- 1Department of Anesthesiology, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 2Anesthesiology and Critical Care Medicine Key Laboratory of Luzhou, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 3Department of Anesthesiology, Hejiang County People’s Hospital, Luzhou, Sichuan, China
- 4Department of Traditional Chinese Medicine, The Affiliated Hospital, Southwest Medical University, Luzhou, China
Sepsis is defined as a condition of immune dysregulation in response to an infection, and sepsis-associated encephalopathy (SAE) is often the initial symptom that manifests in patients with sepsis. This condition is characterized by its high mortality rates and the potential to cause significant disability among survivors. Despite its severity, the underlying pathophysiologic mechanisms that contribute to the development of SAE are not yet fully understood. Additionally, there are no established strict diagnostic criteria or potent treatment options available for this condition. However, an increasing body of evidence suggests that an imbalance in the gut microbiota is associated with SAE, potentially through the gut-brain axis (GBA). The GBA axis refers to the bidirectional communication between the gut microbiota and the central nervous system. In this review, we discuss the changes in the gut microbiota in SAE and the mechanisms of the GBA axis, involving neural, immune, endocrine, and neurotransmitter pathways. Finally, we conclude by evaluating the preclinical and clinical evidence for fecal microbiota transplantation and probiotics in SAE. Targeting the GBA axis will be an actionable target to ameliorate the development and progression of SAE.
1 Introduction
Sepsis is defined as life-threatening multiorgan dysfunction due to the body’s dysregulated response to infection, and acute brain dysfunction arising from sepsis is termed sepsis-associated encephalopathy (SAE). SAE has the potential to manifest as the initial symptom of sepsis and is observed in as many as 70% of sepsis patients (Martin-Loeches et al., 2024; Manabe and Heneka, 2022; Gofton and Young, 2012). Sepsis contributes to nearly 20% of the annual global mortality, with a rate of over 20 deaths occurring per minute. SAE has been demonstrated to be correlated with elevated mortality ratios, increased consumption of intensive care unit (ICU) resources, and extended hospital lengths of stay (Kempker and Martin, 2020; Sonneville et al., 2017). Therefore, new insights into the pathogenesis of SAE are likely to furnish a novel therapeutic direction.
In the body, the gut microbiota and the brain can communicate bidirectionally via the gut-brain axis (GBA), regulating the development and function of the immune, metabolic, and nervous systems and influencing host behavior (Morais et al., 2021). Dysbiosis of the gut microbiota is not only associated with cognitive changes (Xiang et al., 2024; Zhao et al., 2025; Grabrucker et al., 2023), but also plays a key role in the progression of sepsis (Sun et al., 2023). Gut microbiota dysbiosis increases the risk of sepsis and death, while germ-free mouse models of sepsis showed a high pathogen burden and death rate (Liang et al., 2022). Therefore, understanding the relationship between gut microbiota and SAE is crucial for further understanding the pathogenesis and treatment of SAE.
In this review, we provide an in-depth overview of the alterations in gut microbiota in sepsis and SAE exposure. We also elaborate on the impacts of gut microbiota on multiple aspects, such as impairment of barrier function, disruption of the neuroendocrine system, facilitation of neuroinflammation, induction of metabolic dysregulation, and interference with neurotransmitter function. Furthermore, we engage in a detailed discussion of targeted therapeutic approaches aimed at modulating the gut microbiota.
2 Dysbiosis of the gut microbiota and SAE
Numerous studies have demonstrated that gut microbiota exhibits a remarkable susceptibility to sepsis. A two-sample Mendelian randomization study presented the initial suggestive evidence of a causal link between the beneficial or deleterious impacts of gut microbiota on the risk of sepsis. The findings indicated that an augmented abundance of β – Proteobacteria, Vibrio desulfuricans, Catenibacterium, and Hungatella was inversely correlated with the sepsis risk. In contrast, Clostridiaceae 1, Alloprevotella, the Lachnospiraceae ND3007 group, and Terrisporobacter were potentially identified as risk factors for sepsis (Chen et al., 2023). Besides, the intestinal abundance of enteric microbiota enterotypes or enterococci could potentially function as a biomarker for predicting poor prognosis in ICU patients with sepsis (Kullberg et al., 2021). In addition, antibiotic use, host genetics, and comorbidities can also perturb the gut microbiota of sepsis. For example, the use of antibiotics in septic mice may cause intestinal microecological disorders, resulting in an increase in the inflammation-related pathogenic bacteria Desulfovibrio (Han et al., 2021). In two large retrospective studies, admission to the hospital for an infection-related complication and antibiotic exposure significantly increased the risk for subsequent sepsis-related hospitalization within 90 days of the index hospitalization (Baggs et al., 2018; Prescott et al., 2015). Depletion of commensal anaerobic gut microbes by anti-anaerobic antibiotics influences systemic immunity and is associated with increased mortality in patients with sepsis (Kullberg et al., 2025). These demonstrate that the progression of sepsis is associated with perturbations in the gut microbiota at both compositional and functional levels.
Many studies have shown that the gut microbiota also changes during SAE in preclinical models, which is mainly characterized by a decrease in the species diversity of the gut microbiota, a decrease in the abundance of the dominant groups’ Firmicutes and Bacteroidetes, and an increase in the abundance of Proteobacteria (Table 1). Besides, the separation between sepsis and non-sepsis brain specimens as a group was driven primarily by differences in relative abundance of Haemophilus, Neisseria, and Moraxella species, with brain specimens of individual patients with sepsis dominated by gut-associated taxa, such as Enterobacteriaceae sp. and Bacteroides sp. (Singer et al., 2018). Additionally, studies have shown that patients with biliary tract infections and intestinal infections caused by Staphylococcus aureus, Enterococcus faecium, Acinetobacter spp., Pseudomonas aeruginosa, and Stenotrophomonas maltophilia were more prone to develop SAE (Zhang et al., 2012). Furthermore, another analysis identified coagulase-negative staphylococci as an independent risk factor for SAE (HR = 1.919, P < 0.001), but not for mortality. Methicillin-resistant S. aureus (MRSA) was linked to increased mortality in SAE patients (HR = 3.423, P < 0.001) (Fei et al., 2025). These suggest that there may be an association between gut microbiota dysbiosis and SAE, and that sepsis-induced gut microbiota dysbiosis may be involved in the etiology of SAE (Figure 1).
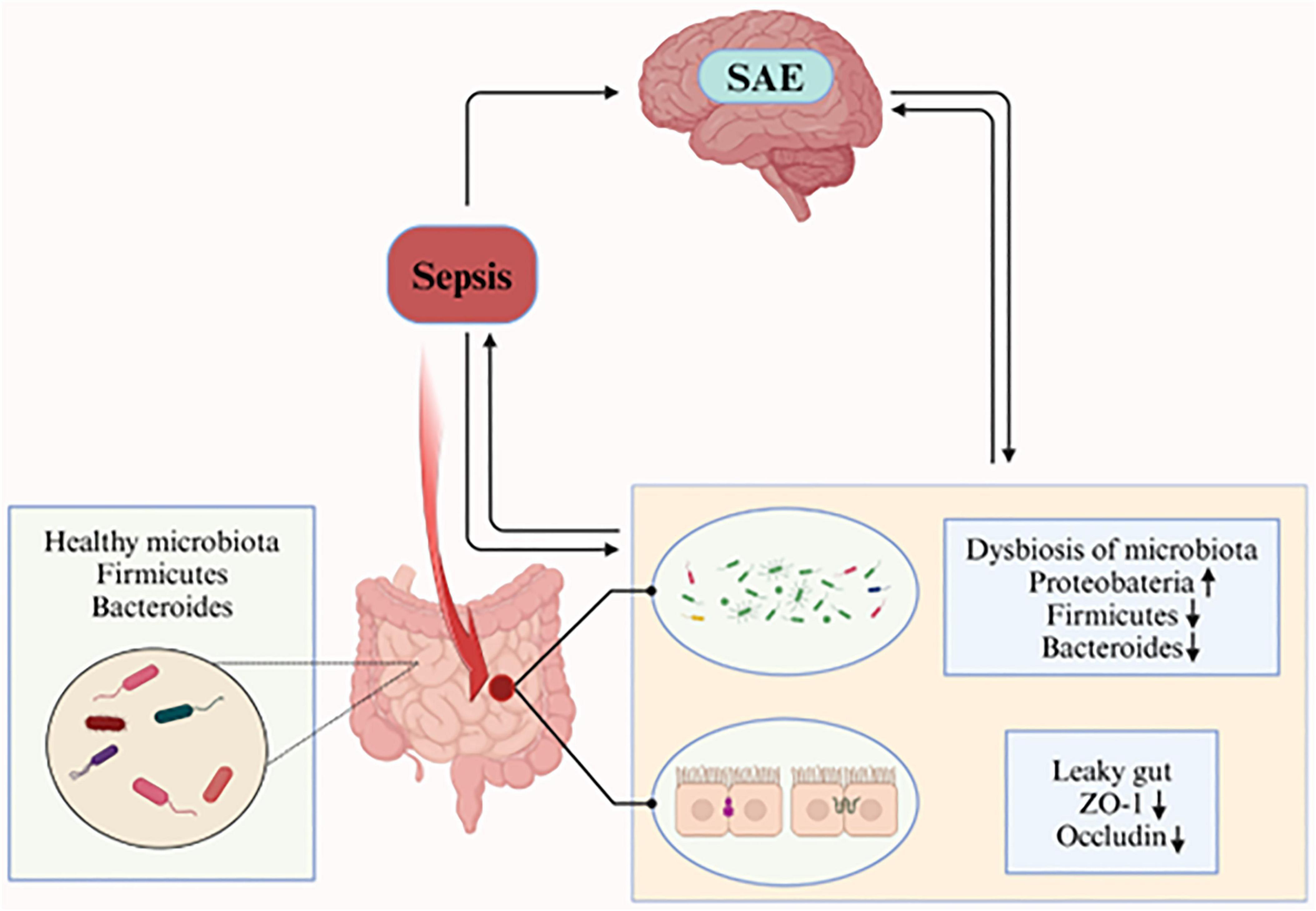
Figure 1. The healthy gut microbiota is dominated by the phyla Bacteroidetes and Firmicutes. During sepsis, gut microbiota composition is perturbed, characterized by increased relative abundance of Proteobacteria and decreased relative abundance of Firmicutes and Bacteroidetes. Concurrently, intestinal epithelial barrier integrity is compromised, with reduced expression of tight junction proteins ZO-1 and occludin (critical components of the epithelial barrier). These result in increased intestinal permeability (“leaky gut”), which exacerbates systemic inflammation, thereby leading to the occurrence of SAE. (Created in https://BioRender.com.)
3 Crosstalk between gut microbiota dysbiosis and SAE
Current studies have demonstrated that SAE is a multifactorial disease, and its pathogenesis potentially involves blood–brain barrier (BBB) disruption, neuroinflammation, microcirculatory dysfunction, neurotransmitter imbalance, and mitochondrial dysfunction (Manabe and Heneka, 2022; Pan et al., 2022; Krzyzaniak et al., 2023; Catarina et al., 2021; Huang et al., 2021; Iacobone et al., 2009). Previous studies indicate that gut microbiota exerts a significant effect on neurodevelopment and cognitive function through the GBA axis. Its abnormal changes are closely associated with many neurological disorders, especially cognitive dysfunction (Xiao et al., 2022; Sampson et al., 2016; Matheson and Holsinger, 2023). As described above, gut microbiota’s species and functions change significantly in SAE. Therefore, we reviewed the possible pathogenesis of gut microbiota dysbiosis involved in SAE by regulating the GBA axis. This review aims to offer insights for better SAE diagnosis and treatment.
3.1 Gut microbiota dysbiosis and barrier dysfunction
Loss of BBB integrity is a key cause of SAE and subsequent systemic damage (Gu et al., 2021) (Figure 2). The BBB consists of cerebrovascular endothelial cells (BECs), astrocytes, pericytes, and extracellular matrix, and this structure prevents entry of neurotoxic plasma components, blood cells, and pathogens into the brain (Obermeier et al., 2013; Sweeney et al., 2019). The BECs of the BBB are unique because of their continuous intercellular tight junctions (TJs) (Obermeier et al., 2013). Gut microbiota dysbiosis not only produces neurotoxic factors but also affects the permeability of the intestinal mucosa, leading to “leaky gut.” Inflammation caused by “leaky gut” eventually leads to “leaky brain,” i.e., increased permeability of the BBB (Lu J. et al., 2022; Hu et al., 2016). MRI imaging in SAE models reveals significant cerebral edema (Towner et al., 2018; Bozza et al., 2010). Additionally, reduced expression of the TJ proteins ZO-1 and occludin was found in brain tissues in a mouse model of SAE (Li et al., 2023). Meanwhile, disruption of the BBB is found to be associated with a loss of cerebral endothelial expression of occludin in autopsies of sepsis-related deaths (Erikson et al., 2020). Increased BBB permeability, loss of TJ proteins, and endothelial cell degeneration allow a large number of inflammatory factors and neurotoxins to enter and damage brain tissue. This process activates brain immune cells to mediate inflammatory responses, further exacerbating BBB disruption and ultimately leading to the progression of SAE (Gu et al., 2021; Sweeney et al., 2019; Lu J. et al., 2022).
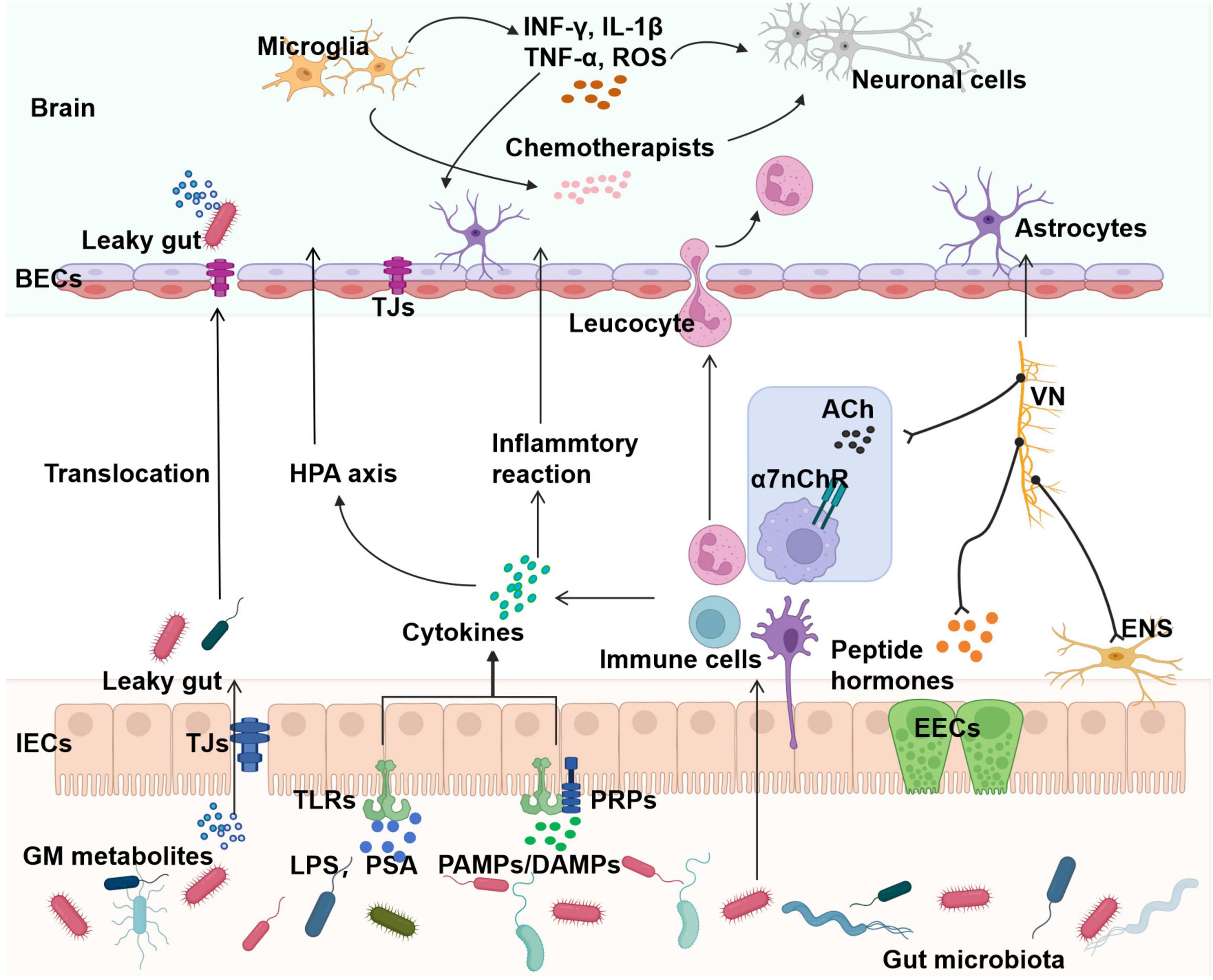
Figure 2. Involvement of gut microbiota in the pathogenesis of SAE. Gut microbiota dysbiosis drives SAE pathogenesis through four interconnected pathways: I. Barrier dysfunction: sepsis alters gut microbiota/metabolites, increasing intestinal and BBB permeability via reduced tight junctions (ZO-1 and occludin), allowing pathogen translocation into systemic and CNS circulation. II. Neuroendocrine axis dysregulation: gut microbiota produce hormones (e.g., serotonin) that modulate intestinal metabolism and brain function via the vagus nerve and the cholinergic anti-inflammatory pathway. III. Immune dysregulation: dysbiosis triggers systemic inflammation (TNF-α and IL-6), which crosses the BBB to activate CNS microglia/astrocytes, amplifying neuroinflammation via reactive ROS. IV. Metabolic/neurotransmitter disorders: altered microbial metabolites (e.g., SCFAs) exacerbate barrier dysfunction and disrupt neurotransmitter balance (e.g., serotonin and dopamine). Collectively, these mechanisms position the gut microbiota as a key therapeutic target for SAE, emphasizing the need for precision microbiome interventions. ROS, reactive oxygen species; BECs, cerebrovascular endothelial cells; TJs, tight junctions; HPA, hypothalamic-pituitary-adrenal; VN, vagus nerve; CAP, cholinergic anti-inflammatory pathway; IECs, intestinal epithelial cells; EECs, enteroendocrine cells; TLRs, Toll-like receptors; PRPs, pattern recognition receptors; PSA, polysaccharide A; PAMPs, pathogen-associated molecular patterns; DAMPs, danger-associated molecular patterns. (Created in https://BioRender.com.)
3.2 Gut microbiota dysbiosis and neuro-endocrine disorders
The gut microbiota is involved in SAE pathogenesis through the vagus nerve. In LPS-induced SAE rat models, gut microbiota composition changed. Meanwhile, EEG activity increased, shown as higher reactivity, abnormal θ/δ rhythms, seizures, and periodic discharges. This suggests gut microbiota disruptions and cognitive problems in LPS-treated rats. Fecal microbiota transplantation (FMT) improves gut dysbiosis and cognitive function via vagus nerve-mediated mechanisms. For example, FMT in SAE rats improved gut dysbiosis, inflammation, and brain function. But after vagotomy, this improvement reversed. Additionally, studies have shown that FMT inhibits hippocampal inflammation mediator release and microglial activation via the vagus nerve, alleviating SAE-related nerve issues (Li et al., 2018). Another investigation demonstrated that intestinal dysbiosis occurring in the post-sepsis period precipitates red light exposure-induced cognitive impairment and anxiety-mimicking behaviors, with the underlying mechanism involving subdiaphragmatic vagal nerve signaling pathways (Xie et al., 2020).
Impaired neurotransmission in SAE is most commonly characterized by dysregulation of cholinergic pathways, resulting in acetylcholine deficiency and delirium-like symptoms (Krzyzaniak et al., 2023). Vagal afferents, in response to inflammation, activate vagal efferent nerves through a mechanism known as the cholinergic anti-inflammatory pathway (CAP). The CAP is an important pathway by which gut microbiota affects brain function. This cholinergic pathway is mediated primarily by nicotinic-type acetylcholine receptors on tissue macrophages, which inhibit the production of tumor necrosis factor (TNF)-α by macrophages (Wang H. et al., 2022; Andersson, 2005; Bonaz et al., 2018). It has been shown that electrical stimulation of the vagus nerve triggers anti-inflammatory effects through cholinergic pathways, improves brain function, and inhibits SAE by attenuating systemic inflammatory responses and neuroinflammation (Li et al., 2015).
In addition, a specialized sensory enteroendocrine cell (EEC) exists in the intestinal epithelium. The gut microbiota and bacterial products can bind to a series of receptors expressed by EECs to induce the release of peptide hormones, including enteric glucagon hormones (glucagon-like peptide-1 and gastric inhibitory peptide), neuropeptides (cholecystokinin and peptide YY), and 5-hydroxytryptophan (5-HT), which can act locally on intestinal neurons and mediate the regulation of gut metabolism by the GBA axis via vagal afferent pathways (Wang et al., 2020; Lach et al., 2018; Parker et al., 2020). It was found that the combination of Ghrelin and growth hormone (GH) treatment significantly inhibited the upregulation of transforming growth factor (TGF)-β expression in septic rats, and Ghrelin administration alone also reduced the levels of TNF-α and interleukin (IL)-6 in the plasma and peritoneal fluid of septic rats, and the vagotomy attenuated this beneficial effect. Whereas in vitro experiments, the administration of Ghrelin alone or in combination with GH did not ameliorate the levels of inflammatory factors in the LPS-induced cells (Zhou et al., 2020; Wu et al., 2007). The discrepancy between in vivo and in vitro may be related to the activation of the microbiota-neuro-endocrine axis during in vivo action, while the in vitro environment has limited ecological complexity and lacks a synergistic interaction mechanism.
Taken together, these suggest that the vagus nerve and the body’s endocrine system are involved in the information network composition of the GBA axis, and gut microbiota dysbiosis may contribute to SAE development via the GBA axis by modulating the neuroendocrine pathway, with the vagus nerve being a key part (Figure 2). The vagal pathway may be a potential therapeutic direction for the treatment of SAE by regulating gut flora.
3.3 Gut microbiota dysbiosis and neuroinflammation
Neuroinflammation is the main pathological process in SAE and is manifested by microglia activation, astrocyte proliferation, and infiltration of peripheral inflammatory mediators and immune cells (Gao Y. et al., 2022). During sepsis progression, specific pattern recognition receptors (PRPs) bind to pathogen-associated molecular patterns (PAMPs) of microorganisms and/or to danger-associated molecular patterns (DAMPs) of damaged tissues, stimulating the release of inflammatory mediators and amplifying the local inflammatory response (Hu et al., 2019). Inflammatory mediators modulate β-adrenergic, γ-aminobutyric acidergic or cholinergic neurotransmission and secretion of corticotropin-releasing factor, adrenocorticotropic hormone, and vasopressin, which affects neuroendocrine pathways, leads to severe systemic reactions, and consequently exacerbates SAE (Zhang et al., 2014). In addition, pro-inflammatory cytokines enter the central nervous system, thereby activating microglia (Lei et al., 2022). Activated microglia trigger a dual inflammatory pathway: releasing pro-inflammatory mediators (IFN-γ, IL-1β, TNFα, and ROS) to activate astrocytes, and producing chemokines to recruit leukocytes into the CNS. Both mechanisms synergistically exacerbate neuroinflammation, culminating in neuronal cell death, compromise of BBB integrity, and cerebral injury. Additionally, upregulated expression of inflammatory mediators within the microenvironment sustains microglial activation through autocrine signaling, establishing a self-reinforcing loop that amplifies neuroinflammatory damage (Lu J. et al., 2022; Parker et al., 2020; Ye et al., 2019).
The gut microbiota dysbiosis exacerbates sepsis-induced systemic inflammatory response. Intracerebral dissemination of polymicrobial organisms of intestinal origin has been detected in mice with experimental sepsis and in patients who died of sepsis, and the structure of the associated bacterial community is strongly correlated with the severity of neuroinflammation in SAE (Singer et al., 2018). Besides, gut microbiota dysbiosis stimulates the secretion of pro-inflammatory cytokines IL-1β, IL-6, and IL-18 by intestinal epithelial cells (IECs), intestinal dendritic cells, and macrophages. Gut microbiota and their constituent components (polysaccharide A and LPS) can interact with IECs Toll-like receptors (TLRs) to induce the production of cytokines such as TNF-α, IL-6, and IL-1, IL-12, and IL-10 (Li and Zhou, 2016; Zhao et al., 2018; Round and Mazmanian, 2010; Sun et al., 2020). The release of these inflammatory mediators into the body circulation induces a peripheral inflammatory infiltrate that drives a systemic inflammatory response and may further exacerbate SAE. Among them, TNF-α and IL-6 are the most important inflammatory factors in the early stage of sepsis: TNF-α causes neutrophil infiltration, brain tissue edema, and BBB dysfunction, and IL-6 indirectly mediates the hypothalamic-pituitary-adrenal axis by affecting the expression of cyclooxygenase 2 (COX2) and prostaglandin synthesis. This pro-inflammatory milieu leads to behavioral alterations, fever, and severe neurological impairments, which result in temporary and permanent cognitive deficits in survivors of sepsis, due to brain edema and neuronal apoptosis (Catarina et al., 2021; Huang et al., 2021). In addition, systemic attack by live bacteria, mainly Gram-negative Escherichia coli and Salmonella typhimurium, can cause microglia activation (Hoogland et al., 2015), and their main cell wall component, endotoxin, can bind to the TLRs on the surface of microglia, activate nuclear factor-κB (NF-κB), and cause inflammatory cascade responses that ultimately lead to neuroinflammation (Jamar et al., 2021; Zheng et al., 2021).
In summary, in sepsis models, gut microbiota dysbiosis leads to imbalance in the GBA immune pathways (Figure 2), resulting in activation of peripheral immune cells and release of inflammatory factors, exacerbating the systemic inflammatory response. At the same time, the integrity of the BBB is damaged, and inflammatory mediators can enter the brain and activate microglia and astrocytes, further promoting the development of neuroinflammation and ultimately leading to SAE.
3.4 Gut microbiota dysbiosis and metabolic dysfunction
Gut microbiota dysbiosis can be involved in the development of SAE by modulating metabolic pathways in the GBA axis. Studies have shown that Sphingorhabdus is negatively correlated with 2-ketobutyric acid, 9-decenoic acid, and L-leucine. It is positively correlated with glycylvaline. The Eubacterium hallii group is positively correlated with 2-methoxy-3-methylamine, acetaminophen, and synephrine acetonide. These correlations play a role in the development of SAE (Wang H. et al., 2022).
Gut microbiota may be involved in the pathophysiological process of SAE by regulating short-chain fatty acids (SCFAs), which are metabolites produced by the fermentation of indigestible dietary fiber by gut microflora (van der Hee and Wells, 2021). Acetate, propionate, and butyrate are the most abundant SCFAs in the human body (Martin-Gallausiaux et al., 2021). A significant decrease in the abundance of some beneficial bacteria positively associated with the production of SCFAs and cognitive function and an increase in the abundance of harmful bacteria associated with infection and cognitive dysfunction were found in a mouse model of sepsis, and cognitive dysfunction was significantly reversed in SAE mice after administration of SCFAs (Li et al., 2023). Decreased concentrations of acetate, propionate, and butyrate (major SCFAs) were found in experimental SAE models (Giridharan et al., 2022; Chen et al., 2021), and the KAT5 inhibitor NU9056 could achieve protective effects against BBB disruption and cognitive dysfunction in SAE mice by modulating the composition of the gut microbiota and up-regulating the concentrations of acetate, propionate, and butyrate (Chen et al., 2021). Based on this, it can be hypothesized that sepsis alters the composition and metabolism of the gut microbiota in mice and reduces the production of SCFAs, which is associated with cognitive impairment in SAE.
On the other hand, SCFAs also play an active role in SAE treatment. SCFAs can improve the integrity of the BBB by up-regulating the expression of ZO-1 and occludin to ameliorate the cognitive impairment in SAE (Li et al., 2023), and also reduce the over-activation of microglia and that of the production of pro-inflammatory cytokines IL-1β and IL-6, and decrease the levels of JNK, NF-κB, p65, and their phosphorylation levels in the mouse brain to achieve neuroprotective effects in SAE mice (Liu et al., 2021). In addition, butyrate partially activates GPR109A receptor on microglia, leading to activation of downstream Nrf2/HO-1 signaling pathway, thereby reducing oxidative stress response and neural lesion, ultimately alleviating the long-term cognitive impairment of SAE mice (Zhang et al., 2022). While these preclinical studies have yielded remarkable results, their translatability to human subjects requires further validation, particularly through multicenter, large-scale clinical trials.
Gut microbiota dysbiosis can also contribute to the development of SAE by affecting the balance of tryptophan metabolism. In the gut, the three main metabolic pathways of tryptophan [the production of indole derivatives, 5-HT, and kynurenine proton (Kyn)] are directly or indirectly controlled by the microbiota (Agus et al., 2018). Tryptophan can be metabolized by intestinal microorganisms to indoles and their derivatives such as indole-3-aldehyde (IAld), indole-3-acetic acid (IAA), and indole-3propionic acid (IPA). Tryptophan metabolites produced by commensal flora can control CNS inflammation through aryl hydrocarbon receptor (AhR)-mediated activation of microglia and transcriptional programs in astrocytes (Rothhammer et al., 2018). It was found that IPA was more enriched in the feces of SER mice than in SES mice, alleviated anxiety and spatial memory dysfunction in septic mice, significantly inhibited activation of NLRP3 inflammasome vesicles and IL-1β secretion in LPS-stimulated microglia, and that these effects were attenuated by antagonism of AhR. It suggests that IPA of gut microbial origin may be a potential therapeutic agent for the prevention of neuroinflammation in SAE (Fang et al., 2022).
In addition, approximately 90% of tryptophan is metabolized along the kynurenine pathway (Kennedy et al., 2017). Kynurenine can be converted into two distinct intermediates via two different pathways: the neurotoxic 3-hydroxykynurenine (3-HAA) and quinolinic acid (QA), and the neuroprotective kynurenic acid (KA) (Gao et al., 2016). Exploration of metabolic fingerprinting has revealed that L-kynurenine is progressively upregulated in sepsis and is strongly associated with the diagnosis and risk stratification of sepsis (Lu G. et al., 2022). CLP-induced sepsis results in markedly impaired hippocampus-dependent cognitive deficits, accompanied by increased kynurenine levels, elevated kynurenine/tryptophan ratios, reduced tryptophan expression, and decreased brain-derived neurotrophic factor (BDNF) concentrations. Single peripheral administration of the indoleamine-2,3-dioxygenase (IDO) metabolite L-kynurenine induces cognitive deficits similar to those caused by CLP, whereas the IDO inhibitor 1-methyl-D-tryptophan attenuates neuroinflammation by inhibiting pro-inflammatory cytokine release and kynurenine production, thereby protecting against sepsis-induced cognitive deficits in mice (Gao et al., 2016). Similarly, exogenous administration of kynurenic acid (KYNA) and its synthetic analogs (SZR-72 and SZR-104) exerts neuroprotective effects in experimental SAE by reducing peripheral neutrophil extracellular trap (NET) formation, attenuating BBB permeability alterations, and mitigating CNS mitochondrial dysfunction (Poles et al., 2021). Collectively, these findings highlight IDO-dependent neurotoxic kynurenine metabolism as a key contributor to sepsis-induced cognitive deficits and a potential target for SAE treatment.
Overall, sepsis disrupts gut microbiota composition, reduces SCFA-producing bacteria, and decreases systemic SCFA concentrations. These changes may aggravate intestinal and BBB disruption, thereby facilitating toxin translocation into the brain and promoting neuroinflammation, ultimately leading to SAE. Alterations in intestinal microbiota metabolism and their metabolites are involved in the etiology of SAE (Figure 2). Therefore, modulation of the gut microbiota and its metabolites may play a therapeutic role in SAE management.
3.5 Gut microbiota dysbiosis and neurotransmitter disorders
Gut microbiota synthesize and/or stimulate neurotransmitters, including gastrointestinal neurotransmitters such as γ-aminobutyric acid (GABA), 5-HT, norepinephrine, dopamine, acetylcholine, and histamine, via dietary amino acid metabolism (Snigdha et al., 2022). Gut microbiota dysbiosis leads to alterations in neurotransmitter levels. Decreased 5-HT levels in hippocampus, brainstem and frontal lobe were observed in mouse models of SAE induced by LPS and CLP (Zhang et al., 2024). Besides, an imbalance in the branched-chain to aromatic amino acid ratio occurs in early SAE, with elevated blood aromatic amino acids potentially increasing central nervous system uptake, leading to cerebral neurotransmitter dysfunction (e.g., impaired neurotransmitter synthesis or aberrant signaling) (Basler et al., 2002). Increased levels of the excitatory neurotransmitter glutamate (Glu) (Tang et al., 2023) and reduced BDNF (Gao L. et al., 2022) were observed in the hippocampus of experimental sepsis mouse models. Additionally, elevated Glu and reduced levels of the inhibitory neurotransmitter GABA were detected in SAE rats (Tang et al., 2023; Xi et al., 2021). Besides, late inflammation was associated with lower levels of BDNF and worse cognitive performance 30 days after sepsis (Biff et al., 2013). Administration of oral antimicrobials to SPF mice transiently altered the composition of the microbiota and increased exploratory behavior and hippocampal expression of BDNF (Bercik et al., 2011). Combined use of soybean embryo ethanol extract and Lactobacillus gasseri NK109 potently enhanced hippocampal BDNF expression, and the number of BDNF-positive neuron cells, while reducing LPS-induced cognitive impairment and colitis in mice (Biff et al., 2013; Yun et al., 2024).
In summary, gut microbiota dysbiosis exerts bidirectional effects on SAE pathogenesis. On the one hand, sepsis-induced alterations in gut microbiota composition and metabolites promote intestinal barrier and BBB permeability, trigger peripheral and central immune cell activation, and initiate inflammatory cascade responses. These processes further exacerbate barrier damage, forming a vicious cycle that drives immune cell infiltration and inflammatory mediator influx into the brain, ultimately leading to neuroinflammation and SAE development. Additionally, gut microbiota dysbiosis disrupts host metabolism and neurotransmitter homeostasis, contributing to SAE progression. Conversely, gut microbiota and their metabolites may exert protective effects by restoring intestinal barrier and BBB integrity, inhibiting immune activation, and reducing neuroinflammation.
However, there are differences in gut microbiota changes between septic rodents and septic patients. In a neuroinflammatory model of CLP-induced sepsis in rats, an upregulated Firmicutes/Bacteroidetes ratio in the gut was observed (Zhao et al., 2022), while the ratio was significantly lower on day 3 of sepsis diagnosis (Luan et al., 2024). Additionally, fundamental differences between rodents and humans include, but are not limited to, the divergence of the transcriptomic response, the mismatch of temporal response patterns, differences in both innate and adaptive immunity, and heterogeneity within the human population in comparison to the homogeneity of highly inbred mouse strains (Stortz et al., 2017). These factors may lead to the conclusion that gut microbiota dysbiosis promotes the development of SAE not being fully applicable to humans. While some correlative studies can be performed in human sepsis, the initial testing of pharmacological agents and determination of the mechanistic basis of their action are not possible with human subjects. Therefore, future efforts could focus on developing new preclinical animal models [e.g., humanized mice and human organoid mice (Cai et al., 2023)] to facilitate clinical translation.
4 Potential therapeutic interventions for SAE targeting the gut microbiota
In the human body, the gut microbiota is dynamic and diverse, and external interventions can be applied to modulate its composition and function. Therefore, the gut microbiota represents a promising therapeutic target for SAE. Emerging evidence supports the efficacy of microbiota-targeted therapies, including FMT and probiotics, in ameliorating SAE outcomes.
4.1 Fecal microbiota transplantation
Fecal microbiota transplantation involves transferring a healthy gut microbiota from a donor to a patient’s gastrointestinal tract to restore microbial homeostasis and improve clinical outcomes (Dodiya et al., 2022). Preclinical studies suggest FMT may be effective in ameliorating sepsis and SAE symptoms. FMT reduces morbidity and mortality in septic mice while restoring gut microbiota abundance and diversity. It improves intestinal barrier function by downregulating epithelial cell apoptosis, enhancing mucus layer composition, upregulating TJ proteins (e.g., ZO-1 and occludin), and reducing intestinal permeability and inflammation (Gai et al., 2021). Additionally, FMT regulates gut microbiota dysbiosis, activates CAPs, and ameliorates cognitive dysfunction in septic rats (Li et al., 2019). In SAE rats, FMT alleviates hippocampal injury by inhibiting inflammatory cytokine secretion, reducing IBA-1 expression, correcting neurotransmitter imbalances, and suppressing M1 macrophage polarization in mesenteric lymph nodes (MLNs) (Xi et al., 2021). Comparative studies show FMT outperforms prebiotics, probiotics, and synbiotics in restoring gut microbiota composition and improving cognitive function in septic rats (Li et al., 2021). These findings demonstrate that FMT has shown positive outcomes in correcting intestinal dysbiosis and improving SAE animals. However, in clinical disease models, there are still gaps in the treatment of SAE with FMT, which can be further explored.
In recent years, the concept of whole intestinal microbiota transplantation (WIMT) has been put forward. WIMT involves transferring microbiota from the jejunum, ileum, cecum, and colon, introducing more small intestine-derived microorganisms and related microbial functions into the recipient’s intestine. Compared with FMT, it can remodel the entire gut microbiota, improves intestinal morphology, and reduces systemic inflammation in recipients (Li et al., 2020). In DSS-induced IBD mice, WIMT demonstrates superior therapeutic effects compared to FMT, which may be attributable to the enrichment of metabolic pathways involving SCFAs and Bifidobacterium (Yang et al., 2023). Given these advantages, future studies should investigate the therapeutic potential of WIMT in SAE. Additionally, with the continuous deepening of research and the advancement of technology, metagenomics sequencing-based establishment of fecal biobanks and targeted infusion of fecal components (Bacteria, Virome, or bacteriophage) (Yu et al., 2023; Wu et al., 2023) hold enormous therapeutic potential.
4.2 Probiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer health benefits to the host” (Hill et al., 2014). This definition emphasizes their microbial nature, viability, and health-promoting properties. Preclinical studies demonstrate that prophylactic administration of Lactobacillus rhamnosus GG (LGG) protects against sepsis in rats (Yılmaz and Erdem, 2020). LGG supplementation significantly alleviated sepsis-caused decreases in hippocampal BDNF expression and p-TrkB phosphorylation levels, preserving neuronal survival and improving cognitive impairments in mice with sepsis (Wang et al., 2024b). A 4-week continuous gavage of LGG reversed gut microbiota dysbiosis in septic mice, reducing potentially pathogenic bacteria and increasing beneficial species, while rebalancing lipid and bile acid metabolism. These effects restored intestinal barrier function, attenuated inflammation, and reduced mortality (Chen et al., 2020). A randomized trial showed that multispecies probiotics restored gut microbiota composition in early sepsis, enriching Lactobacillus and enhancing microbial functional diversity (Stadlbauer et al., 2019). Mechanistically, probiotics alleviate SAE-related brain dysfunction by inhibiting pro-inflammatory cytokines (e.g., TNF-α and IL-6) through microbiota modulation. In a mouse model, 1-month administration of probiotics containing Clostridium butyricum (Cb) improved cognitive function, reduced neuronal damage, suppressed neuroinflammation, and increased BDNF levels (Liu et al., 2020). Collectively, probiotics represent a promising therapeutic strategy for SAE by restoring gut microbiota and metabolic homeostasis, improving cognitive outcomes, and reducing sepsis mortality. However, a RCT of the probiotic Bifidobacterium breve as prophylaxis in over 1,000 very preterm infants at high risk of sepsis did not demonstrate reduction in sepsis incidence or mortality compared to placebo (Costeloe et al., 2016). Meanwhile, concurrent broad-spectrum antibiotic use in patients with sepsis may limit probiotic colonization and beneficial effects (Manzanares et al., 2016). Therefore, the clinical application of probiotics should be comprehensively judged by integrating patients’ individual characteristics, disease types, and strain-specific properties.
4.3 Challenges and limitations
Despite the promising results of microbiota-targeted therapies in preclinical models, their clinical application remains ambiguous with mixed prospects. Currently, FMT has achieved remarkable efficacy in treating various gastrointestinal diseases (Wang et al., 2024a; Costello et al., 2019), and has also demonstrated therapeutic potential in neurological disorders such as Parkinson’s disease (Cheng et al., 2023; Bruggeman et al., 2024), amyotrophic lateral sclerosis (Feng et al., 2024), and autism spectrum disorder (Kang et al., 2020). However, a significant limitation of current research is the lack of standardized FMT treatment protocols, including donor/recipient screening, infusion volume and frequency of fecal matter, administration routes and timing, and whether to combine with other drug therapies (Porcari et al., 2023; Ooijevaar et al., 2019; Carlson, 2020). Among them, the efficacy and safety of FMT are highly dependent on donor screening. Despite the critical importance of donor screening in FMT, identifying and recruiting appropriate donors remains fraught with challenges. First, high-quality donor screening protocols are associated with substantial financial costs (Bénard et al., 2022). In a Dutch cohort, disqualification of potential donors often resulted from the presence of protozoa Dientamoeba fragilis and Blastocystis spp. (Bénard et al., 2022). Moreover, the elevated donor attrition rate is predominantly driven by the onerous stool sample donation protocol, behavioral limitations imposed on donors, and substantial time obligations (Cammarota et al., 2019; McSweeney et al., 2020). Additionally, the safety of FMT in clinical application requires further clarification. A global analysis of FMT safety showed that the most common short-term FMT-related adverse events are diarrhea, abdominal discomfort/pain/cramping, with severe cases involving infection or death (Wang Y. et al., 2022). Another real-world data shows that the risk of developing new medical conditions beyond 12 months after FMT is low (Yau et al., 2024). However, FMT may cause the transmission and clearance of potential carcinogenic bacteria. Four of the 11 patients demonstrated potential engraftment after FMT of donor strains harboring virulence factors (Drewes et al., 2019). This suggests that further studies on appropriate screening measures for FMT donors and the long-term consequences and/or benefits of FMT are warranted.
Although probiotics have shown certain potential in clinical treatment and health maintenance, their practical application is subject to numerous limitations. First, empirical probiotic supplementation may be limited by mucosal colonization resistance (Zmora et al., 2018). Second, there is currently no consensus on optimal probiotic strains, dosages, and treatment durations. Moreover, probiotics have not been proven to have long-term beneficial effects, are only effective under certain conditions and may even cause side effects. C. butyricum, a probiotic commonly prescribed in Asia, occasionally leads to bacteremia (Sada et al., 2024; Jiang et al., 2025), but the prevalence and characteristics of C. butyricum bacteremia and its bacteriologic and genetic underpinnings remain unknown (Sada et al., 2024). Additionally, a markedly higher risk of Lactobacillus bacteremia for ICU patients treated with probiotics compared to those not treated (Yelin et al., 2019). Therefore, the selection of probiotics should be based on individualized assessment, combined with treatment objectives, while avoiding the abuse of probiotics.
5 Conclusion
Sepsis-associated encephalopathy is one of the most common critical encephalopathies, with disease severity closely linked to patient prognosis and quality of life. Current evidence indicates that sepsis-induced gut microbiota dysbiosis contributes to SAE pathogenesis by disrupting intestinal barrier and BBB integrity, amplifying systemic inflammation and neuroinflammation, and promoting metabolic and neurotransmitter imbalances. This mechanism provides novel therapeutic targets for SAE. Microbiota-based therapies, including FMT and probiotics, play a crucial role in ameliorating SAE symptoms and improving patient outcomes. However, optimal criteria for treatment timing, dosage, and strain selection remain unclear. Future research should focus on elucidating the gut microbiota’s contribution to SAE development, with in-depth investigations into gut microbial metabolites offering potential insights into pathogenesis and therapeutic innovation.
Author contributions
NW: Writing – original draft. SD: Writing – original draft. WL: Writing – original draft. JZ: Funding acquisition, Writing – review & editing. YC: Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Sichuan Science and Technology Program (No. 25NSFSC1924), Luzhou Science and Technology Program (No. 2023SYF099), and Hejiang County People’s Hospital-Southwest Medical University Science and Technology strategic cooperation project (No. 2021HJXNYD03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Andersson, J. (2005). The inflammatory reflex–introduction. J. Intern. Med. 257, 122–125. doi: 10.1111/j.1365-2796.2004.01440.x
Baggs, J., Jernigan, J., Halpin, A., Epstein, L., Hatfield, K., and McDonald, L. (2018). Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin. Infect. Dis. 66, 1004–1012. doi: 10.1093/cid/cix947
Basler, T., Meier-Hellmann, A., Bredle, D., and Reinhart, K. (2002). Amino acid imbalance early in septic encephalopathy. Intensive Care Med. 28, 293–298. doi: 10.1007/s00134-002-1217-6
Bénard, M., de Bruijn, C., Fenneman, A., Wortelboer, K., Zeevenhoven, J., Rethans, B., et al. (2022). Challenges and costs of donor screening for fecal microbiota transplantations. PLoS One 17:e0276323. doi: 10.1371/journal.pone.0276323
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609, 609.e1–3. doi: 10.1053/j.gastro.2011.04.052
Biff, D., Petronilho, F., Constantino, L., Vuolo, F., Zamora-Berridi, G., Dall’Igna, D., et al. (2013). Correlation of acute phase inflammatory and oxidative markers with long-term cognitive impairment in sepsis survivors rats. Shock 40, 45–48. doi: 10.1097/SHK.0b013e3182959cfa
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12:49. doi: 10.3389/fnins.2018.00049
Bozza, F., Garteiser, P., Oliveira, M., Doblas, S., Cranford, R., Saunders, D., et al. (2010). Sepsis-associated encephalopathy: A magnetic resonance imaging and spectroscopy study. J. Cereb. Blood Flow Metab. 30, 440–448. doi: 10.1038/jcbfm.2009.215
Bruggeman, A., Vandendriessche, C., Hamerlinck, H., De Looze, D., Tate, D., Vuylsteke, M., et al. (2024). Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): A double-blind, placebo-controlled, randomised, phase 2 trial. EClinicalMedicine 71:102563. doi: 10.1016/j.eclinm.2024.102563
Cai, L., Rodgers, E., Schoenmann, N., and Raju, R. (2023). Advances in rodent experimental models of sepsis. Int. J. Mol. Sci. 24:9578. doi: 10.3390/ijms24119578
Cammarota, G., Ianiro, G., Kelly, C., Mullish, B., Allegretti, J., Kassam, Z., et al. (2019). International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 68, 2111–2121. doi: 10.1136/gutjnl-2019-319548
Carlson, P. (2020). Regulatory considerations for fecal microbiota transplantation products. Cell Host Microbe 27, 173–175. doi: 10.1016/j.chom.2020.01.018
Catarina, A., Branchini, G., Bettoni, L., De Oliveira, J., and Nunes, F. (2021). Sepsis-associated encephalopathy: From pathophysiology to progress in experimental studies. Mol. Neurobiol. 58, 2770–2779. doi: 10.1007/s12035-021-02303-2
Chen, J., Zeng, L., Zhao, Y., Tang, H., Lei, H., Wan, Y., et al. (2023). Causal effects of gut microbiota on sepsis: A two-sample Mendelian randomization study. Front. Microbiol. 14:1167416. doi: 10.3389/fmicb.2023.1167416
Chen, L., Li, H., Chen, Y., and Yang, Y. (2020). Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 78:110863. doi: 10.1016/j.nut.2020.110863
Chen, L., Qing, W., Yi, Z., Lin, G., Peng, Q., and Zhou, F. (2021). NU9056, a KAT 5 inhibitor, treatment alleviates brain dysfunction by inhibiting NLRP3 inflammasome activation, affecting gut microbiota, and derived metabolites in LPS-treated mice. Front. Nutr. 8:701760. doi: 10.3389/fnut.2021.701760
Cheng, Y., Tan, G., Zhu, Q., Wang, C., Ruan, G., Ying, S., et al. (2023). Efficacy of fecal microbiota transplantation in patients with Parkinson’s disease: Clinical trial results from a randomized, placebo-controlled design. Gut Microbes 15:2284247. doi: 10.1080/19490976.2023.2284247
Costello, S., Hughes, P., Waters, O., Bryant, R., Vincent, A., Blatchford, P., et al. (2019). Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 321, 156–164. doi: 10.1001/jama.2018.20046
Costeloe, K., Hardy, P., Juszczak, E., Wilks, M., and Millar, M. (2016). Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 387, 649–660. doi: 10.1016/S0140-6736(15)01027-2
Dodiya, H., Lutz, H., Weigle, I., Patel, P., Michalkiewicz, J., Roman-Santiago, C., et al. (2022). Gut microbiota-driven brain Aβ amyloidosis in mice requires microglia. J. Exp. Med. 219:e20200895. doi: 10.1084/jem.20200895
Drewes, J., Corona, A., Sanchez, U., Fan, Y., Hourigan, S., Weidner, M., et al. (2019). Transmission and clearance of potential procarcinogenic bacteria during fecal microbiota transplantation for recurrent Clostridioides difficile. JCI Insight 4:e130848. doi: 10.1172/jci.insight.130848
Erikson, K., Tuominen, H., Vakkala, M., Liisanantti, J., Karttunen, T., Syrjälä, H., et al. (2020). Brain tight junction protein expression in sepsis in an autopsy series. Crit. Care 24:385. doi: 10.1186/s13054-020-03101-3
Fang, H., Fang, M., Wang, Y., Zhang, H., Li, J., Chen, J., et al. (2022). Indole-3-propionic acid as a potential therapeutic agent for sepsis-induced gut microbiota disturbance. Microbiol. Spectr. 10:e0012522. doi: 10.1128/spectrum.00125-22
Fei, Y., Hao, Z., Zheng, X., Ji, X., and Zhao, W. (2025). Microbiological and clinical predictors of sepsis-associated encephalalopathy in bloodstream infections: A retrospective cohort study. Front. Cell. Infect. Microbiol. 15:1548370. doi: 10.3389/fcimb.2025.1548370
Feng, R., Zhu, Q., Wang, A., Wang, H., Wang, J., Chen, P., et al. (2024). Effect of fecal microbiota transplantation on patients with sporadic amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled trial. BMC Med. 22:566. doi: 10.1186/s12916-024-03781-6
Gai, X., Wang, H., Li, Y., Zhao, H., He, C., Wang, Z., et al. (2021). Fecal microbiota transplantation protects the intestinal mucosal barrier by reconstructing the gut microbiota in a murine model of sepsis. Front. Cell. Infect. Microbiol. 11:736204. doi: 10.3389/fcimb.2021.736204
Gao, Y., Liu, Y., Zhang, X., Shou, S., and Chai, Y. (2022). Insight into regulatory T cells in sepsis-associated encephalopathy. Front. Neurol. 13:830784. doi: 10.3389/fneur.2022.830784
Gao, L., Wang, Z., Mu, Y., Liu, Z., and Pang, L. (2022). Emodin promotes autophagy and prevents apoptosis in sepsis-associated encephalopathy through activating BDNF/TrkB signaling. Pathobiology 89, 135–145. doi: 10.1159/000520281
Gao, R., Kan, M., Wang, S., Yang, R., and Zhang, S. (2016). Disrupted tryptophan metabolism induced cognitive impairment in a mouse model of sepsis-associated encephalopathy. Inflammation 39, 550–560. doi: 10.1007/s10753-015-0279-x
Giridharan, V., Generoso, J., Lence, L., Candiotto, G., Streck, E., Petronilho, F., et al. (2022). A crosstalk between gut and brain in sepsis-induced cognitive decline. J. Neuroinflamm. 19:114. doi: 10.1186/s12974-022-02472-4
Gofton, T., and Young, G. (2012). Sepsis-associated encephalopathy. Nat. Rev. Neurol. 8, 557–566. doi: 10.1038/nrneurol.2012.183
Grabrucker, S., Marizzoni, M., Silajdžić, E., Lopizzo, N., Mombelli, E., Nicolas, S., et al. (2023). Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 146, 4916–4934. doi: 10.1093/brain/awad303
Gu, M., Mei, X., and Zhao, Y. (2021). Sepsis and cerebral dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox. Res. 39, 489–503. doi: 10.1007/s12640-020-00270-5
Han, C., Guo, N., Bu, Y., Peng, Y., Li, X., Ma, X., et al. (2021). Intestinal microbiota and antibiotic-associated acute gastrointestinal injury in sepsis mice. Aging (Albany NY) 13, 10099–10111. doi: 10.18632/aging.202768
Hill, C., Guarner, F., Reid, G., Gibson, G., Merenstein, D., Pot, B., et al. (2014). Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hoogland, I., Houbolt, C., van Westerloo, D., van Gool, W., and van de Beek, D. (2015). Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 12:114. doi: 10.1186/s12974-015-0332-6
Hu, Q., Ren, H., Li, G., Wang, D., Zhou, Q., Wu, J., et al. (2019). STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine 41, 497–508. doi: 10.1016/j.ebiom.2019.02.055
Hu, X., Wang, T., and Jin, F. (2016). Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 59, 1006–1023. doi: 10.1007/s11427-016-5083-9
Huang, Y., Chen, R., Jiang, L., Li, S., and Xue, Y. (2021). Basic research and clinical progress of sepsis-associated encephalopathy. J. Intensive Med. 1, 90–95. doi: 10.1016/j.jointm.2021.08.002
Iacobone, E., Bailly-Salin, J., Polito, A., Friedman, D., Stevens, R., and Sharshar, T. (2009). Sepsis-associated encephalopathy and its differential diagnosis. Crit. Care Med. 37, S331–S336. doi: 10.1097/CCM.0b013e3181b6ed58
Jamar, G., Ribeiro, D., and Pisani, L. (2021). High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit. Rev. Food Sci. Nutr. 61, 836–854. doi: 10.1080/10408398.2020.1747046
Jiang, Y., Yang, J., Liu, Y., Liu, Y., Zhou, W., Yang, B., et al. (2025). A neonate with meningitis caused by probiotic-related Clostridium butyricum. Int. J. Infect. Dis. 155:107900. doi: 10.1016/j.ijid.2025.107900
Kang, D., Adams, J., Vargason, T., Santiago, M., Hahn, J., and Krajmalnik-Brown, R. (2020). Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere 5, e314–e320. doi: 10.1128/mSphere.00314-20
Kempker, J., and Martin, G. S. (2020). A global accounting of sepsis. Lancet 395, 168–170. doi: 10.1016/S0140-6736(19)33065-X
Kennedy, P., Cryan, J., Dinan, T., and Clarke, G. (2017). Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112, 399–412. doi: 10.1016/j.neuropharm.2016.07.002
Krzyzaniak, K., Krion, R., Szymczyk, A., Stepniewska, E., and Sieminski, M. (2023). Exploring neuroprotective agents for sepsis-associated encephalopathy: A comprehensive review. Int. J. Mol. Sci. 24:10780. doi: 10.3390/ijms241310780
Kullberg, R., Haak, B., Chanderraj, R., Prescott, H., Dickson, R., and Wiersinga, W. (2025). Empirical antibiotic therapy for sepsis: Save the anaerobic microbiota. Lancet Respir. Med. 13, 92–100. doi: 10.1016/S2213-2600(24)00257-1
Kullberg, R., Wiersinga, W., and Haak, B. (2021). Gut microbiota and sepsis: From pathogenesis to novel treatments. Curr. Opin. Gastroenterol. 37, 578–585. doi: 10.1097/MOG.0000000000000781
Lach, G., Schellekens, H., Dinan, T., and Cryan, J. (2018). Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics 15, 36–59. doi: 10.1007/s13311-017-0585-0
Lei, W., Ren, Z., Su, J., Zheng, X., Gao, L., Xu, Y., et al. (2022). Immunological risk factors for sepsis-associated delirium and mortality in ICU patients. Front. Immunol. 13:940779. doi: 10.3389/fimmu.2022.940779
Li, N., Li, Z., Xiang, H., Wang, X., Zhang, X., and Li, J. (2015). [Protective effects of vagus nerve stimulation on rats with sepsis-associated encephalopathy]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 27, 509–513. doi: 10.3760/cma.j.issn.2095-4352.2015.06.018
Li, N., Zuo, B., Huang, S., Zeng, B., Han, D., Li, T., et al. (2020). Spatial heterogeneity of bacterial colonization across different gut segments following inter-species microbiota transplantation. Microbiome 8:161. doi: 10.1186/s40168-020-00917-7
Li, Q., and Zhou, J. (2016). The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 324, 131–139. doi: 10.1016/j.neuroscience.2016.03.013
Li, S., Guo, H., Xu, X., Hua, R., Zhao, Q., Li, J., et al. (2021). Therapeutic methods for gut microbiota modification in lipopolysaccharide-associated encephalopathy. Shock 56, 824–831. doi: 10.1097/SHK.0000000000001758
Li, S., Lv, J., Li, J., Zhao, Z., Guo, H., Zhang, Y., et al. (2018). Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci. Lett. 662, 98–104. doi: 10.1016/j.neulet.2017.10.008
Li, S., Xu, N., Hua, R., Niu, X., Lyu, C., Li, M., et al. (2019). [Fecal microbiota transplantation regulates the cholinergic anti-inflammatory pathway in cerebral cortex of septic rats through intestinal microbiota]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 31, 1102–1107. doi: 10.3760/cma.j.issn.2095-4352.2019.09.009
Li, Z., Zhang, F., Sun, M., Liu, J., Zhao, L., Liu, S., et al. (2023). The modulatory effects of gut microbes and metabolites on blood-brain barrier integrity and brain function in sepsis-associated encephalopathy. PeerJ 11:e15122. doi: 10.7717/peerj.15122
Liang, H., Song, H., Zhang, X., Song, G., Wang, Y., Ding, X., et al. (2022). Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerg. Microbes Infect. 11, 815–828. doi: 10.1080/22221751.2022.2045876
Liao, H., Li, H., Bao, H., Jiang, L., Du, J., Guo, Y., et al. (2022). Short chain fatty acids protect the cognitive function of sepsis associated encephalopathy mice via GPR43. Front. Neurol. 13:909436. doi: 10.3389/fneur.2022.909436
Liu, J., Jin, Y., Li, H., Yu, J., Gong, T., Gao, X., et al. (2020). Probiotics exert protective effect against sepsis-induced cognitive impairment by reversing gut microbiota abnormalities. J. Agric. Food Chem. 68, 14874–14883. doi: 10.1021/acs.jafc.0c06332
Liu, J., Jin, Y., Ye, Y., Tang, Y., Dai, S., Li, M., et al. (2021). The neuroprotective effect of short chain fatty acids against sepsis-associated encephalopathy in mice. Front. Immunol. 12:626894. doi: 10.3389/fimmu.2021.626894
Lu, J., Hou, W., Gao, S., Zhang, Y., and Zong, Y. (2022). The role of gut microbiota-gut-brain axis in perioperative neurocognitive dysfunction. Front. Pharmacol. 13:879745. doi: 10.3389/fphar.2022.879745
Lu, G., Zhou, J., Yang, T., Li, J., Jiang, X., Zhang, W., et al. (2022). Landscape of metabolic fingerprinting for diagnosis and risk stratification of sepsis. Front. Immunol. 13:883628. doi: 10.3389/fimmu.2022.883628
Luan, F., Zhou, Y., Ma, X., Li, Y., Peng, Y., Jia, X., et al. (2024). Gut microbiota composition and changes in patients with sepsis: Potential markers for predicting survival. BMC Microbiol. 24:45. doi: 10.1186/s12866-024-03188-6
Manabe, T., and Heneka, M. (2022). Cerebral dysfunctions caused by sepsis during ageing. Nat. Rev. Immunol. 22, 444–458. doi: 10.1038/s41577-021-00643-7
Manzanares, W., Lemieux, M., Langlois, P., and Wischmeyer, P. (2016). Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit. Care 19:262. doi: 10.1186/s13054-016-1434-y
Martin-Gallausiaux, C., Marinelli, L., Blottière, H., Larraufie, P., and Lapaque, N. (2021). SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49. doi: 10.1017/S0029665120006916
Martin-Loeches, I., Singer, M., and Leone, M. (2024). Sepsis: Key insights, future directions, and immediate goals. A review and expert opinion. Intensive Care Med. 50, 2043–2049. doi: 10.1007/s00134-024-07694-z
Matheson, J., and Holsinger, R. (2023). The role of fecal microbiota transplantation in the treatment of neurodegenerative diseases: A review. Int. J. Mol. Sci. 24:1001. doi: 10.3390/ijms24021001
McSweeney, B., Allegretti, J., Fischer, M., Xu, H., Goodman, K., Monaghan, T., et al. (2020). In search of stool donors: A multicenter study of prior knowledge, perceptions, motivators, and deterrents among potential donors for fecal microbiota transplantation. Gut Microbes 11, 51–62. doi: 10.1080/19490976.2019.1611153
Morais, L., Schreiber, H., and Mazmanian, S. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Obermeier, B., Daneman, R., and Ransohoff, R. (2013). Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596. doi: 10.1038/nm.3407
Ooijevaar, R., Terveer, E., Verspaget, H., Kuijper, E., and Keller, J. (2019). Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 70, 335–351. doi: 10.1146/annurev-med-111717-122956
Pan, S., Lv, Z., Wang, R., Shu, H., Yuan, S., Yu, Y., et al. (2022). Sepsis-induced brain dysfunction: Pathogenesis, diagnosis, and treatment. Oxid. Med. Cell Longev. 2022:1328729. doi: 10.1155/2022/1328729
Parker, A., Fonseca, S., and Carding, S. (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157. doi: 10.1080/19490976.2019.1638722
Pinitchun, C., Panpetch, W., Bhunyakarnjanarat, T., Udompornpitak, K., Do, H., Visitchanakun, P., et al. (2024). Aging-induced dysbiosis worsens sepsis severity but is attenuated by probiotics in D-galactose-administered mice with cecal ligation and puncture model. PLoS One 19:e0311774. doi: 10.1371/journal.pone.0311774
Poles, M., Nászai, A., Gulácsi, L., Czakó, B., Gál, K., Glenz, R., et al. (2021). Kynurenic acid and its synthetic derivatives protect against sepsis-associated neutrophil activation and brain mitochondrial dysfunction in rats. Front. Immunol. 12:717157. doi: 10.3389/fimmu.2021.717157
Porcari, S., Benech, N., Valles-Colomer, M., Segata, N., Gasbarrini, A., Cammarota, G., et al. (2023). Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe 31, 712–733. doi: 10.1016/j.chom.2023.03.020
Prescott, H., Dickson, R., Rogers, M., Langa, K., and Iwashyna, T. (2015). Hospitalization type and subsequent severe sepsis. Am. J. Respir. Crit. Care Med. 192, 581–588. doi: 10.1164/rccm.201503-0483OC
Rothhammer, V., Borucki, D., Tjon, E., Takenaka, M., Chao, C., Ardura-Fabregat, A., et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. doi: 10.1038/s41586-018-0119-x
Round, J., and Mazmanian, S. (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209. doi: 10.1073/pnas.0909122107
Sada, R., Matsuo, H., Motooka, D., Kutsuna, S., Hamaguchi, S., Yamamoto, G., et al. (2024). Clostridium butyricum bacteremia associated with probiotic use, Japan. Emerg. Infect. Dis. 30, 665–671. doi: 10.3201/eid3004.231633
Sampson, T., Debelius, J., Thron, T., Janssen, S., Shastri, G., Ilhan, Z., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
Singer, B., Dickson, R., Denstaedt, S., Newstead, M., Kim, K., Falkowski, N., et al. (2018). Bacterial Dissemination to the Brain in Sepsis. Am. J. Respir. Crit. Care Med. 197, 747–756. doi: 10.1164/rccm.201708-1559OC
Snigdha, S., Ha, K., Tsai, P., Dinan, T., Bartos, J., and Shahid, M. (2022). Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol. Ther. 231:107978. doi: 10.1016/j.pharmthera.2021.107978
Sonneville, R., de Montmollin, E., Poujade, J., Garrouste-Orgeas, M., Souweine, B., Darmon, M., et al. (2017). Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 43, 1075–1084. doi: 10.1007/s00134-017-4807-z
Stadlbauer, V., Horvath, A., Komarova, I., Schmerboeck, B., Feldbacher, N., Klymiuk, I., et al. (2019). Dysbiosis in early sepsis can be modulated by a multispecies probiotic: A randomised controlled pilot trial. Benef. Microbes 10, 265–278. doi: 10.3920/BM2018.0067
Stortz, J., Raymond, S., Mira, J., Moldawer, L., Mohr, A., and Efron, P. (2017). Murine models of sepsis and trauma: Can we bridge the gap? ILAR J. 58, 90–105. doi: 10.1093/ilar/ilx007
Sun, L., Li, J., and Nie, Y. (2020). Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. (Engl) 133, 826–833. doi: 10.1097/CM9.0000000000000706
Sun, S., Wang, D., Dong, D., Xu, L., Xie, M., Wang, Y., et al. (2023). Altered intestinal microbiome and metabolome correspond to the clinical outcome of sepsis. Crit. Care 27:127. doi: 10.1186/s13054-023-04412-x
Sweeney, M., Zhao, Z., Montagne, A., Nelson, A., and Zlokovic, B. (2019). Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Tang, F., Chen, L., Gao, H., Lei, Y., Pan, L., Xiao, D., et al. (2023). Munc18-1 contributes to hippocampal injury in septic rats through regulation of syntanxin1a and synaptophysin and glutamate levels. Neurochem. Res. 48, 791–803. doi: 10.1007/s11064-022-03806-7
Towner, R., Saunders, D., Smith, N., Towler, W., Cruz, M., Do, S., et al. (2018). Assessing long-term neuroinflammatory responses to encephalopathy using MRI approaches in a rat endotoxemia model. Geroscience 40, 49–60. doi: 10.1007/s11357-018-0009-z
van der Hee, B., and Wells, J. (2021). Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29, 700–712. doi: 10.1016/j.tim.2021.02.001
Wang, Y., Zhang, S., Borody, T., and Zhang, F. (2022). Encyclopedia of fecal microbiota transplantation: A review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 135, 1927–1939. doi: 10.1097/CM9.0000000000002339
Wang, H., Wang, Q., Chen, J., and Chen, C. (2022). Association among the gut microbiome, the serum metabolomic profile and RNA m6A methylation in sepsis-associated encephalopathy. Front. Genet. 13:859727. doi: 10.3389/fgene.2022.859727
Wang, L., Zhao, R., Li, X., Shao, P., Xie, J., Su, X., et al. (2024b). Lactobacillus rhamnosus GG improves cognitive impairments in mice with sepsis. PeerJ 12:e17427. doi: 10.7717/peerj.17427
Wang, L., Xu, Y., Li, L., Yang, B., Zhao, D., Ye, C., et al. (2024a). The impact of small intestinal bacterial overgrowth on the efficacy of fecal microbiota transplantation in patients with chronic constipation. mBio 15:e0202324. doi: 10.1128/mbio.02023-24
Wang, S., Yu, Y., and Adeli, K. (2020). Role of gut microbiota in neuroendocrine regulation of carbohydrate and lipid metabolism via the microbiota-gut-brain-liver axis. Microorganisms 8:527. doi: 10.3390/microorganisms8040527
Wu, D., Zhang, C., Liu, Y., Yao, J., Yang, X., Wu, S., et al. (2023). Beyond faecal microbiota transplantation, the non-negligible role of faecal virome or bacteriophage transplantation. J. Microbiol. Immunol. Infect. 56, 893–908. doi: 10.1016/j.jmii.2023.02.005
Wu, R., Dong, W., Cui, X., Zhou, M., Simms, H., Ravikumar, T., et al. (2007). Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann. Surg. 245, 480–486. doi: 10.1097/01.sla.0000251614.42290.ed
Xi, S., Wang, Y., Wu, C., Peng, W., Zhu, Y., and Hu, W. (2021). Intestinal epithelial cell exosome launches IL-1β-mediated neuron injury in sepsis-associated encephalopathy. Front. Cell. Infect. Microbiol. 11:783049. doi: 10.3389/fcimb.2021.783049
Xiang, Q., Yu, M., Cai, Q., Hu, M., Rao, B., Liang, X., et al. (2024). Multi-omics insights into the microbiota-gut-brain axis and cognitive improvement post-bariatric surgery. J. Transl. Med. 22:945. doi: 10.1186/s12967-024-05757-9
Xiao, W., Su, J., Gao, X., Yang, H., Weng, R., Ni, W., et al. (2022). The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 10:62. doi: 10.1186/s40168-022-01255-6
Xie, B., Zhang, Y., Qi, H., Yao, H., Shang, Y., Yuan, S., et al. (2020). Red light exaggerated sepsis-induced learning impairments and anxiety-like behaviors. Aging (Albany NY) 12, 23739–23760. doi: 10.18632/aging.103940
Xu, K., Huang, Q., Lyu, Y., Wang, S., Lu, Y., and Qian, G. (2024). Phosphatidylserine improves aging sepsis survival, modulates gut microbiome, and prevents sepsis-associated encephalopathy. Biomed. Pharmacother. 178:117200. doi: 10.1016/j.biopha.2024.117200
Yang, Y., He, J., Wang, Y., Liang, L., Zhang, Z., Tan, X., et al. (2023). Whole intestinal microbiota transplantation is more effective than fecal microbiota transplantation in reducing the susceptibility of DSS-induced germ-free mice colitis. Front. Immunol. 14:1143526. doi: 10.3389/fimmu.2023.1143526
Yau, Y., Lau, L., Lui, R., Wong, S., Guo, C., Mak, J., et al. (2024). Long-term safety outcomes of fecal microbiota transplantation: Real-world data over 8 years from the Hong Kong FMT registry. Clin. Gastroenterol. Hepatol. 22:611–620.e12. doi: 10.1016/j.cgh.2023.09.001
Ye, B., Tao, T., Zhao, A., Wen, L., He, X., Liu, Y., et al. (2019). Blockade of IL-17A/IL-17R pathway protected mice from sepsis-associated encephalopathy by inhibition of microglia activation. Mediat. Inflamm. 2019:8461725. doi: 10.1155/2019/8461725
Yelin, I., Flett, K., Merakou, C., Mehrotra, P., Stam, J., Snesrud, E., et al. (2019). Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 25, 1728–1732. doi: 10.1038/s41591-019-0626-9
Yılmaz, M., and Erdem, A. (2020). The protective role of probiotics in sepsis-induced rats. Ulus Travma Acil Cerrahi Derg 26, 843–846. doi: 10.14744/tjtes.2020.70440
Yu, Y., Wang, W., and Zhang, F. (2023). The next generation fecal microbiota transplantation: To transplant bacteria or virome. Adv. Sci. 10:e2301097. doi: 10.1002/advs.202301097
Yun, S., Lee, D., Park, H., and Kim, D. (2024). Heat-processed soybean germ extract and Lactobacillus gasseri NK109 supplementation reduce LPS-induced cognitive impairment and colitis in mice. Nutrients 16:2736. doi: 10.3390/nu16162736
Zhang, C., Tian, F., Peng, J., Wang, X., Li, J., Zhang, L., et al. (2024). Serotonergic neurotransmission mediated cognitive dysfunction in two mouse models of sepsis-associated encephalopathy. CNS Neurosci. Ther. 30:e14655. doi: 10.1111/cns.14655
Zhang, H., Xu, J., Wu, Q., Fang, H., Shao, X., Ouyang, X., et al. (2022). Gut microbiota mediates the susceptibility of mice to sepsis-associated encephalopathy by butyric acid. J. Inflamm. Res. 15, 2103–2119. doi: 10.2147/JIR.S350566
Zhang, L., Wang, X., Ai, Y., Guo, Q., Huang, L., Liu, Z., et al. (2012). Epidemiological features and risk factors of sepsis-associated encephalopathy in intensive care unit patients: 2008-2011. Chin. Med. J. 125, 828–831. doi: 10.3760/cma.j.issn.0366-6999.2012.05.018
Zhang, Q., Sheng, Z., and Yao, Y. (2014). Septic encephalopathy: When cytokines interact with acetylcholine in the brain. Mil. Med. Res. 1:20. doi: 10.1186/2054-9369-1-20
Zhao, H., Lyu, Y., Zhai, R., Sun, G., and Ding, X. (2022). Metformin mitigates sepsis-related neuroinflammation via modulating gut microbiota and metabolites. Front. Immunol. 13:797312. doi: 10.3389/fimmu.2022.797312
Zhao, H., Zhou, X., Song, Y., Zhao, W., Sun, Z., Zhu, J., et al. (2025). Multi-omics analyses identify gut microbiota-fecal metabolites-brain-cognition pathways in the Alzheimer’s disease continuum. Alzheimers Res. Ther. 17:36. doi: 10.1186/s13195-025-01683-0
Zhao, Y., Chen, F., Wu, W., Sun, M., Bilotta, A., Yao, S., et al. (2018). GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 11, 752–762. doi: 10.1038/mi.2017.118
Zheng, Z., Tu, J., Li, X., Hua, Q., Liu, W., Liu, Y., et al. (2021). Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 91, 505–518. doi: 10.1016/j.bbi.2020.11.007
Zhou, M., Aziz, M., Ochani, M., and Wang, P. (2020). Correction of immunosuppression in aged septic rats by human ghrelin and growth hormone through the vagus nerve-dependent inhibition of TGF-β production. Mol. Med. 26:71. doi: 10.1186/s10020-020-00195-x
Keywords: sepsis-associated encephalopathy, sepsis, gut microbiota, fecal microbiota transplantation, probiotics
Citation: Wei N, Dai S, Li W, Zhou J and Chen Y (2025) Gut microbiota and sepsis-associated encephalopathy: pathogenesis and precision therapies. Front. Neurosci. 19:1596467. doi: 10.3389/fnins.2025.1596467
Received: 19 March 2025; Accepted: 12 June 2025;
Published: 08 July 2025.
Edited by:
Pan Pan, People’s Liberation Army General Hospital, ChinaReviewed by:
Qiyang Li, Southern Medical University, ChinaJohn Sieh Dumbuya, Affiliated Hospital of Guangdong Medical University, China
Copyright © 2025 Wei, Dai, Li, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhou, anVuemhvdUBzd211LmVkdS5jbg==; Ye Chen, Y2hlbnllMDExN0Bzd211LmVkdS5jbg==
 Na Wei
Na Wei Shiyu Dai
Shiyu Dai Wei Li1,2,3
Wei Li1,2,3 Jun Zhou
Jun Zhou