- 1Department of Neurology, Renmin Hospital of Wuhan University, Hubei General Hospital, Wuhan, China
- 2Department of Neurology, Wuhan No. 9 Hospital, Wuhan, China
- 3Department of Neurology, Ezhou Central Hospital, Ezhou, China
Nitrous oxide (N2O), commonly known as laughing gas, is widely used in clinical practice and food industry. However, an increasing number of young people have been abusing N2O for recreational purpose, resulting in many functional disorders and sometimes irreversible nerve damage. We present the case of a 20-year-old N2O abuser who gradually developed peripheral neuropathy after continuously inhaling N2O for 2 months. The neurological symptoms of the patient had kept exacerbation for the next 2 months until she came for medical care sitting in a wheelchair. We suggested the patient halting N2O intake and supplementing methylcobalamine according to the standardized protocol. Her symptoms had partly recovered during the following 2 weeks but remained unchanged in another 2 weeks. Antibodies against ganglioside complexes were detected and anti-GM1 IgM antibodies were positive in both cerebrospinal fluid and serum. Intravenous immunoglobulin was given as an additional treatment and the patient's symptoms had significantly recovered further. The patient discharged walking by herself. Then she has been continuously followed up in outpatient department for the next 4 months and taking steroid hormone as well as methylcobalamine. Her symptoms gradually disappeared and all the electrophysiological parameters significantly improved. With this case we were able to show that N2O-related peripheral neuropathy is not only a metabolic disorder but also an immune-mediated disease. N2O intake can trigger a mimic Guillain-Barré syndrome.
Introduction
Nitrous oxide (N2O), commonly known as laughing gas, is a colorless, non-irritating gas with a sweetish smell. It is widely used as an anesthetic in clinical practice and can also be easily obtained in the catering industry for whipping cream preparation and in the motor industry as a fuel booster (1). In the recent years, an increasing number of young people have been abusing N2O for recreational purpose. In the UK, N2O was the eighth most commonly used substance and its lifetime prevalence was about 38.6% (2). The N2O abuse can cause hypotension, lung injury, apnea, and any other accidental injuries with a large dose in a short time. The chronic toxicities of N2O are megaloblastic anemia, psychiatric syndromes and neurological injuries, including subacute combined degeneration, myeloneuropathy, myelopathy and peripheral neuropathy (3). To date, the mechanism of N2O toxicity has not been clearly elucidated and vitamin B12 deficiency is the most extensively studied mechanism. Vitamin B12 is an important cofactor for methionine synthetase and methylmalonyl coenzyme A mutase and its deficiency can lead to a decrease of methionine, tetrahydrofolate, and S-adenosylmethionine, and an increase of homocysteine, 5-methyltetrahydrofolate, and methylmalonic acid, resulting in nerve demyelination and injury (4). In addition, N2O can lead to NMDA antagonism, alteration of cerebral blood flow, and inhibition of the synthesis and release of xanthine and monoamines (5). Here we present the case of a 20-year-old N2O abuser mimicking Guillain-Barré syndrome (GBS) with significant immune disturbances.
Case description
The patient was a 20-year-old camgirl without any other medical history and occasionally inhaled N2O for fun in the last 2 years (Figure 1A). She had kept inhaling N2O (about 10 whippets daily) after a failed investment 4 months ago. Two months later she gradually felt numbness and weakness in her lower limbs. She didn't pay attention to it and kept inhaling N2O without any treatments. The numbness and weakness kept being heavier and her upper limbs also suffered. The superficial sensation had significantly decreased of both hands and legs below knees. She couldn't feed herself with hands (muscular strength: 3/5) nor stand up (proximal muscular strength: 3/5; distal muscular strength: 0/5), and came for medical care sitting in a wheelchair. Her limbs' muscular tone had decreased and all the tendon reflexes were disappeared while the overall muscle bulks were normal. The deep sensations of vibration, position, and movement stayed normal. Romberg sign and Babinski sign were negative. She had no defecation problems, psychiatric disorders, headache, epilepsy, cognition impairment, or any other neurological symptoms. She also had no history of vaccine injection, enteritis, or influenza in the last year.
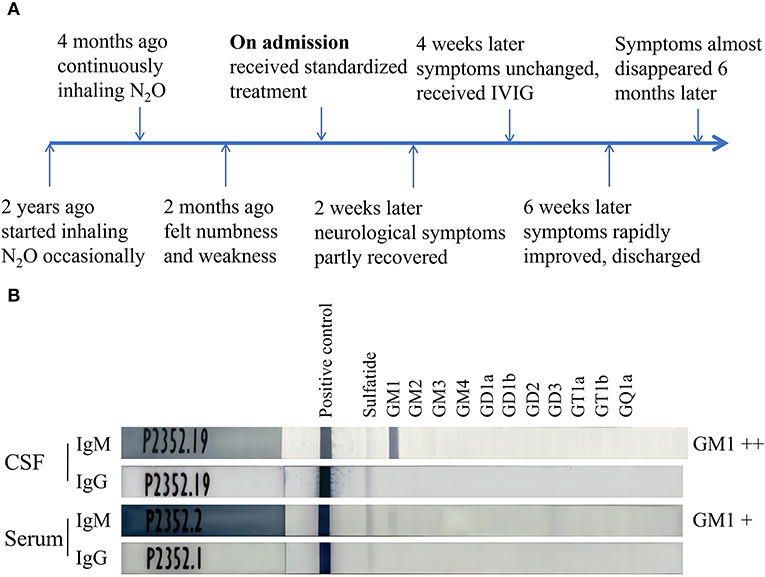
Figure 1. Timeline of the case and ELISA results of antibodies against ganglioside complexes. (A) The patient started inhaling N2O occasionally 2 years ago, felt numbness and weakness 2 months ago, significantly improved after receiving the treatment of IVIG, and almost recovered 6 months after the admission. (B) ELISA tests indicated the anti-GM1 IgM antibodies were positive in both CSF and serum while anti-GM1 IgG antibodies were negative. IVIG, intravenous immunoglobulin; CSF, cerebrospinal fluid.
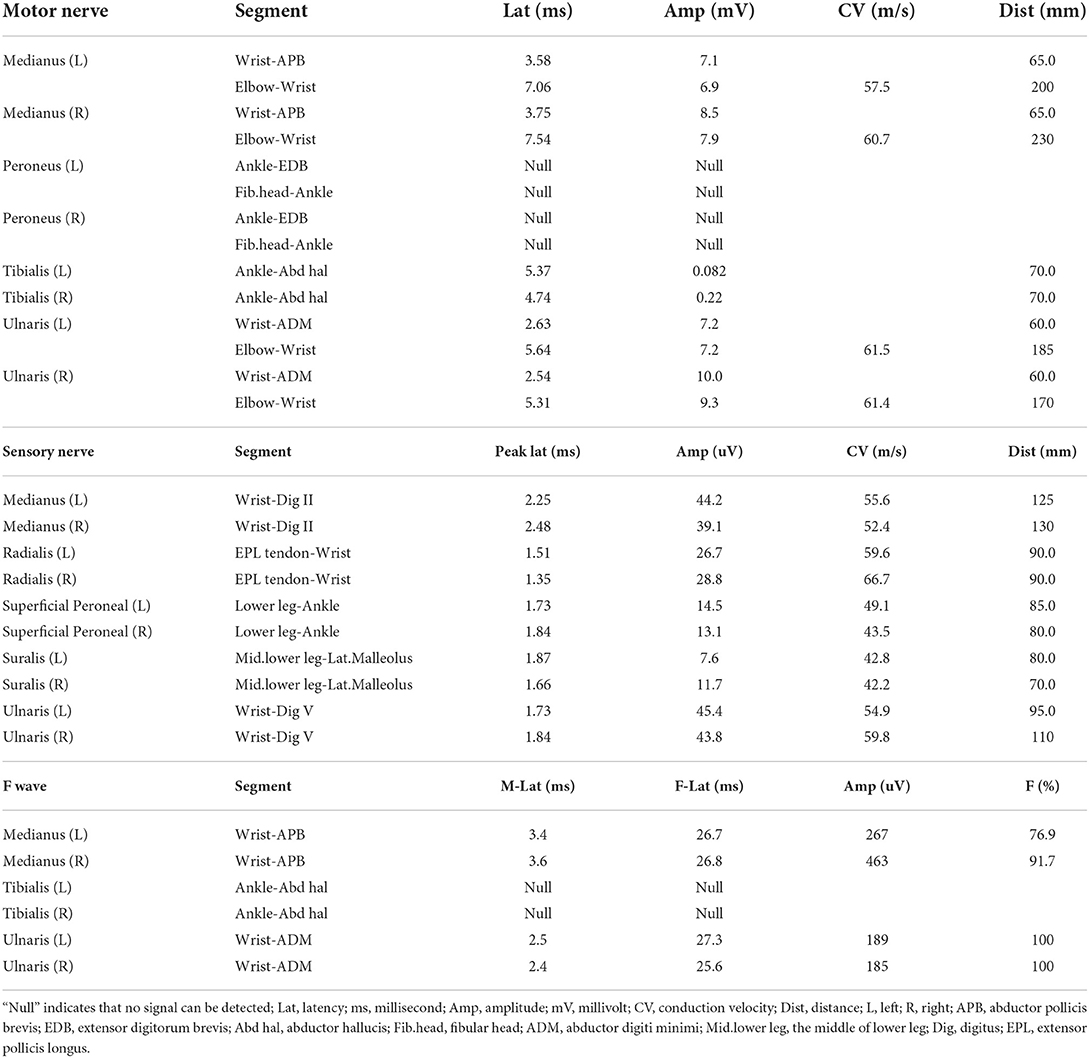
Blood tests indicated slightly decreased hemoglobin (129 g/L) and increased mean corpuscular volume (97.8 fL). Plasma homocysteine level was significantly elevated (24.67 μmol/L) while the serum levels of folic acid, vitamin B12, and any other vitamins were normal. Magnetic resonance imaging hadn't found demyelination or any other abnormal focuses in both brain and spinal cord. Electromyography examination showed extensive peripheral nerve damage, involving motor nerve, sensory nerve, and nerve root. Axonal injuries were especially obvious and nerve damages of the lower limbs were significant heavier than that of upper limbs (Table 1).
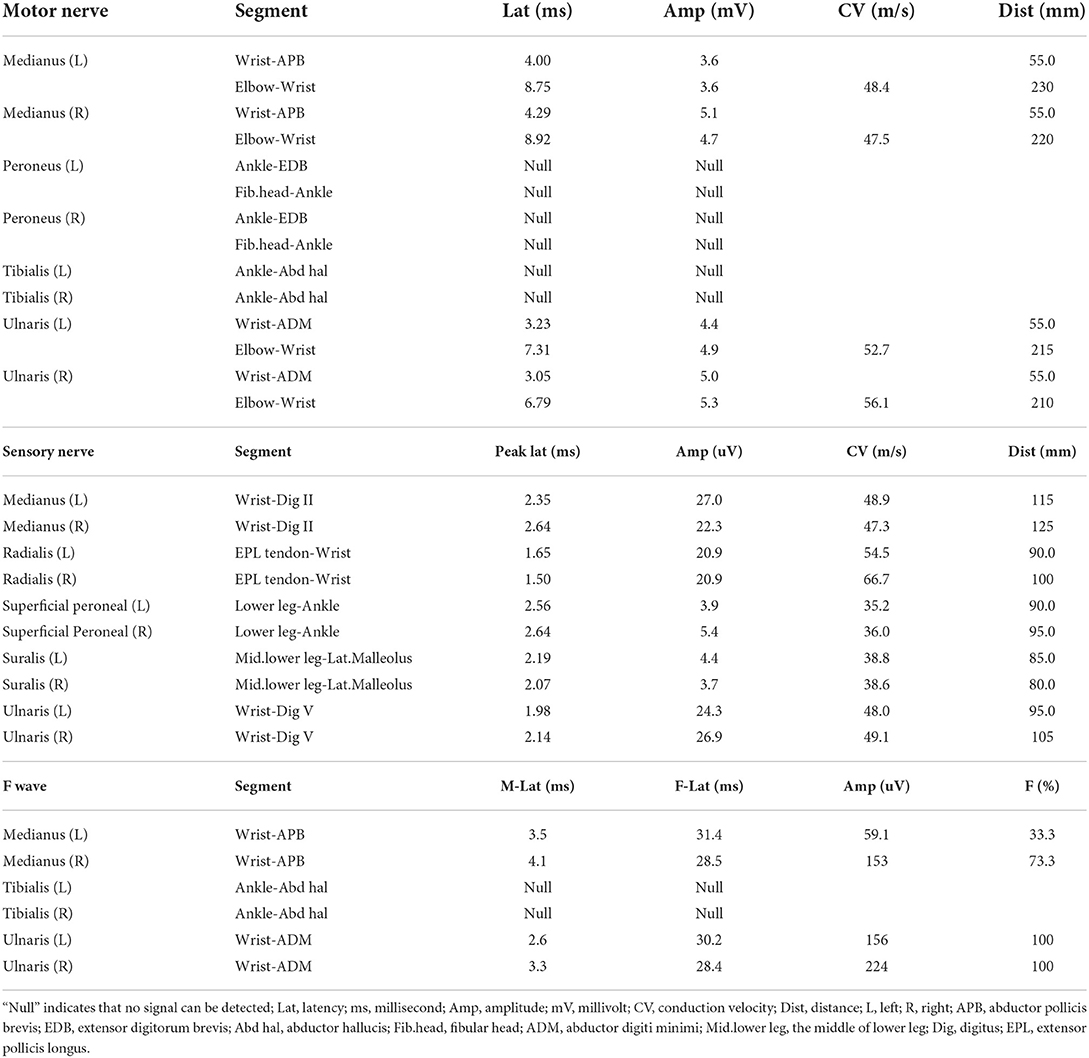
Table 1. Electrophysiological parameters of the case before the treatment with intravenous immunoglobulin.
N2O-related peripheral neuropathy was diagnosed according to the medical history of N2O abuse, typical clinical manifestations, and the above auxiliary examinations. Guillain-Barré syndrome (especially acute motor axonal neuropathy, AMAN) should also be considered as differential diagnosis. After all, the patient was suggested to halt N2O intake and use methylcobalamine for supplementation according to the standardized protocol. The patient's symptoms had partly recovered during the following 2 weeks. The muscular strength of upper limbs increased to 4/5 while superficial sensation and muscular strength of lower limbs recovered slightly. These syndromes remained unchanged after another 2 weeks.
To obtain optimal treatments further, lumbar puncture was performed and cerebrospinal fluid (CSF) was obtained for tests. The leukocyte count of CSF was 3 cells per μl while the protein level was 0.23 g/L. Antibodies against ganglioside complexes were also detected using a commercial ELISA kit and anti-GM1 IgM antibodies were positive in both serum and CSF while anti-GM1 IgG antibodies were negative (Figure 1B). Intravenous immunoglobulin (0.4g/kg × 5 days) was then given as an additional treatment. The patient's symptoms had significantly improved a week later. Her muscular strength of upper limbs and proximal lower limbs increased to 5/5 when the distal lower limbs increased to 1/5. Her superficial sensation had also partly recovered. The patient discharged from the hospital and could walk independently with a steppage gait and take good care of herself.
Then she has been continuously followed up in outpatient department. Considering the amazing treatment outcome of immunoglobin, she accepted the injection of methylprednisolone (1,000 mg qd × 5 days) and orally took prednisone (60 mg qd × 5 days, 30 mg × 5 days, 15 mg qd × 5 days, 10 mg × 5 days) as follows. After that, she has been continuously taking prednisone 5 mg daily. Four months after the discharge from hospital, her numbness gradually disappeared and the muscular strength of distal lower limbs increased to 4/5. Her gait was almost normal and all the electrophysiological parameters significantly improved. The latencies of motor nerves, sensory nerves, and F waves had extensively decreased while the amplitudes had increased. The incidences of F waves also had significantly increased (Table 2). The patient and her parents were aware of the whole process and pleased with the treatment outcome in spite of the injury induced by lumbar puncture, high price of immunoglobulin, and potential side effects of prednisone.
Discussion
Commonly, recreational N2O abuse is recognized to cause metabolic disorders with vitamin B12 deficiency. However, vitamin B12 deficiency is not sufficient to account for N2O-induced peripheral neuropathy as these patients showed prominent motor superexcitability changes and less prominent sensory superexcitability changes in nerve excitability test when compared to patients with vitamin B12 deficiency (6). Here, we present the case of a 20-year-old N2O abuser suffering peripheral neuropathy mimicking GBS with immune disturbances.
Peripheral neuropathy is the most common N2O-related neurological disorder with a morbidity up to 97% (7). These patients primarily exhibited limb numbness or weakness, especially the lower limbs. Decreased muscle strength, superficial sensory disturbances, and decreased tendon reflex were the most common neurological signs according to the published report (7). Increased plasma homocysteine level is more sensitive than plasma vitamin B12 level for clinical diagnosis, as N2O mainly consumes vitamin B12 in tissue but not blood (3). Electromyography of these patients indicated mixed axonal and demyelination injury, including motor and sensory nerves simultaneously. Abnormal F wave and H reflex were also found in the majority of N2O abuser (8). All in all, the case we presented was a typical N2O-related peripheral neuropathy according to these characteristics.
However, without the medical history of N2O abuse, these patients were usually misdiagnosed as GBS (9). The absent medical history of vaccine injection, enteritis, or influenza before the onset didn't support the diagnosis of GBS. The course of disease progression of the patient was also significantly longer than the natural history of GBS (2–4 weeks). To our knowledge, GBS is more likely to occur in the middle-aged or aged population but not the youth. The electrophysiological features of N2O abuser were dramatically different from those in acute inflammatory demyelinating polyradiculoneuropathy (a GBS variant), but exactly similar to another GBS variant (AMAN). It's difficult to differentiate N2O abuse and AMAN according to those electrophysiological findings (10). Meanwhile, the injury severity of lower limbs is usually heavier than the upper limbs in N2O abuser while it is similar in patient with GBS (11). The difference in the distal and proximal compound muscle action potential amplitudes of the upper limbs can be a parameter for differential diagnosis between the N2O abuser and AMAN patients, as the axonal injury was more severe in AMAN patients than N2O abuser, especially upper limbs (10). GBS is an immune-mediated disease while N2O-related neurological syndromes are deemed as metabolic disorders. Albuminocytologic dissociation and antibodies against ganglioside complexes in CSF were the specific characteristics of GBS. However, the potential pathogenesis seemed to be more complicated as the motor neuropathy of young N2O abuser remained disabled with methylcobalamine supplementation in follow-up research (12).
Here, although the patient was suggested to halt N2O intake and supplement methylcobalamine according to the current standardized protocol, her syndromes had only partly recovered without further progress. Lumbar puncture was performed and CSF was tested. Albuminocytologic dissociation was not found in CSF while we were the first to report positive anti-GM1 IgM antibodies in both CSF and serum. Gangliosides are specifically enriched in nervous system plasma membranes while GM1 is mainly expressed at nodes of Ranvier and motor nerve terminals (13). When GM1 or its mimicry, Campylobacter jejuni strains, are exposed to the autoimmune system, anti-GM1 antibodies are produced and activate the complement cascade, leading to either reversible conduction failure or axonal degeneration in AMAN patients (14). Anti-GM1 IgM antibodies have also been found in multifocal motor neuropathy, autoimmune limbic encephalitis, Bickerstaff brainstem encephalitis, and any other patients with neuronal or glial damage, resulting in the exposure of GM1 to autoimmune system (15). The disturbed immunological system of the patient with positive anti-GM1 IgM antibodies were probably primarily caused by N2O or secondary to the peripheral neuropathy by N2O. After all, the favorable prognosis with intravenous immunoglobin and steroid hormone further validated our supposes that immunological system was involved in the N2O-related peripheral neuropathy (12). The effect of N2O on the neuroimmune system is possibly through vitamin B12 deficiency or any other unclear pathways to be clarified in future (16).
Conclusion
With this case we were able to show that recreational N2O abuse can cause immune-mediated disorder besides metabolic disorder. N2O-related peripheral neuropathy is a mimic of GBS with many similar characteristics. We therefore encouraged all clinicians to perform CSF tests of antibodies against ganglioside complexes when encountering N2O abusers. The potential immunological pathogenesis should be clarified to find novel treatments for these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Local Ethics Committee of Renmin Hospital of Wuhan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
M-XD and TL designed the study and analyzed the patient data. QW, J-FX, LH, and YY collected the original clinical data. M-XD wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Funding
This work was supported by the Open Fund of Hubei Key Laboratory of Renmin Hospital of Wuhan University (2021KFY040).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1033327/full#supplementary-material
References
1. Inquimbert C, Maitre Y, Moulis E, Gremillet V, Tramini P, Valcarcel J, et al. Recreational nitrous oxide use and associated factors among health profession students in France. Int J Environ Res Public Health. (2022) 19:5237. doi: 10.3390/ijerph19095237
2. Kaar SJ, Ferris J, Waldron J, Devaney M, Ramsey J, Winstock AR. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. (2016) 30:395–401. doi: 10.1177/0269881116632375
3. Bao L, Li Q, Li Q, Chen H, Zhang R, Shi H, et al. Clinical, electrophysiological and radiological features of nitrous oxide-induced neurological disorders. Neuropsychiatr Dis Treat. (2020) 16:977–84. doi: 10.2147/NDT.S236939
4. Largeau B, Karam A, Potey C, Caous AS, Tard C, Carton L, et al. Myeloneuropathy induced by recreational nitrous oxide use with variable exposure levels. Eur J Neurol. (2022) 29:2173–80. doi: 10.1111/ene.15370
5. Xiang Y, Li L, Ma X, Li S, Xue Y, Yan P, et al. Recreational nitrous oxide abuse: prevalence, neurotoxicity, and treatment. Neurotox Res. (2021) 39:975–85. doi: 10.1007/s12640-021-00352-y
6. Tani J, Weng HY, Chen HJ, Chang TS, Sung JY, Lin CS, et al. Elucidating unique axonal dysfunction between nitrous oxide abuse and vitamin B12 deficiency. Front Neurol. (2019) 10:704. doi: 10.3389/fneur.2019.00704
7. Yu M, Qiao Y, Li W, Fang X, Gao H, Zheng D, et al. Analysis of clinical characteristics and prognostic factors in 110 patients with nitrous oxide abuse. Brain Behav. (2022) 12:e2533. doi: 10.1002/brb3.2533
8. Li Y, Dong J, Xu R, Feng F, Kan W, Ding H, et al. Clinical epidemiological characteristics of nitrous oxide abusers: a single-center experience in a hospital in China. Brain Behav. (2021) 11:e2416. doi: 10.1002/brb3.2416
9. Beal JC, Cheng Y, Merchant S, Zarnegar R. An acute, severe axonal sensorimotor polyneuropathy in the setting of nitrous oxide abuse. Neurohospitalist. (2020) 10:293–6. doi: 10.1177/1941874420910648
10. Li Y, Zhang X, Zhao C. Electrophysiological characteristics of patients with nitrous oxide abuse. Neurol Res. (2021) 43:793–801. doi: 10.1080/01616412.2021.1935101
11. Jiang J, Shang X, Wang X, Chen H, Li W, Wang Y, et al. Nitrous oxide-related neurological disorders: clinical, laboratory, neuroimaging, and electrophysiological findings. Brain Behav. (2021) 11:e2402. doi: 10.1002/brb3.2402
12. Berling E, Fargeot G, Aure K, Tran TH, Kubis N, Lozeron P, et al. Nitrous oxide-induced predominantly motor neuropathies: a follow-up study. J Neurol. (2022) 269:2720–6. doi: 10.1007/s00415-021-10858-2
13. McGonigal R, Campbell CI, Barrie JA, Yao D, Cunningham ME, Crawford CL, et al. Schwann cell nodal membrane disruption triggers bystander axonal degeneration in a Guillain-Barré syndrome mouse model. J Clin Investig. (2022) 132:e158524. doi: 10.1172/JCI158524
14. Yanaka S, Yogo R, Watanabe H, Taniguchi Y, Satoh T, Komura N, et al. On-membrane dynamic interplay between anti-GM1 IgG antibodies and complement component C1q. Int J Mol Sci. (2019) 21:147. doi: 10.3390/ijms21010147
15. Oktar AÇ, Selçuk Ö, Tunç C, Işildak S, Sanli E, Yilmaz V, et al. Serum anti-ganglioside antibodies in patients with autoimmune limbic encephalitis. Turk J Med Sci. (2021) 51:2570–4. doi: 10.3906/sag-2101-348
Keywords: nitrous oxide, peripheral neuropathy, ganglioside complexes, Guillain-Barré syndrome, immunoglobin, steroid hormone
Citation: Dong M-X, Wang Q, Xu J-F, Hu L, Yu Y and Li T (2022) Case report: Recreational nitrous oxide abuse triggered peripheral neuropathy possibly through the immune-mediated pathogenesis. Front. Neurol. 13:1033327. doi: 10.3389/fneur.2022.1033327
Received: 31 August 2022; Accepted: 23 September 2022;
Published: 14 November 2022.
Edited by:
Giovanni Meola, University of Milan, ItalyReviewed by:
Yuan Xue, Shandong Provincial Hospital Affiliated to Shandong First Medical University, ChinaJowy Tani, Taipei Medical University, Taiwan
Copyright © 2022 Dong, Wang, Xu, Hu, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Li, bHRsbEAxNjMuY29t
†These authors have contributed equally to this work
 Mei-Xue Dong
Mei-Xue Dong Qing Wang2†
Qing Wang2†