- 1Department of General Practice, Xuanwu Hospital of Capital Medical University, Beijing, China
- 2Radiological Sciences, Division of Clinical Neuroscience, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom
- 3Department of Neurology, Xuanwu Hospital of Capital Medical University, Beijing, China
- 4National Clinical Research Center for Geriatric Disorders, Beijing, China
- 5Center of Alzheimer’s Disease, Beijing Institute for Brain Disorders, Beijing, China
Background: Previous reports on APOE ε4 allele distribution in different populations have been inconclusive. The Subjective Cognitive Decline-Questionnaire 9 (SCD-Q9) was developed to identify those at risk of objective cognitive impairment [OCI; including mild cognitive impairment (MCI) and dementia groups), but its association with APOE ε4 and discriminatory powers for SCDwith subtle cognitive decline (SCDs) and OCI in memory clinics are unclear.
Objectives: To investigate demographic distribution of APOE ε4, its association with SCD-Q9 scores, and its ability to discriminate SCDs and OCI groups from normal control (NC).
Methods: A total of 632 participants were recruited (NC = 243, SCDs = 298, OCI = 91). APOE ε4 allele distribution and association with SCD-Q9 scores were calculated and the effects on cognitive impairment were analyzed. Receiver operating characteristic (ROC) analysis was applied to identify discriminatory powers for NC, SCDs, and OCI.
Results: Total APOE ε4 frequency was 13.1%. This did not vary by demography but was higher in patients with OCI. The SCD-Q9 scores were higher in APOE ε4 carriers than non-carriers in the OCI group. The area under the curve (AUC) for discriminating from OCI using APOE ε4 were 0.587 and 0.575, using SCD-Q9 scores were 0.738 and 0.571 for NC and SCDs groups, respectively. When we combined APOE ε4 and SCD-Q9 scores into the model, the AUC increased to 0.747 for discriminating OCI from NC. However, when OCI group was split into MCI and dementia groups, only total SCD-Q9 score was the independent affecting factor of MCI.
Conclusion: This study demonstrated that the distribution of APOE ε4 alleles did not vary with different demographic characteristics in a large-scale cohort from a memory clinic. APOE ε4 alleles may be associated with scores of SCD-Q9 reflecting the degree of cognitive complaints but their additional contribution to SCD-Q9 scores is marginal in discriminating between NC, SCDs, and OCI.
Introduction
Previous studies have reported that mutation of Apolipoprotein E (APOE) and regulation of its expression have an important connection with Alzheimer’s disease (AD) dementia (Kim and Tsai, 2009) because of the pivotal role of APOE in lipoprotein metabolism in the brain. Among the three alleles of APOE (ε2, ε3, and ε4), presence of ε4 can increase the risk of AD by approximately 3- (single allele) to 15-fold (double alleles) (Saunders et al., 1993; Kim and Tsai, 2009; Koffie et al., 2012).
An earlier meta-analysis and review of the distributions of APOE alleles showed that APOE ε4 alleles were the second most common allele (besides APOE ε3) (Farrer et al., 1997; Abondio et al., 2019), but distributions vary with age, gender, and ethnicity (Eisenberg et al., 2010; Kern et al., 2015; Le Couteur et al., 2020). Among cognitively normal subjects aged 21–97 years, APOE ε4 carriers were younger than non-carriers, but no significant differences were found in education level and gender (Caselli et al., 2009). Farrer et al. (1997) demonstrated that APOE ε4 was associated with the risk of AD from 40 to 90 years, but the relationship diminished after 70 years of age. They also reported that the frequency of the APOE ε4 allele in males was lower than that in females among the elderly without dementia (Lehmann et al., 2006). In addition, previous studies investigating different ethnic groups revealed that Asian populations might have lower APOE ε4 frequencies than Oceania in the natural population (Singh et al., 2006; Eisenberg et al., 2010), and Chinese cohorts presented relatively low frequencies of APOE ε4 alleles (Katzman et al., 1997), but the conclusions need to be confirmed by more research.
More recently, researchers have also focused on understanding the relevance of APOE ε4 prevalence and the early stages of cognitive impairment, such as subjective cognitive decline (SCD), which is an intermediate stage between mild cognitive impairment (MCI) and normal cognition. A systematic review (Ali et al., 2018) (n = 36 articles) showed that the frequency of APOE ε4 was significantly lower in healthy controls than in groups with objective cognitive impairment (OCI), including MCI and AD dementia, but showed no difference in the SCD group, suggesting that APOE ε4 may not be directly related to the development of SCD. However, another earlier meta-analysis included a total of 28 studies that indicated a weak positive correlation between APOE ε4 and SCD (Zhang et al., 2017). This inconsistency may be due to different study designs (e.g., age) and the smaller sample size, especially in memory clinics, most of which evaluated less than 100 subjects (Striepens et al., 2010; Fortea et al., 2011). However, memory clinics, as the primary setting for individuals with memory complaints seeking care, are crucial for the early identification of those at risk of cognitive decline.
Additionally, since the standardization of SCD with cognitive complaints but unimpaired cognition (Jessen et al., 2014) in 2014, there is growing evidence demonstrating that subtle cognitive decline is already present in populations with SCD (Kielb et al., 2017; Jessen et al., 2018; Hao et al., 2019). To improve the likelihood of preclinical AD diagnosis, a more sensitive and reliable neuropsychological standard for subtle cognitive decline (Jak/Bondi) was developed in 2015 (Edmonds et al., 2015). However, the relationship between SCDwith subtle cognitive decline (SCDs) diagnosis based on the two updated criteria and APOE ε4 within a large cohort has not been reported.
Finally, in recent years the SCD-Questionnaire 9 (SCD-Q9), developed as an easy and quick screening tool, was used to identify patients with SCD at risk of OCI at an early stage using advanced statistical methods (Gifford et al., 2015). Since its development, several studies (Alber et al., 2018; Bott et al., 2018; Kumar et al., 2018; Hao et al., 2020) have applied it to assess changes in memory complaints and defined SCD. Previous evidence has also demonstrated the links between the subjective observations included in SCD-Q9 and objective pathological alterations (Amariglio et al., 2012). APOE ε4 as an objective biomarker of AD has been universally accepted. However, the association between SCD-Q9 scores and APOE ε4 and their predictive powers for OCI with SCD complaints are not clear. Elucidation of this association would help us better understand the biomarkers of AD reflected by subjective cognitive complaints. The combination of the two methods may help us to more quickly and accurately identify early AD patients and could also reduce the economic burden of the society and families.
Therefore, our current investigation studies larger cohorts in memory clinic settings, mainly aimed to (1) investigate the distribution characteristics of APOE ε4 alleles in different demography and cognitive impairment groups diagnosed based on updated criteria (SCD-I) (Jessen et al., 2014) and Jak-Bondi (Edmonds et al., 2015); (2) analyze the association of APOE ε4 and scores of SCD-Q9 reflecting cognitive impairment; and (3) assess the discriminatory powers of APOE ε4 alleles themselves and their combination with scores of SCD-Q9 for diagnosing cognitive impairment.
Materials and Methods
Participants
Subject Recruitment
Six hundred thirty-two individuals participated our study, SCDs, MCI, and AD dementia patients were recruited at first routine visits to the memory clinic of the Neurology Department, Xuanwu Hospital, Capital Medical University and normal control (NC) subjects were recruited from communities in Beijing, China from March, 2017 to January, 2020. The details of the study, including its purpose, procedure and contact information, was advertised in the memory clinic and via broadcasting at large-scale gatherings in the communities. People were asked for their consent to join the study.
Study Procedure and Subject Selection Criteria
All the subjects underwent a series of clinical and standardized neuropsychological evaluations, including the sociodemographic characteristics, medical history, lifestyles, and a neuropsychological test battery which contains Chinese version of Mini-Mental State Examination (MMSE) (Katzman et al., 1988), Montreal Cognitive Assessment – Basic (MoCA-B) (Chen et al., 2016b), Clinical Dementia Rating Scale (CDR) (Morris, 1993), Activities of Daily Living (ADL) (He et al., 1990), Memory: Auditory Verbal Learning Test (AVLT)-Long Delay Recall and Recognition (Guo et al., 2007), Executive function: Shape Trail Making Test-A and B (STT-A and STT-B) (Zhao et al., 2013b), Language: Animal Fluency Test (AFT) (Zhao et al., 2013a), Boston Naming Test (BNT) (Guo et al., 2006), Hamilton Anxiety Scale (HAMA) (Tang and Zhang, 1984), and Hamilton Depression Scale (HAMD) (Hamilton, 1980).
Inclusion Criteria for All Subjects
Jak/Bondi criteria as follows (Bondi et al., 2014; Edmonds et al., 2015) were used for the diagnosis of MCI and SCDs.
Mild cognitive impairment was assigned when (1) the answer needed to be “yes” to the question “Do you have a problem with your memory?”; (2) scores of two measures in the same cognitive domain were >1.0 standard deviation (SD) below the normative mean; or (3) scores of at least one measure in each of the three cognitive domains (Memory, Execution, and Language) were >1.0 SD below the normative mean; (4) failure to meet the criteria of dementia; and (5) ADL had to be normal.
For the diagnosis of SCDs, the following requirement was to be met: (1) the answers needed to be “yes” to both of the questions “Do you have a problem with your memory?” and “Are you concerned about your memory?”; (2) subtle cognitive decline was observed in the neuropsychological examination, indicated by the decreased score of two measures in different cognitive domains (>1.0 SD below the normative mean); (3) failure to meet the criteria of MCI; and (4) ADL was normal.
The diagnosis of mild AD dementia fulfilled standardized diagnostic criteria (Mckhann et al., 1984; American Psychiatric Association, 1994; Dubois et al., 2007): (1) met the diagnostic criteria of dementia; (2) gradual and progressive decline in memory function over more than 6 months; (3) impaired episodic memory revealed by the objective testing listed above; (4) impaired basic and elementary functioning for ADL; (5) CDR = 1; and (6) hippocampal atrophy confirmed by structural magnetic resonance imaging (MRI).
Groupings
The population was divided into three groups according to these listed diagnostic criteria: (1) NC was assigned when participants did not have SCD complaints (the answers needed to be “no” to both of the questions “Do you have a problem with your memory?” and “Are you concerned about your memory?”) and mild AD dementia, MCI, or SCDs, and had normal ADL scores; (2) SCDs group; and (3) the OCI group included people who were diagnosed with MCI and mild AD dementia.
Exclusion Criteria for All Subjects
(a) A history of stroke; (b) severe depression (HAMD >30), and other psychiatric disorders or current psychotropic drugs treatment; (c) other central nervous system diseases that could cause cognitive decline (e.g., brain tumors, Parkinson’s disease, encephalitis, or epilepsy); (d) other systemic diseases which could cause cognitive decline (e.g., alcoholism, thyroid dysfunction, severe anemia, syphilis, HIV, or vitamin B12 abnormalities); (e) a history of psychosis or congenital mental growth retardation; (f) cognitive decline caused by traumatic brain injury; (g) use of anti-dementia agents in SCDs, MCI, and; or (h) those who could not complete neuropsychological tests or with contraindication to MRI.
APOE Genotyping
DNA sequences for each subject were extracted for SNPs rs7412 and rs429358 from the APOE ε2/ε3/ε4 haplotype. APOE was genotyped using the standard Sanger sequencing method (Sangon, Shanghai, China) with the following primers: 5′-ACGCGGGCACGGCTGTCCAAGG-3′ (forward) and 5′-GGCGCTCGCGGATGGCGCTGA-3′ (reverse). APOE was amplified using the following conditions: 1 cycle of 98°C for 10 s, 35 cycles of 72°C for 5 s, and 1 cycle of 72°C for 5 min. PCR was performed in a final volume of 30 μl containing 10 pmol of forward and reverse primers, and 50 ng of genomic DNA template using PrimeSTAR HS DNA Polymerase with the GC Buffer (Takara Bio).
Statistical Analysis
We conducted all analyses using the Statistical Package for the Social Sciences version 17.0 (SPSS Inc., Chicago, IL, United States). Descriptive statistics (APOE alleles and scores of SCD-Q9) were calculated by percentages or mean ± SD (x ± S) or median (percentile 25, 75). The x2 or T-test or Mann–Whitney test was used to assess group differences, and p < 0.05 was considered to be statistically significant. For three groups comparison, p < 0.05 was considered to be statistically significant and corrected p’ value (p < 0.017) was used in the partitions of Pearson’s Chi-square statistics. To examine the potential affecting factors of SCDs and OCI, we performed univariate and binary logistic regression analysis (Supplementary Materials 1). More specifically, we used APOE ε4 alleles and scores of SCD-Q9 that significantly differed between two groups as the independent variables, and “diagnosis” as the dependent variable. Besides, odds ratios (ORs) were calculated for the two variables. p < 0.05 was required for variables to be in the model. Finally, we obtained the receiver operating characteristic (ROC) curves and calculated area under the curves (AUCs) for the factors.
Results
Distribution of APOE ε4 Alleles and Genotypes in the Total Population
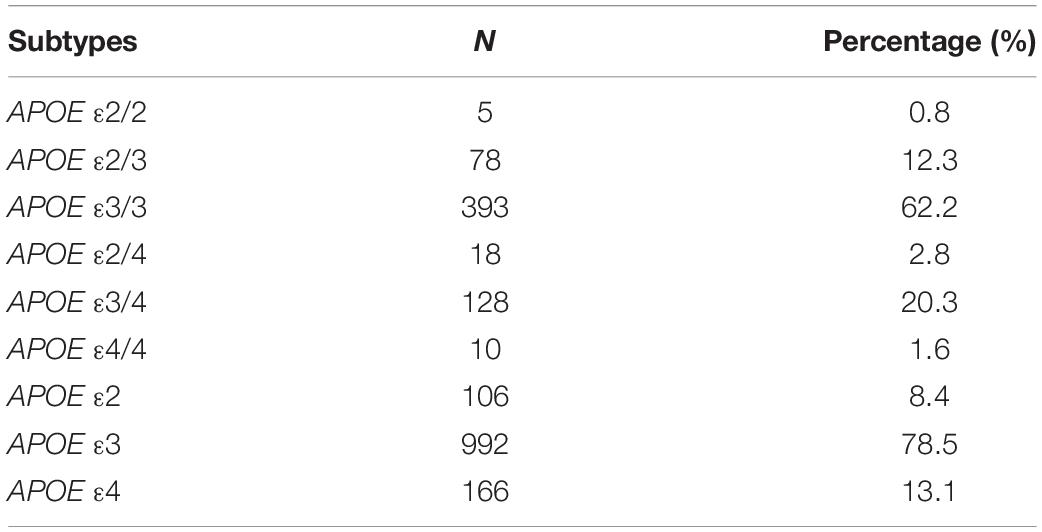
In total, 632 individuals were recruited in our study, including 218 (34.5%) males and 414 (65.5%) females, and the mean age and education years were 65.4 ± 6.76 and 12.4 ± 3.21 years, respectively. The proportions of APOE genotypes were listed in Table 1.
The proportions of APOE ε3/4 and ε2/4 were 20.3% and 2.8%, respectively. The proportion of homozygous ε4 was <2.0%. For the frequencies of the APOE alleles ε4 was 13.1% (see Table 1).
The Demography of APOE ε4 Carriers and Non-carriers
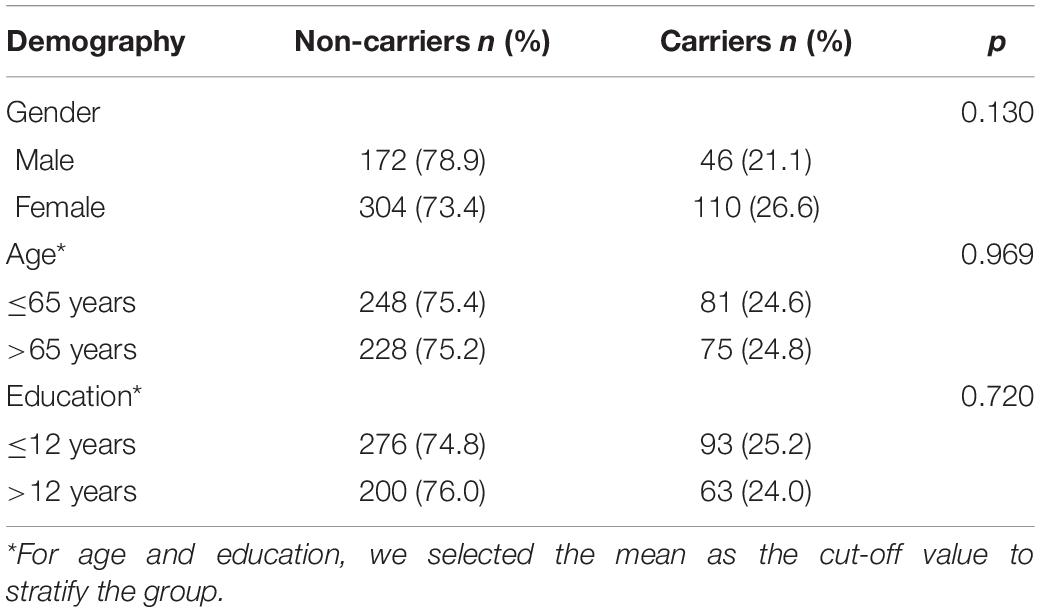
For age, gender, and years of education, we did not find significant differences between APOE ε4 carriers and non-carriers (p > 0.05) (see Table 2).
Distribution of APOE ε4 and Subjective Cognitive Decline-Questionnaire 9 Scores of Carriers and Non-carriers in Normal Control, SCDs, and Objective Cognitive Impairment Groups
The results showed that the difference in the proportion of APOE ε4 carriers among the three groups (NC, SCDs, and OCI) was significant (p = 0.004). Further pairwise comparison showed that the OCI group had a higher proportion of APOE ε4 carriers than the NC (p = 0.001) and SCDs groups (p = 0.005) at corrected test level p′, but no significant difference was found between the other two groups (p = 0.487) (see Table 3). When the total population was divided into male and female subgroups, the latter showed consistent results with the total population (see Table 4), but we did not find any significant differences among NC, SCDs, and OCI groups in the male subgroup (see Table 5).
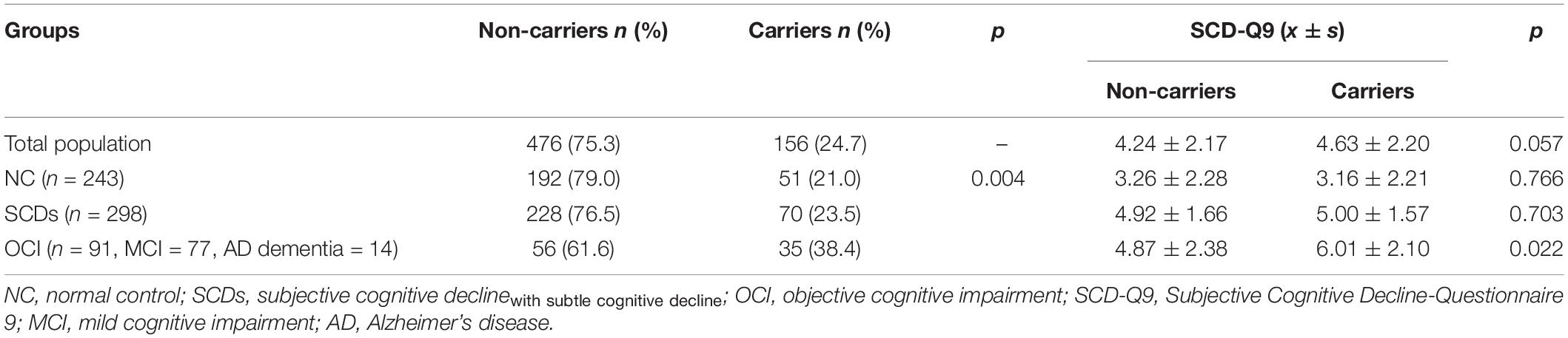
Table 3. Distribution of APOE ε4 and SCD-Q9 scores of carriers and non-carriers in NC, SCDs, and OCI groups.
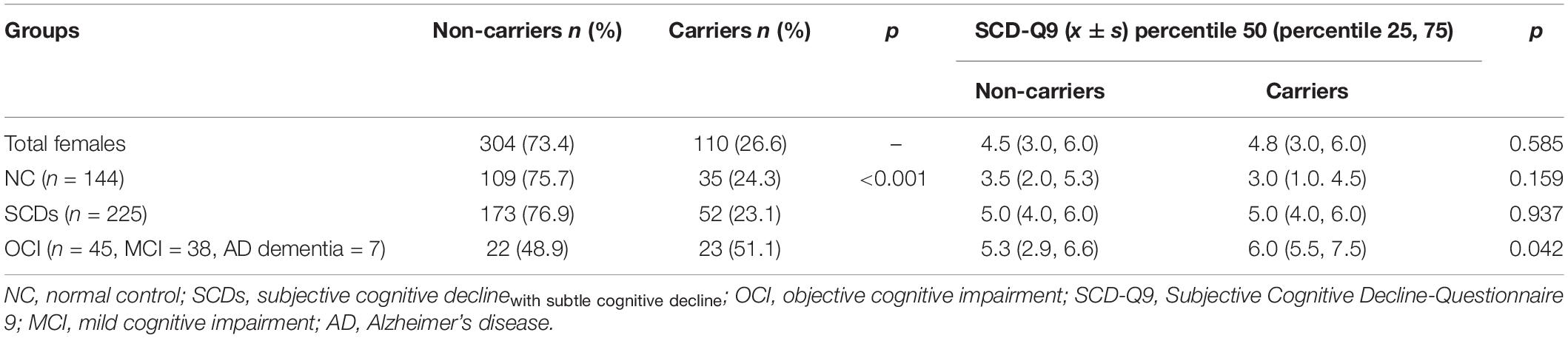
Table 4. Distribution of APOE ε4 and SCD-Q9 scores of carriers and non-carriers in NC, SCDs, and OCI groups in females.
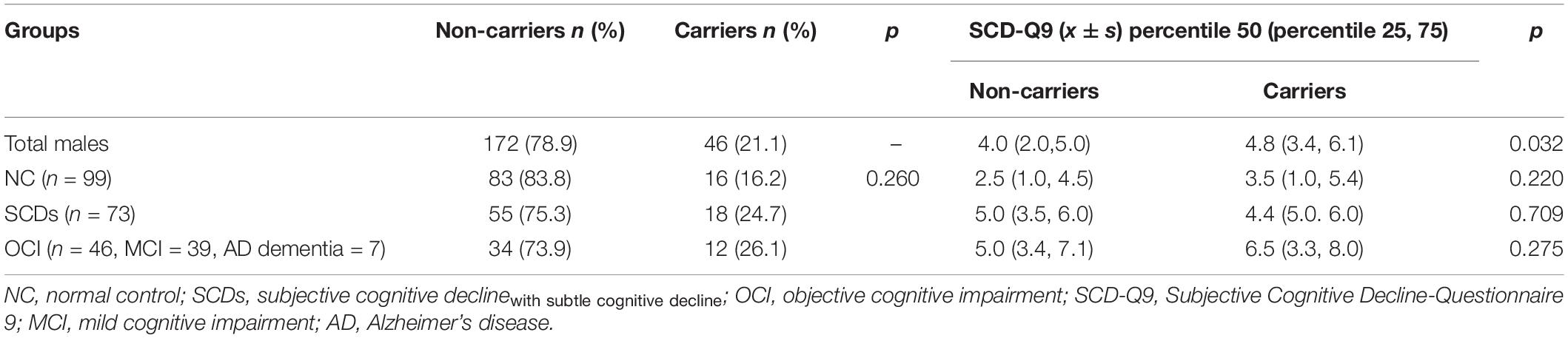
Table 5. Distribution of APOE ε4 and SCD-Q9 scores of carriers and non-carriers in NC, SCDs, and OCI groups in males.
For SCD-Q9 scores, we also found that APOE ε4 carriers scored higher than non-carriers in the OCI group (p = 0.022). However, there were no significant differences in SCD-Q9 scores in the total population (p = 0.057), NC (p = 0.766), and SCDs groups (p = 0.703) between ε4 carriers and non-carriers (see Table 3).Then, we divided the total population into female and male subgroups. For the female subgroup, the results were consistent with the total population (see Table 4). However, a significant difference was found in SCD-Q9 scores between ε4 carrier and non-carriers in the males (p = 0.032), which was different from the female subgroup and the total population. Also, we did not find any significant difference in SCD-Q9 scores between ε4 carriers and non-carriers in different cognitive groups, including NC (p = 0.220), SCDs (p = 0.709), and OCI groups (p = 0.275) (see Table 5).
Logistic Regression Models for Normal Control, SCDs, and Objective Cognitive Impairment Groups
The results of the binary logistic regression analysis for NC and OCI indicate carrier status of APOE ε4 and total SCD-Q9 score were independent risk factors of OCI [OR: 2.050, 95% CI (confidential interval): 1.161–3.620, p = 0.013, and OR: 1.444, 95% CI: 1.285–1.622, p < 0.001, respectively]. When we split OCI group into MCI and AD dementia groups, only total SCD-Q9 score was the independent risk factor of MCI [OR: 1.390, 95% CI: 1.232–1.568, p < 0.001], whereas carrier status of APOE ε4 did not show any relationship (p = 0.191).
Our results also showed that carrying the APOE ε4 allele (OR: 1.960, 95% CI: 1.184–3.243, p = 0.009) was a risk factor for OCI compared with SCDs, whereas scores of SCD-Q9 did not show any relationship (p = 0.153). When we split OCI group into MCI and AD dementia groups, carrying the APOE e4 allele and total SCD-Q9 score were not affecting factors for MCI compared with SCD (p = 0.265 and p = 0.792, respectively).
Receiver Operating Characteristics of Normal Control, SCDs, and Objective Cognitive Impairment Groups
Based on the results of logistic analysis, we calculated the AUCs of APOE ε4 itself for group discrimination, which was 0.587 (95% CI: 0.517–0.658, p = 0.014) between NC and OCI groups and 0.575 (95% CI: 0.506–0.644, p = 0.031) between SCDs and OCI groups (see details in Figure 1). In the female subgroup, the AUCs were 0.634 (95% CI: 0.537–0.731, p = 0.007) and 0.506 (95% CI: 0.445–0.567, p = 0.846) between NC and OCI groups and SCDs and OCI groups, respectively. In males, the AUCs were 0.550 (95% CI: 0.447–0.653, p = 0.337) between NC and OCI groups and 0.542 (95% CI: 0.455–0.630, p = 0.342) between SCDs and OCI groups.
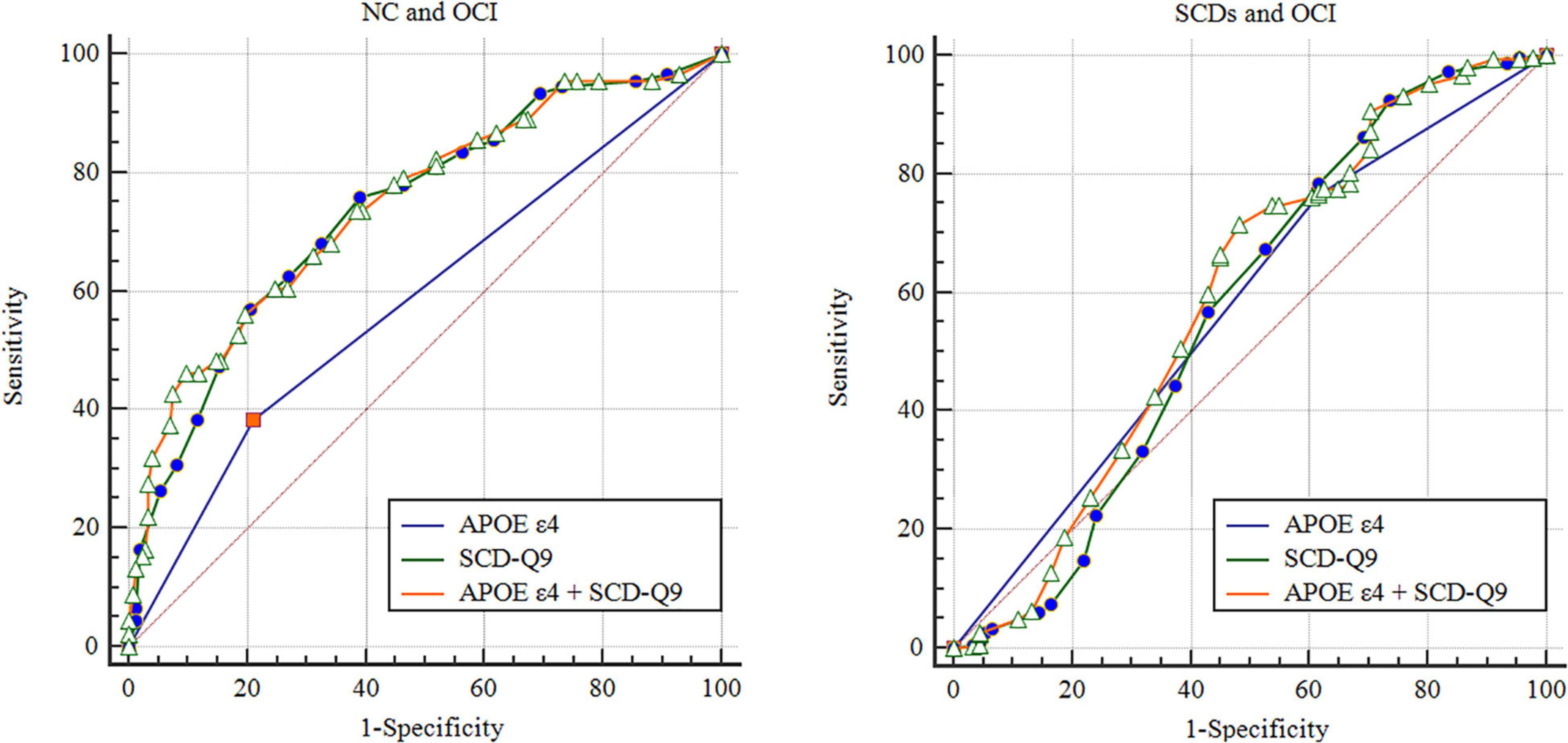
Figure 1. Receiver operating characteristic of NC, SCDs, and OCI groups. NC, normal control; SCDs, subjective cognitive declinewith subtle cognitive decline; OCI, objective cognitive impairment; SCD-Q9, Subjective Cognitive Decline-Questionnaire 9; ROC, receiver operating characteristic.
The AUCs of SCD-Q9 alone were 0.727 (95% CI: 0.682–0.771, p < 0.001) for the NC and SCDs groups, 0.738 (95% CI: 0.678–0.799, p < 0.001) for the NC and OCI groups, and 0.571 (95% CI: 0.494–0.648, p = 0.040) for the SCDs and OCI groups, respectively (see details in Figure 1).
Then, we included scores of SCD-Q9 and APOE ε4 carrier status together in the model and calculated AUCs for group discrimination. The AUCs increased to 0.747 (95% CI: 0.685–0.808, p < 0.001) for the NC and OCI groups, and 0.593 (95% CI: 0.518–0.668, p < 0.001) for the SCDs and OCI groups, respectively (see details in Figure 1). In females, the AUCs increased to 0.758 (95% CI: 0.670–0.846, p < 0.001) for the NC and OCI groups and 0.707 (95% CI: 0.649–0.764, p < 0.001) for the SCDs and OCI groups. In males, the AUCs were 0.753 (95% CI: 0.668–0.839, p < 0.001) for the NC and OCI groups and 0.756 (95% CI: 0.684–0.827, p < 0.001) for the SCDs and OCI groups.
We also performed binary logistic regression analysis and calculated the AUCs of all the related factors [including demographics (age, gender, and education years), HAMD and HAMA scores] for NC, SCDs, and OCI groups (see details in Supplementary Material 2,3).
Discussion
In the current study, we first reported the distribution characteristics of APOE ε4 alleles in a Chinese memory clinic with a larger cohort. To the best of our knowledge, this is also the first study to reveal the associations of APOE ε4 and SCD-Q9 scores in subjective and OCI diagnosed based on the combination of SCD-I (Jessen et al., 2014) and the Jak/Bondi standards (Edmonds et al., 2015). These results will help us better understand the variation in the unfavorable effect of APOE ε4 on the disease progression of AD and identify individuals with higher risk of cognitive decline in order to intervene earlier and treat more effectively.
Our findings showed that the frequency of APOE ε4 was 13.1%, following the APOE ε3 (78.5%), as the second most common allele. This is similar to the previous report based on the worldwide distribution (Farrer et al., 1997), showing APOE ε4 and APOE ε3 with a frequency of 13.7 and 77.9%, respectively. However, compared with a study in China by Li et al. (2002), we reported a higher frequency of APOE ε4 (13.1 vs. 9.7%), which may be due to the different recruitment protocol and more patients with OCI participating in our study. Participants with more memory impairment were included in our investigation, which may have resulted in a higher carrier rate of APOE ε4. Recently, a higher frequency of APOE ε4 alleles (19.6%) was reported in a population with cognitive impairment from a Chinese memory clinic, which supports our assumption (Wang et al., 2014). Moreover, compared with Oceania, South Africa, and Europe, such as Australia (26.0%) (Kamboh et al., 1991), Khoi San (37.0%) (Sandholzer et al., 1995), and NE England (15.4%), respectively (Mastana et al., 1998), a numeric lower frequency of APOE ε4 alleles was found in our study, which was also consistent with the previous conclusions that the frequency of APOE ε4 varied among different ethnicities and that Asian populations have a relatively lower ε4 frequency (Singh et al., 2006; Eisenberg et al., 2010). This also may be one of the reasons that the worldwide distribution of APOE ε4 appeared at a relatively low level.
Second, we analyzed the distribution characteristics of the APOE ε4 alleles in different demographics. The results showed that their frequencies did not vary with age, which was in agreement with the previous studies (Nakayama and Kuzuhara, 1999; Thelma et al., 2001). However, results from Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohorts and Uniform Data Set of the Alzheimer’s Disease (UDS) Centers and Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL), both demonstrated significant correlations between APOE ε4 and aging (Heffernan et al., 2016). This contradiction may be attributed to uncertainties inherent in the study design. For instance, several confounding factors of AD in the UDS and ADNI datasets, such as severe heart disease and diabetes, were excluded from our study. Studies have also reported that the frequencies of APOE ε4 decreased with aging (Jian-Gang et al., 1998; Bonham et al., 2016; Liu and Caselli, 2018; Bellou et al., 2020; Le Couteur et al., 2020) and the risk mainly declined after 75 years of age (Bickeboller et al., 1997; Jian-Gang et al., 1998; Bonham et al., 2016; Liu and Caselli, 2018). In the current study, only 9.3% of individuals older than 75 years agreed to participate in our investigation which may have contributed to this discrepancy. At present, knowledge of education and APOE ε4 alleles is limited. To the best of our knowledge, only one study previously investigated this association. Their results indicated that the relationship was not correlated (Caselli et al., 2009) which is consistent with findings from the current study. Lastly, we did not find a significant difference between gender and APOE ε4 allele distribution, which is consistent with previous findings (Combarros et al., 1998; Vaisi-Raygani et al., 2007; Tsolaki et al., 2018).
Finally, our study reported the distribution of APOE ε4 alleles in different cognitive groups and their associations with the SCD-Q9 scores. The most robust findings have demonstrated that the presence of the APOE ε4 allele imparts a genetic risk for the development of cognitive impairment, specifically that related to AD and vascular dementia (Allan and Ebmeier, 2011; Pink et al., 2015; Chen et al., 2016a; Jiang et al., 2016; Liu et al., 2016). Ali et al. (2018) suggested that the frequencies of carrying the APOE ε4 allele were comparable between healthy controls and SCD samples but were significantly higher in objectively impaired samples (i.e., MCI and AD dementia). In our study, the frequencies of APOE ε4 allele in the NC and SCDs groups were lower than that of the OCI groups in the total population and the female subgroup, but we did not find a difference between the NC and SCDs groups, which was in line with Ali et al.’s (2018) conclusion. In addition, a previous study found that individuals with subjective memory complaints (SMC) with no objective memory impairment did not differ from the NC group in terms of the frequency of APOE ε4 alleles (Lautenschlager et al., 2005). The results of our study did not provide supportive evidence for a positive association between APOE ε4 and SCD. However, a study has previously reported higher APOE ε4 frequency in the SCD group than in normal controls (Jessen et al., 2018), and this inconsistency may be due to different study populations (i.e., gender and ethnicity) and diagnostic criteria. The German Center for Neurodegenerative Diseases (DZNE)-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) enrolled subjects who speak fluent German and defined SCD based on the SCD-I diagnostic frame, which differs from ours. Also, the relatively higher prevalence of SCD but lower conversion rate to OCI in China due to low level of education and income (Wang et al., 2000; Rohr et al., 2020; Si et al., 2020) may result in different conclusions.
In our study, a significant difference in SCD-Q9 scores was found between APOE ε4 allele carriers and non-carriers in OCI in the total population and female subgroup. These results indicate that APOE ε4 alleles may be partially reflected in SCD-Q9 scores in patients with OCI in total population and females. Meanwhile, APOE ε4 allele carriers also presented a higher score of SCD-Q9 than non-carriers in the total population and SCDs group, but the differences were not significant. This is the first attempt to explore the relationship between APOE ε4 and SCDs diagnosed based on the combination standards of SCD-I and Jak/Bondi, which needs to be further verified by follow-up studies. Further attention should also be paid to other ethnicities and cohorts to verify this association in the future. Finally, the results of logistic regression analysis showed that APOE ε4 allele was risk factor for the OCI group (MCI and AD dementia) but not for the NC and SCDs groups, although the predictive powers were smaller. However, we found AUCs of SCD-Q9 alone were 0.727 for the NC and SCDs groups, 0.738 for the NC and OCI groups, and 0.571 for the SCDs and OCI groups, respectively. When APOE ε4 carrier status and SCD-Q9 scores together were added to the model, the AUC increased to 0.747 for NC and OCI groups, and 0.593 for SCDs and OCI groups, suggesting that the predictive power of APOE ε4 is limited, especially when OCI group was split into MCI and dementia groups, their discriminating powers for MCI and NC were marginal, but could be increased by combining with scores of SCD-Q9. Previous studies reported that APOE ε4 status appeared to be a more predictive risk factor for progression from MCI to AD dementia than family history, age, gender, or education (Fleisher et al., 2007), but it was only a useful predictor of progression from 70 to 85 years of age while controlling for education, memory scores, and gender (Devanand et al., 2005). Another study reported that ε4 carriers with SMC showed altered AD-related cerebrospinal fluid and fluorodeoxyglucose-positron emission tomography (PET) measures (Mosconi et al., 2008); In addition, they demonstrated that aging, APOE ε4, and SMC were associated with high Aβ burden, indicating that selection based on the presence of SMC and APOE ε4 may help identify healthy elderly participants with high Aβ burden eligible for secondary prevention trials (Zwan et al., 2016). As encouraging as these results may be, the exact role played by APOE ε4 in the development of AD dementia or other OCI continues to be unclear due to a lack of convergent evidence and considerable sample heterogeneity (Ali et al., 2018). Consequently, further investigation is warranted before APOE ε4 genetic testing can be recommended for wide-scale clinical adoption as a viable diagnostic tool for pathological cognitive decline.
It should be noted that there were obvious limitations associated with this study. (1) Our study is a cross-sectional survey, and follow-up studies should be performed to further confirm the conclusions; (2) the diagnosis of subjective and OCI was not validated by other tests. For instance, it lacks the completeness of Aβ-PET, cerebrospinal fluid tau, or Aβ examinations, given that only parts of the included population underwent Aβ-PET; (3) finally, this study focused on APOE ε4 alleles; thus, no evidence was provided for other related biomarkers and imaging approaches; and (4) the small sample size of OCI group in current study restricts us to further confirm the relationship between APOE ε4 and SCD-Q9 after control the amount of cognitive impairment, and a larger cohort with MCI and mild AD dementia patients was needed to verify our conclusion.
Conclusion
In summary, we reported the distribution characteristics of APOE ε4 alleles in different demographics and levels of cognition, and their associations with scores of SCD-Q9 with a larger cohort from a Chinese memory clinic. The findings of this study indicate that clinicians should be attentive to the distributed variation of APOE ε4 alleles and their unfavorable effects on OCI with SCD complaints, but their additional contribution to SCD-Q9 scores is marginal in discriminating individuals with cognitive impairment from normal controls.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Xuanwu Hospital Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LH: conceiving, implementing, statistics, and writing the manuscript. JJ: revising the manuscript. YX and YH: conceiving and revising the manuscript. YH: providing funding support. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant 61633018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.829031/full#supplementary-material
References
Abondio, P., Sazzini, M., Garagnani, P., Boattini, A., Monti, D., Franceschi, C., et al. (2019). The genetic variability of APOE in different human populations and its implications for longevity. Genes (Basel) 10:222.
Alber, J., Mcgarry, K., Noto, R. B., and Snyder, P. J. (2018). Use of eflornithine (DFMO) in the treatment of early Alzheimer’s disease: a compassionate use, single-case study. Front. Aging Neurosci. 10:60. doi: 10.3389/fnagi.2018.00060
Ali, J. I., Smart, C. M., and Gawryluk, J. R. (2018). Subjective cognitive decline and APOE varepsilon4: a systematic review. J. Alzheimers Dis. 65, 303–320.
Allan, C. L., and Ebmeier, K. P. (2011). The influence of ApoE4 on clinical progression of dementia: a meta-analysis. Int. J. Geriatr. Psychiatry 26, 520–526. doi: 10.1002/gps.2559
Amariglio, R. E., Becker, J. A., Carmasin, J., Wadsworth, L. P., Lorius, N., Sullivan, C., et al. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: American Psychiatric Association.
Bellou, E., Baker, E., Leonenko, G., Racher-Smith, M., Daunt, P., Menzies, G., et al. (2020). Age-dependent effect of APOE and polygenic component on Alzheimer’s disease. Neurobiol. Aging 93, 69–77. doi: 10.1016/j.neurobiolaging.2020.04.024
Bickeboller, H., Campion, D., Brice, A., Amouyel, P., Hannequin, D., Didierjean, O., et al. (1997). Apolipoprotein E and Alzheimer disease: genotype-specific risks by age and sex. Am. J. Hum. Genet. 60, 439–446.
Bondi, M. W., Edmonds, E. C., Jak, A. J., Clark, L. R., Delano-Wood, L., McDonald, C. R., et al. (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimers Dis. 42, 275–289. doi: 10.3233/JAD-140276
Bonham, L. W., Geier, E. G., Fan, C. C., Leong, J. K., Besser, L., Kukull, W. A., et al. (2016). Age-dependent effects of APOE epsilon4 in preclinical Alzheimer’s disease. Ann. Clin. Transl. Neurol. 3, 668–677. doi: 10.1002/acn3.333
Bott, N., Kumar, S., Krebs, C., Glenn, J. M., Madero, E. N., and Juusola, J. L. (2018). A remote intervention to prevent or delay cognitive impairment in older adults: design, recruitment, and baseline characteristics of the virtual cognitive health (VC Health) study. JMIR Res. Protoc. 7:e11368. doi: 10.2196/11368
Caselli, R. J., Dueck, A. C., Osborne, D., Sabbagh, M. N., Connor, D. J., Ahern, G. L., et al. (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 361, 255–263. doi: 10.1056/NEJMoa0809437
Chen, K. L., Xu, Y., Chu, A. Q., Ding, D., Liang, X. N., Nasreddine, Z. S., et al. (2016b). Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Chen, K. L., Sun, Y. M., Zhou, Y., Zhao, Q. H., Ding, D., and Guo, Q. H. (2016a). Associations between APOE polymorphisms and seven diseases with cognitive impairment including Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies in southeast China. Psychiatr. Genet. 26, 124–131. doi: 10.1097/YPG.0000000000000126
Combarros, O., Leno, C., Oterino, A., Berciano, J., Fernandez-Luna, J. L., Fernandez-Viadero, C., et al. (1998). Gender effect on apolipoprotein E epsilon4 allele-associated risk for sporadic Alzheimer’s disease. Acta Neurol. Scand. 97, 68–71. doi: 10.1111/j.1600-0404.1998.tb00611.x
Devanand, D. P., Pelton, G. H., Zamora, D., Liu, X., Tabert, M. H., Goodkind, M., et al. (2005). Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch. Neurol. 62, 975–980. doi: 10.1001/archneur.62.6.975
Dubois, B., Feldman, H. H., Jacova, C., Dekosky, S. T., Barberger-Gateau, P., Cummings, J., et al. (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3
Edmonds, E. C., Delano-Wood, L., Galasko, D. R., Salmon, D. P., Bondi, M. W., and Alzheimer’s Disease Neuroimaging Initiative (2015). Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J. Alzheimers Dis. 47, 231–242. doi: 10.3233/JAD-150128
Eisenberg, D. T., Kuzawa, C. W., and Hayes, M. G. (2010). Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am. J. Phys. Anthropol. 143, 100–111. doi: 10.1002/ajpa.21298
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356.
Fleisher, A. S., Sowell, B. B., Taylor, C., Gamst, A. C., Petersen, R. C., Thal, L. J., et al. (2007). Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 68, 1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c
Fortea, J., Sala-Llonch, R., Bartres-Faz, D., Lladó, A., Solé-Padullés, C., Bosch, B., et al. (2011). Cognitively preserved subjects with transitional cerebrospinal fluid ss-amyloid 1-42 values have thicker cortex in Alzheimer’s disease vulnerable areas. Biol. Psychiatry 70, 183–190. doi: 10.1016/j.biopsych.2011.02.017
Gifford, K. A., Liu, D., Romano, R. R., Jones, R. N., and Jefferson, A. L. (2015). Development of a subjective cognitive decline questionnaire using item response theory: a pilot study. Alzheimers Dement. (Amst.) 1, 429–439. doi: 10.1016/j.dadm.2015.09.004
Guo, Q. H., Hong, Z., Shi, W. X., and Lv, C. Z. (2006). Boston naming test in Chinese elderly, patient with mild cognitive impairment and Alzheimer’s dementia. Chin. Ment. Health J. 20, 81–84.
Guo, Q. H., Sun, Y. W., Yu, P. M., Hong, Z., and Lv, C. Z. (2007). Norm of auditory verbal learning test in the normal aged in china community. Chin. J. Clin. Psychol. 15, 132–134.
Hao, L. X., Sun, Y., Li, Y., Wang, J. Y., Wang, Z. C., Zhang, Z. Y., et al. (2020). Demographic characteristics and neuropsychological assessments of subjective cognitive decline (SCD) (plus). Ann. Clin. Transl. Neurol. 7, 1002–1012. doi: 10.1002/acn3.51068
Hao, L. X., Xing, Y., Li, X. Y., Mu, B., Zhao, W. N., Wang, G. B., et al. (2019). Risk factors and neuropsychological assessments of subjective cognitive decline (plus) in Chinese memory clinic. Front. Neurosci. 13:846. doi: 10.3389/fnins.2019.00846
He, Y. L., Qu, G. Y., Xiong, X. Y., Chi, Y. F., Zhang, M. Y., and Zhang, M. Q. (1990). Assessment of the ability of daily life of the elderly. J. Gerontol. 266–269.
Heffernan, A. L., Chidgey, C., Peng, P., Masters, C. L., and Roberts, B. R. (2016). The neurobiology and age-related prevalence of the epsilon4 allele of apolipoprotein E in Alzheimer’s disease cohorts. J. Mol. Neurosci. 60, 316–324. doi: 10.1007/s12031-016-0804-x
Jessen, F., Amariglio, R. E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., et al. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852. doi: 10.1016/j.jalz.2014.01.001
Jessen, F., Spottke, A., Boecker, H., Brosseron, F., Buerger, K., Catak, C., et al. (2018). Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res. Ther. 10:15. doi: 10.1186/s13195-017-0314-2
Jiang, S., Tang, L., Zhao, N., Yang, W., Qiu, Y., and Chen, H. Z. (2016). A systems view of the differences between APOE epsilon4 carriers and non-carriers in Alzheimer’s disease. Front. Aging Neurosci. 8:171. doi: 10.3389/fnagi.2016.00171
Jian-Gang, Z., Yong-Xing, M., Chuan-Fu, W., Pei-Fang, L., Song-Bai, Z., Nui-Fan, G., et al. (1998). Apolipoprotein E and longevity among Han Chinese population. Mech. Ageing Dev. 104, 159–167. doi: 10.1016/s0047-6374(98)00067-0
Kamboh, M. I., Serjeantson, S. W., and Ferrell, R. E. (1991). Genetic studies of human apolipoproteins. XVIII. Apolipoprotein polymorphisms in Australian Aborigines. Hum. Biol. 63, 179–186.
Katzman, R., Zhang, M. Y., Chen, P. J., Gu, N., Jiang, S., Saitoh, T., et al. (1997). Effects of apolipoprotein E on dementia and aging in the Shanghai Survey of Dementia. Neurology 49, 779–785. doi: 10.1212/wnl.49.3.779
Katzman, R., Zhang, M. Y., Ouang-Ya-Qu, Wang, Z. Y., Liu, W. T., Yu, E., et al. (1988). A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 41, 971–978. doi: 10.1016/0895-4356(88)90034-0
Kern, S., Mehlig, K., Kern, J., Zetterberg, H., Thelle, D., Skoog, I., et al. (2015). The distribution of apolipoprotein E genotype over the adult lifespan and in relation to country of birth. Am. J. Epidemiol. 181, 214–217. doi: 10.1093/aje/kwu442
Kielb, S., Rogalski, E., Weintraub, S., and Rademaker, A. (2017). Objective features of subjective cognitive decline in a United States national database. Alzheimers Dement. 13, 1337–1344. doi: 10.1016/j.jalz.2017.04.008
Kim, D., and Tsai, L. H. (2009). Bridging physiology and pathology in AD. Cell 137, 997–1000. doi: 10.1016/j.cell.2009.05.042
Koffie, R. M., Hashimoto, T., Tai, H. C., Kay, K. R., Serrano-Pozo, A., Joyner, D., et al. (2012). Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135(Pt. 7), 2155–2168. doi: 10.1093/brain/aws127
Kumar, S., Tran, J., Moseson, H., Tai, C., Glenn, J. M., Madero, E. N., et al. (2018). The impact of the virtual cognitive health program on the cognition and mental health of older adults: pre-post 12-month pilot study. JMIR Aging 1:e12031. doi: 10.2196/12031
Lautenschlager, N. T., Flicker, L., Vasikaran, S., Leedman, P., and Almeida, O. P. (2005). Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am. J. Geriatr. Psychiatry 13, 731–734. doi: 10.1176/appi.ajgp.13.8.731
Le Couteur, D. G., Stanaway, F., Waite, L. M., Cullen, J., Lindley, R. I., Blyth, F. M., et al. (2020). Apolipoprotein E and health in older men: the concord health and ageing in men project. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1858–1862. doi: 10.1093/gerona/glaa105
Lehmann, D. J., Refsum, H., Nurk, E., Warden, D. R., Tell, G. S., Vollset, S. E., et al. (2006). Apolipoprotein E epsilon4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. J. Neurol. Neurosurg. Psychiatry 77, 902–908. doi: 10.1136/jnnp.2005.077818
Li, X., Zhao, D., Liu, J., Liu, S., Liu, J., Qin, L. P., et al. (2002). The study on the effects of apolipoprotein E gene polymorphism on serum lipids and it’s frequency distribution in Beijing natural population. J. Cardiovasc. Pulm. Dis. 21, 193–197.
Liu, L., and Caselli, R. J. (2018). Age stratification corrects bias in estimated hazard of APOE genotype for Alzheimer’s disease. Alzheimers Dement. (N Y) 4, 602–608. doi: 10.1016/j.trci.2018.09.006
Liu, Y., Tan, L., Wang, H. F., Liu, Y., Hao, X. K., Tan, C. C., et al. (2016). Multiple effect of APOE genotype on clinical and neuroimaging biomarkers across Alzheimer’s disease spectrum. Mol. Neurobiol. 53, 4539–4547. doi: 10.1007/s12035-015-9388-7
Mastana, S. S., Calderon, R., Pena, J., Reddy, P. H., and Papiha, S. S. (1998). Anthropology of the apoplipoprotein E (apo E) gene: low frequency of apo E4 allele in Basques and in tribal (Baiga) populations of India. Ann. Hum. Biol. 25, 137–143. doi: 10.1080/03014469800005512
Mckhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Morris, J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/wnl.43.11.2412-a
Mosconi, L., De Santi, S., Brys, M., Tsui, W. H., Pirraglia, E., Glodzik-Sobanska, L., et al. (2008). Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry 63, 609–618. doi: 10.1016/j.biopsych.2007.05.030
Nakayama, S., and Kuzuhara, S. (1999). Apolipoprotein E phenotypes in healthy normal controls and demented subjects with Alzheimer’s disease and vascular dementia in Mie Prefecture of Japan. Psychiatry Clin. Neurosci. 53, 643–648. doi: 10.1046/j.1440-1819.1999.00619.x
Pink, A., Stokin, G. B., Bartley, M. M., Roberts, R. O., Sochor, O., Machulda, M. M., et al. (2015). Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology 84, 935–943. doi: 10.1212/WNL.0000000000001307
Rohr, S., Pabst, A., Riedel-Heller, S. G., Jessen, F., Turana, Y., Handajani, Y. S., et al. (2020). Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res. Ther. 12:167. doi: 10.1186/s13195-020-00734-y
Sandholzer, C., Delport, R., Vermaak, H., and Utermann, G. (1995). High frequency of the apo epsilon 4 allele in Khoi San from South Africa. Hum. Genet. 95, 46–48. doi: 10.1007/BF00225073
Saunders, A. M., Strittmatter, W. J., Schmechel, D., George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. doi: 10.1212/wnl.43.8.1467
Si, T., Xing, G., and Han, Y. (2020). Subjective cognitive decline and related cognitive deficits. Front. Neurol. 11:247. doi: 10.3389/fneur.2020.00247
Singh, P. P., Singh, M., and Mastana, S. S. (2006). APOE distribution in world populations with new data from India and the UK. Ann. Hum. Biol. 33, 279–308. doi: 10.1080/03014460600594513
Striepens, N., Scheef, L., Wind, A., Popp, J., Spottke, A., Cooper-Mahkorn, D., et al. (2010). Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement. Geriatr. Cogn. Disord. 29, 75–81. doi: 10.1159/000264630
Tang, Y. H., and Zhang, M. Y. (1984). Hamilton anxiety scale (HAMA). Shanghai Arch. Psychiatry 12–13.
Thelma, B. K., Juyal, R. C., Dodge, H. H., Pandav, R., Chandra, V., and Ganguli, M. (2001). APOE polymorphism in a rural older population-based sample in India. Hum. Biol. 73, 135–144. doi: 10.1353/hub.2001.0012
Tsolaki, A. C., Gatzima, O., Daniilidou, M., Lazarou, E., Bamidis, P. D., Verykouki, E., et al. (2018). Prevalence of apolipoprotein E polymorphisms in Alzheimer’s disease, mild cognitive impairment, and healthy elderly: a Northern Greece study. Neurodegener. Dis. 18, 216–224. doi: 10.1159/000491764
Vaisi-Raygani, A., Kharrazi, H., Rahimi, Z., and Pourmotabbed, T. (2007). Frequencies of apolipoprotein E polymorphism in a healthy Kurdish population from Kermanshah, Iran. Hum. Biol. 79, 579–587. doi: 10.1353/hub.2008.0003
Wang, P. N., Wang, S. J., Fuh, J. L., Teng, E. L., Liu, C. Y., Lin, C. H., et al. (2000). Subjective memory complaint in relation to cognitive performance and depression: a longitudinal study of a rural Chinese population. J. Am. Geriatr. Soc. 48, 295–299. doi: 10.1111/j.1532-5415.2000.tb02649.x
Wang, X., Wang, H. L., Li, H. Y., Li, T., and Yu, X. (2014). Frequency of the apolipoprotein E epsilon4 allele in a memory clinic cohort in Beijing: a naturalistic descriptive study. PLoS One 9:e99130. doi: 10.1371/journal.pone.0099130
Zhang, T., Liu, S. L., Zhang, Y. J., Guan, Y. L., Wang, X. D., Zhao, L., et al. (2017). Apolipoprotein E e4 allele is associated with subjective cognitive decline: a meta-analysis. Neuroepidemiology 49, 165–173. doi: 10.1159/000482018
Zhao, Q. H., Guo, Q. H., Li, F., Zhou, Y., Wang, B., and Hong, Z. (2013b). The Shape Trail Test: application of a new variant of the Trail making test. PLoS One 8:e57333. doi: 10.1371/journal.pone.0057333
Zhao, Q. H., Guo, Q. H., and Hong, Z. (2013a). Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neurosci. Bull. 29, 75–82. doi: 10.1007/s12264-013-1301-7
Keywords: APOE ε4, distribution, demography, SCD-Q9, discrimination
Citation: Hao L, Jia J, Xing Y and Han Y (2022) APOE ε4 Allele Distribution and Association With Scores of Subjective Cognitive Decline Questionnaire 9 in a Large Chinese Memory Clinic Cohort. Front. Neurosci. 16:829031. doi: 10.3389/fnins.2022.829031
Received: 04 December 2021; Accepted: 16 May 2022;
Published: 03 June 2022.
Edited by:
Hamid R. Sohrabi, Murdoch University, AustraliaReviewed by:
Dongming Cai, Icahn School of Medicine at Mount Sinai, United StatesMartin Vyhnalek, Charles University, Czechia
Copyright © 2022 Hao, Jia, Xing and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Xing, WXVlLlhpbmdAbm90dGluZ2hhbS5hYy51aw==; Ying Han, aGFueWluZ0B4d2guY2NtdS5lZHUuY24=
 Lixiao Hao
Lixiao Hao Jianguo Jia
Jianguo Jia Yue Xing
Yue Xing Ying Han
Ying Han