- 1Department of Neurology, Affiliated Hospital of Yangzhou University, Yangzhou, China
- 2School of Nursing and School of Public Health, Yangzhou University, Yangzhou, China
- 3Division of Satoyama Nursing and Telecare, Nagano College of Nursing, Komagane, Japan
- 4Department of the Advanced Biomedical Research, Interdisciplinary Graduate School of Medicine, University of Yamanashi, Chuo, Japan
- 5Department of Biomedical Science and Institute of Bioscience and Biotechnology, Kangwon National University, Chuncheon-si, Gangwon-do, Republic of Korea
- 6Department of Anatomy, Medical College, Yangzhou University, Yangzhou, China
- 7Jiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases, Yangzhou University, Yangzhou, China
Objective: This systematic review was performed to identify the role of cognitive reserve (CR) proxies in the functional outcome and mortality prognostication of patients after acute ischemic stroke.
Methods: PubMed, Embase, Web of Science, and Cochrane Library were comprehensively searched by two independent reviewers from their inception to 31 August 2022, with no restrictions on language. The reference lists of reviews or included articles were also searched. Cohort studies with a follow-up period of ≥3 months identifying the association between CR indicators and the post-stroke functional outcome and mortality were included. The outcome records for patients with hemorrhage and ischemic stroke not reported separately were excluded. The Quality In Prognosis Studies (QUIPS) tool was used to assess the quality of included studies.
Results: Our search yielded 28 studies (n = 1,14,212) between 2004 and 2022, of which 14 were prospective cohort studies and 14 were retrospective cohort studies. The follow-up period ranged from 3 months to 36 years, and the mean or median age varied from 39.6 to 77.2 years. Of the 28 studies, 15 studies used the functional outcome as their primary outcome interest, and 11 of the 28 studies included the end-point interest of mortality after ischemic stroke. In addition, two of the 28 studies focused on the interest of functional outcomes and mortality. Among the included studies, CR proxies were measured by education, income, occupation, premorbid intelligence quotient, bilingualism, and socioeconomic status, respectively. The quality of the review studies was affected by low to high risk of bias.
Conclusion: Based on the current literature, patients with ischemic stroke with higher CR proxies may have a lower risk of adverse outcomes. Further prospective studies involving a combination of CR proxies and residuals of fMRI measurements are warranted to determine the contribution of CR to the adverse outcome of ischemic stroke.
Systematic review registration: PROSPERO, identifier CRD42022332810, https://www.crd.york.ac.uk/PROSPERO/.
Introduction
It is well established that stroke is one of the leading causes of death and long-term disability worldwide among adults (1). Especially, older patients aged ≥75 years are at an increased risk of suffering from stroke during the last decades of their life (2), which imposes an enormous burden on global public health (3). Among stroke survivors, they were more likely to present significant deficits in multiple domains, including motor and cognitive impairment, disability, and psychological disorders (4, 5). Previous studies demonstrated that the first 3 months after ischemic stroke is a critical period of recovery, followed by a stable stage (6, 7). Although numerous studies have been conducted to predict adverse clinical and functional outcomes after ischemic stroke (8–10), further studies are essential to understand the underlying factors of inter-individual heterogeneity that contributed to unfavorable stroke outcomes. The term conserve reserve (CR) was theoretically constructed to explain the inter-individual discrepancies between the severity of brain pathology and clinical manifestations (11). A consensus was reached on the definition of CR in a recent whitepaper, defining CR as an active model of reserve acquired from various lifetime experiences (i.e., education attainment, intellectual activity, occupation history, and other environmental factors) via shaping the brain's network efficiency, processing capacity, and flexibility to protect against brain aging, pathology, or brain insult (12, 13). As measuring CR directly is full of challenges, sociobehavioral proxy indicators are commonly used to indirectly estimate CR, including education, occupation, leisure activities, premorbid intelligence quotient (IQ), socioeconomic status (SES), and/or bilingualism (14).
The concept of CR is well validated in patients with stroke as the study has found that the degree of cognitive impairment varied widely among individuals, despite comparable levels of pathology (13). An increasing number of studies have been carried out to examine the effects of potential CR proxies on the prediction and recovery of cognitive impairment after stroke (15–17). Previous studies provided some indication that CR may act as a crucial role in stroke recovery (17). Nevertheless, recent reviews or original studies focused only on the effect of educational attainment as an indicator of CR on post-stroke cognition, neglecting other functional stroke outcomes (i.e., disability, psychological disorders, and motor impairment) (16, 18). To the best of our knowledge, no systematic review or meta-analysis was conducted to methodically summarize the impact of CR sociobehavioral proxies on post-stroke functional outcomes and mortality.
To comprehensively assess the impact of CR sociobehavioral proxies on ischemic stroke outcomes, we performed a systematic review to identify the association of CR proxies with stroke outcomes, taking inter-individual variability into consideration.
Methods
Search strategy
The study was performed in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Data Sheet 1). A systematic and comprehensive literature search was conducted in PubMed, Embase, Web of Science, and Cochrane Library, from their inception to 31 August 2022. We used two keywords, namely, “ischemic stroke” and “cognitive reserve” that were cross-searched by two independent reviewers. The phrase “cognitive reserve” as this term is sometimes used interchangeably with education, occupation, IQ, bilingualism, leisure activities, and socioeconomic status (14, 19, 20). In addition, our study was designed to focus on the prognosis of ischemic stroke. Keywords such as “prognosis” or “stroke outcome” were used for retrieval. No language limitations were used. The complete list of keywords for each literature search is available in Supplementary Data Sheet 2 (Table S1).
Studies selection
Two authors (YY and XY) independently identified the article, abstract, and keywords of each article and evaluated the eligibility. Any discrepancies were discussed and resolved by a third referee (ZS). Studies that met the following criteria were included in this systematic analysis: (1) human study (participants age ≥18 years old); (2) cohort study (prospective cohort study or retrospective cohort study); (3) CR proxies as the exposure of interest (i.e., education, occupation, IQ, bilingualism, leisure activities, and socioeconomic status); (4) mortality and functional outcomes (i.e., post-stroke cognitive impairment or post-stroke depression) as the end-point of interest; (5) minimum follow-up period ≥3 months. The following studies were excluded: studies focusing on the transient ischemic attack (TIA) or with a combined record of patients with hemorrhagic and ischemic stroke, not first-ever patients with stroke, conference abstracts, letters, comments, editorials, and case reports. We also excluded systematic reviews and/or meta-analysis, but their reference lists were searched to identify primary studies.
Data extraction
Data from the studies included were independently extracted by three authors (TC, WS, and WZ) through a standardized electronic form. We collected the following data elements for this study: study characteristics (first author, publication year, country, journal, and study design), demographic data (age and proportion of women), population recruitment interval, stroke types, the length of follow-up, the number of patients in the cohorts/number of participants with poor outcome (n total/n outcomes), cognitive reserve indicators, outcome definition and assessment, the type of statistical model, main findings, and the relationship between cognitive reserve and the outcome.
Outcome definition
The prespecified primary outcome of interest was unfavorable functional outcomes, including post-stroke cognitive impairment or dementia, disability, and psychological dysfunction (depression or anxiety). Similarly, we considered the secondary outcome as death after a first-ever stroke. The modified Rankin Scale (mRS), the Barthel Index (BI), the Mini-Mental State Examination (MMSE), and the Hospital Anxiety and Depression Scale (HADS) were the common instruments to assess disability, motor impairment, cognitive dysfunction, and psychological disorders after ischemic stroke.
Quality assessment
To critically appraise and evaluate the methodological quality of included studies, the Quality in Prognosis Studies (QUIPS) tool which is an optimal assessment tool was used, allowing an evaluation of risk bias and consideration of different CR proxies as prognostic factors (21). Accordingly, we evaluated the potential bias of each study in terms of study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, as well as statistical analysis and reporting. The outcomes were divided into low-, medium-, and high-risk biases.
Results
Literate search
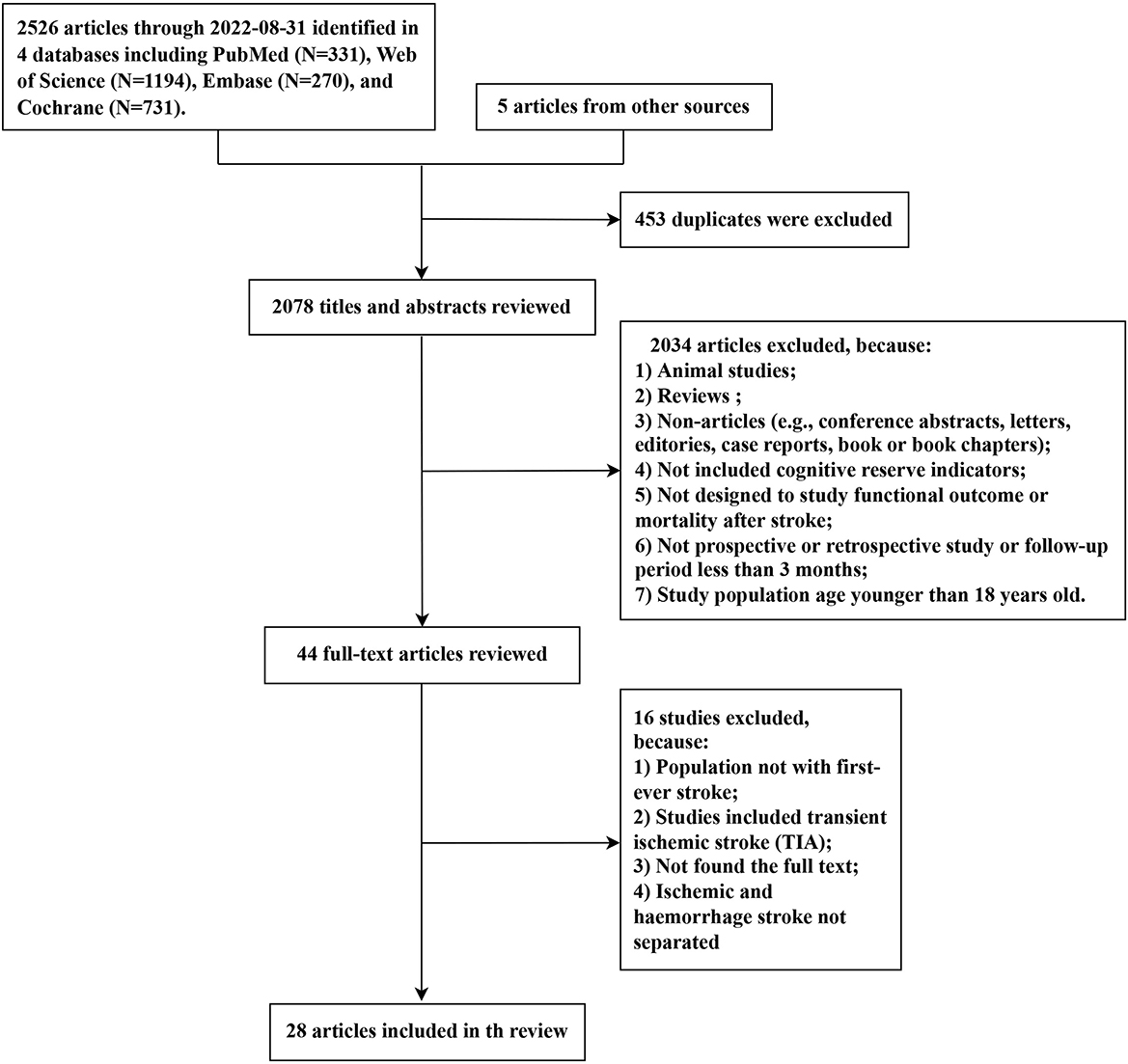
The selection procedure for the systematic review is illustrated in Figure 1. The search yielded 2,526 articles from four databases (PubMed: 331, Web of Science: 1,194, Embase: 270, and Cochrane: 731). A total of five additional studies were identified from the references of relevant reviews. Among the 2,531 studies, we eliminated 453 duplicated articles and retained 44 articles for the full-text review after rigorously screening titles and abstracts. Ultimately, 28 articles met the eligibility criteria and were included in this systematic review. Given that the result data of meta-analyses demonstrated significant heterogeneity between studies, we attempted to present the synthesis of the studies in a narrative review format.
Characteristics of included studies
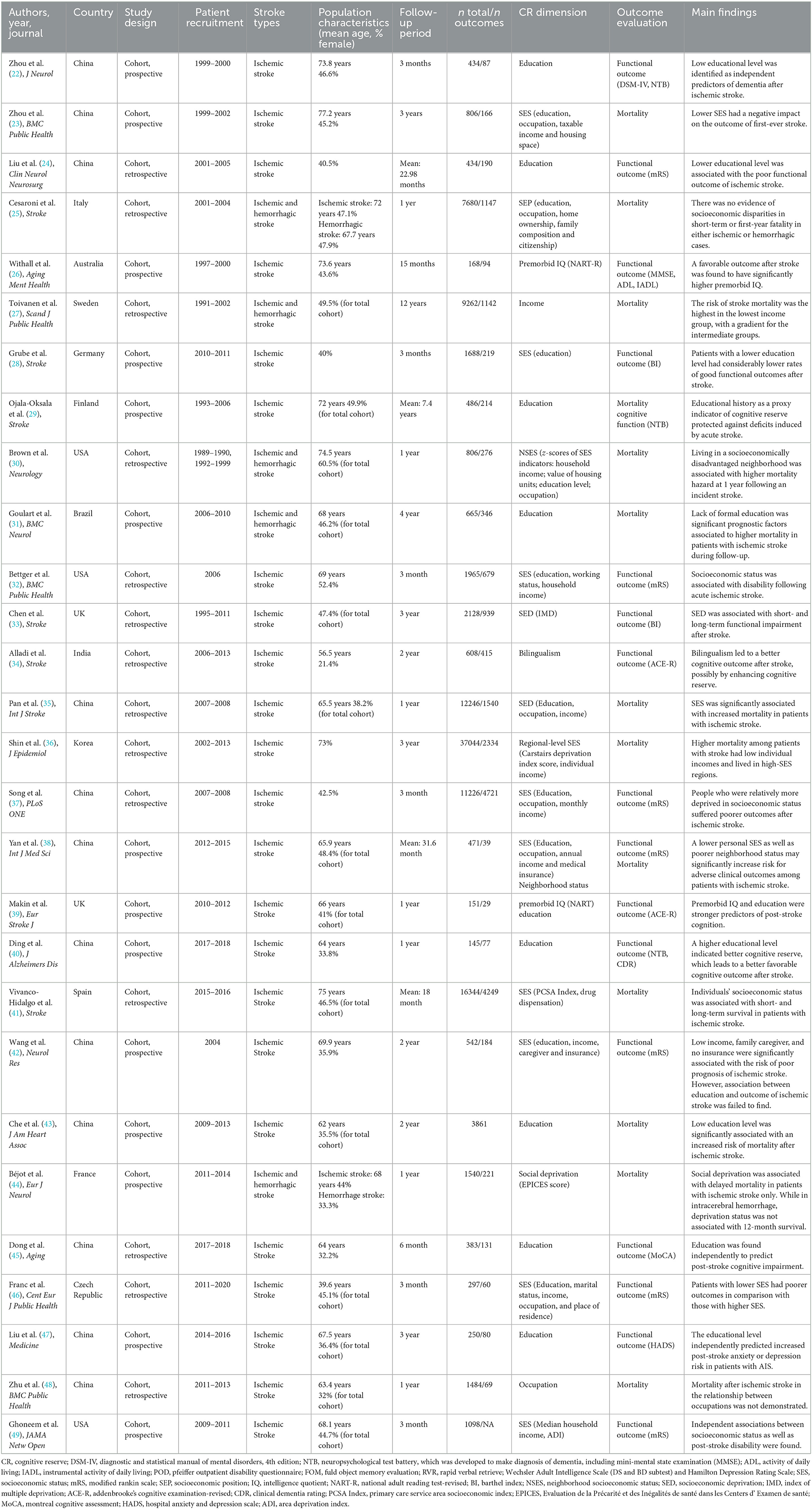
The basic characteristics of the 28 included studies are summarized in Table 1. Studies in this review encompassed a total of 1,14,212 patients with stroke and were published between 2004 and 2022, with an average or median age varied from 39.6 to 77.2 years. The follow-up period ranged from 3 months to 36 years. A total of 12 studies were conducted in China (22–24, 35, 37, 38, 40, 42, 43, 45, 47, 48), three studies in the USA (30, 32, 49), two studies in the UK (33, 39), and one study each in Italy (25), Australia (26), Sweden (27), Germany (28), Finland (29), Brazil (31), India (34), Korea (36), Spain (41), France (44), and Czech Republic (46). A total of 15 studies (22, 24, 26, 28, 29, 32–34, 37, 39, 40, 42, 45–47, 49) involving 21,517 patients were pooled for an evaluation of functional outcome, eight of which (22, 26, 28, 39, 40, 42, 47, 49) adopted prospective designs. A total of 11 studies (23, 25, 27, 30, 31, 35, 36, 41, 43, 44) reported the outcome of mortality, of which four studies (23, 31, 43, 44) were designed as prospective studies, and the rest were retrospective studies. In addition, data from two prospective studies (29, 38) were pooled for an assessment of mortality and functional outcomes. Furthermore, the data of two studies (35, 37) were from the same retrospective cohort study but reported different outcomes of stroke, so we included them both. A total of 23 studies (22–24, 26, 28, 29, 32–35, 48) enrolled patients with first-episode ischemic stroke, and five studies (25, 27, 30, 31, 44) selected patients with ischemic and hemorrhagic stroke but reported separately. In addition, 16 studies (23, 25, 27, 28, 30, 32, 35–38, 41, 42, 44, 49) defined cognitive reserve indicators as socioeconomic status, eight studies (22, 24, 29, 31, 40, 43, 45, 47) used education attainment, two studies (26, 39) measured premorbid IQ, and one study each estimated bilingualism (34) and occupation (48).
Quality assessment
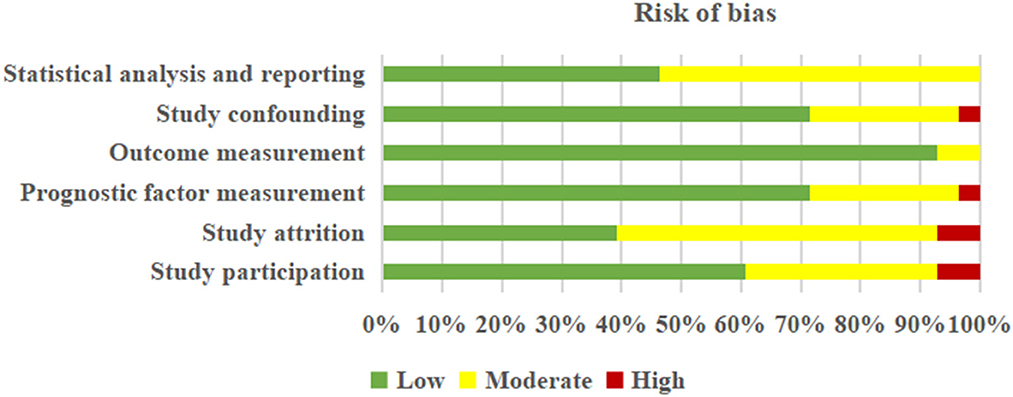
Detailed information about the judgment of each “risk of bias” domain is tabulated in Supplementary Data Sheet 2 (Table S2). Based on the QUIPS tool, no studies were excluded. In short, five (27, 29, 31, 32, 34) out of 28 studies were rated as having a high risk of bias, eight (22, 24, 26, 39, 45–48) as moderate, and 15 (23, 25, 28, 30, 33, 35–38, 40–44, 49) as low. Overall, the risk of six domains was judged to vary from low to high-risk bias, respectively, in “study participation” (low risk: 60.71%, moderate risk: 32.13%, and high risk: 7.14%), “study attrition” (low risk: 39.29%, moderate risk: 53.57%, and high risk: 7.14%), “prognosis factor measurement” (low risk: 71.43%, moderate risk: 25.00%, and high risk: 3.57%), “outcome measurement” (low risk: 92.86% and moderate risk: 7.14%), “study confounding” (low risk: 71.43%, moderate risk: 25.00%, and high risk: 3.57%), and “statistical analysis and reporting” (low risk: 46.43% and moderate risk: 53.57%) (Figure 2).
Association of cognitive reserve with the functional outcome of ischemic stroke
A total of 17 studies reported the functional outcome at the end of follow-up, with the endpoint interest of cognitive dysfunction, disability, motor impairment, depression, or anxiety after ischemic stroke (22, 24, 26, 28, 29, 32–34, 37–40, 42, 45–47). A summary of the findings in the included literature that identified the association between CR and functional outcomes is demonstrated in Supplementary Data Sheet 2 (Table S3).
Out of the seven studies using the modified Rankin Scale (mRS), the association between post-stroke disability and cognitive reserve was identified (24, 32, 37, 38, 42, 46, 49). Liu et al. (24) adopted a multivariate logistic regression model and found a significant relationship between poor outcomes and lower educational levels. Among the included literature, the definition of SES varied widely. A total of two studies used a composition of education, occupation, and income (32, 37). Only one study additionally included medical insurance, neighborhood status (38), and another study included marital status and place of residence (46). Another study measured a combination of education, income, caregiver, and insurance (42), and one study evaluated median household income and the area deprivation index (ADI), which combined 17 weighted census indicators (e.g., measures of education, employment, housing quality, and poverty) (49). Regardless of the definition of SES and the type of regression model used, a lower SES significantly increased the risk of adverse clinical outcomes among patients with ischemic stroke.
A total of two studies used the Barthel Index (BI) to confirm the relationship between SES and motor impairment after ischemic stroke (28, 33). Grube et al. (28) defined SES measured by education as CR proxies and found that the lower the education attainment, the worse the functional outcome. In addition, the index of multiple socioeconomic deprivations based on patient postcodes as an SES indicator was found to be strongly associated with short- and long-term motor impairment after stroke (33). Both studies adopted the multivariate logistic regression model.
A total of six studies used various cognitive testing scales to examine the correlation between CR proxies and the post-stroke cognitive outcome (22, 29, 34, 39, 40, 45). The included studies most frequently used education level as CR indicators (n = 4) and found that the higher the education level, the better the cognitive outcome (22, 29, 40, 45). Among the four studies, three of them adopted the logistic regression model (22), binomial logistic regression model and multivariate logistic regression model respectively, whereas the other study did not provide the method used (29). Using bilingualism as an indicator of CR, it is found that bilingualism contributes to better cognitive outcomes, based on the logistic regression model (34). Makin et al. (39) defined composition of premorbid IQ and education as CR indicators and found that both of them were stronger predictors of post-stroke cognition through the logistic and linear regression model.
A study, using a combination index of the mini-mental state examination (MMSE) scores, the activity of daily living (ADL) scores, and the instrument activity of daily living (IADL) scores, identified the association between premorbid IQ and the clinical outcome after ischemic stroke. Based on the multivariate logistic regression model, the study found that better outcomes after stroke had significantly higher premorbid IQ (26).
Association of cognitive reserve with the mortality of ischemic stroke
A total of 13 studies reported the mortality outcome at the end of the follow-up (23, 25, 27, 29–31, 35, 36, 38, 41, 43, 44, 48). A total of three studies defined education as CR proxies and found that educational history was associated with lower mortality after ischemic stroke through the Cox regression model and Cox proportional hazard model (31, 43). One each study regarded income or occupation as CR indicators, and both of them used the Cox proportional hazard model, but the results were different (27, 48). The association of lower income with the increase in the risk of stroke mortality was found in the study by Toivanen et al. (27). Nevertheless, Zhu et al. (48) failed to find any relationship between occupation and mortality after ischemic stroke. A total of eight studies assessed whether SES as a proxy for CR was significantly associated with mortality after stroke, based on the various definitions of SES (23, 25, 30, 35, 36, 38, 41, 44). Pan et al. (35) defined a composition indicator of education, occupation, and income as SES, and Zhou et al. (23) additionally included housing space as one of the indicators. Brown et al. (30) included the value of the housing unit, and Yan et al. (38) also included medical insurance. Similarly, it was concluded that lower SES had a negative impact on the outcome of first-ever stroke. Moreover, Shin et al. (36) calculated the Carstairs deprivation index score and individual income as SES indicators and found that low individual incomes and living in high-SES regions enhanced higher mortality among patients with stroke. Vivanco-Hidalgo et al. (41) considered the Primary Care Service Area Socioeconomic (PCAS) index scores and drug dispensation as SES indicators, finding that an individual's SES was related to survival in patients with ischemic stroke. SES measured by socioeconomic deprivation was strongly correlated with delayed mortality in patients with ischemic stroke (44). Of the eight articles, three studies adopted Cox proportional hazard models (23, 30, 36), and each opted for the logistic regression model (25), multivariate-adjusted logistic regression model (35), and mixed-effects logistic and survival model (41) and multivariate Cox model (38, 44), respectively. Supplementary Data Sheet 2 (Table S4) shows detailed information on the association between CR and mortality after stroke.
Discussion
Eventually, this systematic review included 14 prospective cohort studies and 14 retrospective cohort studies to identify the association between CR proxies and the prognosis of ischemic stroke, as measured by education, income, occupation, premorbid IQ, bilingualism, and SES as CR proxies. The results reveal that lower scores on CR proxies may have an important potential role in predicting motor and cognitive impairment, disability, psychological disorders, and mortality after ischemic stroke.
Consistent with our findings, several previous studies that did not meet the eligibility criteria for the current systematic review also reported that CR characterized by neural reserve and compensation might partially address the gap in the heterogeneity of stroke injury and recovery (11, 15). More specifically, neural networks protect against neurological damage in ischemic stroke by spontaneously utilizing, optimizing, strengthening existing effective cognitive process, or recruiting alternate pathways (11, 50). However, the exact mechanisms of CR remain uncertain. Although the concept of CR is a theoretical construct, various methods attempted to operationalize and measure CR. Education, occupation, leisure activity, and premorbid IQ were commonly used to measure CR indirectly (14, 51). Among the limited methodological studies, most of them investigated only a single CR proxy to reflect the CR level for the feasibility and expedient of operationalization. Furthermore, some of the included studies used different evaluation methods for the indicator itself (e.g., education and SES), which may make a difference in the results of CR. Hence, we should possess a cautious attitude toward these results as CR is constructed by multidimensional components.
A previous study ascertaining the prognostic role of CR in stroke-induced functional impairment and mortality is limited. Low educational attainment was shown to be associated with disability, mortality, worse cognitive function, and higher risk of depression or anxiety in the stable stroke phase (24, 43, 44, 47). Shin et al. (16) found that a higher educational level can predict the recovery of active and stable phases after the stroke onset. Educational history as a proxy indicator of CR is significantly associated with post-stroke cognitive deficits, dementia, and long-term survival, independent of age, gender, stroke severity, and white matter lesions (WML) in mild/moderate ischemic patients with stroke (52). Patients with a higher educational level may have more synapses, larger brains, or more efficient brain networks to tolerate more pathology until reaching a critical threshold and presenting a cognitive deficit later (13, 53).
Heretofore, the evidence that occupation complexity, bilingualism, income, and premorbid IQ as proxies for CR are correlated with the long-term outcome of ischemic stroke is relatively limited. The low complexity of occupation was found to be associated with a high risk of cognitive impairment and decreased the speed of cognitive recovery (16). Alladi et al. (34) found that bilingualism served as a protective role in the development of post-stroke cognitive impairment, independent of age or vascular risk factors. Compared with the highest income group, patients with the lowest income have a considerably higher risk of stroke mortality, with a gradient for the intermediate groups (27). Premorbid IQ as a proxy indicator of CR was found to be a stronger predictor of the long-term post-stroke cognition outcome and late-life depression and dementia (26, 39). The favorable impacts of occupation, bilingualism, income, and premorbid IQ on the cognitive function and mortality of stroke outcomes can be explained by CR theory. Patients with a higher CR level might be more capable of resisting stroke damage by recruiting alternative functional centers and providing easier and faster compensation (15, 54).
In the past decades, there has been a widespread interest in exploring the relationship between SES as a proxy indicator of CR and stroke outcomes. Socioeconomic status is a complex conception that combined economics and sociology to reflect an individual's or family's position, commonly based on education, occupation, and income (55). Studies found that the frequency of motor impairment, mortality, and disability was lower than that in patients with a higher SES level (33, 44, 49, 56). However, the association between SES and stroke mortality was inconsistent. Several studies yielded no correlation between SES and stroke mortality, which may be due to differences in the selected SES indicators or regions (57). To provide robust evidence on the relationship between SES and stroke outcome, Wang et al. (55) summarized the evidence and found that patients with a low SES level had a higher risk of stroke mortality despite the heterogeneity of each SES indicator. The underlying mechanisms between SES and the increased risk of stroke mortality remain unclear. The long-term outcome of stroke may depend on the differences in inter-individual SES level in the initial stroke severity, independent of treatments and symptom duration (49). SES, as a significant component of CR, is influenced by lifetime experiences and decreases structural brain changes through shaping network efficiency, processing capacity, and flexibility (13).
While the exact mechanism of CR on the prognostic performance of long-term outcomes after ischemic stroke has not been clarified clearly, the impact of CR on inferior outcomes might comprise the following underlying mechanisms (15). First, patients with a higher CR level may tolerate more pathology until reaching a critical threshold manifested by cognitive deficits despite comparable stroke severity (58). Second, based on the CR theory, dendritic or plasticity was fostered to improve network efficiency and capacity to resist brain pathology after ischemic stroke (53). Third, higher CR was linked to recruiting alternative neural networks to provide faster compensation for brain injury (54). Overall, CR is a dynamic and modifiable reserve model affected by lifetime intellectual activities (12). In the present studies, the residual method was proposed to directly measure CR, which was calculated through the regression model combined with functional imaging results (12, 59).
Our systematic review reveals that cognitive reserve played a significant role in the prediction of stroke-related impairment and recovery, which resolves a major clinical challenge (60). To reduce the global burden of post-stroke disability and provide precise health promotion or prevention, cognitive reserve as a modifiable conception deserves further investigation.
Strengths and limitations
To the best of our knowledge, this systematic review is the first attempt to comprehensively provide a summary impact of CR sociobehavioral proxies on the prognosis of patients with ischemic stroke. Nevertheless, we acknowledge that our study has several limitations. First, this review only considered sociobehavioral proxies as CR indicators, lacking other potential direct measurements of CR, and most studies merely used a single proxy. Second, given the heterogeneity of CR proxies, follow-up period, population, and stroke outcome, we failed to quantitatively summarize the data and have to perform a descriptive overview. Third, only longitudinal studies were included in this systematic review, and other potentially relevant randomized controlled trials may provide stronger evidence to reveal the underlying mechanism of CR and stroke outcomes. Finally, although some of the included studies were rated as having moderate to high-risk bias according to the QUIPS tool, we made the decision to retain these studies as the difficulty of avoiding bias in literature reviews, indicating that the results should be illustrated with caution.
Conclusion
Our results provide evidence that lower CR proxies may have a significant association with unfavorable outcomes after ischemic stroke. However, given the limitations of this review, the results of this study ought to be treated cautiously. Accordingly, further prospective studies measuring CR with a multidimensional approach are warranted to develop a deeper understanding of the underlying neural mechanism of CR and its contribution to the prognosis of ischemic stroke.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL and YW designed the study and search strategy. YY, YX, and SZ performed the literature search and assessment. CT, SW, and ZW contributed to data extraction. CT wrote the first draft of the manuscript, and all authors provided critical revision and approved the final version.
Funding
This study was supported by the National Key Research Program of China (2016YFE0126000), the general program of natural science research in colleges and universities of Jiangsu Province (21KJD320005), the Open Project of Key Laboratory of Animal Genetic Breeding and Molecular Design in Jiangsu Province (AGBMD2021), and the projects supported by the Six Talent Peaks in Jiangsu Province (WSN-082).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1100469/full#supplementary-material
References
1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Vaartjes I, O'Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. (2013) 44:591–7. doi: 10.1161/STROKEAHA.112.677724
4. Ankolekar S, Renton C, Sare G, Ellender S, Sprigg N, Wardlaw JM, et al. Relationship between post-stroke cognition, baseline factors, and functional outcome: data from “efficacy of nitric oxide in stroke” trial. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. (2014) 23:1821–9. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.022
5. Leys D, Hénon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. (2005) 4:752–9. doi: 10.1016/S1474-4422(05)70221-0
6. Creutzfeldt CJ, Holloway RG, Walker M. Symptomatic and palliative care for stroke survivors. J Gen Intern Med. (2012) 27:853–60. doi: 10.1007/s11606-011-1966-4
7. Lee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, et al. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehabil Res. (2015) 38:173–80. doi: 10.1097/MRR.0000000000000108
8. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. (2009) 8:1006–18. doi: 10.1016/S1474-4422(09)70236-4
9. Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. (2008) 63:272–87. doi: 10.1002/ana.21393
10. Hillis AE, Tippett DC. Stroke recovery: surprising influences and residual consequences. Adv Med. (2014) 2014:263. doi: 10.1155/2014/378263
11. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc JINS. (2002) 8:448–60. doi: 10.1017/S1355617702813248
12. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dement J Alzheimer's Assoc. (2020) 16:1305–11. doi: 10.1016/j.jalz.2018.07.219
13. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cognit Sci. (2013) 17:502–9. doi: 10.1016/j.tics.2013.08.012
14. Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc JINS. (2011) 17:593–601. doi: 10.1017/S1355617710001748
15. Umarova RM. Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: implications for understanding neglect. Cortex J Devot Study Nerv Syst Behav. (2017) 97:327–38. doi: 10.1016/j.cortex.2016.12.006
16. Shin M, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, et al. Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke: the Kosco study. Stroke. (2020) 51:99–107. doi: 10.1161/STROKEAHA.119.026829
17. Umarova RM, Sperber C, Kaller CP, Schmidt CSM, Urbach H, Klöppel S, et al. Cognitive reserve impacts on disability and cognitive deficits in acute stroke. J Neurol. (2019) 266:2495–504. doi: 10.1007/s00415-019-09442-6
18. MacPherson SE, Healy C, Allerhand M, Spanò B, Tudor-Sfetea C, White M, et al. Cognitive reserve and cognitive performance of patients with focal frontal lesions. Neuropsychologia. (2017) 96:19–28. doi: 10.1016/j.neuropsychologia.2016.12.028
19. Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Neuropsychol Dev Cognit Sect B Aging Neuropsychol Cognit. (2016) 23:40–60. doi: 10.1080/13825585.2015.1041450
20. Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol Med. (2006) 36:1065–73. doi: 10.1017/S0033291706007744
21. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
22. Zhou DH, Wang JY Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. (2004) 251:421–7. doi: 10.1007/s00415-004-0337-z
23. Zhou G, Liu X, Xu G, Liu X, Zhang R, Zhu W. The effect of socioeconomic status on 3-year mortality after first-ever ischemic stroke in Nanjing, china. BMC Public Health. (2006) 6:227. doi: 10.1186/1471-2458-6-227
24. Liu X, Lv Y, Wang B, Zhao G, Yan Y, Xu D. Prediction of functional outcome of ischemic stroke patients in northwest china. Clin Neurol Neurosurg. (2007) 109:571–7. doi: 10.1016/j.clineuro.2007.05.008
25. Cesaroni G, Agabiti N, Forastiere F, Perucci CA. Socioeconomic differences in stroke incidence and prognosis under a universal healthcare system. Stroke. (2009) 40:2812–9. doi: 10.1161/STROKEAHA.108.542944
26. Withall A, Brodaty H, Altendorf A, Sachdev PS. Who does well after a stroke? The Sydney stroke study. Aging Mental Health. (2009) 13:693–8. doi: 10.1080/13607860902845525
27. Toivanen S. Income differences in stroke mortality: a 12-year follow-up study of the Swedish working population. Scand J Public Health. (2011) 39:797–804. doi: 10.1177/1403494811418280
28. Grube MM, Koennecke HC, Walter G, Thümmler J, Meisel A, Wellwood I, et al. Association between socioeconomic status and functional impairment 3 months after ischemic stroke: the Berlin stroke register. Stroke. (2012) 43:3325–30. doi: 10.1161/STROKEAHA.112.669580
29. Ojala-Oksala J, Jokinen H, Kopsi V, Lehtonen K, Luukkonen L, Paukkunen A, et al. Educational history is an independent predictor of cognitive deficits and long-term survival in post-acute patients with mild to moderate ischemic stroke. Stroke. (2012) 43:2931–5. doi: 10.1161/STROKEAHA.112.667618
30. Brown AF, Liang LJ, Vassar SD, Merkin SS, Longstreth WT, Ovbiagele B, et al. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. (2013) 80:520–7. doi: 10.1212/WNL.0b013e31828154ae
31. Goulart AC, Fernandes TG, Santos IS, Alencar AP, Bensenor IM, Lotufo PA. Predictors of long-term survival among first-ever ischemic and hemorrhagic stroke in a Brazilian stroke cohort. BMC Neurol. (2013) 13:51. doi: 10.1186/1471-2377-13-51
32. Bettger JP, Zhao X, Bushnell C, Zimmer L, Pan W, Williams LS, et al. The association between socioeconomic status and disability after stroke: Findings from the adherence evaluation after ischemic stroke longitudinal (avail) registry. BMC Public Health. (2014) 14:281. doi: 10.1186/1471-2458-14-281
33. Chen R, Crichton S, McKevitt C, Rudd AG, Sheldenkar A, Wolfe CD. Association between socioeconomic deprivation and functional impairment after stroke: the south london stroke register. Stroke. (2015) 46:800–5. doi: 10.1161/STROKEAHA.114.007569
34. Alladi S, Bak TH, Mekala S, Rajan A, Chaudhuri JR, Mioshi E, et al. Impact of bilingualism on cognitive outcome after stroke. Stroke. (2016) 47:258–61. doi: 10.1161/STROKEAHA.115.010418
35. Pan Y, Song T, Chen R, Li H, Zhao X, Liu L, et al. Socioeconomic deprivation and mortality in people after ischemic stroke: the China national stroke registry. Int J Stroke Off J Int Stroke Soc. (2016) 11:557–64. doi: 10.1177/1747493016641121
36. Shin J, Choi Y, Kim SW, Lee SG, Park EC. Cross-level interaction between individual socioeconomic status and regional deprivation on overall survival after onset of ischemic stroke: national health insurance cohort sample data from 2002 to 2013. J Epidemiol. (2017) 27:381–8. doi: 10.1016/j.je.2016.08.020
37. Song T, Pan Y, Chen R, Li H, Zhao X, Liu L, et al. Is there a correlation between socioeconomic disparity and functional outcome after acute ischemic stroke? PLoS ONE. (2017) 12:e0181196. doi: 10.1371/journal.pone.0181196
38. Yan H, Liu B, Meng G, Shang B, Jie Q, Wei Y, et al. The influence of individual socioeconomic status on the clinical outcomes in ischemic stroke patients with different neighborhood status in shanghai, china. Int J Med Sci. (2017) 14:86–96. doi: 10.7150/ijms.17241
39. Makin SD, Doubal FN, Shuler K, Chappell FM, Staals J, Dennis MS, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J. (2018) 3:145–56. doi: 10.1177/2396987317750517
40. Ding MY, Xu Y, Wang YZ Li PX, Mao YT Yu JT, et al. Predictors of cognitive impairment after stroke: a prospective stroke cohort study. J Alzheimer's Dis JAD. (2019) 71:1139–51. doi: 10.3233/JAD-190382
41. Vivanco-Hidalgo RM, Ribera A, Abilleira S. Association of socioeconomic status with ischemic stroke survival. Stroke. (2019) 50:3400–7. doi: 10.1161/STROKEAHA.119.026607
42. Wang S, Shen B, Wei L, Wu M, Wang J. Association between socioeconomic status and prognosis after ischemic stroke in south china. Neurol Res. (2019) 41:916–22. doi: 10.1080/01616412.2019.1630165
43. Che B, Shen S, Zhu Z, Wang A, Xu T, Peng Y, et al. Education level and long-term mortality, recurrent stroke, and cardiovascular events in patients with ischemic stroke. J Am Heart Assoc. (2020) 9:e016671. doi: 10.1161/JAHA.120.016671
44. Béjot Y, Bourredjem A, Mimeau E, Joux J, Lannuzel A, Misslin-Tritsch C, et al. Social deprivation and 1-year survival after stroke: a prospective cohort study. Eur J Neurol. (2021) 28:800–8. doi: 10.1111/ene.14614
45. Dong Y, Ding M, Cui M, Fang M, Gong L, Xu Z, et al. Development and validation of a clinical model (dream-ldl) for post-stroke cognitive impairment at 6 months. Aging. (2021) 13:21628–41. doi: 10.18632/aging.203507
46. Franc D, Šanák D, Divišová P, Lysková L, Bártková A, Zapletalová J, et al. Socioeconomic status and lifestyle in young ischaemic stroke patients: a possible relationship to stroke recovery and risk of recurrent event. Central Eur J Public Health. (2021) 29:223–9. doi: 10.21101/cejph.a6697
47. Liu X, Cheng C, Liu Z, Fan W, Liu C, Liu Y. Longitudinal assessment of anxiety/depression rates and their related predictive factors in acute ischemic stroke patients: a 36-month follow-up study. Medicine. (2021) 100:e28022. doi: 10.1097/MD.0000000000028022
48. Zhu Y, Lu Y, Zhou M, Huang P, Zhang P, Guo Y, et al. Occupational class differences in outcomes after ischemic stroke: a prospective observational study. BMC Public Health. (2021) 21:1571. doi: 10.1186/s12889-021-11624-9
49. Ghoneem A, Osborne MT, Abohashem S, Naddaf N, Patrich T, Dar T, et al. Association of socioeconomic status and infarct volume with functional outcome in patients with ischemic stroke. JAMA Netw Open. (2022) 5:e229178. doi: 10.1001/jamanetworkopen.2022.9178
50. Stern Y. Cognitive reserve. Neuropsychologia. (2009) 47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004
51. Chapko D, McCormack R, Black C, Staff R, Murray A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia: a systematic literature review. Aging Mental Health. (2018) 22:915–26. doi: 10.1080/13607863.2017.1348471
52. Dufouil C, Alpérovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. (2003) 60:831–6. doi: 10.1212/01.WNL.0000049456.33231.96
53. Brosnan MB, Demaria G, Petersen A, Dockree PM, Robertson IH, Wiegand I. Plasticity of the right-lateralized cognitive reserve network in ageing. Cerebral Cortex. (2018) 28:1749–59. doi: 10.1093/cercor/bhx085
54. Steffener J, Reuben A, Rakitin BC, Stern Y. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imag Behav. (2011) 5:212–21. doi: 10.1007/s11682-011-9125-4
55. Wang S, Zhai H, Wei L, Shen B, Wang J. Socioeconomic status predicts the risk of stroke death: a systematic review and meta-analysis. Prev Med Rep. (2020) 19:101124. doi: 10.1016/j.pmedr.2020.101124
56. Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. (2015) 14:1206–18. doi: 10.1016/S1474-4422(15)00200-8
57. Arrich J, Lalouschek W, Müllner M. Influence of socioeconomic status on mortality after stroke: retrospective cohort study. Stroke. (2005) 36:310–4. doi: 10.1161/01.STR.0000152962.92621.b5
58. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. (2012) 11:1006–12. doi: 10.1016/S1474-4422(12)70191-6
59. van Loenhoud AC, Wink AM, Groot C, Verfaillie SCJ, Twisk J, Barkhof F, et al. A neuroimaging approach to capture cognitive reserve: application to Alzheimer's disease. Hum Brain Mapp. (2017) 38:4703–15. doi: 10.1002/hbm.23695
Keywords: cognitive reserve, ischemic stroke, mortality, functional outcome, systematic-analysis
Citation: Tao C, Yuan Y, Xu Y, Zhang S, Wang Z, Wang S, Liang J and Wang Y (2023) Role of cognitive reserve in ischemic stroke prognosis: A systematic review. Front. Neurol. 14:1100469. doi: 10.3389/fneur.2023.1100469
Received: 20 November 2022; Accepted: 26 January 2023;
Published: 22 February 2023.
Edited by:
Mandip Singh Dhamoon, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Jörgen Borg, Karolinska Institutet (KI), SwedenJeevitha Mariapun, Monash University Malaysia, Malaysia
Giorgio Arcara, San Camillo IRCCS S.r.l. Società Unipersonale, Italy
Copyright © 2023 Tao, Yuan, Xu, Zhang, Wang, Wang, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingge Wang,  eWluZ2dld2FuZzI3OUBob3RtYWlsLmNvbQ==
eWluZ2dld2FuZzI3OUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Chunhua Tao
Chunhua Tao Yuan Yuan
Yuan Yuan Yijun Xu4
Yijun Xu4 Song Zhang
Song Zhang Yingge Wang
Yingge Wang