- 1Sichuan Philosophy and Social Key Laboratory of Monitoring and Assessing for Rural Land Utilization, School of History, Geography and Tourism, Chengdu Normal University, Chengdu, China
- 2Institute of Soil and Water Conservation, Chinese Academy of Sciences and Ministry of Water Resources, Yangling, China
- 3School of Forestry and Prataculture, Ningxia University, Yinchuan, Ningxia, China
Introduction: Soil enzymes are critical to plant growth and soil carbon turnover. However, the traditional method of assessing enzyme activity per unit of soil may be insufficient; instead, soil-specific enzyme activity per unit of soil organic carbon (SOCE) or microbial biomass carbon (MBCE) has been widely used to characterize soil carbon accumulation.
Methods: We systematically examined the changes in SOCE and MBCE with sand dune fixation (mobile, semi-mobile, semi-fixed, and fixed). We explored the implications of this soil-specific enzyme activity for soil microbial necromass carbon (NC) and soil organic carbon (SOC) accumulation.
Results: We found that β-1, 4-glucosidase, β-D-cellobiosidase, β-1, 4-N-acetylglucosaminidase, and L-leucine aminopeptidase in SOCE and MBCE, the soil enzyme activity coefficient (SEAC), and the geometric mean of enzyme activity (GMEA) were significantly higher in semi-mobile, semi-fixed, and fixed dunes than those in mobile dunes. Furthermore, SOCE, MBCE, SEAC, and GMEA showed significant relationships with microbial NC and SOC. Specifically, soil-specific enzyme activity accounted for 32.2 and 24.1% of microbial NC and SOC variance, respectively.
Conclusion: Dune fixation significantly increases SOCE and MBCE. More importantly, we recommend that changes in SOCE and MBCE should be widely used to assess microbial NC and SOC accumulation in degraded sandy land ecosystems.
1 Introduction
Desertification is an important ecological and environmental problem that severely restricts human survival and threatens sustainable economic and social development (Kéfi et al., 2007; Peters et al., 2012). The Mu Us Sandy Land is an important area for ecological restoration (Li et al., 2017). Since the 1950s, the ecosystem in this region has been severely degraded because of overgrazing (Miao et al., 2016). Local restoration measures, such as fencing, cropland abandonment, and vegetation restoration, have been implemented to restore the ecological environment, which has significantly changed the land-use pattern and affected plant growth and material cycling processes in the local ecosystem (Guo et al., 2023). Currently, mobile (coverage: <5%), semi-mobile (5–20%), semi-fixed (21–50%), and fixed (>50%) dunes with different vegetation coverages have formed (Chen et al., 2022).
Currently, further research has focused on soil absolute enzyme activity (SAE; Nannipieri et al., 2012; Zheng et al., 2025), but in natural soil, enzymes coexist with soil microorganisms and carbon (Stone et al., 2014; Xu et al., 2020). Therefore, soil enzymes cannot be separated from organic matter or microorganism (Liu et al., 2017). Based on this relationship, researchers have introduced the concept of soil-specific enzyme activity [per unit of soil organic carbon (SOCE) or microbial biomass carbon (MBCE)] (Yu et al., 2019). SOCE and MBCE reflect the activity based on organic matter and microorganisms, which providing clear insights into changes in SOC and MBC (Raiesi and Beheshti, 2014).
Some scholars have noted that SOCE and MBCE are regulated by land-use change (Xiao et al., 2021). For example, Zhang et al. (2015) reported consistent changes in both SOCE and SAE in response to different fertilization treatments. Raiesi and Salek-Gilani (2018) found that after cropland abandonment, SAE increased, whereas SOCE decreased with recovery time. The roles of SOCE and MBCE also differ in their responses to environmental changes. For example, Raiesi and Beheshti (2014) indicated that MBCE and SOCE respond consistently to land-use changes. However, Xu et al. (2020) observed that SOCE first increased and then tended to stabilize with recovery time, whereas MBCE gradually decreased with longer recovery times. Consequently, changes in SOCE and MBCE with restoration remain inconclusive.
Soil enzymes are sensitive to sand dune fixation (ecological restoration) (Cao C. et al., 2024). It is generally accepted that sand dune fixation increases plant diversity and vegetation cover (Qiao et al., 2012), which in turn produces more litter that returns to the soil, thereby increasing soil nutrient content and providing more food resources for soil microorganisms. This resource increase boosts enzyme secretion by microorganisms (Cheng et al., 2022). Moreover, sand dune fixation promotes plant growth and microbial activity, thereby promoting interactions among plants, microorganisms, and soil development (Cao et al., 2017; Alamusa et al., 2023). In arid and semi-arid regions, soil water controls microbial activity (Li et al., 2023). By increasing vegetation cover, dune fixation reduces water evaporation and enhances soil water availability (Cao M. et al., 2024). Increases in soil stability and water content further enhance SAE (Xu et al., 2016), but the effect of sand dune fixation on SOCE and MBCE remain poorly understood.
Soil microorganisms regulate soil carbon transformation, in the form of living organisms and microbial necromass carbon (NC; Buckeridge et al., 2022; Xiang et al., 2024). Liang et al. (2019) confirmed that microbial NC, as a stable soil carbon component, contributes more than 50% of SOC accumulation. This process occurs as soil microorganisms continuously grow, metabolize, proliferate, and die, leaving behind cell wall residues (Buckeridge et al., 2022). Chitin from fungal cell walls and peptidoglycan from bacterial cell walls accumulate in soils, which directly contribute to the soil carbon pool (Camenzind et al., 2023). Moreover, Xu et al. (2020) showed that soil-specific enzyme activity may help explain changes in carbon stability. However, the relationship between SOCE, MBCE, and microbial NC, as well as whether SOCE and MBCE can explain changes in microbial NC, requires further exploration.
To explore the correlation between SOCE and MBCE with soil microbial NC during dune fixation, we established study sites with varying dune coverages: mobile, semi-mobile, semi-fixed, and fixed dunes. We aimed to study the effect of dune fixation on SOCE and MBCE and its relationship with plant and microbial community characteristics. We hypothesized that dune fixation would lead to increased SOCE and MBCE. Additionally, we hypothesized that the SOCE and MBCE are significantly positively correlated with microbial NC.
2 Methods
2.1 Study site
The study site is located in Yanchi County, Ningxia Hui Autonomous Region, China (Figure 1). The mean annual precipitation and temperature are 250–350 mm and 6.0 °C–8.5 °C, respectively, and the elevation is 1,200–1,600 m. The wind is mainly northwesterly, with annual average wind speeds of 2.1–3.3 m.s−1, especially from March to May. The soil type is typical eolian sand soil, with loose surface material, rich sand source material, and strong eolian sand activity. The zonal vegetation primarily consists of Agriophyllum squarrosum, Artemisia ordosica, Aster altaicus, Artemisia scoparia, and Caragana microphylla. In the 1950s, the ecosystem of the region was severely degraded owing to overgrazing. Consequently, fencing has been implemented to restore these degraded ecosystems, and grassland communities at different stages of restoration have been formed.
2.2 Experimental setting and sample analysis
Four treatments were established: mobile, semi-mobile, semi-fixed, and fixed dune groups. Three 5 × 5 m plots were set up for each dune type, totaling 12 plots. The slope angle, slope direction, and slope position in each treatment plot remained unchanged. In each plot, three 1 × 1 m quadrats were set evenly along the diagonal, totaling 36 quadrats. A vegetation diversity survey was conducted for each sample quadrat. The soil depth is 0–20 cm. The visible stones and roots in soil samples were removed and sieved using a 2-mm sieve and then divided into three parts: one part was used to determine the microbial community; the second, to determine the enzyme activity; and the third, to determine the microbial NC content.
Microbial community and microbial NC were determined by the Rhonin Biosciences1 and Baihui Organisms2 companies, respectively. Soil enzyme activity of β-1, 4-glucosidase (BG), β-D-cellobiosidase (CBH), β-1, 4-N-acetylglucosaminidase (NAG), L-leucine aminopeptidase (LAP), and acid phosphatase (AP) were determined using 96-microplate enzymic fluorescence assays (German et al., 2011). The soil microbial biomass was determined by chloroform fumigation methods (Vance et al., 1987). The soil pH, organic carbon (SOC), total nitrogen (TN), and total phosphorus (TP) were determined using the pH meter, Walkley and Black, Kjeldahl, and molybdenum blue methods, respectively (Bremner, 1982; Nelson and Sommers, 1982). The detailed methods of soil microbial community, microbial NC, soil microbial biomass, and enzyme activity were described in Zhou et al. (2025).
2.3 Statistical analyses
The Shannon–Weiner diversity, Pielou evenness, and Margalef richness indices were selected to characterize plant diversity, which were calculated as:
The SOCE and MBCE were calculated as follows (Trasar-Cepeda et al., 2008):
The soil enzyme activity coefficient (SEAC) reflects the relative microbial demand for carbon and nitrogen, which was calculated as:
The GMEA was calculated as follows (Hinojosa et al., 2004):
Microbial NC was calculated as follows:
The results of plant diversity and soil physicochemical properties in different dunes are shown in Supplementary Tables S1, S2. SOCE, MBCE, soil nutrients, and plant and microbial diversity were analyzed using a one-way analysis of variance. The Pearson’s correlation was used to evaluate the relationships of SOCE and MBCE with microbial NC, SOC, soil nutrients, and plant and microbial diversity. Random forest analysis was used to assess the relative contributions of SOCE, MBCE, soil nutrients, and plant and microbial diversity to microbial NC and SOC (randomForest package in R version 4.5.0).
3 Results
3.1 Dynamics of SOCE and MBCE
The semi-fixed and fixed dunes had higher BG/SOC and LAP/SOC, compared with the semi-mobile and mobile dunes (Figures 2a,d). Furthermore, the fixed dunes had higher CBH/SOC, and NAG/SOC than the semi-mobile and mobile dunes, and the fixed dunes also had higher AP/SOC than other dune types (Figures 2b,c,e).
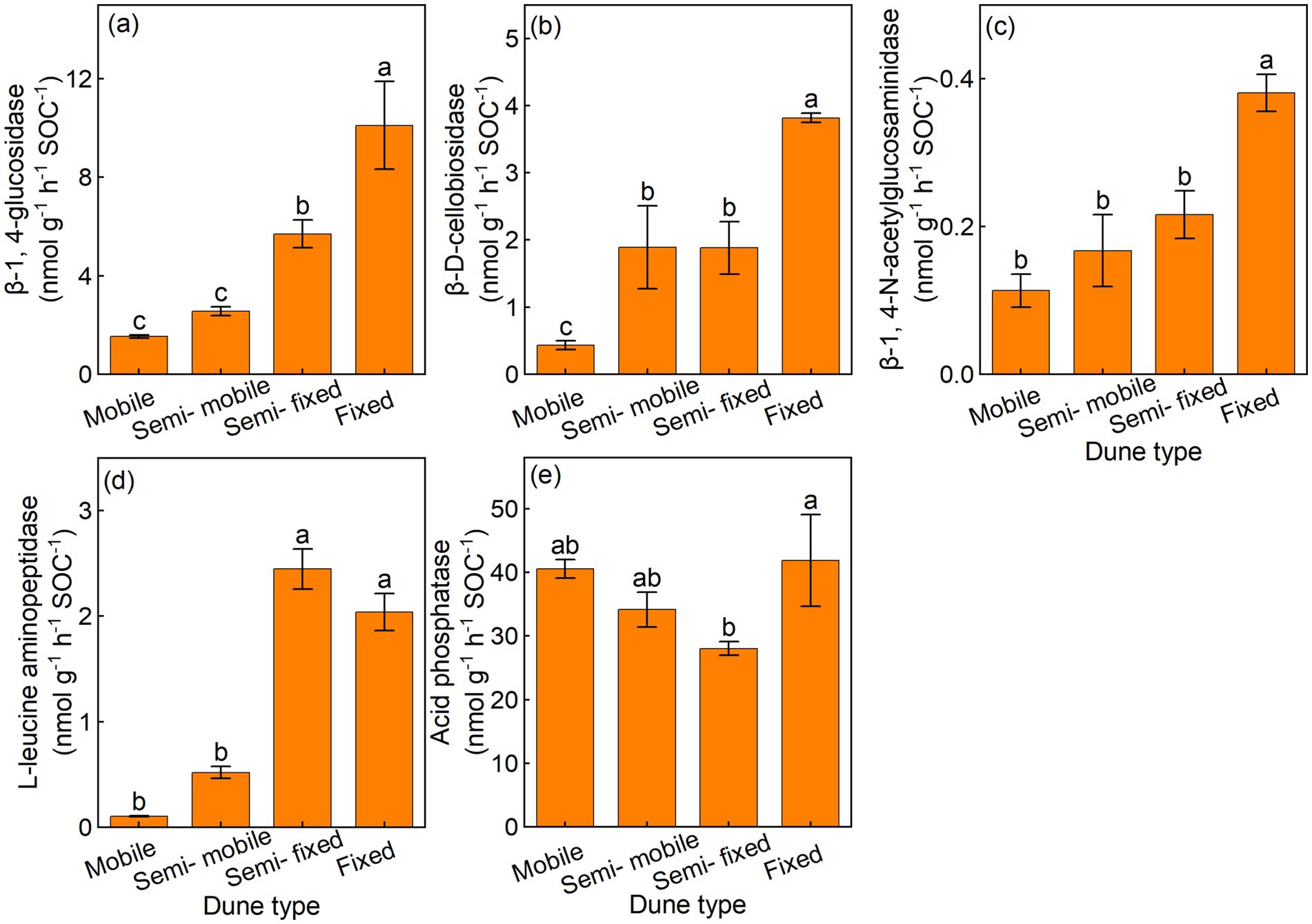
Figure 2. Mean (±) of soil-specific enzyme activity per unit of soil organic carbon in (a) β-1, 4-glucosidase (BG/SOC), (b) β-D-cellobiosidase (CBH/SOC), (c) β-1, 4-N-acetylglucosaminidase (NAG/SOC), (d) L-leucine aminopeptidase (LAP/SOC), and (e) acid phosphatase (AP/SOC) of different dune types.
Additionally, the semi-fixed and fixed dunes had higher BG/MBC and LAP/MBC, compared with the semi-mobile and mobile dunes (Figures 3a,d). The CBH/MBC and NAG/MBC were lowest in mobile dunes, and the semi-fixed and fixed dunes had lower AP/MBC, compared with the semi-mobile and mobile dunes (Figures 3b,c,e).
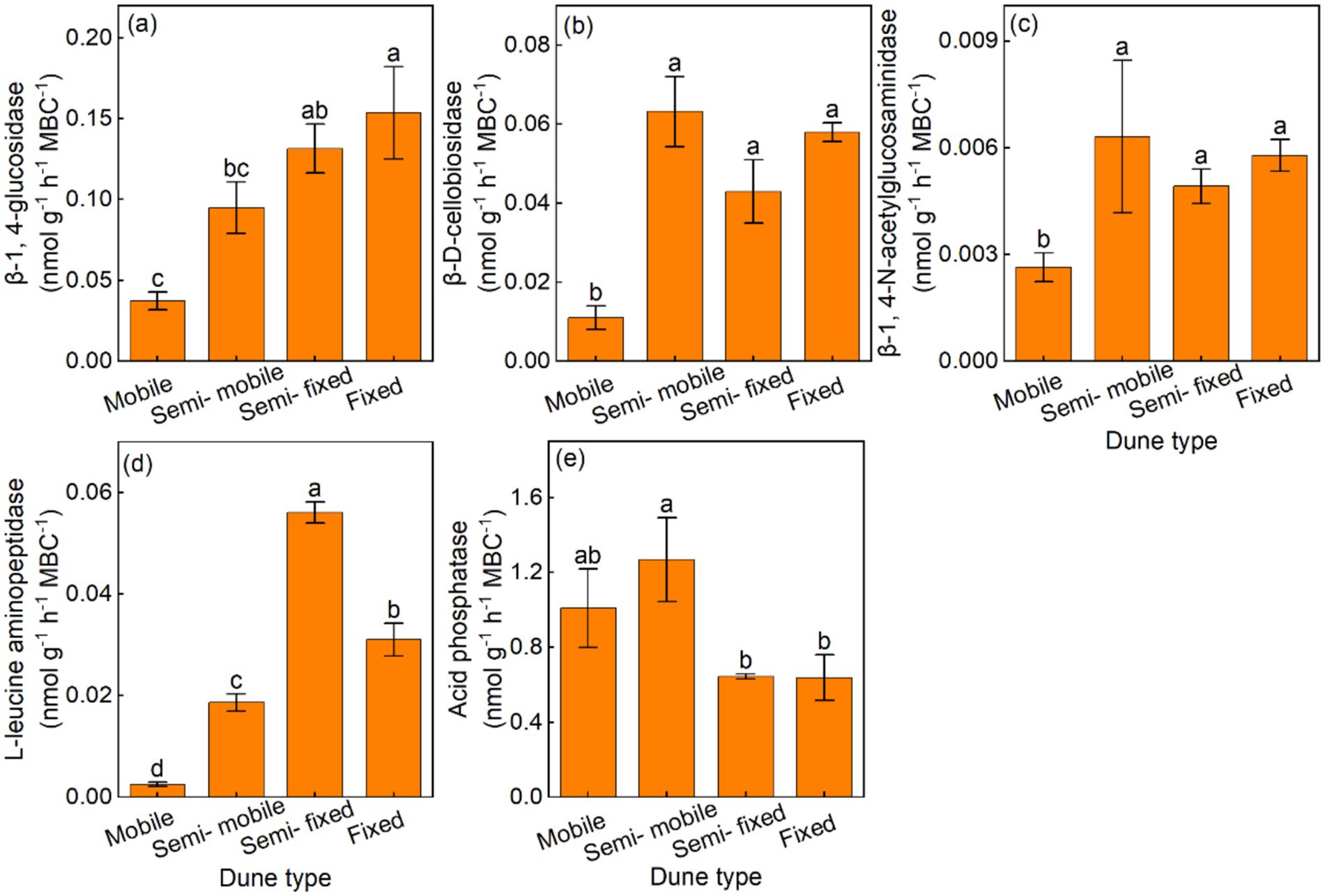
Figure 3. Mean (±) of soil-specific enzyme activity per unit of microbial biomass carbon in (a) β-1, 4-glucosidase (BG/MBC), (b) β-D-cellobiosidase (CBH/MBC), (c) β-1, 4-N-acetylglucosaminidase (NAG/MBC), (d) L-leucine aminopeptidase (LAP/MBC), and (e) acid phosphatase (AP/MBC) of different dune types.
3.2 Dynamics of the SEAC and GMEA
The fixed dunes had higher BG coefficients than other dune types, whereas the mobile dunes had lower CBH, NAG, and LAP coefficients than other dune types (Figures 4a–d). The GMEA of the fixed dunes was approximately twice that of the semi-fixed dunes, four-fold that of the semi-mobile, and 20 times that of the mobile dunes (Figure 5a).
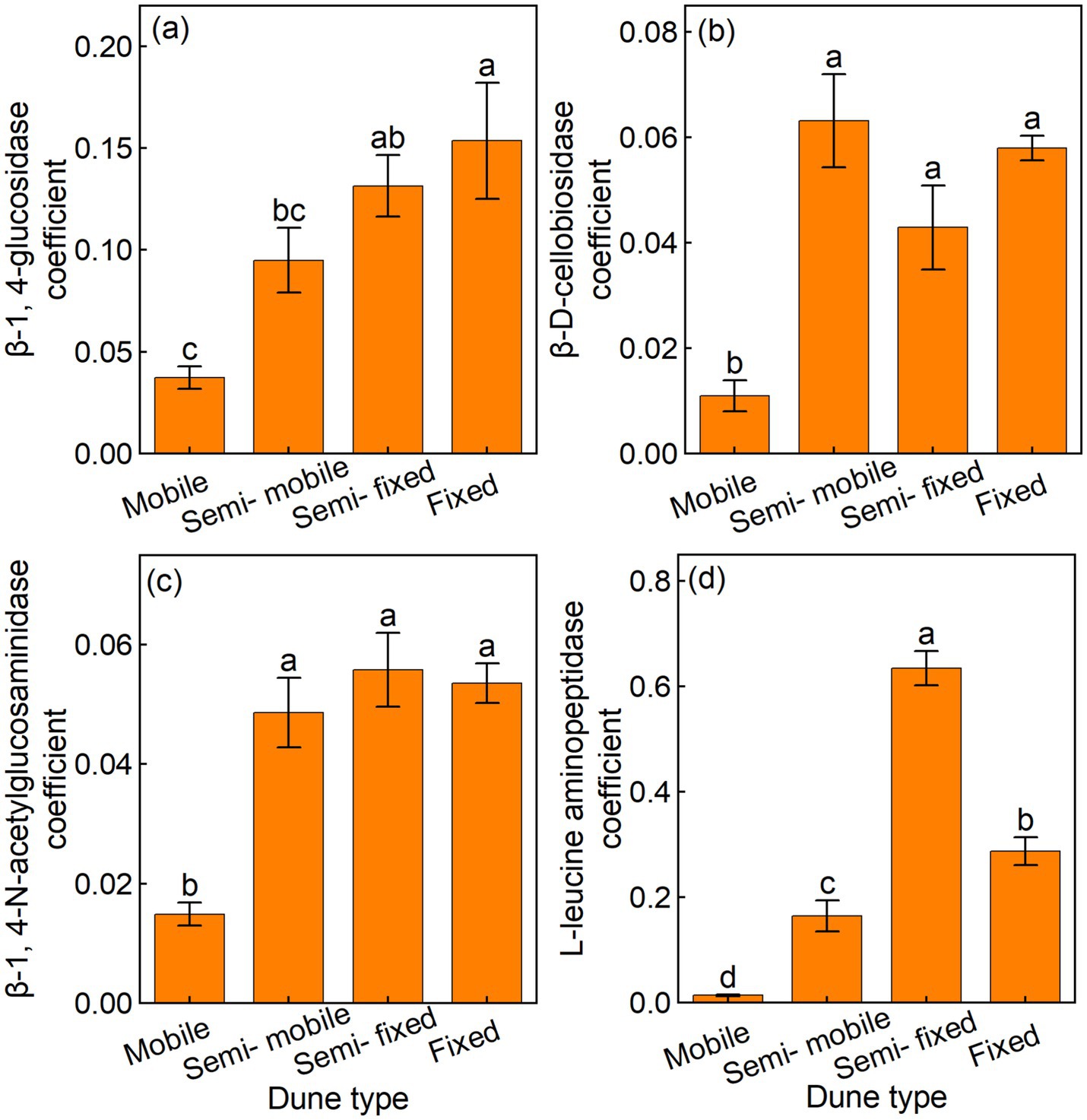
Figure 4. Mean (±) of soil enzyme activity coefficient in (a) β-1, 4-glucosidase (BG), (b) β-D-cellobiosidase (CBH), (c) β-1, 4-N-acetylglucosaminidase (NAG), and (d) L-leucine aminopeptidase (LAP) of different dune types.
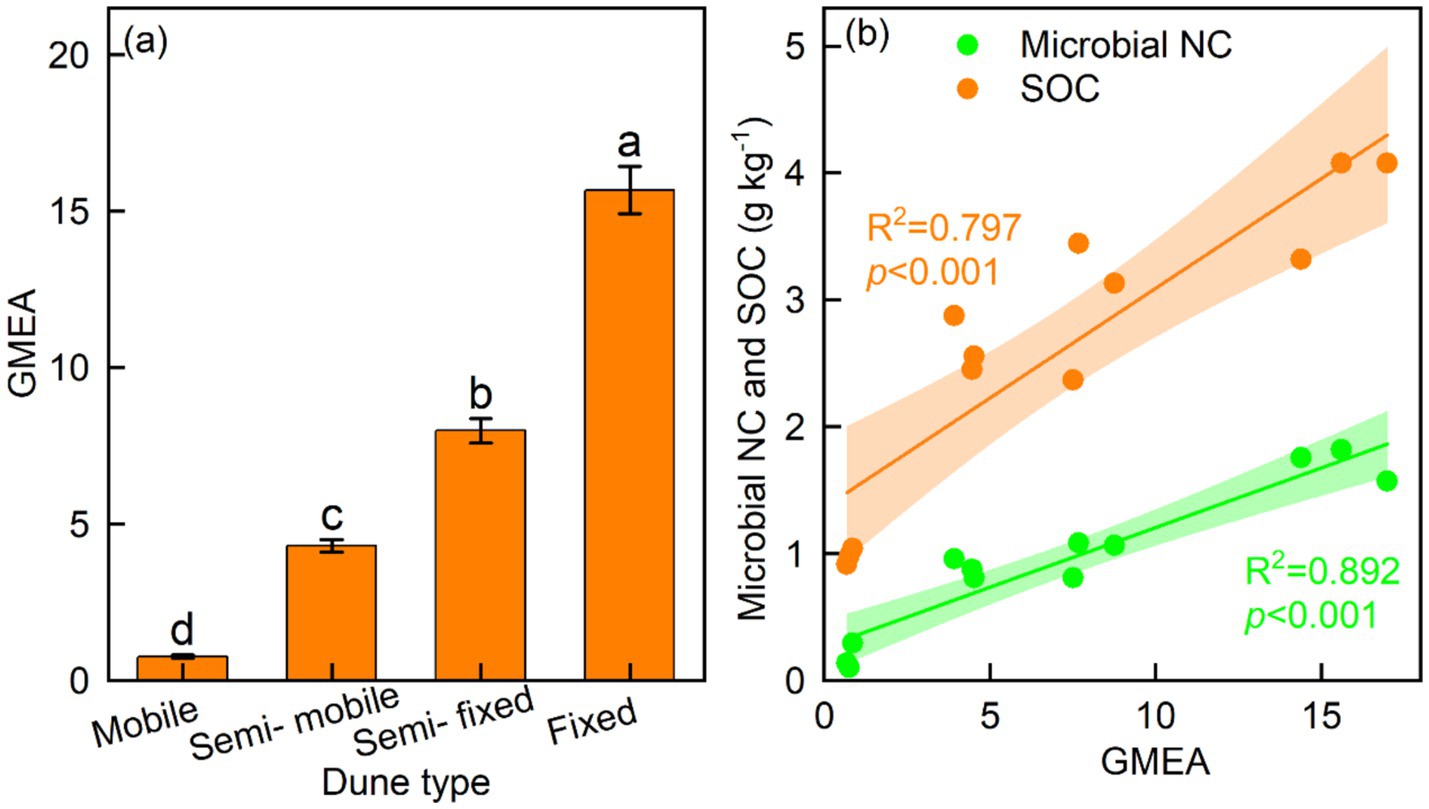
Figure 5. Mean (± SE) of (a) geometric mean of enzyme activity (GMEA) and (b) relationship of GMEA with soil microbial necromass carbon (NC) and soil organic carbon (SOC).
3.3 Correlations of soil-specific enzyme activity with plant and soil variables
The SOCE was positively correlated with the MBCE and the enzyme activity coefficients overall. The SOCE and MBCE were positively correlated with the plant diversity indices (i.e., the Shannon–Weiner diversity, Pielou evenness, and Margalef richness indices), soil nutrients (i.e., TN and TP), and soil microbial properties (i.e., MBC, MBN, bacterial diversity, bacterial richness, fungal diversity, and fungal richness) (Table 1).
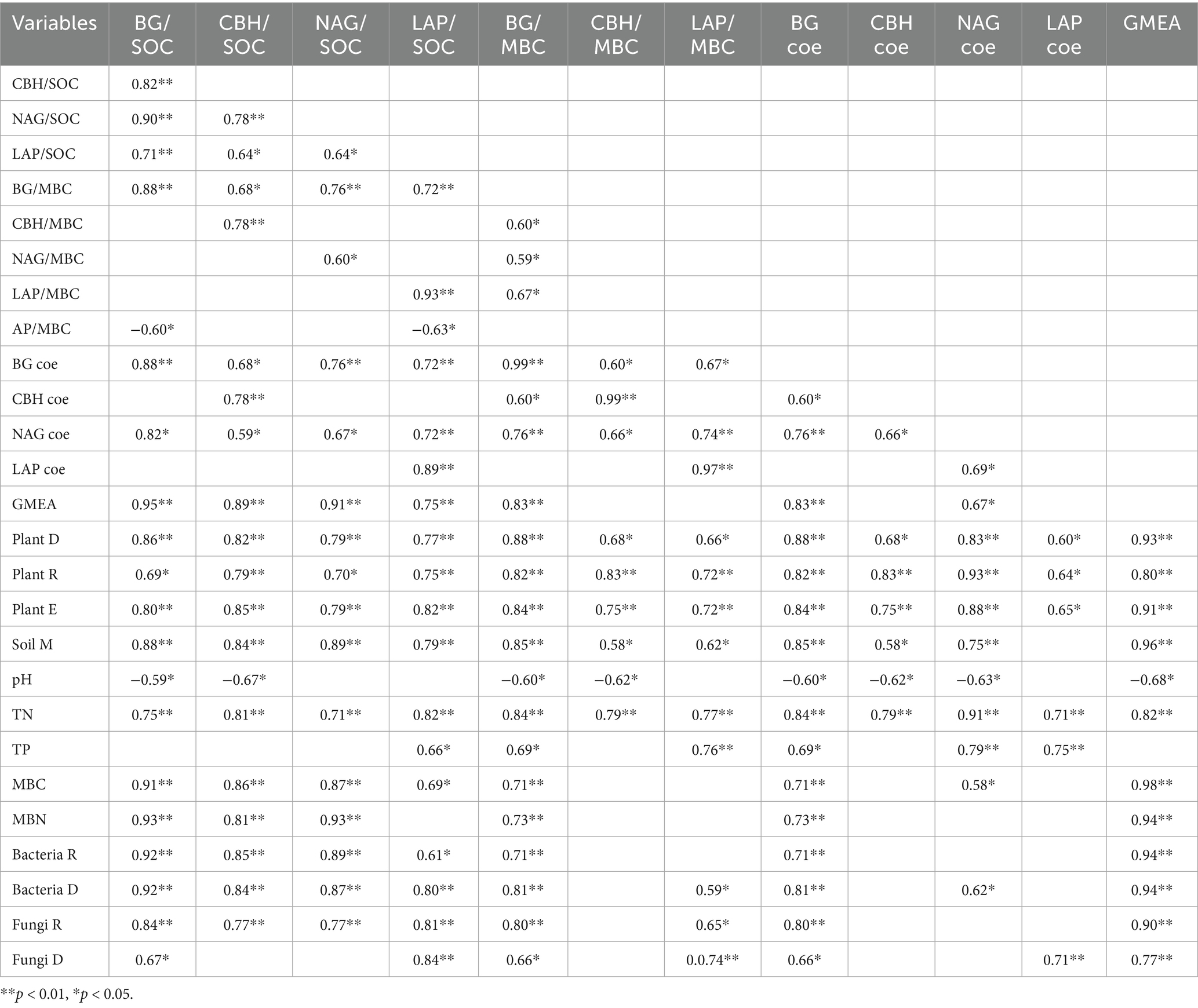
Table 1. Pearson’s correlation of soil extracellular enzyme activity coefficient with plant and soil variables.
3.4 Correlations of soil-specific enzyme activity with microbial NC and SOC
The BG/SOC, CBH/SOC, NAG/SOC, LAP/SOC, BG/MBC, and CBH/MBC were linearly related to microbial NC and SOC, whereas NAG/MBC was quadratically related to microbial NC and SOC (Figures 6a–g). LAP/MBC was linearly related to SOC, whereas LAP/MBC was quadratically related to microbial NC (Figure 6h). The BG, CBH, and NAG coefficients were linearly related to microbial NC and SOC, while the LAP coefficient was quadratically related to microbial NC and SOC (Figures 7a–d). In addition, GMEA was linearly related to microbial NC and SOC (Figure 5b).
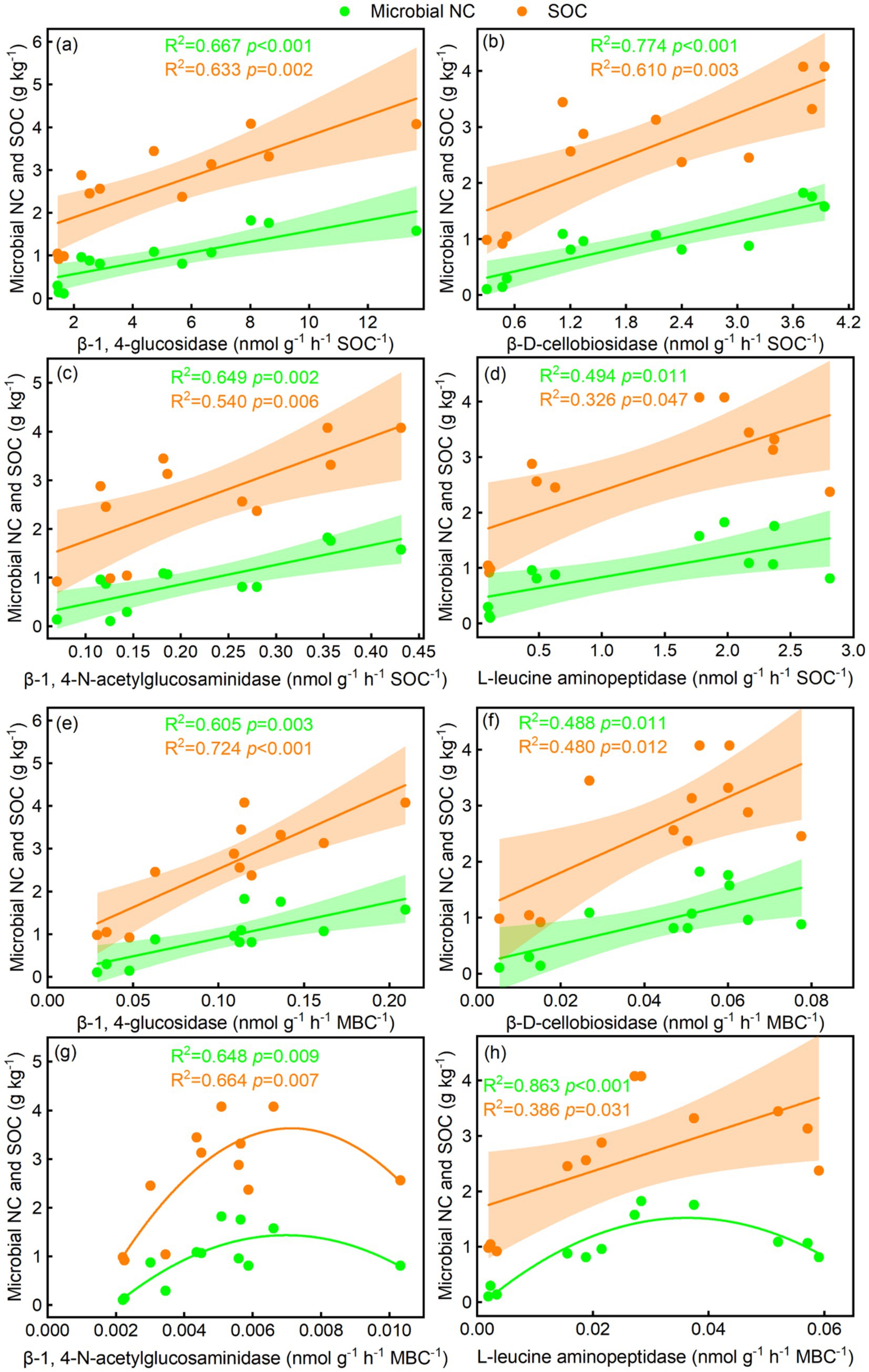
Figure 6. Relationships of soil-specific enzyme activity per unit of (a–d) soil organic carbon and (e–h) microbial biomass carbon with soil microbial necromass carbon (NC) and SOC.
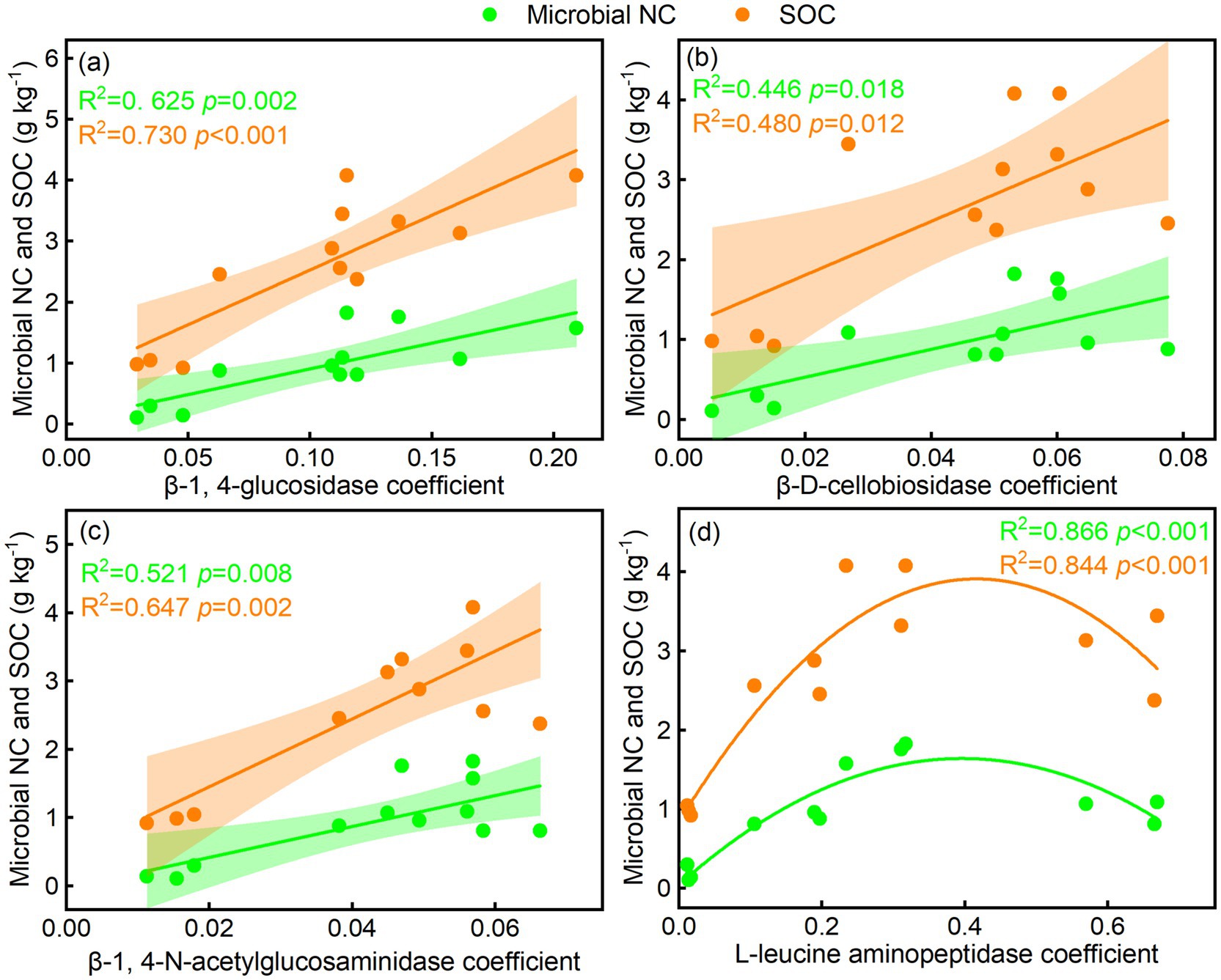
Figure 7. Relationships of soil enzyme activity coefficient with (a–d) soil microbial necromass carbon (NC) and soil organic carbon (SOC).
The microbial NC was mainly influenced by BG/SOC (6.7%), plant evenness (6.7%), bacterial richness (6.5%), GMEA (6.5%), plant richness (6.4%), MBC (6.2%), plant diversity (6.2%), soil moisture (6.1%), CBH/SOC (5.8%), bacterial diversity (5.6%), fungal richness (5.1%), TN (5.0%), the LAP coefficient (4.6%), the BG coefficient (4.6%), and LAP/SOC (4.0%), which cumulatively explained 86.0% of the variance in microbial NC (Figure 8a). The SOC was mainly influenced by fungal richness (7.1%), plant richness (5.7%), BG/SOC (5.2%), the LAP coefficient (5.1%), GMEA (5.1%), soil moisture (M) (4.8%), bacterial richness (4.8%), bacterial diversity (4.7%), fungal diversity (4.6%), LAP/SOC (4.5%), LAP/MBC (4.2%), and TN (4.1%), which cumulatively explained 59.9% of the variance in SOC (Figure 8b).
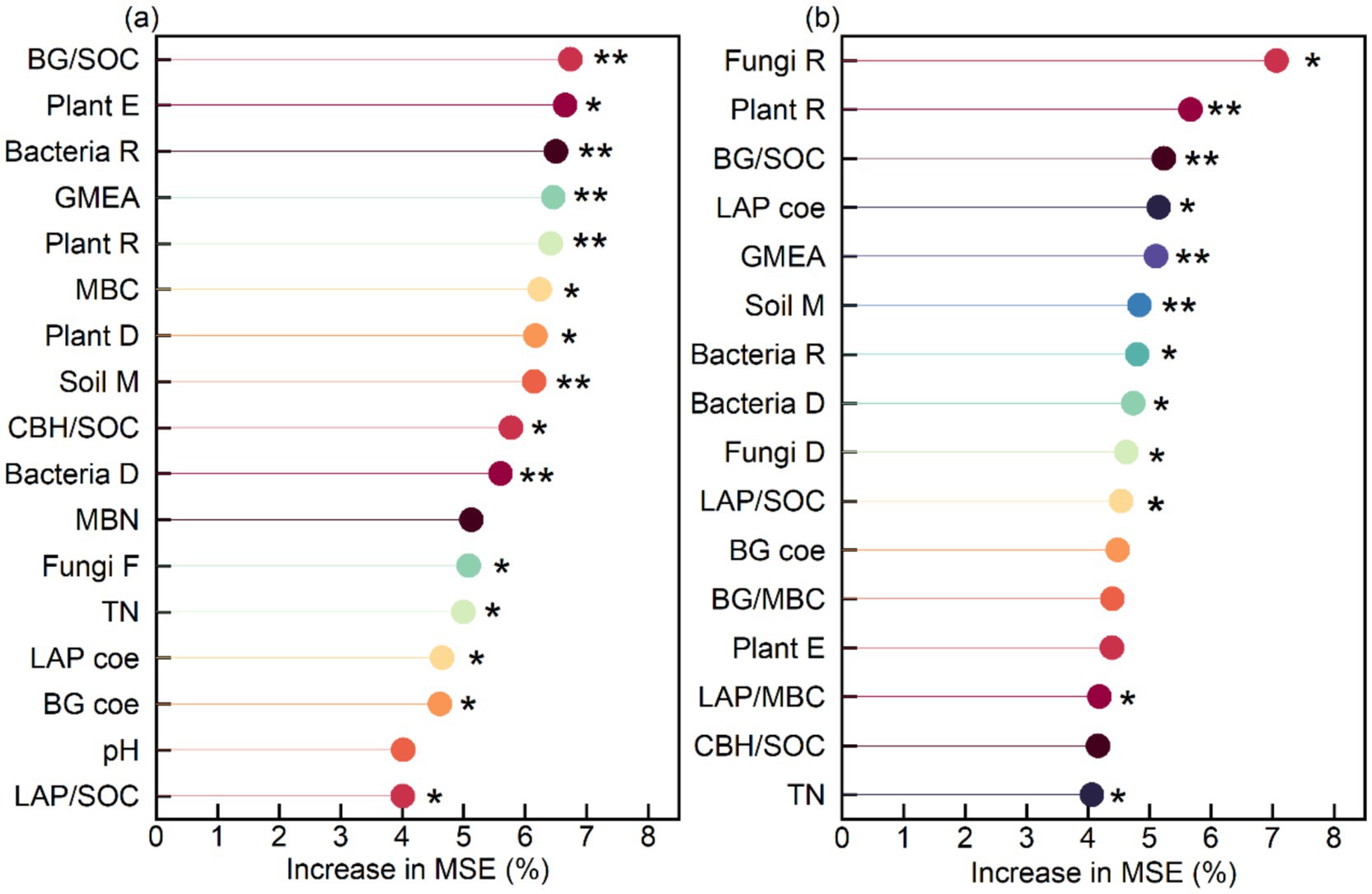
Figure 8. Main predictor importance (% of increase of MSE) of soil-specific enzyme activity and soil variables on (a) soil microbial necromass carbon and (b) soil organic carbon by random forest modeling analysis. **p < 0.01, *p < 0.05.
4 Discussion
4.1 Effect of dune fixation on SOCE
The traditional absolute enzyme activity (SAE) appears to be insufficient to characterize the accumulation of SOC (Xu et al., 2020). Consequently, SOCE has been widely used to characterize soil carbon turnover (Zhang et al., 2015; Xiao et al., 2021). As indicated by our hypothesis, sand fixation (ecological restoration) promoted an overall increase in SOCE (except for AP), indicating an increase in the enzyme production capacity of soil microorganisms. Although both SOC and soil absolute enzymes had positive effects on dune fixation, the percentage change in soil absolute enzymes (except AP) was higher than that of SOC, which led to an increase in SOCE.
There are three possible mechanisms for this: (1) Dune fixation increases plant diversity (Supplementary Table S2) and plant biomass (Qiao et al., 2012), which increases the production of dead leaves that enter the soil and provide energy and food for microorganisms (Cheng et al., 2022), thereby increasing SAE in the soil (Supplementary Table S2). (2) Differences in vegetation types caused by dune fixation significantly affect the connections between plant rhizospheres, thereby improving the soil porosity, soil bulk density, and aggregates (Alamusa et al., 2023). Furthermore, the increase in plant diversity and coverage leads to the secretion of large amounts of organic matter from the root system, which improves the soil physical structure (Jones et al., 2004; Liu et al., 2005; Liu et al., 2009; Cao et al., 2017) and increases the microbial activity (Supplementary Table S2), enzyme secretion, and SOCE (Xu et al., 2020). (3) The plant cover acts as a shade for the soil surface and effectively reduces the impact of rain and wind erosion, thereby protecting the environmental conditions of the soil (Cao M. et al., 2024; Xu et al., 2024), and providing a stable environment for the secretion of soil enzymes, leading to a higher SOCE. In addition, we were surprised to find that the SOCE of phosphatase enzymes was different from that of carbon and nitrogen enzymes, showing that AP/SOC in semi-fixed dunes was lower than that in mobile and semi-mobile dunes. This may be attributed to the efficiency of the phosphatases released by microorganisms decreasing during the late recovery period (Li et al., 2018), whereas SOC continued to increase (Xu et al., 2021), thereby reducing the AP/SOC. Overall, this study provides direct evidence that SOCE is sensitive to dune fixation and shows potential as a sensitive index for characterizing SOC accumulation.
4.2 Effect of dune fixation on MBCE, SEAC, and GMEA
The percentage increase in MBC during dune fixation was smaller than that of enzyme activity (Supplementary Table S1), which, in turn, led to an increase in MBCE (except for AP), confirming our hypothesis. The MBCE characterizes the metabolic activity and catalytic efficiency of enzymes produced by microbial communities (Waldrop et al., 2000). The changes in MBCE were consistent with the changes in soil nutrient content (Supplementary Table S2), indicating that, with the accumulation of soil nutrients, the metabolic activity and enzyme production ability of soil microorganisms gradually increased. During dune fixation, changes in plant diversity and biomass, accompanied by changes in soil physical and chemical properties, are important causes of changes in soil enzyme activity (Cheng et al., 2022; Naeimi et al., 2023). The increase in plant productivity produces more litter, which is conducive to the improvement of soil nutrient accumulation (Taylor et al., 2024; Yang et al., 2024). Soils with adequate nutrients tend to have higher soil microbial diversity and activity, which in turn enhances enzyme secretion (Moharana et al., 2024; Zhang et al., 2024), thereby increasing the MBCE (Raiesi and Beheshti, 2015). This conclusion was confirmed by the positive correlations between MBCE and plant diversity, soil nutrient content, and microbial diversity (Table 1). However, other studies have indicated that ecological restoration reduces MBCE (Raiesi and Salek-Gilani, 2018; Xu et al., 2020). The reason for these differences may be related to differences in the ecological community types. The research of Raiesi and Salek-Gilani (2018) and Xu et al. (2020) investigated an area with relatively abundant rainfall and found that the growth and reproduction of plants and microorganisms were less affected by water (Ghorbani et al., 2023). By contrast, this study was conducted in an extremely arid environment (with an average annual rainfall of 250–350 mm), and the reproduction of microorganisms was limited by water, resulting in a more significant impact of ecological restoration on microorganisms (Chang et al., 2024; Zhao et al., 2024). This study indicated that dune fixation significantly increased the metabolic activity of soil microorganisms and increased the production of phosphatase enzymes; MBCE was a sensitive index reflecting the interaction between soil enzyme activity and microorganisms.
The soil enzyme activity coefficient and GMEA are common indices for integrating soil enzyme data and information and are also important indicators of soil quality (Hinojosa et al., 2004). Dune fixation significantly increased the soil enzyme activity coefficient and GMEA. The increase in the GMEA was mainly due to an increase in enzyme activity associated with increased dune fixation. As explained earlier, dune fixation increases plant diversity and productivity (Qiao et al., 2012), leading to increased litter production and root turnover, which, in turn, leads to increased soil organic carbon and nutrient content (Cao M. et al., 2024). The increase in the organic substrates available to microorganisms enhances the activity of soil microorganisms and accelerates the secretion of enzymes (Cao et al., 2017; Naeimi et al., 2023). Therefore, overall, dune fixation accelerated the production of soil enzymes in this arid ecosystem.
4.3 Relationships of soil-specific enzyme activity with microbial NC and SOC
Absolute soil enzymes can explain the accumulation of microbial NC and SOC (Bai et al., 2024; Raza et al., 2024). However, the precise relationships of soil-specific enzyme activity with microbial NC and SOC are still not fully understood. Our study found a significant correlation between SOCE, MBCE, the enzyme activity coefficient, and GMEA with microbial NC and SOC. Additionally, dune fixation promoted the accumulation of microbial NC and SOC (Supplementary Table S2), indicating that higher soil-specific enzyme activity supports this process.
Although earlier studies have identified microbial traits as major drivers of microbial NC accumulation (Han et al., 2024), our study indicated that soil-specific enzyme activity accounted for up to 32.2% of the variations in microbial NC, compared to 6.7% for BG/SOC. The contributions of plant diversity and microbial communities were 19.3 and 23.4%, respectively. For SOC, soil-specific enzyme activity, plant diversity, and microbial community composition accounted for 24.1, 5.7, and 21.2%, respectively. These observations suggest that soil-specific enzymatic activity is crucial for accumulating both microbial NC and SOC. We highlight two key mechanisms behind these results. First, an increase in soil-specific enzyme activity can modulate the hydrothermal conditions and physical structure of the soil (Bharti et al., 2024), which affects the accumulation process of soil carbon. Second, vegetation input directly influences the dynamic changes in SOC (Zhou et al., 2019). However, both the quantity and quality of plant litter affect how microorganisms use substrates (Shigyo et al., 2024), thus influencing SOC distribution (Wiesmeier et al., 2019). Higher soil-specific enzyme activity supports plant growth and promotes the accumulation of both aboveground and subsurface biomass (Yu et al., 2019), leading to more plant litter and further promoting the accumulation of microbial NC and SOC (Xiao et al., 2021). In addition, soil-specific enzyme activity enhances the carbon use efficiency of soil microorganisms through both direct effects (enhanced microbial activity) and indirect effects (affecting root growth and its secretions) (Liu et al., 2017). Therefore, in arid regions, dune fixation produces higher aboveground biomass and litter input, collectively promoting soil microbial NC and SOC accumulation through increased root growth and more active microbial processes.
5 Conclusion
Our study confirmed that dune fixation increased the soil-specific enzyme activity (including SOCE and MBCE), the enzyme activity coefficient, and GMEA; among them, the GMEA of the fixed dunes was approximately twice that of the semi-fixed dunes, four-fold that of the semi-mobile, and 20 times that of the mobile dunes. This was mainly attributed to the increase in plant diversity, plant biomass, soil moisture, and soil nutrients. Moreover, SOCE and MBCE were significantly correlated with microbial NC and SOC. Although soil microbial communities and plant diversity largely influenced microbial NC and SOC, soil-specific enzyme activity explained even more variation, accounting for 32.2% of microbial NC and 24.1% of SOC. Therefore, this study provides strong evidence that SOCE and MBCE are sensitive indicators in responses to dune restoration, making them useful for explaining changes in microbial NC and SOC during ecological restoration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QQ: Investigation, Conceptualization, Funding acquisition, Writing – review & editing, Methodology, Data curation, Writing – original draft. XH: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Philosophy and Social Key Laboratory of Monitoring and Assessing for Rural Land Utilization, Chengdu Normal University (NDZDS202504) and the Talent Recruiting Program of Chengdu Normal University (YJRC202447).
Acknowledgments
Thanks to Baihui Organisms (http://m.baihuitech.cn/) for providing the amino sugar and microbial community determination.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1687297/full#supplementary-material
Footnotes
References
Alamusa, Y., Su, Y., Yin, J., Zhou, Q., and Wang, Y. (2023). Effect of sand-fixing vegetation on the hydrological regulation function of sand dunes and its practical significance. J. Arid. Land 15, 52–62. doi: 10.1007/s40333-023-0002-y
Bai, X., Zhai, G., Zhai, Y., Li, H., An, S., Rafiq, A., et al. (2024). Mechanism of microbial necromass formation during decomposition of Stipa bungeana above-ground residues. Catena 245:108283. doi: 10.1016/j.catena.2024.108283
Bharti, P., Das, A., Kumar, S., and Rakshit, R. (2024). Assessment of soil specific enzyme activities in aggregates size fractions: a case study from subtropical agro-ecosystem. Eurasian Soil Sci. 57, 646–656. doi: 10.1134/S1064229323602627
Bremner, J. (1982). “Nitrogen - total” in Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, A. L. Page, R. H. Miller, and D. R. Keeney (Madison, WI: American Society of Agronomy) vol. 72, 532–535.
Buckeridge, K. M., Creamer, C., and Whitaker, J. (2022). Deconstructing the microbial necromass continuum to inform soil carbon sequestration. Funct. Ecol. 36, 1396–1410. doi: 10.1111/1365-2435.14014
Camenzind, T., Mason-Jones, K., Mansour, I., Rillig, M. C., and Lehmann, J. (2023). Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat. Geosci. 16, 115–122. doi: 10.1038/s41561-022-01100-3
Cao, M., Lu, P., Ma, F., Yang, L., Yu, J., Xia, Z., et al. (2024). Holocene aeolian environmental dynamics in fixed and semi-fixed deserts over the arid Central Asia revealed by comprehensive sand-dune records. Catena 246:108368. doi: 10.1016/j.catena.2024.108368
Cao, C., Zhang, Y., and Cui, Z. (2024). Successions of bacterial and fungal communities in biological soil crust under sand-fixation plantation in Horqin Sandy land, Northeast China. Forests 15:1631. doi: 10.3390/f15091631
Cao, C., Zhang, Y., Cui, Z., Feng, S., Wang, T., and Ren, Q. (2017). Soil bacterial community responses to revegetation of moving sand dune in semi-arid grassland. Appl. Microbiol. Biotechnol. 101, 6217–6228. doi: 10.1007/s00253-017-8336-z
Chang, W., Song, Q., Zheng, X., Li, C., Wang, L., Li, H., et al. (2024). Leaf trait variations and correlations across four forests with similar mean annual precipitation in northern China. Ecol. Indic. 165:112199. doi: 10.1016/j.ecolind.2024.112199
Chen, Y., Gao, G., Wang, L., Ding, G., Zhang, Y., and Zhao, Y. (2022). Wind erodibility of Arenosols and its driving factors during sand dune fixation: a wind tunnel experiment. Catena 214:106237. doi: 10.1016/j.catena.2022.106237
Cheng, J., Zhang, Y., Wang, H., Cui, Z., and Cao, C. (2022). Sand-fixation plantation type affects soil phosphorus transformation microbial community in a revegetation area of Horqin Sandy land, Northeast China. Ecol. Eng. 180:106644. doi: 10.1016/j.ecoleng.2022.106644
German, D. P., Weintraub, M. N., Grandy, A. S., Lauber, C. L., Rinkes, Z. L., and Allison, S. D. (2011). Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43, 1387–1397. doi: 10.1016/j.soilbio.2011.03.017
Ghorbani, M., Amirahmadi, E., Konvalina, P., Moudry, J., Kopecky, M., and Hoang, T. N. (2023). Carbon pool dynamic and soil microbial respiration affected by land use alteration: a case study in humid subtropical area. Land 12:459. doi: 10.3390/land12020459
Guo, Z., Zhang, H., Li, J., Chen, T., Wang, H., and Zhang, Y. (2023). Distribution of soil microorganisms in different complex soil layers in mu us sandy land. PLoS One 18:e0283341. doi: 10.1371/journal.pone.0283341
Han, B., Yao, Y., Wang, Y., Su, X., Ma, L., Chen, X., et al. (2024). Microbial traits dictate soil necromass accumulation coefficient: a global synthesis. Glob. Ecol. Biogeogr. 33, 151–161. doi: 10.1111/geb.13776
Hinojosa, M. B., García-Ruíz, R., Viñegla, B., and Carreira, J. A. (2004). Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcollar toxic spill. Soil Biol. Biochem. 36, 1637–1644. doi: 10.1016/j.soilbio.2004.07.006
Jones, M. L. M., Wallace, H. L., Norris, D., Brittain, S. A., Haria, S., Jones, R. E., et al. (2004). Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition. Plant Biol. 6, 598–605. doi: 10.1055/s-2004-821004
Kéfi, S., Rietkerk, M., Alados, C. L., Pueyo, Y., Papanastasis, V. P., ElAich, A., et al. (2007). Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature 449, 213–217. doi: 10.1038/nature06111
Li, Y., Cao, Z., Long, H., Liu, Y., and Li, W. (2017). Dynamic analysis of ecological environment combined with land cover and NDVI changes and implications for sustainable urban-rural development: the case of mu us Sandy land, China. J. Clean. Prod. 142, 697–715. doi: 10.1016/j.jclepro.2016.09.011
Li, J., Tong, X., Awasthi, M. K., Wu, F., Ha, S., Ma, J., et al. (2018). Dynamics of soil microbial biomass and enzyme activities along a chronosequence of desertified land revegetation. Ecol. Eng. 111, 22–30. doi: 10.1016/j.ecoleng.2017.11.006
Li, W., Wang, J., Jiang, L., Lv, G., Hu, D., Wu, D., et al. (2023). Rhizosphere effect and water constraint jointly determined the roles of microorganism in soil phosphorus cycling in arid desert regions. Catena 222:106809. doi: 10.1016/j.catena.2022.106809
Liang, C., Amelung, W., Lehmann, J., and Kaestner, M. (2019). Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 25, 3578–3590. doi: 10.1111/gcb.14781
Liu, X., Guo, K., Huang, L., Ji, Z., Jiang, H., Li, H., et al. (2017). Responses of absolute and specific enzyme activity to consecutive application of composted sewage sludge in a Fluventic Ustochrept. PLoS One 12:e0177796. doi: 10.1371/journal.pone.0177796
Liu, L. Y., Skidmore, E., Hasi, E., Wagner, L., and Tatarko, J. (2005). Dune sand transport as influenced by wind directions, speed and frequencies in the Ordos plateau, China. Geomorphology 67, 283–297. doi: 10.1016/j.geomorph.2004.10.005
Liu, R., Zhao, H., Zhao, X., Zuo, X., and Drake, S. (2009). Soil macrofaunal response to sand dune conversion from mobile dunes to fixed dunes in Horqin sandy land, northern China. Eur. J. Soil Biol. 45, 417–422. doi: 10.1016/j.ejsobi.2009.06.006
Miao, Y., Jin, H., and Cui, J. (2016). Human activity accelerating the rapid desertification of the mu us Sandy lands, North China. Sci. Rep. 6:23003. doi: 10.1038/srep23003
Moharana, T., Patnaik, A., Mishra, C. S. K., Behera, B. P., and Samal, R. R. (2024). High-density polyethylene microplastics in agricultural soil: impact on microbes, enzymes, and carbon-nitrogen ratio. J. Environ. Qual. 53, 711–726. doi: 10.1002/jeq2.20610
Naeimi, M., Chu, J., Khosroshahi, M., and Zenouzi, L. K. (2023). Soil stabilization for dunes fixation using microbially induced calcium carbonate precipitation. Geoderma 429:116183. doi: 10.1016/j.geoderma.2022.116183
Nannipieri, P., Giagnoni, L., Renella, G., Puglisi, E., Ceccanti, B., Masciandaro, G., et al. (2012). Soil enzymology: classical and molecular approaches. Biol. Fertil. Soils 48, 743–762. doi: 10.1007/s00374-012-0723-0
Nelson, D., and Sommers, L. (1982). “Total carbon, organic carbon and organic matter” in Methods of Soil Analysis Part 2. Chemical and Microbial Properties. Madison, WI: ASA-SSSA.
Peters, D. P. C., Yao, J., Sala, O. E., and Anderson, J. P. (2012). Directional climate change and potential reversal of desertification in arid and semiarid ecosystems. Glob. Change Biol. 18, 151–163. doi: 10.1111/j.1365-2486.2011.02498.x
Qiao, J., Zhao, W., Xie, X., Liu, G., Ye, X., Chu, Y., et al. (2012). Variation in plant diversity and dominance across dune fixation stages in the Chinese steppe zone. J. Plant Ecol. 5, 313–319. doi: 10.1093/jpe/rtr032
Raiesi, F., and Beheshti, A. (2014). Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of Northwest Iran. Appl. Soil Ecol. 75, 63–70. doi: 10.1016/j.apsoil.2013.10.012
Raiesi, F., and Beheshti, A. (2015). Microbiological indicators of soil quality and degradation following conversion of native forests to continuous croplands. Ecol. Indic. 50, 173–185. doi: 10.1016/j.ecolind.2014.11.008
Raiesi, F., and Salek-Gilani, S. (2018). The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 126, 140–147. doi: 10.1016/j.apsoil.2018.02.022
Raza, S., Sommer, R., and Margenot, A. J. (2024). Do soil enzyme activities explain stimulated carbon mineralization following liming? Soil Biol. Biochem. 194:109416. doi: 10.1016/j.soilbio.2024.109416
Shigyo, N., Umeki, K., and Hirao, T. (2024). Soil microbial identity explains home-field advantage for litter decomposition. New Phytol. 243, 2146–2156. doi: 10.1111/nph.19769
Stone, M. M., DeForest, J. L., and Plante, A. F. (2014). Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo critical zone observatory. Soil Biol. Biochem. 75, 237–247. doi: 10.1016/j.soilbio.2014.04.017
Taylor, K. M., Nelsen, T. S., Scow, K. M., and Lundy, M. E. (2024). No-till annual wheat increases plant productivity, soil microbial biomass, and soil carbon stabilization relative to intermediate wheatgrass in a Mediterranean climate. Soil Tillage Res. 235:105874. doi: 10.1016/j.still.2023.105874
Trasar-Cepeda, C., Leirós, M. C., and Gil-Sotres, F. (2008). Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 40, 2146–2155. doi: 10.1016/j.soilbio.2008.03.015
Vance, E. D., Brookes, P. C., and Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 19, 703–707. doi: 10.1016/0038-0717(87)90052-6
Waldrop, M. P., Balser, T. C., and Firestone, M. K. (2000). Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 32, 1837–1846. doi: 10.1016/S0038-0717(00)00157-7
Wiesmeier, M., Urbanski, L., Hobley, E., Lang, B., von Luetzow, M., Marin-Spiotta, E., et al. (2019). Soil organic carbon storage as a key function of soils - a review of drivers and indicators at various scales. Geoderma 333, 149–162. doi: 10.1016/j.geoderma.2018.07.026
Xiang, X., De, K., Lin, W., Feng, T., Li, F., and Wei, X. (2024). Indirect influence of soil enzymes and their stoichiometry on soil organic carbon response to warming and nitrogen deposition in the Tibetan plateau alpine meadow. Front. Microbiol. 15:1381891. doi: 10.3389/fmicb.2024.1381891
Xiao, L., Liu, G., Li, P., and Xue, S. (2021). Dynamics of soil specific enzyme activities and temperature sensitivities during grassland succession after farmland abandonment. Catena 199:105081. doi: 10.1016/j.catena.2020.105081
Xu, H., Qu, Q., Chen, Y., Liu, G., and Xue, S. (2021). Responses of soil enzyme activity and soil organic carbon stability over time after cropland abandonment in different vegetation zones of the loess plateau of China. Catena 196:104812. doi: 10.1016/j.catena.2020.104812
Xu, H., Qu, Q., Lu, B., Li, P., Xue, S., and Liu, G. (2020). Response of soil specific enzyme activity to vegetation restoration in the loess hilly region of China. Catena 191:104564. doi: 10.1016/j.catena.2020.104564
Xu, H., Qu, Q., Xue, S., and Wang, M. (2024). A global analysis of the effects of forest thinning on soil N stocks and dynamics. Catena 246:108411. doi: 10.1016/j.catena.2024.108411
Xu, S., Zhang, L., Zhou, L., Mi, J., McLaughlin, N. B., and Liu, J. (2016). Effect of synthetic and natural water absorbing soil amendments on soil microbiological parameters under potato production in a semi-arid region. Eur. J. Soil Biol. 75, 8–14. doi: 10.1016/j.ejsobi.2016.04.002
Yang, Y., Yang, J., Dong, Q., Li, D., Tan, B., Wu, Q., et al. (2024). Heavy nitrogen application rate and long-term duration decrease the soil organic carbon and nitrogen sequestration rates in forest ecosystems. Forests 15:1585. doi: 10.3390/f15091585
Yu, P., Tang, X., Zhang, A., Fan, G., and Liu, S. (2019). Responses of soil specific enzyme activities to short-term land use conversions in a salt-affected region, northeastern China. Sci. Total Environ. 687, 939–945. doi: 10.1016/j.scitotenv.2019.06.171
Zhang, X., Dong, W., Dai, X., Schaeffer, S., Yang, F., Radosevich, M., et al. (2015). Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci. Total Environ. 536, 59–67. doi: 10.1016/j.scitotenv.2015.07.043
Zhang, L., Xiong, S., Shen, Y., You, C., Li, H., Wang, L., et al. (2024). Changes in soil microbial community and function across stand age of Cryptomeria japonica var. sinensis plantations in subtropical China. Appl. Soil Ecol. 203:105645. doi: 10.1016/j.apsoil.2024.105645
Zhao, S., van der Heijden, M. G. A., Banerjee, S., Liu, J., Gu, H., Zhou, N., et al. (2024). The role of halophyte-induced saline fertile islands in soil microbial biogeochemical cycling across arid ecosystems. Commun. Biol. 7:1061. doi: 10.1038/s42003-024-06741-1
Zheng, T., Huang, X., Zhou, X., Wu, J., Kamran, M. A., Yu, X., et al. (2025). Biochar and Bacillus subtilis co-drive dryland soil microbial community and enzyme responses. Front. Microbiol. 16:1603488. doi: 10.3389/fmicb.2025.1603488
Zhou, H., Qu, Q., Xu, H., Wang, M., and Xue, S. (2025). Effects of vegetation restoration on soil microbial necromass carbon and organic carbon in grazed and degraded sandy land. J. Environ. Manag. 382:125380. doi: 10.1016/j.jenvman.2025.125380
Keywords: degeneration, soil enzyme activity, grassland, recovery, microorganisms, soil nutrients
Citation: Qu Q and Hai X (2025) Soil-specific enzyme activity provides novel insight into the soil microbial necromass accumulation during sand dune fixation. Front. Microbiol. 16:1687297. doi: 10.3389/fmicb.2025.1687297
Edited by:
Jiaoyang Zhang, Anhui Agricultural University, ChinaReviewed by:
Baoshan Zhang, Northeast Forestry University, ChinaSining Liu, Sichuan Agricultural University, China
Copyright © 2025 Qu and Hai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Qu, eWx4bnFxQG53YWZ1LmVkdS5jbg==
 Qing Qu
Qing Qu Xuying Hai3
Xuying Hai3