- 1Life Science and Clinical Medicine Research Center, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, China
- 2Drug Clinical Trial Management Office, The Second Affiliated Hospital Of Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Background: The effectiveness of statins in preventing post-stroke depression (PSD) remains controversial. Lower testosterone levels caused by statins could potentially increase the risk of PSD. Low-dose statins could offer better benefits due to their lesser impact on testosterone levels. Repetitive transcranial magnetic stimulation (rTMS) has been shown to reduce PSD in the acute stage. The combination of low-dose statins and rTMS may provide a promising therapeutic option for patients with PSD.
Methods: Data were collected from ischemic stroke patients. Unsupervised machine learning methods were employed to explore the risk factor of PSD. Our prospective cohort study collected data from ischemic stroke patients over 3 years. The patients’ conditions determined the prescription of statins. The outcomes measured included the incidence of PSD, favorable functional outcome (FFO) at 6- and 12-months post-onset.
Results: A total of 545 patients were included in the study. The mean age was 60.99 ± 12.768 years, and 31.9% of the participants were female. The combination of low-dose statins and rTMS subgroup had the lowest incidence of PSD at both 6 and 12 months (p < 0.001). In multivariable logistic regression, the combination of low-dose statins and rTMS was inversely related to PSD at 6 months (OR = 0.370, p = 0.003) and 12 months (OR = 0.386, p = 0.004).
Conclusion: The combination of statins and the rTMS group related the lower incidence of PSD in stroke patients. Combining low-dose statins and rTMS could reduce PSD and further improve the prognosis of ischemic stroke patients.
Introduction
Post-stroke depression (PSD) is a common complication among ischemic stroke patients. These patients have worse functional outcomes and higher mortality rates than those without PSD (1). Antidepressants are the primary treatment for PSD patients. However, longer treatment cycles and more side effects affect the compliance of PSD patients (2). Additionally, an increasing number of PSD patients develop tolerance to antidepressants (2). These PSD patients require a safer and simpler treatment.
Statins could affect the incidence of PSD of stroke patients (3–6). Different studies have reached distinct conclusions about the effect. One Asian study suggests that statins increase the incidence rate of PSD (4). Other studies have shown the opposite result, indicating that statins decrease the incidence rate of PSD (5, 6). The various effects of statins and different types of studies could partly explain these contradictory conclusions (7). Several studies have demonstrated that reduced testosterone levels may elevate the risk of PSD (8, 9). Therefore, low-dose statins may offer greater benefits to PSD patients, as they exert a less pronounced effect on testosterone levels (8, 9).
Statins may have varying effects on PSD depending on the stage of stroke. The use of statins before a stroke may reduce the risk of PSD, whereas post-stroke use could potentially increase it (10, 11). Thus, identifying an effective treatment strategy for patients in the acute phase of stroke with PSD is essential. Our analysis of a retrospective cohort, utilizing unsupervised machine learning methods, revealed that stroke patients receiving statins during the acute phase in conjunction with rTMS exhibited a lower incidence of PSD. Several studies have indicated that high-frequency rTMS is an effective treatment for PSD, particularly for patients in the acute phase of stroke (12–14). rTMS may counteract the adverse effects of statins in acute PSD patients, while statins could provide longer-term benefits for those in the chronic phase of PSD. Consequently, combining statins with rTMS may provide a comprehensive solution to these challenges.
This study aims to explore whether the combination of statins and rTMS can decrease PSD in ischemic stroke patients. Additionally, we aim to analyze further whether the combination of low-dose statins and rTMS better affects PSD patients.
Methods
Cohort 1: retrospective explored cohort
Patients
The study cohort was a retrospective observational cohort. The cohort comprised consecutive patients with ischemic stroke. Patients were recruited from the Neurology and Rehabilitation Departments of the Affiliated Hospital of Youjiang Medical University for Nationalities between January 1, 2018, and May 30, 2019.
The inclusion criteria included ischemic stroke patients aged 18 years or older who had undergone head CT or MRI examinations, met the WHO ischemic stroke diagnostic criteria and received conventional medicine and rehabilitation therapy post-admission. The exclusion criteria were as follows: (1) patients with a recent history of depression before onset; (2) patients unable to be evaluated for depression due to aphasia, disturbance of consciousness, cognitive disorder, or other conditions; (3) patients who taken other lipid-lowering drugs such as fenofibrate; (4) patients with intracerebral hemorrhage, subarachnoid hemorrhage, or severe systemic disease; (5) Patients who are intolerant to statins or rTMS treatment; (6) patients who withdrew from the study or could not provide outcome events.
Data collected
Baseline patient data were collected from electronic medical records, including demographic information, vital signs, and laboratory data. The data collected at admission included age, gender, heart rate, and blood pressure. Critical laboratory data collected at admission included PLT (platelet), INR (international normalized ratio), ALT (alanine aminotransferase), AST (aspartate aminotransferase), CRP (C-reactive protein), among others. Blood lipid levels, including TC (total cholesterol), TG (triglycerides), HDL-C (high-density lipoprotein cholesterol), and LDL-C (low-density lipoprotein cholesterol), were recorded at admission and discharge. Comorbidities, such as renal insufficiency, epilepsy, and pneumonia, were also documented. Medical histories and medication profiles were obtained using structured questionnaires completed by patients or their relatives. Two experienced neurologists, blinded to the patient’s conditions and outcomes, collected and evaluated these data.
Post-stroke depression
The diagnosis of post-stroke depression (PSD) was identified using ICD-9-CM codes 296.2, 296.3, 300.4, or 311. The Mini International Neuropsychiatric Interview (MINI) (6–8), a structured diagnostic psychiatric interview based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), was also used. According to these criteria, patients were diagnosed with major depression if they presented one core symptom and at least four additional depressive symptoms. Alternatively, patients were diagnosed with minor depression if they exhibited at least one core symptom and between two and four additional symptoms. PSD was defined as encompassing both major and minor depressive disorders.
Classify data by unsupervised machine learning
We utilized Python 3.8 for our analysis. For cluster analysis, we first standardized all data using the StandardScaler module from the sklearn library, and then classified data using Hierarchical Clustering methods (AgglomerativeClustering module, sklearn library). The heatmap illustrated the distinct characteristics among the classified patient groups. We compared outcome events across the different identified groups. Grouping can yield clinical significance by clarifying differences in PSD among patient groups. Subsequently, we applied chi-square tests and t-tests to further analyze the factors within the selected groups. Significant differences were found in statin use and rTMS across the Hierarchical Clustering groups. We subsequently validated the impact of statin use and rTMS on the incidence of PSD.
Cohort 2: prospective validated cohort
Study subjects
This study was a prospective cohort study. Ischemic stroke patients were recruited from the neurology and rehabilitation departments of affiliated hospitals of Youjiang Medical University for Nationalities. Patients were enrolled from January 2020 to December 2022 and followed up until December 2023.
Patients who took statins after admission were assigned to the statin group and the remaining patients were assigned to the control group. The study included only patients who did not take statins before admission. Patients were prescribed statins and specific types based on the guidelines for the management of stroke (15).
The inclusion criteria and exclusion criteria were similar as cohort 1.
Baseline data
Baseline patient data were similar as cohort 1.
We evaluated patients’ NIHSS (National Institutes of Health Stroke Scale) scores and mRS (Modified Rankin Scale) scores at admission. Two experienced neurologists, blinded to patients’ conditions and outcomes, collected and evaluated these clinical scale scores.
Statin use
We collected data on patients’ statin use during hospitalization and follow-up. Low-dose and medium-high-dose statins were defined according to the ACC/AHA guidelines on statin intensity (16). The types of statins used in our cohort included atorvastatin, rosuvastatin, simvastatin, and others. Statins were classified into lipophilic (atorvastatin, simvastatin, pitavastatin, lovastatin) and hydrophilic (rosuvastatin) categories. For patients whose statin type or dosage was adjusted during their course of treatment due to changes in their condition, we typically record the statin type or dosage used at the first administration during hospitalization. If the duration of statin use after the change in type or dosage exceeds 70% of the total usage time, the changed type or dosage will be recorded. Lower statin compliance was defined as using statins continuously for less than 40% of the follow-up period. The cohort did not include patients with lower statin compliance. When patients experience significant side effects from statin use and need to discontinue the medication, they are withdrawn from the cohort.
rTMS
Multiple rTMS protocols have been proposed for treating post-stroke depression; this study adopted the treatment approaches from several well-powered studies with robust conclusions (12–14). All patients underwent rTMS therapy for 4 weeks, with five sessions per week, totaling 20 sessions. rTMS was administered to the left dorsolateral prefrontal cortex (DLPFC) at 10 Hz, with magnetic stimulation strength set at 80–100% of the motor threshold level. Each daily treatment included 20 sequences (20 min), each consisting of 4 s of continuous stimulation followed by a 56-s interval (12–14). When patients experience intolerable side effects from transcranial magnetic stimulation therapy or are deemed unsuitable for further treatment by a physician, they will be withdrawn from the cohort.
Outcome events
The primary outcome was the incidence rate of PSD at 6- and 12-months post-onset. The secondary outcomes included favorable functional outcomes (FFO) at 6- and 12-months post-onset. A favorable functional outcome was a modified Rankin Scale (mRS) score of less than 3. Outcome events were collected post-discharge through face-to-face interviews.
Statistical analysis
Statistical analyses were conducted using SPSS 23.0 for Windows. The threshold for statistical significance was set at p < 0.05.
Baseline character
Continuous variables (e.g., blood pressure, laboratory measures) normally distributed between two groups were analyzed using a t-test and expressed as mean ± standard deviation (SD). Continuous variables (e.g., NIHSS) that were abnormally distributed between two groups were analyzed using the Mann–Whitney U test and expressed as the median and interquartile range (IQR). Categorical variables (e.g., gender, history of disease) and ordinal variables (e.g., mRS) were analyzed using the chi-square test and expressed as numbers and percentages.
Outcome variables
A chi-square test was employed to compare the incidence rates of PSD, optimism, and FFO between the two groups. Additionally, we compared the incidence of post-stroke depression across various subgroups. These subgroups were categorized by different doses, type of statins and whether patients underwent repetitive transcranial magnetic stimulation treatment.
We employed multivariate logistic regression analysis to control for confounding factors. In cases where the extreme data exhibited significant imbalance, subgroup analysis and propensity score matching were considered for data processing. However, since the validation cohort did not show a notable imbalance in extreme risk factors, only multivariate logistic regression was used for data analysis.
Logistic regression was used to analyze the relationship between the combination of low-dose statins and rTMS, or other risk factors, and the incidence rate of PSD at different time points. Eligible factors for multivariable regression were screened as follows: (1) a p-value of less than 0.05 in univariable logistic regression; (2) factors selected using LASSO regression; (3) factors meeting criteria (1) and (2). The eligible data were then analyzed using multivariable logistic regression. Odds ratios (ORs), 95% confidence intervals (CIs), and p-values were calculated using logistic regression.
Results
Cohort 1
The exploratory cohort included 117 patients. Thirty-two were female (27.4%), and the mean age was 63.15 ± 11.002 years.
The heatmap suggested that two groups (HCgroup1 and HCgroup2) were optimal for hierarchical clustering methods (Figure 1). The heatmap also indicated significant distinctions between statins and rTMS (Figure 1). Comparing the incidence of PSD between the two groups, HCgroup1 (3.4%) had a significantly lower rate of PSD (p < 0.001) than HCgroup2 (17.7%).
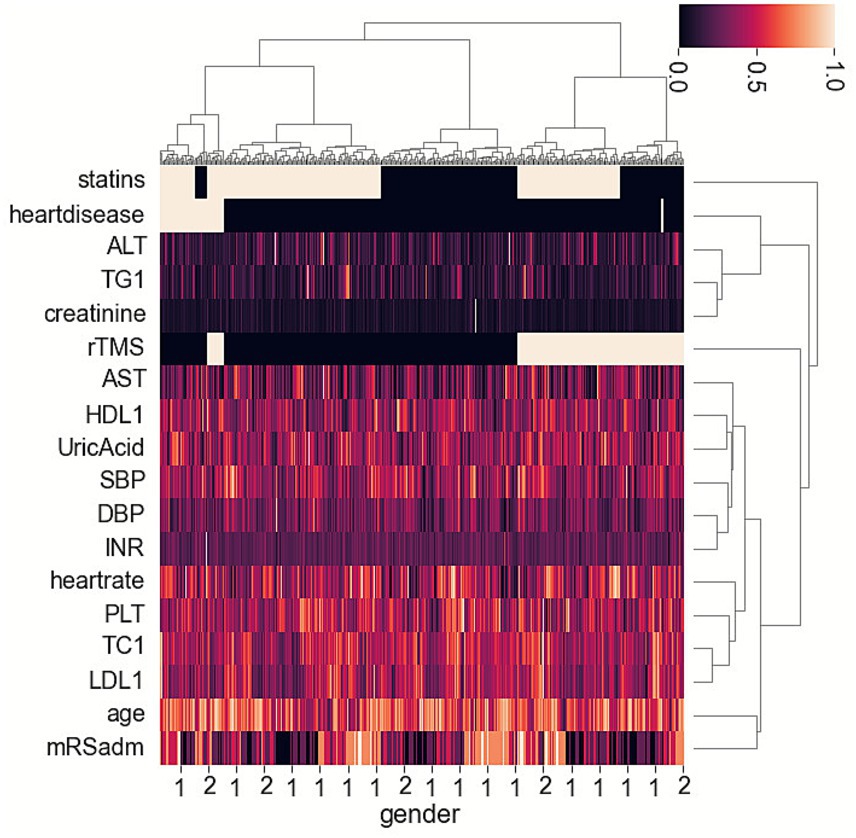
Figure 1. Heatmap showing hierarchical clustering methods for post-stroke depression and associated risk factors in ischemic stroke patients.
When comparing data between HCgroup1 and HCgroup2, it was found that HCgroup1 had more patients taking statins (90.5% vs. 52.1%, p < 0.001), more patients using rTMS (61.9% vs. 33.3%, p = 0.015), fewer patients experiencing severe conditions (mRS at admission ≥ 4) (9.5% vs. 29.2%, p = 0.003). The results were similar to another study, indicating that patients with more severe conditions at admission had a higher incidence of PSD (15). However, the relationship between a higher rate of statin uses or rTMS and a lower rate of PSD requires further validation.
Cohort 2
Baseline characteristics
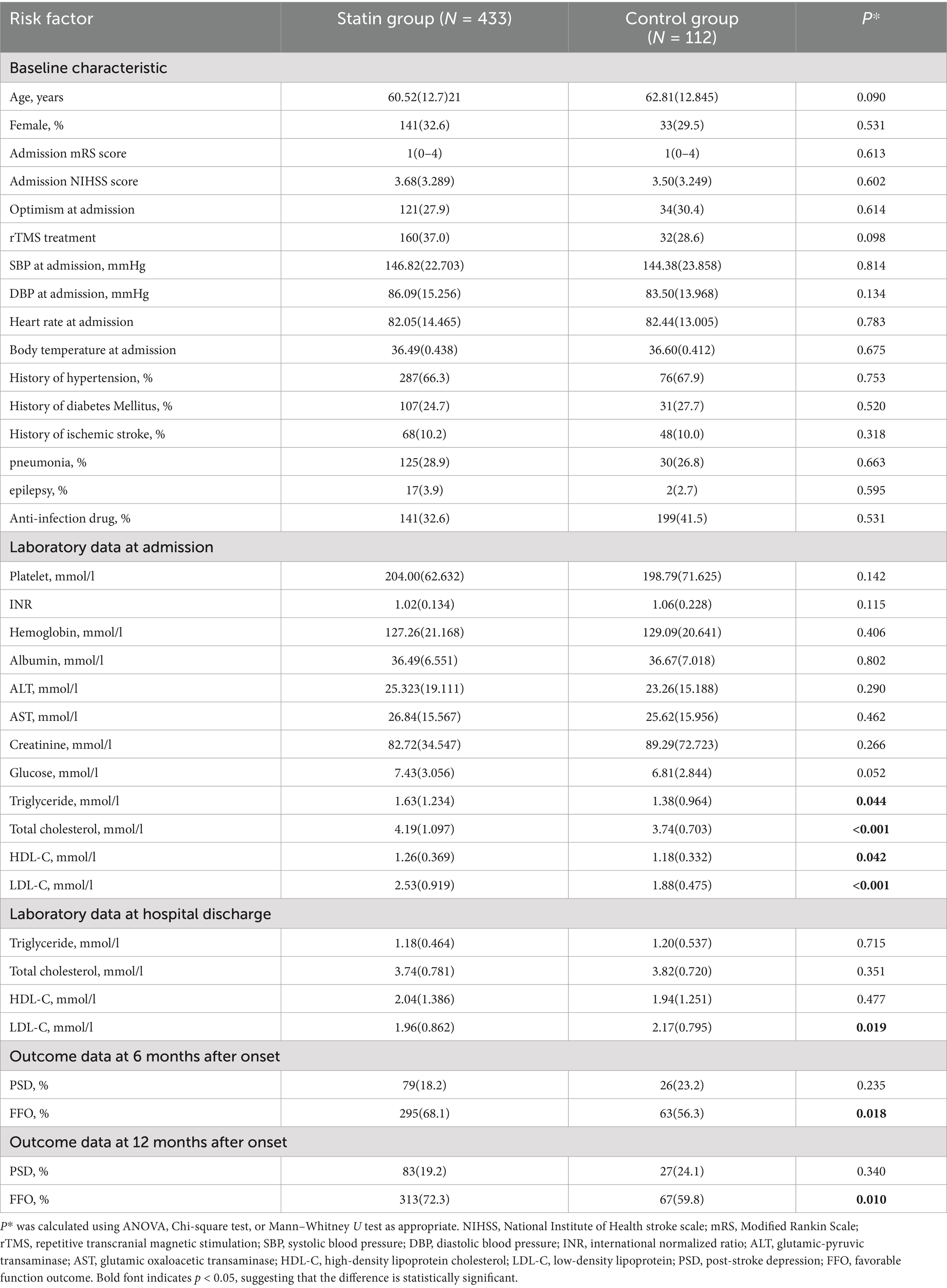
Initially, we recruited 647 eligible patients for the study. Among them, 102 patients were lost to follow-up or had missing data on statin treatment and PSD. Consequently, our study included 545 patients, 433 in the statin group and 112 in the control group.
The mean age of the cohort was 60.99 ± 12.768 years, with 31.9% of patients being female (174 patients). The statin group had higher TC, TG, LDL-C, and HDL-C levels at admission than the control group. These TC, TG, and LDL-C levels in the statin group all decreased and were lower than those in the control group at discharge. Other features showed no significant differences (Table 1).
Chi-square test for PSD and FFO
The statin group (18.2%) exhibited a lower incidence of PSD 6 months post-onset compared to the control group (23.2%) (p = 0.235). The statin group (19.2%) showed a lower incidence of PSD at 12 months post-onset than the control group (24.1%) (p = 0.340). The statin group (68.2%) exhibited a higher rate of FFO at 6 months post-onset compared to the control group (56.3%) (p = 0.018). The statin group (72.3%) showed a higher rate of FFO at 12 months post-onset than the control group (59.8%) (p = 0.010) (Table 1).
In a more detailed subgroup, the low-dose statins with the rTMS subgroup exhibited the lowest incidence of PSD, while the medium and high-dose statins with the rTMS subgroup and the control subgroup had similar incidences of PSD. There were significant differences in the incidence of post-stroke depression between patients treated with different types of statins and those in the control group (Table 2).
Logistic regression for PSD
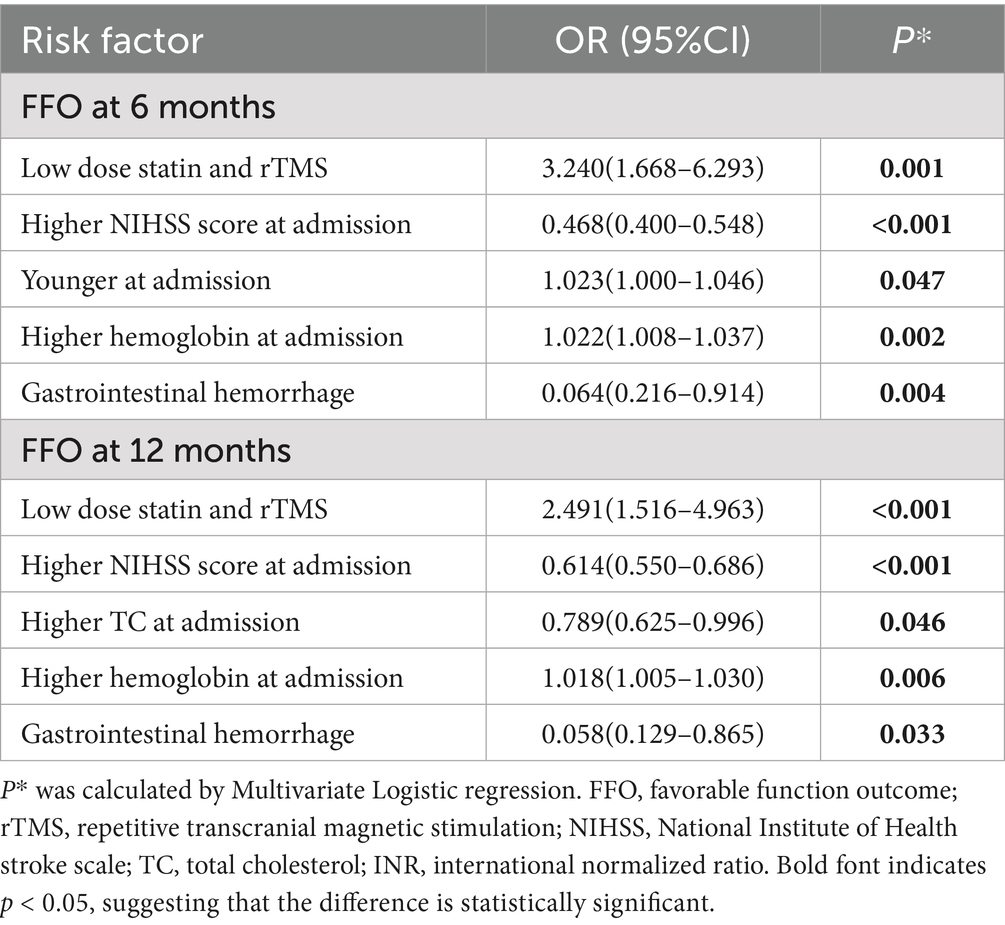
Univariable logistic regression indicated that the combination of low-dose statin and rTMS was associated with PSD at 6 months (OR = 0.425 [0.224–0.807], p = 0.009) and PSD at 12 months (OR = 0.464 [0.249–0.865], p = 0.016), and FFO at 6 months (OR = 1.567 [1.012–2.426], p = 0.044) and FFO at 12 months (OR = 1.710 [1.163–2.554], p = 0.031).
Multivariable logistic regression indicated higher NIHSS scores at admission (OR = 1.127, p < 0.001) were associated with PSD at 6 months. The combination of low-dose statin and rTMS (OR = 0.370, p = 0.003) and higher heart rate (OR = 0.341, p = 0.028) were inversely associated with PSD at 6 months. Higher NIHSS scores at admission (OR = 1.130, p < 0.001) were associated with PSD at 12 months. The combination of low-dose statin and rTMS (OR = 0.386, p = 0.004), higher heart rate (OR = 0.263, p = 0.013), and lower urea nitrogen levels (OR = 0.910, p = 0.034) were inversely associated with PSD at 12 months. The relationships between risk factors and FFO at 6 and 12 months are detailed in Table 3.
Discussion
In the study, we used unsupervised machine learning to analyze risk and protective factors for PSD in an ischemic stroke patient cohort. The heatmap of hierarchical clustering methods indicated a significant difference in the use of statins and rTMS between the two groups. The group with a lower incidence of PSD had more patients taking statins or using rTMS. The validated cohort analysis revealed the statins group exhibited a lower incidence of PSD and a higher incidence FFO at 6 and 12 months. Comparing subgroup data, the low-dose statins with the rTMS subgroup had the lowest incidence of PSD, while the medium- and high-dose statins subgroup had the highest incidence of PSD. The combination of low-dose statin and rTMS was associated with lower PSD and higher levels of FFO at both 6 and 12 months.
In a study, we utilized unsupervised machine learning to explore factors influencing the prognosis of ischemic stroke patients undergoing transcranial magnetic stimulation therapy (17). In cohort 1, Using unsupervised machine learning to identify risk factors for PSD, we found that the use of statins among stroke patients was linked to a lower incidence of post-stroke depression. Moreover, stroke patients taking statins who also received rTMS exhibited an even lower incidence of depression. This suggests that combining both treatments may have a synergistic effect in reducing post-stroke depression. Therefore, we validated this conclusion in cohort 2, which served as the validation cohort.
The cohort 2 suggested that the statins group had a better prognosis. Our previous studies also reached a similar conclusion regarding the combination of statins and rTMS for ischemic stroke patients (17). However, we found a complex association when we analyzed the relationship between PSD and statins. This complexity was particularly evident when we analyzed the distribution of PSD in different subgroups by taking statins and using rTMS. The low-dose statins subgroup and the combination of low-dose statins and rTMS showed more significant benefits in decreasing PSD. However, the medium and high-dose statin subgroups exhibited a reversed effect, increasing PSD. The dosage of statins affected the incidence of PSD. This conclusion is not supported by similar research, as prior studies have not performed a detailed analysis of the impact of different statin dosages on post-stroke depression. Some studies concluded that different types of statins had varying effects on PSD (6, 18). Our study did not observe any differences in the effects among various types of statins, which may be due to differences in patient numbers, regional distribution across studies, and the higher proportion of patients in our study using low-dose statins.
The pleiotropic effects of statins might cause a complex relationship. Statins have an anti-inflammatory effect (7). Some studies suggest that brain inflammation following a stroke is related to symptoms of PSD (3, 19). However, the anti-inflammatory effects were not the only mechanisms by which statins influence PSD. Additionally, the lipid-lowering effects of statins also have an impact on PSD (8, 9). One study found that low cholesterol levels are related to post-stroke emotional disturbances (9). Another study suggested that low cholesterol levels might be related to the incidence of PSD (8). Therefore, the different effects of statins could have a comprehensive relationship with PSD. Low-dose statins with a lower lipid-lowering effect might be more beneficial for PSD patients.
Some studies found that rTMS reduced PSD (13, 14). Additionally, rTMS decreased PSD through various mechanisms, such as adjusting connectivity alterations in the depression circuit (2, 12). Therefore, rTMS and statins had specific mechanisms for reducing PSD. The combination of statins and rTMS exhibited an additive effect in the mechanism. rTMS is only administered to patients during hospitalization. Therefore, the effect of rTMS on PSD may not be persistent. The effect of statins could be long-lasting due to their convenience for patients after discharge. Therefore, the combination of statins and rTMS had an additive effect at different stages of stroke recovery. This is particularly evident in the combination of low-dose statins and rTMS, as demonstrated by the study’s results.
Our study had several limitations. First, this study is a cohort study, which may have selection bias. Therefore, we conducted multivariate analysis and subgroup analysis of confounding factors to ensure the reliability of the conclusions. Second, the number of patients in the medium to high-dose statins and rTMS subgroup and the rTMS-only subgroup was smaller. However, the statistical difference remained significant. Therefore, the results remain credible. Finally, the effects of rTMS in combination with different treatment parameters may vary. The duration of the effects following rTMS treatment also requires further investigation. Therefore, further research is needed to explore whether varying treatment protocols influence the combination treatment.
In conclusion, taking statins decreases the incidence of PSD in ischemic stroke patients. Patients in the statins combined with the rTMS group had a lower incidence of PSD. Combining low dose statins and rTMS could reduce PSD and further improve the prognosis of ischemic stroke patients. The conclusions of this study require further validation through large-scale, multicenter clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Youjiang Medical University for Nationalities (Approval number: 2019(013)). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
CCu: Writing – review & editing, Writing – original draft, Resources, Conceptualization, Supervision, Funding acquisition, Methodology, Software. CCa: Conceptualization, Investigation, Project administration, Writing – review & editing, Methodology. QY: Software, Data curation, Methodology, Investigation, Writing – review & editing. HG: Writing – review & editing, Methodology, Investigation, Data curation. TL: Writing – review & editing, Methodology, Investigation, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Specific Research Project of Guangxi for Research Bases and Talents (AD23026241).
Acknowledgments
We are grateful to Jiaona Lan for providing valuable suggestions for the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer WC declared a past collaboration with the author CCu to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1649263/full#supplementary-material
References
1. Teoh, V, Sims, J, and Milgrom, J. Psychosocial predictors of quality of life in a sample of community-dwelling stroke survivors: a longitudinal study. Top Stroke Rehabil. (2009) 16:157–66. doi: 10.1310/tsr1602-157
2. Blumberger, DM, Vila-Rodriguez, F, and Thorpe, KE. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. (2018) 391:E24–4. doi: 10.1016/s0140-6736(18)31323-0
3. Wium-Andersen, IK, Wium-Andersen, MK, Jorgensen, MB, and Osler, M. Anti-inflammatory treatment and risk for depression after first-time stroke in a cohort of 147 487 Danish patients. J Psychiatry Neurosci. (2017) 42:320–30. doi: 10.1503/jpn160244
4. Kang, JH, Kao, LT, Lin, HC, Tsai, MC, and Chung, SD. Statin use increases the risk of depressive disorder in stroke patients: a population-based study. J Neurol Sci. (2015) 348:89–93. doi: 10.1016/j.jns.2014.11.013
5. Kim, JM, Stewart, R, Kang, HJ, Bae, KY, Kim, SW, Shin, IS, et al. A prospective study of statin use and poststroke depression. J Clin Psychopharmacol. (2014) 34:72–9. doi: 10.1097/jcp.0000000000000051
6. Kang, HJ, Bae, KY, Kim, SW, Kim, JT, Park, MS, Cho, KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. (2016) 72:156–60. doi: 10.1016/j.psyneuen.2016.07.001
7. McFarland, AJ, Anoopkumar-Dukie, S, Arora, DS, Grant, G, McDermott, C, Perkins, A, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. (2014) 15:20607–37. doi: 10.3390/ijms151120607
8. Swiger, KJ, and Martin, SS. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf. (2015) 38:519–26. doi: 10.1007/s40264-015-0296-6
9. Choi, MH, Lim, TS, Yoon, BS, Son, KS, Hong, JM, and Lee, JS. Low testosterone level as a predictor of poststroke emotional disturbances: anger proneness and emotional incontinence. J Stroke Cerebrovasc Dis. (2018) 27:3549–54. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.014
10. Li, YB, Guo, YJ, Zhou, MK, Ma, M, Fang, J, and He, L. Paradoxical effect of statin medication on depressive disorder in first-ever ischemic stroke patients: possible antidepressant-like effect prestroke and the opposite in continuous medication poststroke. Int Clin Psychopharmacol. (2021) 36:147–53. doi: 10.1097/yic.0000000000000352
11. Wimmer, I, Zrzavy, T, and Lassmann, H. Neuroinflammatory responses in experimental and human stroke lesions. J Neuroimmunol. (2018) 323:10–8. doi: 10.1016/j.jneuroim.2018.07.003
12. Liu, CM, Wang, MZ, Liang, X, Xue, J, and Zhang, G. Efficacy and safety of high-frequency repetitive transcranial magnetic stimulation for Poststroke depression: a systematic review and Meta-analysis. Arch Phys Med Rehabil. (2019) 100:1964–75. doi: 10.1016/j.apmr.2019.03.012
13. Duan, HR, Yan, X, Meng, SF, Qiu, L, Zhang, J, Yang, C, et al. Effectiveness evaluation of repetitive transcranial magnetic stimulation therapy combined with mindfulness-based stress reduction for people with post-stroke depression: a randomized controlled trial. Int J Environ Res Public Health. (2023) 20:20. doi: 10.3390/ijerph20020930
14. Ross, RE, VanDerwerker, CJ, George, MS, and Gregory, CM. Feasibility of performing a multi-arm clinical trial examining the novel combination of repetitive transcranial magnetic stimulation and aerobic exercise for post-stroke depression. Top Stroke Rehabil. (2023) 30:649–62. doi: 10.1080/10749357.2023.2165258
15. Sacco, RL, Adams, R, Albers, G, Alberts, MJ, Benavente, O, Furie, K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack – a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on stroke – co-sponsored by the council on cardiovascular radiology and intervention – the American Academy of Neurology affirms the value of this guideline. Stroke. (2006) 37:577–617. doi: 10.1161/01.Str.0000199147.30016.74
16. Stone, NJ, Robinson, JG, Lichtenstein, AH, Bairey Merz, CN, Blum, CB, Eckel, RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. (2014) 129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a
17. Cui, CH, Li, CH, Long, TH, Lao, Z, and Xia, T. Unsupervised machine learning revealed that repeat transcranial magnetic stimulation is more suitable for stroke patients with statin. Neurol Ther. (2024) 13:857–68. doi: 10.1007/s40120-024-00615-8
18. Cham, S, Koslik, HJ, and Golomb, BA Mood, personality, and behavior changes during treatment with statins: a case series Drug Saf Case Rep 2016 3:1 doi: 10.1007/s40800-015-0024-2
Keywords: post-stroke depression, statins, repetitive transcranial magnetic stimulation, incidence rate and prognosis, machine learning
Citation: Cui C, Cai C, Yin Q, Guan H and Long T (2025) Low-dose statins combined with repetitive transcranial magnetic stimulation reduce post-stroke depression. Front. Neurol. 16:1649263. doi: 10.3389/fneur.2025.1649263
Edited by:
Anna Bersano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, ColombiaWeicong Chen, Qingcheng District People’s Hospital, China
Qingyang Zhan, Shanghai Third Rehabilitation Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, China
Copyright © 2025 Cui, Cai, Yin, Guan and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaohua Cui, Y2NoYWl3cEAxNjMuY29t
†These authors have contributed equally to this work
 Chaohua Cui
Chaohua Cui Chuhua Cai2†
Chuhua Cai2† Tonghua Long
Tonghua Long