- 1Department of Medical Genetics and Center for Rare Diseases, Second Affiliated Hospital, Zhejiang University School of Medicine and Zhejiang Key Laboratory of Rare Diseases for Precision Medicine and Clinical Translation, Hangzhou, China
- 2Department of Neurology, Lishui Hospital, Zhejiang University School of Medicine, Lishui, China
- 3Department of Neurology, First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 4Department of Neurology, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China
- 5Research Centre for Intelligent Healthcare, Faculty of Health and Life Sciences, Coventry University, Coventry, United Kingdom
Objective: This study aims to investigate the association of Fc receptor-like 3 (FCRL3) gene variants with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) in a Chinese population cohort.
Methods: In Stage 1, 154 MS patients, 109 NMOSD patients, and 301 normal controls were recruited, Sequenom MassARRAY technology was used for genotyping single nucleotide polymorphisms (SNPs). Stage 2 involved an independent cohort of 95 MS patients, 139 NMOSD patients, and 226 normal controls. Two FCRL3 SNPs (rs7528684 and rs11264799) were determined using allele-specific polymerase chain reaction (PCR) with specific primers.
Results: Allele C of rs7528684 emerged as a protective factor for MS. Allele A of rs11264799 exhibited no significant effect on MS or NMOSD. A notable disparity in rs7528684 genotype distribution was observed between oligoclonal band (OCB)-positive and OCB-negative MS patients. Allele C of rs7528684 exhibited an association with OCB-positive MS patients.
Conclusion: The findings suggest that the FCRL3 variant (rs7528684) is associated with MS rather than NMOSD. FCRL3 might significantly contribute to OCB synthesis, while the underlying mechanisms warrant further elucidation.
1 Introduction
Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) are autoimmune inflammatory demyelinating conditions affecting the central nervous system (CNS) that primarily afflict young females (1). While the precise pathogenesis of MS and NMOSD remains incompletely elucidated, it is associated with a spectrum of genetic and environmental risk factors (2, 3). NMOSD often affects only the optic nerve and spinal cord, and can involve vision problems in both eyes. In comparison, MS can affect other locations in the CNS but often one eye. Despite these nuances, the similarities between NMOSD and MS in symptom and pathology make the accurate diagnosis difficult (4). Moreover, their clinical manifestations and pathological profiles exhibit considerable diversity across different populations, posing challenges in the accurate diagnosis.
Supplementary examinations, e.g., cerebrospinal fluid (CSF) analysis and imaging play a vital role in definitive diagnosis of NMOSD and MS (5). CSF analysis is instrumental in detecting intrathecal immunoglobulin synthesis, notably through assessing the immunoglobulin G (IgG) index and CSF-specific oligonucleotide bands (OCB), providing pivotal insights into central nervous system inflammation (6). In contrast to MS, NMOSD exhibits a higher prevalence among East Asians and other non-white populations (7, 8). Most NMOSD patients demonstrate the presence of a serum autoantibody specific to the astrocyte water channel protein aquaporin-4 (AQP4) (9, 10). Revised NMOSD criteria classify the condition into AQP4 antibody-positive (AQP4+) and AQP4 antibody-negative (AQP4-) subtypes, with AQP4 + patients showing a higher propensity for relapses compared to AQP4- individuals (11). Despite the unequivocal role of B cells in antibody production, the genetic underpinnings of this antibody production largely remain elusive.
A recent multi-center genome-wide association study (GWAS) identified several novel loci associated with MS, among which the HLA DRB1*1501 allele showed the strongest association (12). Located in the lq21 region, FCRL3 encodes a Fc receptor-like (FCRL) molecule exhibiting significant structural homology with Fc region immunoglobulin receptors, implicating its role in various autoimmune diseases such as psoriasis, Sjogren’s syndrome, and autoimmune thyroid disorders (13–15). While the G allele of rs3761959 in FCRL3 was initially identified as a risk factor for MS in the primary GWAS phase, subsequent replication across diverse ethnic groups yielded inconclusive results, underscoring the influence of genetic diversity (12). Previous case–control studies in the Spanish population revealed that the C allele of rs7528684, situated in the promoter region of FCRL3, acted as a protective factor against MS (16, 17). Interestingly, rs7528684 has exhibited strong association with rheumatoid arthritis (RA) and anti-cyclic citrullinated peptide (CCP) antibody positivity in the Japanese population (18), hinting at FCRL3’s potential involvement in antibody production in autoimmune diseases. A previous study identified the G allele in the −1901A > G polymorphism and the T allele in the -658C > T polymorphism as genetic risk factors for NMO in the Chinese population. Another study found four SNPs in the FCRL3 gene (FCRL3_3C, 5C, 6A, 8G) that may increase the risk of NMO in the Han Chinese population (19, 20). However, despite these findings, no study to date has investigated the link between FCRL3 variants and the presence of antibodies in MS and NMOSD. Moreover, the diverse associations of FCRL3 variants with MS susceptibility in prior studies highlight the complexity and variability within this genetic context (16, 17, 21).
In this study, our objective was to investigate the potential association of FCRL3 variants with both MS and NMOSD among individuals within the Chinese population. Additionally, we aimed to assess the relationship between FCRL3 variants and the presence of OCB as well as AQP4 antibody (AQP4-ab).
2 Materials and methods
2.1 Subjects
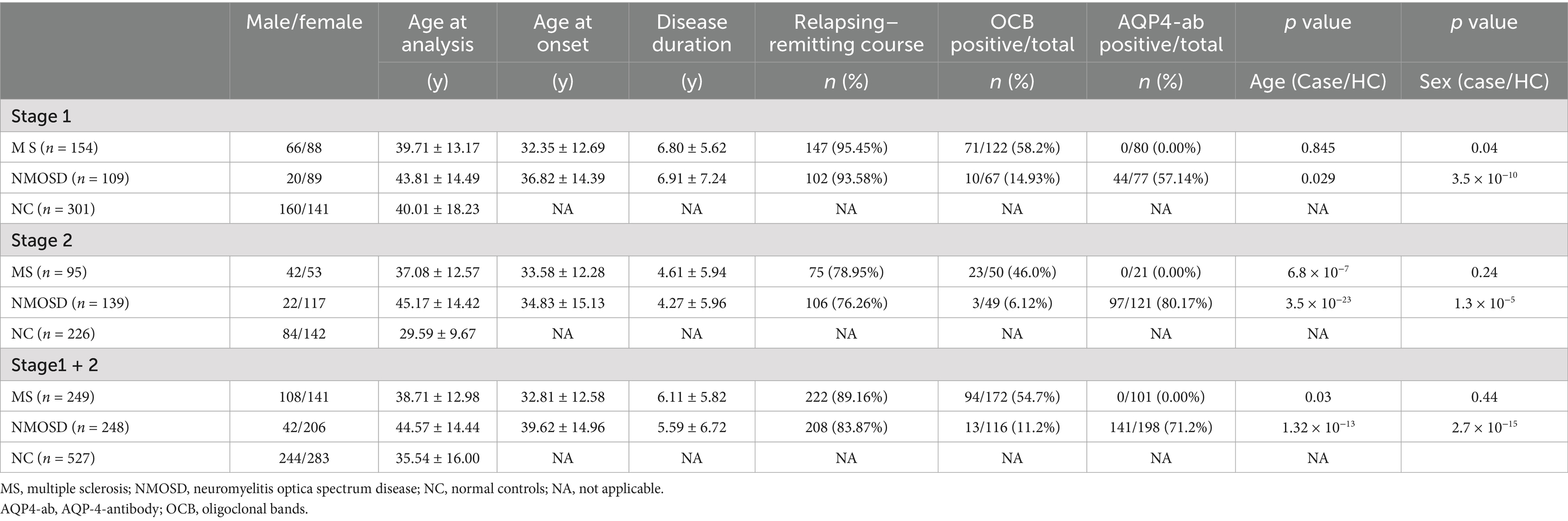
Table 1 provides an overview of the general clinical characteristics of the subjects involved in this study. For Stage 1, we recruited 154 MS patients (88 females and 66 males), 109 NMOSD patients (89 females and 20 males), and 301 normal controls (141 females and 160 males) from the First Affiliated Hospital of Fujian Medical University and Huashan Hospital of Fudan University between October 25, 2007, and February 4, 2012. Among these individuals, the positive rate of oligoclonal bands in MS patients was 58.2%, while the positive rate of AQP4 antibody in NMOSD patients was 57.14%.
For Stage 2, a separate cohort consisting of 95 MS patients (53 females and 42 males), 139 NMOSD patients (117 females and 22 males), and 226 normal controls (142 females and 84 males) was enrolled from the Second Affiliated Hospital of Zhejiang University School of Medicine and the First Affiliated Hospital of Fujian Medical University between March 6, 2013, and March 31, 2021. In this stage, the positive rate of oligoclonal bands in MS patients was 46.0%, whereas the positive rate of AQP4 antibody in NMOSD patients increased to 80.17%. All participants met the current diagnostic criteria for either MS or NMOSD (5, 11).
Detection of AQP4 antibodies was conducted using an indirect immunofluorescence assay with a Euroimmun (Germany) kit, following the manufacturer’s instructions. Transfected and non-transfected AQP4 cells were incubated with diluted serum and FITC-labeled goat anti-human IgG successively. A positive result was indicated by distinct fluorescence on the surface of AQP4-transfected cells, matching the AQP4 expression pattern, with no fluorescence in non-transfected cells. Oligoclonal band detection was conducted using the isoelectric focusing technique followed by agarose gel electrophoresis. Ethical approval for this study was obtained from the Ethics Committees of the mentioned research centers, and informed consent was obtained from all participants or their guardians.
2.2 Two-stage SNP genotyping
Genomic DNA extraction from peripheral blood mononuclear cells was conducted using the QIAamp DNA Blood Minikit (QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. All participants underwent genotyping utilizing the Sequenom MassArray system. Four FCRL3 variants (rs7528684, rs11264799, rs3761959, and rs7522061), previously associated with MS (12), were selected from the GWAS database.
Stage 1 involved genotyping these four SNPs in 154 MS patients, 109 NMOSD patients, and 301 controls at the Fudan-Van Andel Research Institute (VARI) Center (School of Life Science, Fudan University, China). This was carried out using the matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) platform (MassArray TM, Sequenom Inc., San Diego, CA, USA), following a previously established method (22). PCR and extension primers were designed using MassArray Assay Design 3.1 software (Sequenom, San Diego, CA, USA) (Supplementary Table 1).
Based on Stage 1 results, two SNPs of FCRL3 were chosen for replication in Stage 2, encompassing 95 MS patients, 139 NMOSD patients, and 226 normal controls. This replication stage employed allele-specific PCR with the designated primers (Supplementary Table 2).
2.3 Statistical analysis
The Hardy–Weinberg equilibrium was tested using the Chi-squared test. To evaluate differences in allele and genotype frequencies between patients and controls, the Chi-squared test or Fisher’s exact test was employed. Utilizing a binary logistic regression model adjusted for age and gender, odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were estimated.
Linkage disequilibrium (LD) was determined using SHEsis software (23) available at http://analysis2.bio-x.cn/SHEsisMain.htm. All statistical analyses were conducted using SPSS 23.0 software. Statistical significance was set at a p-value less than 0.05.
3 Results
3.1 Genotyping of four SNPs in MS, NMOSD and control groups
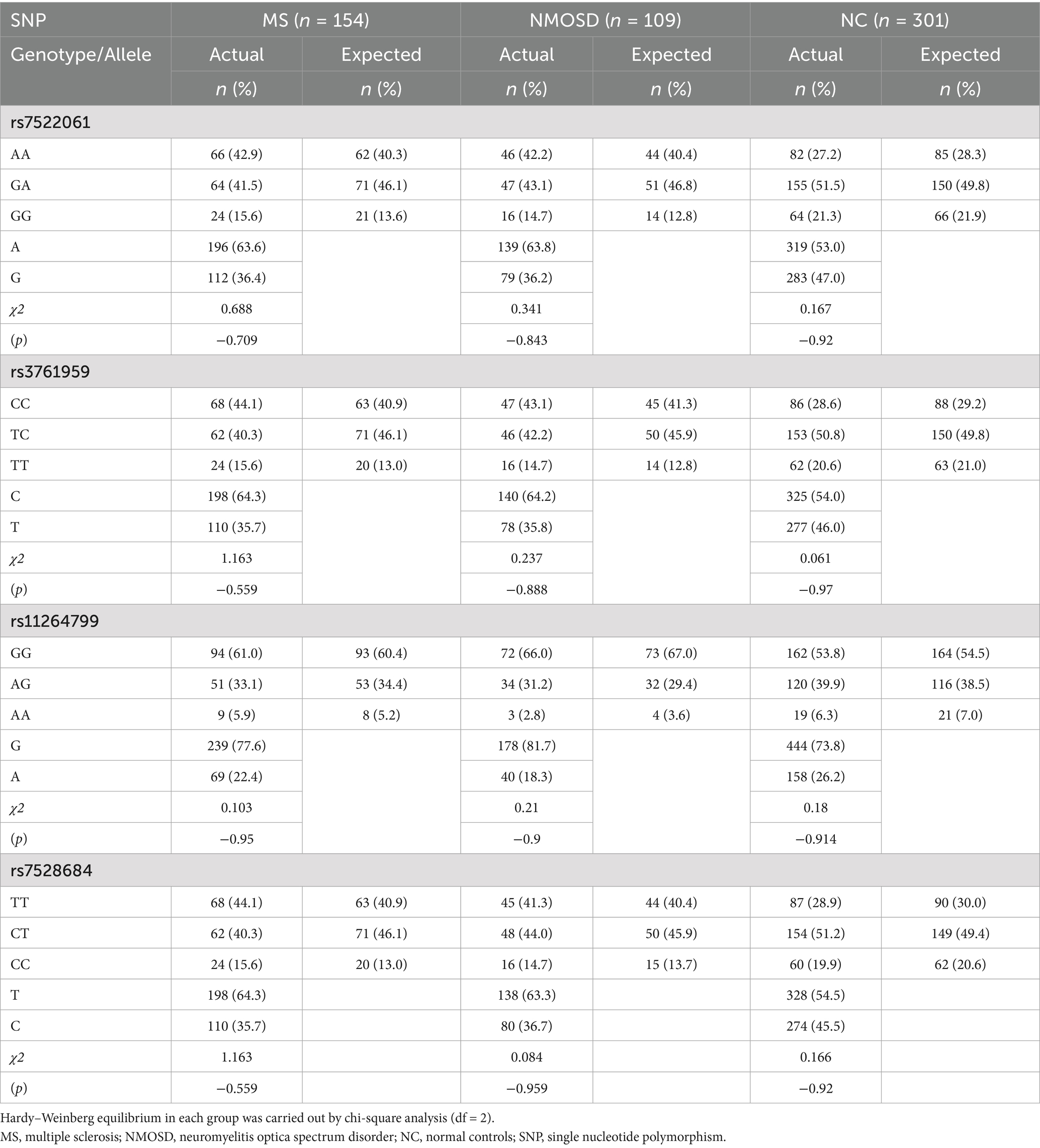
The four SNPs (rs7528684, rs11264799, rs3761959, and rs7522061) within FCRL3 demonstrated Hardy–Weinberg equilibrium in both MS/NMOSD cases and controls (p > 0.05) (Table 2). Comparing the gene frequency distribution of these four SNPs between the MS group and the control group revealed significant associations: rs7522061 (p = 0.0003, OR = 0.593), rs3761959 (p = 0.0005, OR = 0.601), rs7528684 (p = 0.0007, OR = 0.616), rs11264799 (p = 0.189, OR = 0.778). The genomic locations of these selected FCRL3 variants were as follows: rs7522061 located in exon 3, rs3761959 in intron 2, and rs11264799 along with rs7528684 in the promoter region (−110 position and −169 position, respectively).
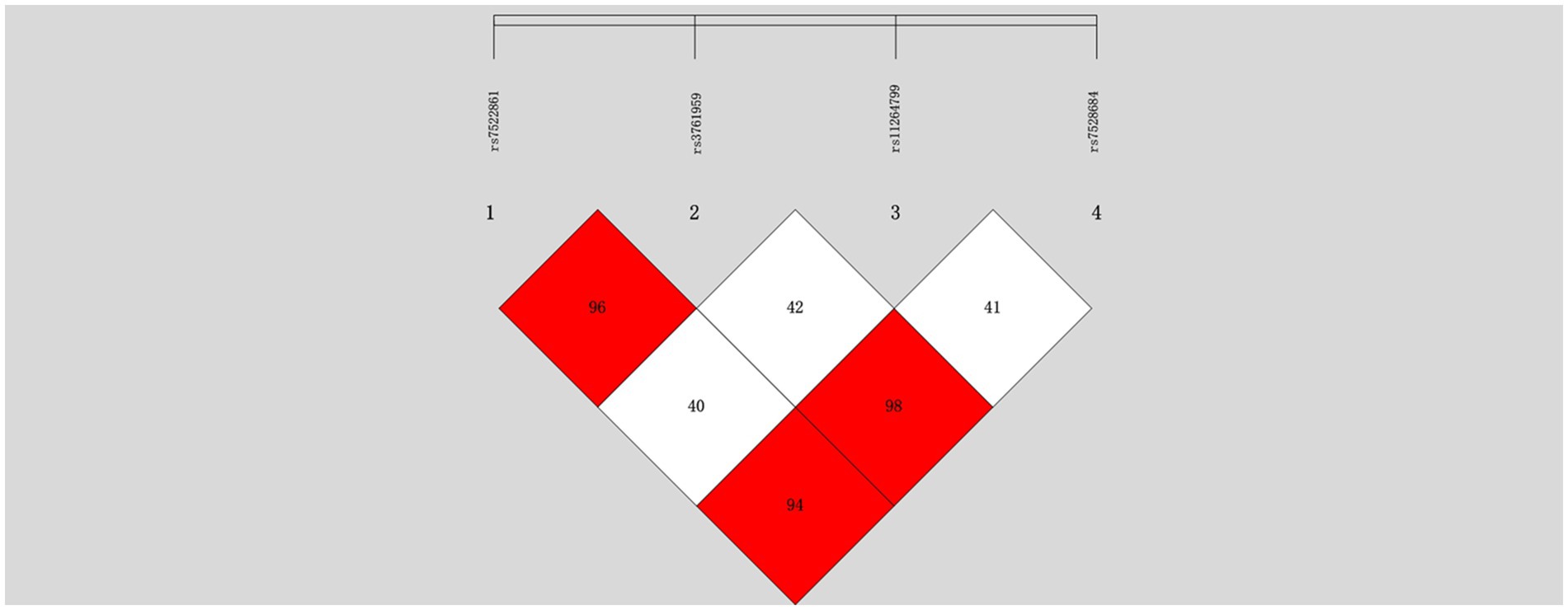
We observed that rs7528684, positioned in the promoter of FCRL3, exhibited high linkage disequilibrium (LD) compared to the other two SNPs (rs7522061 and rs3761959). However, rs11264799, another SNP in the promoter region, displayed relatively lower LD with the aforementioned three SNPs (mean R2 = 0.41) in the controls (Figure 1). Consequently, statistical analyses were focused on rs7528684 and rs11264799.
3.2 FCRL3 is associated with MS in the Chinese population
In Stage 1 (Table 3), the less frequent occurrence of allele C of rs7528684 was noted in both MS and NMOSD patients compared to controls (MS vs. controls: p = 0.005, OR = 0.665, 95% CI: 0.501–0.883; NMOSD vs. controls: p = 0.024, OR = 0.694, 95% CI: 0.505–0.954). Conversely, allele A of rs11264799 was less common in NMOSD (p = 0.020, OR = 0.631, 95% CI: 0.428–0.931) but not in MS when compared to controls. After adjusting for age and gender in a logistic regression model, allele C of rs7528684 demonstrated protective effects for MS in the genotype of dominant model (CT + CC vs. TT, p = 0.002, OR = 0.528, 95% CI: 0.352–0.793) but not for NMOSD. Similarly, allele A of rs11264799 acted as a protective factor for NMOSD in the dominant model (AG + AA vs. GG, p = 0.005, OR = 0.492, 95% CI: 0.300–0.806) but not for MS.
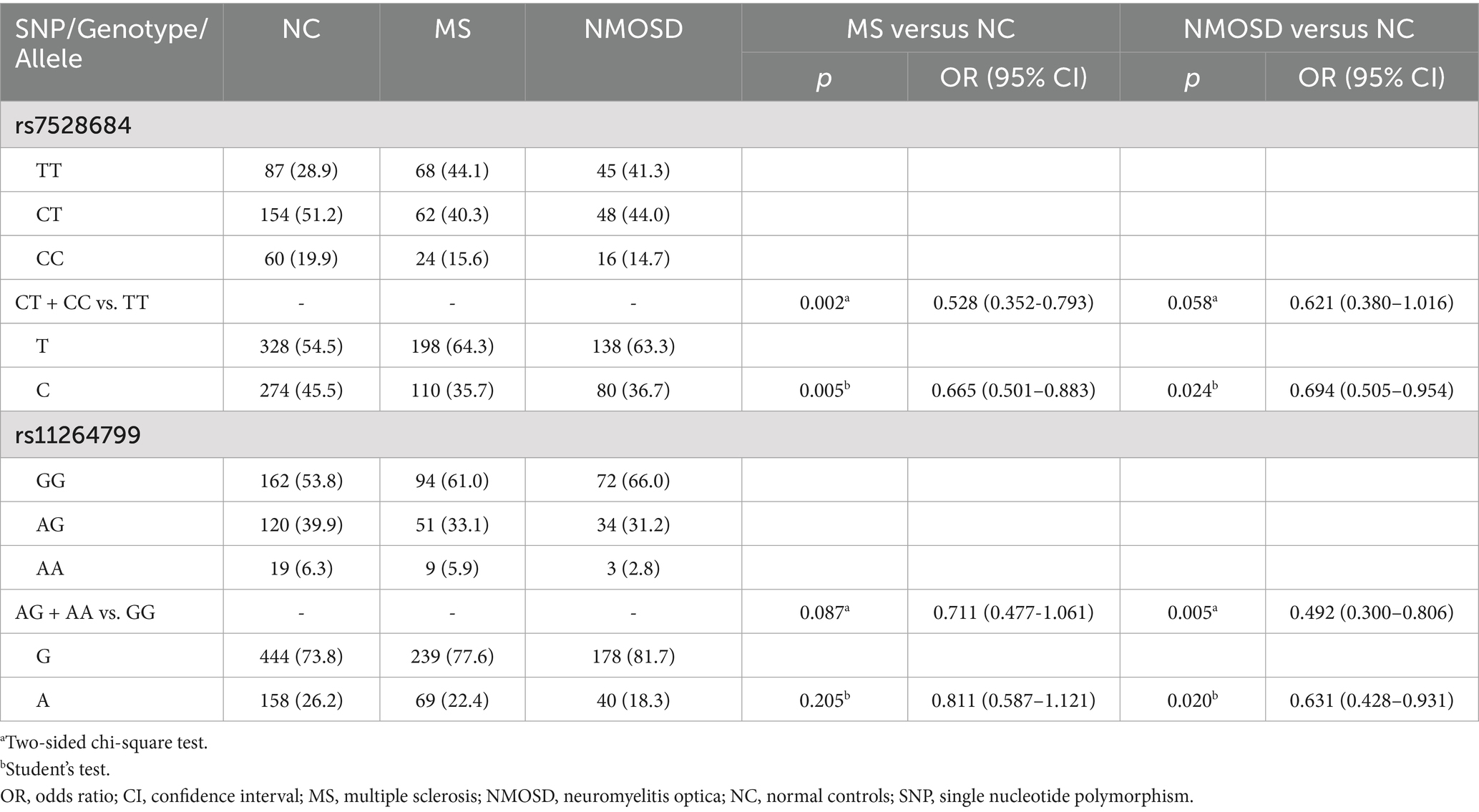
Table 3. Genotype and allele frequencies of rs7528684 and rs11264799 in MS, NMOSD and controls in Stage 1.
Consequently, rs7528684 and rs11264799 were selected for confirmation in Stage 2. In Stage 2 (Table 4), allele C of rs7528684 remained less prevalent in MS patients compared to controls (p = 0.015, OR = 0.647, 95% CI: 0.455–0.920), maintaining its protective association in the dominant model (CT + CC vs. TT, p = 0.004, OR = 0.466, 95% CI: 0.276–0.788), consistent with Stage 1 findings. However, no significant association was found between NMOSD and controls. Considering both Stage 1 and Stage 2 collectively (Table 5), the association of rs7528684 strengthened, showing a significant protective association with MS (p = 1.93 × 10−4, OR = 0.659, 95% CI: 0.529–0.821). Nevertheless, allele A of rs11264799 did not exhibit significant differences in either MS or NMOSD compared to controls (MS vs. controls: p = 0.102, OR = 0.808, 95% CI: 0.625–1.044; NMOSD vs. controls: p = 0.077, OR = 0.792, 95% CI: 0.612–1.025).
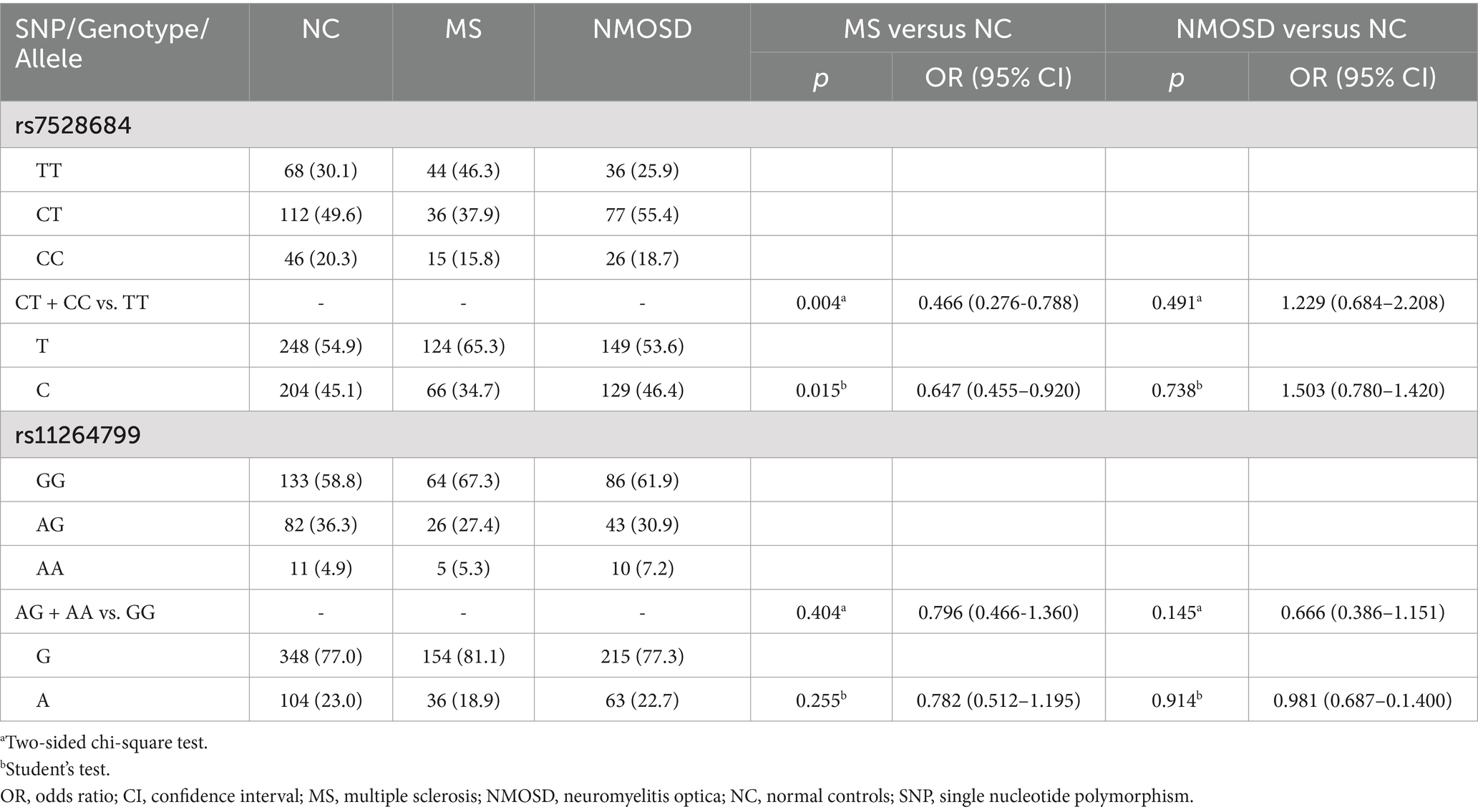
Table 4. Genotype and allele frequencies of rs7528684 and rs11264799 in MS, NMOSD and controls in Stage 2.
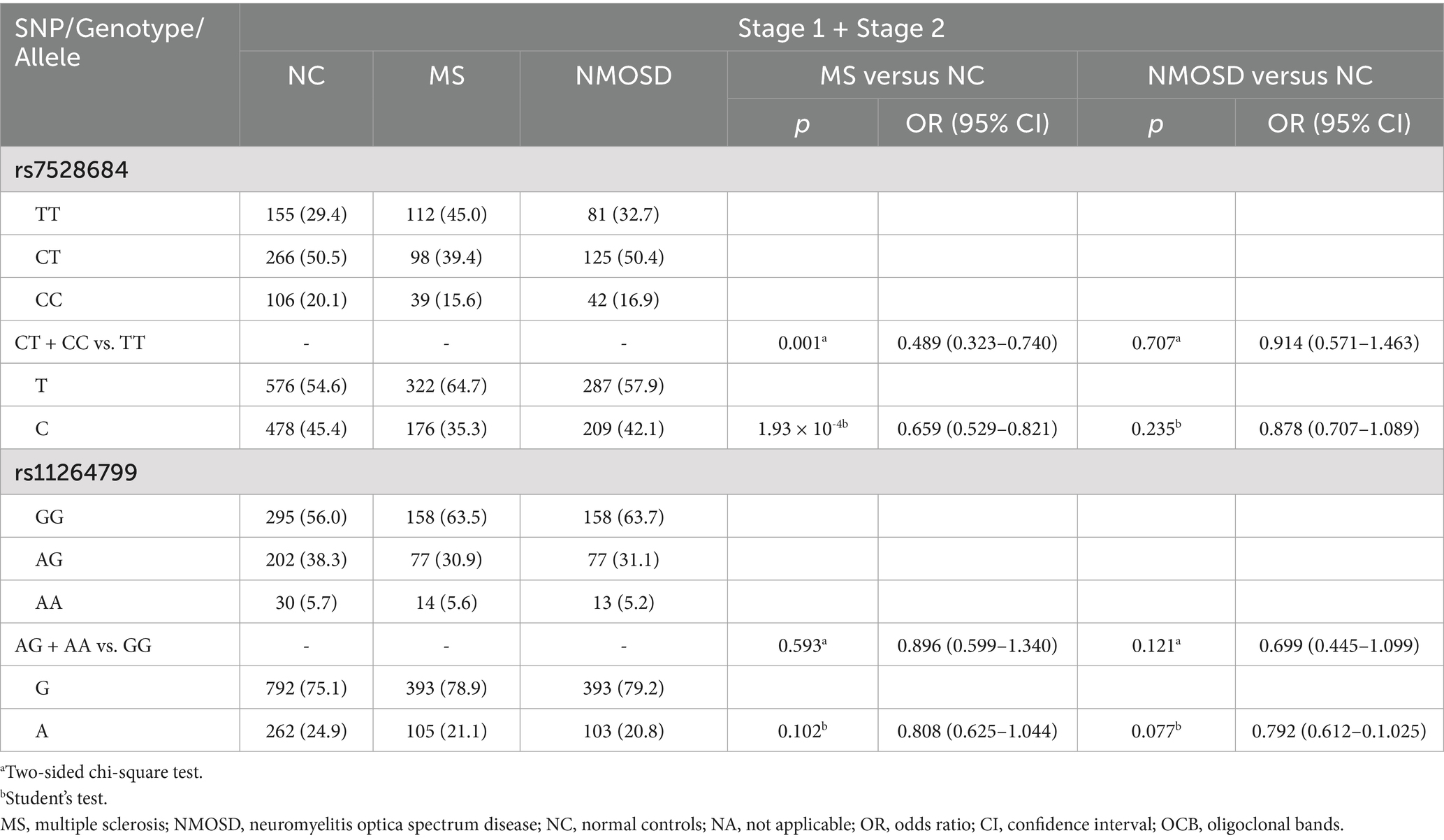
Table 5. Genotype and allele frequencies of rs7528684 and rs11264799 in MS, NMOSD and controls in two Stages.
3.3 FCRL3 is associated with the presence of OCB in MS
Stratification based on the presence of OCB in CSF among MS patients was conducted to further explore the association between immune response and genotype. Table 6 displays a significant disparity in the genotype distribution of rs7528684 between OCB-positive and OCB-negative MS patients (p = 0.001). Notably, the C allele of rs7528684 exhibited an association with OCB positivity in MS (p = 1.4 × 10−5; OR = 0.37; 95% CI: 0.235 to 0.583). Moreover, this allele demonstrated association solely with OCB-positive MS when compared to controls (p = 4.1 × 10–7; OR = 0.413; 95% CI: 0.291 to 0.586).
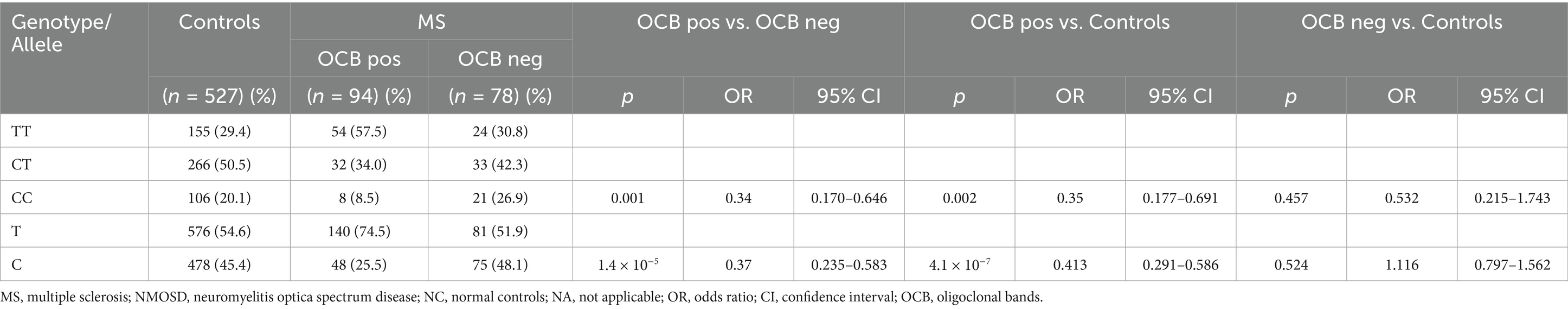
Table 6. Genotype and allele frequencies of rs7528684 in all MS and controls in stage 1 and 2 divided by OCB status.
4 Discussion
This investigation delves into the correlation between four FCRL3 SNPs and MS/NMOSD within the Chinese population. Notably, we observed that genotype and allele frequencies of rs7528684 were protective against MS, specifically highlighting the protective role of the C allele. Confirming our findings, a combined analysis of two distinct cohorts further emphasized this association, aligning with prior research (17). However, divergent findings in other studies have indicated associations of this variant with increased susceptibility to MS (16, 21).
The investigation of OCB positivity in MS, predominantly observed in Caucasian populations (85–98%), contrasted with lower incidences in Asian cohorts (21–63%) (24–28). Our study’s OCB prevalence (54.7%) aligns with previous reports, hinting at potential immunogenetic influences on OCB production. Notably, the association of DRB1*15 with increased OCB frequency across diverse populations, including Japan, Sardinia, Sweden, and Spain, has been reported (29). Our findings further suggest an inverse association between the C allele of rs7528684 in FCRL3 and the presence of OCB in MS patients. These results propose FCRL3 as an immunogenetic factor potentially involved in OCB production.
The involvement of FCRL3, primarily expressed in mature B cells within lymph nodes, suggests a potential functional role in B cell maturation regulation. Its cytoplasmic domain, comprising immune receptor tyrosine activating motif (ITAM) and immune receptor tyrosine inhibiting motif (ITIM), hints at its influence on B cell signal transduction by either activating or inhibiting cellular pathways (30). FCRL3 as a functional molecule within the immune system implies its potential role in autoimmune disease pathology, necessitating further investigation to elucidate genotype–phenotype correlations. Notably, the rs7528684 C allele exhibited a strong inverse association with insulinoma-associated antigen 2 (IA2), an anti-islet autoantibody linked to Type 1 diabetes (31). Studies have indicated that the C allele of rs7528684 could enhance nuclear factor-kappa B (NF-κB) binding affinity, subsequently elevating FCRL3 expression (18, 32). Studies reveal lower FCRL3 and interleukin-10 (IL-10) levels in MS patients, yet both are more elevated during remission than in the acute phase. This implies FCRL3 is pivotal for immune protection in MS. It activates the SHP-1 and p38 MAPK pathways, boosting IL-10 secretion and curbing inflammatory factor release (32). FCRL3 expression in B cells is influenced by stimuli such as lipopolysaccharides and CpG, impacting B cell survival, proliferation, and antibody production (33). In MS patients, abnormal B cell activation is linked to oligoclonal band formation. FCRL3 dysfunction may cause excessive B cell activation, driving oligoclonal band formation and disease progression (34).
Regarding rs11264799, the initial Stage 1 analysis suggested the A allele as a protective factor for NMOSD but not for MS, failing confirmation in Stage 2. This outcome contradicts a previous study (19), potentially indicating its role in disease susceptibility when triggered or in interaction with other disease-specific genetic variants or uneven distribution within the population. The observed protective effects of rs7528684 and rs11264799 on MS and NMOSD might be attributed to disease complexity, intricate genetic responses, and disease heterogeneity. These complexities necessitate further exploration to comprehend their precise roles in disease pathogenesis and their potential as therapeutic targets.
This study presents preliminary evidence suggesting that FCRL3 polymorphisms may protect against MS, possibly through neuroprotective mechanisms associated with this genetic location. However, there are several methodological limitations that need to be addressed. First, the small sample size may have restricted the statistical power of our findings, potentially hindering the identification of subtle genetic factors. It is crucial to carry out future replication studies involving larger and more diverse cohorts to confirm these results. Second, as the concept of Myelin Oligodendrocyte Glycoprotein (MOG) antibodies had not been established at the time, we did not conduct further subgroup analyses in AQP4- patients. Third, prospective cohorts with sex-stratified recruitment should be established and subgroup analyses performed to uncover potential gender-specific genetic effects, as the retrospective design revealed an uneven sex distribution within the case group. This raises significant concerns given the well-documented sexual dimorphism in MS epidemiology. Furthermore, due to incomplete clinical information, we were unable to examine the correlation between oligoclonal-IgG bands and clinical data, preventing us from considering all relevant factors that may impact oligoclonal-IgG bands. Regarding disease-modifying therapies (DMT), many patients were not receiving these treatments as the first oral DMT in China was only introduced in 2018 (35). In the future, the correlation between Oligoclonal-IgG bands and clinical information, such as age at onset, gender, years of disease at specimen collection, EDSS, number of recurrences, and the presence or absence of DMT, should be analyzed simultaneously. Such efforts could further clarify how FCRL3-modulated immune pathways contribute to the pathogenesis of MS, thereby enhancing our understanding of this complex disorder.
Exploring the functional role of FCRL3 variants stands as an important and intriguing area further investigation.
5 Conclusion
FCRL3 variants show associations MS and NMOSD within the Chinese population, underscoring the need for future studies to validate these findings across broader ethnic cohorts. Moreover, FCRL3 might serve as a significant contributor to OCB synthesis, warranting detailed exploration to unravel the precise underlying mechanisms. These findings warrant validation in larger cohorts.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/snp/, 90.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Fujian Medical University, Huashan Hospital of Fudan University and the Second Affiliated Hospital of Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
H-FH: Data curation, Formal analysis, Methodology, Writing – original draft. Q-BL: Data curation, Formal analysis, Methodology, Writing – original draft. Y-FX: Data curation, Formal analysis, Writing – review & editing. G-XZ: Data curation, Writing – review & editing. H-PL: Formal analysis, Writing – review & editing. HY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing. Z-YW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the research foundation for distinguished scholar of Zhejiang University to Zhi-Ying Wu (188020–193810101/089) and the National Natural Science Foundation of China (82201513).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1552149/full#supplementary-material
References
1. Ochi, H, and Fujihara, K. Demyelinating diseases in Asia. Curr Opin Neurol. (2016) 29:222–8. doi: 10.1097/WCO.0000000000000328
2. Jasiak-Zatonska, M, Kalinowska-Lyszczarz, A, Michalak, S, and Kozubski, W. The immunology of Neuromyelitis Optica-current knowledge, clinical implications, controversies and future perspectives. Int J Mol Sci. (2016) 17:273. doi: 10.3390/ijms17030273
3. Munger, K, and Ascherio, A. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. (2016) 36:103–14. doi: 10.1055/s-0036-1579693
4. Seok, JM, Cho, W, Chung, YH, Ju, H, Kim, ST, Seong, J-K, et al. Differentiation between multiple sclerosis and neuromyelitis optica spectrum disorder using a deep learning model. Sci Rep. (2023) 13:11625. doi: 10.1038/s41598-023-38271-x
5. Thompson, AJ, Banwell, BL, Barkhof, F, Carroll, WM, Coetzee, T, Comi, G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
6. Zhang, X, Hao, H, Jin, T, Qiu, W, Yang, H, Xue, Q, et al. Cerebrospinal fluid oligoclonal bands in Chinese patients with multiple sclerosis: the prevalence and its association with clinical features. Front Immunol. (2023) 14:1280020. doi: 10.3389/fimmu.2023.1280020
7. Hor, JY, Asgari, N, Nakashima, I, Broadley, SA, Leite, MI, Kissani, N, et al. Epidemiology of Neuromyelitis Optica Spectrum disorder and its prevalence and incidence worldwide. Front Neurol. (2020) 11:501. doi: 10.3389/fneur.2020.00501
8. Tian, DC, Li, Z, Yuan, M, Zhang, C, Gu, H, Wang, Y, et al. Incidence of neuromyelitis optica spectrum disorder (NMOSD) in China: a national population-based study. Lancet Reg Health West Pac. (2020) 2:100021. doi: 10.1016/j.lanwpc.2020.100021
9. Lennon, VA, Wingerchuk, DM, Kryzer, TJ, Pittock, SJ, Lucchinetti, CF, Fujihara, K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
10. Wingerchuk, DM, Lennon, VA, Lucchinetti, CF, Pittock, SJ, and Weinshenker, BG. The spectrum of neuromyelitis optica. Lancet Neurol. (2007) 6:805–15. doi: 10.1016/S1474-4422(07)70216-8
11. Wingerchuk, DM, Banwell, B, Bennett, JL, Cabre, P, Carroll, W, Chitnis, T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
12. Sawcer, S, Hellenthal, G, Pirinen, M, Spencer, CC, Patsopoulos, NA, Moutsianas, L, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. (2011) 476:214–9. doi: 10.1038/nature10251
13. Capon, F, Semprini, S, Chimenti, S, Fabrizi, G, Zambruno, G, Murgia, S, et al. Fine mapping of the PSORS4 psoriasis susceptibility region on chromosome 1q21. J Invest Dermatol. (2001) 116:728–30. doi: 10.1046/j.1523-1747.2001.01311.x
14. Dai, KZ, Harbo, HF, Celius, EG, Oturai, A, Sorensen, PS, Ryder, LP, et al. The T cell regulator gene SH2D2A contributes to the genetic susceptibility of multiple sclerosis. Genes Immun. (2001) 2:263–8. doi: 10.1038/sj.gene.6363774
15. Agarwal, S, Kraus, Z, Dement-Brown, J, Alabi, O, Starost, K, and Tolnay, M. Human fc receptor-like 3 inhibits regulatory T cell function and binds secretory IgA. Cell Rep. (2020) 30:1292–1299.e3. doi: 10.1016/j.celrep.2019.12.099
16. Martinez, A, Mas, A, de Las Heras, V, Bartolome, M, Arroyo, R, Fernandez-Arquero, M, et al. FcRL3 and multiple sclerosis pathogenesis: role in autoimmunity? J Neuroimmunol. (2007) 189:132–6. doi: 10.1016/j.jneuroim.2007.06.018
17. Matesanz, F, Fernandez, O, Milne, RL, Fedetz, M, Leyva, L, Guerrero, M, et al. The high producer variant of the fc-receptor like-3 (FCRL3) gene is involved in protection against multiple sclerosis. J Neuroimmunol. (2008) 195:146–50. doi: 10.1016/j.jneuroim.2008.01.004
18. Kochi, Y, Yamada, R, Suzuki, A, Harley, JB, Shirasawa, S, Sawada, T, et al. A functional variant in FCRL3, encoding fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. (2005) 37:478–85. doi: 10.1038/ng1540
19. Wang, X, Tao, Y, Yan, Q, Wang, W, Meng, N, Li, X, et al. Significant association between fc receptor-like 3 polymorphisms (−1901A>G and -658C>T) and Neuromyelitis Optica (NMO) susceptibility in the Chinese population. Mol Neurobiol. (2016) 53:686–94. doi: 10.1007/s12035-014-9036-7
20. Lan, W, Fang, S, Zhang, H, Wang, DTJ, and Wu, J. The fc receptor-like 3 polymorphisms (rs7528684, rs945635, rs3761959 and rs2282284) and the risk of Neuromyelitis Optica in a Chinese population. Medicine. (2015) 94:e1320. doi: 10.1097/MD.0000000000001320
21. Yuan, M, Wei, L, Zhou, R, Bai, Q, Wei, Y, Zhang, W, et al. Four FCRL3 gene polymorphisms (FCRL3_3, _5, _6, _8) confer susceptibility to multiple sclerosis: results from a Case-Control study. Mol Neurobiol. (2016) 53:2029–35. doi: 10.1007/s12035-015-9149-7
22. Buetow, KH, Edmonson, M, MacDonald, R, Clifford, R, Yip, P, Kelley, J, et al. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci USA. (2001) 98:581–4. doi: 10.1073/pnas.98.2.581
23. Li, Z, Zhang, Z, He, Z, Tang, W, Li, T, Zeng, Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis. Cell Res. (2009) 19:519–23. doi: 10.1038/cr.2009.33
24. Schwenkenbecher, P, Konen, FF, Wurster, U, Jendretzky, KF, Gingele, S, Suhs, KW, et al. The persisting significance of Oligoclonal bands in the dawning era of kappa free light chains for the diagnosis of multiple sclerosis. Int J Mol Sci. (2018) 19:796. doi: 10.3390/ijms19123796
25. Simonsen, CS, Flemmen, HO, Lauritzen, T, Berg-Hansen, P, Moen, SM, and Celius, EG. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult Scler J Exp Transl Clin. (2020) 6:1291. doi: 10.1177/2055217319901291
26. Lu, T, Zhao, L, Sun, X, Au, C, Huang, Y, Yang, Y, et al. Comparison of multiple sclerosis patients with and without oligoclonal IgG bands in South China. J Clin Neurosci. (2019) 66:51–5. doi: 10.1016/j.jocn.2019.05.025
27. Niino, M, Sato, S, Fukazawa, T, Yoshimura, S, Hisahara, S, Matsushita, T, et al. Latitude and HLA-DRB1 alleles independently affect the emergence of cerebrospinal fluid IgG abnormality in multiple sclerosis. Mult Scler. (2015) 21:1112–20. doi: 10.1177/1352458514560924
28. Yoshimura, S, Isobe, N, Matsushita, T, Masaki, K, Sato, S, Kawano, Y, et al. Genetic and infectious profiles influence cerebrospinal fluid IgG abnormality in Japanese multiple sclerosis patients. PLoS One. (2014) 9:e95367. doi: 10.1371/journal.pone.0095367
29. Romero-Pinel, L, Martinez-Yelamos, S, Bau, L, Matas, E, Gubieras, L, Maria Pujal, J, et al. Association of HLA-DRB1*15 allele and CSF oligoclonal bands in a Spanish multiple sclerosis cohort. Eur J Neurol. (2011) 18:1258–62. doi: 10.1111/j.1468-1331.2011.03379.x
30. Ehrhardt, GRLC, Zhang, S, Aksu, G, Jackson, T, and Haga, C. Fc receptor–like proteins (FCRL): immunomodulators of B cell function. Adv Exp Med Biol. (2017) 596:155–62. doi: 10.1007/0-387-46530-8_14
31. Plagnol, V, Howson, JM, Smyth, DJ, Walker, N, Hafler, JP, Wallace, C, et al. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. (2011) 7:e1002216. doi: 10.1371/journal.pgen.1002216
32. Cui, X, Liu, CM, and Liu, QB. FCRL3 promotes IL-10 expression in B cells through the SHP-1 and p38 MAPK signaling pathways. Cell Biol Int. (2020) 44:1811–9. doi: 10.1002/cbin.11373
33. Liu, QB, Zhou, RH, and Liu, CM. TLR9/FCRL3 regulates B cell viability, apoptosis, and antibody and IL-10 production through ERK1/2, p38, and STAT3 signaling pathways. In Vitro Cell Dev Biol Anim. (2022) 58:702–11. doi: 10.1007/s11626-022-00720-8
34. Yu, K, Jiang, R, Li, Z, Ren, X, Jiang, H, and Zhao, Z. Integrated analyses of single-cell transcriptome and Mendelian randomization reveal the protective role of FCRL3 in multiple sclerosis. Front Immunol. (2024) 15:1428962. doi: 10.3389/fimmu.2024.1428962
35. National Medical Products Administration (2018) Import drug approval document: Teriflunomide tablets [in Chinese]. NMPA announcement no. 2018-03-21. Available online at: http://www.nmpa.gov.cn (Accessed March 28, 2018).
Keywords: multiple sclerosis, neuromyelitis optica spectrum disorders, FCRL3, variant, association
Citation: Huang H-F, Liu Q-B, Xu Y-F, Zhao G-X, Liu H-P, Yu H and Wu Z-Y (2025) FCRL3 genetic variants drive autoimmune pathogenesis in multiple sclerosis and neuromyelitis optica spectrum disorders. Front. Neurol. 16:1552149. doi: 10.3389/fneur.2025.1552149
Edited by:
Patricia Coyle, Stony Brook University, United StatesReviewed by:
Chris Bundell, Pathwest Laboratory Medicine, AustraliaShuhei Nishiyama, Harvard Medical School, United States
Copyright © 2025 Huang, Liu, Xu, Zhao, Liu, Yu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Yu, aGFveXV6anVAemp1LmVkdS5jbg==; Zhi-Ying Wu, emhpeWluZ3d1QHpqdS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Hai-Peng Liu, orcid.org/0000-0002-4212-2503
 Hui-Fen Huang
Hui-Fen Huang Qi-Bing Liu3†
Qi-Bing Liu3† Yong-Feng Xu
Yong-Feng Xu Hai-Peng Liu
Hai-Peng Liu Hao Yu
Hao Yu Zhi-Ying Wu
Zhi-Ying Wu