the phylogenetic tree. Many strains that were historically classified as Enterobacter have since been reassigned to Kluyvera. This taxonomic ambiguity not only increases the difficulty of clinical identification but may also lead to an underestimation of the epidemiological significance of Kluyvera species (Farmer et al., 1981; Pavan et al., 2005; Brady et al., 2013). However, due to the high degree of phenotypic similarity with other members of the Enterobacteriaceae, accurate identification of Kluyvera species in clinical microbiology laboratories remains a diagnostic challenge (Bellaoui et al., 2009).
Although Kluyvera spp. are infrequently isolated in medical microbiology and human infections have historically been considered rare with unclear clinical significance, increasing reports indicate that these organisms not only possess opportunistic pathogenic potential but may also be involved in the dissemination of antibiotic resistance genes (Altun Koroglu et al., 2010; Stock, 2005). Antimicrobial resistance in K. ascorbata has become a major challenge in antimicrobial therapy. Numerous studies have demonstrated that K. ascorbata has developed resistance to multiple classes of antibiotics, including commonly used classes such as beta-lactams, carbapenems, aminoglycosides, fluoroquinolones, and tetracyclines (Rodríguez et al., 2004; Rodriguez et al., 2018; Mutoh et al., 2019).
Notably, NDM-1 (New Delhi metallo-β-lactamase-1) is an enzyme capable of degrading various antibiotics, particularly carbapenems. This enables strains carrying the blaNDM-1 gene to exhibit significant multidrug resistance (Gao et al., 2023; Marquez-Ortiz et al., 2017). The rapid and widespread dissemination of NDM-1 has positioned it as a critical concern in the global antibiotic resistance crisis (Guo et al., 2011; Heng et al., 2024). This phenomenon presents considerable challenges to clinical treatment and poses substantial threats to public health and safety (Adams et al., 2020; Hirabayashi et al., 2021; Liu et al., 2023). Although K. ascorbata is not considered a highly pathogenic bacterium in the traditional sense, it may serve as a reservoir of antibiotic resistance genes in the environment or intestinal microbiota, contributing to the interspecies dissemination of resistance determinants such as blaNDM-1. Kluyvera species often harbor mobile genetic elements, including plasmids, integrons, and transposons, which enable the horizontal transfer of resistance genes to other Enterobacteriaceae pathogens, such as Escherichia coli and Klebsiella pneumoniae. Therefore, its potential “silent role” in the dissemination network of NDM-1 resistance should not be overlooked (Raro et al., 2019; Li et al., 2019).
This study focused on K. ascorbata L4110hy, the first K. ascorbata strain identified to carry the blaNDM-1 gene in China. The present research not only elucidates the drug resistance background of K. ascorbata in clinical isolates and clarifies its resistance mechanisms, which are crucial for effective clinical treatment and infection control. Therefore, it is essential to implement a continuous monitoring program for the prevalence and spread of blaNDM-1 and other drug-resistant genes conferring carbapenemase-like activity in K. ascorbata and other bacterial populations to prevent potential public health crises.
2 Materials and methods
2.1 Sample collection and bacterial culture
In 2021, a carbapenem-resistant strain of K. ascorbata (strain L4110hy) was isolated from a stool sample of a male patient with severe diarrhea at a teaching hospital in Zhejiang Province. The strain was incubated on MacConkey agar plates at 37°C for 18 to 24 h. Subsequently, individual colonies were screened using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) and polymerase chain reaction (PCR) was performed to detect the carbapenemase gene blaNDM-1 in the isolates.
2.2 Location of the blaNDM-1 gene and transferability of plasmid carrying blaNDM-1
The number and size of plasmids in strain L4110hy were determined using the S1 nuclease pulsed-field gel electrophoresis (S1-PFGE) method. The location of the blaNDM-1 resistance gene was assessed by Southern blotting and hybridization with an NDM-1-specific probe, using Salmonella H9812 as a reference (Ge et al., 2023). In the conjugation experiments, rifampicin-resistant Pseudomonas aeruginosa PAO, Pseudomonas aeruginosa Ri, and Escherichia coli EC600 were used as recipients, and the plasmid transferability using the broth method (Chen et al., 2024; Liu et al., 2025). The recipient bacteria and target single colony were inoculated into 5 mL LB broth and cultured at 37°C with shaking for approximately 6 h. The donor and recipient bacteria were evenly mixed in a 2:1 ratio in 5 mL LB broth and incubated at 37°C for approximately 18 h. Transconjugants were selected on Mueller-Hinton medium containing [400 mg/L rifampicin and 2 mg/L meropenem] and [2 mg/L meropenem, and 2 mg/L mucin]incubated at 37°C for 18 h. Successful transfer was verified by detecting the blaNDM-1 target gene using polymerase chain reaction (PCR). Additionally, strain identification was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS), and drug sensitivity assays were conducted to confirm the successful transfer of the plasmids. The transfer efficiency was calculated by dividing the number of transconjugants (CFU/mL) by the number of donor bacteria (CFU/mL) (Zhang et al., 2023).
2.3 Drug sensitivity test
The minimum inhibitory concentration (MIC) of the fixed strain L4110hy against a range of antimicrobial drugs was determined using two complementary methods: agar dilution and microdilution method. The results of the drug sensitivity experiments were interpreted in accordance with the guidelines established by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee for Antimicrobial Susceptibility Testing (EUCAST)1. The interpretation of tigecycline, omadacycline, and eravacycline was based on definitions provided by the FDA (Tigecycline Injectable Products, Omadacycline Injectable and Oral Products, and Eravacycline Injectable Products). E. coli ATCC25922 was used as a quality control strain.
2.4 Whole-genome sequencing and bioinformatics analysis
To obtain the complete genome sequence of the strain, we initially extracted the genomic DNA using the QIAGEN Bacterial DNA kit. Subsequently, DNA sequencing was performed using both the Illumina NovaSeq 6,000 platform and Oxford Nanopore technology. The sequenced fragments were assembled using Unicycler v0.4.7 software2, and the assembled genome was annotated using the RAST 2.0 online tool3.
To gain a deeper understanding of the genetic characteristics of the strain, we identified insertion elements (IS) using the ISfinder4 database and detected acquired antibiotic resistance genes (ARGs) and plasmid incompatibility types through the ResFinder5 and PlasmidFinder6 databases (Carattoli et al., 2014; Zankari et al., 2012).
The oriTfinder tool was employed to investigate the origin of transfer (oriT7) within the DNA sequences of bacterial mobile genetic elements. Concurrently, the VFDB.18 database was used to identify virulence factors. For further insight into the plasmid sequences, we compared them with the GenBank database using the BLASTN tool9 (Liu et al., 2024).
The BLAST Ring Image Generator (BRIG) and EasyFig software were utilized to construct the circular map for plasmid comparison and to perform comparative mapping of the genetic environment surrounding the blaNDM-1 gene, respectively (Liu et al., 2024; Luo et al., 2024; Liu et al., 2024; Chen et al., 2024). Based on the blast analysis results of pL4110, 72 plasmid sequences were downloaded from the NCBI database. All sequences were annotated using Prokka, and a phylogenetic tree was constructed using the maximum likelihood method in MEGA 11 software.
3 Results
3.1 Isolation and characterization of L4110hy carrying blaNDM-1
In 2021, a male patient was admitted to a teaching hospital in Zhejiang Province for the treatment of severe diarrhea and vomiting. During his treatment, a bacterial strain designated L4110hy was isolated from the patient’s fecal sample. Mass spectrometry identified the strain as K. ascorbata, which demonstrated resistance to carbapenems, as documented in clinical reports. Subsequent PCR testing confirmed the presence of the blaNDM-1 gene, marking the first instance of NDM-1-producing K. ascorbata identified in China.
3.2 Drug sensitivity results of L4110hy
L4110hy and EC600-pL4110-NDM-1 exhibited decreased susceptibility to imipenem, meropenem, ceftriaxone, cefotaxime, ceftazidime, and piperacillin-tazobactam; however, these strains remained susceptible to aztreonam. In contrast, all strains showed good sensitivity to amikacin, gentamicin, chloramphenicol, and trimethoprim-sulfamethoxazole. Notably, the EC600 strain demonstrated significantly higher sensitivity to fluoroquinolones (such as ciprofloxacin and levofloxacin) and chloramphenicol, with greater sensitivity compared to both L4110hy and EC600-pL4110-NDM-1. Their antimicrobial susceptibility testing results are shown in Table 1.
Table 1
| Antibiotics | MIC value (μg/mL) | ||
|---|---|---|---|
| L4110hy | EC600-pL4110-NDM-1 | EC600 | |
| Aztreonam | 0.5 | 0.5 | 0.06 |
| Imipenem | 4 | 4 | 0.125 |
| Meropenem | 4 | 4 | 0.06 |
| Ceftriaxone sodium | 64 | 128 | 0.125 |
| Cefotaxime sodium | 128 | 128 | 0.125 |
| Ceftazidime | 128 | 128 | 0.5 |
| Levofloxacin | 0.25 | 0.25 | 0.06 |
| Ciprofloxacin | 0.25 | 0.25 | 0.008 |
| Amikacin | 4 | 2 | 1 |
| Gentamicin | 1 | 1 | 1 |
| Piperacillin Sodium and Tazobactam Sodium | 128 | 128 | 4 |
| Fosfomycin + Glucose 6-phosphate | 1 | 1 | 1 |
| Chloramphenicol | 32 | 32 | 8 |
| Trimethoprim-sulfamethoxazole | ≤0.125 | ≤0.125 | ≤0.125 |
| Cefepime | 8 | 8 | 0.06 |
| Ceftazidime/Avibactam | >128 | >128 | 0.25 |
| Tigecycline | 0.5 | 0.5 | 0.125 |
Results of drug sensitization experiments.
3.3 Genomic characterization of L4110hy
Table 2 provides a summary of the genomic characterization of L4110hy, which contains a circular chromosome of 4,845,762 bp with an average GC content of 54%. Additionally, the genome comprises six plasmid sequences, with lengths ranging from 1,554 bp to 301,733 bp. The resistance genes carried by the strain were identified through the analysis of antibiotic resistance genes (ARGs) using the ResFinder tool. The chromosome of L4110hy carries the blaCTX-M-77 resistance gene, while the plasmid carries the blaNDM-1 resistance gene. The chromosome harbors the virulence factor nlpI, while the plasmid carries the virulence factors clpK1 and terC.
Table 2
| Contig | Total number of bases (bp) | G + C content (%) | Plasmid replicon type | Transfer region | Resistance genes | Virulence factors |
|---|---|---|---|---|---|---|
| K. ascorbata strain L4110hy 1 | 4,845,762 | 54 | NA | / | bla CTX-M-77 | nlpI |
| K. ascorbata strain L4110hy 2 | 301,733 | 48 | Col440I, IncFIB(K), IncHIIA, IncHIIB(R27) | IncHII1B(R27) | / | clpK1, terC |
| K. ascorbata strain L4110hy 3 | 52,121 | 52 | IncFIA(HI1), IncR | / | bla NDM-1 | / |
| K. ascorbata strain L4110hy 4 | 4,019 | 46 | NA | / | / | / |
| K. ascorbata strain L4110hy 5 | 2,723 | 46 | Col440I | / | / | / |
| K. ascorbata strain L4110hy 6 | 2,206 | 46 | NA | / | / | / |
| K. ascorbata strain L4110hy 7 | 1,554 | 47 | NA | / | / | / |
Genomic characterization of L4110hy.
3.4 Characterization of plasmids containing blaNDM-1
The blaNDM-1 resistance gene was located on a plasmid with a total length of 52,121 bp (Figure 1), as determined by both ResFinder and S1-PFGE analyses. This plasmid was classified as an IncR-IncFIA composite plasmid. BLASTN searches were performed using the plasmid pL4410-NDM-1, which contains the blaNDM-1 resistance gene, as a reference sequence in the NCBI database. By searching against the core plasmid region in GenBank, the pL4110-NDM-1 backbone and Enterobacter hormaechei strains pEXB34 (PQ760046.1), pAVS0889 (CP092044.1), Klebsiella pneumoniae strains pKPS1023 (CP186455.1), pKPS571 (CP186467.1), pKP4930 (CP186394.1), pKP304 (CP178194.1) showed very high nucleotide identity (>99%), Moreover, their Query Cover exceeds 45%. Genetic environment analysis showed that ISKpn19 was located upstream and downstream of the drug resistance gene blaNDM-1 (Figures 2A,B). The phylogenetic tree constructed based on the core genome in this study reveals the evolutionary position of the host strains carrying the pL4110-NDM-1 plasmid and its genetic association with closely related species. This plasmid forms an independent branch in the phylogenetic tree and is critically clustered closely with a group of K. pneumoniae plasmids, suggesting that the L4110hy strain is substantially phylogenetically close to the K. pneumoniae branch. Combining the clustering results, it can be speculated that the origin or early evolutionary process of pL4110-NDM-1 is significantly associated with a specific plasmid pool of Klebsiella pneumoniae (Figure 2C). To analyze the phylogenetic relationship between strain L4110hy and other K. ascorbata strain, we used the 16S sequence of strain L4110hy as a template for BLAST alignment in the NCBI database. The analysis results are shown in Figure 2D. Strain L4110hy is closely related to AM933756.1. Strain AM933756.1 is from Argentina. Through genomic analysis, we found that it does not carry the blaNDM-1 resistance gene. Strain L4110hy is closely related to it, and the uneven distribution of the blaNDM-1 gene strongly suggests that this resistance gene is not an inherent part of the genetic background of the K. ascorbata species, but was recently acquired through a horizontal gene transfer mechanism. After analyzing the resistance genes of K. pneumoniae CP149890.1, CP122470.1, CP099407.1, CP109609.1, CP109615.1, and Raoultella planticola CP040185.1, it was found that these plasmids do not carry blaNDM-1 (Supplementary Table 1).
Figure 1
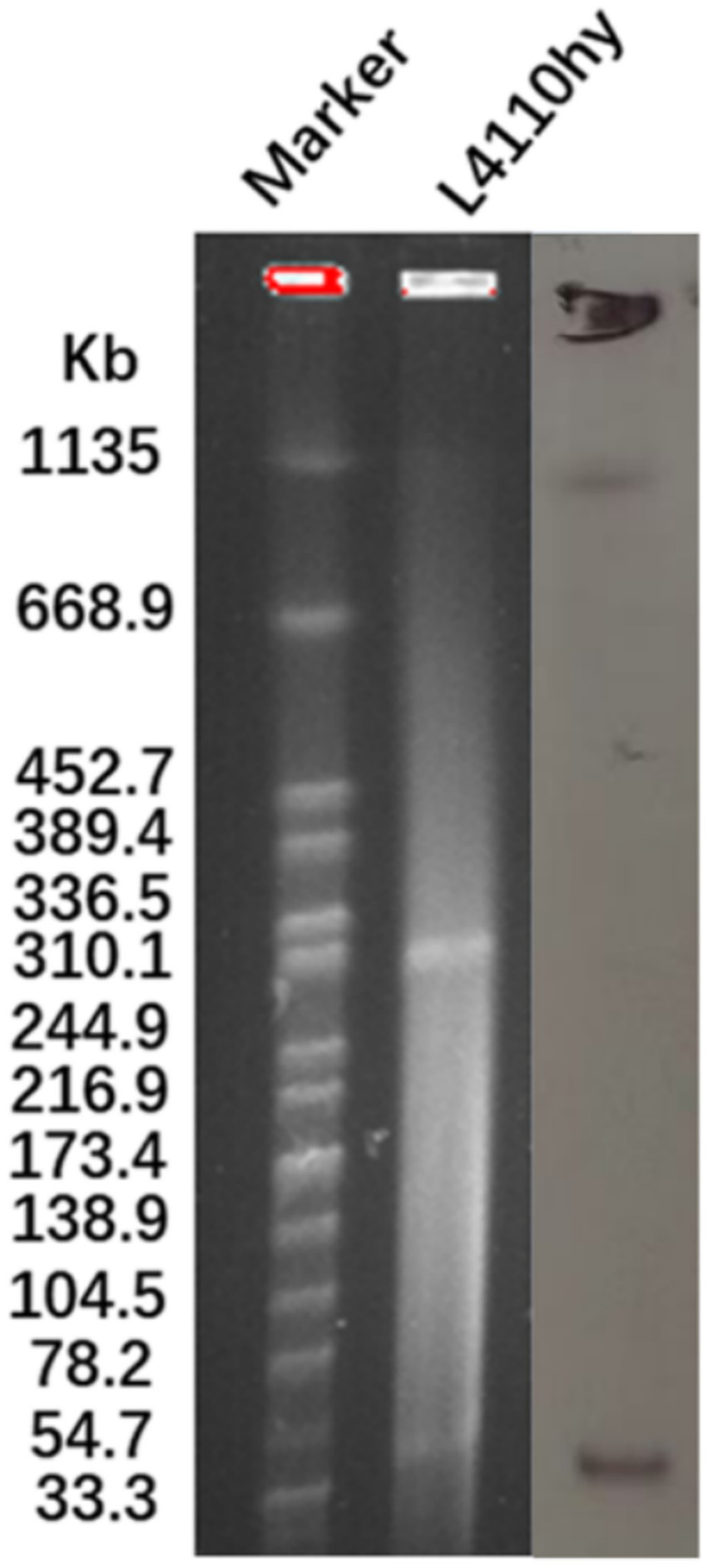
The plasmid size of L4110hy was determined using S1-PFGE with Salmonella enterica serotype Braenderup H9812 as a size marker. Subsequently, Southern blot hybridization was performed using an NDM-1-specific probe.
Figure 2
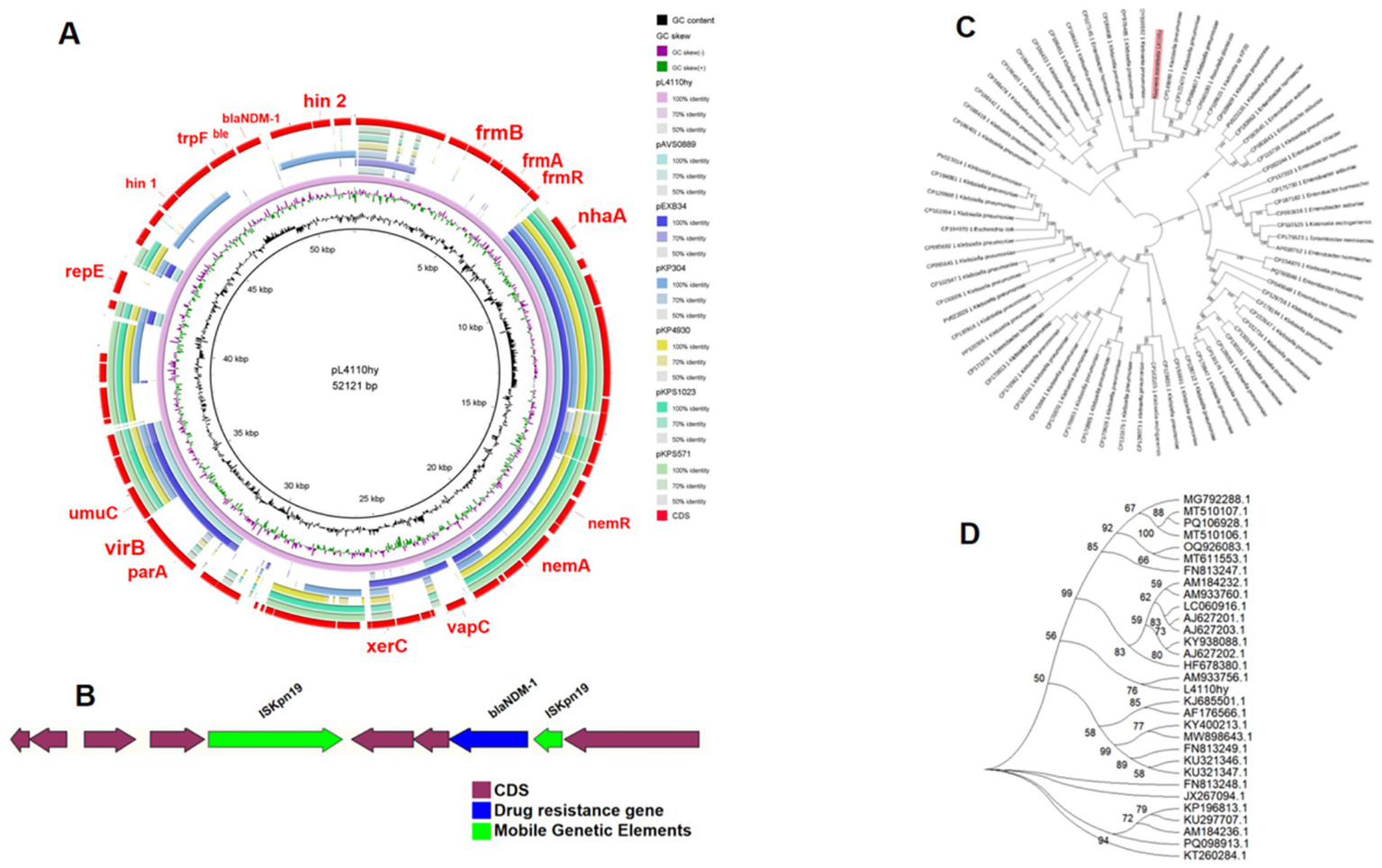
(A) Circular comparative alignment of plasmid pL4110-NDM-1 with four reference plasmids from K. ascorbata using BRIG. Although the nucleotide identity of these plasmids in the blaNDM-containing region exceeded 99% compared with pL4110-NDM-1, the overall query coverage was only over 45%, indicating the existence of structural diversity beyond the resistance modules. (B) Genetic context of blaNDM-1 showing ISKpn19 elements flanking the resistance gene, suggestive of transposon-mediated mobility. (C) Through BLAST analysis, 72 plasmid sequences similar to pL4110-NDM-1 were screened. A phylogenetic analysis of L4110hy was performed using the maximum likelihood method, and the phylogenetic analysis and visualization were carried out on MEGA11. (D) Phylogenetic relationship between strain L4110hy and other K. ascorbata strains.
The blaCTX-M-77 is located on chromosome 4,845,762 bp in length, and a BLASTN search was performed using the L4110hy chromosome of the chromosome containing the blaCTX-M-77 in the NCBI database as the reference sequence. A BLASTN search was performed using the core sequence region encompassing blaCTX-M-77 and its flanking genetic context from the L4110hy chromosome as the query sequence in the GenBank database. The results showed that the L4110hy chromosome shared very high query coverage and nucleotide identity (all above 95%) with the chromosomes of K. ascorbata strain Colony288 (CP078559.1), strain Colony413 (CP070603.1), and strain SK (CP096201.1). Environmental analyses indicated the presence of IS3 and IS1A insertion sequences downstream of the blaCTX-M-77 resistance gene (Figure 3). As shown in Figures 3A–C, comparative circular alignment of the L4110hy chromosome with three reference strains (K. ascorbata Colony288, Colony413, and SK) revealed strong structural conservation in the chromosomal region harboring blaCTX-M-77, with both query coverage and nucleotide identity exceeding 95%. Despite this high conservation, detailed genetic context analysis (Figure 3C) identified two well-characterized insertion sequences, IS3 and IS1A, located downstream of blaCTX-M-77, oriented in tandem. These mobile genetic elements are known mediators of non-homologous recombination and can facilitate horizontal gene transfer and genomic rearrangement. The presence of IS3 and IS1A flanking the blaCTX-M-77 gene suggests that this resistance determinant may have been mobilized from an extrachromosomal element, such as a plasmid or integrative transposon, and stably integrated into the K. ascorbata chromosome. Moreover, such chromosomal integration could confer enhanced genetic stability compared to plasmid-borne resistance genes, especially under non-selective environmental conditions.
Figure 3
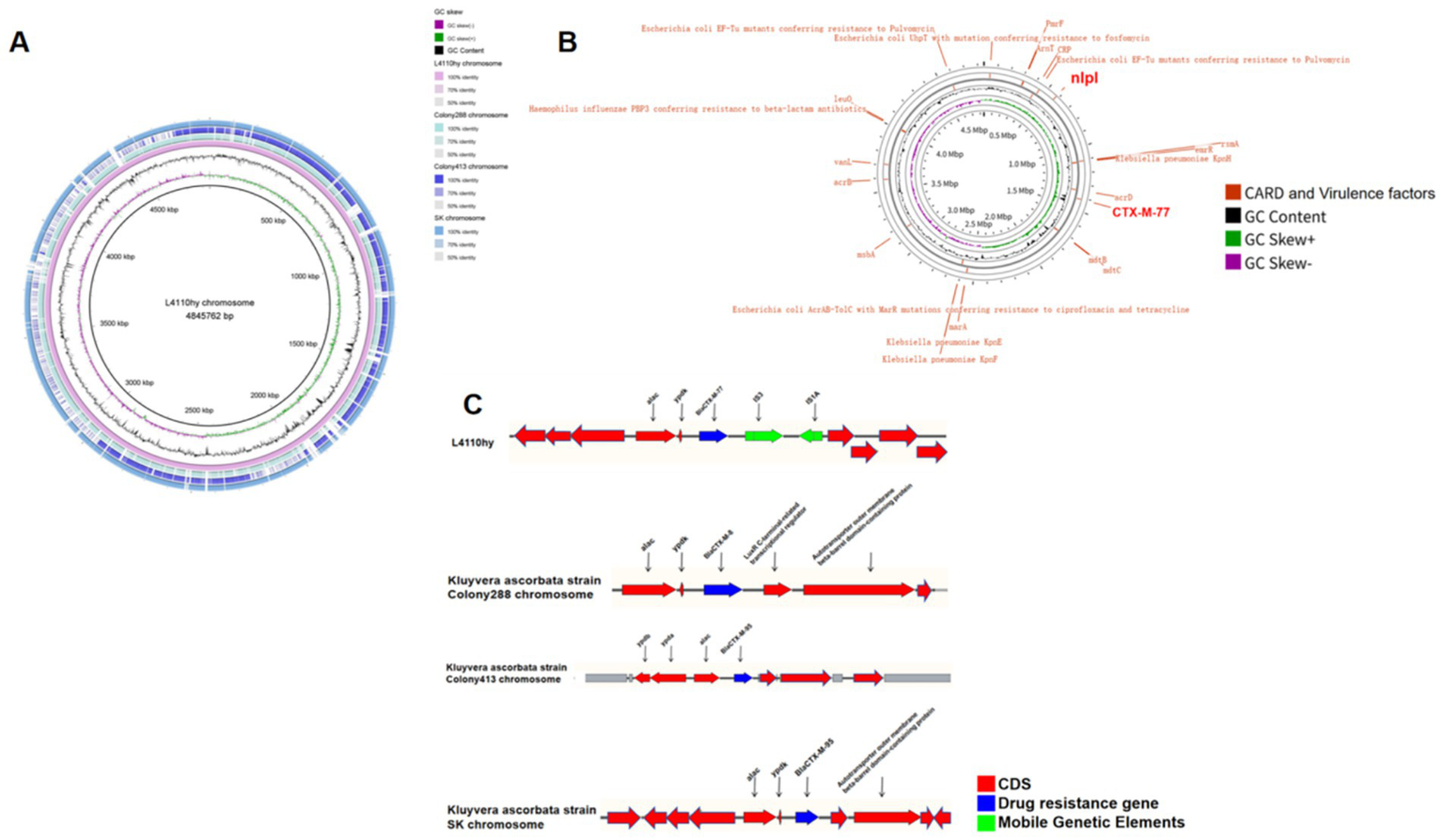
Chromosomal structure and comparative analysis of L4110hy. (A,B) Genome-wide circular alignment of the L4110hy chromosome with three K. ascorbata reference strains. The alignment highlights conserved regions (marked in blue) and divergent regions (marked in red), with a focus on the core genomic structure and the genetic loci potentially involved in horizontal gene transfer. Notably, the area surrounding blaCTX-M-77 is marked to show the genomic conservation across strains. (C) Genetic context of the blaCTX-M-77 resistance gene on the L4110hy chromosome. The resistance gene is located between the insertion sequences IS3 and IS1A, which are known to mediate horizontal gene transfer. The locations of these insertion sequences are indicated with arrows pointing to their respective positions upstream and downstream of blaCTX-M-77.
3.5 Bacterial coupling assay
The EC600-pL4110-NDM-1 coupler was selected for identification and confirmed as E. coli using MALDI-TOF mass spectrometry. Subsequently, polymerase chain reaction (PCR) was performed to detect the blaNDM-1 resistance gene, yielding a positive result. This confirmed that the plasmid carrying the blaNDM-1 resistance gene from the donor strain L4110hy had been successfully transferred to the recipient strain E. coli EC600 (the successful transfer experiment of the EC600 recipient strain was conducted using selective antibiotics: 400 mg/L rifampicin and 2 mg/L meropenem), with a conjugation efficiency of 3.87 × 10−4. Meanwhile, the transfer experiments with P. aeruginosa PAO1 and Ri as recipient strains were unsuccessful.
4 Discussion
This study reports for the first time the discovery of blaNDM-1 in K. ascorbata, a finding that marks the first recorded case in China. This result significantly expands the known host range of blaNDM-1, emphasizing the potential role of this less-studied species of the Enterobacteriaceae family as a hidden reservoir of carbapenem resistance. As a metal-β-lactamase, blaNDM-1 can severely impair the efficacy of carbapenem antibiotics, making it one of the major driving factors of the global antibiotic resistance crisis (Jia et al., 2025). Particularly in China, with the increasing prevalence of multidrug-resistant strains, K. ascorbata serves as an emerging carrier of resistance genes, posing a potential threat to public health. Our study highlights the need for monitoring non-traditional pathogens, especially in high-selective-pressure environments like hospitals, to effectively prevent the further spread of antibiotic resistance genes.
Genome analysis revealed that blaNDM-1 is carried on a unique IncR-IncFIA (H11) chimeric plasmid. IncR plasmids are known for their efficient transferability and frequent association with multidrug resistance, while IncFIA plasmids ensure stable replication in the Enterobacteriaceae family, helping maintain the persistence of resistance traits (Zhang et al., 2025; Chatzidimitriou et al., 2025). The combination of these two replicons likely enhances the horizontal transmission and long-term stability of blaNDM-1. Although the resistance gene locus is highly conserved compared to reference plasmids, the overall structure of the pL4110-NDM-1 plasmid differs, reflecting recombination and gene acquisition events. This showcases the structural plasticity and evolutionary adaptability of plasmids carrying blaNDM-1.
The gene environment of blaNDM-1 in K. ascorbata differs significantly from that in other known hosts such as K. pneumoniae and E. coli. Typically, blaNDM-1 is located within the Tn125 transposon element, which is flanked by ISAba125 insertion sequences, with trpF and dsbD genes often located downstream (Zhang et al., 2024). These structures facilitate stable transmission and easy transfer, particularly in plasmid-mediated spread. However, in K. ascorbata, the surrounding genetic environment of blaNDM-1 lacks these classic features, notably the absence of the trpF and dsbD genes downstream. This absence suggests that blaNDM-1 may have been mobilized by a different mechanism. This observation is closely associated with the presence of the ISKpn19 insertion sequence, indicating that ISKpn19 may play a key role in the mobilization of blaNDM-1.
ISKpn19, a typical member of the IS3 family, promotes the horizontal spread of adjacent genes through a “cut-and-paste” or replicative transposition mechanism (Zi et al., 2024). In K. ascorbata, ISKpn19 is not only located near blaNDM-1 but may also facilitate the cross-plasmid transfer of blaNDM-1. Compared to the ISAba125 insertion sequence in Tn125, the mechanism of ISKpn19 is more flexible, promoting recombination and spread of resistance genes through various insertion and transposition events. Specifically, ISKpn19’s insertion sequence can guide the cross-plasmid transfer of blaNDM-1, potentially even integrating blaNDM-1 into the chromosome, thus accelerating its horizontal spread among bacterial host (Wang et al., 2025).
This phenomenon is not commonly seen in other known blaNDM-1 carriers such as K. pneumoniae or E. coli, which typically rely on the stability of the Tn125 element to maintain long-term transmission of resistance traits (Marquez-Ortiz et al., 2017; Yao et al., 2025). In contrast, Tn125 relies on ISAba125 and other classic transposon sequences to facilitate stable replication and transmission of blaNDM-1 (Wu et al., 2019). However, the lack of ISAba125 does not destabilize the spread of blaNDM-1; instead, ISKpn19 might provide an alternative mechanism for mobilization via replicative transposition or the “cut-and-paste” mechanism. This could potentially increases the recombination frequency of blaNDM-1 between plasmids but may also promote the evolution and spread of resistance genes through multiple pathways (Wu et al., 2016; Zhao et al., 2021).
In comparison, the blaCTX-M-77 gene is located on the chromosome, surrounded by IS3 and IS1A insertion sequences. Chromosomal integration provides a stable inheritance mechanism and reduces the adaptive cost associated with plasmid maintenance. While blaNDM-1 on plasmids requires selective pressure to be maintained, it can spread rapidly. These findings reveal a dual evolutionary strategy in K. ascorbata: acquiring blaNDM-1 through plasmid-mediated transfer allows for rapid environmental adaptation, while chromosomal integration of blaCTX-M-77 ensures long-term stability. This complementary arrangement of resistance genes enables the bacteria to quickly respond to changes in antibiotic pressure and ensures the persistence of resistance traits (Zhao et al., 2021; Partridge et al., 2018; Oliveira et al., 2024; Liu et al., 2020; San Millan and MacLean, 2017).
From a clinical perspective, this dual strategy is concerning. The co-existence of plasmid-borne blaNDM-1 and chromosomal blaCTX-M-77 weakens the effectiveness of carbapenems and broad-spectrum cephalosporins, leaving limited treatment options, with polymyxin and tigecycline being among the few remaining choices. Furthermore, the presence of the novel IncR-IncFIA plasmid carrying blaNDM-1 may facilitate the rapid spread of carbapenem resistance, especially in hospital environments with high antibiotic pressure and patient-to-patient transmission risk, such as intensive care units. In our study, we observed a conjugative transfer efficiency of 3.87 × 10−4, confirming that this plasmid can transfer blaNDM-1 to recipient strains, further supporting its potential for rapid transmission in clinical settings (Wu et al., 2019; Kim et al., 2025; Yengui et al., 2025).
Genomic analysis of the L4110hy strain identified several virulence-related genes, including nlpI, clpK1, and terC. Among them, nlpI encodes the NlpI protein, an outer membrane lipoprotein that plays a role in cell wall stability and peptidoglycan degradation; its presence may reduce the efficacy of antibacterial agents by enhancing cell wall robustness (Wilson and Bernstein, 2016). clpK1 belongs to the heat shock protein family and functions as a molecular chaperone, helping bacteria maintain protein homeostasis under high-temperature and host-induced stress conditions, thereby enhancing bacterial survival (Gurung and Biswas, 2022). terC is associated with tellurite resistance and confers the ability to withstand oxidative stress and metal toxicity (Whelan et al., 1995). These genes are associated with stress tolerance, protein homeostasis, and heavy metal resistance, possibly indirectly supporting the persistence of bacteria in harsh environments. The distribution of virulence factors, particularly on plasmids, suggests functional diversification, which may enhance the bacteria’s environmental adaptability and pathogenic potential. The presence of these virulence factors highlights the multifactorial nature of bacterial survival and resistance.
This study provides the first evidence of blaNDM-1 in K. ascorbata, expanding the host range of this gene and highlighting the need for monitoring non-traditional pathogens, particularly in high-selective-pressure environments like hospitals, to prevent the spread of resistance genes. Future research should focus on the transmission mechanisms of blaNDM-1, particularly the role of small mobile elements (such as transposons and insertion sequences) in this process, and further investigate their synergistic effects with other resistance genes. Studying the ISKpn19 insertion sequence and its role in resistance gene transfer will help unveil the evolutionary dynamics of resistance genes and their impact on public health. In conclusion, this study reveals the transmission mechanism of blaNDM-1 in K. ascorbata and provides theoretical support for future antibiotic resistance monitoring and control strategies.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found: https://www.ncbi.nlm.nih.gov/genbank/, the BioSample accession number of: SAMN44237076.
Ethics statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (reference number: 2018–752). The Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine also approved the waiver of informed consent for participation in the study due to its retrospective design. Patient data were anonymized prior to analysis. The study procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki.
Author contributions
DZha: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. DZho: Writing – review & editing, Conceptualization, Formal analysis. SZ: Methodology, Writing – review & editing. XZ: Writing – review & editing, Conceptualization, Formal analysis. QH: Writing – review & editing. YL: Writing – review & editing. SL: Validation, Writing – review & editing. HH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project of the Jilin Province’s Training Program of Innovation and Entrepreneurship for Undergraduates (grant no. 202410201105). Science and Technology projects in Jilin Province Department of Education (grant no. JJKH20240092KJ). Jilin Province Health and Family Planning Commission (grant no. 2022JC021), The Natural Science Project of the Science and Technology Department in Jilin Province, China (YDZJ202501ZYTS160).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1666215/full#supplementary-material
Footnotes
2.^ https://github.com/rrwick/Unicycler
5.^ https://cge.food.dtu.dk/services/ResFinder/
6.^ https://cge.food.dtu.dk/services/PlasmidFinder/
References
1
Adams M. D. Pasteran F. Traglia G. M. Martinez J. Huang F. Liu C. et al . (2020). Distinct mechanisms of dissemination of NDM-1 Metallo-β-lactamase in Acinetobacter species in Argentina. Antimicrob. Agents Chemother.64:e00324-20. doi: 10.1128/AAC.00324-20
2
Akiki M. Ali R. Jamil A. Slim J. Miller R. (2023). Kluyvera ascorbata: an unusual cause of septic shock in a patient with urothelial Cancer. Cureus15:e51057. doi: 10.7759/cureus.51057
3
Altun Koroglu O. Yalaz M. Ozalkaya E. Polat D. C. Akısu M. Kultursay N. et al . (2010). Kluyvera cryocrescens sepsis in a preterm infant. Jpn. J. Infect. Dis.63, 195–196. doi: 10.7883/yoken.63.195
4
Bellaoui N. Belabbes H. Lahsoune M. El Mdagrhri N. (2009). Kluyvera bacteriemia: an epidemic in a Moroccan teaching hospital. Med. Mal. Infect.39, 133–135. doi: 10.1016/j.medmal.2008.09.027
5
Brady C. Cleenwerck I. Venter S. Coutinho T. De Vos P. et al . (2013). Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. Nimipressuralis and E. amnigenus into Lelliottia gen. Nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. Gergoviae and E. pyrinus into Pluralibacter gen. Nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. Oryzae and E. arachidis into Kosakonia gen. Nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. Helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. Nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst. Appl. Microbiol.36, 309–319. doi: 10.1016/j.syapm.2013.03.005
6
Carattoli A. Zankari E. García-Fernández A. Voldby Larsen M. Lund O. Villa L. et al . (2014). In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother.58, 3895–3903. doi: 10.1128/AAC.02412-14
7
Chatzidimitriou M. Tsolakidou P. Kyriazidi M. A. Chatzopoulou F. Varlamis S. Mavridou M. et al . (2025). Identification of NDM-1 producing and colistin resistant Klebsiella pneumoniae ST11: a highly drug-resistant strain detected in intensive care unit of a Greek tertiary care hospital. Acta Microbiol. Immunol. Hung.72, 16–22. doi: 10.1556/030.2025.02499
8
Chen R. Li C. Xu H. Liu R. Ge H. Qiao J. et al . (2024). First documentation of a clinical multidrug-resistant Enterobacter chuandaensis ST2493 isolate co-harboring Bla(NDM-1) and two Bla(KPC-2) bearing plasmids. Sci. Rep.14:26817. doi: 10.1038/s41598-024-78163-2
9
Chen H. Xu H. Liu R. Shen J. Zheng B. Li L. (2024). Coexistence of Bla(IMP-4) and Bla(SFO-1) in an IncHI5B plasmid harbored by tigecycline-non-susceptible Klebsiella variicola strain. Ann. Clin. Microbiol. Antimicrob.23:24. doi: 10.1186/s12941-024-00680-9
10
Farmer J. J. Fanning G. R. Huntley-Carter G. P. Holmes B. Hickman F. W. Richard C. et al . (1981). Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J. Clin. Microbiol.13, 919–933. doi: 10.1128/jcm.13.5.919-933.1981
11
Gao Y. du P. Zhang P. Wang J. Liu Z. Fanning S. et al . (2023). Dynamic evolution and transmission of a Bla (NDM-1)-bearing fusion plasmid in a clinical Escherichia coli. Microbiol. Res.275:127450. doi: 10.1016/j.micres.2023.127450
12
Ge H. Qiao J. Zheng J. Xu H. Liu R. Zhao J. et al . (2023). Emergence and clonal dissemination of KPC-3-producing Pseudomonas aeruginosa in China with an IncP-2 megaplasmid. Ann. Clin. Microbiol. Antimicrob.22:31. doi: 10.1186/s12941-023-00577-z
13
Guo Y. Wang J. Niu G. Shui W. Sun Y. Zhou H. et al . (2011). A structural view of the antibiotic degradation enzyme NDM-1 from a superbug. Protein Cell2, 384–394. doi: 10.1007/s13238-011-1055-9
14
Gurung V. Biswas I. (2022). ClpX/P-dependent degradation of novel substrates in Streptococcus mutans. J. Bacteriol.204:e0059421. doi: 10.1128/jb.00594-21
15
Heng H. Yang X. Zhang H. Sun R. Ye L. Li J. et al . (2024). Early detection of OXA-232-producing Klebsiella pneumoniae in China predating its global emergence. Microbiol. Res.282:127672. doi: 10.1016/j.micres.2024.127672
16
Hirabayashi A. Yahara K. Mitsuhashi S. Nakagawa S. Imanishi T. Ha V. T. T. et al . (2021). Plasmid analysis of NDM metallo-β-lactamase-producing Enterobacterales isolated in Vietnam. PLoS One16:e0231119. doi: 10.1371/journal.pone.0231119
17
Jia L. Zhang G. Li X. Wang H. Zhao J. Huang X. et al . (2025). ESBL-producing Klebsiella Pneumoniae isolated from dairy cows with pneumonia: insight into their epidemiology, genetic features and antimicrobial resistance profiles. Microb. Pathog.207:107909. doi: 10.1016/j.micpath.2025.107909
18
Kim H. Bell T. Lee K. Jeong J. Bardwell J. C. A. Lee C. (2025). Identification of host genetic factors modulating β-lactam resistance in Escherichia coli harbouring plasmid-borne β-lactamase through transposon-sequencing. Emerg Microbes Infect14:2493921. doi: 10.1080/22221751.2025.2493921
19
Lee J. Hwang J. H. Jo D. S. Lee H. S. Hwang J. H. (2019). Kluyvera ascorbata as a pathogen in adults and children: clinical features and antibiotic susceptibilities in a single center study. Jpn. J. Infect. Dis.72, 142–148. doi: 10.7883/yoken.JJID.2018.375
20
Li Y. Luo L. Xiao Z. Wang G. Li C. Zhang Z. et al . (2019). Characterization of a Carbapenem-resistant Kluyvera Cryocrescens isolate carrying Bla (ndm-1) from hospital sewage. Antibiotics8:149. doi: 10.3390/antibiotics8030149
21
Liu R. Chen Y. Xu H. Zhang H. Liu Y. Liu X. et al . (2024). Fusion event mediated by IS903B between chromosome and plasmid in two MCR-9- and KPC-2-co-producing Klebsiella pneumoniae isolates. Drug Resist. Updat.77:101139. doi: 10.1016/j.drup.2024.101139
22
Liu L. Feng Y. Wei L. Qiao F. Zong Z. (2020). Precise species identification and taxonomy update for the genus Kluyvera with reporting Kluyvera sichuanensis sp. nov. Front. Microbiol.11:579306. doi: 10.3389/fmicb.2020.579306
23
Liu K. D. Jin W. J. Li R. B. Zhang R. M. Sun J. Liu Y. H. et al . (2023). Prevalence and molecular characteristics of mcr-1-positive Escherichia coli isolated from duck farms and the surrounding environments in coastal China. Microbiol. Res.270:127348. doi: 10.1016/j.micres.2023.127348
24
Liu Y. Liu X. Liu R. Xu H. Chen M. Qian J. et al . (2025). First characterization of four repeat regions with the Bla (NDM-1) carried on an IncFII plasmid in Enterobacter hormaechei. iScience28:112369. doi: 10.1016/j.isci.2025.112369
25
Liu X. Liu Y. Ma X. Chen R. Li C. Fu H. et al . (2024). Emergence of plasmid-borne tet(X4) resistance gene in clinical isolate of eravacycline- and omadacycline-resistant Klebsiella pneumoniae ST485. Microbiol Spectr12:e0049624. doi: 10.1128/spectrum.00496-24
26
Luo Q. Lu P. Chen Y. Shen P. Zheng B. Ji J. et al . (2024). ESKAPE in China: epidemiology and characteristics of antibiotic resistance. Emerg Microbes Infect13:2317915. doi: 10.1080/22221751.2024.2317915
27
Marquez-Ortiz R. A. Haggerty L. Olarte N. Duarte C. Garza-Ramos U. Silva-Sanchez J. et al . (2017). Genomic epidemiology of NDM-1-encoding plasmids in Latin American clinical isolates reveals insights into the evolution of multidrug resistance. Genome Biol. Evol.9, 1725–1741. doi: 10.1093/gbe/evx115
28
Mutoh Y. Kobe T. Hirano T. Ichihara T. Takenaka H. Niinomi T. et al . (2019). The first case of third-generation cephalosporins resistant Kluyvera ascorbata biliary tract infection in Japan: a case report and review of the literature. IDCases15:e00498. doi: 10.1016/j.idcr.2019.e00498
29
Ochi F. Tauchi H. Mizumoto M. Miyamoto H. Ishii E. (2017). Kluyvera ascorbata infection in a neonate. Pediatr. Int.59, 640–641. doi: 10.1111/ped.13267
30
Oliveira R. P. da Silva J. S. da Silva G. C. Rosa J. N. Bazzolli D. M. S. Mantovani H. C. (2024). Prevalence and characteristics of ESBL-producing Escherichia coli in clinically healthy pigs: implications for antibiotic resistance spread in livestock. J. Appl. Microbiol.135:58. doi: 10.1093/jambio/lxae058
31
Partridge S. R. Kwong S. M. Firth N. Jensen S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev.31:e00088-17. doi: 10.1128/CMR.00088-17
32
Pavan M. E. Franco R. J. Rodriguez J. M. Gadaleta P. Abbott S. L. Janda J. M. et al . (2005). Phylogenetic relationships of the genus Kluyvera: transfer of Enterobacter intermedius izard et al. 1980 to the genus Kluyvera as Kluyvera intermedia comb. nov. and reclassification of Kluyvera cochleae as a later synonym of K. intermedia. Int. J. Syst. Evol. Microbiol.55, 437–442. doi: 10.1099/ijs.0.63071-0
33
Raro O. H. F. de Lima-Morales D. Barth A. L. Paim T. G. Mott M. P. Riche C. V. W. et al . (2019). Putative horizontal transfer of carbapenem resistance between Klebsiella pneumoniae and Kluyvera ascorbata during abdominal infection: a case report. Infect. Control Hosp. Epidemiol.40, 494–496. doi: 10.1017/ice.2019.26
34
Rodriguez M. M. Ghiglione B. Power P. Naas T. Gutkind G. (2018). Proposing Kluyvera georgiana as the origin of the plasmid-mediated resistance gene fosA4. Antimicrob. Agents Chemother.62:e00710-18. doi: 10.1128/AAC.00710-18
35
Rodríguez M. M. Power P. Radice M. Vay C. Famiglietti A. Galleni M . (2004). Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob. Agents Chemother.48, 4895–4897. doi: 10.1128/AAC.48.12.4895-4897.2004
36
San Millan A. MacLean R. C. (2017). Fitness costs of plasmids: a limit to plasmid transmission. Microbiol Spectr5:MTBP-0016-2017. doi: 10.1128/microbiolspec.MTBP-0016-2017
37
Stock I. (2005). Natural antimicrobial susceptibility patterns of Kluyvera ascorbata and Kluyvera cryocrescens strains and review of the clinical efficacy of antimicrobial agents used for the treatment of Kluyvera infections. J. Chemother.17, 143–160. doi: 10.1179/joc.2005.17.2.143
38
Ulloa-Clavijo C. Suárez-Laurés A. Viejo de la Cuadra G. Galván L. Martínez-Suárez C. Sánchez-Álvarez E. (2023). Kluyvera ascorbata sepsis in a patient on hemodialysis. Nefrologia43, 792–794. doi: 10.1016/j.nefroe.2021.09.013
39
Wang Q. Zhou L. Chen X. Yao J. Sun X. Peng K. et al . (2025). Global emergence and transmission dynamics of carbapenemase-producing Citrobacter freundii sequence type 22 high-risk international clone: a retrospective, genomic, epidemiological study. Lancet Microbe:101149. doi: 10.1016/j.lanmic.2025.101149
40
Whelan K. F. Colleran E. Taylor D. E. (1995). Phage inhibition, colicin resistance, and tellurite resistance are encoded by a single cluster of genes on the IncHI2 plasmid R478. J. Bacteriol.177, 5016–5027. doi: 10.1128/jb.177.17.5016-5027.1995
41
Wilson M. M. Bernstein H. D. (2016). Surface-exposed lipoproteins: an emerging secretion phenomenon in gram-negative Bacteria. Trends Microbiol.24, 198–208. doi: 10.1016/j.tim.2015.11.006
42
Wu W. Espedido B. Feng Y. Zong Z. (2016). Citrobacter freundii carrying blaKPC-2 and blaNDM-1: characterization by whole genome sequencing. Sci. Rep.6:30670. doi: 10.1038/srep30670
43
Wu W. Feng Y. Tang G. Qiao F. McNally A. Zong Z. (2019). NDM Metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev.32, e00115-18. doi: 10.1128/CMR.00115-18
44
Yao J. Hu Y. Wang X. Sheng J. Zhang Y. Zhao X. et al . (2025). Carbapenem-resistant Morganella morganii carrying Bla(KPC-2) or Bla(NDM-1) in the clinic: one-decade genomic epidemiology analysis. Microbiol Spectr13:e0247624. doi: 10.1128/spectrum.02476-24
45
Yengui M. Trabelsi R. Gdoura R. Hamieh A. Zerrouki H. Rolain J. M. et al . (2025). Antibiotic resistance profiles of gram-negative bacteria in southern Tunisia: focus on ESBL, carbapenem and colistin resistance. Infect. Genet. Evol.133:105787. doi: 10.1016/j.meegid.2025.105787
46
Zankari E. Hasman H. Cosentino S. Vestergaard M. Rasmussen S. Lund O. et al . (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother.67, 2640–2644. doi: 10.1093/jac/dks261
47
Zhang D. Li S. Zhang X. Zheng S. Zhou D. Hou Q. et al . (2025). Epidemiological and biological characteristics of IncR plasmids as multihost antibiotic resistance carriers. Front. Microbiol.16:1622352. doi: 10.3389/fmicb.2025.1622352
48
Zhang Y. Liu M. Zhang J. Wu J. Hong L. Zhu L. Q. et al . (2024). Large-scale comparative analysis reveals phylogenomic preference of Bla(NDM-1) and Bla(KPC-2) transmission among Klebsiella pneumoniae. Int. J. Antimicrob. Agents64:107225. doi: 10.1016/j.ijantimicag.2024.107225
49
Zhang X. Peng L. Ke Y. Zhao D. Yu G. Zhou Y. et al . (2023). Emergence of a clinical isolate of E. Coil ST297 co-carrying Bla(NDM-13) and mcr-1.1 in China. J. Infect. Public Health16, 1813–1820. doi: 10.1016/j.jiph.2023.09.007
50
Zhao Q. Y. Zhu J. H. Cai R. M. Zheng X. R. Zhang L. J. Chang M. X. et al . (2021). IS26 IS responsible for the evolution and transmission of Bla(NDM)-harboring plasmids in Escherichia coli of poultry origin in China. mSystems6:e0064621. doi: 10.1128/mSystems.00646-21
51
Zi P. Fang M. Yang H. Zheng J. Ma N. Liu Q. (2024). Characterization of an NDM-1-producing Citrobacter koseri isolate from China. Infect Drug Resist17, 61–67. doi: 10.2147/IDR.S435771
Summary
Keywords
Kluyvera ascorbata , NDM-1, CTX-M-77, IncR, IncFIA(HI1)
Citation
Zhang D, Zhou D, Zheng S, Zhang X, Hou Q, Liu Y, Li S and Han H (2025) Emergence of NDM-1 on IncR-IncFIA (HI1) plasmid in carbapenem-resistant Kluyvera ascorbata from China. Front. Microbiol. 16:1666215. doi: 10.3389/fmicb.2025.1666215
Received
15 July 2025
Accepted
25 August 2025
Published
11 September 2025
Volume
16 - 2025
Edited by
Taru Singh, Amity University, India
Reviewed by
Shijun Sun, First Affiliated Hospital of Zhengzhou University, China
Renqiao Wen, Sichuan University, China
Tian Jiang, Wenzhou Medical University, China
Updates
Copyright
© 2025 Zhang, Zhou, Zheng, Zhang, Hou, Liu, Li and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiming Han, hanhuiming@beihua.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.