- 1Institute of Maternal, Child and Adolescent Health, School of Public Health, Lanzhou University, Lanzhou, China
- 2The Affiliated Dongguan Songshan Lake Central Hospital, Guangdong Medical University, Dongguan, China
Background: Preeclampsia (PE), a leading cause of maternal and fetal morbidity, remains poorly understood mechanistically. While metal elements like manganese (Mn) are critical for placental function, their interplay with gut microbiota in PE pathogenesis is underexplored. This study evaluates placental heavy metal exposure—particularly Mn—and its interaction with gut microbiota in modulating PE risk.
Methods: The study included 21 healthy pregnant women (Control group), and 21 pregnant women diagnosed with PE (PE group). Placental samples were collected to measure metal elements concentrations, while fecal samples were obtained to assess gut microbiota composition. Associations between gut microbiota, PE, and placental Mn levels were analyzed using the Analysis of Composition of Microbiomes with Bias Correction 2 method. Additionally, KEGG pathway enrichment analysis was conducted to identify metabolic pathways linked to PE and Mn levels.
Results: Mn levels were significantly lower in the PE group compared to the Control group (p = 0.002). Gut microbiota diversity showed no significant differences between groups, but specific genera were linked to PE and Mn levels: Campylobacter and Porphyromonas were positively correlated with PE and negatively with Mn, while Coprobacillus showed the opposite pattern. KEGG pathway enrichment analysis identified eight metabolic pathways negatively associated with PE and positively linked to Mn, including the degradation of aromatic compounds.
Conclusion: Our findings suggest that Mn may serve as a protective factor against PE within a certain concentration range. Interactions between Mn and specific bacterial genera (Coprobacillus, Campylobacter, and Porphyromonas) appear to influence PE development by altering gut microbiota metabolic activities. These findings underscore the potential significance of the gut microbiota-Mn interplay in PE pathogenesis.
Introduction
Preeclampsia (PE) is a multisystem pregnancy disorder involving varying degrees of placental malperfusion, which leads to the release of soluble factors into the maternal circulation. These factors cause maternal vascular endothelial injury, leading to hypertension and damage to multiple organs (Chiang et al., 2024; Chappell et al., 2021). As a major contributor to maternal and perinatal morbidity and mortality, PE imposes a significant global health burden. The global incidence of PE is estimated to be 4.5%, which is similar to its incidence in China (Abalos et al., 2013; Li et al., 2021). PE is associated with adverse fetal outcomes, such as fetal growth restriction (FGR), preterm delivery, and stillbirth (Li et al., 2021; Harmon et al., 2015; Poon et al., 2019). It also has long-term health consequences for both pregnant women and their offspring, including cardiovascular disease, diabetes, dyslipidemia, and, in children, attention-deficit/hyperactivity disorder (ADHD) (Pittara et al., 2021). Due to its serious consequences, preeclampsia has been extensively studied. Research has identified maternal vascular malperfusion as a key factor in its pathogenesis (Ernst, 2018; Khong et al., 2016). However, the upstream causes remain unclear.
Recent studies have focused on the association between preeclampsia and metal elements, especially manganese (Mn). Mn is an essential trace element involved in enzyme synthesis, activation, and the regulation of glucose and lipid metabolism (Li and Yang, 2018). Most studies have reported a negative correlation between Mn levels and preeclampsia, though the underlying mechanisms remain unclear (Chen et al., 2024; Liu et al., 2019; Liu et al., 2020; Borghese et al., 2023; Enebe et al., 2023). One possible explanation is that Mn is a key component of superoxide dismutase (SOD), an enzyme that neutralizes reactive oxygen species (e.g., superoxide anions) linked to hypertension, thus reducing preeclampsia risk (Li and Yang, 2018; Schlichte et al., 2021). Other metal elements, including Nickel (Ni), copper (Cu), arsenic (As), cadmium (Cd), and lead (Pb), have also been reported to be associated with PE (He et al., 2024). Notably, most current studies primarily focus on detecting metal elements in serum, with only a few examining their levels in the placenta. Therefore, one of the objectives of this study is to explore the association between metal elements, particularly Mn in the placenta, and preeclampsia, as well as their underlying mechanisms.
In addition to manganese, the gut microbiota is also considered closely linked to preeclampsia. The gut microbiota plays a vital role in human metabolism and immune regulation and is strongly associated with the development of diseases such as preeclampsia (Zambella et al., 2024; Zong et al., 2023; Deady et al., 2024). A 2021 study revealed that preeclamptic patients exhibited significantly lower relative abundances of Varibaculum, Prevotella, Lactobacillus, and Porphyromonas in their gut microbiota compared to healthy pregnant women. These bacteria can produce short-chain fatty acids (SCFAs), such as butyrate and propionate. These metabolites support intestinal barrier integrity and help modulate the immune system (Huang et al., 2021). Similarly, another study highlighted Limosilactobacillus Fermentum as a key bacterium linked to severe preeclampsia (Liu et al., 2024). It may mitigate severe preeclampsia by enhancing arginine and proline metabolism and influencing the flagellar assembly functions of the gut microbiota. However, most studies on the gut microbiota and preeclampsia emphasize bacterial relative abundances, with research on absolute abundances remaining limited (Huang et al., 2021; Wang et al., 2020; Zhao et al., 2023; Meijer et al., 2023; Chang et al., 2020). Furthermore, these studies frequently employ Linear Discriminant Analysis Effect Size (LefSe), a method sensitive to sparse data and unable to adjust for confounding factors like age and body mass index (BMI). Thus, more robust analytical methods are crucial for investigating gut microbiota differences between preeclamptic and healthy pregnant women.
Although emerging evidence above suggests that both the gut microbiota and trace elements contribute to the pathogenesis of preeclampsia, their intricate interplay remains unclear. Current studies have revealed that metal exposure, such as manganese, can affect gut microbiota composition, potentially promoting or preventing the development of certain diseases (Zhu et al., 2024). However, human studies on this topic remain limited, especially in vulnerable groups like pregnant women. Therefore, this study compares gut microbiota and metal element levels between healthy pregnant women and those with preeclampsia. Particular attention is given to the relationship among preeclampsia, gut microbiota, and placental manganese levels. Our findings may provide new insights into the mechanisms underlying preeclampsia.
Methods
Participants
Participants were recruited from Dongguan Songshan Lake Central Hospital, Guangdong Province, China, between November 2022 and October 2023. Eligible participants were pregnant women aged 18 years or older, with a gestational age of more than 32 weeks, residing in Dongguan for over 1 year, and willing to provide informed consent.
The exclusion criteria were as follows: (1) A history of preexisting conditions such as hypertension, diabetes mellitus, coronary heart disease, cerebral infarction, kidney disease, cancer, glucose-6-phosphate dehydrogenase deficiency, or other metabolic or immune disorders; (2) A history of acute gastroenteritis, bacterial urinary tract infections, inflammatory bowel disease, or other chronic conditions that may affect gut microbiota; (3) A family history of hypertension (including gestational hypertension), coronary artery disease, diabetes mellitus, or kidney disease; (4) Use of mineral supplements, antibiotics, probiotics, prebiotics, proton pump inhibitors (PPIs), or other medications that may influence body metal element levels or gut microbiota after 28 weeks of gestation; (5) Twin or multiple pregnancies, assisted reproduction, severe memory impairment, mental or neurological disorders, or a history of seizures or loss of consciousness.
A total of 42 pregnant women were included in the study: 21 were healthy, and 21 were diagnosed with preeclampsia based on the Diagnosis and Treatment of Hypertension and Preeclampsia in Pregnancy: A Clinical Practice Guideline in China (2020) (Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association, 2020).
Written informed consent was obtained from all participants. To ensure privacy, personal identifiers were replaced with codes consisting of the participant’s initials and admission numbers during chemical and microbiological analyses. For statistical analyses, only these codes were used, with all personal information excluded. The study protocol was approved by the Ethics Committee of Dongguan Songshan Lake Central Hospital.
Data collection
Demographic data, including age, height, pre-pregnancy weight, pre-pregnancy body mass index (BMI), delivery weight, and delivery BMI, as well as clinical data such as biochemical markers [e.g., Total Protein (TP) and Aspartate Aminotransferase (AST)] and hematological markers [e.g., White Blood Cell Count (WBC) and Red Blood Cell Count (RBC)], were obtained from hospital medical records. For statistical analysis, delivery BMI was used in place of pre-pregnancy BMI. Similarly, clinical measurements closest to the time of delivery were selected to ensure temporal consistency, as placental samples were collected postpartum.
Placental tissues were collected immediately following delivery. To ensure sample integrity, peripheral margins, necrotic areas, and calcified regions were excluded. After thorough rinsing with sterile saline, two tissue samples—each approximately 2 cm in diameter—were excised from the central and peripheral regions of each placenta. The final metal concentration for each placenta was calculated as the average of these two samples. All specimens were immediately flash-frozen in liquid nitrogen and stored at −80 °C until analysis. For metal extraction, tissues were subjected to microwave-assisted acid digestion using nitric acid (HNO₃) and hydrogen peroxide (H₂O₂) to fully decompose organic material and release metal ions into solution. Metal concentrations, including manganese (Mn), nickel (Ni), copper (Cu), arsenic (As), cadmium (Cd), and lead (Pb), were subsequently quantified using liquid chromatography–inductively coupled plasma mass spectrometry (LC-ICP-MS). Results were reported in micrograms per gram (μg/g) of tissue.
Approximately 5 grams of fresh stool were collected from each participant within 1 week prior to their estimated due date and placed into sterile collection tubes. The samples were transported to the laboratory within 2 h and immediately stored at −80 °C for subsequent analysis. Absolute quantitative 16S rRNA gene sequencing was performed using the Accu16S method, which incorporates synthetic DNA sequences as internal standards for precise quantification (Genesky Biotechnologies Inc, n.d.). Details of the instruments and reagents used for gene sequencing are listed in Supplementary Tables S1, S2. All microbial analyses in this study were conducted based on absolute copy numbers.
Statistical analysis
Demographic data, clinical data, and placental metal data are expressed as mean (standard deviation). Group differences were assessed using the student t-test for data meeting normality and homogeneity of variance. For data that violated these assumptions, the Mann–Whitney U test was applied. Normality was evaluated using the Shapiro–Wilk test, and homogeneity of variance was assessed with the Levene test.
Group differences in α-diversity were analyzed using linear regression if the data met the normality assumption, or a Generalized Linear Model (GLM) if the data did not meet the normality assumption. p-values were adjusted for multiple comparisons using the Bonferroni correction (False Discovery Rate, FDR) to assess the significance of species diversity. Differences in β-diversity were evaluated using Principal Coordinates Analysis (PCoA). Both α-diversity and β-diversity analyses were adjusted for age and delivery BMI.
Gut microbiota analyses were performed at the genus level. Associations between bacterial genera and preeclampsia or placental metal elements were evaluated using Analysis of Composition of Microbiomes with Bias Correction 2 (ANCOM-BC2) (Lin and Peddada, 2024), with p-values adjusted by the Benjamini-Hochberg method. Differential KEGG (Kyoto Encyclopedia of Genes and Genomes) Orthology genes were identified through the KEGG database (Kanehisa and Goto, 2000), and pathway enrichment analysis was conducted to highlight significant changes in metabolic pathways. Particular attention was given to bacterial genera, KEGG Orthology (KO) genes, and metabolic pathways significantly associated with both preeclampsia and placental manganese levels.
Statistical analyses were performed in R (version 4.4.2). Visualizations were generated using R (version 4.4.2) and Photoshop (version 26.2). All tests were two-tailed, with a p-value < 0.05 considered statistically significant.
Results
Demographic and clinical data
A total of 42 pregnant women were included in the study, with 21 healthy pregnant women serving as the control group and 21 women diagnosed with preeclampsia (PE) comprising the PE group. Their demographic and clinical data are presented in Table 1. The mean age was comparable between the control group (28.90 ± 5.64 years) and the PE group (29.95 ± 5.26 years, p = 0.524). Similarly, no significant differences were observed in height, weight or BMI (p > 0.05). Regarding liver function indicators, the PE group exhibited significantly elevated aspartate aminotransferase (AST) levels compared to the control group (28.87 ± 19.87 U/L vs. 20.70 ± 2.97 U/L, p = 0.018). However, alanine aminotransferase (ALT) and the AST/ALT ratio did not differ significantly between the groups. In terms of hematological parameters, there was a significant increase in lymphocyte ratio (LYMPH) in the PE group compared to the control group (0.27 ± 0.26 vs. 0.19 ± 0.11, p = 0.024), whereas other indices, including white blood cell count (WBC), red blood cell count (RBC), hematocrit (HCT), and neutrophil ratio (NEUT), showed no statistically significant differences (p > 0.05).
Placental metal element levels
The levels of various metal elements were compared between healthy pregnant women and women with preeclampsia (Figure 1; Supplementary Table S3). Mn levels were significantly lower in the PE group than in the Control group (0.60 ± 0.31 μg/g vs. 0.86 ± 0.30 μg/g, p = 0.002). No significant differences were found for Ni (p = 0.063), Cu (p = 0.506), As (p = 0.640), Cd (p = 0.367), or Pb (p = 0.372).
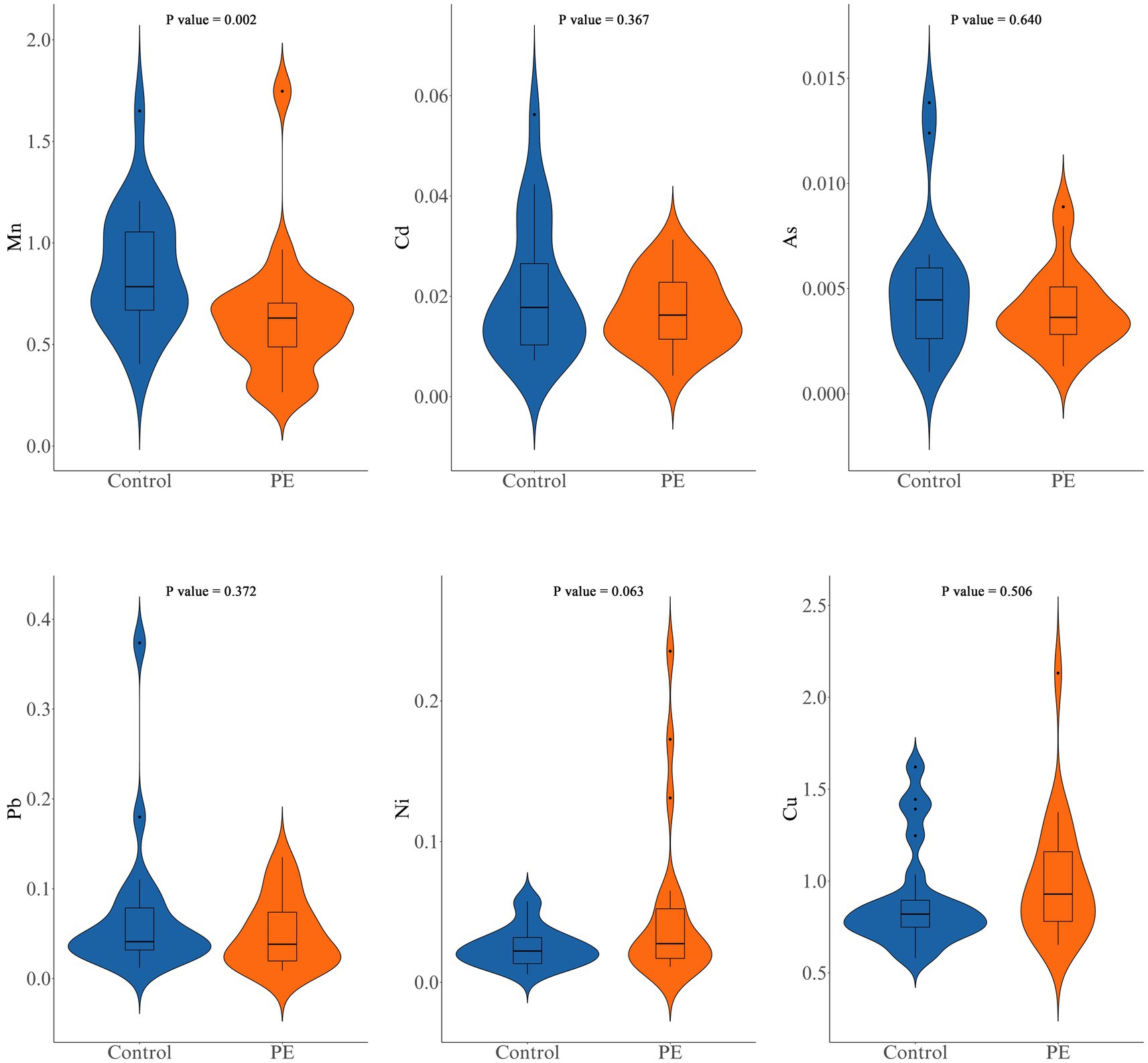
Figure 1. Comparison of manganese (Mn), cadmium (Cd), arsenic (As), lead (Pb), nickel (Ni), and copper (Cu) levels between the control group and the PE group.
Gut microbiota diversity analysis
The gut microbiota diversity between the two groups was compared in terms of both α- and β-diversity. For α-diversity (Figures 2A–F), the following indices were analyzed: Observed Species (the count of distinct species observed), Chao 1 Estimator and Abundance-based Coverage Estimator (both estimators of species richness that account for unobserved species), Shannon Diversity Index and Simpson’s Diversity Index (which measure both diversity and evenness within a community), and Coverage (estimating the proportion of the total species that are represented in the sample). None of these indices showed statistically significant differences between the two groups (all p > 0.05), suggesting no significant differences in α-diversity. For β-diversity (Figure 2G), PCoA demonstrated no clear separation between the groups (p = 0.194), with the first principal coordinate accounting for 8.4% of the variance and the second 7.6%. These results suggest that β-diversity may also lack significant differences between healthy pregnant women and those with preeclampsia.
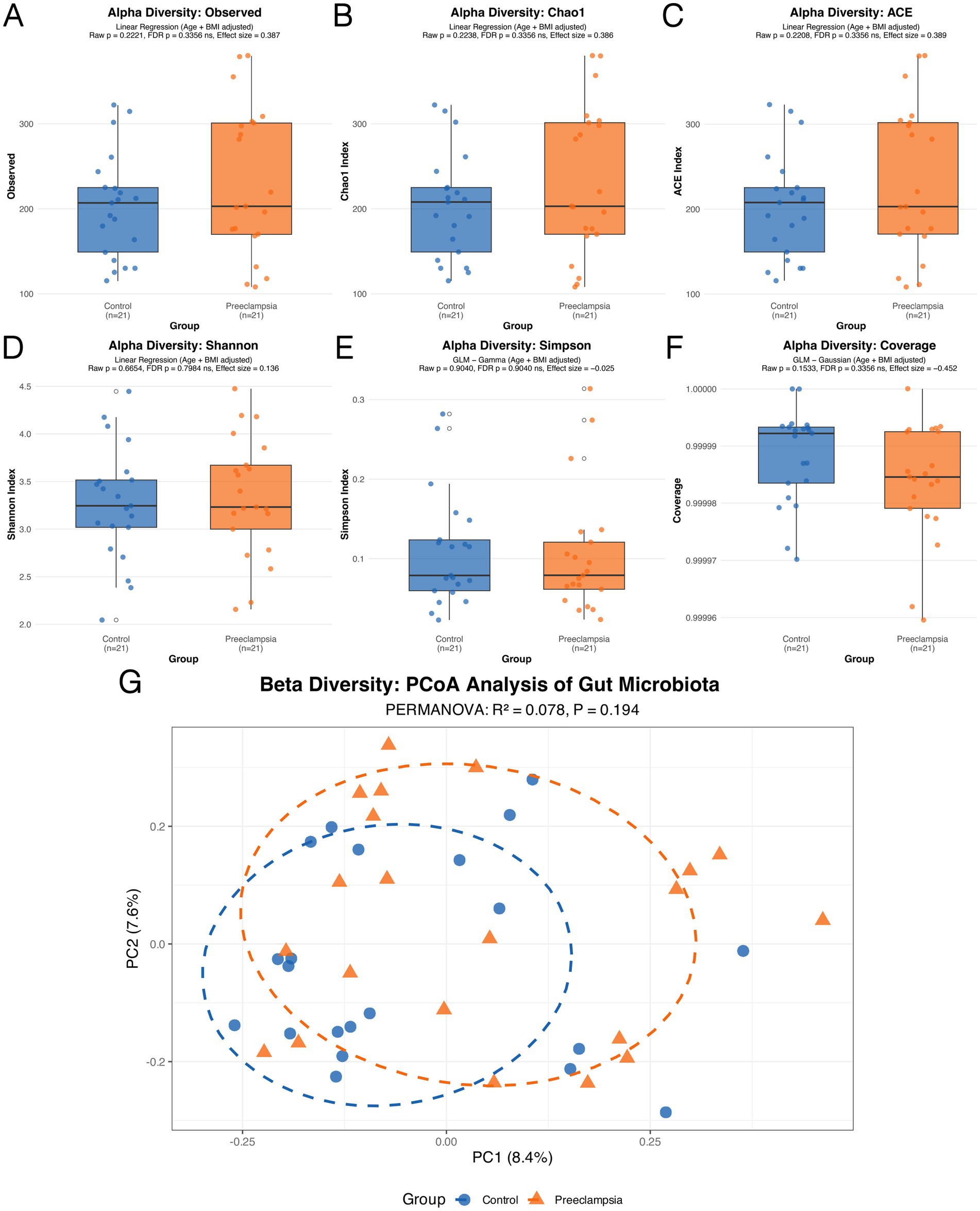
Figure 2. Comparison of gut microbiota α-diversity (A–F) and β-diversity (G) between the Control group and the PE group.
Differential bacteria at the genus level
In this study, we investigated the relationships between the gut microbiota, preeclampsia, and placental Mn levels at the genus level, employing the ANCOM-BC2 analytical method, which was adjusted for age and delivery BMI. The results are shown in Figure 3 and Supplementary Table S4. Our findings highlighted distinct microbial compositions associated with PE and placental Mn levels. Four bacterial genera were identified as having significant correlations with PE and Mn levels. Campylobacter, Porphyromonas, and UCG-009 showed positive associations with PE but negative associations with Mn levels. Conversely, Coprobacillus was negatively correlated with PE and positively correlated with Mn levels. The specific log2 fold changes (log2FC) and FDR underscore the robustness and significance of these associations. These results indicate specific dysbiosis patterns in the gut microbiota related to PE and Mn.
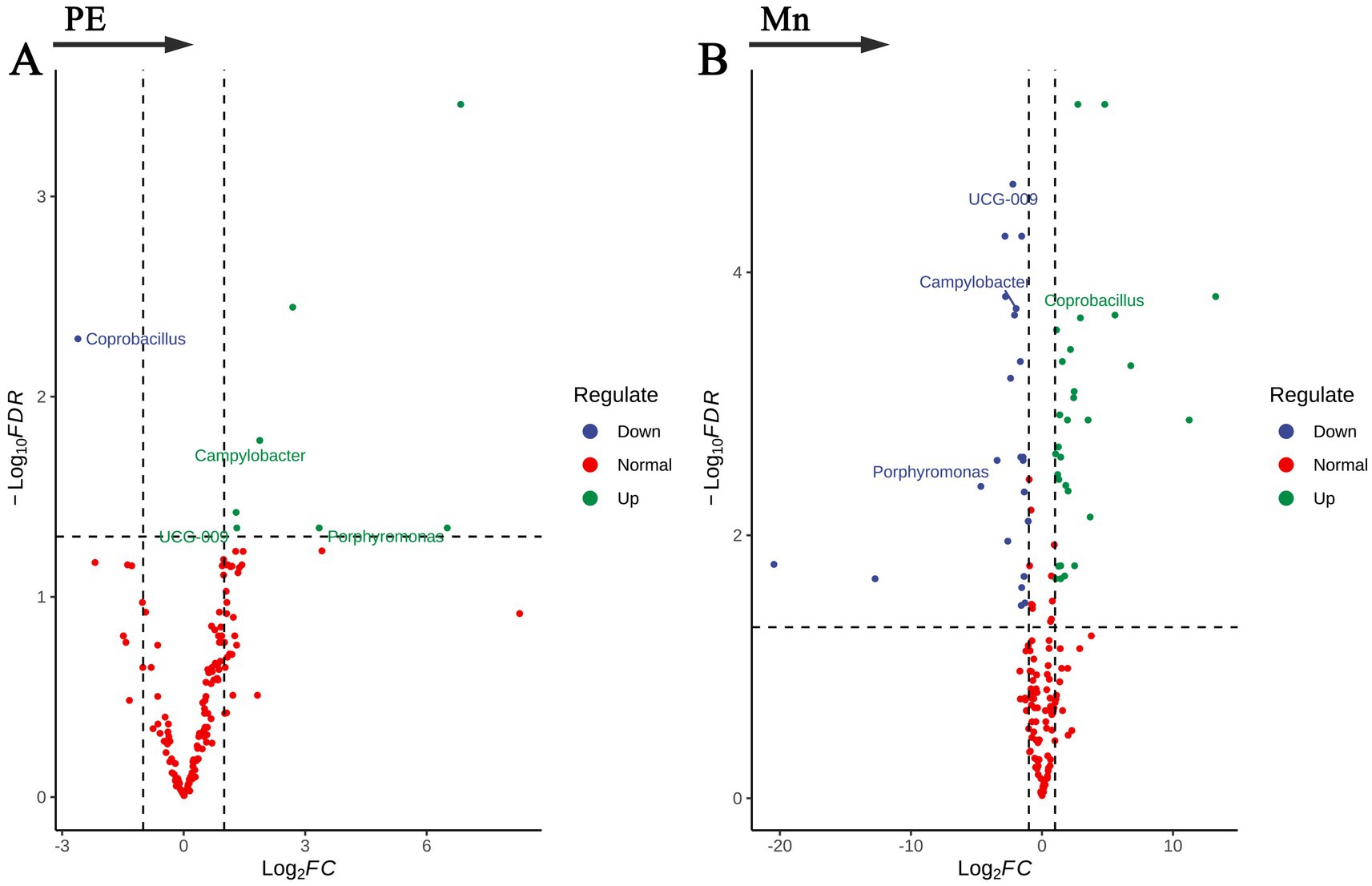
Figure 3. Volcano plots of bacterial genera significantly associated with (A) preeclampsia and (B) placental manganese levels.
KEGG pathway enrichment analysis
The potential functional capacities of the gut microbiota in relation to preeclampsia and placental manganese levels were explored by mapping microbial genes to the KEGG databases, with the findings detailed in Figure 4 and Supplementary Table S5. We identified 81 distinct KEGG Orthology (KO) genes: 29 exhibited a positive correlation with preeclampsia but a negative correlation with Mn; conversely, 52 showed a negative correlation with preeclampsia and a positive correlation with Mn. Subsequent KEGG pathway enrichment analysis of these KO genes revealed eight metabolic pathways negatively associated with preeclampsia yet positively associated with Mn. These pathways include: Pinene, camphor, and geraniol degradation; Dioxin degradation; Xylene degradation; Caprolactam degradation; Degradation of aromatic compounds; Glycine, serine, and threonine metabolism; Benzoate degradation; Methane metabolism. These results underscore metabolic alterations within the gut microbiota in response to fluctuations in both preeclampsia and manganese levels. These findings lay a foundation for further investigation into the role of microbial genes in influencing host metabolic processes, thereby elucidating the complex pathophysiology of preeclampsia associated with manganese.
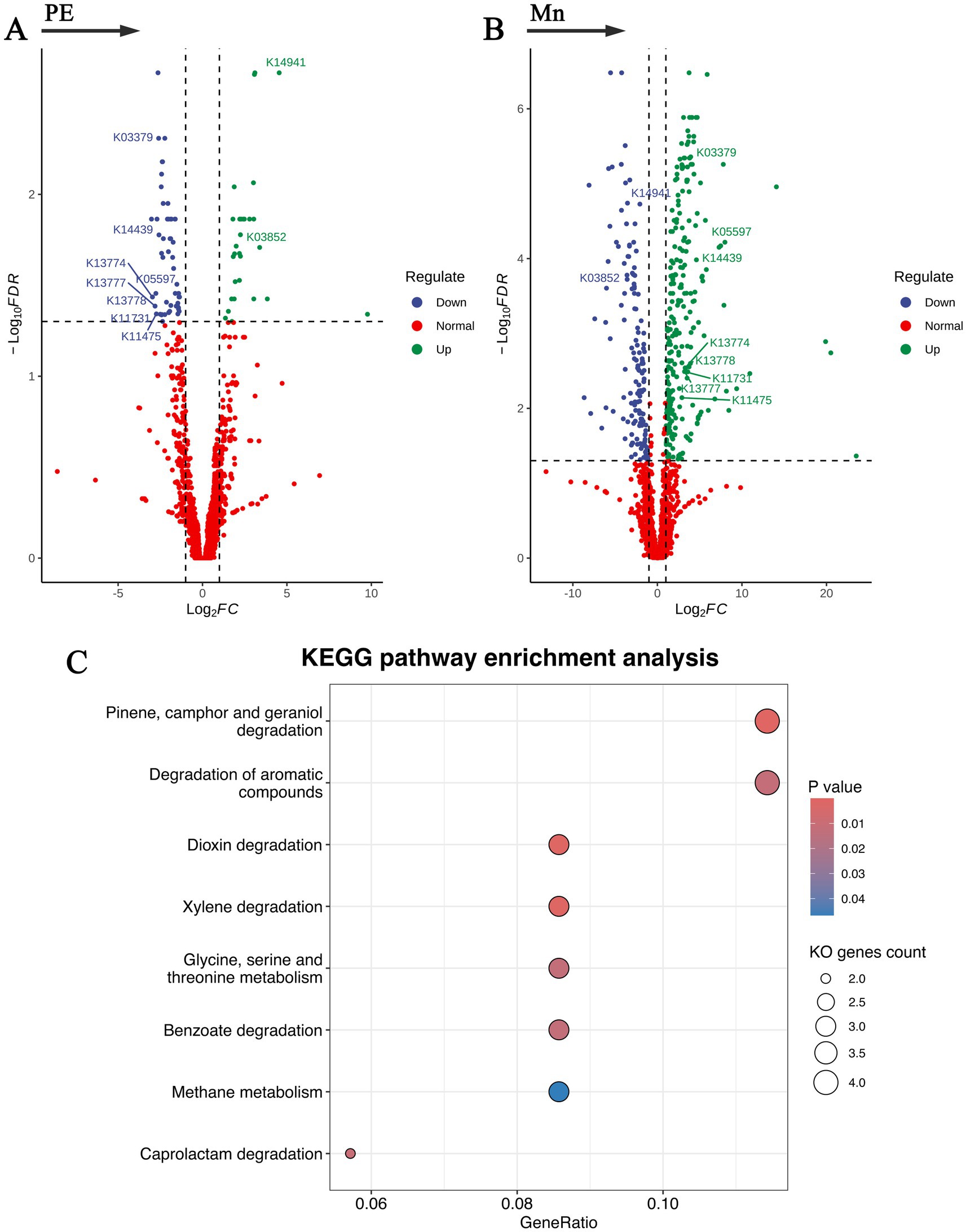
Figure 4. Functional capacities of the gut microbiota in relation to preeclampsia and placental manganese levels. (A,B) Volcano plots of KO genes significantly associated with preeclampsia and placental manganese levels. (C) Bubble plot of KEGG pathway enrichment analysis for the selected KO genes.
Discussion
This study investigates the relationship between placental trace element levels, specifically manganese, gut microbiota, and preeclampsia. We identified a negative correlation between placental Mn levels and preeclampsia. Additionally, our findings suggest that interactions between Mn and four bacterial genera—Coprobacillus, Campylobacter, Porphyromonas, and UCG-009—may influence the development of preeclampsia. This effect may be linked to altered gut microbiota metabolic activities, including the degradation of exogenous organic pollutants and the metabolism of methane, purines, and amino acids.
In this study, six metal elements were measured: Mn, Ni, Cu, As, Cd, and Pb. Among them, Mn is distinct. Previous studies have generally reported positive or null associations between the other metals and preeclampsia (Chen et al., 2024; Borghese et al., 2023; Hernandez-Castro et al., 2024; Sandoval-Carrillo et al., 2016), whereas Mn has been widely recognized as a protective factor. A recent meta-analysis (Wu et al., 2024), encompassing 18 observational studies, evaluated the relationship between maternal Mn levels and PE. The analysis revealed that lower serum Mn levels were associated with a higher risk of PE, regardless of the geographic region or the timing of serum Mn collection. Our study similarly supports this association. However, we chose to analyze placental Mn levels rather than serum levels. Although the protective role of Mn in PE is supported by a growing body of evidence, the underlying mechanisms remain unclear. One widely accepted hypothesis suggests that Mn contributes to the activity of manganese superoxide dismutase (Mn-SOD), a critical antioxidant enzyme. Mn-SOD mitigates oxidative stress by scavenging reactive oxygen species (ROS), thereby alleviating inflammation and reducing placental damage (Schlichte et al., 2021; Jahan et al., 2023; Grzeszczak et al., 2023; Tossetta et al., 2023; Liu et al., 2022; Matsubara et al., 2015). Additionally, ROS may impair blood pressure regulation by promoting endothelial dysfunction (Silva et al., 2012), while Mn-SOD can alleviate preeclampsia by counteracting this effect (Glover et al., 2014; Tomimatsu et al., 2019; Gong et al., 2020).
Besides Mn-SOD, our findings suggest that manganese may indirectly mitigate the progression of preeclampsia by altering the composition of the gut microbiota. Increasing evidence highlights that exposure to metal elements, such as Mn, can influence gut microbiota composition (Li et al., 2021; Peng et al., 2024). However, most studies have been conducted in animal models. Research on the relationship between Mn and the gut microbiota in humans—especially in pregnant women—remains limited. Our study identified associations between Mn and 63 bacterial genera, four of which—Coprobacillus, Campylobacter, Porphyromonas, and UCG-009—were also associated with preeclampsia. In particular, “UCG-009” refers to an unclassified genus group with minimal research available. Therefore, in this paragraph, let us focus on the other three genera. Campylobacter and Porphyromonas are common human pathogens. In our study, both genera were positively associated with preeclampsia and negatively correlated with placental manganese (Mn) levels. Campylobacter is a well-established cause of gastroenteritis and has been linked to Guillain-Barré syndrome (Butzler, 2004; Nachamkin et al., 1998). Similarly, Porphyromonas is associated with periodontal diseases such as periodontitis and is considered a risk factor for adverse pregnancy outcomes, including preeclampsia (Slots, 1999; Chopra et al., 2020). A shared characteristic of the two pathogens is their capacity to provoke inflammatory responses, resulting in elevated levels of pro-inflammatory cytokines in the body (Zhang et al., 2022; Young et al., 2007; Mukherjee et al., 2024; Kang et al., 2022). This pro-inflammatory activity may underlie their association with preeclampsia, highlighting the potential role of inflammatory mechanisms in the disease’s pathogenesis. In contrast, Coprobacillus demonstrated a positive correlation with placental Mn levels and a negative association with preeclampsia. Previous studies suggest that this genus produces SCFAs, which are known to benefit human health and can reduce inflammation (Yang et al., 2024; Yu et al., 2021; Hu et al., 2023; Liu et al., 2022).
The three genera—Coprobacillus, Campylobacter, and Porphyromonas—highlighted in our study have been rarely reported in prior research. In another study conducted in Liaocheng, China, Akkermansia muciniphila, a bacterium known for producing SCFAs, was found to be reduced in PE patients. Animal experiments further showed that supplementation with Akkermansia muciniphila, propionic acid, or butyric acid alleviated several features of preeclampsia in rats (Jin et al., 2022). Similarly, a study in Guangzhou, China, found that not only was Akkermansia reduced in PE patients, but another beneficial bacterium, Faecalibacterium—also known for butyrate production—was diminished as well (Chen et al., 2020). An Australian study also reported a reduction in the butyrate-producing genus Coprococcus among PE patients (Altemani et al., 2021). However, our study did not detect significant differences in the abundance of Akkermansia, Faecalibacterium, or Coprococcus. These discrepancies could stem from variations in the timing of fecal sample collection or differences in microbial composition analysis methods. Despite these inconsistencies, both our findings and prior research consistently underscore a reduction in SCFA-producing, inflammation-regulating beneficial bacteria in PE patients. This supports the hypothesis that SCFAs may exert a protective effect against PE (Mackay and Marques, 2022; Cui et al., 2023).
KEGG metabolic pathway prediction based on Accu16S data suggested eight potentially downregulated pathways in patients with PE. Six of these predicted pathways—dioxin degradation, xylene degradation, caprolactam degradation, benzoate degradation, pinene/camphor/geraniol degradation, and aromatic compound degradation—are related to microbial xenobiotic metabolism and may be involved in the degradation of exogenous organic pollutants. Such pollutants have been reported to activate the aryl hydrocarbon receptor (AHR), thereby contributing to inflammation and oxidative stress (Vogel et al., 2020). The other two predicted pathways—glycine, serine, and threonine metabolism, and methane metabolism—also have potential links to inflammation and oxidative stress (Ye et al., 2020; Aguayo-Cerón et al., 2023; Egbujor et al., 2024). Nevertheless, it should be emphasized that these findings are based on predictive functional profiling of microbial communities rather than direct metabolomic measurements. Direct evidence linking these pathways or metabolites to preeclampsia remains limited. Further validation, particularly through metabolomic analyses and mechanistic studies in animal models, is warranted to clarify these potential associations.
The primary strength of this study is its comprehensive approach, which adopts a tripartite perspective to investigate the interplay among placental manganese levels, gut microbiota composition, and preeclampsia. Rather than focusing on a single factor, such as gut microbiota or manganese, our study integrates these factors to provide novel insights into the potential mechanisms by which manganese may mitigate the risk of preeclampsia through modulating gut microbiota composition. Furthermore, we employed ANCOM-BC2, a robust analytical method published in Nature Methods in 2024 (Lin and Peddada, 2024), to precisely evaluate differences in gut microbiota composition. Despite these strengths, our study has several limitations. First, the sample size was relatively small, with only 21 pregnant women in the PE group and 21 in the control group, which may limit statistical power. Second, placenta and fecal samples were collected at delivery or within 1 week before delivery, when preeclampsia was already established. Therefore, the observed microbial and metabolic alterations may represent consequences of the disease rather than predisposing risk factors, limiting causal interpretation. To address these issues, future studies should adopt larger, multi-center cohorts with longitudinal sampling starting early in pregnancy to validate our findings and clarify temporal and causal relationships.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://db.cngb.org/data_resources/?query=CNP0006841, China National GeneBank DataBase (CNGBdb), accession code CNP0006841.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Dongguan Songshan Lake Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TD: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft. XH: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. SA: Methodology, Supervision, Writing – review & editing. YP: Data curation, Funding acquisition, Project administration, Resources, Writing – review & editing. WZ: Data curation, Investigation, Methodology, Writing – review & editing. SH: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing. YD: Conceptualization, Data curation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present study was supported by the Major Scientific Research Project Cultivation Fund of Songshan Lake Central Hospital [grant number SZR003] and Dongguan Social Science and Technology Development Key Project (2022) [grant number 20221800906202].
Acknowledgments
The authors express gratitude to our patients and all participants for their contributions to data collection. We also thank the medical staff at the study hospital for their assistance in recruiting subjects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1674549/full#supplementary-material
References
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D., and Say, L. (2013). Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7. doi: 10.1016/j.ejogrb.2013.05.005
Aguayo-Cerón, K. A., Sánchez-Muñoz, F., Gutierrez-Rojas, R. A., Acevedo-Villavicencio, L. N., Flores-Zarate, A. V., Huang, F., et al. (2023). Glycine: the smallest anti-inflammatory micronutrient. Int. J. Mol. Sci. 24:11236. doi: 10.3390/ijms241411236
Altemani, F., Barrett, H. L., Gomez-Arango, L., Josh, P., David McIntyre, H., Callaway, L. K., et al. (2021). Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer coprococcus in their gut microbiota. Pregnancy Hypertens. 23, 211–219. doi: 10.1016/j.preghy.2021.01.002
Borghese, M. M., Fisher, M., Ashley-Martin, J., Fraser, W. D., Trottier, H., Lanphear, B., et al. (2023). Individual, independent, and joint associations of toxic metals and manganese on hypertensive disorders of pregnancy: results from the MIREC Canadian pregnancy cohort. Environ. Health Perspect. 131:47014. doi: 10.1289/EHP10825
Butzler, J. P. (2004). Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10, 868–876. doi: 10.1111/j.1469-0691.2004.00983.x
Chang, Y., Chen, Y., Zhou, Q., Wang, C., Chen, L., di, W., et al. (2020). Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. 134, 289–302. doi: 10.1042/CS20191253
Chappell, L. C., Cluver, C. A., Kingdom, J., and Tong, S. (2021). Pre-eclampsia. Lancet 398, 341–354. doi: 10.1016/S0140-6736(20)32335-7
Chen, X., Li, P., Liu, M., Zheng, H., He, Y., Chen, M. X., et al. (2020). Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69, 513–522. doi: 10.1136/gutjnl-2019-319101
Chen, Y., Pu, Y., Liu, H., Cao, A., du, Y., He, S., et al. (2024). A study on the mediating role of serum hormones in the effects of heavy metals on preeclampsia. Environ. Pollut. 360:124721. doi: 10.1016/j.envpol.2024.124721
Chiang, Y. T., Seow, K. M., and Chen, K. H. (2024). The pathophysiological, genetic, and hormonal changes in preeclampsia: a systematic review of the molecular mechanisms. Int. J. Mol. Sci. 25:4532. doi: 10.3390/ijms25084532
Chopra, A., Radhakrishnan, R., and Sharma, M. (2020). Porphyromonas gingivalis and adverse pregnancy outcomes: a review on its intricate pathogenic mechanisms. Crit. Rev. Microbiol. 46, 213–236. doi: 10.1080/1040841X.2020.1747392
Cui, J., Wang, J., and Wang, Y. (2023). The role of short-chain fatty acids produced by gut microbiota in the regulation of pre-eclampsia onset. Front. Cell. Infect. Microbiol. 13:1177768. doi: 10.3389/fcimb.2023.1177768
Deady, C., McCarthy, F. P., Barron, A., McCarthy, C. M., O’Keeffe, G. W., and O’Mahony, S. M. (2024). An altered gut microbiome in pre-eclampsia: cause or consequence. Front. Cell. Infect. Microbiol. 14:1352267. doi: 10.3389/fcimb.2024.1352267
Egbujor, M. C., Olaniyan, O. T., Emeruwa, C. N., Saha, S., Saso, L., and Tucci, P. (2024). An insight into role of amino acids as antioxidants via NRF2 activation. Amino Acids 56:23. doi: 10.1007/s00726-024-03384-8
Enebe, J. T., Dim, C. C., and Omeke, A. C. (2023). Maternal antioxidant micronutrient deficiencies among pre-eclamptic women in Enugu, Nigeria: a cross-sectional analytical study. J. Int. Med. Res. 51:3000605231209159. doi: 10.1177/03000605231209159
Ernst, L. M. (2018). Maternal vascular malperfusion of the placental bed. APMIS 126, 551–560. doi: 10.1111/apm.12833
Genesky Biotechnologies Inc. Accu16S®bacterial absolute quantification sequencing. Available online at: http://www.geneskybiotech.com/114/ (Accessed December 25, 2024).
Glover, M., Hebert, V. Y., Nichols, K., Xue, S. Y., Thibeaux, T. M., Zavecz, J. A., et al. (2014). Overexpression of mitochondrial antioxidant manganese superoxide dismutase (MnSOD) provides protection against AZT- or 3TC-induced endothelial dysfunction. Antivir. Res. 111, 136–142. doi: 10.1016/j.antiviral.2014.09.010
Gong, J. H., Lo, K., Liu, Q., Li, J., Lai, S., Shadyab, A. H., et al. (2020). Dietary manganese, plasma markers of inflammation, and the development of type 2 diabetes in postmenopausal women: findings from the women’s health initiative. Diabetes Care 43, 1344–1351. doi: 10.2337/dc20-0243
Grzeszczak, K., Łanocha-Arendarczyk, N., Malinowski, W., Ziętek, P., and Kosik-Bogacka, D. (2023). Oxidative stress in pregnancy. Biomolecules 13:1768. doi: 10.3390/biom13121768
Harmon, Q. E., Huang, L., Umbach, D. M., Klungsøyr, K., Engel, S. M., Magnus, P., et al. (2015). Risk of fetal death with preeclampsia. Obstet. Gynecol. 125, 628–635. doi: 10.1097/AOG.0000000000000696
He, J., Pu, Y., Du, Y., Liu, H., Wang, X., He, S., et al. (2024). An exploratory study on the association of multiple metals in serum with preeclampsia. Front. Public Health 12:1336188. doi: 10.3389/fpubh.2024.1336188
Hernandez-Castro, I., Rifas-Shiman, S. L., Lin, P. I. D., Chavarro, J. E., Gold, D. R., Zhang, M., et al. (2024). First trimester prenatal metal mixtures, vitamins, and hypertensive disorders of pregnancy in the project viva cohort. Environ. Int. 190:108909. doi: 10.1016/j.envint.2024.108909
Hu, H., Zhu, H., Yang, H., Yao, W., and Zheng, W. (2023). In vitro fermentation properties of magnesium hydride and related modulation effects on broiler cecal microbiome and metabolome. Front. Microbiol. 14:1175858. doi: 10.3389/fmicb.2023.1175858
Huang, L., Cai, M., Li, L., Zhang, X., Xu, Y., Xiao, J., et al. (2021). Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 21:265. doi: 10.1186/s12866-021-02327-7
Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association (2020). Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China. Chin. J. Obstet.Gynecol. 55, 227–238. doi: 10.3760/cma.j.cn112141-20200114-00039
Jahan, F., Vasam, G., Green, A. E., Bainbridge, S. A., and Menzies, K. J. (2023). Placental mitochondrial function and dysfunction in preeclampsia. Int. J. Mol. Sci. 24:4177. doi: 10.3390/ijms24044177
Jin, J., Gao, L., Zou, X., Zhang, Y., Zheng, Z., Zhang, X., et al. (2022). Gut dysbiosis promotes preeclampsia by regulating macrophages and trophoblasts. Circ. Res. 131, 492–506. doi: 10.1161/CIRCRESAHA.122.320771
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Kang, S., Dai, A., Wang, H., and Ding, P. H. (2022). Interaction between autophagy and porphyromonas gingivalis-induced inflammation. Front. Cell. Infect. Microbiol. 12:892610. doi: 10.3389/fcimb.2022.892610
Khong, T. Y., Mooney, E. E., Ariel, I., Balmus, N. C. M., Boyd, T. K., Brundler, M. A., et al. (2016). Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab Med. 140, 698–713. doi: 10.5858/arpa.2015-0225-CC
Li, C. Y., Li, X. Y., Shen, L., and Ji, H. F. (2021). Regulatory effects of transition metals supplementation/deficiency on the gut microbiota. Appl. Microbiol. Biotechnol. 105, 1007–1015. doi: 10.1007/s00253-021-11096-2
Li, F., Qin, J., Zhang, S., and Chen, L. (2021). Prevalence of hypertensive disorders in pregnancy in China: a systematic review and meta-analysis. Pregnancy Hypertens. 24, 13–21. doi: 10.1016/j.preghy.2021.02.001
Li, L., and Yang, X. (2018). The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxidative Med. Cell. Longev. 2018:7580707. doi: 10.1155/2018/7580707
Lin, H., and Peddada, S. D. (2024). Multigroup analysis of compositions of microbiomes with covariate adjustments and repeated measures. Nat. Methods 21, 83–91. doi: 10.1038/s41592-023-02092-7
Liu, L., Chen, X., Liu, L., and Qin, H. (2022). Clostridium butyricum potentially improves immunity and nutrition through alteration of the microbiota and metabolism of elderly people with malnutrition in long-term care. Nutrients 14:3546. doi: 10.3390/nu14173546
Liu, T., Hivert, M. F., Rifas-Shiman, S. L., Rahman, M. L., Oken, E., Cardenas, A., et al. (2020). Prospective association between manganese in early pregnancy and the risk of preeclampsia. Epidemiology 31, 677–680. doi: 10.1097/EDE.0000000000001227
Liu, M., Sun, X., Chen, B., Dai, R., Xi, Z., and Xu, H. (2022). Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 23:15893. doi: 10.3390/ijms232415893
Liu, X., Zeng, X., Li, X., Xin, S., Zhang, F., Liu, F., et al. (2024). Landscapes of gut bacterial and fecal metabolic signatures and their relationship in severe preeclampsia. J. Transl. Med. 22:360. doi: 10.1186/s12967-024-05143-5
Liu, T., Zhang, M., Guallar, E., Wang, G., Hong, X., Wang, X., et al. (2019). Trace minerals, heavy metals, and preeclampsia: findings from the Boston birth cohort. J. Am. Heart Assoc. 8:e012436. doi: 10.1161/JAHA.119.012436
Mackay, C. R., and Marques, F. Z. (2022). Dysbiosis in preeclampsia and treatment with short chain fatty acids. Circ. Res. 131, 507–509. doi: 10.1161/CIRCRESAHA.122.321701
Matsubara, K., Higaki, T., Matsubara, Y., and Nawa, A. (2015). Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 16, 4600–4614. doi: 10.3390/ijms16034600
Meijer, S., Pasquinelli, E., Renzi, S., Lavasani, S., Nouri, M., Erlandsson, L., et al. (2023). Gut micro- and mycobiota in preeclampsia: bacterial composition differences suggest role in pathophysiology. Biomolecules 13:346. doi: 10.3390/biom13020346
Mukherjee, S., Chopra, A., Karmakar, S., and Bhat, S. G. (2024). Periodontitis increases the risk of gastrointestinal dysfunction: an update on the plausible pathogenic molecular mechanisms. Crit. Rev. Microbiol. 51, 187–217. doi: 10.1080/1040841X.2024.2339260
Nachamkin, I., Allos, B. M., and Ho, T. (1998). Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 11, 555–567. doi: 10.1128/CMR.11.3.555
Peng, Z., Liao, Y., Yang, W., and Liu, L. (2024). Metal(loid)-gut microbiota interactions and microbiota-related protective strategies: a review. Environ. Int. 192:109017. doi: 10.1016/j.envint.2024.109017
Pittara, T., Vyrides, A., Lamnisos, D., and Giannakou, K. (2021). Pre-eclampsia and long-term health outcomes for mother and infant: an umbrella review. BJOG 128, 1421–1430. doi: 10.1111/1471-0528.16683
Poon, L. C., Shennan, A., Hyett, J. A., Kapur, A., Hadar, E., Divakar, H., et al. (2019). The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 145, 1–33. doi: 10.1002/ijgo.12802
Sandoval-Carrillo, A., Méndez-Hernández, E. M., Antuna-Salcido, E. I., Salas-Pacheco, S. M., Vázquez-Alaniz, F., Téllez-Valencia, A., et al. (2016). Arsenic exposure and risk of preeclampsia in a mexican mestizo population. BMC Pregnancy Childbirth 16:153. doi: 10.1186/s12884-016-0946-4
Schlichte, S. L., Pekas, E. J., Bruett, T. J., Kosmacek, E. A., Hackfort, B. T., Rasmussen, J. M., et al. (2021). Sympathoinhibition and vasodilation contribute to the acute hypotensive response of the superoxide dismutase mimic, MnTnBuOE-2-PyP5+, in hypertensive animals. Adv. Redox Res. 3:100016. doi: 10.1016/j.arres.2021.100016
Silva, B. R., Pernomian, L., and Bendhack, L. M. (2012). Contribution of oxidative stress to endothelial dysfunction in hypertension. Front. Physiol. 3:441. doi: 10.3389/fphys.2012.00441
Slots, J. (1999). Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease: introduction. Periodontol 2000 20, 7–13. doi: 10.1111/j.1600-0757.1999.tb00155.x
Tomimatsu, T., Mimura, K., Matsuzaki, S., Endo, M., Kumasawa, K., and Kimura, T. (2019). Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int. J. Mol. Sci. 20:4246. doi: 10.3390/ijms20174246
Tossetta, G., Fantone, S., Piani, F., Crescimanno, C., Ciavattini, A., Giannubilo, S. R., et al. (2023). Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells 12:1545. doi: 10.3390/cells12111545
Vogel, C. F. A., Van Winkle, L. S., Esser, C., and Haarmann-Stemmann, T. (2020). The aryl hydrocarbon receptor as a target of environmental stressors - implications for pollution mediated stress and inflammatory responses. Redox Biol. 34:101530. doi: 10.1016/j.redox.2020.101530
Wang, J., Shi, Z. H., Yang, J., Wei, Y., Wang, X. Y., and Zhao, Y. Y. (2020). Gut microbiota dysbiosis in preeclampsia patients in the second and third trimesters. Chin. Med. J. 133, 1057–1065. doi: 10.1097/CM9.0000000000000734
Wu, A., Li, J., Yuan, J., Zhang, N., Zhang, Y., Li, M., et al. (2024). Association of blood manganese and preeclampsia: a systematic review and meta-analysis. Biol. Trace Elem. Res. 202, 1843–1855. doi: 10.1007/s12011-023-03796-9
Yang, C., Chen, J., Zhou, H., Zeng, D., Wan, H., and Yang, J. (2024). Therapeutic effect of yinhuapinggan granules mediated through the intestinal flora in mice infected with the H1N1 influenza virus. Front. Microbiol. 15:1394304. doi: 10.3389/fmicb.2024.1394304
Ye, Z. H., Ning, K., Ander, B. P., and Sun, X. J. (2020). Therapeutic effect of methane and its mechanism in disease treatment. J Zhejiang Univ Sci B 21, 593–602. doi: 10.1631/jzus.B1900629
Young, K. T., Davis, L. M., and DiRita, V. J. (2007). Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5, 665–679. doi: 10.1038/nrmicro1718
Yu, L., Duan, H., Kellingray, L., Cen, S., Tian, F., Zhao, J., et al. (2021). Lactobacillus plantarum-mediated regulation of dietary aluminum induces changes in the human gut microbiota: an in vitro colonic fermentation study. Probiotics Antimicrob. Proteins 13, 398–412. doi: 10.1007/s12602-020-09677-0
Zambella, E., Peruffo, B., Guarano, A., Inversetti, A., and Di Simone, N. (2024). The hidden relationship between intestinal microbiota and immunological modifications in preeclampsia pathogenesis. Int. J. Mol. Sci. 25:10099. doi: 10.3390/ijms251810099
Zhang, L., Liu, F., Xue, J., Lee, S. A., Liu, L., and Riordan, S. M. (2022). Bacterial species associated with human inflammatory bowel disease and their pathogenic mechanisms. Front. Microbiol. 13:801892. doi: 10.3389/fmicb.2022.801892
Zhao, Y., Wang, B., Zhao, X., Cui, D., Hou, S., and Zhang, H. (2023). The effect of gut microbiota dysbiosis on patients with preeclampsia. Front. Cell. Infect. Microbiol. 12:1022857. doi: 10.3389/fcimb.2022.1022857
Zhu, Q., Chen, B., Zhang, F., Zhang, B., Guo, Y., Pang, M., et al. (2024). Toxic and essential metals: metabolic interactions with the gut microbiota and health implications. Front. Nutr. 11:1448388. doi: 10.3389/fnut.2024.1448388
Keywords: manganese, preeclampsia, gut microbiota, metabolic pathways, placenta
Citation: Ding T, Huang X, Ai S, Pu Y, Zhao W, He S and Dang Y (2025) Association of placental manganese levels, maternal gut microbiota, and preeclampsia: a tripartite perspective. Front. Microbiol. 16:1674549. doi: 10.3389/fmicb.2025.1674549
Edited by:
Svetlana Khaiboullina, University of Nevada, Reno, United StatesReviewed by:
Silvia Giugliano, Humanitas University, ItalyShoshannah Eggers, The University of Iowa, United States
Copyright © 2025 Ding, Huang, Ai, Pu, Zhao, He and Dang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuzhen He, Mjg5NjAxMDI4NEBxcS5jb20=; Yuhui Dang, ZGFuZ3loQGx6dS5lZHUuY24=
†These authors have contributed equally to this work
 Tianze Ding
Tianze Ding Xiaoli Huang2†
Xiaoli Huang2† Shiwei Ai
Shiwei Ai Yudong Pu
Yudong Pu Yuhui Dang
Yuhui Dang