- 1Department of Physiology and Cell Biology, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 2Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 3Department of Psychology, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 4Ilse Katz Institute for Nanoscale Science and Technology, Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 5Faculty of Engineering, Holon Institute of Technology, Holon, Israel
- 6National Center for Autism Research, Ben-Gurion University of the Negev, Be’er Sheva, Israel
Studies in rodent models suggest that calls emitted by isolated pups serve as an early behavioral manifestation of communication deficits and autistic like behavior. Previous studies in our labs showed that gestational exposure to the pesticide chlorpyrifos (CPF) and the Mthfr-knock-out mice are associated with impaired social preference and restricted or repetitive behavior. To extend these studies, we examine how pup communication via ultrasonic vocalizations is altered in these ASD models. We implemented an unsupervised hierarchical clustering method based on the spectral properties of the syllables in order to exploit syllable classification to homogeneous categories while avoiding over-categorization. Comparative exploration of the spectral and temporal aspects of syllables emitted by pups in two ASD models point to the following: (1) Most clusters showed a significant effect of the ASD factor on the start and end frequencies and bandwidth and (2) The highest percent change due to the ASD factor was on the bandwidth and duration. In addition, we found sex differences in the spectral and temporal properties of the calls in both control groups as well as an interaction between sex and the gene/environment factor. Considering the basal differences in the characteristics of syllables emitted by pups of the C57Bl/6 and Balb/c strains used as a background in the two models, we suggest that the above spectral-temporal parameters start frequency, bandwidth, and duration are the most sensitive USV features that may represent developmental changes in ASD models.
Introduction
Autism spectrum disorder (ASD) refers to a multi-faceted developmental syndrome whose symptoms include impoverished verbal and non-verbal communication, atypical social interactions, and repetitive behaviors. As in other complex behavioral disorders, genetic and pharmacological mouse models are used to investigate the genetic and neurological substrates of the syndrome. Recent research indicates that signs of ASD can be detected in babies even before the emergence of verbal speech. Retrospective analysis of canonical and non-canonical vocalization in infants, who were later diagnosed with ASD, showed lower production of vocalizations compared to typically developing babies (Patten et al., 2014). Siblings of children diagnosed with ASD, considered high risk for ASD, produced fewer canonical syllables, consonant types and speech-like vocalizations, and more non-speech vocalizations than children at low risk for ASD (Paul et al., 2011). Pre-speech vocalizations in infants are modified by interaction with the caregiver, causing perturbation of social dynamics in babies who were later diagnosed with ASD. For example, adults reacted more to non-speech-like vocalizations for the babies who were later diagnosed with ASD because their canonical babbling was impoverished (Warlaumont et al., 2014).
Ultrasonic vocalizations (USV) production in isolated mouse pups provides a potential assay for assessing communication deficits in mouse models of ASD. Pup calls enhance maternal approach and retrieval behaviors (Ehret, 2005). The maternal response suggests that USVs have adaptive communicative functions. In addition, responsivity of the dam has established USVs as “an important indication of responsivity to social stimuli” (Kazdoba et al., 2016) reinforced by post-isolation reunion with the dam and litter (Hofer and Shair, 1991).
Epidemiological evidence favors a genetic etiology for ASD (Satterstrom et al., 2020); however, incomplete concordance between monozygotic twins suggests that there is also an environmental component (Hallmayer et al., 2011). Longitudinal studies on toddlers who were exposed to pesticides in utero in agricultural and urban communities in the US and China pointed to an association between levels of organophosphate (OP) pesticide metabolites and impaired social development in the toddlers (Rauh et al., 2006; Eskenazi et al., 2007; Wang et al., 2017). Impaired social skills in black participants and in boys aged 7–9 years were associated with gestational exposure to OP pesticides (Furlong et al., 2014; Shelton et al., 2014). These studies point to a possible contribution of gestational OP pesticide exposure to ASD symptoms.
The Methylenetetrahydrofolate reductase (Mthfr) gene (Entrez Gene ID: 4524) encodes for an essential enzyme in the C1 metabolic pathway. The Mthfr 677C > T polymorphism (rs1801133), which shows suppressed activity of this enzyme, is markedly higher among ASD patients and their mothers than in the general population (Goin-Kochel et al., 2009; Mohammad et al., 2009; Schmidt et al., 2011); see also meta-analysis by Rai (2016).
Previous work in our labs showed that gestational exposure to the pesticide chlorpyrifos (C9H11Cl3NO3PS, CID2730, CPF) (Lan et al., 2017, 2019) and the Mthfr heterozygosity (Mthfr+/−) in mice (Sadigurschi and Golan, 2018; Orenbuch et al., 2019; Agam et al., 2020) are both associated with impaired social preference and restricted or repetitive behavior, establishing them as mouse models of ASD with face validity. Mouse pups in both models present with developmental delay, in agreement with a neurodevelopmental delay observed in part of ASD patients (Furlong et al., 2014; Shelton et al., 2014).
Gestational administration of the pesticide CPF (1 mg/kg) in the second-third trimester to mouse dams induced a reduction in the number of USV calls and longer latencies to produce calls, with no difference in call duration or the highest or mean peak frequency in pups (Morales-Navas et al., 2020). A similar regimen of in utero CPF (6 mg/kg) tested in BTBR mice and CPF-oxon in C57Bl6 mice did not alter the number of isolation-induced USV calls compared to vehicle treated pups (Laviola et al., 2006; De Felice et al., 2015), but did reduce the number of USVs in CD1 outbred pups (Venerosi et al., 2009).
Studies that analyzed USV in ASD mouse models suggest that quantitative changes in USV production that were described in various ASD genetic models are variable. For example, the Engrailed-2 null (Brielmaier et al., 2012), the Avpr1b (Scattoni et al., 2008a), and the FMR1 knock-out (KO) pups (Nolan et al., 2017, 2020) did not produce significantly fewer isolation calls than the control wild-type mice. On the other hand, other genotypes such as BTBR (Scattoni et al., 2008b) and FMR1-KO on postnatal day 7 (P7) (Lai et al., 2014) that clearly showed impaired social behavior show greater production of USV than the control genotype. Thus, despite deficits in social and repetitive behavior in the different mouse models, the quantity of USVs can be greater than, less than, or not different from the USV production in the control genotype (Lai et al., 2014). Considering the variability of phenotypes in ASD, and mismatch between the severity of communication impairment and rigid behaviors, it is not surprising to find vast variability when considering only the number of calls produced. Call duration has also been suggested as a valid variable to distinguish between ASD-model mice and wild-type controls as demonstrated by the BTBR strain, FMR1-KO (Lai et al., 2014; Nolan et al., 2020), and females of the ProSap1/Shank 2 (Ey et al., 2013) genetic models of ASD.
Considering the variable phenotypes in ASD models and the emerging finding in babies’ spectral properties, the major goal was to describe common and diverse USV features of a genetic and an environmental ASD mouse model using a spectral-temporal analysis of the characteristics of the USVs in order to compare the two models. To do this, we used a novel, unsupervised spectral analysis of the call syllables which allowed us to define strain and sex differences as well as the ASD-relevant features that have not been described previously.
Materials and Methods
Experimental Design
Methylenetetrahydrofolate Reductase Mice
Mice on a Balb/cAnNCrlBR background were studied (Chen et al., 2001) to assess the effect of maternal Mthfr+/− genotype vs. offspring genotype. Mthfr+/+ [wild-type (Wt)] and Mthfr+/− [heterozygote (Het)] female mice were mated with Wt males to create three groups defined by genotype and maternal genotype as follows: Wt offspring from Wt mothers (Wt:Wt, n = 23), Wt offspring from Mthfr+/− mothers (Het:Wt, n = 18), and Mthfr+/− offspring from Mthfr+/− mothers (Het:Het, n = 21). Mthfr−/− mice are not viable.
C57Bl6J (B6) Mice
Dams and sires for breeding were purchased from Envigo, Israel. Dams were divided into three treatment groups for pesticide exposure (CPF) during gestation: Vehicle (VEH, corn oil, n = 17), 2.5 mg/kg CPF (CPF-L, n = 22), or 5 mg/kg CPF (CPF-H, n = 20).
Sample size was chosen based on our previous experiments. An illustration of the study design and description of experimental groups can be seen in Supplementary Figure 1A.
The mouse colonies were maintained on a 12:12 h light/dark schedule, temperature 21–23°C with ad libitum food and water. All procedures were performed according to the guidelines of the Israeli Council on Animal Care and approved by the Animal Care and Use Committee of Ben-Gurion University of the Negev (protocols IL-16-07-14 and IL-66-11-13).
Prenatal Exposure
Chlorpyrifos (CPF, 99.5% purity, Chem Service, Inc) suspended in corn oil (Willi Food, Yavneh, Israel) or vehicle control was administered by gavage to B6 pregnant females daily from gestation day (GND) 12–15 in a volume of 0.1 ml/10 g body weight using a 22-gauge stainless steel feeding tube (Solomon Instech, Inc). The dams showed no signs of cholinergic toxicity (Ricceri et al., 2007; Lan et al., 2017).
Genotyping
Mthfr and Wt mice were genotyped using polymerase chain reaction, as previously described (Chen et al., 2001).
Ultrasonic Vocalization
Acoustic Recording
Ultrasonic signals were recorded using Avisoft Bioacoustics (Berlin, Germany) system including UltraSoundGate 116Hm with the Ultrasound Microphone CM16/CMPA, using the Avisoft Recorder 4.2.17 – Bioacoustics recording software. Recordings were set at a sampling frequency of 250 kHz in a trigger mode using a threshold of 0.5% of the signal’s energy in the range of 10–250 kHz.
ASD Models
Methylenetetrahydrofolate reductase model: two to three pups per litter were recorded on P4, 6, 8, 10, and 12. Sex and genotype were defined when pups reached P30. CPF model: One male and one female pup from each litter, marked by snipping the end of the tails on P1, were randomly chosen. Pups were recorded on P2, 5, 8, and 14. To enable comparison between the environmental and genetic model, the third recording sessions that took place at P8 were analyzed for the current study, as in other studies on analysis of pup USV communication (De Felice et al., 2015). Data collected on other postnatal days will be used for further developmental studies. In total, the numbers of mice analyzed for the current study were as follows: Mthfr, 31 each sex; CPF, 31 female and 28 male.
Each pup was separated from the litter and placed in a transparent plastic cup (11 cm high and 10 cm diameter). The microphone was placed 10 cm above the pup. After a 10-min isolation session, the pup was placed back in the home cage with the litter and the area cleaned with ethanol (70%) between pups.
Ultrasonic Vocalization Analysis
Call variables were extracted by SASLab Pro (Avisoft Bioacoustics®, Berlin, Germany) by researchers blind to the group identity with the following setting: Single threshold of −73 dB, 5 ms hold time, and eight regular intervals of duration resulting in nine frequency samples for each syllable F1–F9 (see an example in Supplementary Figure 1B). Larger number of frequency samples (up to 30) for USV detection did not increase detection rate. The following variables were used to compare USV syllables between groups: Start Frequency, defined as mean frequency at start of the syllable - F1; End Frequency, defined as mean frequency at the end of the syllable – F9; Mean Frequency (mean of all nine samples); Bandwidth (the range of syllable’s frequencies calculated by the highest frequency sample minus the lowest frequency sample of the syllable); and Duration (the time of F9–F1).
Cluster Analysis
Hierarchical clustering was applied using frequency values of F1-F9 as the features for clustering (Partek® software). Euclidean distance, as point distance metric, and Wards linkage as cluster distance metric were applied. The difference in the characteristics between neighboring clusters in each strain was calculated by SPSS (Kolmogorov-Smirnov, K-S test) for the five variables: start frequency, end frequency, mean frequency, bandwidth, and duration. A significant difference in at least four out of five variables was sufficient to accept the cluster as a distinct cluster. In both strains, eight distinct clusters were identified (inclusion of other parameters such as duration, bandwidth, and mean frequency did not contribute and require modification of data in order to use a single scale).
Statistical Analysis
All calls emitted by the study pups during 10 min maternal isolation session were analyzed: 5837 syllables from the Mthfr model and 10715 from the CPF model. Statistical analyses were performed by SPSS23 software (IBM) to test the effect of the fixed factors: strain, sex, and experimental group. Analysis of parametric variables was performed by univariate (general linear model) test and test of normality. Non-parametric analysis was performed using the Kolmogorov-Smirnov (K-S) test. Syllable distribution among clusters was compared using Chi-square test.
Results
Different mouse strains present significant variability in USV properties including number of calls, syllable duration, and distinct developmental time course (Scattoni et al., 2008b), in parallel with strain differences observed in other aspects of social behavior (Nadler et al., 2004; Panksepp et al., 2007; Defensor et al., 2011). Therefore, prior to the comprehensive analysis of environmental and genetic mouse models of autism, which are based on different strains of mice, we compared the two control groups (Wt:Wt for the Mhtfr model and oil gavage for the CPF model) for each of the models, including both sexes.
To explore USV syllables properties, five variables for each syllable were tested for normality using ShapiroWilk test; start, end, mean frequencies, bandwidth, and duration all differ from normal distribution (The distributions of these variables in the control group of the ASD models tested B6 and Balb/c strains are presented in Supplementary Figure 2).
A significant sex difference was observed in both strains for each of the variables, as shown in Supplementary Table 1. B6 and Balb/c strains differ in all variables tested: start, end and mean frequencies, duration, and bandwidth. Longer syllable duration and wider bandwidth were observed in mice of the Balb/c strain, compared to the B6 with a median value in Balb/c mice twice that of the B6. Significant differences between strains were observed also in the start and end frequencies as well as the mean frequency of syllables.
The Effect of Chlorpyrifos and Methylenetetrahydrofolate Reductase on Ultrasonic Vocalization Spectral and Temporal Characteristics
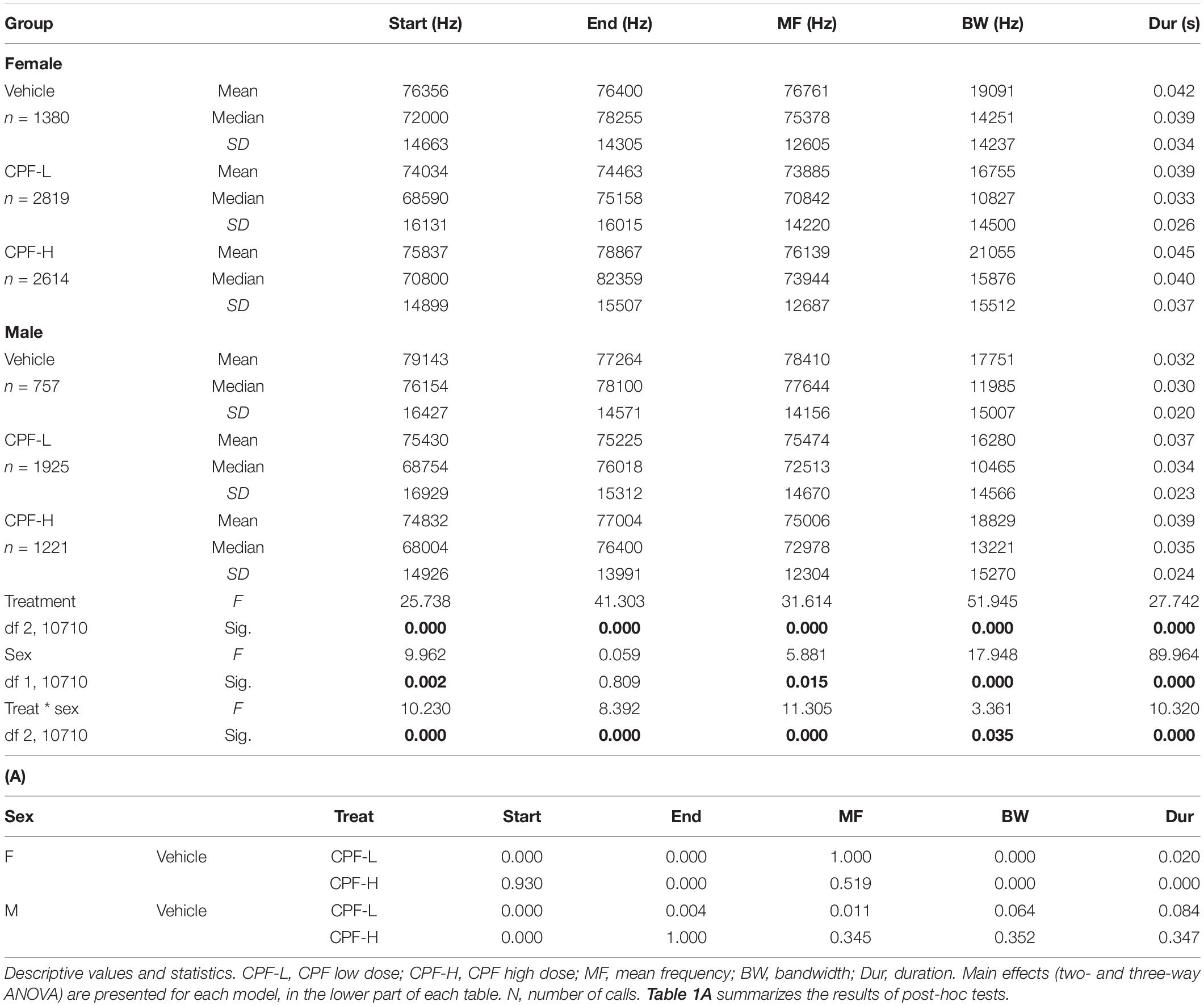
The CPF and Mthfr models were analyzed compared to their respective control groups with sex and treatment/genotype as independent variables. An effect of in utero exposure to CPF at high and low doses was observed, with an interaction between sex and treatment for all variables (Table 1). In females, the effect of the low dose was significant for the start and end frequencies, bandwidth, and duration (Table 1A), and the high CPF dose increased bandwidth, end frequency, and duration as shown in Table 1A. In the males, both doses of CPF were associated with a lower start frequency and the low dose was also associated with lower mean and end frequencies (Table 1).
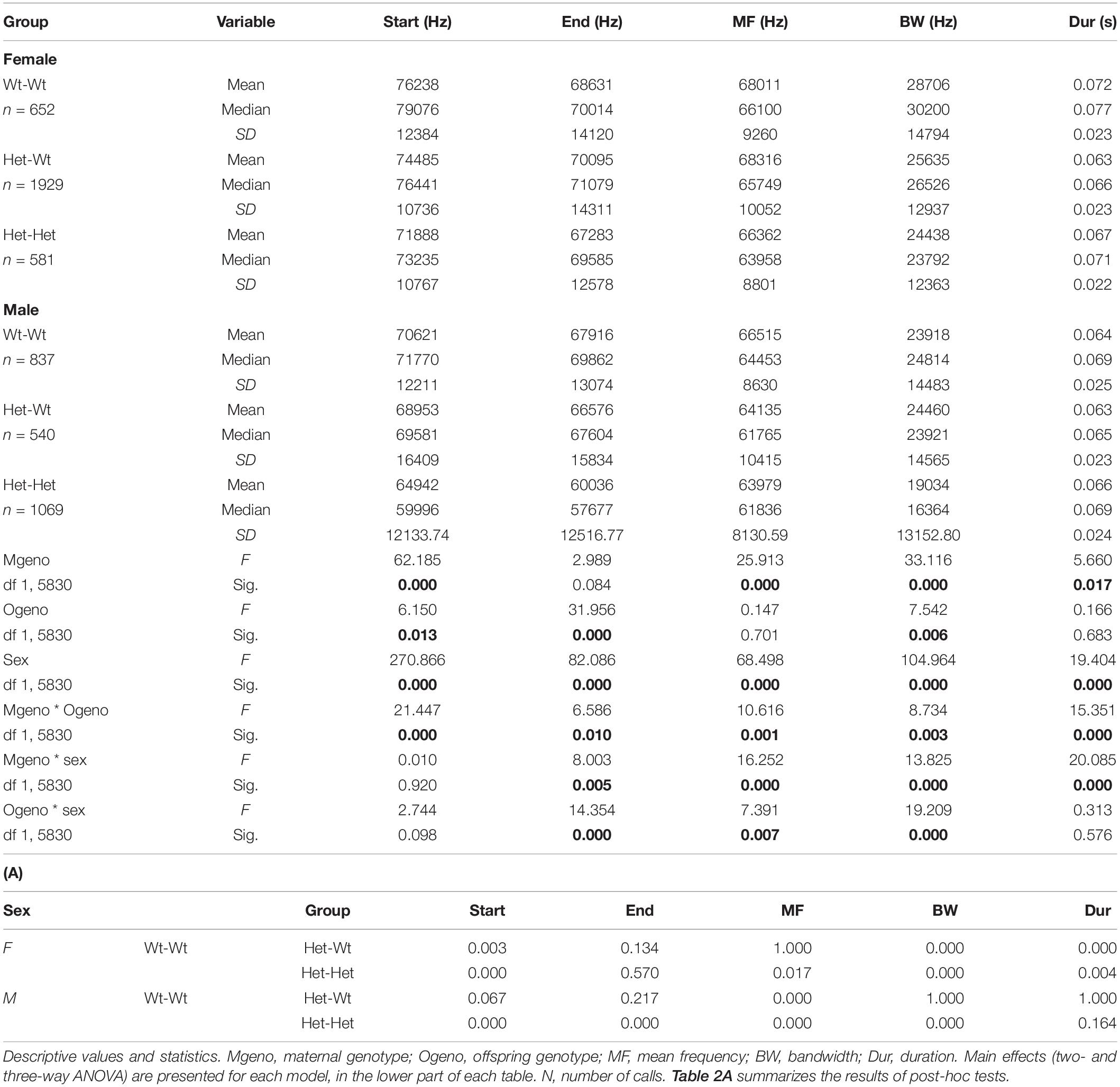
In the Mthfr model, offspring and maternal genotypes interacted to affect all variables, and each of these factors interacted with sex to affect the end frequency, mean frequency, and bandwidth. Duration was affected by a maternal genotype x sex interaction (see statistical values in Table 2).
In the Mthfr+/− females, which emit syllables with higher frequencies (start, end, and mean frequency) than males, an interaction between maternal x offspring genotype was observed, where maternal Mthfr+/+ (Wt-Wt) genotype had higher frequencies, compared to pups with maternal Mthfr+/− genotype, and the offspring Mthfr+/− genotype (Het-Het) had lower mean and end frequencies, compared to pups with Mthfr+/+ genotype (Wt-Wt and Het-Wt). In contrast, the start frequency and bandwidth were lower in the mice with maternal Mthfr+/− genotype and further reduced by offspring Mthfr+/− genotype such that the Mthfr+/− pups of Mthfr+/− dams showed the lowest start frequencies. In the males, maternal and offspring Mthfr+/− genotype lowered the frequencies of the start, end, and mean frequency of the syllables. In both sexes, the bandwidth was lowered by maternal Mthfr+/− genotype and further lowered by offspring Mthfr+/− genotype (Table 2). Post-hoc analysis revealed that differences in the start frequency, mean frequency, and bandwidth between the control Wt:Wt group and test groups are found in most comparisons, while an effect of genotype on duration was found only in female pups (Table 2A).
Next, we examined the distribution of syllable frequencies and range, focusing on the start and end frequencies, and the bandwidth for the effects of sex and the impact of the relevant model variable (CPF treatment or Mthfr+/− genotype). The sex and model differences are illustrated in Figure 1; rows 1–3 CPF model and rows 4–6 Mthfr model. Compared to the males, the female mice were more affected by prenatal CPF for the start frequency, end frequency, and syllable duration (Figures 1A–C, rows 1–3, left side). In the Mthfr+/− males, the distributions of start and end of the syllable were shifted to lower frequencies with shorter duration compared to Wt:Wt (Figures 1A,B, rows 4–6, right side). Comparison and statistical analysis of the effects of treatment and genotype on syllable distribution are shown in Tables 1, 2, respectively.
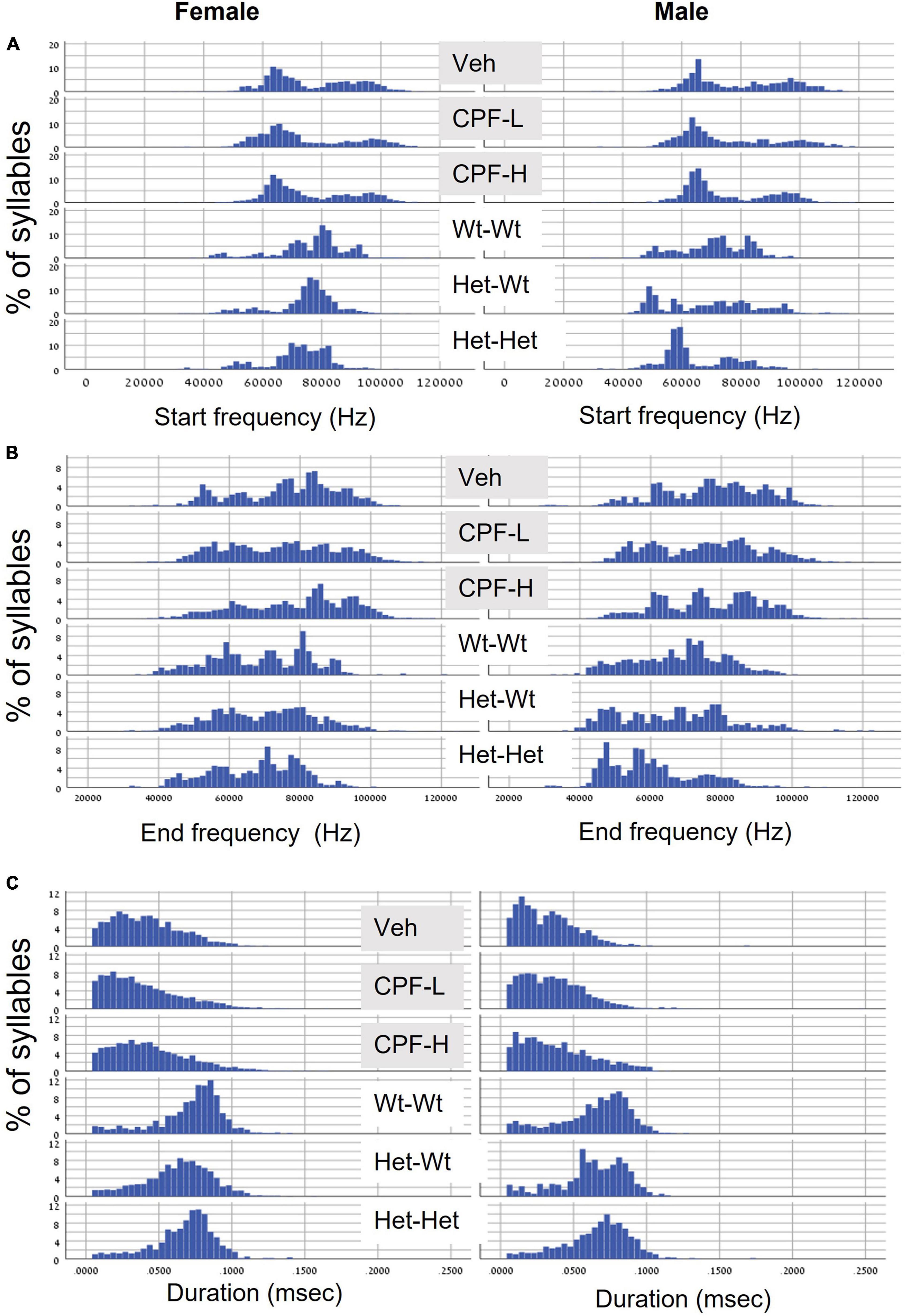
Figure 1. CPF treatment and Mthfr+/– genotype alters the distribution of isolation induced syllables in female and male pups. (A) Start frequency. (B) End frequency. (C) Duration. For each variable, the following groups are presented from top to bottom: Vehicle (Veh), CPF low dose (CPF-L), CPF high dose (CPF-H), Wt:Wt, Het-Wt, and Het-Het.
Unsupervised Clustering of Isolation Induced Ultrasonic Vocalizations
In order to further analyze the spectral characteristics of the calls, the range of syllables was analyzed and classified on the basis of the frequencies of the different types of calls. This resulted in large variety of syllables that might not have been detected if the syllables were analyzed under a single distribution. This analysis allowed us to detect changes that occurred only in one type of syllable or tendencies to emit a particular type of syllable as a result of the relevant ASD factor (CPF treatment and Mthfr+/− genotype, compared to their controls).
To categorize all syllables with no a priori bias, we used an unsupervised method based on the USV spectral properties, as described above. Each syllable was equally divided into nine frequencies which were submitted to hierarchical clustering. Hierarchical clustering using Euclidean distance as point distance metric and Wards linkage as cluster distance metric resulted in eight distinct clusters in each model. The dendrogram generated by the Partek® software was colored to illustrate the different clusters (Figure 2). The heat-maps indicate the values at 9 points along the syllables’ main frequency from the beginning of the syllable (upper part of the heat-map) to the end of the syllable (lower part of the heat-map), ranging from high frequencies in blue to low frequencies in yellow. Syllables with a single component and narrow bandwidth in a frequency range around 80,000 kHz are shown at the right end of the heat-map, whereas clusters with frequency range around 50,000 kHz and narrow bandwidth are at the left side of the heat-maps. Examples of syllables from each cluster are shown below each heat-map for each of the two ASD models. Syllables containing more than a single component appear at the central region of the heat-maps. The heat-map demonstrates the presence of frequency steps and the relative proportion between the length of each component of the syllables. This presentation highlights differences between the models/strains that are most obvious in heat-maps. For example, the heat map illustrates the higher syllable complexity in the Mthfr model, compared to the CPF model. The differences between the clusters are supported by the differences in start, end, mean frequencies, bandwidth, and duration (see descriptive data in the Supplementary Tables 2A,B). The median values and range of each cluster characteristics are shown in the box plots in Figure 3. Note that although the color code is similar for the CPF and the Mthfr models, these clusters do not represent similar syllables.
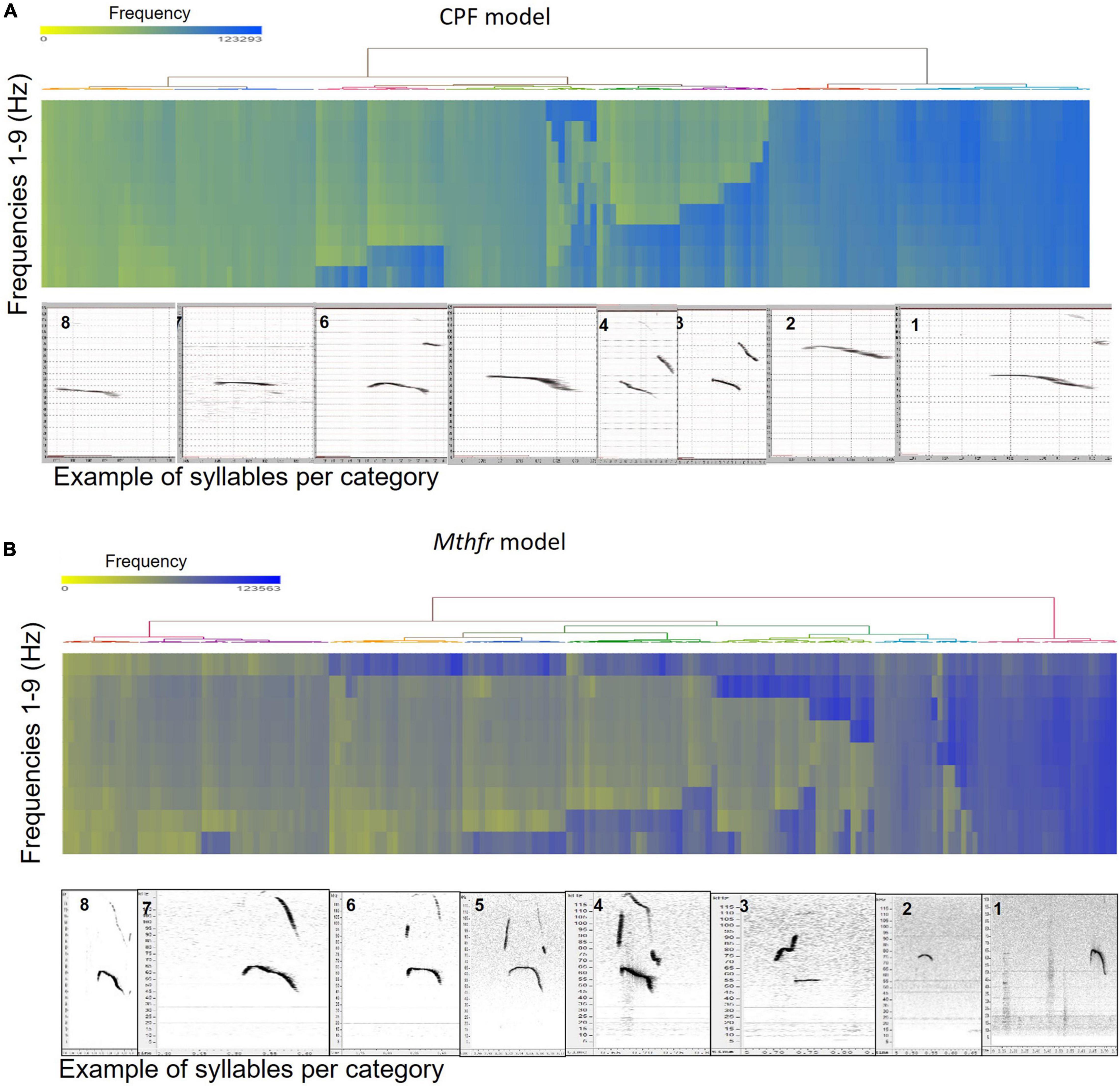
Figure 2. Hierarchical clustering of syllable spectral properties resulted in eight distinct clusters for each ASD model. Hierarchical clustering dendrogram and heat-map for the CPF model (A) and the Mthfr model (B). Frequencies in the heat map range from high frequencies in blue to low frequencies in yellow. Examples of syllables from each cluster, as generated by Avisoft SASLab Pro ver5.2.11 software, numbered as in the text and tables, are shown below each heat-map.
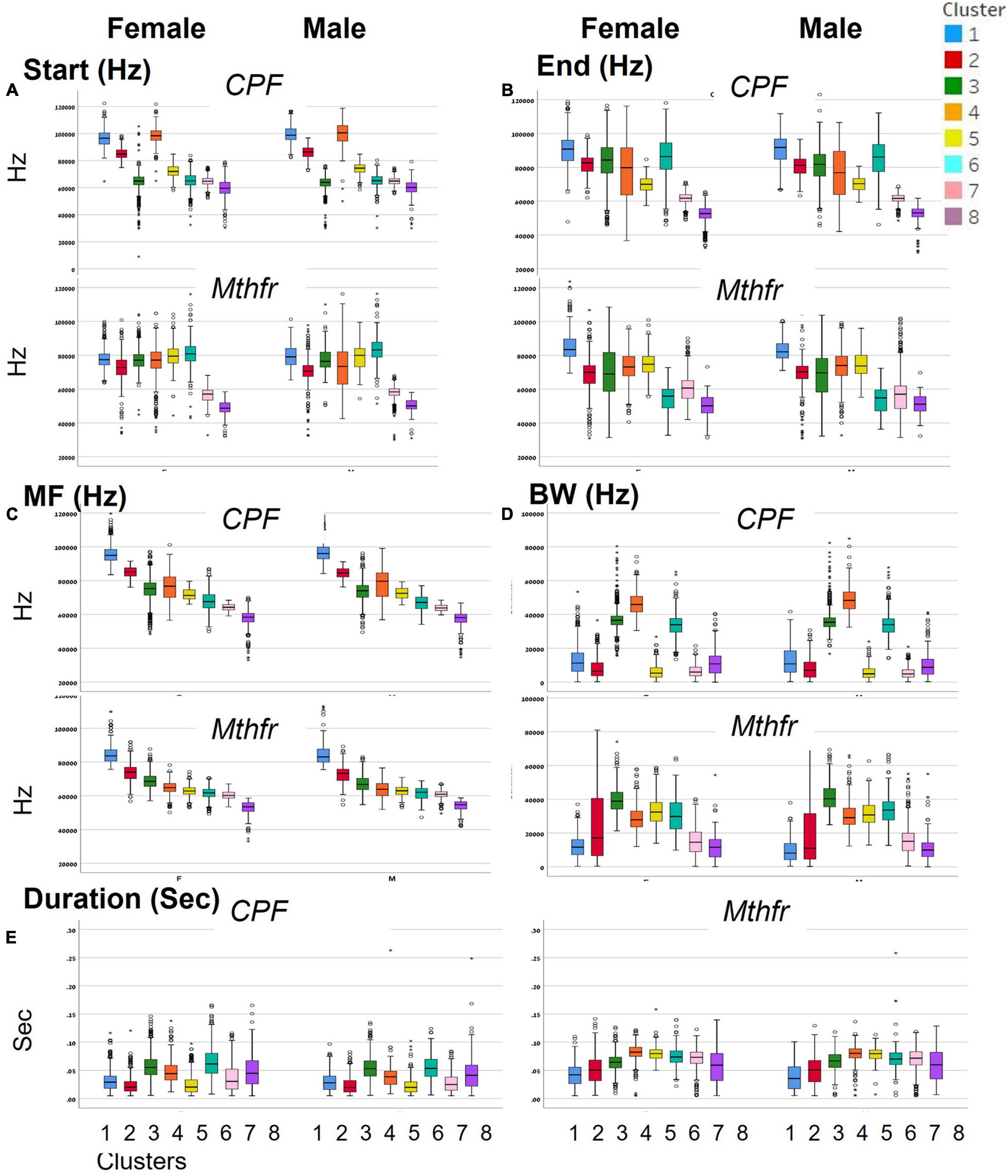
Figure 3. Distinct spectral and temporal characteristics of the syllable clusters. Spectral and temporal characteristics of the syllables in each of the clusters for female and male of CPF and Mthfr models: (A) start frequency, (B) end frequency, (C) mean frequency, (D) bandwidth, and (E) duration. Median, confidence interval (95 and 5%) and outliers are presented. Note that each ASD model has a unique cluster distribution; the number and color code for Mthfr model do not overlap with the same characteristics in the CPF model.
In female and male mice of the CPF model, there is a clear difference in the start frequency between clusters 1–3 and 4–6, with clusters 3, 6, 7, and 8 showing lower variability. In the Mthfr model, start frequency of clusters 1–6 are characterized by values around 80,000 kHz while the start frequency of clusters 7 and 8 is around 60,000 kHz. Compared to the start frequency, the end frequency is more variable in both models, with similar patterns in male and female in the two models, as can be seen in Figures 3A,B. In both models, the mean frequency of the clusters declines from cluster 1 to 8, with each cluster having lower mean frequency than the previous. The highest frequency is around 95,000 kHz in the CPF model and 80,000 kHz in the Mthfr model, while the lowest frequencies are 50000–55000 kHz in both models. In contrast to the gradual decline of the mean frequency from cluster 1 to 8, the bandwidth values vary among clusters. In the CPF model, clusters 1, 2 5, 7, and 8 are at the range of 10,000–20,000 kHz, whereas clusters 3, 4, and 6 range around 40,000 kHz. In the Mthfr model, clusters 1, 7, and 8 range below 20,000 kHz, cluster 2 had a large variability, and clusters 3–6 vary between 30,000–40,000 kHz (Figure 3D). Thus, in both models, clusters 3–6 contain syllables with two or three distinct components, as can be observed also in the heat-maps presented in Figure 2. As for the duration (Figure 3E), the clusters in CPF models show a trend similar to that of the bandwidth, where syllables with low bandwidth in the lower frequency range are also shorter and those in the higher frequency range bandwidth are longer. In addition, there are significant sex differences (Supplementary Table 2A). In the Mthfr model, in clusters 1 and 2, syllable duration is 0.051 s or below, whereas in all other clusters syllables are mostly longer than 0.051 s with minimal differences between male and female (Supplementary Table 2B).
The Effect of Chlorpyrifos and Methylenetetrahydrofolate Reductase on Syllable Properties in Each Cluster
Following characterization of eight distinct syllable clusters in each ASD model, the effects of the independent factors on syllable properties, compared to control, in each of the different clusters were examined. The effect of CPF treatment is shown in Tables 3A,B for female and male mice, respectively. In general, CPF had a greater effect on female pups (Table 3), such that in clusters 1, 3, 6, 7, and 8 at least 4 out of 5 variables were significantly affected. This was more apparent in the CPF-L group (Table 3). The greater effect of the CPF-L group was also apparent in the males with more significant changes in clusters 1, 2, 3, 7, and 8.
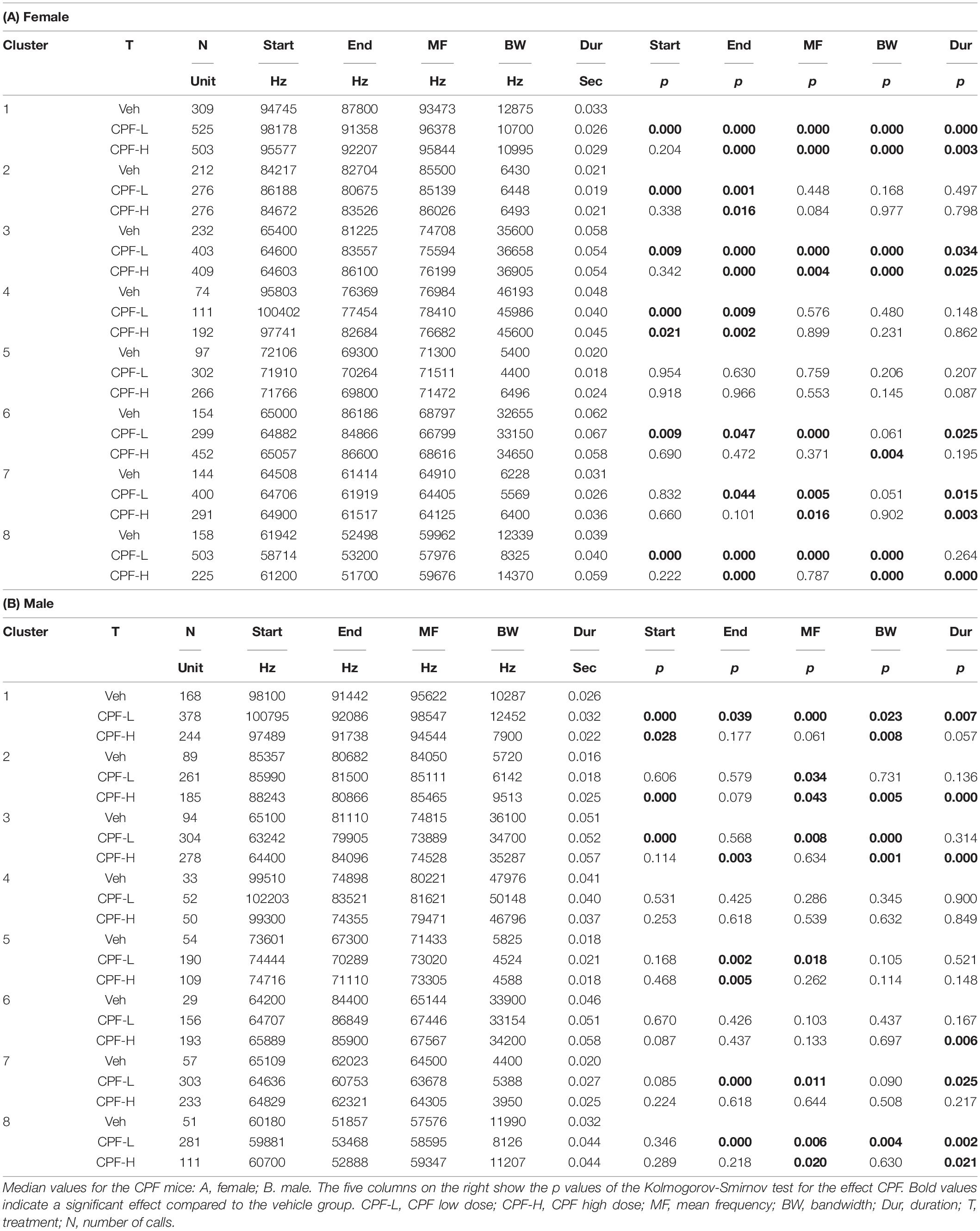
Table 3. The effect of CPF exposure on spectral and temporal properties of isolation induced syllables of the different syllable clusters.
Since in the Mthfr model most of the significant effects were observed in males, we present the data for males only (Table 4). Maternal and offspring genotype affected syllable variables as follows: an effect of maternal and offspring Mthfr+/− genotypes was noticed in clusters 2, 3, 6, 7, and 8 with higher number of differences detected due to offspring genotype (Wt:Wt vs. Het:Het). In addition, the variables start frequency and bandwidth were affected in a higher number of clusters compared to the rest of the variables.
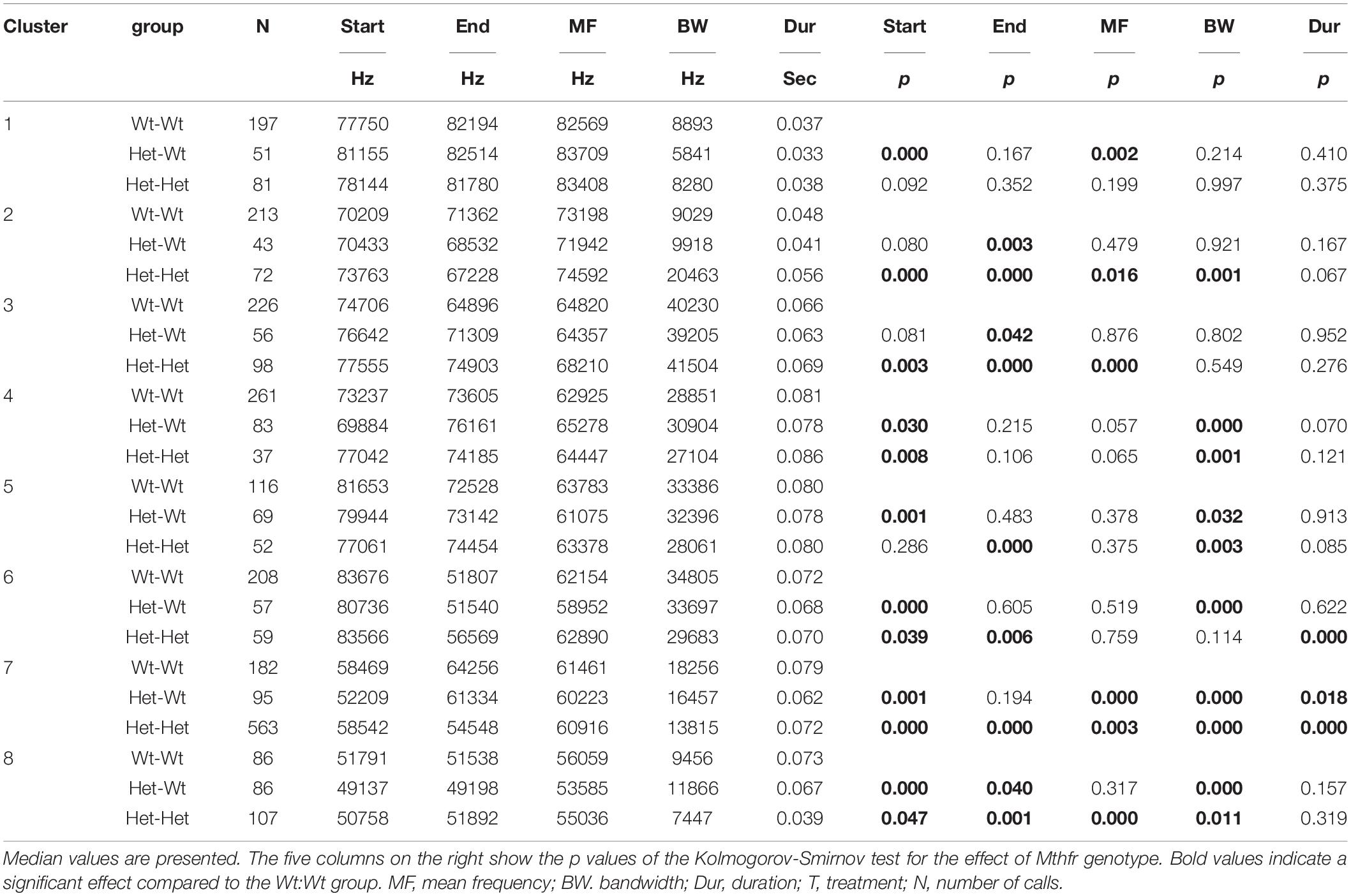
Table 4. The effect of Mthfr genotype on spectral and temporal properties of isolation induced syllables of the different syllable clusters in male pups.
Thus, in both models, the start and end frequencies and the bandwidth were related to the ASD factor.
When evaluating in both models the effects of the independent factors on syllable variables in each cluster, two qualitative features are salient: (1) the start frequency and bandwidth were the variables that were affected by the ASD factor in the greatest number of clusters and (2) bandwidth and duration were the variables that show the highest percent change compared to controls group (Tables 3, 4).
The Effect of Chlorpyrifos and Methylenetetrahydrofolate Reductase on Syllable Usage
Last, we examined the proportional production of the different syllables, categorized by cluster number. The percent of syllables from each cluster emitted by the pups of the different groups is shown in Figure 4. In both models, a chi square test for the distribution of the percent of calls from each cluster revealed significant differences compared to the model’s control group (Veh and Wt:Wt). In the CPF model, compared to the vehicle group, mice of both sexes that were exposed to both doses of CPF emitted a lower proportion of high frequency syllables (clusters 1, 2, 3). Mice that were exposed to the low dose (CPF-L) showed a higher proportion of low frequency cluster 5 syllables. Both doses of CPF also increased the proportion of low frequency clusters 7 in males. In the females, only the low dose CPF-L mice showed a higher proportion of clusters 7 and 8. CPF-H females had a 30–50% higher proportion of low frequency and two components syllables (clusters 4, 5, and 6) (Figure 4A). Interestingly, the male offspring of maternal Mthfr+/− genotype (Het-Wt and even more in the Het-Het pups) showed a similar tendency, such that there was a lower proportion of syllables with high frequency in syllables of both simple (Clusters 1 and 2) or complex (3, 4, 5, and 6) structure (20–75% of control). Maternal Mthfr+/− genotype also was associated with a marked increase in the proportions of low frequency calls (clusters 7 and 8). Moreover, an additional effect of offspring genotype was shown in cluster 7, where male Het:Het mice showed a 400% increase in the proportion of those syllables (Figure 4B). Altogether, CPF and Mthfr genotype decreased the proportion of syllables at the range of 70,000 kHz and above and increased the use of syllables with a range around 50,000 kHz.
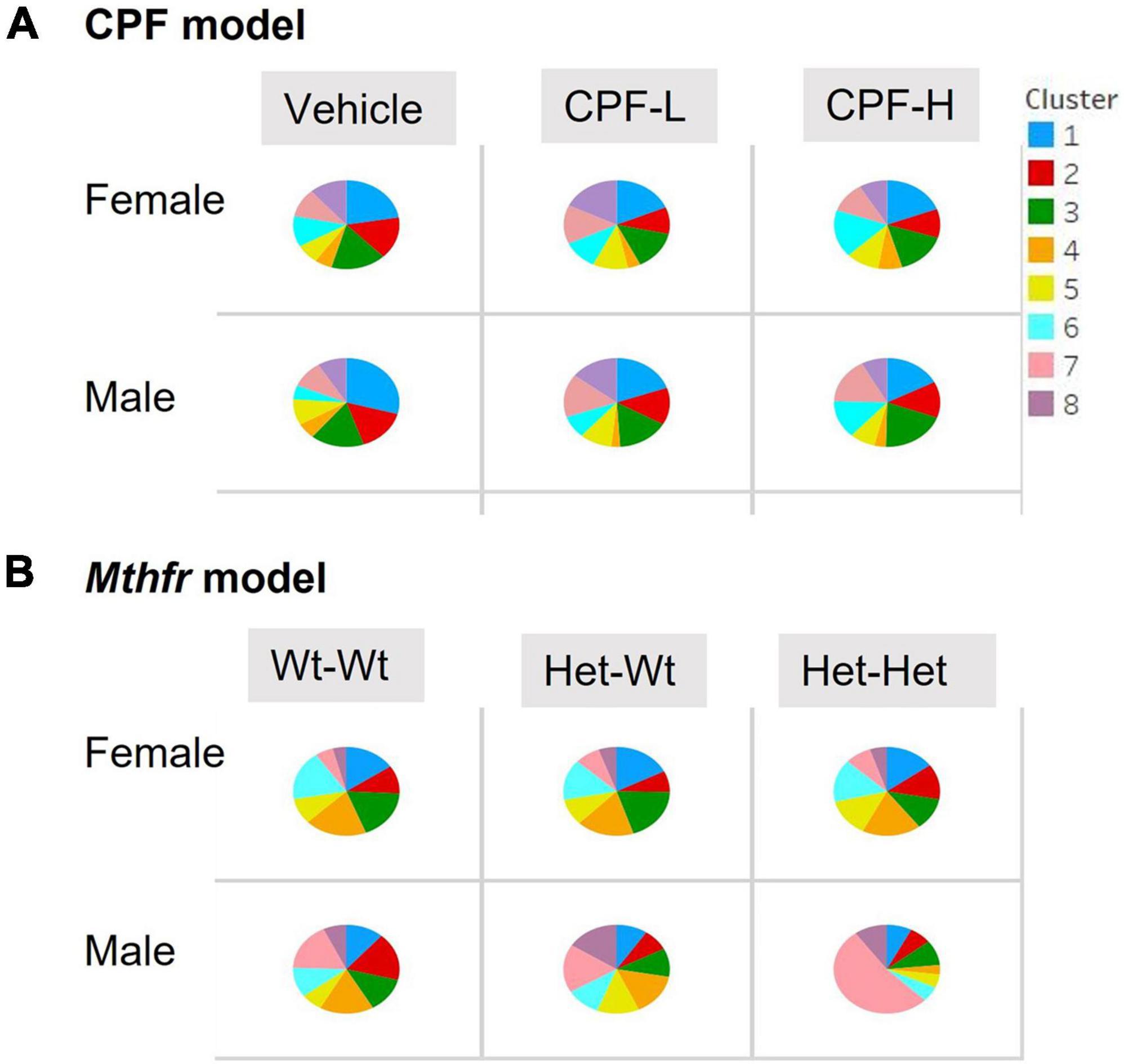
Figure 4. CPF and Mthfr+/– altered the proportion of syllables per cluster emitted by the pups. The proportion of syllables emitted by each experimental group is presented for females and males: (A) CPF model and (B) Mthfr model. Different clusters are color coded as in Figure 4. Note that each ASD model has a unique cluster distribution; the number and color code for Mthfr model do not overlap with the same characteristics in the CPF model.
Discussion
Comparative exploration of the spectral and temporal aspects of isolation USVs in pups of the two ASD models shows that: (1) the start frequency, end frequency, and bandwidth were the variables most significantly affected by the ASD factor in most clusters and (2) bandwidth and duration showed the largest differences between ASD and control groups. These findings were distinct for syllables with a simple structure, i.e., a single main component. By analyzing syllable characteristics for two distinct mouse models, we found similar communication deficits, despite the fact that the two models were derived from different strains that had discrete basal USV characteristics. We suggest that these variables are the most sensitive to the developmental changes in brain circuitry in ASD models.
In addition to the number of syllables, syllable duration is the most frequently used measure to characterize isolation induced calls in pups as summarized by Young et al. (2010), and Lai et al. (2014). Shorter syllable duration compared to their controls was previously reported in the C57-htr1a; C57-cacna1c; FVB MALTT, and C57-Ext1c; ProSap1/Shank 2 (Ey et al., 2013) strains, whereas C57-Tsc2 het and male FMR1-KO pups (Nolan et al., 2020) had longer syllable durations. Most of these studies do not distinguish between syllable types. Mice exposed to CPF had shorter syllable durations regardless of syllable type or sex similar to FMR1-KO mice (Lai et al., 2014). This was more pronounced in the females’ simple syllables in the cluster analysis. A similar observation was obtained in the Mthfr model, where syllable cluster analysis enabled detection of shorter duration in simple syllables with low frequency (clusters 6–8) of the experimental groups vs. the control. Improvement in the resolution and specificity of the effect to a particular syllable type was reported for the BTBR mouse strain (Scattoni et al., 2008b), where the duration of harmonic and composite, but not other syllables, differs compared to other strains. Thus, when the difference between the control and ASD model mouse occurs in the predominant syllable type, changes in the spectral and temporal properties of all syllables can be detected.
Various classification schemes of syllable types and characteristic specifications were suggested by Scattoni et al. (2008a, b), Young et al. (2010), and Ey et al. (2013), enhancing the ability to detect specific alterations in model pups, and detection of inter-strain differences. Classification of multiple (10–100) syllable types performed by the observer or software designed for that task (Van Segbroeck et al., 2017; Coffey et al., 2019) provides the desired enhanced resolution. Alternative strategies of analysis rely on the use of fewer and more general categories of calls as reported by several groups in recent years (Agarwalla et al., 2020; Nolan et al., 2020; Yang et al., 2021). Aiming to exploit syllable classification to homogeneous categories while at the same time avoiding over-categorization, we implemented an unsupervised method of hierarchical clustering based on the spectral properties of the syllables. The number of clusters was restricted to 8, as a compromise between overfitting of clusters to syllables and the need to have a comprehensive portrayal of the range of clusters. Although only spectral information was used for classification, the clusters were distinct in their temporal properties (i.e., duration) as evident in Figure 3E and Tables 3, 4 confirming the relevance of the cluster analysis to create meaningful categories of syllables. The unbiased classification enabled the detection of the effect of CPF treatment on the mean frequency, and syllable bandwidth, that were not detected before in the CPF model (Berg et al., 2020; Morales-Navas et al., 2020).
Although we did not find any deficits in the latency to pup retrieval in either the CPF or Mthfr model at a young age, a more careful analysis of the dams’ response to recorded USVs, without the olfactory cues that the “lost” pup provides, will shed light on the functional significance of the “ASD-like” spectro-temporal properties. In the TBX1 genetic model, dams responded more to the location of signals that had sequence properties of WT mice, suggesting that the higher order characteristics of the call sequence affect maternal response (Kato et al., 2021).
Another aspect shown to affect quantity and properties of isolation calls is related to anxiety as shown in early works (Olivier et al., 1998) that tested the effect of anxiolytic drugs on USV emission. Benzodiazepines significantly affect the number of calls emitted (Hodgson et al., 2008; Takahashi et al., 2009), suggesting the involvement of the GABAergic system in the regulation of this behavior. Changes in cellular organization of Parvalbumin interneurons and decreased levels of GABAergic proteins were reported in the Mthfr deficient mouse (Sadigurschi and Golan, 2018; Orenbuch et al., 2019) and may influence isolation calls emitted by Mthfr deficient pups. Interestingly, enhanced anxiety was observed in adult offspring to Mthfr deficiency dams (Sadigurschi and Golan, 2018). GABA has also been implicated in the effects of exposure to CPF on anxiety, while perinatal CPF increased anxiety in female mice, but reduced anxiety in zebrafish larvae (Braquenier et al., 2010; Venerosi et al., 2010; Richendrfer et al., 2012; Perez-Fernandez et al., 2020), although the underlying mechanism is unknown. The GABAergic pathway and parvalbumin interneurons undergo dramatic developmental maturation at the first postnatal week (Rivera et al., 1999; Huang et al., 2007) that may affect USV development. Support for the role of GABA in the development of USVs is provided by the finding that premature hypothalamic Agrp-GABAergic neurons, prior to the maturation of the GABA pathway, activate the neuronal circuitry regulating the emission of isolation induced USVs. These neurons are not only involved in the stimulation of USV emission but also affect the repertoire of syllables emitted by the pups (Zimmer et al., 2019). This emphasizes the relation between the maturation of the GABAergic system and aspects of pup-caregiver bonding, supporting the modulation of GABA pathway as a shared origin for the communication deficit in ASD mice models. Changes in the GABA pathway were shown in several mouse models of ASD, including BTBR strain (Han et al., 2012, 2014; Silverman et al., 2015), the FMR1 (Silverman et al., 2015), SCN1A (Han et al., 2012), and others. Although the GABA pathway was less well studied in rodents following prenatal CPF exposure, two recent publications support this direction; mice exposure to CPF, gestation up to the age of weaning, led to decreased expression of the Gabbr2 (Pallotta et al., 2017) and to increased extracellular GABA concentration in the cerebellum of male, but not female rats (Gómez-Giménez et al., 2018). Further studies are required to link these changes with the modulation of isolation calls emitted by pups in murine models of ASD.
Last, the effect of sex on the number of isolation calls and their characteristics varies between studies. Although early reports do not find an effect of sex (Scattoni et al., 2008b; Lai et al., 2014) the possibility of sex specific effects in the context of ASD promoted analysis of this variable. FMR1-KO (Reynolds et al., 2016; Nolan et al., 2020), PTEN-KO (Binder and Lugo, 2017), and FoxP1-KO mice (Fröhlich et al., 2017) present sex-specific effects on the number and properties of isolation-induced calls. We found a sex difference in the spectral and temporal properties of the calls in both control groups indicating a sex difference in each of the background strains. The interaction between each ASD factor with sex parallels similar complex interactions with sex on social variables in the CPF and Mthfr models (Sadigurschi and Golan, 2018; Lan et al., 2019).
One limitation of the current study is the use of different background strains for the different ASD models. While on one hand, the method applied for syllable classification enabled the detection of similar trends in the two models despite of the strain difference, generalization of the findings to other ASD models remains to be done in future studies.
In summary, genetic and environmental ASD factors had parallel effects on the development of USVs. Further research is needed to explore the association between USV and core behavioral deficits in mouse ASD models.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by Israel Council on Animal Care and approved by the Animal Care and Use Committee of Ben-Gurion University of the Negev, Israel.
Author Contributions
IS performed experiments, developed method for analysis, and analyzed the data. SG, ER, and MW performed experiments. VC-C guided and supervised analysis methods and manuscript critical review. DL performed methods development and manuscript review. OK and HG conceptualized and planned the experiments, supervised experiments and analysis, funded the study, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Zlotowski Center for Neuroscience, summer fellowship to IS and Israel Science Foundation to HG (grant number 515/17) and OK (grant number 1530/14).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Prof. Yossi Yovel from Tel Aviv University for his professional guidance and support in all methodological aspects of ultrasonic vocalization recording. Mthfr+/− mice were kindly provided by Rima Rozen (McGill University, Montreal, QC, Canada).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.769670/full#supplementary-material
References
Agam, G., Taylor, Z., Vainer, E., and Golan, H. M. (2020). The influence of choline treatment on behavioral and neurochemical autistic-like phenotype in Mthfr-deficient mice. Transl. Psychiatry 10:316. doi: 10.1038/s41398-020-01002-1
Agarwalla, S., Arroyo, N. S., Long, N. E., O’Brien, W. T., Abel, T., and Bandyopadhyay, S. (2020). Male-specific alterations in structure of isolation call sequences of mouse pups with 16p11.2 deletion. Genes Brain Behav. 19:e12681. doi: 10.1111/gbb.12681
Berg, E. L., Ching, T. M., Bruun, D. A., Rivera, J. K., Careaga, M., Ellegood, J., et al. (2020). Translational outcomes relevant to neurodevelopmental disorders following early life exposure of rats to chlorpyrifos. J. Neurodev. Disord. 12:40. doi: 10.1186/s11689-020-09342-1
Binder, M. S., and Lugo, J. N. (2017). NS-Pten knockout mice show sex- and age-specific differences in ultrasonic vocalizations. Brain Behav. 7;e00857. doi: 10.1002/brb3.857
Braquenier, J. B., Quertemont, E., Tirelli, E., and Plumier, J. C. (2010). Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicol. Teratol. 32, 234–239. doi: 10.1016/j.ntt.2009.08.008
Brielmaier, J., Matteson, P. G., Silverman, J. L., Senerth, J. M., Kelly, S., Genestine, M., et al. (2012). Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One 7:e40914. doi: 10.1371/journal.pone.0040914
Chen, Z., Karaplis, A. C., Ackerman, S. L., Pogribny, I. P., Melnyk, S., Lussier-Cacan, S., et al. (2001). Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 10, 433–443.
Coffey, K. R., Marx, R. G., and Neumaier, J. F. (2019). DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 44, 859–868. doi: 10.1038/s41386-018-0303-6
De Felice, A., Scattoni, M. L., Ricceri, L., and Calamandrei, G. (2015). Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One 10:e0121663. doi: 10.1371/journal.pone.0121663
Defensor, E. B., Pearson, B. L., Pobbe, R. L., Bolivar, V. J., Blanchard, D. C., and Blanchard, R. J. (2011). A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav. Brain Res. 217, 302–308. doi: 10.1016/j.bbr.2010.10.033
Ehret, G. (2005). Infant rodent ultrasounds – a gate to the understanding of sound communication. Behav. Genet. 35, 19–29. doi: 10.1007/s10519-004-0853-8
Eskenazi, B., Marks, A. R., Bradman, A., Harley, K., Barr, D. B., Johnson, C., et al. (2007). Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 115, 792–798. doi: 10.1289/ehp.9828
Ey, E., Torquet, N., Le Sourd, A., Leblond, C. S., Boeckers, T. M., Faure, P., et al. (2013). The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav. Brain Res. 256, 677–689. doi: 10.1016/j.bbr.2013.08.031
Fröhlich, H., Rafiullah, R., Schmitt, N., Abele, S., and Rappold, G. A. (2017). Foxp1 expression is essential for sex-specific murine neonatal ultrasonic vocalization. Hum. Mol. Genet. 26, 1511–1521. doi: 10.1093/hmg/ddx055
Furlong, M. A., Engel, S. M., Barr, D. B., and Wolff, M. S. (2014). Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ. Int. 70, 125–131. doi: 10.1016/j.envint.2014.05.011
Goin-Kochel, R. P., Porter, A. E., Peters, S. U., Shinawi, M., Sahoo, T., and Beaudet, A. L. (2009). The MTHFR 677C–>T polymorphism and behaviors in children with autism: exploratory genotype-phenotype correlations. Autism Res. 2, 98–108.
Gómez-Giménez, B., Felipo, V., Cabrera-Pastor, A., Agustí, A., Hernández-Rabaza, V., and Llansola, M. (2018). developmental exposure to pesticides alters motor activity and coordination in rats: sex differences and underlying mechanisms. Neurotox. Res. 33, 247–258. doi: 10.1007/s12640-017-9823-9
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102. doi: 10.1001/archgenpsychiatry.2011.76
Han, S., Tai, C., Jones, C., Scheuer, T., and Catterall, W. (2014). Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron 81, 1282–1289. doi: 10.1016/j.neuron.2014.01.016
Han, S., Tai, C., Westenbroek, R. E., Yu, F. H., Cheah, C. S., Potter, G. B., et al. (2012). Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489, 385–390. doi: 10.1038/nature11356
Hodgson, R. A., Guthrie, D. H., and Varty, G. B. (2008). Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol. Biochem. Behav. 88, 341–348.
Hofer, M. A., and Shair, H. N. (1991). Independence of ultrasonic vocalization and thermogenic responses in infant rats. Behav. Neurosci. 105, 41–48.
Huang, Z. J., Di Cristo, G., and Ango, F. (2007). Development of GABA innervation in the cerebral and cerebellar cortices. Nat. Rev. Neurosci. 8, 673–686.
Kato, R., Machida, A., Nomoto, K., Kang, G., Hiramoto, T., Tanigaki, K., et al. (2021). Maternal approach behaviors toward neonatal calls are impaired by mother’s experiences of raising pups with a risk gene variant for autism. Dev. Psychobiol. 63, 108–113.
Kazdoba, T. M., Leach, P. T., and Crawley, J. N. (2016). Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 15, 7–26. doi: 10.1111/gbb.12256
Lai, J. K., Sobala-Drozdowski, M., Zhou, L., Doering, L. C., Faure, P. A., and Foster, J. A. (2014). Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav. Brain Res. 259, 119–130.
Lan, A., Kalimian, M., Amram, B., and Kofman, O. (2017). Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environ. Health 16:43. doi: 10.1186/s12940-017-0251-3
Lan, A., Stein, D., Portillo, M., Toiber, D., and Kofman, O. (2019). Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav. Brain Funct. 15:2. doi: 10.1186/s12993-019-0153-3
Laviola, G., Adriani, W., Gaudino, C., Marino, R., and Keller, F. (2006). Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced stereotypies in reeler mutant mice. Psychopharmacology (Berl) 187, 331–344. doi: 10.1007/s00213-006-0426-z
Mohammad, N. S., Jain, J. M., Chintakindi, K. P., Singh, R. P., Naik, U., and Akella, R. R. (2009). Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiatr. Genet. 19, 171–176. doi: 10.1097/YPG.0b013e32832cebd2
Morales-Navas, M., Castaño-Castaño, S., Pérez-Fernández, C., Sánchez-Gil, A., Teresa Colomina, M., Leinekugel, X., et al. (2020). Similarities between the effects of prenatal chlorpyrifos and valproic acid on ultrasonic vocalization in infant wistar ratsint. J. Environ. Res. Public. Health. 17:6376. doi: 10.3390/ijerph17176376
Nadler, J. J., Moy, S. S., Dold, G., Trang, D., Simmons, N., Perez, A., et al. (2004). Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3, 303–314. doi: 10.1111/j.1601-183X.2004.00071.x
Nolan, S. O., Hodges, S. L., and Lugo, J. N. (2020). High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes Brain Behav. 19:e12611.
Nolan, S. O., Reynolds, C. D., Smith, G. D., Holley, A. J., Escobar, B., Chandler, M. A., et al. (2017). Deletion of Fmr1 results in sex-specific changes in behavior. Brain Behav. 7:e00800.
Olivier, B., Molewijk, E., van Oorschot, R., van der Heyden, J., Ronken, E., and Mos, J. (1998). Rat pup ultrasonic vocalization: effects of benzodiazepine receptor ligands. Eur. J. Pharmacol. 358, 117–128.
Orenbuch, A., Fortis, K., Taesuwan, S., Yaffe, R., Caudill, M. A., and Golan, H. M. (2019). Prenatal nutritional intervention reduces autistic-like behavior rates among mthfr-deficient mice. Front. Neurosci. 13:383. doi: 10.3389/fnins.2019.00383
Pallotta, M. M., Ronca, R., Carotenuto, R., Porreca, I., Turano, M., Ambrosino, C., et al. (2017). Specific effects of chronic dietary exposure to chlorpyrifos on brain gene expression-a mouse study. Int. J. Mol. Sci. 18:2467. doi: 10.3390/ijms18112467
Panksepp, J. B., Jochman, K. A., Kim, J. U., Koy, J. J., Wilson, E. D., Chen, Q., et al. (2007). Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One 2:e351. doi: 10.1371/journal.pone.0000351
Patten, E., Belardi, K., Baranek, G. T., Watson, L. R., Labban, J. D., and Oller, D. K. (2014). Vocal patterns in infants with autism spectrum disorder: canonical babbling status and vocalization frequency. J. Autism Dev. Disord. 44, 2413–2428. doi: 10.1007/s10803-014-2047-4
Paul, R., Fuerst, Y., Ramsay, G., Chawarska, K., and Klin, A. (2011). Out of the mouths of babes: vocal production in infant siblings of children with ASD. J. Child Psychol. Psychiatry 52, 588–598. doi: 10.1111/j.1469-7610.2010.02332.x
Perez-Fernandez, C., Morales-Navas, M., Guardia-Escote, L., Garrido-Cárdenas, J. A., Colomina, M. T., Giménez, E., et al. (2020). Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: a locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem. Toxicol. 135:110865.
Rai, V. (2016). Association of methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism with autism: evidence of genetic susceptibility. Metab. Brain Dis. 31, 727–735.
Rauh, V. A., Garfinkel, R., Perera, F. P., Andrews, H. F., Hoepner, L., Barr, D. B., et al. (2006). Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118, e1845–e1859. doi: 10.1542/peds.2006-0338
Reynolds, C. D., Nolan, S. O., Jefferson, T., and Lugo, J. N. (2016). Sex-specific and genotype-specific differences in vocalization development in FMR1 knockout mice. Neuroreport 27, 1331–1335.
Ricceri, L., Cutuli, D., Venerosi, A., Scattoni, M. L., and Calamandrei, G. (2007). Neonatal basal forebrain cholinergic hypofunction affects ultrasonic vocalizations and fear conditioning responses in preweaning rats. Behav. Brain Res. 183, 111–117.
Richendrfer, H., Pelkowski, S. D., Colwill, R. M., and Creton, R. (2012). On the edge: pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav. Brain Res. 228, 99–106.
Rivera, C., Voipio, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K., et al. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. doi: 10.1038/16697
Sadigurschi, N., and Golan, H. M. (2018). Maternal and offspring MTHFR genotypes interact in a mouse model to induce ASD-like behavior. Genes Brain Behav. 18:e12547.
Satterstrom, F. K., Kosmicki, J. A., Wang, J., Breen, M. S., De Rubeis, S., An, J. Y., et al. (2020). Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584.e23.
Scattoni, M. L., McFarlane, H. G., Zhodzishsky, V., Caldwell, H. K., Young, W. S., Ricceri, L., et al. (2008a). Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav. Brain Res. 187, 371–378.
Scattoni, M. L., Gandhy, S. U., Ricceri, L., and Crawley, J. N. (2008b). Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One 3:e3067. doi: 10.1371/journal.pone.0003067
Schmidt, R. J., Hansen, R. L., Hartiala, J., Allayee, H., Schmidt, L. C., Tancredi, D. J., et al. (2011). Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22, 476–485.
Shelton, J. F., Geraghty, E. M., Tancredi, D. J., Delwiche, L. D., Schmidt, R. J., Ritz, B., et al. (2014). Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ. Health Perspect. 122, 1103–1109. doi: 10.1289/ehp.1307044
Silverman, J. L., Pride, M. C., Hayes, J. E., Puhger, K. R., Butler-Struben, H. M., Baker, S., et al. (2015). GABAB receptor agonist r-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology 40, 2228–2239. doi: 10.1038/npp.2015.66
Takahashi, A., Yap, J. J., Bohager, D. Z., Faccidomo, S., Clayton, T., Cook, J. M., et al. (2009). Glutamatergic and GABAergic modulations of ultrasonic vocalizations during maternal separation distress in mouse pups. Psychopharmacology (Berl) 204, 61–71. doi: 10.1007/s00213-008-1437-8
Van Segbroeck, M., Knoll, A. T., Levitt, P., and Narayanan, S. (2017). MUPET-mouse ultrasonic profile ExTraction: a signal processing tool for rapid and unsupervised analysis of ultrasonic vocalizations. Neuron 94, 465–485.e5.
Venerosi, A., Ricceri, L., Rungi, A., Sanghez, V., and Calamandrei, G. (2010). Gestational exposure to the organophosphate chlorpyrifos alters social-emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology (Berl) 208, 99–107. doi: 10.1007/s00213-009-1713-2
Venerosi, A., Ricceri, L., Scattoni, M. L., and Calamandrei, G. (2009). Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ. Health 8:12.
Wang, Y., Zhang, Y., Ji, L., Hu, Y., Zhang, J., Wang, C., et al. (2017). Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ. Int. 108, 119–126. doi: 10.1016/j.envint.2017.08.010
Warlaumont, A. S., Richards, J. A., Gilkerson, J., and Oller, D. K. (2014). A social feedback loop for speech development and its reduction in autism. Psychol. Sci. 25, 1314–1324. doi: 10.1177/0956797614531023
Yang, X., Guo, D., Li, K., and Shi, L. (2021). Altered postnatal developmental patterns of ultrasonic vocalizations in Dock4 knockout mice. Behav. Brain Res. 406, 113232.
Young, D. M., Schenk, A. K., Yang, S. B., Jan, Y. N., and Jan, L. Y. (2010). Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc. Natl. Acad. Sci. U.S.A. 107, 11074–11079.
Keywords: autism, MTHFR, chlorpyrifos, GABA, ultrasonic vocalization, communication, newborn
Citation: Shekel I, Giladi S, Raykin E, Weiner M, Chalifa-Caspi V, Lederman D, Kofman O and Golan HM (2021) Isolation-Induced Ultrasonic Vocalization in Environmental and Genetic Mice Models of Autism. Front. Neurosci. 15:769670. doi: 10.3389/fnins.2021.769670
Received: 02 September 2021; Accepted: 18 October 2021;
Published: 22 November 2021.
Edited by:
Anthony LaMantia, Virginia Tech, United StatesReviewed by:
Keiko Iwata, University of Fukui, JapanChiara Verpelli, Institute of Neuroscience, National Research Council, Consiglio Nazionale delle Ricerche (CNR), Italy
Copyright © 2021 Shekel, Giladi, Raykin, Weiner, Chalifa-Caspi, Lederman, Kofman and Golan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ora Kofman, a29mbWFuQGJndS5hYy5pbA==; Hava M. Golan, aGF2YWdAYmd1LmFjLmls
†These authors share senior authorship
 Itay Shekel1,2
Itay Shekel1,2 Vered Chalifa-Caspi
Vered Chalifa-Caspi Ora Kofman
Ora Kofman Hava M. Golan
Hava M. Golan