- 1The Jackson Laboratory, Bar Harbor, ME, United States
- 2Department of Biomedical Sciences, Marshall University, Huntington, WV, United States
- 3Department of Psychological Science, Ball State University, Muncie, IN, United States
Substance use disorders are prevalent and present a tremendous societal cost but the mechanisms underlying addiction behavior are poorly understood and few biological treatments exist. One strategy to identify novel molecular mechanisms of addiction is through functional genomic experimentation. However, results from individual experiments are often noisy. To address this problem, the convergent analysis of multiple genomic experiments can discern signal from these studies. In the present study, we examine genetic loci that modulate the locomotor response to cocaine identified in the recombinant inbred (BXD RI) genetic reference population. We then applied the GeneWeaver software system for heterogeneous functional genomic analysis to integrate and aggregate multiple studies of addiction genomics, resulting in the identification of Rab3b as a functional correlate of the locomotor response to cocaine in rodents. This gene encodes a member of the RAB family of Ras-like GTPases known to be involved in trafficking of secretory and endocytic vesicles in eukaryotic cells. The convergent evidence for a role of Rab3b includes co-occurrence in previously published genetic mapping studies of cocaine related behaviors; methamphetamine response and cocaine- and amphetamine-regulated transcript prepropeptide (Cartpt) transcript abundance; evidence related to other addictive substances; density of polymorphisms; and its expression pattern in reward pathways. To evaluate this finding, we examined the effect of RAB3 complex perturbation in cocaine response. B6;129-Rab3btm1Sud Rab3ctm1sud Rab3dtm1sud triple null mice (Rab3bcd–/–) exhibited significant deficits in habituation, and increased acute and repeated cocaine responses. This previously unidentified mechanism of the behavioral predisposition and response to cocaine is an example of many that can be identified and validated using aggregate genomic studies.
Introduction
Addiction presents a substantial threat to public health, with 15.3 million persons world-wide experiencing a drug use disorder (WHO, 2017). Substance use disorders are complex behavioral processes with varying but largely unknown molecular etiology. The self-initiated first use of drugs and progression to addiction are distinct traits that are highly heritable (Ducci and Goldman, 2012). Such heritable traits can be exploited to identify new biological mechanisms underlying the complex processes that determine addiction behavior, which, in turn, may reveal biomarkers of addiction and/or therapeutic targets for treatment. However, identifying and characterizing the specific effects of genes and variants associated with addiction behavior has been challenging due to the difficulty of modeling addiction in experimental systems (Crabbe et al., 2013), and the significant power requirements necessary for human genetic analysis. Genetic and genomic strategies are promising but often yield noisy data with numerous false positives and false negative results. To overcome these barriers, complex and diverse data sets can be aggregated and analyzed using advanced systems genetics approaches to discover the biological mechanisms that are associated with addiction across experimental contexts.
The locomotor activating and sensitizing effect of psycho-stimulants such as cocaine is a well-established behavioral assay for acute drug response, often assayed using the open field device. Open field testing of locomotion has been effective in high-throughput mouse phenotyping experiments to map and identify biological mechanisms underlying response to novel environments and cocaine behavioral responses (Wiltshire et al., 2015; Yazdani et al., 2015). Studies in humans and non-human primates have established a relationship between cocaine sensitization with clinical symptomology (Bradberry, 2008). Cocaine-induced behavioral sensitization is a measure of drug-induced plasticity, specifically adaptation of the mesocorticolimbic dopamine (DA) system (Liu et al., 2018). Locomotion as a behavioral assay of cocaine use was employed to identify Cyfip2 (Kumar et al., 2013) via quantitative trait locus (QTL) mapping in a reduced complexity cross, as a causative variant for acute and sensitized cocaine response phenotypes. Through the use of a systems genetic analysis of cocaine self-administration in mice, Cyfip2 has recently been associated with a homolog of a psychostimulant addiction candidate, Fam53b (Dickson et al., 2016), the ortholog of which was initially identified in a human cocaine dependence GWAS study (Gelernter et al., 2014), suggesting that a common and conserved biological mechanism supports sensitization and drug use. These findings indicate that locomotor activation and sensitization to cocaine is an effective phenotypic construct to identify conserved functional genetic correlates of addiction-related behavior.
These assays have been deployed in numerous genetic studies of psychostimulant and other drugs, but efforts to identify trait-relevant genes and mechanisms in model organisms has been a lengthy process. The widespread utilization of QTL mapping and functional genomics has resulted in large numbers of QTLs, harboring numerous candidate genes, but for many of these studies, particularly hundreds of legacy studies, the challenging task of identifying the causative genes remains. In a large study using the C57BL/6J x DBA/2J recombinant inbred (BXD RI) genetic reference population and phenotype data from over 250 measures related to multiple behavioral assays across multiple batteries, Philip et al. (2010) identified two QTLs (Chr 4 and Chr 15) related to locomotor activation and sensitization. Typical of many genetic analyses in two-progenitor mapping panels, these genetic loci are large and require refinement. The interval of the previously reported QTL was 20 Mbp and contained numerous protein-coding genes.
Heterogeneous functional genomic experiments can be integrated to prioritize the many genes in the interval based on diverse evidence sources. GeneWeaver1, facilitates convergent analyses across multiple genomic experiments, platforms and species (Baker et al., 2011, 2016). The GeneWeaver platform objectively integrates diverse experimental outcomes in silico through advanced statistical techniques to provide plausible evidence for previously unknown roles for genes involved in addiction-related behaviors. Genomic databases include published and user submitted experimental data as well as data from multiple large-scale public data resources. We have previously used GeneWeaver to identify a gene, Ap3m2, underlying two overlapping biological phenomena, alcohol withdrawal and alcohol preference (Bubier et al., 2014), that we have validated using Ap3m2 null mice. These and other studies (for review, Bubier et al., 2015) demonstrate the effectiveness of this system to find a convergent signal in noisy functional genomics data.
In the present study, we sought to refine the large QTLs previously detected for cocaine-induced locomotor activation (Philip et al., 2010) using refined QTL analysis followed by gene prioritization using heterogeneous functional genomic studies. We prioritized positional candidates using convergent evidence from a database of publicly available genotypes, expression data, sequence variation, ontology annotations and genome-wide experiments from the addiction genomics literature to nominate the most likely candidate(s) in an interval for experimental evaluation. We identified a promising mechanistic candidate gene, Rab3b, and evaluated its role in cocaine-induced locomotor activation and sensitization using a gene knockout approach.
Materials and Methods
QTL Mapping in the BXD RI
Quantitative trait locus (QTL) mapping of the previously identified acute locomotor response to cocaine (Philip et al., 2010) was performed in combined male and female mice from the BXD recombinant inbred strain panel using GeneNetwork (RRID:SCR_002388) (Wang et al., 2003; Chesler and Williams, 2004; Philip et al., 2010). The original analysis of these data was based on an earlier marker map and performed a single-locus scan. To confirm the original result, and more precisely model the effect and locations of variation influencing this trait, R/QTL (RRID:SCR_009085) was utilized for additional QTL model fitting (Broman et al., 2003) of multiple loci. One thousand permutations were performed for each mapping analysis to obtain genome-wide suggestive and significant thresholds. Ninety-five percent confidence intervals (CI) were obtained by extracting 1.5-LOD drop around the peak marker for statistically suggestive and/or significant QTLs.
Integrative Functional Genomic Analysis of the Candidate Genes Using GeneWeaver Database
The candidate genes residing within the 1.5 LOD drop interval of detected QTLs were imported into GeneWeaver (RRID:SCR_003009). At the time of the analysis, the GeneWeaver database contained ∼100,000 gene sets. The database consists of diverse curated gene sets. Mammalian phenotype (MPO, RRID:SCR_004855) and gene ontology (GO, RRID:SCR_006447) annotations and previously published QTLs were obtained from the Mouse Genome Database (MGD, RRID:SCR_012953). Published gene expression studies were obtained from either the Neuroscience Information Framework (Gardner et al., 2008) (RRID:SCR_00483) or directly from publications. Basal gene expression from striatal and nucleus accumbens obtained from the Allen Brain Atlas (RRID:SCR_005984), polymorphic genes among the BXD RI reference panel, and BXD gene expression correlates for striatal and nucleus accumbens to locomotor response after the first and second i.p. injection of 10 mg/kg of cocaine were generated using GeneNetwork. To find convergence of experimentally derived gene associations from genomewide experiments, the database was queried for “Cocaine, Nicotine, or Methamphetamine” followed by manual review to omit false positive search results, e.g., those for which cocaine, nicotine, or methamphetamine was mentioned in the publication abstract but was not relevant to the specific gene set. This convergent analysis resulted in the retrieval of 113 data sets.
Gene Prioritization
GeneWeaver’s GeneSet graph tool represents the collections of gene lists as a bipartite graph, from which high-degree vertices can be identified. This structure is analyzed using the Hierarchical Similarity (HiSim) graph tool to enumerate all intersections among gene sets and highlight high order intersections containing positional candidates for prioritization of candidate genes within the two chromosomal intervals. This was performed by interrogating each of the two candidate gene lists, one from each locus, with selected gene sets describe above. HiSim was used to group experimentally derived gene-set results based on the genes they contain. For a collection of input gene sets, this tool constructs a graph of hierarchical relationships in which each terminal node represents individual gene sets and each parent node represents gene–gene set bicliques found among combinations of these sets using the maximal biclique enumeration algorithm (MBEA) (Zhang et al., 2014). The resulting graph structure was determined solely from the gene-set intersections of every populated combination of gene sets. In terms of gene sets, the smallest intersections (fewest gene sets, most genes) were at the lower levels, and the largest intersections (most gene sets, fewest genes) were at the top of the graph. To prune the hierarchical similarity graph, bootstrapping was performed. The graph in the present analysis was sampled with replacement at 75% for 1,000 iterations; node-node parent-child relationships occurring in greater than 50% of the results were included in the bootstrapped graph.
Animals
Generation of mice with targeted deletions in Rab3a; Rab3b; Rab3c, and/or Rab3d function has been previously described (Schluter et al., 2004). Rab3a heterozygous and Rab3bcd triple null mice were obtained from cryopreservation at The Jackson Laboratory (Strain Name: B6;129-Rab3btm1Sud Rab3atm1Sud Rab3dtm1Rja Rab3ctm1Sud/J; RRID:IMSR_JAX:006375) by IVF of a B6J oocyte. The quadruple knock-out mice are lethal. The frozen sperm came from a donated mouse that had been backcrossed two or three times to C57BL/6J. The IVF pups that were homozygous wild type for Rab3a and heterozygous for Rab3bcd deletion (Rab3bcd+/–) were selected. The heterozygous mice were bred to each other to produce triple null (Rab3bcd–/–) offspring. Ninety-six mice (eight in each group) subdivided into three genotype groups [Rab3bcd+/+(C57BL/6J), Rab3bcd+/–, - Rab3bcd–/–] each sex and two treatments (saline or cocaine) were tested. Age- and sex-matched mice were randomly assigned to the following groups: Rab3bcd+/+ (C57BL/6J) RRID:IMSR_JAX:000664); Rab3bcd+/–; and Rab3bcd–/–. As the −/− mice were on a B6/129 background, the inbred Rab3bcd+/+ C57BL/6J and the Rab3bcd+/– heterozygous mice on a mixed B6/129 background can both be considered as controls. The heterozygous mice would have a mixed background more similar to the −/− mice and these mice did not differ from the inbred C57BL/6J mice. The lack of a difference between the heterozygous mice and the C57BL/6J support the use of C57BL/6J as sufficient genetic controls. The mice were between 76 and 116 days of age at the start of behavioral testing. All mice were maintained at The Jackson Laboratory in climate- and 12 h light cycle-controlled rooms and provided acidified water and 5K52 chow (LabDiet/PMI Nutrition, St. Louis, MO, United States) ad libitum. The Jackson Laboratory Animal Care and Use Committee approved all protocols involving mice.
Behavioral Analysis
Prior to testing, mice were randomly assigned either to a saline (SAL) or cocaine (COCA) group. Mice were brought from their home room into the test room and allowed to habituate for 1 h. Mice were administered 10 mg/kg of saline or cocaine by ip injection. Immediately post-injection, mice were placed in an open field box (39 cm × 39 cm × 39 cm) and locomotor activity was recorded over 20 min using the EthoVision XT 8 (RRID:SCR_000441) video tracking and analysis system (Noldus Information Technology, Wageningen, Netherlands). On days 1 and 2 of testing all mice received saline. Day 1 measurements define locomotor activity in response to injection stress and novel environment, while Day 2 measurements determine locomotor activity within a familiar environment and attenuated injection stress. On days 3, 5, 7, and 9, mice received their assigned saline or cocaine injection and the development of cocaine sensitization was measured relative to activity observed on Day 3. Tester was blind to all genotypes and treatments. Data were binned into intervals of 5 min each.
Tissue Collection
Whole striatal samples were collected to evaluate expression of Cartpt. In order to minimize differences in gene expression due to stress from handling and injection, mice were sacrificed via decapitation within 24 h of the last behavioral trial. In addition to mice that were subjected to cocaine or saline treatment, mice for behavioral testing, a behaviorally and drug naïve group of mice (n = 8) were also sacrificed via decapitation to obtain basal levels of Cartpt and housekeeping genes. Striatal tissue was collected under a dissecting scope and total striatal RNA was isolated.
qRT-PCR
Striatal tissue samples were stored in RNAlater (Life Technologies, Carlsbad, CA, United States), homogenized and total RNA was isolated by standard TRIzol® Plus (Life Technologies, Carlsbad, CA, United States) methods that included DNase digestion. The quality of the isolated RNA was assessed using an Agilent 2100 Bioanalyzer instrument (RRID:SCR_018043) and the RNA 6000 Nano LabChip assay. 500 ng of total RNA was then reverse transcribed with random decamers and M-MLV reverse transcriptase (RT) using the Message Sensor RT Kit (Life Technologies, Carlsbad, CA, United States).
Statistical Analysis
Statistical analyses were performed in JMP 9.0 (RRID:SCR_014242 SAS Institute Inc., Raleigh, NC, United States). To analyze the locomotor data, we performed a 3 × 2 × 2 × 6 mixed-model ANOVA using distance traveled over 20 min as the dependent measure. Between-subjects factors were Rab3bcd genotype (+/+, +/−, −/−), drug (saline and cocaine), and sex. Sessions (1–6) was a within-subjects factor. To dissociate the effects of Rab3bcd complex deletion on habituation and cocaine sensitization we performed a 3 × 2 × 2 × 6 mixed-model ANCOVA using distance traveled on session 1 and session 2 as covariates and using the same dependent measure and independent factors used in the initial ANOVA.
Results
QTL Mapping
Locomotor activity in response to cocaine (i.p. injection of 10 mg/kg of cocaine) was genetically mapped to loci on Chr 4 and 15 in a previous study (Philip et al., 2010). The data from this study was remapped using software allowing for the inclusion of multiple chromosomal effects in the same model, ensuring improved accuracy of the QTL locations. The overall QTL model that best predicted locomotor response following repeated injection of cocaine (GeneNetwork Dataset GN11796; Figure 1A) was a female-specific two-QTL additive model involving Chr 4 (Cocia15 Cocaine Induced Activation 15, rs13477919 @ 104.96 Mb) and Chr 15 (Cocia16 Cocaine Induced Activation 16 rs13482528 @ 38.82 Mb), which together accounted for 34.28% of the trait variance (Figure 1B). A 1.5 LOD window was used to define the 95% confidence interval (CI). The Chr 4 CI spans 101.10 (rs13477873) – 114.51 (gnf04.110.583) Mb (Figure 1C) while the Chr 15 CI spans 33.43 (rs8267966) – 45.11 (rs13482547) Mb (Figure 1D). The Chr 4 and Chr 15 QTLs accounted for 11.38 and 15.60% of the trait variance, respectively. Furthermore, at each of these loci the DBA2/J (D2) allele is the increaser allele (Supplementary Figures S1A,B). No significant interactions among these loci were detected. A genome-wide pair scan was also performed, resulting in the detection of a significant Chr 4 (rs13477881 @ 103.31) × Chr 5 (rs3723202 @ 91.57) interaction (Supplementary Figure S1C, p < 0.001). Inclusion of this interaction term increases the percent variance accounted for by the model to 55.40%. Positional candidate genes underlying each of these regions were retrieved from the Mouse Genome Informatics (MGI) database2 (Eppig et al., 2012)]. Each gene list was uploaded to GeneWeaver.org (Baker et al., 2016) for integrative functional genomic analysis.
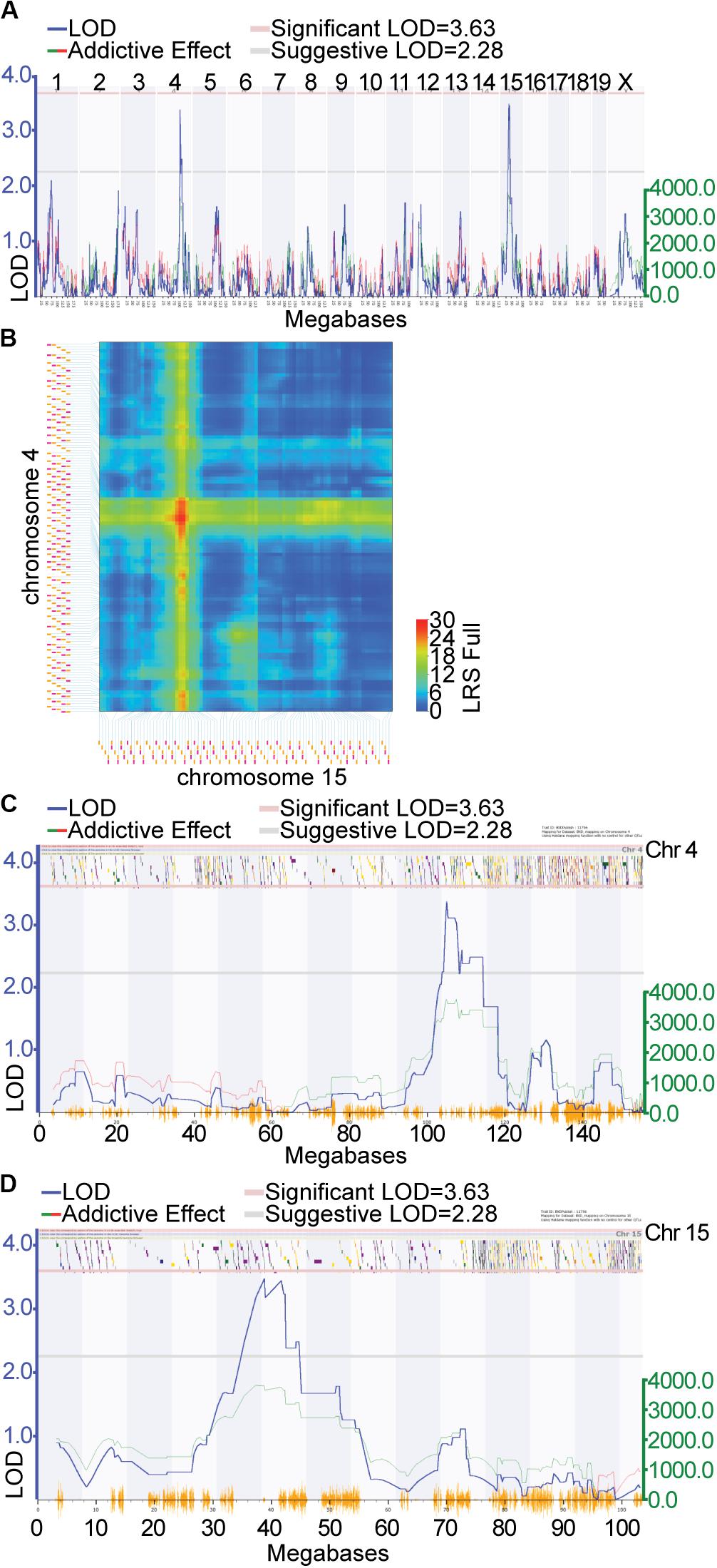
Figure 1. Quantitative trait locus (QTL) mapping of cocaine response in the expanded BXD RI genetic reference population: QTL mapping of locomotor response following the second injection of cocaine in females resulted in the detection of suggestive QTLs on Chr 4 and Chr 15. (A) Green lines indicate regions where DBA/2J is associated with increased phenotypic effect the increaser allele, red lines indicate that C57BL/6J is associated with the increased phenotypic effect. (B) The two QTL additive model involving Chr 4 and Chr 15. Additive the black horizontal lines represent the 1.5 LOD support interval on Chr 4 (C) and Chr 15 (D). The LOD support interval on Chr 4 spans 101.10 (rs13477873) 114.51 (gnf04.110.583) Mb, while on Chr 15 it spans 33.43 (rs8267966) 45.11 (rs13482547) Mb. 108 and 61 positional protein coding candidate genes reside in the Chr 4 and Chr 15 intervals, respectively.
Integrative Functional Genomic Analysis of the Candidate Genes Reveals Rab3b as a Likely Candidate Gene for Locomotor Response to Cocaine
A search of keywords pertaining to cocaine, methamphetamine, heroin and nicotine was performed in GeneWeaver [05-01-2012] and 3,315 sets were retrieved (Philip, 2012). This was followed by a closer inspection of the descriptive meta-content associated with the search results, and removal of redundant or misidentified gene sets (e.g., abstract referenced cocaine, gene set was from untreated controls). A total of 113 gene sets with an average of 66.5 genes per gene set and spanning 19 publications (Figure 2A and Supplementary Table S1) were retained for analysis. The HiSim analysis tool was used to construct an empirically derived hierarchy of the input gene sets for both the Chr 4 and Chr 15 candidate gene list. This allowed us to identify the most highly connected positional candidate genes among a large number of genomic data sets, suggesting that it is the one most frequently implicated in functionally relevant studies. The gene with the highest degree (7) of connectivity from either the Chr 4 (Figure 2B) or Chr 15 positional candidates was Rab3b (Chr 4 108.87–108.94 MBp): a member of the RAS oncogene family thought to function in protein metabolism and cell junction dynamics (Stenmark and Olkkonen, 2001; Takai et al., 2001). Rab3b is expressed in the dorsal and ventral striatum, down-regulated in C57BL/6J and up-regulated in C3H/HeJ after exposure to another psychostimulant, nicotine GS:14888 (Wang et al., 2008), and is differentially expressed in dopamine receptor 1 mutants when compared to their wild-type controls after saline treatment GS:87050 (Zhang et al., 2005). Furthermore, Rab3b is a candidate gene for three previously published QTLs, all of which were mapped in the BXD RI reference population, derived from the inbred progeny of a cross of the C57BL/6J and DBA/2 strains. These three traits mapped to Rab3b were climbing behaviors in response to methamphetamine GS:84160 (Grisel et al., 1997), repeated movements following cocaine GS:84158 (Jones et al., 1999) and hypothalamic CART peptide (Carpt) abundance GS:83998 (Boone et al., 2008). In each of the three QTLs, the DBA/2-derived allele increases activity and peptide release, which also occurs as the allelic effect of the cocaine response QTL on Chr 4 (Cocia15).
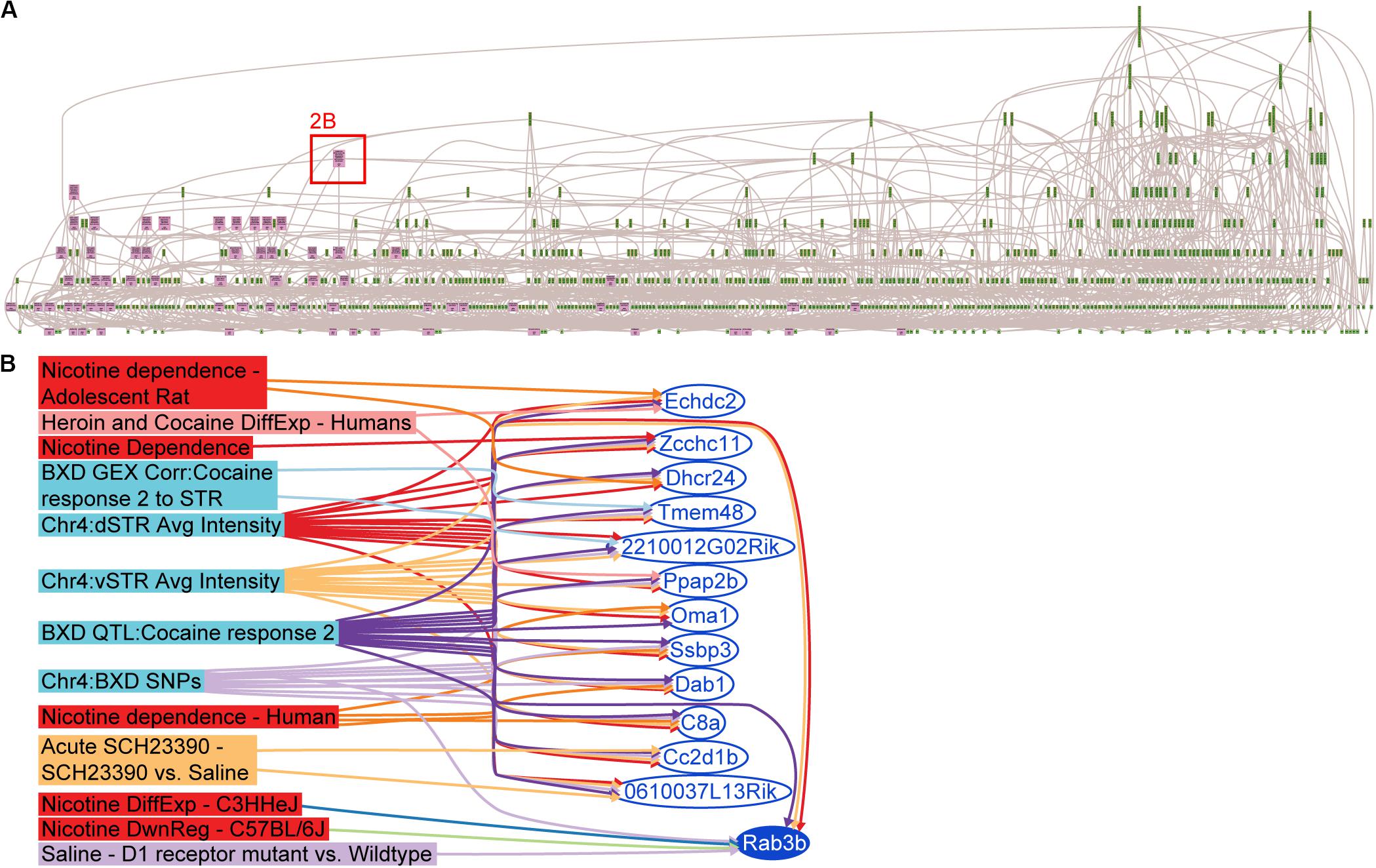
Figure 2. Integrative functional analysis in GeneWeaver. (A) Hierarchical Similarity tool depicting a hierarchical representation of gene sets used in the integrative analysis. Nodes at the top are highly connected genes. Pink colored nodes represent nodes containing one or more QTL candidate genes. Rab3b is revealed as the most highly connected QTL candidate gene. (B) GeneSet graph representing the underlying interconnections among the gene sets containing Rab3b.
To evaluate this candidate experimentally, Rab3b targeted mice were characterized for cocaine response. We did not observe any locomotor, cocaine or habituation difference in the Rab3b KO (Supplementary Figure S2). Previous studies have suggested that compensatory effects of Rab complex proteins limit the effect of individual gene deletions (Schluter et al., 2004; Tsetsenis et al., 2011), thus a triple knock-out approach was necessary to effectively inactivate this functionality. Accordingly, Rab3b, Rab3c and Rab3d (B6;129-Rab3btm1Sud Rab3ctm1sud Rab3dtm1sud) (Schluter et al., 2004; Tsetsenis et al., 2011) was obtained from The Jackson Laboratory for experiments.
Assessment of Cocaine Response in Rab3bcd Triple Null Mice
A 9 days cocaine sensitization paradigm modeled after the work of Phillips et al. (1998) was administered. Briefly, on days 1 and 2 of testing, all mice received saline. Day 1 measurements define locomotor activity in response to injection stress and novel environment, while Day 2 measurements depict locomotor activity within a familiar environment (habituation) and attenuated injection stress. On Days 3, 5, 7, and 9, mice received their assigned saline or cocaine injection i.p., blinded to investigator.
There were significant main effects of genotype [F(2,83) = 10.68, p < 0.0001], drug [F(1,83) = 194.69, p < 0.0001], and session [F(5,79) = 101.31, p < 0.0001], as well as a significant three-way interactions among these factors [F(10,158) = 1.98, p = 0.03]. To determine the nature of the three-way interaction, we examined performance of each of the genotype-drug subgroups on all sessions (Figures 3A–C). Rab3bcd−/− mutants exhibited significantly greater cocaine activation (p < 0.05) relative to wild type and heterozygote mice on all but the second cocaine session (Figure 3B). However, Rab3bcd−/− mutants also traveled a significantly greater distance relative to wild type and heterozygote mice on several sessions in the saline condition beginning with the first saline injection (Figure 3C), suggesting an effect of Rab3bcd complex perturbation on habituation to a novel environment.

Figure 3. Cocaine induced locomotor activation in Rab3bcd mutants, heterozygotes, and wild types. (A) Rab3bcd–/– mutants exhibited significantly greater cocaine sensitization (p < 0.05) relative to wild types and heterozygotes on all but the second cocaine session (B). However, Rab3bcd–/– mutants also traveled a significantly greater distance relative to wild types and heterozygotes on several sessions in the saline condition beginning with the first saline injection (C), suggesting an effect of Rab3bcd on habituation to the apparatus or injection stress. After adjusting for the effect of habituation (D–F) by including distance traveled on the first two sessions as covariates, the significant effect of Rab3bcd on cocaine sensitization persisted, whereas significant differences in distance traveled between wild types, heterozygotes, and mutants in the saline condition were no longer observed. These data indicate significant effects of Rab3bcd on both habituation and cocaine sensitization. *p < 0.05.
To dissociate the effects of Rab3b manipulation on habituation and cocaine sensitization, we performed a 3 × 2 × 2 × 6 mixed-model ANCOVA using distance traveled on session 1 and session 2 as covariates (Figures 3D–F). The main effects of genotype [F(2,81) = 3.69, p = 0.02], drug [F(1,81) = 355.83, p < 0.0001], and session [F(4, 78) = 6.04, p = 0.0003], as well as the three-way interaction between these factors [F(8,156) = 2.24, p = 0.02] remained significant. Post hoc tests indicated that after adjusting for the effects of habituation, effects of Rab3b on cocaine activation persisted (Figure 3E), whereas the previously significant differences in distance traveled between wild types, heterozygote, and mutant mice were no longer observed (Figure 3F).
Cartpt Transcript Abundance in Rab3bcd–/– and C57BL/6J
The GeneWeaver search revealed a previously published QTL mapping study involving cocaine- and amphetamine-regulated transcript peptide transcript abundance (Boone et al., 2008) and the Cocia15 QTL. Cartpt is involved in reward- and feeding-related behaviors. Studies in rats have shown that CART mRNA expression after acute cocaine self-administration is upregulated in brain regions of the mesocorticolimbic dopamine system (Douglass and Daoud, 1996). To assess the effects of Rab3bcd variation on Cartpt expression, we analyzed Cartpt expression using ΔCt values to detect genotype (Rab3bcd–/– and C57BL/6J) × treatment (naïve, SAL and COCA) effects. The genotype x treatment effect was suggestive [F(2,2) = 2.58; pgenotype × treatment < 0.08] (Table 1). No significant differences were observed across genotypes for the naïve group, suggesting that Rab3bcd deletion had no effect on expression of Cartpt. However, planned contrasts revealed differences in Cartpt expression following saline or cocaine treatment. Following saline or cocaine treatment Cartpt is differentially expressed across the two genotypes [SAL: F(1,42) = 10.03, pgenotype < 0.003; COCA: F(1,42) = 11.10, pgenotype < 0.002]. Within genotype contrasts failed to detect any significant differences across the two treatments [Rab3bcd–/–: F(1,42) = 1.76, ptreatment < 0.19; C57BL/6J: F(1,42) = 1.84, ptreatment < 0.18] (Table 2). These results suggest that disruption of the Rab3 complex induces dysregulation of Cartpt in response to injection of either saline or cocaine.
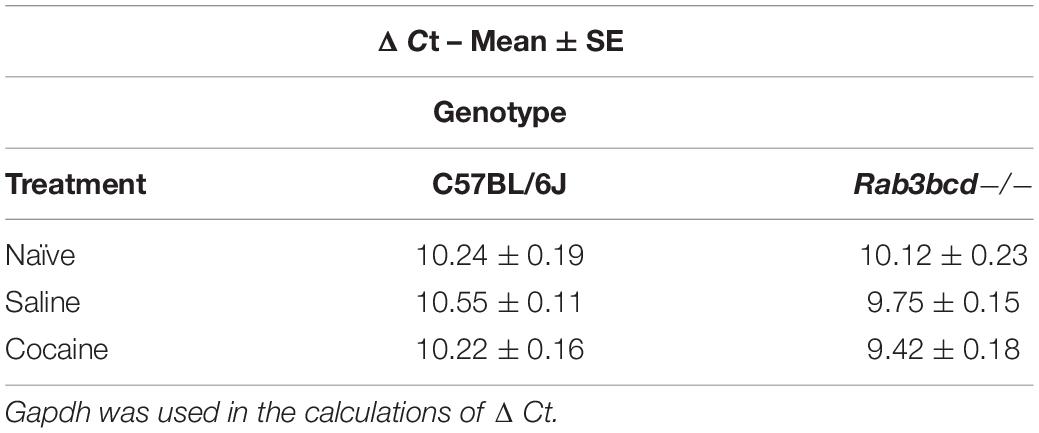
Table 1. Cartpt ΔΔCt values for C57BL/6J and Rab3bcd−/− among naïve, saline, and cocaine treated mice.
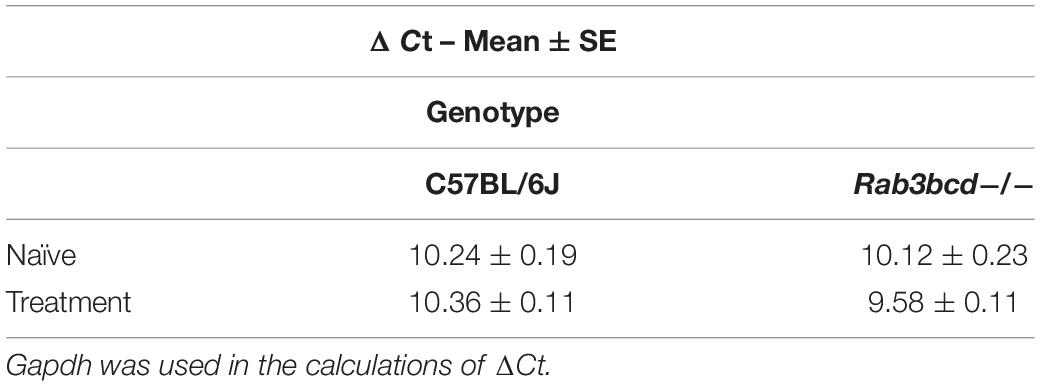
Table 2. Cartpt ΔCt values for C57BL/6J and Rab3bcd−/− among naïve and challenged (saline and cocaine treated) mice.
Discussion
Here we present the discovery of a role for the RAB3B complex in habituation and cocaine-induced activation using the GeneWeaver integrative functional genomic analysis of the candidate genes for a previously mapped cocaine-induced activation QTL within the BXD RI genetic reference population (Philip et al., 2010). We have shown that by incorporating multiple independent lines of evidence from convergent studies it is possible to readily interrogate a QTL interval, to arrive at highly likely candidate genes. In the integrative functional genomic analysis described in this report, we have incorporated empirical information derived from 21 published studies. The approach is relatively unbiased in that the genome-wide studies integrated into the analysis evaluate virtually all positional candidates in the QTL interval. Using these genomic data sets, we have identified a highly likely gene, Rab3b, which resides in the Cocia15 QTL. RAB proteins belong to the family of Ras-like GTPases, and are primarily involved in trafficking of secretory and endocytic vesicles in eukaryotic cells (Novick and Zerial, 1997; Martinez and Goud, 1998). Sixty known RAB proteins exist, with the RAB3 subfamily being the most abundant and expressed at variable levels in the brain (Stenmark and Olkkonen, 2001; Takai et al., 2001; Gurkan et al., 2005). The RAB3 protein subfamily consists of four homologous isoforms, namely, RAB3A, 3B, 3C, and 3D. RAB3A, 3B and 3C co-localize on presynaptic vesicles, while RAB3D localizes to secretory vesicles in exocrine glands (Fischer von Mollard et al., 1990; Nonet et al., 1997; Schluter et al., 2002). The RAB3 subfamily is involved in regulated exocytosis and in docking, priming and fusion stages of the synaptic vesicle cycle. Specifically, in vitro studies have revealed that RAB3 proteins inhibit Ca2+-triggered exocytosis in PC12 cells (Schluter et al., 2002). Rab3b is expressed in neurons but not astrocytes or oligodendrocytes (Cahoy et al., 2008). The integrative functional genomic evidence presented here from multiple independent studies has suggested variation in the RAB3B subunit as a source of phenotypic variation of multiple traits but deletion of this gene is likely developmentally compensated by other subunits due to functional redundancy, with RAB3A minimally needed for survival (Schluter et al., 2004; Chung et al., 2009). Because of this functional redundancy among RAB3 members, a triple knock-out approach was necessary to effectively inactivate this functionality. Of these genes only Rab3b resides in the QTL interval on Chr 4. Our Cocia15 QTL for cocaine response harboring Rab3b, has also been detected in three previous BXD mapping studies, namely, CART (Carpt) transcript abundance (Boone et al., 2008), methamphetamine-related climbing behavior (Grisel et al., 1997) and cocaine-related behavior (repeated movements at 5 mg/kg) (Jones et al., 1999). Rab3b is expressed in the striatum, nucleus accumbens and the ventral tegmental area of mice3, all of which are addiction-relevant regions of the brain. Each of these brain regions contains dopaminergic neurons and plays a central role in cocaine-mediated behaviors. In vitro and in vivo studies involving overexpression of Rab3b in dopamine neurons of the rat substantia nigra (SN) has revealed that overexpression of Rab3b increases dopamine content, uptake, number and size of synaptic vesicles, and levels of presynaptic proteins (Chung et al., 2009). The role of Rab3b in Ca2+-triggered exocytosis, localization on synaptic vesicles, expression in addiction-relevant tissues and appearance in multiple independent QTL mapping studies that target the dopaminergic system makes it a highly likely candidate gene underlying the cocaine-induced locomotor response QTL.
The effects of RAB complex manipulation on habituation to the open field, behavioral response to repeated cocaine injection, and the effects on CART expression which are induced by either saline or cocaine injection suggest that the complex is involved in response to salient or novel experiences, and the potential reinforcement of exposure to certain novel experiences. This is supported by the same locus on Chr 4 being identified when the saline controls were mapped in the original dataset (Philip et al., 2010). A significant literature supports the hypothesis that exposure to novelty and sensory stimuli are reinforcing across species reviewed in Jaegle et al. (2019). Moreover, multiple studies reveal the predictive relationship of novelty seeking and sensation seeking on drug use and effect (Piazza et al., 1990; Belin and Deroche-Gamonet, 2012; Dickson et al., 2015, 2016). The present findings provide causal evidence that the RAB complex is part of a larger network underlying these associations.
At the Chr 4 QTL, the C57BL/6 allele is associated with lower activity and response to cocaine than the DBA/2 allele. Without baseline expression differences, it is difficult to predict the direction of effect in a knock-out on a C57BL/6J background. However, multiple genetic factors can impact the direction of the effects of the mutation on behavior including the interactions of the complex proteins, the interactions of genetic background and the mutations effects on other interacting mechanisms. Therefore, our experimental validation is limited to a confirmation of the involvement of Rab3 complex in this behavior, and specifically of Rab3b due to the QTL mapping result. Future work could refine this by identifying and modeling the precise allelic variant involved in Rab3b abundance or function.
The putative mechanism of genetic variation in the mouse can ultimately be discerned from the location of the variants affecting Rab3b. The SNPs that exist in Rab3b between C57BL/6 and DBA/2 founders of the BXD RI strains are either intronic or 5′ and 3′ intergenic. The Sanger re-sequencing project, using heterogeneous stock QTL mapping results, has shown that functional variants at small effect QTLs (<4%) are most likely to be intergenic, and that functional variants at large effect QTLs (>4%) are most likely to be intronic (Keane et al., 2011). Cocia15 QTL harboring Rab3b is a large effect QTL accounting for 11.38% of the trait variation. There is no cis-eQTL for Rab3b detected in the BXD RI panels including using RNA-seq data sets and exome microarrays4 (not shown). We tested for differential splicing of the Rab3b-L (OTTMUST00000018722) and Rab3b-S (OTTMUST00000018724) transcripts, and detected no alternate splicing between the drug naive founders (data not shown). The absence of a detectable Rab3b eQTL does not preclude genomic variation in expression regulation. One potential explanatory SNP that exists in B6 vs. D2 is at rs32262045. Importantly this SNP lies within a region of Rab3b known to display cocaine-induced epigenetic changes in 5-hydroxymethylcytosine (5hmC) (Feng et al., 2015). These types of cocaine-induced DNA methylation changes in genomic regions are correlated with changes in local gene expression. Another promising causal variant is SNP rs32532179 which lies within a LINE/L1 (Long interspersed nuclear element) element of 738 bp LINE (Okudaira et al., 2014). Repetitive elements, in particular LINE1 have recently been shown to be subject to activation or epigenetic regulation through H3K9Me3. These SNPs leave open the possibility that an epigenetic mechanism; with complex developmental and spatial regulation underlies the behavioral phenotype; likely due to exposure to some other environmental factor earlier on. GeneNetwork captures many brain regions but we know it is not exhaustive. Further, the altered differential expression of Rab3b during embryogenesis due to B6/D2 allelic variation can produce the observed phenotypic differences. This would be consistent with the known embryonic lethality of the Rab3 quadruple mutant (B6;129-Rab3btm1Sud Rab3atm1Sud Rab3dtm1Rja Rab3ctm1Sud/J). Engineering of these variants in cellular and mouse experiments can provide conclusive experimental identification of the regulatory variant, and how it responds to repeated experience.
In summary, our findings demonstrate the use of integrative functional genomics as a tool to identify a novel biological mechanism of cocaine-induced locomotor activation, habituation and other effects of exposure. Using the integrative functional genomic analysis we identified and validated of Rab3b as a candidate gene for cocaine-induced locomotor activation based on genetic and functional evidence. Future studies into this mechanism may elucidate its precise role in the response to novel stimuli. Further application of the integrative functional genomics approach may enable discovery of mechanism of other complex trait variation from aggregate data from current and legacy genetics and genomics.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by The Jackson Laboratory Animal Care and Use Committee approved all protocols involving mice.
Author Contributions
JB, VP, GM, and EC: conception or design of the work. JB, VP, and GM: data collection. JB, VP, PD, and EC: data analysis and interpretation and drafting and revising the article. JB, VP, PD, GM, and EC: final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Funding
Funding for this project comes from NIAAA R01 AA018776 (supported by NIAAA and NIDA), R01 DA 037927, P50 DA39841 to EC, and U01 DA043809 to JB. PD was funded by NIDA K99 DA043573 during preparation of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Raymond F. Robledo technical assistance or all the brain dissections. We would also like to thank Dr. Brynn H. Voy, Dr. Arnold M. Saxton, and Dr. Matthew A. Cooper for their helpful comments on this research. Finally, we thank Stephen Krasinski for assistance with this manuscript. Portions of this manuscript have been published as part of VP dissertation work and referenced in the text. This manuscript has been released as a pre-print at https://doi.org/10.1101/2020.04.21.048405, Bubier et al., 2020.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2020.00721/full#supplementary-material
Footnotes
References
Baker, E., Bubier, J. A., Reynolds, T., Langston, M. A., and Chesler, E. J. (2016). GeneWeaver: data driven alignment of cross-species genomics in biology and disease. Nucleic Acids Res. 44, D555–D559.
Baker, E. J., Jay, J. J., Bubier, J. A., Langston, M. A., and Chesler, E. J. (2011). GeneWeaver: a web-based system for integrative functional genomics. Nucleic Acids Res. 40, D1067–D1076.
Belin, D., and Deroche-Gamonet, V. (2012). Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb. Perspect. Med. 2:a011940. doi: 10.1101/cshperspect.a011940
Boone, E. M., Hawks, B. W., Li, W., and Garlow, S. J. (2008). Genetic regulation of hypothalamic cocaine and amphetamine-regulated transcript (CART) in BxD inbred mice. Brain Res. 1194, 1–7. doi: 10.1016/j.brainres.2007.11.074
Bradberry, C. W. (2008). Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 19, 113–128.
Broman, K. W., Wu, H., Sen, S., and Churchill, G. A. (2003). R/qtl: QTL mapping in experimental crosses. Bioinformatics 19, 889–890. doi: 10.1093/bioinformatics/btg112
Bubier, J. A., Jay, J. J., Baker, C. L., Bergeson, S. E., Ohno, H., and Metten, P. (2014). Identification of a QTL in mus musculus for alcohol preference, withdrawal, and Ap3m2 expression using integrative functional genomics and precision genetics. Genetics 197, 1377–1393. doi: 10.1534/genetics.114.166165
Bubier, J. A., Philip, V. M., Dickson, P. E., Mittleman, G., and Chesler, E. J. (2020). Discovery of a role for Rab3b in habituation and cocaine induced locomotor activation in mice using heterogeneous functional genomic analysis. BioRxiv [Preprint] doi: 10.1101/2020.04.21.048405
Bubier, J. A., Phillips, C. A., Langston, M. A., Baker, E. J., and Chesler, E. J. (2015). GeneWeaver: finding consilience in heterogeneous cross-species functional genomics data. Mamm. Genome 26, 556–566. doi: 10.1007/s00335-015-9575-x
Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Zamanian, J. L., Christopherson, K. S., et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278. doi: 10.1523/jneurosci.4178-07.2008
Chesler, E. J., and Williams, R. W. (2004). Brain gene expression: genomics and genetics. Int. Rev. Neurobiol. 60, 59–95. doi: 10.1016/s0074-7742(04)60003-1
Chung, C. Y., Koprich, J. B., Hallett, P. J., and Isacson, O. (2009). Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. Proc. Natl. Acad. Sci. U.S.A. 106, 22474–22479. doi: 10.1073/pnas.0912193106
Crabbe, J. C., Kendler, K. S., and Hitzemann, R. J. (2013). Modeling the diagnostic criteria for alcohol dependence with genetic animal models. Curr. Top. Behav. Neurosci. 13, 187–221. doi: 10.1007/978-3-642-28720-6_162
Dickson, P. E., Miller, M. M., Calton, M. A., Bubier, J. A., Cook, M. N., Goldowitz, D., et al. (2016). Systems genetics of intravenous cocaine self-administration in the BXD recombinant inbred mouse panel. Psychopharmacology 233, 701–714. doi: 10.1007/s00213-015-4147-z
Dickson, P. E., Ndukum, J., Wilcox, T., Clark, J., Roy, B., Zhang, L., et al. (2015). Association of novelty-related behaviors and intravenous cocaine self-administration in diversity outbred mice. Psychopharmacology 232, 1011–1024. doi: 10.1007/s00213-014-3737-5
Douglass, J., and Daoud, S. (1996). Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene 169, 241–245. doi: 10.1016/0378-1119(96)88651-3
Ducci, F., and Goldman, D. (2012). The genetic basis of addictive disorders. Psychiatr. Clin. N. Am. 35, 495–519. doi: 10.1016/j.psc.2012.03.010
Eppig, J. T., Blake, J. A., Bult, C. J., Kadin, J. A., and Richardson, J. E. (2012). The mouse genome database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic Acids Res. 40, D881–D886.
Feng, J., Shao, N., Szulwach, K. E., Vialou, V., Huynh, J., Zhong, C., et al. (2015). Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat. Neurosci. 18, 536–544.
Fischer von Mollard, G., Mignery, G. A., Baumert, M., Perin, M. S., Hanson, T. J., Burger, P. M., et al. (1990). rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 87, 1988–1992. doi: 10.1073/pnas.87.5.1988
Gardner, D., Akil, H., Ascoli, G. A., Bowden, D. M., Bug, W., Donohue, D. E., et al. (2008). The neuroscience information framework: a data and knowledge environment for neuroscience. Neuroinformatics 6, 149–160. doi: 10.1007/s12021-008-9024-z
Gelernter, J., Sherva, R., Koesterer, R., Almasy, L., Zhao, H., Kranzler, H. R., et al. (2014). Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol. Psychiatry 19, 717–723. doi: 10.1038/mp.2013.99
Grisel, J. E., Belknap, J. K., O’Toole, L. A., Helms, M. L., Wenger, C. D., and Crabbe, J. C. (1997). Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J. Neurosci. 17, 745–754. doi: 10.1523/jneurosci.17-02-00745.1997
Gurkan, C., Lapp, H., Alory, C., Su, A. I., Hogenesch, J. B., and Balch, W. E. (2005). Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol. Biol. Cell 16, 3847–3864. doi: 10.1091/mbc.e05-01-0062
Jaegle, A., Mehrpour, V., and Rust, N. (2019). Visual novelty, curiosity, and intrinsic reward in machine learning and the brain. Curr. Opin. Neurobiol 58, 167–174. doi: 10.1016/j.conb.2019.08.004
Jones, B. C., Tarantino, L. M., Rodriguez, L. A., Reed, C. L., McClearn, G. E., Plomin, R., et al. (1999). Quantitative-trait loci analysis of cocaine-related behaviours and neurochemistry. Pharmacogenetics 9, 607–617.
Keane, T. M., Goodstadt, L., Danecek, P., White, M. A., Wong, K., Yalcin, B., et al. (2011). Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294.
Kumar, V., Kim, K., Joseph, C., Kourrich, S., Yoo, S. H., Huang, H. C., et al. (2013). C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342, 1508–1512. doi: 10.1126/science.1245503
Liu, J., Gandhi, P. J., Pavuluri, R., Shelkar, G. P., and Dravid, S. M. (2018). Glutamate delta-1 receptor regulates cocaine-induced plasticity in the nucleus accumbens. Transl. Psychiatry 8:219.
Nonet, M. L., Staunton, J. E., Kilgard, M. P., Fergestad, T., Hartwieg, E., Horvitz, H. R., et al. (1997). Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 17, 8061–8073. doi: 10.1523/jneurosci.17-21-08061.1997
Novick, P., and Zerial, M. (1997). The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol 9, 496–504. doi: 10.1016/s0955-0674(97)80025-7
Okudaira, N., Ishizaka, Y., and Nishio, H. (2014). Retrotransposition of long interspersed element 1 induced by methamphetamine or cocaine. J. Biol. Chem. 289, 25476–25485. doi: 10.1074/jbc.m114.559419
Philip, V. M. (2012). An Integrative Functional Genomics Approach Towards Quantitative Trait Gene Nomination in Existing and Emerging mouse Genetic Reference Populations. PhD dissesrtation, University of Tennessee Knoxville, UT. 206.
Philip, V. M., Duvvuru, S., Gomero, B., Ansah, T. A., Blaha, C. D., Cook, M. N., et al. (2010). High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 9, 129–159. doi: 10.1111/j.1601-183x.2009.00540.x
Phillips, T. J., Huson, M. G., and McKinnon, C. S. (1998). Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J. Neurosci. 18, 3023–3034. doi: 10.1523/jneurosci.18-08-03023.1998
Piazza, P. V., Deminiere, J. M., Maccari, S., Mormede, P., Le Moal, M., and Simon, H. (1990). Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav. Pharmacol. 1, 339–345.
Schluter, O. M., Khvotchev, M., Jahn, R., and Sudhof, T. C. (2002). Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 277, 40919–40929. doi: 10.1074/jbc.m203704200
Schluter, O. M., Schmitz, F., Jahn, R., Rosenmund, C., and Sudhof, T. C. (2004). A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 24, 6629–6637. doi: 10.1523/jneurosci.1610-04.2004
Takai, Y., Sasaki, T., and Matozaki, T. (2001). Small GTP-binding proteins. Physiol. Rev. 81, 153–208.
Tsetsenis, T., Younts, T. J., Chiu, C. Q., Kaeser, P. S., Castillo, P. E., and Sudhof, T. C. (2011). Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc. Natl. Acad. Sci. U.S.A. 108, 14300–14305. doi: 10.1073/pnas.1112237108
Wang, J., Gutala, R., Hwang, Y. Y., Kim, J. M., Konu, O., Ma, J. Z., et al. (2008). Strain- and region-specific gene expression profiles in mouse brain in response to chronic nicotine treatment. Genes Brain Behav. 7, 78–87.
Wang, J., Williams, R. W., and Manly, K. F. (2003). WebQTL: web-based complex trait analysis. Neuroinformatics 1, 299–308. doi: 10.1385/ni:1:4:299
Wiltshire, T., Ervin, R. B., Duan, H., Bogue, M. A., Zamboni, W. C., Cook, S., et al. (2015). Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav. 14, 271–280. doi: 10.1111/gbb.12209
Yazdani, N., Parker, C. C., Shen, Y., Reed, E. R., Guido, M. A., Kole, L. A., et al. (2015). Hnrnph1 is a quantitative trait gene for methamphetamine sensitivity. PLoS Genet. 11:e1005713. doi: 10.1371/journal.pgen.1005713
Zhang, D., Zhang, L., Tang, Y., Zhang, Q., Lou, D., Sharp, F. R., et al. (2005). Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology 30, 1443–1454. doi: 10.1038/sj.npp.1300680
Keywords: genetic, QTL, genomics, cocaine sensitization, habituation
Citation: Bubier JA, Philip VM, Dickson PE, Mittleman G and Chesler EJ (2020) Discovery of a Role for Rab3b in Habituation and Cocaine Induced Locomotor Activation in Mice Using Heterogeneous Functional Genomic Analysis. Front. Neurosci. 14:721. doi: 10.3389/fnins.2020.00721
Received: 21 April 2020; Accepted: 16 June 2020;
Published: 09 July 2020.
Edited by:
Igor Ponomarev, Texas Tech University Health Sciences Center, United StatesReviewed by:
Megan K. Mulligan, The University of Tennessee Health Science Center (UTHSC), United StatesCamron D. Bryant, Boston University, United States
Copyright © 2020 Bubier, Philip, Dickson, Mittleman and Chesler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elissa J. Chesler, ZWxpc3NhLmNoZXNsZXJAamF4Lm9yZw==
†These authors have contributed equally to this work and share first authorship
 Jason A. Bubier
Jason A. Bubier Vivek M. Philip
Vivek M. Philip Price E. Dickson1,2
Price E. Dickson1,2 Guy Mittleman
Guy Mittleman Elissa J. Chesler
Elissa J. Chesler