- 1The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 2Suzhou Industrial Park Xinghai Hospital, Suzhou, Jiangsu, China
Cognitive impairment (CI) is common, with diverse underlying causes, symptoms, and imaging features. It often leads to disability and loss of independence. Early diagnosis and assessment of CI are crucial for the prognosis improvement. Conventional diagnostic methods for CI are hindered by subjectivity and imprecision. Radiomics, a sophisticated and objective methodology, has been increasingly utilized in CI in recent times. This article describes the methodology of radiomics and reviews the application of radiomics in the prediction and evaluation of cognitive impairment related to neurological diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), cerebral small vessel disease (CSVD), and stroke. It can provide imaging markers for the early diagnosis and risk stratification of cognitive impairment.
1 Background
As the global population ages, the incidence of cognitive impairment (CI) and dementia is increasing significantly. Studies indicate that approximately one in five individuals over the age of 65 will experience varying degrees of cognitive decline (Han et al., 2020). CI encompasses a complex symptomatology associated with various diseases and may lead to disability and compromised independent living capacity in affected individuals. Growing evidence suggests that dementia can be significantly mitigated through early diagnosis and intervention (Reuben et al., 2024). Traditional diagnostic methods for cognitive impairment (CI) primarily rely on clinical assessments and standardized scales, which are often constrained by subjectivity and limited precision. In this context, radiomics emerges as an advanced and complementary technique for the diagnosis and evaluation of CI.
2 Overview of radiomics
2.1 Introduction
Radiomics is an imaging technology, which extracts a large number of quantitative features based on the medical imaging data through computer science and statistical methods to reveal potential associations between images and diseases, and to support the diagnosis, prognosis assessment and treatment of diseases. It is firstly proposed by Lambin et al. (2012). This technology excels in identifying imaging features or biomarkers associated with specific diseases through systematic analysis of high-dimensional imaging data and textural features (Gillies et al., 2016). Radiomics analysis encompasses a diverse spectrum of analytical approaches, including voxel-based methods, connectivity-based techniques, and pattern recognition-based strategies. The radiomics analysis process typically involves several key steps: image data acquisition, region of interest (ROI) segmentation, feature extraction, feature selection, model training, and ultimately classification or prediction (Bera et al., 2022; Lambin et al., 2012), Figure 1 depicts a radiomics workflow diagram and provides additional methodological details.
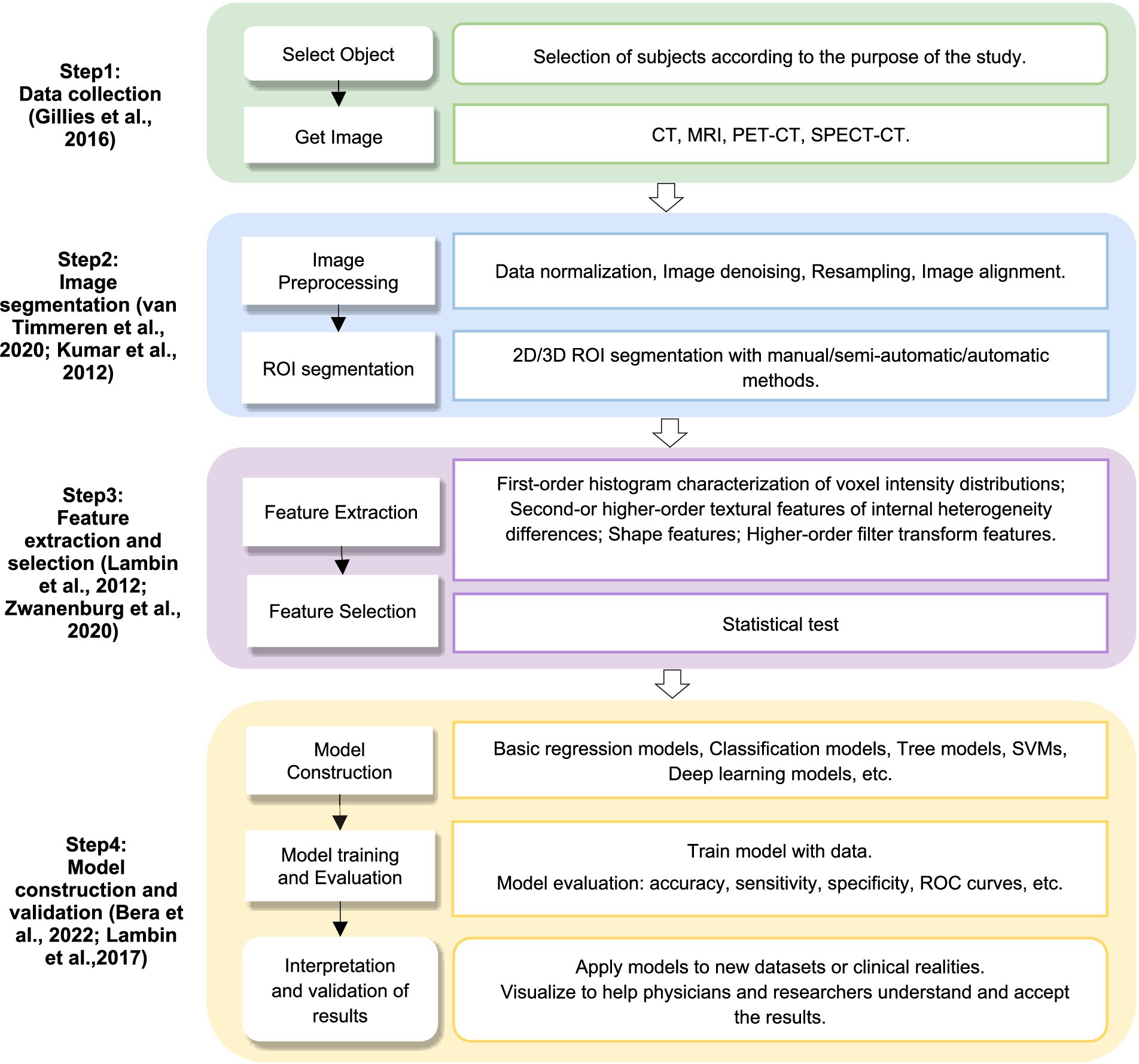
Figure 1. Radiomics workflow. CT, computed tomography; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography; SPECT–CT, single-photon emission computed tomography-computed tomography; ROI, region of interest; SVM, support vector machine; ROC, receiver operation characteristic.
2.2 Segmentation of region of interest
Segmentation of ROI facilitates targeted analysis of specific brain regions or structures. The methodologies for ROI segmentation can be categorized into several distinct approaches (Cardenas et al., 2019; van Timmeren et al., 2020): (1) Manual labeling: Researchers manually delineate the boundaries of the ROI by selecting and segmenting specific regions. This approach is well-suited for limited datasets and less complex anatomical structures. However, it exhibits limitations in both computational efficiency and anatomical precision when dealing with large datasets or complex brain regions (Kumar et al., 2012; Zwanenburg et al., 2020). (2) Standard templates: This method employs validated standard brain templates, such as those from the Montreal Neurological Institute (MNI) (Pan et al., 2022), whereby researchers spatially normalize these templates to individual brain images and segment the ROI based on predefined regions. While effective for normative brain regions, this method may not account for individual neuroanatomical variations, thereby potentially compromising segmentation accuracy in specific brain regions. (3) Automatic segmentation algorithms: These algorithms implement advanced image processing techniques to autonomously localize and segment the ROI through the application of mathematical models and statistical methods. These algorithms substantially improve efficiency and consistency of segmentation but encounter significant limitations when applied to complex brain regions or in the presence of substantial pathological changes (Puzio et al., 2025; Yu et al., 2025). (4) Hybrid method: This integrative approach synergistically combines the strengths of manual labeling and automatic segmentation algorithms. For example, initial manual contours can inform and constrain the automatic segmentation process, with subsequent refinements and iterative optimizations based on the algorithm’s results (Parmar et al., 2014).
The selection of an appropriate method should be determined by the research objectives, the intrinsic characteristics of the data, and the available research resources. Segmentation accuracy and methodological reproducibility constitute the fundamental criteria for evaluating the performance of these segmentation approaches.
2.3 Extraction of features and construction of prediction model
Features can be broadly categorized into qualitative and quantitative categories (Cheung et al., 2022). Qualitative features include specific location, size, morphology, etc. Quantitative features can be systematically subdivided into the following four types: (1) Morphological Features: These features elucidate the three-dimensional shape and structural features of the lesion (Kumar et al., 2012); (2) First-Order Features (Histogram Features): These features represent the distribution of the lesion across different gray levels, providing quantitative metrics regarding of the lesion’s intensity characteristics (Zwanenburg et al., 2020); (3) Second-Order Features: These quantify the heterogeneity of the lesion by examining the spatial relationships between voxels or pixels, thereby capturing complex textural patterns and architectural information (Zwanenburg et al., 2020); (4) Higher-Order features: These comprise advanced mathematical transformations including Wavelet transforms, Laplace filters, and Gabor filters, etc. which facilitate multi-scale analysis of the lesion at a more sophisticated level, elucidating subtle patterns and structures (Kumar et al., 2012; Zhang et al., 2022; Zwanenburg et al., 2020). When performing radiomics analysis, the focus is on identifying various image attributes including shape, intensity, texture, gradient, and wavelet. Furthermore, the incorporation of non-image data encompassing clinical information and biogenetic data should be systematically evaluated to optimize model performance and clinical relevance.
The construction of the prediction model often employs algorithmic models based on machine learning, such as convolutional neural networks, support vector machines (SVM), and random forests, representing a critical component in the radiomics workflow (Lambin et al., 2017; Rathore et al., 2017). Sensitivity, specificity, accuracy, and the receiver operating characteristic (ROC) curve analysis constitute the standard metrics for rigorous evaluation of the model (Bera et al., 2022; Lambin et al., 2017). Ultimately, these metrics serve as the cornerstone for validating the reliability and performance of our predictive models in clinical applications.
3 Advances in radiomics of Alzheimer’s disease
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, which has a serious impact not only on the patient’s daily life but the psychological and economic status of the patient’s family (Alzheimer’s Association, 2016). Mild cognitive impairment (MCI) is characterized as the transitional stage from normal cognition to AD (Dubois et al., 2016) with approximately 10–20% of MCI patients progressing to dementia annually (Langa, Levine, 2014). Studies have demonstrated that therapeutic intervention during the MCI stage may delay the onset of irreversible dementia (Mueller et al., 2005). Therefore, there exists an urgent need for reliable biomarkers to facilitate early screening and diagnosis of AD. However, volumetric alterations of the brain regions remain subtle and challenging to detect during the early stage of AD. Therefore, the identification of alternative biomarkers for the early prediction of MCI-to-AD conversion is imperative.
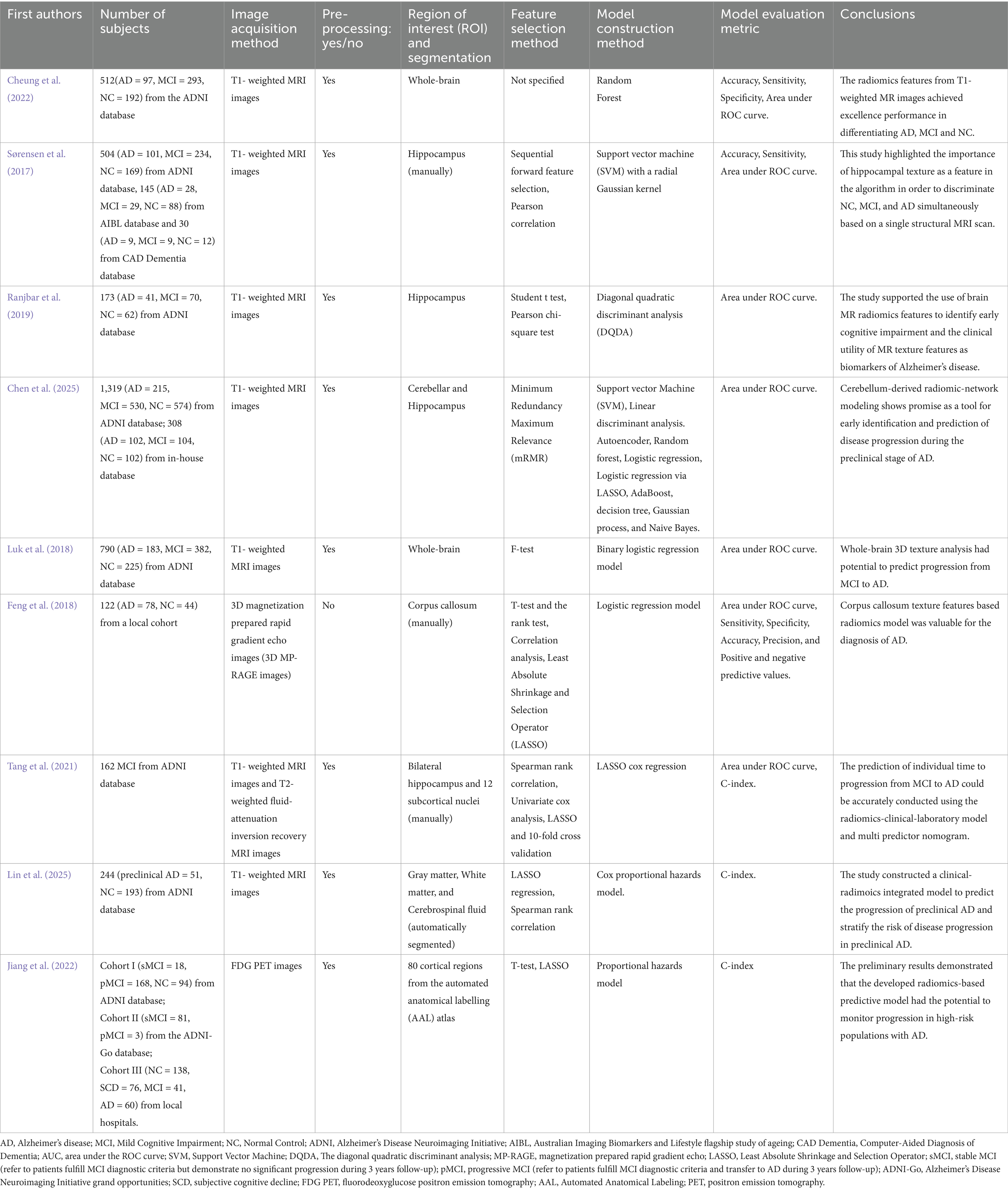
In recent years, neuroimaging measures (volume, thickness, surface morphometry) derived from structural magnetic resonance imaging (MRI), functional MRI, and positron emission tomography (PET) have been proposed as reliable biomarkers of AD. Radiomics analysis incorporating these parameters has been demonstrated significant potential for the automated diagnosis of MCI progression to AD. Cheung et al. constructed a model using brain region volumes and radiomics features separately (Cheung et al., 2022). A total of 107 whole-brain radiomics features were extracted using voxel-based morphometry (VBM) and the DARTEL algorithm from structural MRI data. Their findings indicated that features of the whole-brain derived from T1-weighted MR images achieved excellent performance in differentiating between AD and MCI. Besides, the hippocampus are also often used as the ROI in studies of cognitive disorders. Sorensen and colleagues (Sørensen et al., 2017) used hippocampal texture, hippocampal shape and cortical thickness to differentiate between MCI and AD based on T1-weighted structural MRI scans. A total of 215 radiomics texture features were extracted from the hippocampus, with sequential forward feature selection subsequently applied for feature selection and model construction. The analysis revealed hippocampal texture as the most significant discriminative feature, suggesting its diagnostic utility operates independently of hippocampal volume. Ranjbar et al. (2019) received similar conclusions. In their analysis of 173 subjects comprising AD, MCI, and healthy control groups, hippocampus texture features acquired the favorable performance in distinguishing AD from controls with an area under curve (AUC) of 0.89. While the hippocampus have remained a focal region in AD radiomics research, Chen et al. (2025) explored the potential of cerebellar-derived radiomics for predicting AD progression over a 6-year follow-up with integrated machine learning models. Notably, the cerebellar models outperformed hippocampal models in distinguishing MCI and in predicting transitions from normal cognition to MCI. Key predictors included textural features in the right III and left I and II lobules, as well as network properties in Vermis I and II, which were significantly associated with cognitive decline in AD. In conclusion, the whole-brain, hippocampus and cerebellum are regarded as ROI in different researches, more studies are required to ensure the optimal choice.
In terms of the model construction, several machine learning methods have been used for distinguishing MCI conversion to AD. Luk et al. (2018) utilized SVM in distinguishing MCI conversion to AD and achieved high accuracy. Feng et al. (2018) constructed a logistic regression model using t-test, correlation analysis, and LASSO screening, achieving an AUC of 0.720. Tang’s team (Tang et al., 2021) followed 162 patients with MCI, and after 5 years of follow-up, 68 patients transitioned to AD. They built a radiomics model using LASSO Cox regression analysis. The C-index was 0.950 (0.929–0.971). Lin et al. (2025) developed and validated a clinical-radiomics integrated nomogram model. The radiomics features from T1-weighted MRI images combined with clinical factors identified through univariate and multivariate Cox regression, were used to construct clinical, radiomics, and integrated models. The integrated model demonstrated superior predictive performance, achieving a C-index of 0.903 (95% CI: 0.870–0.936) in the training cohort and 0.813 (95% CI: 0.734–0.892) in the validation cohort. In a different imaging modality, Jiang et al. (2022) proposed a workflow for radiomics predictive modeling analysis of AD using fluorodeoxyglucose positron emission tomography (FDG PET) images. A total of 34,400 quantitative features were extracted from 80 cortical regions in each subject. The machine learning methods they used were t-test and LASSO. Three models were constructed: a clinical model, a standardized uptake value ratio (SUVR) Cox model, and a radiomics-based predictive model (RPM model). This study confirmed that metabolic profiles of AD pathologically susceptible regions are more effective in predicting progression to AD, and that metabolic abnormalities in these regions are better characterized by high-dimensional radiomics features (Table 1 presents a summary of the above studies).
The application of radiomics in AD can provides insights into the mechanisms and pathological processes of AD, which facilitates early diagnosis and prediction. Recent studies highlight the potential of radiomics and machine learning in predicting AD progression from MCI using neuroimaging biomarkers. Key brain regions including the hippocampus, cerebellum, and precuneus provide discriminative features. While hippocampal textural features outperforms volumetry, emerging biomarkers in the cerebellum and precuneus challenge traditional AD paradigms. However, current limitations include small sample sizes, unclear biological correlates of radiomic features, and limited clinical generalizability. Future research should focus on longitudinal validation, multimodal integration, and explainable AI to enhance translational utility.
4 Advances in radiomics for predicting cognitive impairment in Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that primarily affects dopamine-producing neurons in the specific region of the brain called the substantia nigra (SN). Diagnosis is typically based on clinical history and motor symptoms (Chu et al., 2024). However, the early-stages of PD often present diagnostic challenges due to heterogeneous clinical presentations and overlapping symptoms with other neurological disorders. These challenges are particularly pronounced when evaluating cognitive impairments associated with PD, which are often subtle and difficult to differentiate. To address these complexities, radiomics has emerged as a promising approach for early diagnosis through the extraction and analysis of high-throughput quantitative imaging features. This advanced approach not only facilitates in the detection of PD but also holds potential for identifying and characterizing cognitive impairments, thereby enhancing both diagnostic accuracy and disease management.
Cognitive impairment is one of the most common non-motor symptoms of PD (Aarsland et al., 2017). PD dementia (PDD) occurs in more than 80% of PD patients with disease duration exceeding 20 years (Hely et al., 2008). PDD primarily affects cognitive domains such as attention, executive function, and working memory. PD-MCI is prevalent in 19–42% of PD patients (Aarsland et al., 2009; Yarnall et al., 2014) and it is usually considered a risk factor for PDD (Pedersen et al., 2013). However, substantial heterogeneity exists in the cognitive trajectories of PD-MCI, some patients progress to PDD, while others remain stable or even return to normal cognition (Pedersen et al., 2013). Therefore, the risk stratification of PD-MCI convention to PDD is essential, as high-risk patients may require more aggressive treatment and could potentially benefit from disease-modifying therapies.
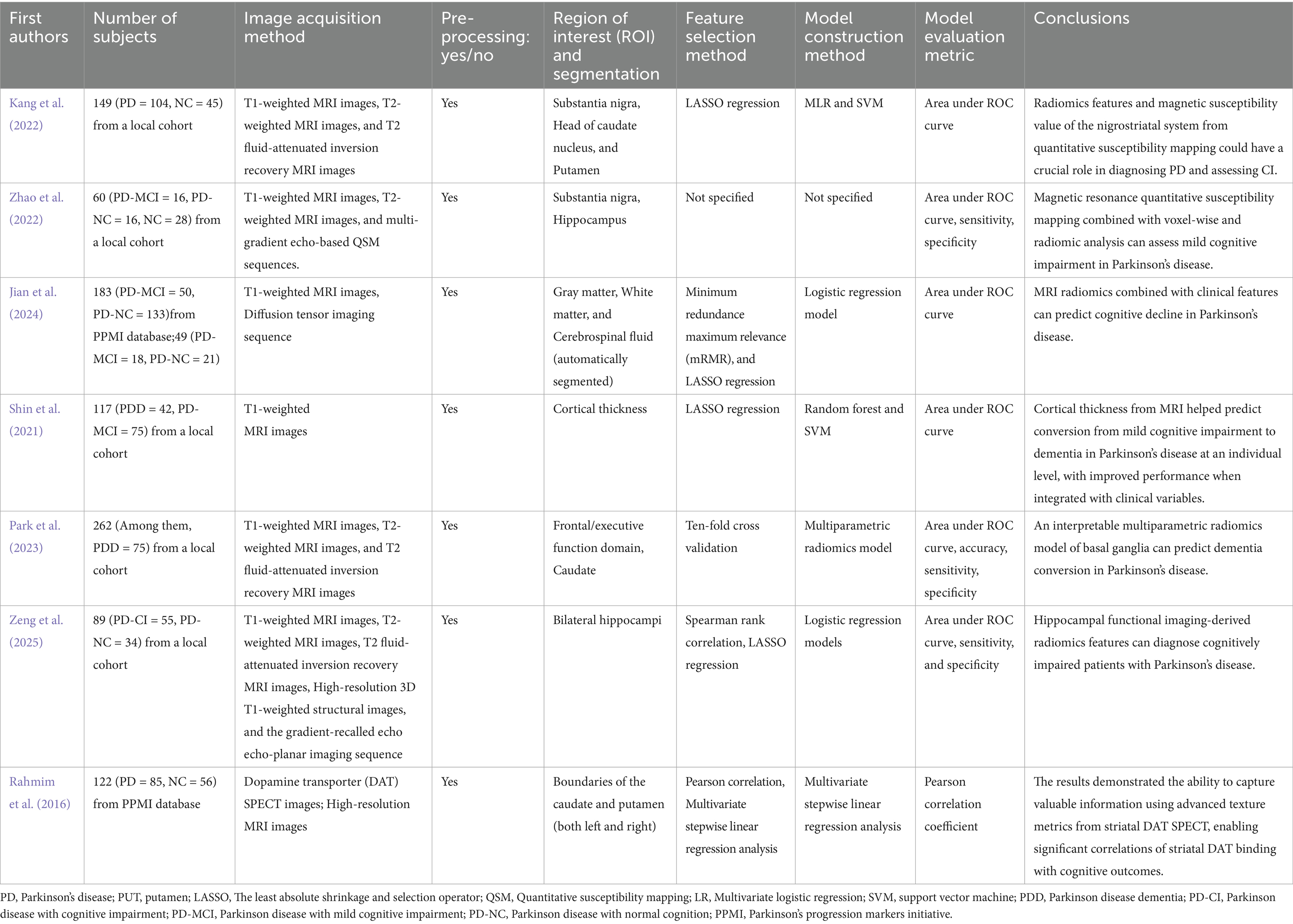
Conventional structural MRI sequences were used in the evaluation of PD related cognitive impairment. Shin et al. (2021) developed a machine-learning-based predictive model to assess the risk of progression to dementia in patients with PD-MCI using cortical thickness measurements based on T1-weighted MR images. Train random forest and SVM models were used to construct the model. The study demonstrated that radiomics features derived from the cortical thickness on conventional structural MRI sequences could predict the progression from MCI to dementia in individual PD patients. More recently, advanced multiparametric radiomics models have further improved predictive accuracy and interpretability. Park et al. (2023) analyzed T1, T2, and T2-FLAIR sequences from basal ganglia MRI and extracted more than 1,200 radiomic features. Their multiparametric radiomics model achieved an AUC of 0.928, identifying key radiomic features associated with dementia progression.
Increased iron deposition in the SN system is one of the most common features of PD. Evidence suggests that cognitive impairment is often secondary to abnormal brain tissue iron deposition and this mechanism may also contribute to the pathology of PDD (Uchida et al., 2019). Therefore, imaging markers that quantify iron deposition have received increasing attention in recent years (An et al., 2018). Magnetic susceptibility value (MSV) derived from quantitative susceptibility mapping (QSM) can quantify iron deposition while radiomics can analyze the distribution of iron deposition. Kang et al. (2022) explored whether MSV and radiomics features could serve as imaging markers for the objective assessment of CI in PD patients. The study used SN, head of the caudate nucleus (HCN), and putamen as the ROI. Multivariate logistic regression and SVM models were developed. The study revealed that characteristics including mean absolute difference, variance, and gray-scale variance of the HCN in PD patients were negatively correlated with the Montreal Cognitive Assessment (MoCA) scores. Similar to the previous study, Zhao et al. (2022) used QSM to evaluate iron deposition and microstructural changes in 60 patients with PD (16 with MCI). They performed voxel-based and radiomic analysis of subcortical nuclei (substantia nigra, basal ganglia) to extract texture and susceptibility-related features. A SVM classifier was able to distinguish cognitively normal PD patients from PD-MCI ones with 83% accuracy, suggesting that QSM-based radiomics represents a sensitive biomarker for early cognitive impairment in PD.
Diffusion tensor imaging (DTI) scan was also used in the prediction of PD-MCI and PDD. In a multicenter study conducted by Jian et al. (2024), the investigators developed an MRI-based radiomics model based on T1-weighted and DTI. They extracted 3,396 radiomic features focusing on gray and white matter regions. The feature selection method was LASSO regression and the model construction method was random forest model. The model achieved an AUC of 0.86. Zeng et al. (2025) investigated hippocampal functional imaging with resting-state fMRI in 89 PD patients (55 with cognitive impairment). Their research results suggest the logistic regression model achieved 88.9% accuracy, emphasizing hippocampal functional connectivity detected by resting-state fMRI as a key predictor of cognitive decline of PD patients.
Beyond structural changes, cognitive impairment in PD has also been linked to disruptions in specific neural circuits. Previous research suggests that CI is associated with dysfunction in frontal-striatal circuits, frontal-basal ganglia circuits, and frontal-thalamic circuits in patients with PD (Apostolova et al., 2010; Kim et al., 2012). Supporting this, functional and molecular imaging studies have further explored radiomic correlates of CI. For instance, Rahmim et al. used single-photon emission computed tomography (SPECT) radiomic features to investigate the correlation between striatal dopamine transporter and PD-MCI (Rahmim et al., 2016). This study suggested that SPECT could also be used as the tool for objectively assessing CI in PD patients (Table 2 presents a summary of the above studies).
In conclusion, radiomics shows promise in predicting cognitive impairment in PD, capturing subtle brain changes and enhancing diagnostic precision. Integration with clinical data improves predictive accuracy, but challenges persist. Model heterogeneity and lack of interpretability hinder clinical adoption. Future research should address these issues to develop robust, interpretable, and generalizable radiomics models for PD cognitive impairment prediction.
5 Advances in radiomics for predicting cognitive impairment associated with cerebrovascular disease
As life expectancy continues to increase, age-related diseases have become one of the most significant health challenges worldwide. Cerebral small vessel disease (CSVD) is a common age-related condition that affects 80% of the elderly population are suffering (Zhang et al., 2023). CSVD is recognized as one of the major causes of stroke and vascular cognitive dysfunction, significantly impacting patients’ quality of life (Cannistraro et al., 2019). The pathogenesis of CSVD-related cognitive dysfunction remains incompletely understood, however, early intervention can delay or even reverse its progression (Ngandu et al., 2015).
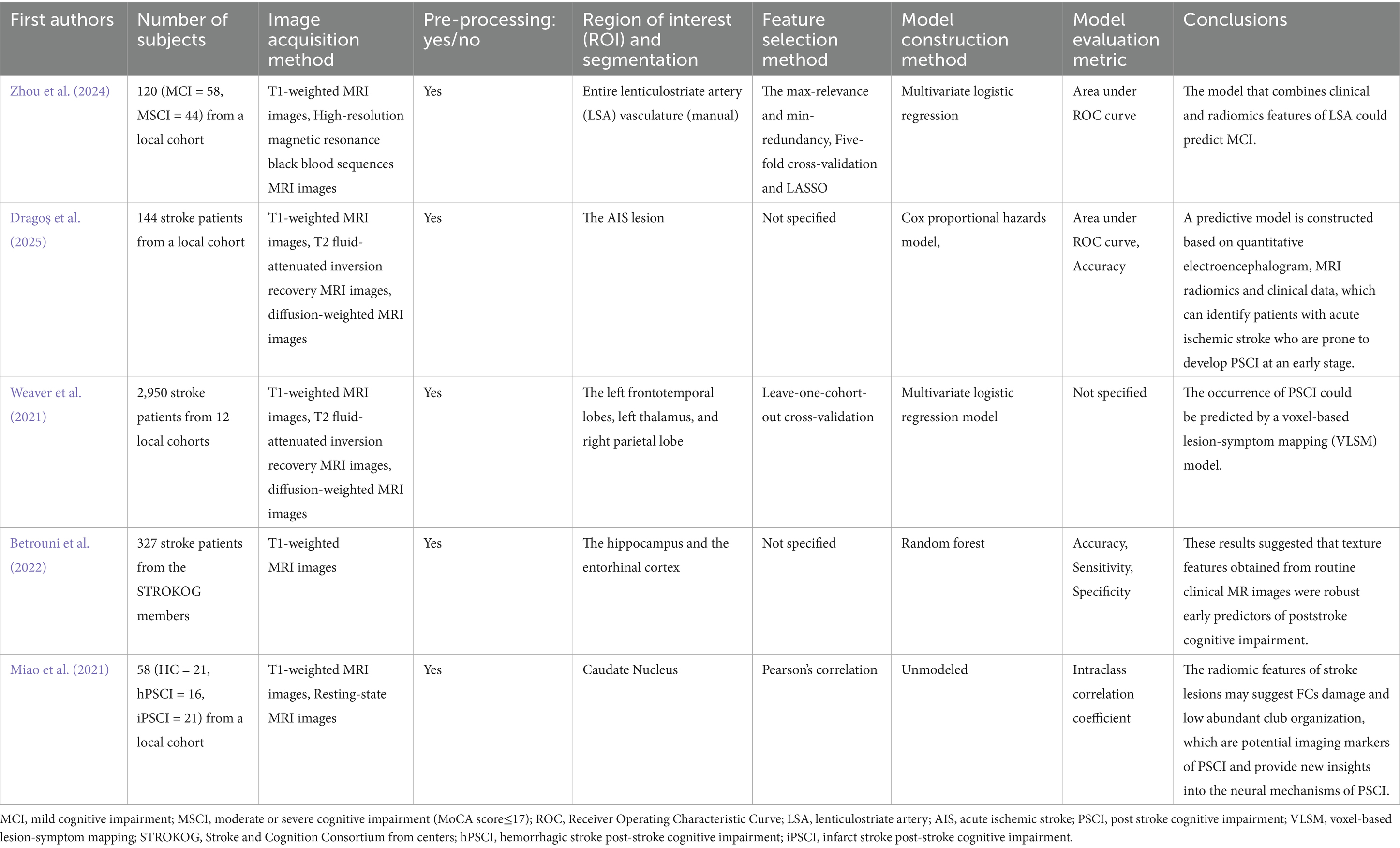
With advances in neuroimaging techniques, multiparametric MRI plays a crucial in the early diagnosis of CSVD-related cognitive dysfunction. These techniques can non-invasively assess neuroimaging indices of brain structure, function, perfusion, and metabolism. Comprehensive analysis of multiparametric MRI data using machine learning can facilitate accurate diagnosis and prognostic assessment of CSVD at the individual level. Türe et al. (2000) reported that the majority of pathological changes associated with CSVD occur in the basal ganglia and corona radiata regions supplied by the lenticulostriate artery (LSA). These regions affect basic motor, sensory, and visual functions. Additionally, a recent study (Decavel et al., 2012) reported that cognitive deficits occur in approximately 30–80% of LSA infarction cases, necessitating analysis of the potential relationship between LSA and cognitive deficits. Zhou et al. (2024) collected data from 102 CSVD patients with cognitive dysfunction and developed a model that combined LSA radiomics with clinical features using high-resolution magnetic resonance black blood sequences, which aimed to analyze the potential relationship between corresponding LSA imaging markers and cognitive dysfunction. The study selected four clinical features (left hemisphere stems, left hemisphere branches, bilateral stems, total vessel count) and six wavelet transform features using LASSO regression analysis. Three models were constructed based on clinical features, radiomics features, and combined clinical-radiomics features, respectively. The study demonstrated that the combined model achieved the best predictive performance, with the radiomics model also outperformed the clinical model.
Post-stroke cognitive impairment (PSCI) is a major component of stroke sequelae (Pantoni, Salvadori, 2021), which includes ischemic PSCI (iPSCI) and hemorrhagic PSCI (hPSCI) each with distinct pathogenesis. iPSCI stems from ischemic cerebral infarction disrupting the cortico-subcortical network, particularly in the frontal-executive circuit. In contrast, hPSCI results from direct tissue destruction via hematoma mass effect with secondary iron-mediated neurotoxicity, neuroinflammation and subarachnoid hemorrhage (SAH)-associated delayed cerebral ischemia. Despite their distinct mechanisms, both iPSCI and hPSCI represent critical forms of post-stroke cognitive dysfunction requiring early intervention to prevent irreversible decline. Studies have shown that the interval between the onset of stroke and PSCI development represents a therapeutic window for early intervention to preserve cognitive function (Brainin et al., 2015). Therefore, identifying reliable predictors of PSCI is essential for improving long-term stroke outcomes. For iPSCI, radiomics analysis of white matter integrity can capture subtle microstructural changes prior to cognitive decline (Habes et al., 2016). In hPSCI, perihematoma lesion heterogeneity quantified through CT/MRI radiomics (Haider et al., 2023) may predict the risk of iron-mediated neurotoxicity. Such imaging biomarkers may enable targeted interventions during this vulnerable stage, ultimately improving long-term cognitive outcomes.
The texture features derived from routine clinical MR images can represent robust early predictors of PSCI. Betrouni et al. (2022) analyzed texture features of the hippocampus and entorhinal cortex to predict cognitive status 6 months post-stroke using a random forest model based on T1-weighted images. The model achieved an accuracy of 0.90 ± 0.05, sensitivity was 0.92 ± 0.04, specificity was 0.93 ± 0.02, and AUC for the subject’s working characteristics was 0.90 ± 0.03 in the study.
Several studies have leveraged radiomics and functional connectivity (FC) analyses to uncover early predictive markers of PSCI. Dragoș et al. (2025) enrolled 144 patients to investigate the predictive role of FC and MRI radiomic markers in evaluating PSCI 1 year after acute ischemic stroke (AIS). The study employed MRI and electroencephalogram (EEG) processing techniques to assess brain and cognitive reserve. By integrating these measures with predictive models based on quantitative EEG (QEEG), MRI radiomics, and clinical data, the study sought to identify early predictive factors for PSCI using Cox proportional hazards models. The integration of FC and radiomics biomarkers offers potential for enhanced predictive accuracy, possibly facilitating more effective therapeutic approaches for PSCI. Besides, Weaver et al. (2021) conducted a large multicohort study including 2,950 patients were enrolled, with MRI imaging data and cognitive assessments were obtained up to 15 months after AIS onset. The study selected the infarcts in the left frontotemporal lobe, right parietal lobe, and left thalamus as the ROI and got the conclusion that the occurrence of PSCI could be predicted using a voxel-based lesion-symptom mapping (VLSM) model. This study underscores the importance of lesion location in predicting PSCI, complementing the findings of Dragoș et al. by emphasizing the structural correlates of PSCI. Miao et al. (2021) combined resting-state functional magnetic resonance imaging (rs-fMRI) with radiomics features to investigate FC of low-degree rich club organization and the caudate nucleus. The results revealed that changes in functional connectivity of low-degree rich club organization and the caudate nucleus were correlated with 3D shape features and first-order statistics of stroke lesions. These radiomic features may indicate disruption of FC and low-degree rich club organization, serving as potential imaging markers of PSCI and providing new insights into its neural mechanisms (Table 3 presents a summary of the above studies).
These studies demonstrate the potential of radiomics as a robust tool for predicting cognitive outcomes following stroke. By leveraging multiparametric MRI and machine learning, radiomics models can identify early predictive markers of cognitive dysfunction. The integration of radiomics with functional connectivity and clinical data enhances predictive accuracy, providing insights into the neural mechanisms underlying cognitive decline. However, challenges remain, including the need for larger, diverse cohorts to validate the findings and the complexity of interpreting radiomics features in the clinical background.
6 Discussion
The above studies highlight the potential of radiomics to extract high-dimensional quantitative features from medical images. The recent studies integrate radiomics with clinical and neuropsychological data, thereby enhance the predictive accuracy of the models. This multidimensional approach allows for a more comprehensive assessment of cognitive decline, capturing both imaging-derived and clinical risk factors. The application of machine learning techniques in radiomics analysis enables the development of sophisticated predictive models. These models can identify complex patterns and relationships within the data, facilitating early diagnosis and risk stratification of cognitive impairment.
The existing studies also exhibit several limitations. First, significant heterogeneity exists in the imaging protocols, patient populations, and cognitive assessment tools employed across studies. Standardization of imaging protocols and cognitive assessments is essential to enhance the comparability of radiomics-based studies. Second, many studies are limited by small sample sizes, which may diminish both statistical power and generalizability of the findings. Larger, prospective studies are needed to validate the predictive value of radiomics biomarkers. Third, while little studies employ longitudinal follow-up, many existing researches are cross-sectional, thereby restrict the ability to understand the dynamic changes of cognitive decline. Fourth, despite the high predictive accuracy of radiomics models, their interpretability remains challenging. Future researches should focus on developing interpretable radiomics models that can be easily integrated into clinical practice.
7 Conclusion
The strength of radiomics lies in its capacity to systematically and efficiently extract complex information from medical images, thereby providing novel perspectives and valuable tools for the realms of medical diagnosis and disease management. Radiomics-derived imaging biomarkers, particularly those targeting cognitive dysfunction, represent important clinical applications. These biomarkers enable physicians to efficiently differentiate patients with cognitive disorders across a spectrum of neurological conditions, facilitating timely and appropriate interventions. Nevertheless, the field of radiomics continues to face several challenges, including index standardization and normalization, limited sample sizes, and data consistency of data across multicenter studies. Therefore, further research and technological advancement are required to enhance the effectiveness and reliability of radiomics in cognitive impairment research.
Author contributions
HX: Conceptualization, Methodology, Writing – original draft. XH: Methodology, Writing – original draft. WZ: Formal analysis, Writing – original draft. XG: Formal analysis, Writing – review & editing. XC: Writing – review & editing. TL: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by Jiangsu Young Talent Program (JSSA2024YB03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Brønnick, K., Larsen, J. P., Tysnes, O. B., and Alves, G. (2009). Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72, 1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb
Aarsland, D., Creese, B., Politis, M., Chaudhuri, K. R., Ffytche, D. H., Weintraub, D., et al. (2017). Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 13, 217–231. doi: 10.1038/nrneurol.2017.27
Alzheimer’s Association (2016). 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 12, 459–509. doi: 10.1016/j.jalz.2016.03.001
An, H., Zeng, X., Niu, T., Li, G., Yang, J., Zheng, L., et al. (2018). Quantifying iron deposition within the substantia nigra of Parkinson's disease by quantitative susceptibility mapping. J. Neurol. Sci. 386, 46–52. doi: 10.1016/j.jns.2018.01.008
Apostolova, L. G., Beyer, M., Green, A. E., Hwang, K. S., Morra, J. H., Chou, Y. Y., et al. (2010). Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov. Disord. 25, 687–695. doi: 10.1002/mds.22799
Bera, K., Braman, N., Gupta, A., Velcheti, V., and Madabhushi, A. (2022). Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19, 132–146. doi: 10.1038/s41571-021-00560-7
Betrouni, N., Jiang, J., Duering, M., Georgakis, M. K., Oestreich, L., Sachdev, P. S., et al. (2022). Texture features of magnetic resonance images predict Poststroke cognitive impairment: validation in a multicenter study. Stroke 53, 3446–3454. doi: 10.1161/strokeaha.122.039732
Brainin, M., Tuomilehto, J., Heiss, W. D., Bornstein, N. M., Bath, P. M., Teuschl, Y., et al. (2015). Post-stroke cognitive decline: an update and perspectives for clinical research. Eur. J. Neurol. 22:e213-226, 229–238. doi: 10.1111/ene.12626
Cannistraro, R. J., Badi, M., Eidelman, B. H., Dickson, D. W., Middlebrooks, E. H., and Meschia, J. F. (2019). CNS small vessel disease: a clinical review. Neurology 92, 1146–1156. doi: 10.1212/wnl.0000000000007654
Cardenas, C. E., Yang, J., Anderson, B. M., Court, L. E., and Brock, K. B. (2019). Advances in auto-segmentation. Semin. Radiat. Oncol. 29, 185–197. doi: 10.1016/j.semradonc.2019.02.001
Chen, Y., Qi, Y., Hu, Y., Qiu, X., Qiu, T., Li, S., et al. (2025). Integrated cerebellar radiomic-network model for predicting mild cognitive impairment in Alzheimer's disease. Alzheimers Dement. 21:e14361. doi: 10.1002/alz.14361
Cheung, E. Y. W., Chau, A. C. M., and Tang, F. H.On Behalf Of The Alzheimer's Disease Neuroimaging, I (2022). Radiomics-based artificial intelligence differentiation of neurodegenerative diseases with reference to the volumetry. Life (Basel). 12:514. doi: 10.3390/life12040514
Chu, Y., Hirst, W. D., Federoff, H. J., Harms, A. S., Stoessl, A. J., and Kordower, J. H. (2024). Nigrostriatal tau pathology in parkinsonism and Parkinson's disease. Brain 147, 444–457. doi: 10.1093/brain/awad388
Decavel, P., Vuillier, F., and Moulin, T. (2012). Lenticulostriate infarction. Front. Neurol. Neurosci. 30, 115–119. doi: 10.1159/000333606
Dragoș, H. M., Stan, A., Popa, L. L., Pintican, R., Feier, D., Drăghici, N. C., et al. (2025). Functional connectivity and MRI Radiomics biomarkers of cognitive and brain Reserve in Post-Stroke Cognitive Impairment Prediction-a Study Protocol. Life (Basel) 15:131. doi: 10.3390/life15010131
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., et al. (2016). Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323. doi: 10.1016/j.jalz.2016.02.002
Feng, Q., Chen, Y., Liao, Z., Jiang, H., Mao, D., Wang, M., et al. (2018). Corpus callosum radiomics-based classification model in Alzheimer's disease: a case-control study. Front. Neurol. 9:618. doi: 10.3389/fneur.2018.00618
Gillies, R. J., Kinahan, P. E., and Hricak, H. (2016). Radiomics: images are more than pictures, they are data. Radiology 278, 563–577. doi: 10.1148/radiol.2015151169
Habes, M., Erus, G., Toledo, J. B., Zhang, T., Bryan, N., Launer, L. J., et al. (2016). White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 139, 1164–1179. doi: 10.1093/brain/aww008
Haider, S. P., Qureshi, A. I., Jain, A., Tharmaseelan, H., Berson, E. R., Zeevi, T., et al. (2023). Radiomic markers of intracerebral hemorrhage expansion on non-contrast CT: independent validation and comparison with visual markers. Front. Neurosci. 17:1225342. doi: 10.3389/fnins.2023.1225342
Han, Y., Zhou, A., Li, F., Wang, Q., Xu, L., and Jia, J. (2020). Apolipoprotein E ε4 allele is associated with vascular cognitive impairment no dementia in Chinese population. J. Neurol. Sci. 409:116606. doi: 10.1016/j.jns.2019.116606
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Jian, Y., Peng, J., Wang, W., Hu, T., Wang, J., Shi, H., et al. (2024). Prediction of cognitive decline in Parkinson's disease based on MRI radiomics and clinical features: a multicenter study. CNS Neurosci. Ther. 30:e14789. doi: 10.1111/cns.14789
Jiang, J., Wang, M., Alberts, I., Sun, X., Li, T., Rominger, A., et al. (2022). Using radiomics-based modelling to predict individual progression from mild cognitive impairment to Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging 49, 2163–2173. doi: 10.1007/s00259-022-05687-y
Kang, J. J., Chen, Y., Xu, G. D., Bao, S. L., Wang, J., Ge, M., et al. (2022). Combining quantitative susceptibility mapping to radiomics in diagnosing Parkinson's disease and assessing cognitive impairment. Eur. Radiol. 32, 6992–7003. doi: 10.1007/s00330-022-08790-8
Kim, J. S., Oh, Y. S., Lee, K. S., Kim, Y. I., Yang, D. W., and Goldstein, D. S. (2012). Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 79, 1323–1331. doi: 10.1212/WNL.0b013e31826c1acd
Kumar, V., Gu, Y., Basu, S., Berglund, A., Eschrich, S. A., Schabath, M. B., et al. (2012). Radiomics: the process and the challenges. Magn. Reson. Imaging 30, 1234–1248. doi: 10.1016/j.mri.2012.06.010
Lambin, P., Leijenaar, R. T. H., Deist, T. M., Peerlings, J., de Jong, E. E. C., van Timmeren, J., et al. (2017). Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762. doi: 10.1038/nrclinonc.2017.141
Lambin, P., Rios-Velazquez, E., Leijenaar, R., Carvalho, S., van Stiphout, R. G., Granton, P., et al. (2012). Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48, 441–446. doi: 10.1016/j.ejca.2011.11.036
Langa, K. M., and Levine, D. A. (2014). The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312, 2551–2561. doi: 10.1001/jama.2014.13806
Lin, S., Xue, M., Sun, J., Xu, C., Wang, T., Lian, J., et al. (2025). MRI Radiomics nomogram for predicting disease transition time and risk stratification in preclinical Alzheimer's disease. Acad. Radiol. 32, 951–962. doi: 10.1016/j.acra.2024.08.059
Luk, C. C., Ishaque, A., Khan, M., Ta, D., Chenji, S., Yang, Y. H., et al. (2018). Alzheimer's disease: 3-dimensional MRI texture for prediction of conversion from mild cognitive impairment. Alzheimers Dement (Amst) 10, 755–763. doi: 10.1016/j.dadm.2018.09.002
Miao, G., Rao, B., Wang, S., Fang, P., Chen, Z., Chen, L., et al. (2021). Decreased functional connectivities of low-degree level rich club organization and caudate in post-stroke cognitive impairment based on resting-state fMRI and radiomics features. Front. Neurosci. 15:796530. doi: 10.3389/fnins.2021.796530
Mueller, S. G., Weiner, M. W., Thal, L. J., Petersen, R. C., Jack, C. R., Jagust, W., et al. (2005). Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's disease neuroimaging Initiative (ADNI). Alzheimers Dement. 1, 55–66. doi: 10.1016/j.jalz.2005.06.003
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/s0140-6736(15)60461-5
Pan, Y., Liu, S., Zeng, Y., Ye, C., Qiao, H., Song, T., et al. (2022). A multi-atlas-based [18F]9-Fluoropropyl-(+)-Dihydrotetrabenazine positron emission tomography image segmentation method for Parkinson's disease quantification. Front. Aging Neurosci. 14:902169. doi: 10.3389/fnagi.2022.902169
Pantoni, L., and Salvadori, E. (2021). Location of infarcts and post-stroke cognitive impairment. Lancet Neurol. 20, 413–414. doi: 10.1016/s1474-4422(21)00107-1
Park, C. J., Eom, J., Park, K. S., Park, Y. W., Chung, S. J., Kim, Y. J., et al. (2023). An interpretable multiparametric radiomics model of basal ganglia to predict dementia conversion in Parkinson's disease. NPJ Parkinsons Dis. 9:127. doi: 10.1038/s41531-023-00566-1
Parmar, C., Rios Velazquez, E., Leijenaar, R., Jermoumi, M., Carvalho, S., Mak, R. H., et al. (2014). Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One 9:e102107. doi: 10.1371/journal.pone.0102107
Pedersen, K. F., Larsen, J. P., Tysnes, O. B., and Alves, G. (2013). Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 70, 580–586. doi: 10.1001/jamaneurol.2013.2110
Puzio, T., Matera, K., Karwowski, J., Piwnik, J., Białkowski, S., Podyma, M., et al. (2025). Deep learning-based automatic segmentation of brain structures on MRI: a test-retest reproducibility analysis. Comput. Struct. Biotechnol. J. 28, 128–140. doi: 10.1016/j.csbj.2025.04.007
Rahmim, A., Salimpour, Y., Jain, S., Blinder, S. A., Klyuzhin, I. S., Smith, G. S., et al. (2016). Application of texture analysis to DAT SPECT imaging: relationship to clinical assessments. Neuroimage Clin. 12, e1–e9. doi: 10.1016/j.nicl.2016.02.012
Ranjbar, S., Velgos, S. N., Dueck, A. C., Geda, Y. E., and Mitchell, J. R. (2019). Brain MR radiomics to differentiate cognitive disorders. J. Neuropsychiatry Clin. Neurosci. 31, 210–219. doi: 10.1176/appi.neuropsych.17120366
Rathore, S., Habes, M., Iftikhar, M. A., Shacklett, A., and Davatzikos, C. (2017). A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer's disease and its prodromal stages. NeuroImage 155, 530–548. doi: 10.1016/j.neuroimage.2017.03.057
Reuben, D. B., Kremen, S., and Maust, D. T. (2024). Dementia prevention and treatment: a narrative review. JAMA Intern. Med. 184, 563–572. doi: 10.1001/jamainternmed.2023.8522
Shin, N. Y., Bang, M., Yoo, S. W., Kim, J. S., Yun, E., Yoon, U., et al. (2021). Cortical thickness from MRI to predict conversion from mild cognitive impairment to dementia in Parkinson disease: a machine learning-based model. Radiology 300, 390–399. doi: 10.1148/radiol.2021203383
Sørensen, L., Igel, C., Pai, A., Balas, I., Anker, C., Lillholm, M., et al. (2017). Differential diagnosis of mild cognitive impairment and Alzheimer's disease using structural MRI cortical thickness, hippocampal shape, hippocampal texture, and volumetry. Neuroimage Clin. 13, 470–482. doi: 10.1016/j.nicl.2016.11.025
Tang, L., Wu, X., Liu, H., Wu, F., Song, R., Zhang, W., et al. (2021). Individualized Prediction of Early Alzheimer’s Disease Based on Magnetic Resonance Imaging Radiomics, Clinical, and Laboratory Examinations. A 60-Month Follow-Up Study. J Magn Reson Imaging 54, 1647–1657. doi: 10.1002/jmri.27689
Türe, U., Yaşargil, M. G., Al-Mefty, O., and Yaşargil, D. C. (2000). Arteries of the insula. J. Neurosurg. 92, 676–687. doi: 10.3171/jns.2000.92.4.0676
Uchida, Y., Kan, H., Sakurai, K., Arai, N., Kato, D., Kawashima, S., et al. (2019). Voxel-based quantitative susceptibility mapping in Parkinson's disease with mild cognitive impairment. Mov. Disord. 34, 1164–1173. doi: 10.1002/mds.27717
van Timmeren, J. E., Cester, D., Tanadini-Lang, S., Alkadhi, H., and Baessler, B. (2020). Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging 11:91. doi: 10.1186/s13244-020-00887-2
Weaver, N. A., Kuijf, H. J., Aben, H. P., Abrigo, J., Bae, H. J., Barbay, M., et al. (2021). Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol. 20, 448–459. doi: 10.1016/s1474-4422(21)00060-0
Yarnall, A. J., Breen, D. P., Duncan, G. W., Khoo, T. K., Coleman, S. Y., Firbank, M. J., et al. (2014). Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82, 308–316. doi: 10.1212/wnl.0000000000000066
Yu, Y., Li, G. F., Tan, W. X., Qu, X. Y., Zhang, T., Hou, X. Y., et al. (2025). Towards automatical tumor segmentation in radiomics: a comparative analysis of various methods and radiologists for both region extraction and downstream diagnosis. BMC Med. Imaging 25:63. doi: 10.1186/s12880-025-01596-2
Zeng, W., Liang, X., Guo, J., Cheng, W., Yin, Z., Hong, D., et al. (2025). Hippocampal functional imaging-derived radiomics features for diagnosing cognitively impaired patients with Parkinson's disease. BMC Neurosci. 26:27. doi: 10.1186/s12868-025-00938-8
Zhang, W., Cheng, Z., Fu, F., and Zhan, Z. (2023). Prevalence and clinical characteristics of white matter hyperintensities in migraine: a meta-analysis. Neuroimage Clin. 37:103312. doi: 10.1016/j.nicl.2023.103312
Zhang, X., Zhang, Y., Zhang, G., Qiu, X., Tan, W., Yin, X., et al. (2022). Deep learning with Radiomics for disease diagnosis and treatment: challenges and potential. Front. Oncol. 12:773840. doi: 10.3389/fonc.2022.773840
Zhao, Y., Qu, H., Wang, W., Liu, J., Pan, Y., Li, Z., et al. (2022). Assessing mild cognitive impairment in Parkinson's disease by magnetic resonance quantitative susceptibility mapping combined voxel-wise and Radiomic analysis. Eur. Neurol. 85, 280–290. doi: 10.1159/000522329
Zhou, L., Wu, H., and Zhou, H. (2024). Correlation between cognitive impairment and Lenticulostriate arteries: a clinical and Radiomics analysis. J. Imaging. Inform. Med. 37, 1261–1272. doi: 10.1007/s10278-024-01060-7
Keywords: cognitive impairment, radiomics, Alzheimer’s disease, Parkinson’s disease, cerebral small vessel disease, stroke
Citation: Xiao H, He X, Zhou W, Guo X, Cai X and Li T (2025) The application of radiomics in the diagnosis and evaluation of cognitive impairment related to neurological diseases. Front. Neurosci. 19:1591605. doi: 10.3389/fnins.2025.1591605
Edited by:
Tao Xu, Northwestern Polytechnical University, ChinaReviewed by:
Dang Chun, Sichuan University, ChinaS. Sivaranjini, SRM Institute of Science and Technology, India
Copyright © 2025 Xiao, He, Zhou, Guo, Cai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tan Li, NTA0NTM1NDczQHFxLmNvbQ==; Xiuying Cai, Y3h5OTk5MDg4OEAxNjMuY29t
†These authors have contributed equally to this work
 Haixing Xiao
Haixing Xiao Xinyi He1†
Xinyi He1† Xianghong Guo
Xianghong Guo Tan Li
Tan Li