- 1North Toronto Neurology, Toronto, ON, Canada
- 2Department of Research, JMCC Group, Toronto, ON, Canada
- 3Division of Experimental and Translational Neuroscience, Krembil Brain Institute, University Health Network, Toronto, ON, Canada
- 4Department of Physiology, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 5Department of Pediatrics, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Purpose: Seizure freedom (SF) is the primary goal of epilepsy treatment. More treatments that produce SF in drug-resistant epilepsy (DRE) are needed. Cannabis-based products for medicinal use (CBPMs) containing cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC), administered as oils, have been shown to induce SF in DRE. However, there remains a paucity of published real-world evidence in both pediatrics and adults on SF resulting from CBPM therapy.
Methods: This is a retrospective case series at an outpatient neurology clinic in Toronto, Canada, on patients with DRE who experienced significant SF during CBPM treatment. All patients were treated via the clinic’s stepwise treatment protocol with CBPM oils only. The study describes clinical features of patients and their CBPM-related SF.
Results: We report 19 DRE cases that experienced SF; 15 pediatric, 4 adults. The median cumulative SF duration was 245 days, split between continuous SF periods lasting at least 90 days. Five patients had continuous SF periods lasting ≥ 1 year. Most patients used CBD+THC regimens. Three patients weaned all concomitant ASMs. Adverse events (AEs) were reported by half of the patients.
Conclusion: The results of the study support prioritizing CBPMs in cases of DRE. It also supports research into identifying clinical and biological biomarkers for DRE cases that may achieve SF under CBPM treatment. Lastly, the study supports improving the accessibility of CBPMs, using SF as a primary outcome in future CBPM epilepsy trials, and assessing the role of THC in reducing seizures.
Introduction
Epilepsy is characterized by recurring, spontaneous seizures affecting > 1% of individuals worldwide (GBD 2016 Epilepsy Collaborators, 2019). Poor seizure control has harmful consequences, including impaired quality of life (QoL), high rates of psychiatric comorbidities and mortality, (Mohammadzadeh and Nazarbaghi, 2022) increased financial burden, and a high likelihood of cognitive impairments (Laxer et al., 2016). Conversely, people with epilepsy (PWE) achieving seizure freedom (SF) report significant QoL improvements (Jain et al., 2020) even when compared to PWE with just one seizure within the past 5 years (Josephson et al., 2017). Patients and caretakers also rank SF as their primary treatment outcome (Josephson et al., 2017; Halford and Edwards, 2020).
Medical intervention with anti-seizure medications (ASMs) is the first line of treatment to reduce seizures. Most ASMs modulate neuronal excitability by targeting ion channels and neurotransmitters (Manford, 2017). For example, carbamazepine (CZP) and valproic acid (VPA) are inhibitors of voltage-gated sodium and L-type voltage-gated calcium channels, preventing action potential propagation in neurons (Gambeta et al., 2020). VPA also has a bipartite mechanism on GABA, that works to both reduce the breakdown of and increase the release of GABA, thus shunting spreading depolarization and seizures (Rahman et al., 2025). Though ion-channel based mechanisms like these are appropriate for some seizures, there are few alternative ASMs that operate on separate mechanisms that may be better suited for certain epilepsies, as reflected in the current rates of ASM-induced SF. While 37% (Brodie et al., 2012) to 50.5% (Chen et al., 2018) of patients experience significant SF (i.e., > 1 year) after starting their first ASM, the probability of SF decreases substantially with each successive ASM regimen (Brodie et al., 2012). Chen et al. (2018) found that the second and third regimens provide just an additional 11.6% and 4.4% likelihood of SF, respectively, and that there was a 1.73-fold increase in the probability of uncontrolled seizures occurring with each successive ASM treatment. Further, with about 50% of epilepsy cases having no identifiable etiology (Thijs et al., 2019), ASM selection is challenging and, at times, based on clinical features or practitioner experience alone, rendering SF a difficult outcome to achieve. Thus, many PWE try several different ASMs, which is associated with economic burden (de Kinderen et al., 2014), reduced QoL (Puri et al., 2018), and disruptive ASM-related adverse events (AEs) (Perucca et al., 2018), without reaching SF.
A diagnosis of drug-resistant epilepsy (DRE) is made when a patient fails to respond to more than two appropriately trialed ASMs (Sultana et al., 2021). More than 30% of PWE suffer from DRE (Tang et al., 2017), and its associated increases in risk of comorbidities, number of hospitalizations, and mortality rates compared to non-DRE PWE (Strzelczyk et al., 2017). At least one third of individuals with DRE have a psychiatric comorbidity. The risk of depression is 2.7-fold greater in individuals with epilepsy than in the general population. Psychiatric comorbidities, including depression (major depression and treatment-resistant depression), anxiety/anxiety-related disorders, and functional seizures (psychogenic non-epileptic seizures, PNES) have disproportionate prevalence in the epilepsy population, reaching rates of 23.1, 20.1, and 9–12%, respectively (Mula et al., 2021).
In support of the definition of DRE, Chen et al. (2018) analysis of their DRE cohort of 1,792 pediatric and adult patients demonstrated that, beyond the third ASM, the chance of achieving SF in this population was 1% or less and “cumulative probabilities of seizure freedom [after 2 drug trials] were not significantly different with each successive” ASM. These outcomes have not improved compared to similar research from over two decades ago, indicating a need for a “paradigm shift in treatment and research strategies” (Chen et al., 2018). Non-pharmaceutical options exist for DRE, but these vary in efficacy; have inherent accessibility barriers; and pose independent, significant risks (Solli et al., 2020). Thus, exploring alternative treatments that achieve SF is necessary.
A promising treatment for DRE are cannabis-based products for medicinal use (CBPMs) containing variations of cannabinoids, such as cannabidiol (CBD), Δ-9-tetrahydrocannabinol (THC) and other cannabis plant products in an oil formulation. Cannabinoids act by binding to receptors to modulate the endocannabinoid system, contrasting the mechanisms by which most ASMs operate. Pre-clinical work established CBD’s anti-seizure properties (Jones et al., 2010) while clinical randomized control trials of purified CBD demonstrated effective seizure reduction in patients with Dravet syndrome (Devinsky et al., 2011; Devinsky et al., 2018b; Miller et al., 2020), Lennox-Gastaut syndrome (French et al., 2017; Devinsky et al., 2018a; Thiele et al., 2018), and Tuberous Sclerosis complex (Thiele et al., 2021), with 50% seizure response rates in refractory epilepsy ranging from approximately 35% to 63% (Hausman-Kedem et al., 2018; Lattanzi et al., 2018; McCoy et al., 2018; Devinsky et al., 2019; Devinsky et al., 2020; Lattanzi et al., 2020a; Thiele et al., 2022). These trials led to United States Food and Drug Administration, European Medical Association, and Health Canada approvals for Epidiolex®—a purified CBD product—for DRE in patients 2 years and older suffering from seizures due to these conditions (US Food & Drug, 2018; Ema, 2019; Product information, 2024). Collectively, past RCTs and open-label trials of CBD have demonstrated its contribution to SF in approximately 4% of DRE patients (Table 1).
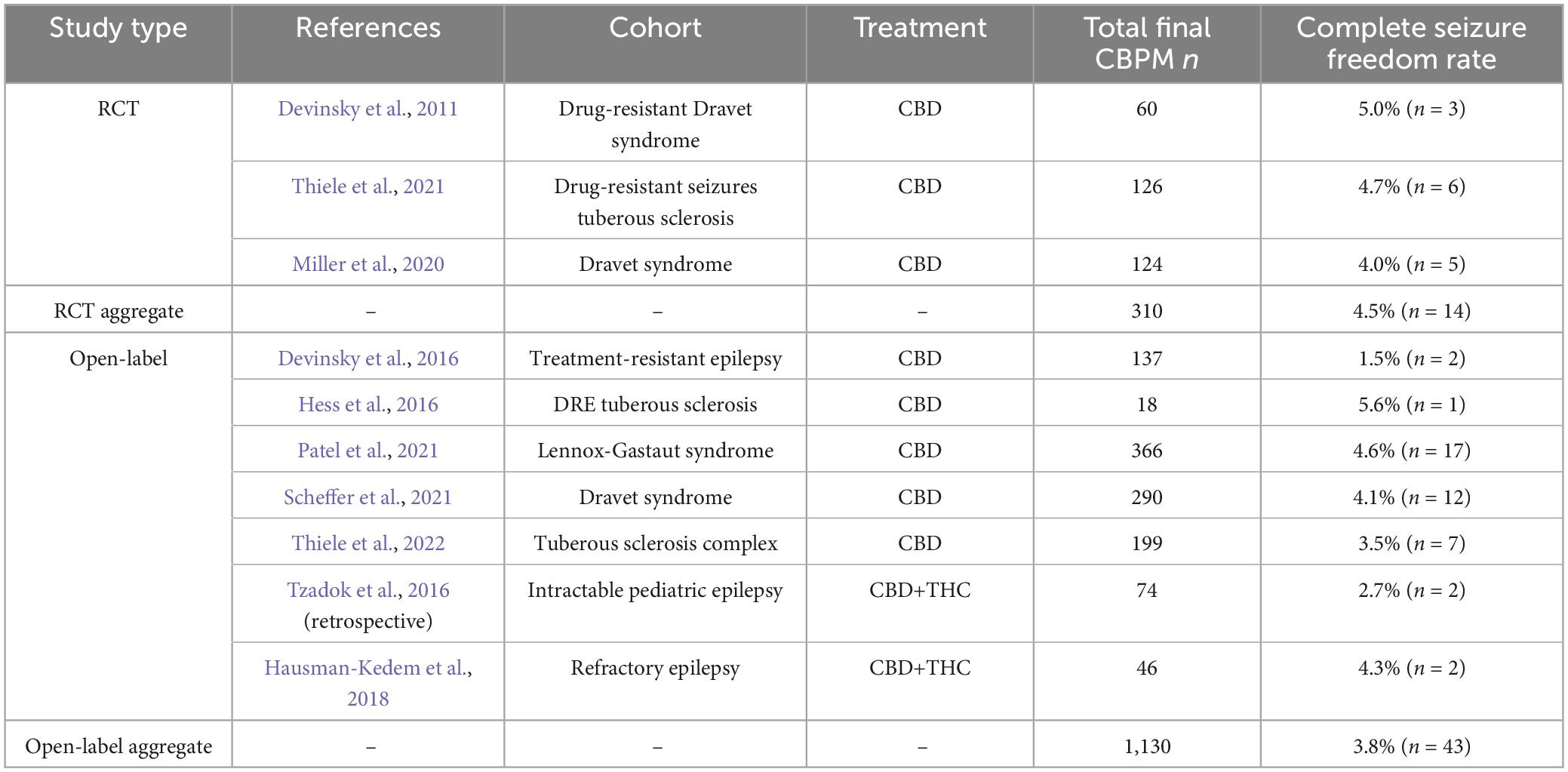
Table 1. Studies assessing oil-based CBPM treatment of epilepsy that report complete seizure freedom rates and have an N > 10, as of October 2023.
Preclinical research regarding the effects of THC on seizures has yielded mixed results. In different rodent studies, seizures were induced with high doses of THC (Malyshevskaya et al., 2017; Anderson et al., 2020) while low-dose THC, co-administered with CBD, improved seizure outcomes (Anderson et al., 2020; Dlugosz et al., 2023). Published open-label and observational studies of THC in humans with epilepsy support the latter finding from animal studies (Pamplona et al., 2018). CBD-rich extracts demonstrate similar degrees of seizure reduction compared to purified CBD in patients diagnosed with Dravet syndrome and other epileptic encephalopathies (Pamplona et al., 2018; Huntsman et al., 2019; Zafar et al., 2021) and clinical reports by Nowicki et al. (2022) and Erridge et al. (2023) presented evidence that add-on THC may contribute to achieving SF in pediatric DRE patients (Nowicki et al., 2022; Erridge et al., 2023).
Despite CBPM research presenting meaningful seizure response rates and significant seizure reductions, there is an absence of research on the practical aspects of achieving SF with CBPMs in real-world settings and the qualities that define this special population. For instance, there is no published data on clinical biomarkers predicting SF, clinical protocols associated with achieving SF, or longitudinal clinical outcomes. Every CBPM epilepsy study to date has reported SF rates as a secondary outcome with little to no attention paid to specific features of this special population.
The aim of this study was to focus on this population and report its clinical features to provide a basis for future research. This may help demarcate this population from those with DRE providing predictive clinical metrics to determine the most robust responders to CBPM therapy in the context of DRE. To achieve this, we analyzed the clinical features of 19 patients with DRE treated with CBPMs who experienced relevant and complete seizure-free periods lasting more than 3 months, with a specific emphasis on patients who experienced SF periods lasting at least 1 year.
Materials and methods
Study design
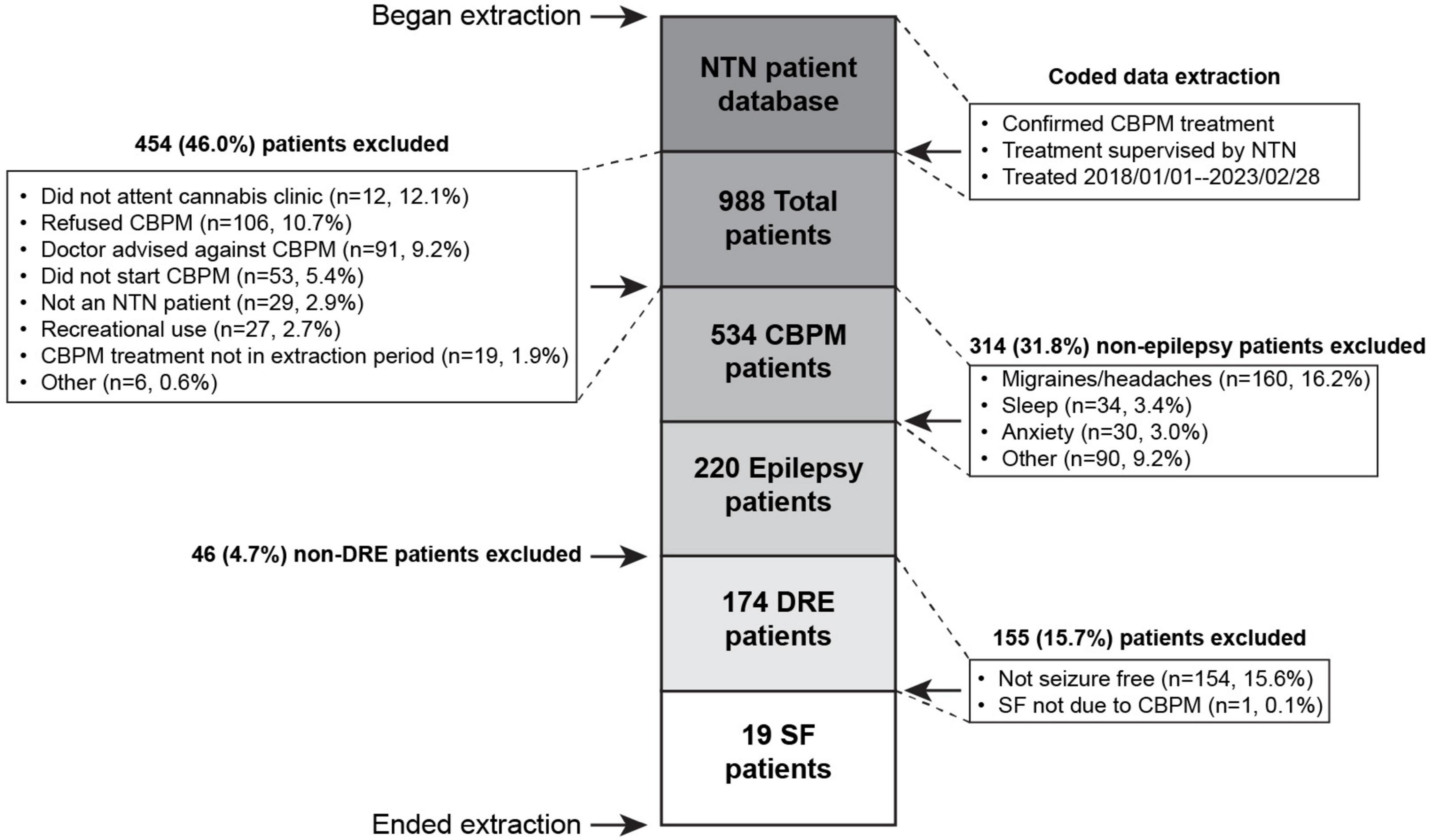
This is a retrospective case series using medical records from an outpatient neurology clinic in Toronto, Ontario (North Toronto Neurology [NTN]; formerly Neurology Centre of Toronto). The study included patients that (1) started CBPMs to improve their seizures between 1st January 2018 and 28th February 2023; (2) were supervised by an NTN neurologist with specialization in pediatric epilepsy; (3) had DRE, as defined by the International League Against Epilepsy (ILAE) (Kwan et al., 2010); and (4) experienced at least one continuous and substantial SF period, marked by freedom of all seizure types (i.e., seizure frequency of 0). A continuous and substantial SF period was defined as at least 90 days in which no seizures were experienced and occurring 90 days after the addition/alteration of CBPMs, without altering other treatments. This timeline was established to reduce the potential of confounding from the “honeymoon effect,” which is associated with several ASMs (Avanzini, 2006; Löscher and Schmidt, 2006; Chen et al., 2018) including CBD (Lattanzi et al., 2020b; Uliel-Sibony et al., 2021; Kühne et al., 2023). We performed further analyses on patients with continuous SF periods of at least 1 year, to align with the ILAE’s definition of SF (Kwan et al., 2010). Patients were excluded if (1) they were using CBPMs for non-seizure conditions; (2) their CBPM treatment was not guided by NTN; (3) they were self-medicating with cannabis products, as reported by the patients; or (4) their SF was due to the alteration/addition of a different treatment. Ethical approval for this study was received by Veritas, a Canadian Independent Review Board (Reference number: 2021-2597-5657-2).
Medical cannabis program
All patients were treated under the NTN Medical Cannabis Program and received authorization to access medical cannabis from the program’s neurologist. The program commences with an education session and an initial consultation with the clinical team. If the patient is medically suitable (no history of unstable/severe cardiac/renal/hepatic impairment; no active/unstable psychiatric condition), they are granted authorization to use CBPMs, adhering to the treatment protocol, and according to the Canadian regulatory framework governing CBPM authorization. The program’s clinical team remained consistent throughout the observation period.
The Medical Cannabis Program treatment protocol for epilepsy is based on published evidence, neurobiological mechanisms, and team-based clinical experience. Its structure aligns with the first principles of epilepsy care, in that CBD and THC are regarded as two distinct ASMs used in combination (Sander, 2004). Prior to commencing treatment, standard and relevant bloodwork and electrocardiogram are ordered. Patient diagnoses and epilepsy evaluations are also confirmed by electroencephalograms (EEGs) prior to commencing treatment. At the initial consultation, the neurologist helps patients parse their seizures into different seizure types. The protocol consists of 5 phases, and follows a step-wise titration of CBD, then THC based upon the patient’s clinical response (Supplementary Figure 1). When THC is added, CBD dosage is reduced. As with management using conventional ASMs, if a patient fails to respond or exhibits only a partial response during each visit, dosing progresses to the next phase. Conversely, if a patient demonstrates an “adequate response,” dosing is maintained. EEGs during and after CBPM treatment were not ordered, due to feasibility issues of a real world study. Adequate response is defined as a change in seizures (frequency, duration or severity) that meets patient goals of care (e.g., seizure freedom/reduction, improved QoL, reduced CBPM/ASM AEs, etc.), and is highly individualized and dynamic which is typical in the DRE population. CBPM progression and discontinuation is weighed against AEs, similar to adding new ASMs to treatment regimens.
Prescribed CBPMs were those available in Ontario, Canada for medical use. They included oil-based isolated cannabinoids or broad/full spectrum. No patients were prescribed dry flower preparations. Epidiolex® (approved in Canada in 2024) was unavailable in Canada during the study period and, therefore, no patients reviewed were using this product.
Seizure tracking
Seizure counts from baseline, during, and after CBMPs were based on information obtained from the caretaker/patient at each FU. To establish baseline numbers, patients/caregivers are instructed to record at least two weeks of baseline seizure frequency and duration with respect to each seizure type. At each subsequent visit and during CBPM treatment, patients/caregivers are instructed to track seizures. The neurologist reviews each seizure type and respective frequencies and durations at FUs. Patients and caregivers were not given standard seizure calendars or trackers. Instead, patients used a variety of tools, ranging from handwritten calendars to seizure-tracking apps.
Data collection/analyses
All relevant data were collected from the NTN electronic medical records, which were stored in a TELUS PS Suite® EMR software hosted locally on NTN servers. Baseline EEGs used to affirm preliminary diagnoses were unavailable for analysis. Eligible patients were identified using PS Suite’s built-in query tools, to generate an anonymized list of all patients who underwent CBPM treatment at NTN during the observation period. Manual and automated data cleaning processes were performed to assess eligibility. A further round of data cleaning was performed by the authors (EL and FC) to affirm SF patient eligibility and data accuracy. Data was only available to research and clinical care teams. Missing data was marked in the dataset, but core data was available for all eligible patients. Extracted data included SF information (e.g., length of SF period; SF dosages; AEs) and secondary outcomes (demographics, epilepsy history, CBPM information, seizure/QOL changes at each follow-up [FU], ASM weaning). Seizure changes, QoL, and AE measures were from patient self-reports during appointments. The main goal of this study was to describe SF in the context of CBPMs. Python and its PANDAs library were used to clean the data. All study outcomes were presented using descriptive statistics performed in Microsoft Excel®; no inferential statistics were performed.
Results
Demographics and epilepsy medical history
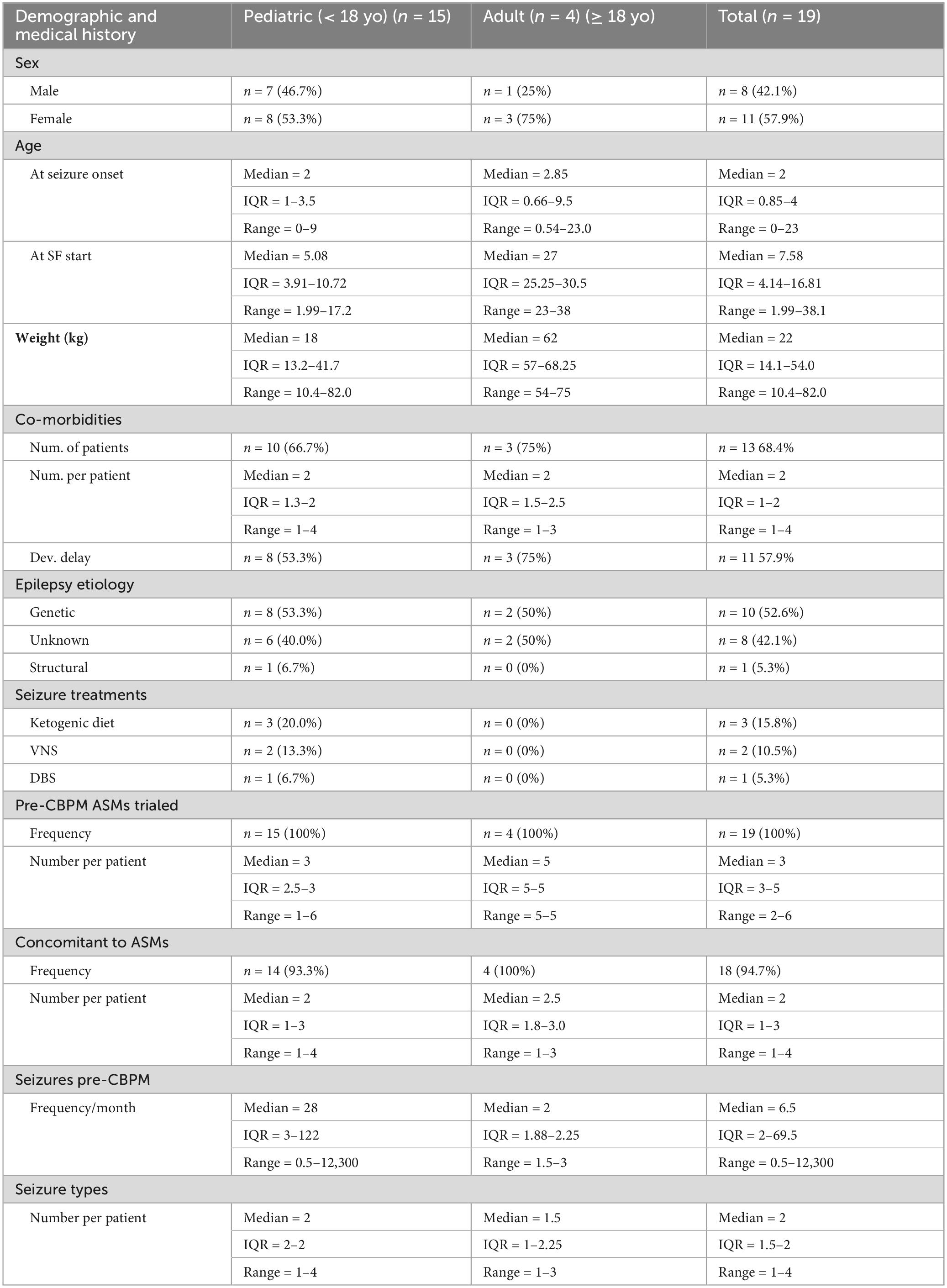
The extraction process (Figure 1) yielded 174 patients with DRE. From these, 19 (10.9% of initial cohort) DRE patients met the SF criteria for this study. One patient experienced SF while on CBPMs, but had to be excluded because their SF occurred immediately after a change in lamotrigine and valproic acid (VPA) dosing, violating exclusion criterion #4. Pediatric patients comprised 79% (n = 15) of SF patients. Table 2 summarizes the demographic information of the SF cohort, while Supplementary Table 1 presents patient-specific demographics. All relevant epilepsy medical history data is summarized in Table 2 and Supplementary Table 1. The most common seizure types were Generalized Tonic-Clonic (n = 13, 69%), Absence (n = 8, 42%), and Myoclonic seizures (n = 3, 16%) (Supplementary Table 1). The cohort represented a wide range of syndromes, seizure types and etiologies. The cohort was treated with a variety of anti-seizure interventions as shown in Table 2 and Supplementary Table 2. During CBPM treatment, 13 patients were taking concomitant ASMs. The median reported seizure frequency per month before CBPMs was 6.50 (range 0.5–12,300), with each patients’ pre-CBPM seizure count reported in Supplementary Table 1. For the full summary of specific CBPM each patient was on, see Supplementary Table 4.
Seizure freedom
Seizure free periods
Table 3 provides summary information on the cohort’s cumulative SF experience. All patients experienced the cessation of all seizure types. The median cumulative duration of reported SF was 245 days (range 90–1,694). These were split between different continuous SF periods, though most patients reported experiencing just one continuous SF period (n = 14), with the median amount of SF periods per patient being 1 (range 1–3). Five pediatric patients had at least one continuous SF period lasting ≥ 1 year, representing 4.0% of the pediatric DRE cohort. As per each patient’s last check-in with their neurologist, 26% (n = 5) remain seizure-free as of October 2024.
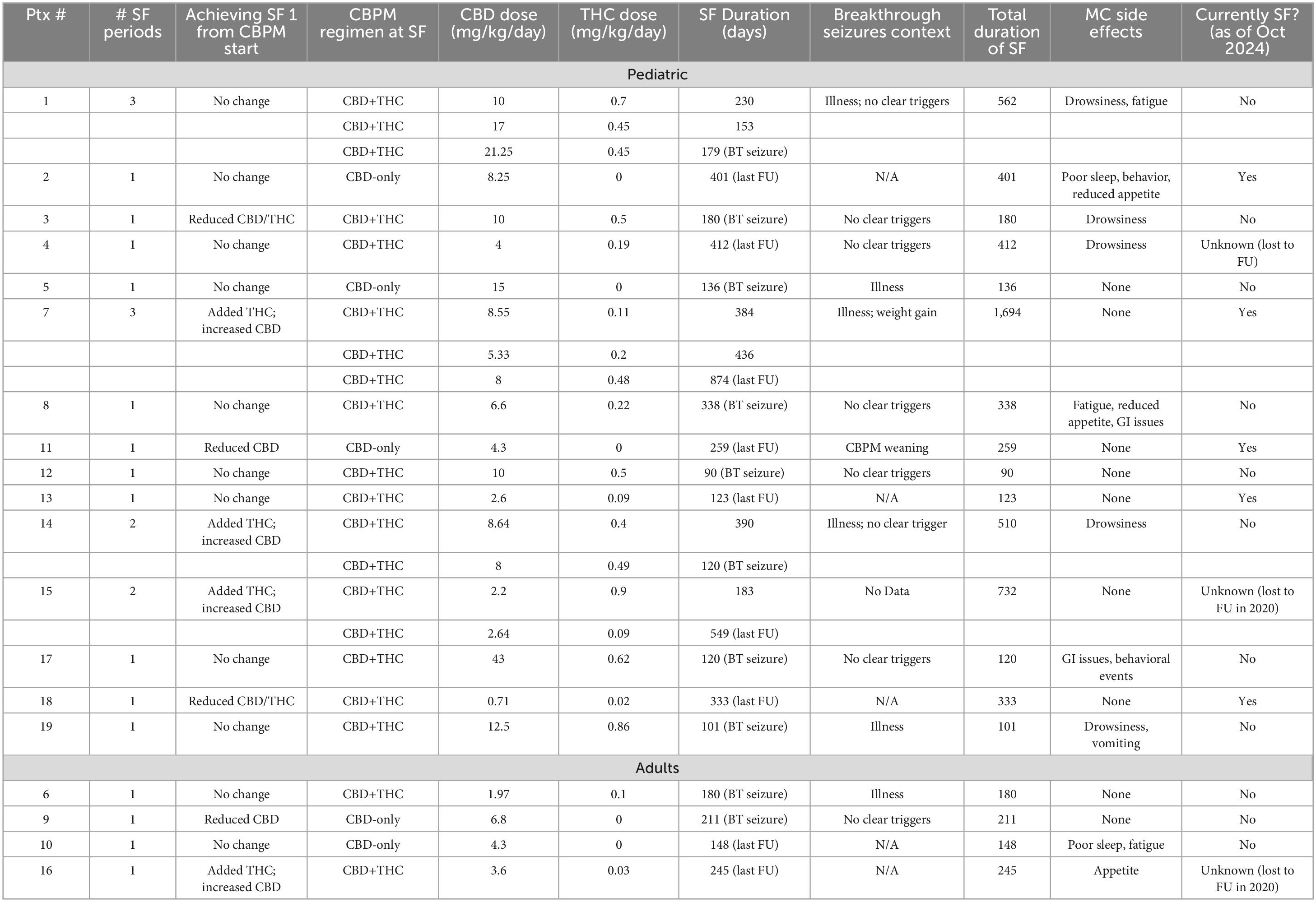
Supplementary Table 3 summarizes data on each continuous SF period, while a patient-specific breakdown of CBPM and SF periods can be found on Table 4. At the beginning of their treatments, 9 patients were on a CBD-only treatment, while the remaining 10 patients were using a combined CBD and THC treatment. Three of the four adults patients (75%) started on CBD only.
Achieving seizure freedom
In the transition between starting CBPMs and achieving the first continuous SF period, 11 patients did not change their treatment or target dosages, as they became seizure-free when they started CBPMs. For instance, patient 7 became seizure-free after one week of CBPM treatment and was no longer exhibiting ataxia. Four patients added THC to their CBD-only treatment and adjusted their CBD dose. This included patient 17, who adjusted their CBD dose and added THC. Patients 12 and 16 added THC to address their aggressive behavior and sleep/anxiety, respectively. Other changes involved adjustments of dosages in both CBD and THC. For instance, patient 13 switched to a different CBD product (i.e., a 1:20 CBD oil to another company’s 1:30 CBD oil). Before achieving SF, Patient 11 had to wean CBPMs briefly due to product unavailability, which led to a significant worsening of seizure frequency. Once the product became available again, patient 11 achieved SF.
CBD and THC dosing associated with SF achievement
Looking at patients’ first continuous SF period (Table 4 and Supplementary Table 3), the median duration of SF was 211 days (range 90–412). Five patients (26.3%) were on CBD only, while 14 (73.7%) were on a combination of CBD and THC. The associated median CBD doses for CBD only and CBD+THC regimens were 6.8 (range 4.3–15) and 7.58 (range 0.71–43) mg/kg/day, respectively. The median THC dose during this first SF period was 0.31 mg/kg/day (range 0.02–0.9). Supplementary Table 3 presents these doses split into the adult and pediatric cohorts.
Breakthrough seizures
Of the patients with multiple SF periods, the median time between the first and second was 106.5 days (range 20–174). Most patients experienced breakthrough seizures (n = 13) (Table 4 and Supplementary Table 3) during their first SF period. Though 6 of these had no clear triggers, multiple events were associated with breakthrough seizures onset, including illness (n = 5), ASM weaning (n = 1), CBPM weaning (n = 1), and weight gain (n = 1). Patients 1 and 14 both noted illness associated with breakthrough seizures, but these were not identified as a definitive trigger in those individuals. Patient 7 had breakthrough seizures following weight gain and intercurrent illnesses. In the transition between the first and second SF periods, CBPM products and/or dosages of CBD and/or THC were adjusted. This includes patient 1, who switched from taking full spectrum CBPMs (Shubie Oil and Banook Oil) to a combination of separate products of purified CBD (Rho Phyto) and THC-rich extract (THC Reign Drops) to achieve their second SF period following breakthrough seizures (Supplementary Table 4). Patient 7, who experienced breakthrough seizures in the context of illness and weight gain, added THC at night (Tilray THC Oil), resulting in their second SF period, which has lasted for > 2 years.
Second SF period following breakthrough seizures
During the patients’ second SF period, no patients were using CBD-only CBPMs (Supplementary Table 3). All of these patients were pediatric. The median duration of the second SF period was 294.5 days (range 120–592). The associated median CBD and THC doses were 6.67 (range 2.64–17) and 0.33 (range 0.09–0.49) mg/kg/day. During their second SF period, patient 15 weaned CBPMs and all ASMs due to the complete remission of seizures. They remained seizure-free as of October 2023. Though patient 4 experienced a second SF period, this was only after adjusting both CBPM and oxcarbazepine dosing, so it was not included in this study due to this study’s fourth exclusion criterion. Extended data on patients’ third continuous SF period can be found in Supplementary Table 3.
Secondary outcomes
Seizure frequency when not seizure-free
The median monthly seizure frequency prior to CBPM treatment was 6.5 (range 0.5–12,300) (Table 2). This decreased to 2 (range 0.25–990) when patients were on CBPMs but not in their SF period (i.e., during their breakthrough seizures or post-SF periods). This was a 69.2% reduction in monthly seizure frequency. Eleven patients (57.9%) experienced an interval breakthrough seizures period during CBPM treatment, of which 4 (21.1%) re-established a second SF period (Table 4 and Supplementary Table 3) using CBPMs.
Quality of life
Seventeen (89.5%) patients reported improvements in QoL; 2 (10.5%) reported no change (Table 3). Ten (52.6%) patients reported an AE when on CBPMs. Five of these patients reported AEs only at the initialization of CBPM treatment. The most commonly reported AEs were sleepiness/drowsiness (n = 5), reduced appetite (n = 3), and increased fatigue (n = 3). Patients 2 and 10 reported sleep issues: both reported sleep maintenance issues, and one reported issues initiating sleep as well (Table 3). The AEs are summarized in Table 3. No severe AEs were reported in this cohort; CBPMs were generally well-tolerated.
Concomitant ASMs
Most patients were taking concomitant clobazam or VPA while seizure-free and did not completely wean off concomitant ASMs. Three (15.8%) patients of the cohort completely weaned off all concomitant ASMs while taking CBPMs. These were patient 2 (clobazam and topiramate); patient 5 (clobazam); and patient 7 (topiramate). Patient 15 weaned off just one of their ASMs (clobazam). Though patient 4 did wean oxcarbazepine, they experienced a BT seizure, and had to re-administer oxcarbazepine.
Discussion
In this RWE case series of 19 patients with DRE who underwent periods of SF (≥ 90 days) during CBPM treatment, we provide practical information on SF in the context of CBPMs. Unlike past trials reporting SF after treatment with pharmaceutical-grade CBD (Devinsky et al., 2011; Devinsky et al., 2016; Devinsky et al., 2018a; Devinsky et al., 2019; Thiele et al., 2019; Patel et al., 2021; Scheffer et al., 2021; Thiele et al., 2021), our SF population was not limited to certain syndromes (i.e., Lennox-Gastaut, Dravet). This indicates CBPM efficacy in other syndromes and is consistent with past research that has shown similarly efficacious effects of CBPMs in other DRE subtypes (Stockings et al., 2018; Espinosa-Jovel et al., 2023; Kühne et al., 2023). Though we did not assess 50% seizure response rates, which has previously been reported to be around 35%–63% (Hausman-Kedem et al., 2018; McCoy et al., 2018; Devinsky et al., 2019; Devinsky et al., 2020; Lattanzi et al., 2020a; Thiele et al., 2022; Espinosa-Jovel et al., 2023), our data showed that ∼4% of our pediatric DRE population achieved at least 1 year of SF.
Patients spent approximately 50% of their total CBPM treatment duration seizure-free, from a median pre-CBPM seizure rate of 6.5 seizures per month. The median duration of the first SF period was 211 days, while the cohort’s median total SF duration was 245 days, ranging from 90 to 1,694 days, spread over 1 to 3 SF periods. These findings set expectations for patients, caregivers and clinicians providing a foundation for future research to explore and detail CBPM-related seizure-free periods more comprehensively. Given the well-known high costs associated with managing seizures in DRE (Widjaja et al., 2021), and resultant SF within our study population, it would be beneficial to assess the economics of a CBPM-related SF period.
Taken together, with similar findings reported in past research (Devinsky et al., 2011; French et al., 2017; Devinsky et al., 2018b; Chakraborty and Hocker, 2019; Devinsky et al., 2019; Thiele et al., 2019; Thiele et al., 2022; Wu et al., 2022), the outcomes of CBPM treatment in our cohort align with the priorities/goals of patients and caretakers, including SF, improved QoL, low AE impact, and complete ASM weaning. Seizure freedom and its associated QoL improvements (Jacoby et al., 2009; Ring et al., 2019) are recognized as the main goals of epilepsy treatment among patients, families, and treatment guidelines (Josephson et al., 2017; Halford and Edwards, 2020). Patients and caretakers also prioritize low AE profiles in ASM trials (Jacoby et al., 2009). These priorities, coupled with our results, highlight the need to further assess the nature and long-term effects of CBPM-related seizure freedom in more rigorous studies. This would help improve CBPM access as a DRE treatment and support the more accurate disclosure of current, established CBPM-related research findings by epileptologists to patients with DRE and their families.
Our cohort’s SF periods were substantial, considering the patients’ DRE statuses, and the difficulty individuals with DRE have in achieving SF. Previous research shows that after trialing 3 ASMs, patients have at most a 1% likelihood of achieving SF as defined by the ILAE (Kwan et al., 2010) (i.e., at least a year without seizures) when trialing further regimens (Chen et al., 2018). Despite the introduction of more than a dozen new ASMs between 2000 and 2018, these likelihoods and SF rates have remained unchanged (Kwan and Brodie Martin, 2000; Chen et al., 2018). In contrast, 4% of our pediatric DRE population that was treated with CBPMs—having tried a median of 3 ASMs prior to CBPM treatment—achieved 1 year of SF. This is a significant improvement from a 1% likelihood of achieving SF with other ASMs and aligns with past CBPM RCTs (Table 1), which reported that 5% of DRE patients treated with CBPMs undergo significant periods of SF.
Given the consistency across independent studies of varying rigor—from observational trials to high-quality randomized control trials—rates of SF freedom reported with CBPMs and observed in the analysis of our data set present a clinical outcome that should be actively considered and evaluated. CBPMs modulate the endocannabinoid system, thereby acting via a unique mechanism in comparison to conventional ASMs. CBD and THC have distinct pharmacological targets on the nervous system. While CBD has a relatively low affinity (4,350 μM Ki) at the CB1 receptor (De Petrocellis et al., 2011), it inhibits fatty acid amide hydrolase (FAAH) enzyme activity, which hydrolyzes the endocannabinoid anandamide, thereby enhancing its signaling. Multi-modal mechanisms described in the literature provide a theoretical and experimentally demonstrated basis for stabilizing neuronal excitatory:inhibitory balance via interactions with the 5-HT1A, 5HT3, TRPV1 and GRP55 receptors in addition to the ENT-1 adenosine transporter (Townsend et al., 2002, Gray and Whalley, 2020). Pre-clinical support has been demonstrated in acute mouse brain slices that inhibiting the 5-HT1A receptor blocks CBD’s anti-convulsant effects (Javadzadeh et al., 2024). THC, on the other hand, is a partial agonist of both the CB1 and CB2 receptors. Cumulatively, CBD and THC have diverse pharmacological effects modulating neurotransmission and inflammation that provide a theoretical neurobiological basis as to why a significant and consistent minority of patients with DRE appear to robustly respond to CBPMs. Though further rigorous research is needed to ascertain the mechanisms of action in CBPM-related seizure freedom and the SF rate in DRE populations, our data supports improving CBPM accessibility to those with DRE. This paper also supports pursuing placebo-controlled studies that assess whether CBPMs should be considered a prioritized pharmacological treatment for DRE alongside epilepsy surgery referral, given the SF findings from this study and previous research. Future research should also actively compare seizure freedom and 50% seizure response rates between CBPMs and ASMs in DRE, to further define CBPMs role in DRE treatment.
The findings of this study and the priorities of PWE also highlight the greater issue regarding the importance of using SF data to assess ASMs. Clinical and research-based assessments of ASMs focus on seizure frequency reductions without reporting SF data (Halford and Edwards, 2020). Seizure frequency reductions certainly have value; however, focus should not be diverted from SF, especially considering that non-seizure-free reductions in seizure frequency are associated with reduced QoL, increased social isolation and stigmatization, increased self-reported mood disorders, greater psychological distress, and lower employment rates than SF (Jacoby et al., 2009; Josephson et al., 2017; Ring et al., 2019). Shifting a greater degree of focus to SF in ASM assessments in DRE would align with patient outcomes and desires, and could facilitate a reframing of ASM testing that previous researchers have called for due to the lack of change in DRE SF rates from ASMs over the past 20 years (Chen et al., 2018; Halford and Edwards, 2020).
Study limitations
The retrospective case series study design risks the inclusion of selection bias and potential confounds. These, combined with the lack of randomization, placebo comparison, and low sample size, prevent us from drawing causal conclusions. For example, due to the design of the study, we were unable to assess and report an accurate 50% seizure responder rate in our total population. The cohort was also heterogeneous in seizure types, etiology, age, treatment protocols, and medical history. Our use of patient chart data also resulted in heterogeneous entries, missing data, and reliance on patient reports. These hinder the ability to draw comparisons between patients, especially since patient reports on seizure tracking may be biased (e.g., placebo effect after CBPM was initiated) and inaccurate. This is further limited by the absence of EEG data in our analyses. Though EEGs were used to confirm baseline diagnoses and seizure types, these were unavailable for analysis, and were not performed during or after CBMP treatment. Thus, we cannot ascertain if patients were experiencing subclinical seizures. However, during SF periods, no patients/caregivers reported clinical signs of worrisome subclinical events (e.g., overly fatigued, unwell, not at baseline level of health). Despite the limitations, it is important to note that the authors have reported a dichotomous variable (i.e., complete absence of seizures) and, therefore, these limitations may not be as impactful in a similar study solely evaluating seizure reduction in a DRE population.
Conclusion
This study reports RWE from 19 patients with DRE who experienced SF due to CBPM therapy. The SF rates observed in this study complement existing literature that reports rates of at least 4%, higher than the 1% observed with established ASMs.
CBPMs are pharmacologically distinct from conventional ASMs, acting through unique mechanisms via modulation of the ECS. Therefore, patients whose epilepsies respond particularly well to CBPMs may represent a distinct cohort with shared neurobiological and clinical features. Given the significant burden that ongoing seizures pose on morbidity, mortality, QoL, and healthcare costs, the authors call on the epilepsy research community to prioritize the identification of this population’s shared characteristics. Focusing on the identification of their chemical and genetic biomarkers may translate clinically in the guidance of treatment choice and the prioritization of CBPMs in treatment pathways for DRE. Given the comparative SF data in the published literature, future double blind, placebo-controlled studies should also assess whether CBPMs should be prioritized as first-line medical therapy for DRE cases that lack established, evidence-based treatment options.
Finally, the RWE presented in this study supports the need for greater accessibility to CBPMs, a comparative economic analysis of the costs associated with DRE patients not treated with CBPMs, and the inclusion of SF data in future CBPM epilepsy trials.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Veritas Institutional Review Board (reference number: 2021-2597-5657-2). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because The IRB deemed that we did not need consent to use retrospective data from patient medical records. The data were completely anonymized, and any potentially identifying personal information was omitted from the manuscript.
Author contributions
FYC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review and editing, Funding acquisition, Resources, Supervision. JD: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review and editing. BSR: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review and editing, Conceptualization, Methodology, Project administration, Supervision, Visualization. KH: Data curation, Investigation, Writing – original draft, Writing – review and editing. ECL: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. Avicanna Inc. paid for publication fees. JMCC Group funded the ethics approval application and renewals.
Acknowledgments
We are gracious to Avicanna Inc. for their support of this research by covering publication costs.
Conflict of interest
FYC is currently employed by Avicanna, and was previously employed by JMCC during the study. BSR held a MITACS Accelerate Fellowship sponsored by Avicanna for unrelated work with a different group. ECL previously held an unpaid advisory role at JMCC Group and has been paid for speaking engagements with Jazz Pharmaceuticals, Avicanna Inc. MGC Pharmaceuticals, and Argent BioPharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Avicanna Inc. and JMCC Group. Avicanna had the following involvement in the study: requesting the collection and publication of Supplementary Table 4 data. The funders had no other involvement in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2025.1570531/full#supplementary-material
Supplementary Figure 1 | Standardized CBPM dosing protocol designed by ECL and the NTN neurological care team.
References
Anderson, L. L., Low, I. K., McGregor, I. S., and Arnold, J. C. (2020). Interactions between cannabidiol and Δ9 -tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome. Br. J. Pharmacol. 177, 4261–4274.
Avanzini, G. (2006). Is tolerance to antiepileptic drugs clinically relevant? Epilepsia 47, 1285–1287. doi: 10.1111/j.1528-1167.2006.00616.x
Brodie, M., Barry, S., Bamagous, G., Norrie, J., and Kwan, P. (2012). Patterns of treatment response in newly diagnosed epilepsy. Neurology 78, 1548–1554. doi: 10.1212/WNL.0b013e3182563b19
Chakraborty, T., and Hocker, S. (2019). Weaning from antiseizure drugs after new onset status epilepticus. Epilepsia 60, 979–985. doi10.1111/epi.14730
Chen, Z., Brodie, M., Liew, D., and Kwan, P. (2018). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30-year longitudinal cohort study. JAMA Neurol. 75, 279–286. doi: 10.1001/jamaneurol.2017.3949
de Kinderen, R., Evers, S., Rinkens, R., Postulart, D., Vader, C., Majoie, M., et al. (2014). Side-effects of antiepileptic drugs: The economic burden. Seizure 23, 184–190. doi: 10.1016/j.seizure.2013.11.009
De Petrocellis, L., Ligresti, A., Moriello, A., Allarà, M., Bisogno, T., Petrosino, S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x
Devinsky, O., Cross, J., Laux, L., Marsh, E., Miller, I., Nabbout, R., et al. (2011). Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med. 376, 2011–2020. doi: 10.1056/NEJMoa1611618
Devinsky, O., Marsh, E., Friedman, D., Thiele, E., Laux, L., Sullivan, J., et al. (2016). Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 15, 270–278. doi: 10.1016/S1474-4422(15)00379-8
Devinsky, O., Nabbout, R., Miller, I., Laux, L., Zolnowska, M., Wright, S., et al. (2019). Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 60, 294–302. doi: 10.1111/epi.14628
Devinsky, O., Patel, A., Cross, J., Villanueva, V., Wirrell, E., Privitera, M., et al. (2018a). Effect of cannabidiol on drop seizures in the lennox-gastaut syndrome. N. Engl. J. Med. 378, 1888–1897. doi: 10.1056/NEJMoa1714631
Devinsky, O., Patel, A., Thiele, E., Wong, M., Appleton, R., Harden, C., et al. (2018b). Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90, e1204–e1211. doi: 10.1212/WNL.0000000000005254
Devinsky, O., Thiele, E., Wright, S., Checketts, D., Morrison, G., Dunayevich, E., et al. (2020). Cannabidiol efficacy independent of clobazam: Meta-analysis of four randomized controlled trials. Acta Neurol Scand. 142, 531–540. doi: 10.1111/ane.13305
Dlugosz, L., Zhou, H., Scott, B., and Burnham, M. (2023). The effects of cannabidiol and Δ9-tetrahydrocannabinol, alone and in combination, in the maximal electroshock seizure model. Epilepsy Res. 190:107087. doi: 10.1016/j.eplepsyres.2023.107087
Ema (2019). Epidyolex. European Medicines Agency. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex (accessed January 17, 2018).
Erridge, S., Holvey, C., Coomber, R., Hoare, J., Khan, S., Platt, M., et al. (2023). Clinical outcome data of children treated with cannabis-based medicinal products for treatment resistant epilepsy-analysis from the UK medical cannabis registry. Neuropediatrics 54, 174–181. doi: 10.1055/a-2002-2119
Espinosa-Jovel, C., Riveros, S., Bolaños-Almeida, C., Salazar, M., Inga, L., and Guío, L. (2023). Real-world evidence on the use of cannabidiol for the treatment of drug resistant epilepsy not related to Lennox-Gastaut syndrome, Dravet syndrome or Tuberous Sclerosis Complex. Seizure 112, 72–76. doi: 10.1016/j.seizure.2023.09.015
French, J., Thiele, E., Mazurkiewicz-Beldzinska, M., Benbadis, S., Marsh, E., Joshi, C., et al. (2017). Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): Results of a multi-center, randomized, double-blind, placebo controlled trial (GWPCARE4) (S21.001). Neurology 88, 1085–1096. doi: 10.1016/S0140-6736(18)30136-3
Gambeta, E., Chichorro, J. G., and Zamponi, G. W. (2020). Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Mol. Pain 16:1744806920901890. doi: 10.1177/1744806920901890
GBD 2016 Epilepsy Collaborators (2019). Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 357–375.
Gray, R. A., and Whalley, B. J. (2020). “The proposed mechanisms of action of CBDin epilepsy”, Epileptic Disord 2020; 22 (Suppl. 1): S10–S15
Halford, J. J., and Edwards, J. C. (2020). Seizure freedom as an outcome in epilepsy treatment clinical trials. Acta Neurol Scand. 142, 91–107. doi: 10.1111/ane.13257
Hausman-Kedem, M., Menascu, S., and Kramer, U. (2018). Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents - An observational, longitudinal study. Brain Dev. 40, 544–551. doi: 10.1016/j.braindev.2018.03.013
Hess, E., Moody, K., Geffrey, A., Pollack, S., Skirvin, L., Bruno, P., et al. (2016). Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 57, 1617–1624. doi: 10.1111/epi.13499
Huntsman, R., Tang-Wai, R., Alcorn, J., Vuong, S., Acton, B., Corley, S., et al. (2019). Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: Preliminary results of the CARE-E study. Front. Neurol. 10:716. doi: 10.3389/fneur.2019.00716
Jacoby, A., Snape, D., and Baker, G. A. (2009). Determinants of quality of life in people with epilepsy. Neurologic Clin. 27, 843–863. doi: 10.1016/j.ncl.2009.06.003
Jain, P., Smith, M., Speechley, K., Ferro, M., Connolly, M., Ramachandrannair, R., et al. (2020). Seizure freedom improves health-related quality of life after epilepsy surgery in children. Dev. Med. Child. Neurol. 62, 600–608. doi: 10.1111/dmcn.14390
Javadzadeh, Y., Santos, A., Aquilino, M., Mylvaganam, S., Urban, K., and Carlen, P. (2024). Cannabidiol exerts anticonvulsant effects alone and in combination with Δ9-THC through the 5-HT1A receptor in the neocortex of mice. Cells 13:466. doi: 10.3390/cells13060466
Jones, N., Hill, A., Smith, I., Bevan, S., Williams, C., Whalley, B., et al. (2010). Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 332, 569–577. doi: 10.1124/jpet.109.159145
Josephson, C., Patten, S., Bulloch, A., Williams, J., Lavorato, D., Fiest, K., et al. (2017). The impact of seizures on epilepsy outcomes: A national, community-based survey. Epilepsia 58, 764–771. doi: 10.1111/epi.13723
Kühne, F., Becker, L., Bast, T., Bertsche, A., Borggraefe, I., Boßelmann, C., et al. (2023). Real-world data on cannabidiol treatment of various epilepsy subtypes: A retrospective, multicenter study. Epilepsia Open 8, 360–370. doi: 10.1002/epi4.12699
Kwan, P., Arzimanoglou, A., Berg, A., Brodie, M., Allen Hauser, W., Mathern, G., et al. (2010). Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51, 1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x
Kwan, P., and Brodie Martin, J. (2000). Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319. doi: 10.1056/NEJM200002033420503
Lattanzi, S., Brigo, F., Trinka, E., Zaccara, G., Cagnetti, C., Del Giovane, C., et al. (2018). Efficacy and safety of cannabidiol in epilepsy: A systematic review and meta-analysis. Drugs 78, 1791–1804. doi: 10.1007/s40265-018-0992-5
Lattanzi, S., Brigo, F., Trinka, E., Zaccara, G., Striano, P., Del Giovane, C., et al. (2020a). Adjunctive cannabidiol in patients with dravet syndrome: A systematic review and meta-analysis of efficacy and safety. CNS Drugs 34, 229–241. doi: 10.1007/s40263-020-00708-6
Lattanzi, S., Trinka, E., Striano, P., Zaccara, G., Del Giovane, C., Nardone, R., et al. (2020b). Cannabidiol efficacy and clobazam status: A systematic review and meta-analysis. Epilepsia 61, 1090–1098. doi: 10.1111/epi.16546
Laxer, K., Trinka, E., Hirsch, L., Cendes, F., Langfitt, J., Delanty, N., et al. (2016). The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 37, 59–70. doi: 10.1016/j.yebeh.2014.05.031
Löscher, W., and Schmidt, D. (2006). Experimental and clinical evidence for loss of effect (tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia 47, 1253–1284. doi: 10.1111/j.1528-1167.2006.00607.x
Malyshevskaya, O., Aritake, K., Kaushik, M., Uchiyama, N., Cherasse, Y., Kikura-Hanajiri, R., et al. (2017). Natural (Δ9-THC) and synthetic (JWH-018) cannabinoids induce seizures by acting through the cannabinoid CB1 receptor. Sci. Rep. 7:10516. doi: 10.1038/s41598-017-10447-2
Manford, M. (2017). Recent advances in epilepsy. J. Neurol. 264, 1811–1824. doi: 10.1007/s00415-017-8394-2
Mattson, R., Cramer, J., and Collins, J. (1992). A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic–clonic seizures in adults. N. Engl. J. Med. 327, 765–71. doi: 10.1056/NEJM199209103271104
McCoy, B., Wang, L., Zak, M., Al-Mehmadi, S., Kabir, N., Alhadid, K., et al. (2018). A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann. Clin. Transl. Neurol. 5, 1077–1088. doi: 10.1002/acn3.621
Miller, I., Scheffer, I., Gunning, B., Sanchez-Carpintero, R., Gil-Nagel, A., Perry, M., et al. (2020). Dose-ranging effect of adjunctive oral Cannabidiol vs Placebo on convulsive seizure frequency in dravet syndrome: A randomized clinical trial. JAMA Neurol. 77, 613–621. doi: 10.1001/jamaneurol.2020.0073
Mohammadzadeh, P., and Nazarbaghi, S. (2022). The prevalence of drug-resistant-epilepsy and its associated factors in patients with epilepsy. Clin. Neurol. Neurosurg. 213:107086. doi: 10.1016/j.clineuro.2021.107086
Mula, M., Kanner, A., Jetté, N., and Sander, J. (2021). Psychiatric comorbidities in people with epilepsy. Neurol Clin. Pract. 11, e112–e120. doi: 10.1212/CPJ.0000000000000874
Nowicki, M., Bourgeois-Tardif, S., Diaz, P., Hebert, F., Sanon, N., Champagne, P., et al. (2022). Potential benefit of add-on Δ9-tetrahydrocannabinol in pediatric drug-resistant epilepsy: A case series. Can. J. Neurol. Sci. 49, 595–597. doi: 10.1017/cjn.2021.151
Pamplona, F. A., da Silva, L. R., and Coan, A. C. (2018). Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front. Neurol. 9:759. doi: 10.3389/fneur.2018.00759
Patel, A., Mazurkiewicz-Bełdzińska, M., Chin, R., Gil-Nagel, A., Gunning, B., Halford, J., et al. (2021). Long-term safety and efficacy of add-on cannabidiol in patients with Lennox-Gastaut syndrome: Results of a long-term open-label extension trial. Epilepsia 62, 2228–2239. doi: 10.1111/epi.17000
Perucca, P., Scheffer, I. E., and Kiley, M. (2018). T*he management of epilepsy in children and adults. Med. J. Aust. 208, 226–233. doi: 10.5694/mja17.00951
Product information (2024). Drug Product Database Online Query. Available online at: https://health-products.canada.ca/dpd-bdpp/dispatch-repartition (accessed Auguest 14, 2024).
Puri, I., Dash, D., Padma, M. V., and Tripathi, M. (2018). Quality of life and its determinants in adult drug refractory epilepsy patients who were not candidates for epilepsy surgery: A correlational study. J. Epilepsy Res. 8, 81–86. doi: 10.14581/jer.18013
Rahman, M., Awosika, A. O., and Nguyen, H. (2025). Valproic Acid. in StatPearls. Treasure Island, FL: StatPearls Publishing.
Ring, A., Jacoby, A., Baker, G., Holmes, E., Hughes, D., Kierans, C., et al. (2019). What really matters? A mixed methods study of treatment preferences and priorities among people with epilepsy in the UK. Epilepsy Behav. 95, 181–191. doi: 10.1016/j.yebeh.2019.03.033
Sander, J. (2004). The use of antiepileptic drugs–principles and practice. Epilepsia 45, (Suppl. 6), 28–34. doi: 10.1111/j.0013-9580.2004.455005.x
Scheffer, I., Halford, J., Miller, I., Nabbout, R., Sanchez-Carpintero, R., Shiloh-Malawsky, Y., et al. (2021). Add-on cannabidiol in patients with Dravet syndrome: Results of a long-term open-label extension trial. Epilepsia 62, 2505–2517. doi: 10.1111/epi.17036
Solli, E., Colwell, N., Say, I., Houston, R., Johal, A., Pak, J., et al. (2020). Deciphering the surgical treatment gap for drug-resistant epilepsy (DRE): A literature review. Epilepsia 61, 1352–1364. doi: 10.1111/epi.16572
Stockings, E., Zagic, D., Campbell, G., Weier, M., Hall, W., Nielsen, S., et al. (2018). Evidence for cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry 89, 741–753. doi: 10.1136/jnnp-2017-317168
Strzelczyk, A., Griebel, C., Lux, W., Rosenow, F., and Reese, J. (2017). The burden of severely drug-refractory epilepsy: A comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using german health insurance data. Front. Neurol. 8:712. doi: 10.3389/fneur.2017.00712
Sultana, B., Panzini, M., Veilleux Carpentier, A., Comtois, J., Rioux, B., Gore, G., et al. (2021). Incidence and prevalence of drug-resistant epilepsy: A systematic review and meta-analysis. Neurology 96, 805–817. doi: 10.1212/WNL.0000000000011839
Tang, F., Hartz, A., and Bauer, B. (2017). Drug-resistant epilepsy: Multiple hypotheses. Few answers. Front. Neurol. 8:301. doi: 10.3389/fneur.2017.00301
Thiele, E., Bebin, E., Bhathal, H., Jansen, F., Kotulska, K., Lawson, J., et al. (2021). Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: A placebo-controlled randomized clinical trial. JAMA Neurol. 78, 285–292. doi: 10.1001/jamaneurol.2020.4607
Thiele, E., Bebin, E., Filloux, F., Kwan, P., Loftus, R., Sahebkar, F., et al. (2022). Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open-label extension trial. Epilepsia 63, 426–439. doi: 10.1111/epi.17150
Thiele, E., Marsh, E., Mazurkiewicz-Beldzinska, M., Halford, J., Gunning, B., Devinsky, O., et al. (2019). Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia 60, 419–428. doi: 10.1111/epi.14670
Thiele, E., Marsh, E. D., French, J., Mazurkiewicz-Beldzinska, M., Benbadis, S., Joshi, C., et al. (2018). Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. doi: 10.1016/S0140-6736(18)30136-3
Thijs, R., Surges, R., O’Brien, T., and Sander, J. (2019). Epilepsy in adults. Lancet 393, 689–701. doi: 10.1016/S0140-6736(18)32596-0
Townsend, D., Thayer, S., and Brown, D. (2002). Cannabinoids throw up a conundrum. Br. J. Pharmacol. 137, 575–577. doi: 10.1038/sj.bjp.0704913
Tzadok, M., Uliel-Siboni, S., Linder, I., Kramer, U., Epstein, O., Menascu, S., et al. (2016). CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure 35, 41–44. doi: 10.1016/j.seizure.2016.01.004
US Food & Drug (2018). Drugs@FDA: FDA-Approved Drugs. Available online at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210365
Uliel-Sibony, S., Hausman-Kedem, M., Fattal-Valevski, A., and Kramer, U. (2021). Cannabidiol-enriched oil in children and adults with treatment-resistant epilepsy-does tolerance exist? Brain Dev. 43, 89–96. doi: 10.1016/j.braindev.2020.06.018
Widjaja, E., Guttmann, A., Tomlinson, G., Snead, I. O. C., and Sander, B. (2021). Economic burden of epilepsy in children: A population-based matched cohort study in Canada. Epilepsia 62, 152–162. doi: 10.1111/epi.16775
Wu, J., Cock, H., Devinsky, O., Joshi, C., Miller, I., Roberts, C., et al. (2022). Time to onset of cannabidiol treatment effect and resolution of adverse events in tuberous sclerosis complex: Post hoc analysis of randomized controlled phase 3 trial GWPCARE6. Epilepsia 63, 1189–1199. doi: 10.1111/epi.17199
Keywords: epilepsy, cannabis, CBD–cannabidiol, THC–tetrahydrocannabinol, seizure freedom, pediatric epilepsy, seizures, cannabis medicine
Citation: Chen FY, Duckman JM, Rabinovitch BS, Hannesson KJ and Lewis EC (2025) 19 patients report seizure freedom with medical cannabis oil treatment for drug-resistant epilepsy: a case series. Front. Neurosci. 19:1570531. doi: 10.3389/fnins.2025.1570531
Received: 05 February 2025; Accepted: 17 April 2025;
Published: 19 May 2025.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Ligia Renata Rodrigues Tavares, São Paulo State University, BrazilArie Weinstock, University at Buffalo, United States
Copyright © 2025 Chen, Duckman, Rabinovitch, Hannesson and Lewis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brenden Samuel Rabinovitch, YnJlbmRlbi5yYWJpbm92aXRjaEB1aG4uY2E=; YnJlbmRlbi5yYWJpbm92aXRjaEBtYWlsLnV0b3JvbnRvLmNh
†Present address: Frank Yizhao Chen, Avicanna Inc., Toronto, ON, Canada
‡These authors share first authorship
 Frank Yizhao Chen
Frank Yizhao Chen Joshua Myles Duckman
Joshua Myles Duckman Brenden Samuel Rabinovitch
Brenden Samuel Rabinovitch Katrin Julia Hannesson
Katrin Julia Hannesson Evan Cole Lewis
Evan Cole Lewis