- 1Clinical Neuroscience, Institute of Neuroscience (IoNS), Université Catholique de Louvain, Brussels, Belgium
- 2BEAMS Department, Université Libre de Bruxelles, Brussels, Belgium
- 3Department of Neurology, Center for Refractory Epilepsy, Cliniques UniversitairesSaint-Luc, Brussels, Belgium
- 4Walloon Excellence in Life Sciences and Biotechnology (WELBIO) Department, WELResearch Institute, Wavre, Belgium
Introduction: One-third of epileptic patients are resistant to conventional treatments. Vagus nerve stimulation is a promising therapy, especially when applied early during seizure onset. This study explores vagus nerve activity (VNA) during seizures in Genetic Absence Epilepsy Rat from Strasbourg (GAERS) model and explores how VNA changes with epilepsy duration.
Methods: Eleven rats (4, 6, and 10 months old, n = 4, 4, 3, respectively) were continuously recorded with electroencephalography, VNA recordings, and video for 24 h. Ictal VNA root mean square (RMS) values preceded by NREM sleep extracted from 11 rats were studied in a total of 620 seizures.
Results: Overall, VNA RMS increased during seizures, with a median rise of 60%. However, this modulation decreased with age, despite stable seizure severity. Significant differences in VNA activity and inter-quartile range were observed between age groups.
Discussion: These results support seizure severity-dependent changes in ictal VNA modulation and point toward the potential of VNA as a biomarker for seizure detection and autonomic dysfunction.
Highlights
• Vagus nerve activity (VNA) recording increases during absences in GAERS.
• Ictal VNA response decreases with age despite stable epilepsy.
• Individual variability of the ictal VNA modifications decreases with age.
1 Introduction
Epilepsy is a neurological disorder marked by recurrent seizures, leading to neurobiological, cognitive, psychological, and social consequences, accompanied by stigma, comorbidities, and high economic costs (Beghi, 2020). Vagus Nerve Stimulation (VNS) is a treatment used for patients with refractory epilepsy (Afra et al., 2021; Ben-Menachem, 2002). VNS consists of an implantable pulse generator that delivers electrical pulses in continuous programmable ON/OFF cycles. VNS response varies, with a 50% seizure reduction in half of the implanted patients (Elliott et al., 2011). However, patient phenotype, epilepsy type or etiology do not predict treatment response (Englot et al., 2016; Labar, 2004). Studies suggest that on-demand VNS at seizure onset can reduce seizure duration or intensity (McLachlan, 1993; Zabara, 1992). The VNS aspire model uses a heart-based seizure detection algorithm to deliver on-demand VNS in addition to continuous stimulation in duty cycles (Boon et al., 2001). However, many patients do not exhibit ictal tachycardia during seizures and exhibit other sympathetic responses, such as increased respiration or blood pressure. Additionally, from infancy to adulthood, tonic-clonic and focal seizures can activate sympathetic responses or lead to parasympathetic dominance, affecting peristalsis, pupil dilation, and heart or respiratory rates (Devinsky, 2004). The vagus nerve, integral part of the parasympathetic system, helps balance sympathetic system dysregulation (Teckentrup and Kroemer, 2024). Thus, developing seizure detection based on parasympathetic modulation during a seizure seems promising.
Seizures affect autonomic function during, after, and between seizures (Devinsky, 2004) in many types of chronic epilepsy, including idiopathic generalized epilepsy (IGE) including childhood absence epilepsy (CAE), a common form of pediatric epilepsy that is typically non-eligible for surgery. Absence seizures are the semiology of several other epilepsy syndromes, such as juvenile absence epilepsy, juvenile myoclonic epilepsy, and myoclonic absence epilepsy (Crunelli and Leresche, 2002; Smyk and van Luijtelaar, 2020). The GAERS model established in 1982 closely mirrors the characteristics of IGE showing similar behavioral and electrophysiological traits to human absence epilepsy, and responds to antiseizure drugs closely parallels clinical observations (Depaulis et al., 2016). Literature suggests that abnormal autonomic regulatory functions are disrupted by epilepsy in TLE and IGE (Shaker et al., 2021) with cases of ictal tachypnea and bradypnea during CAE (Jansen et al., 2013). This makes the GAERS model an interesting tool for studying autonomic changes during seizures.
Autonomic dysfunction in epilepsy—marked by increased sympathetic tone and reduced HRV—can result from chronic seizures, antiseizure treatments, or epilepsy itself (Thijs et al., 2021). Reduced HRV has been observed during interictal discharges in absence epilepsy (Pradhan et al., 2011) and in WAG/Rij rats, a genetic model of absence epilepsy, where aging leads to diminished HRV and increased sympathetic dominance (Mamalyga and Mamalyga, 2019). These findings highlight the interest in studying VNA after an accumulation of seizures to better understand autonomic involvement over time.
We aim to study whether VNA modifications occur during absence seizures in the GAERS model. We hypothesize that autonomic ictal activation during absences can be recorded through continuous VNA recording. Additionally, we hypothesize that ictal VNA modifications will be less prominent with increasing age and cumulative occurrence of absences.
2 Materials and methods
2.1 Animals
All experimental protocols were approved by Brussels Environment ethical committee (2023/UCL/MD/09) and complied with EU directive 2010/63/EU. Female and male GAERS rats [4, 6, and 10 months (n = 4/age)] bred in-house under the License LA 1230664 from a Grenoble Institute of Neuroscience (France) parental stock, was used. Animals were housed in groups of 2–3 under standard conditions (12 h light cycle, 23–24°C, 45%–55% humidity) with ad libitum food and water, and provided with enrichment materials.
2.2 Experimental procedures
Twelve rats were used and recorded (VNA, EEG, and video) for 48 h. The 2 days EEG recordings were used to characterize seizures across the different age groups. Only the first 24 h per rat were used for the VNA analysis.
2.3 Surgical procedures
Anesthesia was induced with 5% sevoflurane in O2 and maintained at 2.5% (3 L/min, Dräger-Vapor2000). Tradamol® (5 mg/kg, s.c.) was given 30 min pre-surgery. Body temperature was maintained at 37°C. Craniotomies were performed with a hand drill, and five stainless-steel screws were implanted for epidural EEG recordings at standard coordinates. To implant the vagus nerve electrode, the left cervical vagus nerve was exposed via blunt dissection. A custom 3-lead micro-cuff electrode (300 μm ID, 100 μm contact width, 4 mm spacing; Chávez Cerda et al., 2022) was placed around the nerve and sealed with surgical silicone. Electrodes were impedance-tested (< 20 kΩ) before implantation. VNA signal quality was confirmed postoperatively via detection of respiration and cardiac-related bursts (Stumpp et al., 2020). Leads were subcutaneously tunneled to the skull and fixed with UV dental cement. Postoperative care included twice-daily Tradamol® and Ketofen® (5 mg/kg each, s.c.) for 3 days, along with dietary enrichment to support recovery.
2.4 Signals recording and processing
Each rat was placed in a custom-built chronic monitoring system inside a Faraday cage to record seizures and VNA (Chávez Cerda et al., 2022). The setup enables free movement via slipring and allows real-time acquisition of VNA recordings, EEG, and video. Signals were amplified (10 MΩ input impedances, 800 V/V gain) with low noise (0.5 μVRMS) and filtered (10 Hz–10 kHz for VNA and 0.1–100 Hz for EEG). Recordings enabled time-aligned acquisition including two VNA channels (20 kS/s sampling rate), two EEG channels (1 kS/s sampling rate), and video (20 fps, Night-Vision Infrared camera, 5-megapixel). In preprocessing, VNA was bandpass-filtered to 300 Hz–3 kHz and EEG to 0.5–70 Hz. The system was previously validated (Chávez Cerda et al., 2022; Smets et al., 2022; Stumpp et al., 2020).
2.5 EEG analysis
Absence seizure onset and offset were identified EEG by characteristic 7–11 Hz slow wave discharge (300–1,000 μV) (Marescaux et al., 1992). We analyzed age-related seizure features in GAERS rats including seizure count per 24 h, duration, and ictal spiking frequency. To assess potential differences at seizure onset and offset spiking frequency was extracted via Fourier transform over the full seizure and the first and last 2 s segments.
2.6 VNA analysis
To analyze VNA changes during seizures, only absences ≥ 5 s and preceded by non-rapid eye movement (NREM) (0.5–4.0 Hz) were included to avoid movement artifacts. Even minor movements during wakefulness, such as grooming or postural adjustments, can introduce significant artifacts in recordings obtained from cuff electrodes. Therefore, to ensure optimal signal quality, we included only those seizures that were preceded by non-rapid eye movement (NREM) sleep in our analysis. For each event, a VNA segment matching the seizure duration and a clean 10 s pre-ictal baseline were extracted. RMS values were computed for both segments, and a VNA ratio (ictal RMS/baseline RMS) was calculated per seizure.
2.7 Statistical analyses
Statistical analyses were performed with GraphPad. Normality was assessed using the Shapiro-Wilcoxon test. Descriptive statistics (mean, SD, SEM) were reported for seizure features across age groups. Group differences were analyzed using the Kruskal-Wallis test with Dunn’s post hoc correction. RMS VNA ratios (median, 25th–75th percentiles) were compared similarly. Significance was set at α < 0.0001 (****). Interquartile ranges were compared using one-way ANOVA with Tukey’s test.
3 Results
3.1 Epilepsy characterization through age
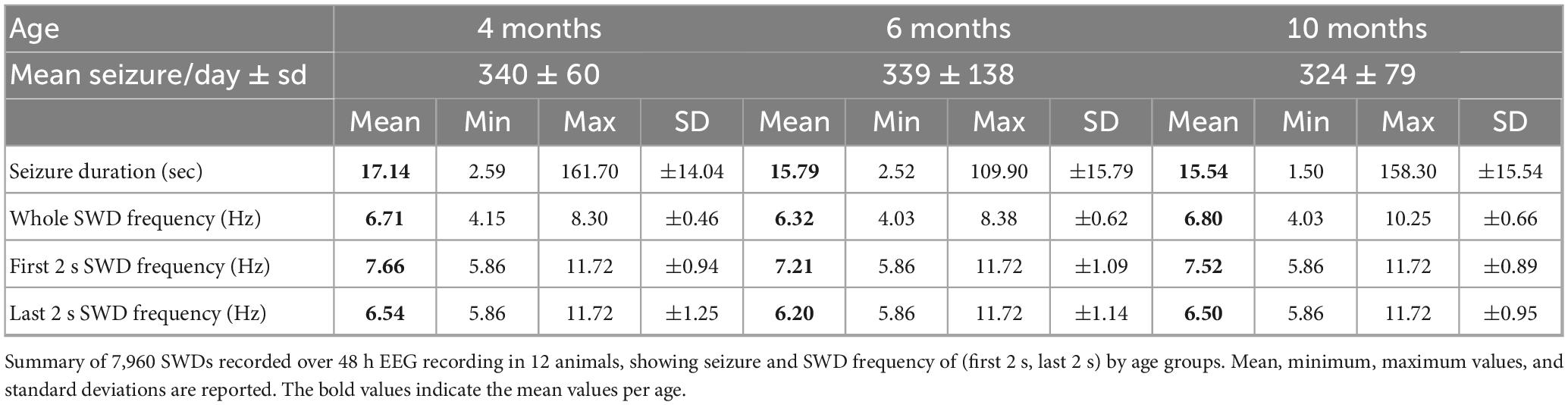
The mean daily seizure counts (340, 339, and 324 for 4, 6, and 10 months-old rats, respectively) and seizure duration were similar across age groups (Table 1). Mean discharge frequencies during the entire seizure and the initial, and final 2 s of SWD also showed no significant differences (Table 1 and Supplementary Table 1).
3.2 VNA modulation during absences
Twelve rats were recorded, but one 10 months-old rat was excluded due to poor-quality VNA leaving 620 seizures from eleven rats for analysis.
Figure 1A illustrates two absence seizures during NREM sleep with co-occurrence increases in VNA (orange and pink) compared to a 10 s baseline. Figure 1B displays RMS ratios indicating a median ratio of 60% VNA increase during seizures. Over 80% of seizures showed at least 20% increase, and 44% exceeded 80% (Supplementary Figure 1).
Figure 1. Vagus nerve activity is modulated during absences in Genetic Absence Epilepsy Rat from Strasbourg (GAERS) rats. (A) Example of vagus nerve activity increase during absence in one GAERS rat. Upper trace: bilateral scalp EEG recording. The onsets of the absences are shown by a red line and the offsets by a yellow line. Lower trace: bipolar vagus nerve activity (VNA) recording. The color of the vagus nerve activity corresponds to the color of the seizures detected on the EEG. The black segment is the pre-ictal phase selected as a 10 s baseline. (B) The root mean square (RMS) of the vagus nerve increases during SWDs compared to baseline. A median ratio of 1.6 is presented with a line, and the quartiles are shown in dashed lines (n = 620 seizures).
3.3 Effect of age on ictal VNA
To assess the effect of epilepsy duration on VNA, VNA recordings of eleven rats aged 4, 6, and 10 months were analyzed. Figure 2A depicts that VNA during seizures was highest 4 months-old rats c with median RMS ratio of 1.98, 1.68, and 1.37 for 4, 6, and 10 months-old GAERS, respectively (Supplementary Table 2). All age comparisons showed significant differences (Supplementary Table 3). Figure 2B reveals significant higher interquartile range in younger rats at 4 months compared to 6 and 10 months (p-value: 0.0352 and 0.0132) (Supplementary Table 4).
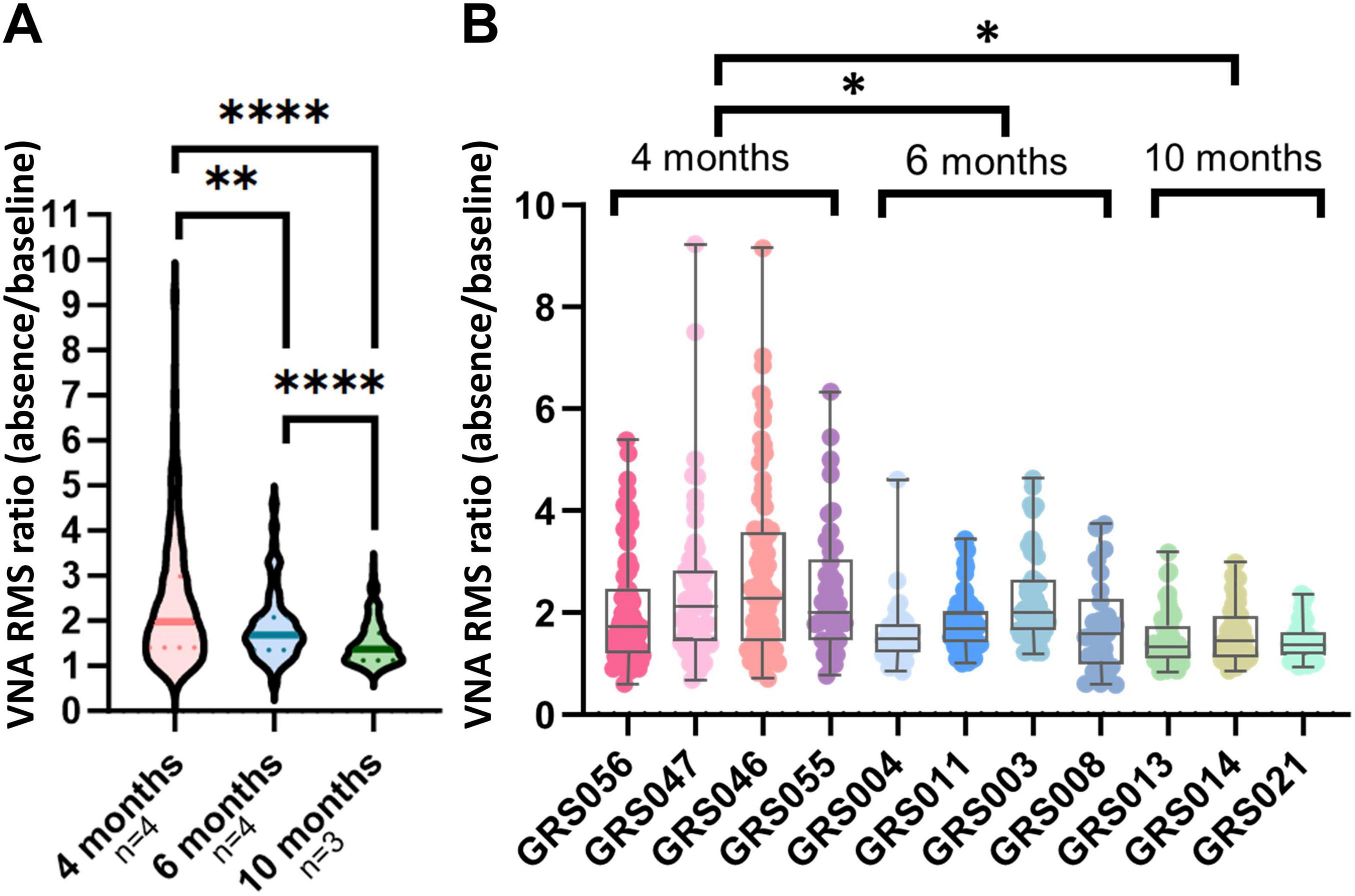
Figure 2. Ictal vagus nerve modulation decreases with age. (A) The root mean square (RMS) ratio of each seizure detected is presented pooled per age group. The line for each violin represents the median value. The dashed lines represent quartiles. Vagus nerve activity during absences in Genetic Absence Epilepsy Rat from Strasbourg (GAERS) decreases with age. (B) The dots representing vagus nerve modulation for each seizure per animal show a larger interquartile range between seizures in the 4 months-old rats compared to 6 and 10 months-old rats. The quartiles and the median are represented by a boxplot for each animal. The min and max values are shown with whiskers. *p < 0.05, **p < 0.01, ***p < 0.001.
4 Discussion
This study demonstrates the feasibility of capturing VNA modulations during absences under chronic monitoring conditions, potentially offering new biomarkers for seizure during seizures to pre-ictal baselines during NREM sleep across three distinct age groups (4, 6, and 10 months). Our results show that VNA increases during absences, with a total mean increase of 60% in 620 seizures from eleven rats. Additionally, our findings revealed a significant age-dependent reduction in VNA during absences, while no discernible differences were observed in electrographic seizure characteristics among the three age groups.
Vagal visceral afferents play a significant role in epilepsy and autonomic alterations, as they convey visceral sensations from internal organs, including upper and lower airways, aortic baro- and chemoreceptors, to the central nervous system (Câmara and Griessenauer, 2015; Ruffoli et al., 2011). In healthy rats and humans, vagus nerve recordings have demonstrated that respiratory and ECG-related burst activity arises from both afferent and efferent components (Macefield et al., 2023; Ottaviani et al., 2020; Sevcencu et al., 2018). Physiological information such as blood pressure, heart, and respiration rate, or physiological behaviors in rats like eating and grooming were already extracted from the VNA (Marmerstein et al., 2022; Ottaviani et al., 2020; Stumpp et al., 2020). It could, therefore, be hypothesized that measuring vagus nerve activity might serve as a biomarker for dysregulation of the autonomic system in certain pathologies. In the context of epilepsy, Harreby et al. demonstrated that during induced pentylenetetrazol seizures under anesthesia, the cardiac-related vagus nerve signal exhibited significant ictal and post-ictal alterations (Harreby et al., 2011). In the same model, spike clustering detection revealed abrupt changes in VNA amplitude and frequency during induced seizures (Stumpp et al., 2021) and enabled earlier seizure detection compared to heart rate-based seizure detection methods (Stumpp et al., 2021). In the current study, the observed VNA augmentation during spontaneous seizures across all animals is coherent with the literature and encourages further research to explore the potential of VNA-based detection using signals obtained under chronic conditions.
Although ictal VNA generally increases, the patterns vary both across and within animals. In 6% of seizures, VNA RMS decreased compared to baseline; in 14%, it showed a slight increase (up to 20%); 80% of seizures showed an increase greater than 20%, and in 44% of cases, the rise exceeded 80%. While autonomic homeostasis relies on parasympathetic and sympathetic balance, seizures often disrupt this equilibrium, triggering autonomic symptoms across cardiovascular, respiratory, gastrointestinal, cutaneous, pupillary, genital, sexual, and urinary domains (Baumgartner et al., 2001). Sakamoto et al. (2008) showed that kainic acid-induced seizures in anesthetized rats increase both parasympathetic and sympathetic activities and impair baroreflex function with altered c-Fos expression in medullary regions. One plausible hypothesis to account for the observed heterogeneity in parasympathetic responses may stem from differences in seizure severity, both within individual animals and across different age cohorts. Severe seizures could induce more significant sympathetic activation and subsequent parasympathetic compensation (Naggar et al., 2022), while milder seizures might necessitate less pronounced restorative efforts. Although all seizures began during NREM sleep, post-ictal states varied, with possible transitions to wakefulness, activity, or REM sleep. This variability may have influenced ictal VNA modulation. However, without EMG data, post-ictal states could not be reliably classified.
Unlike Wag/Rij rats, which display two types of SWDs with type II concentrated in the occipital cortex (Sitnikova and van Luijtelaar, 2007), GAERS seizures after 4 months show consistent electrophysiological and behavioral features. Seizures are driven thalamocortically, originate in the primary somatosensory cortex, and are secondly generalized with stable frequency and duration from 4 months onward (Depaulis et al., 2016). Therefore, seizure severity likely doesn’t explain parasympathetic variability, though differences in onset may contribute. Most GAERS SWDs (91.9%) begin in the primary somatosensory cortex S1 with a preference for more anterior sites, while 8.1% begin simultaneously across cortical sites (Depaulis et al., 2016).
However, it is imperative to note that all seizures examined for VNA modulation were prefaced by a non-rapid eye movement (NREM) sleep period. Consequently, further investigations are warranted to validate the observed increase in VNA activity during absence seizures occurring throughout various phases of the day. In addition, a deeper understanding of VNA modulation in response to common physiological events alongside the progression of epilepsy could significantly enhance the development of reliable VNA-based seizure detection algorithms.
Altered vagus-mediated heart rate variability, together with increased epilepsy severity, is linked to higher SUDEP risk (DeGiorgio et al., 2010). Studies in children with drug-resistant epilepsy showed that non-treated epilepsy increases the risk of comorbidities and mortality due to a cardio-autonomic imbalance and left ventricular dysfunction (Ibrahim et al., 2021; Sakamoto et al., 2008). To explore autonomic alterations in epilepsy, we compared parasympathetic responses during seizures across age groups. In our study, no differences in seizure characteristics were found between age groups, but the median VNA RMS value during absence seizures was significantly lower in 10 months-old rats compared to 4 and 6 months-olds. This suggests that VNA response recorded during absence seizures diminishes with age, consistent with the notion that untreated epilepsy reduces parasympathetic tone (Yuen and Sander, 2017) and increases the risk of comorbidities and mortality due to cardio-autonomic and subclinical left ventricular dysfunction, independent of epilepsy duration, frequency, and seizure type (Ibrahim et al., 2021). However, the age-related changes in vagus nerve activity observed may also be influenced by age-dependent shifts in sympathetic and parasympathetic balance seen in healthy subjects (Pfeifer et al., 1983).
Lastly, we demonstrated that ictal parasympathetic inter-quartile range diminishes with age in GAERS rats, which might suggest reduced vagal responsiveness to sympathetic imbalance. This may reflect a cumulative strain on the parasympathetic system from repeated seizures leading to functional fatigue. Similar findings in WAG/Rij showed age-related reduction in HRV and increased sympathetic dominance (Mamalyga and Mamalyga, 2019). Vagus nerve activity is also subject to physiological modulation during natural behaviors such as feeding (Marmerstein et al., 2022) and may be different according to day/night (Smets et al., 2022). Thus, additional autonomic measures, such as electrocardiograms (ECG), heart rate variability (HRV), or respiratory rate concurrently recorded, could help distinguish seizure-related VNA changes from those driven by broader autonomic fluctuations.
We used an external cuff recording method to enhance translational relevance, though no control electrode devoid of vagus nerve input was included in our measurements. Consequently, it cannot be excluded that the recorded VNA signal may comprise a mixture of physiological and non-physiological signals. However, the potential effects of movement were minimized by focusing exclusively on absence seizures preceded by NREM sleep phases. For broader application to 24 h recording in freely moving animals, additional controls such as electromyography (EMG) and non-vagal contact are needed to isolate true vagus nerve activity, particularly during movement and to generalize the methodology to other epileptic models.
5 Conclusion
This study demonstrates for the first time that recording seizure-related modulations of VNA in chronic conditions in rats is possible. Those observations not only provide general insights into altered vagus nerve activity, as assessed by VNA recording, in relation to seizures, but also encourage further research into the potential of VNA-based detection using signals collected under chronic conditions. Consequently, it could pave the way for future studies measuring vagus nerve activity as a biomarker for autonomic system dysregulation in various pathologies.
Data availability statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon request and with the permission of Université Catholique de Louvain.
Ethics statement
The animal study was approved by Brussels Environment (2023/UCL/MD/09) License Agreement (LA 1230664). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review and editing. EG: Investigation, Methodology, Software, Writing – review and editing. EA: Investigation, Methodology, Writing – review and editing. AS: Writing – review and editing. AA: Investigation, Writing – review and editing. AN: Supervision, Writing – review and editing. RE: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the F.R.S.-FNRS (Fonds National de la Recherche Scientifique, Belgium): EC is a Research Fellow “FRIA-B1” (Grant no: 40021901). EG, AA, and RE are funded by the WELBIO program, Wavre, Belgium.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1568261/full#supplementary-material
References
Afra, P., Adamolekun, B., Aydemir, S., and Watson, G. (2021). evolution of the vagus nerve stimulation (VNS) therapy system technology for drug-resistant Epilepsy. Front. Med. Technol. 3:696543. doi: 10.3389/fmedt.2021.696543
Baumgartner, C., Lurger, S., and Leutmezer, F. (2001). Autonomic symptoms during epileptic seizures. Epileptic Disord. 3, 103–116. doi: 10.1684/j.1950-6945.2001.tb00380.x
Beghi, E. (2020). The epidemiology of epilepsy. Neuroepidemiology 54, 185–191. doi: 10.1159/000503831
Ben-Menachem, E. (2002). Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 1, 477–482. doi: 10.1016/s1474-4422(02)00220-x
Boon, P., Vonck, K., Van Walleghem, P., D’Havé, M., Goossens, L., Vandekerckhove, T., et al. (2001). Programmed and magnet-induced vagus nerve stimulation for refractory epilepsy. J. Clin. Neurophysiol. 18, 402–407. doi: 10.1097/00004691-200109000-00003
Câmara, R., and Griessenauer, C. (2015). “Chapter 27 - anatomy of the vagus nerve,” in Nerves and Nerve Injuries, eds R. S. Tubbs, E. Rizk, M. M. Shoja, and M. Loukas (San Diego: Academic Press).
Chávez Cerda, J., Acedo Reina, E., Smets, H., Verstraeten, M., Vande Perre, L., Díaz Cortés, M., et al. (2022). “Chronic setup system for continuous monitoring of epileptic rats,” in Proceedings of the 2022 IEEE Biomedical Circuits and Systems Conference (BioCAS), (Piscataway, NJ: IEEE).
Crunelli, V., and Leresche, N. (2002). Childhood absence epilepsy: Genes, channels, neurons and networks. Nat. Rev. Neurosci. 3, 371–382. doi: 10.1038/nrn811
DeGiorgio, C., Miller, P., Meymandi, S., Chin, A., Epps, J., Gordon, S., et al. (2010). RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 Inventory. Epilepsy. Behav. 19, 78–81. doi: 10.1016/j.yebeh.2010.06.011
Depaulis, A., David, O., and Charpier, S. (2016). The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J. Neurosci. Methods 260, 159–174. doi: 10.1016/j.jneumeth.2015.05.022
Devinsky, O. (2004). Effects of seizures on autonomic and cardiovascular function. Epilepsy. Curr. 4, 43–46. doi: 10.1111/j.1535-7597.2004.42001.x
Elliott, R., Morsi, A., Kalhorn, S., Marcus, J., Sellin, J., Kang, M., et al. (2011). Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: Long-term outcomes and predictors of response. Epilepsy. Behav. 20, 57–63. doi: 10.1016/j.yebeh.2010.10.017
Englot, D., Rolston, J., Wright, C., Hassnain, K., and Chang, E. (2016). Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery 79, 345–353. doi: 10.1227/NEU.0000000000001165
Harreby, K., Sevcencu, C., and Struijk, J. (2011). Ictal and peri-ictal changes in cervical vagus nerve activity associated with cardiac effects. Med. Biol. Eng. Comput. 49, 1025–1033. doi: 10.1007/s11517-011-0782-7
Ibrahim, A., Soliman, W., Mesbah, B., and Salem, A. (2021). Left ventricular dysfunction and cardiac autonomic imbalance in children with drug-resistant epilepsy. Epilepsy. Res. 176:106709. doi: 10.1016/j.eplepsyres.2021.106709
Jansen, K., Varon, C., Van Huffel, S., and Lagae, L. (2013). Ictal and interictal respiratory changes in temporal lobe and absence epilepsy in childhood. Epilepsy Res. 106, 410–416. doi: 10.1016/j.eplepsyres.2013.07.008
Labar, D. (2004). Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure 13, 392–398. doi: 10.1016/j.seizure.2003.09.009
Macefield, V., Patros, M., Ottaviani, M., and Dawood, T. (2023). Understanding the physiology of the human vagus nerve from intraneural microelectrode recordings. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation. 16:164. doi: 10.1016/j.brs.2023.01.149
Mamalyga, M., and Mamalyga, L. (2019). Department of human and animal anatomy and physiology of Moscow Pedagogical State University, Moscow. Effect of progressive absence epilepsy and its treatment on hemodynamics and autonomic regulation of the heart rhythm. Romanian J. Neurol. 18, 22-8. doi: 10.37897/RJN.2019.1.4
Marescaux, C., Vergnes, M., and Depaulis, A. (1992). Genetic absence epilepsy in rats from Strasbourg–a review. J. Neural Transm. Suppl. 35, 37–69. doi: 10.1007/978-3-7091-9206-1_4
Marmerstein, J., McCallum, G., and Durand, D. (2022). Decoding vagus-nerve activity with carbon nanotube sensors in freely moving rodents. Biosensors (Basel) 12:114. doi: 10.3390/bios12020114
McLachlan, R. (1993). Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia 34, 918–923. doi: 10.1111/j.1528-1157.1993.tb02112.x
Naggar, I., Sakamoto, K., Jones, S., and Stewart, M. (2022). Autonomic nerve activity and cardiovascular changes during discrete seizures in rats. Auton Neurosci. 240:102971. doi: 10.1016/j.autneu.2022.102971
Ottaviani, M., Wright, L., Dawood, T., and Macefield, V. (2020). In vivo recordings from the human vagus nerve using ultrasound-guided microneurography. J. Physiol. 598, 3569–3576. doi: 10.1113/JP280077
Pfeifer, M., Weinberg, C., Cook, D., Best, J., Reenan, A., and Halter, J. (1983). Differential changes of autonomic nervous system function with age in man. Am. J. Med. 75, 249–258. doi: 10.1016/0002-9343(83)91201-9
Pradhan, C., Sinha, S., Thennarasu, K., and Jagadisha, T. (2011). Quantitative analysis of heart rate variability in patients with absence epilepsy. Neurol. India 59, 25–29. doi: 10.4103/0028-3886.76852
Ruffoli, R., Giorgi, F., Pizzanelli, C., Murri, L., Paparelli, A., and Fornai, F. (2011). The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat. 42, 288–296. doi: 10.1016/j.jchemneu.2010.12.002
Sakamoto, K., Saito, T., Orman, R., Koizumi, K., Lazar, J., Salciccioli, L., et al. (2008). Autonomic consequences of kainic acid-induced limbic cortical seizures in rats: Peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia 49, 982–996. doi: 10.1111/j.1528-1167.2008.01545.x
Sevcencu, C., Nielsen, T., Kjaergaard, B., and Struijk, J. J. A. (2018). Respiratory marker derived from left vagus nerve signals recorded with implantable cuff electrodes. Neuromodulation 21, 269–275. doi: 10.1111/ner.12630
Shaker, K., Al Mahdawi, A., and Hamdan, F. (2021). Interictal autonomic dysfunction in patients with epilepsy. Egypt. J. Neurol. Psychiatry Neurosurg. 57:165. doi: 10.1186/s41983-021-00422-0
Sitnikova, E., and van Luijtelaar, G. (2007). Electroencephalographic characterization of spike-wave discharges in cortex and thalamus in WAG/Rij rats. Epilepsia 48, 2296–2311. doi: 10.1111/j.1528-1167.2007.01250.x
Smets, H., Stumpp, L., Chavez, J., Cury, J., Vande Perre, L., Doguet, P., et al. (2022). Chronic recording of the vagus nerve to analyze modulations by the light-dark cycle. J. Neural. Eng. 19:046008. doi: 10.1088/1741-2552/ac7c8f
Smyk, M., and van Luijtelaar, G. (2020). Circadian rhythms and epilepsy: A suitable case for absence epilepsy. Front. Neurol. 11:245. doi: 10.3389/fneur.2020.00245
Stumpp, L., Smets, H., Vespa, S., Cury, J., Doguet, P., Delbeke, J., et al. (2020). Recording of spontaneous vagus nerve activity during Pentylenetetrazol-induced seizures in rats. J. Neurosci. Methods 343:108832. doi: 10.1016/j.jneumeth.2020.108832
Stumpp, L., Smets, H., Vespa, S., Cury, J., Doguet, P., Delbeke, J., et al. (2021). Vagus nerve electroneurogram-based detection of acute pentylenetetrazol induced seizures in rats. Int. J. Neural Syst. 31:2150024. doi: 10.1142/S0129065721500246
Teckentrup, V., and Kroemer, N. (2024). Mechanisms for survival: Vagal control of goal-directed behavior. Trends Cogn. Sci. 28, 237–251. doi: 10.1016/j.tics.2023.11.001
Thijs, R., Ryvlin, P., and Surges, R. (2021). Autonomic manifestations of epilepsy: Emerging pathways to sudden death? Nat. Rev. Neurol. 17, 774–788. doi: 10.1038/s41582-021-00574-w
Yuen, A., and Sander, J. (2017). Can natural ways to stimulate the vagus nerve improve seizure control? Epilepsy. Behav. 67, 105–110. doi: 10.1016/j.yebeh.2016.10.039
Keywords: vagus nerve activity (VNA), GAERS (Genetic Absence Epilepsy Rats from Strasbourg), absence seizure, autonomic dysfunction, parasympathetic, NREM (non REM) sleep, age
Citation: Collard E, Germany Morrison E, Acedo Reina E, S. Dereli A, Apaire A, Nonclercq A and El Tahry R (2025) Vagal nerve signals are modulated by spontaneous seizures in Genetic Absence Epilepsy Rats from Strasbourg. Front. Neurosci. 19:1568261. doi: 10.3389/fnins.2025.1568261
Received: 29 January 2025; Accepted: 11 June 2025;
Published: 07 July 2025.
Edited by:
Recep Avci, The University of Auckland, New ZealandReviewed by:
Glenn D. R. Watson, Duke University, United StatesAnnaelle Devergnas, Yerkes National Primate Research Center, Georgia
Taekyung Kim, Samsung Medical Center, Republic of Korea
Copyright © 2025 Collard, Germany Morrison, Acedo Reina, S. Dereli, Apaire, Nonclercq and El Tahry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elise Collard, ZWxpc2UuY29sbGFyZEB1Y2xvdXZhaW4uYmU=
 Elise Collard
Elise Collard Enrique Germany Morrison
Enrique Germany Morrison Elena Acedo Reina
Elena Acedo Reina Ayse S. Dereli
Ayse S. Dereli Auriane Apaire1,2
Auriane Apaire1,2 Antoine Nonclercq
Antoine Nonclercq