- 1Department of Spine Surgery, Shaoxing Central Hospital, Affiliated Central Hospital of Shaoxing University, Shaoxing, Zhejiang Province, China
- 2Department of Radiology, Ningbo No. 2 Hospital, Ningbo, Zhejiang Province, China
- 3Department of Pediatrics, Shaoxing Central Hospital, Affiliated Central Hospital of Shaoxing University, Shaoxing, Zhejiang Province, China
Spinal cord injury (SCI) is a traumatic neurological disorder with a high incidence and limited clinical treatment options. Ferroptosis, a newly discovered form of programmed cell death, has shown significant research potential in the field of neurological diseases. Stem cells have become an ideal therapeutic option for various diseases due to their multidirectional differentiation potential and paracrine properties. Existing studies have demonstrated that stem cells possess substantial potential in the repair of spinal cord injuries. Recent research has found that stem cell transplantation can improve the pathological process of SCI by regulating the ferroptosis pathway. This review systematically described the molecular mechanisms of ferroptosis in SCI, the biological effects of stem cell therapy for SCI, and the therapeutic potential of stem cell-targeted regulation of ferroptosis. Additionally, we proposed three key research directions: cross-study of ferroptosis signaling pathways and stem cell action mechanisms, optimization strategies for therapeutic stem cells, and multimodal combined treatments based on ferroptosis regulation. This review aimed to provide new theoretical foundations and research perspectives for stem cell therapy in SCI.
1 Introduction
SCI is a serious disabling disease of the central nervous system, leading to motor, sensory, and autonomic dysfunction below the injury site (Anjum et al., 2020). Based on the pathological mechanism, SCI can be divided into primary and secondary injury: the former is caused by direct mechanical force and is usually irreversible, while the latter involves a cascade of dynamic regulatory reactions and plays a key role in the deterioration of neurological function (He et al., 2024). New research suggests that the primary reason for poor regeneration and functional recovery in SCI is “microenvironmental imbalance,” which manifests as an increase in inhibitory factors at the tissue, cell, and molecular levels, and a decrease in promoting factors at different times and locations (Fan et al., 2018). As the core pathological event of secondary injury, programmed cell death significantly impairs neurological function recovery and clinical prognosis by exacerbating microenvironmental damage and neuronal cell death, suggesting that its regulation may become a potential target for SCI treatment (Shi et al., 2021; He et al., 2024).
Epidemiological data show that the global prevalence of SCI has risen steadily over the past 30 years, with incidence rates in various countries ranging from 236 to 1,298 cases per million people (Khorasanizadeh et al., 2019). SCI was once considered an untreatable disease when it was first mentioned in the Edwin Smith Papyrus manuscript in the 17th century BC (Hughes, 1988). Although current clinical treatments primarily involve surgical intervention, drug therapy, hyperbaric oxygen, and physical therapy, the overall efficacy and prognosis remain unsatisfactory (Eli et al., 2021; Fehlings et al., 2017; Gao et al., 2020). This is mainly due to the lack of effective tools for regenerating neural tissue (Jendelova, 2018). Recent studies have identified ferroptosis, an iron-dependent form of programmed cell death driven by membrane lipid peroxidation (Jiang et al., 2021), as playing a crucial role in the pathological process of SCI (Shi et al., 2021). Ferroptosis is regulated by multiple pathways, including cysteine transport, glutathione metabolism, glutathione peroxidase 4 (GPX4) function, and FSP1 protein, and its activation can lead to neuronal dysfunction and increased damage (Li et al., 2022; Ge et al., 2021). Traditional treatments are still ineffective in preventing the occurrence of ferroptosis (Song et al., 2023).
Given the challenges in SCI repair, stem cell therapy has shown unique advantages. Stem cells have significant potential in inhibiting ferroptosis and promoting neural regeneration by differentiating into neurons to replace damaged cells, secreting neurotrophic factors, and regulating the inflammatory microenvironment (Song et al., 2023; Shao et al., 2019). This strategy offers a new research direction for overcoming the existing treatment bottleneck. This review systematically discusses the role of stem cell therapy in SCI treatment, with a focus on ferroptosis as a therapeutic target.
2 Overview of stem cell therapy
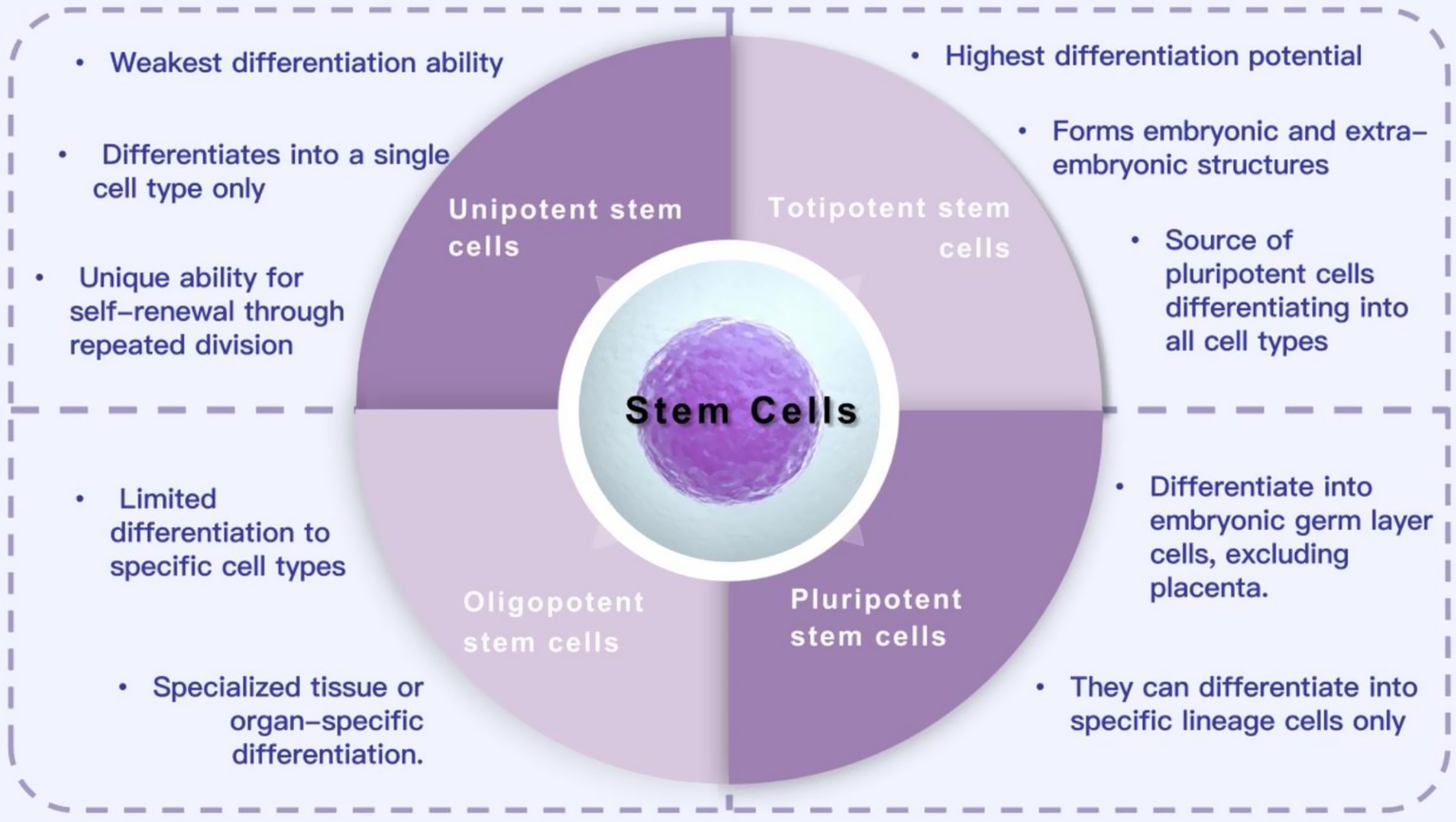
Stem cells are a group of cells with differentiation potential. They have the ability to self-renew, proliferate, and differentiate into various functional cell types through division. They are widely used to generate different tissues and organ systems (Tian et al., 2023). Stem cells come from various sources and have different differentiation potentials. Based on their differentiation ability, they are classified into totipotent, pluripotent, oligopotent, and unipotent stem cells (Kolios and Moodley, 2013; Zakrzewski et al., 2019). Totipotent stem cells have the highest differentiation potential and can form embryos and extraembryonic structures. Pluripotent stem cells (PSCs) can proliferate indefinitely and differentiate into cells of all three germ layers. These characteristics make PSCs an ideal option for cell therapy in various diseases and injuries (Yamanaka, 2020). Oligopotent stem cells can differentiate into multiple cell types, such as myeloid stem cells that can differentiate into white blood cells. Unipotent stem cells have the weakest differentiation ability and can only form one cell type, but they can divide repeatedly. Based on their sources, stem cells can be classified into various types, such as embryonic stem cells, umbilical cord stem cells, bone marrow mesenchymal stem cells, tendon stem cells, dental pulp stem cells, and adipose mesenchymal stem cells (Ankrum et al., 2014; Nombela-Arrieta et al., 2011; Hu et al., 2020; He et al., 2024; Zhang et al., 2023). Currently, stem cells are widely used in regenerative medicine for treating SCI, osteoarthritis, heart disease, retinopathy, diabetes, and other diseases, as well as in tissue engineering and drug testing (Li et al., 2019; Zhang et al., 2015; Mikłosz and Chabowski, 2023; Yu et al., 2022; Cofano et al., 2019; Figure 1).
Various stem cell populations have shown important application potential in SCI regenerative therapy due to their self-renewal characteristics and multi-lineage differentiation potential. Current preclinical research mainly focuses on three types of stem cells: mesenchymal stem cells (MSCs), neural stem cells (NSCs), and PSC-derived cells (Martin-Lopez et al., 2021). Among them, MSCs not only improve the pro-inflammatory microenvironment of the injury site through immunomodulatory effects (Cofano et al., 2019) but also repair neuronal mitochondrial function by mediating mitochondrial transfer, thereby inhibiting the progression of secondary injury (Yao et al., 2023). Additionally, exosomes secreted by MSCs, as intercellular communication mediators, can cross the blood-spinal cord barrier and achieve precise molecular regulation by delivering specific miRNAs, making them unique drug carriers (Liu et al., 2021). NSCs work through a dual mechanism: on one hand, they secrete immunomodulatory and neurotrophic factors; on the other hand, they directly differentiate into neurons and glial cells, significantly improving motor function recovery (Zou et al., 2020; Huang et al., 2021).
PSCs have become a major focus of research due to their ability to differentiate into cells from all three germ layers. They can be induced to produce functional cells from the endoderm, mesoderm, and ectoderm (Tian et al., 2023). As a classic pluripotent cell, embryonic stem cells can differentiate into functional, tissue-specific cells under a specific induction system (Evans and Kaufman, 1981). Transplantation studies in SCI models have confirmed that differentiated neurons and glial cells can accurately integrate into the injured area, effectively promoting the reconstruction of neural circuits (Shao et al., 2019). Germline stem cells also show neural differentiation potential. For example, spermatogonial stem cells can trans-differentiate into functional neurons under specific culture conditions (Glaser et al., 2008). These findings not only reveal the diversity of stem cell mechanisms (including cell replacement, paracrine regulation, and exosome-mediated molecular delivery) but also lay a theoretical foundation for the development of multi-target combined treatment strategies.
The application of stem cell therapy in SCI has developed a multi-level research system from basic studies to clinical translation. Human PSCs have shown significant potential for neural repair in preclinical studies due to their strong neural differentiation ability (Farzaneh et al., 2020). In the non-human primate (common marmoset) SCI model, transplantation of human induced PSC-derived neural precursor cells not only achieves non-tumorigenic differentiation but also significantly improves motor function through axon regeneration mediated by new neurons (Nagoshi et al., 2020). However, clinical translation still faces significant challenges. Existing clinical trial data show that stem cell transplantation therapies (e.g., MSCs, adipose-derived MSCs) have good safety profiles but have not demonstrated significant functional improvements (Oh et al., 2016; Bydon et al., 2024). This discrepancy between preclinical research and clinical application may be due to factors such as mismatches in stem cell survival rate, transplantation timing, and microenvironment regulation, as well as the lack of effective treatment strategies (Yamazaki et al., 2020).
Optimizing the stem cell delivery system (e.g., biomaterial scaffold loading, targeted homing technology) or implementing combined treatment strategies (e.g., co-delivery with neurotrophic factors, electrical stimulation synergy) may help overcome the current treatment bottleneck. These findings provide an important theoretical basis for developing a precise and time-controlled stem cell treatment system.
3 Ferroptosis and SCI
Ferroptosis is an iron-dependent form of programmed cell death driven by phospholipid peroxidation. The core mechanism involves the inactivation of GPX4 or inhibition of the cystine/glutamate antiporter (System Xc−), leading to the collapse of antioxidant defenses, accumulation of lipid peroxidation-derived reactive oxygen species (ROS), and ultimately, oxidative cell death (Li et al., 2020; Liu et al., 2022; Tang et al., 2021). This process relies on the peroxidation of polyunsaturated fatty acids and free radical chain reactions involving iron ions. Ferroptosis plays physiological roles in tumor suppression, immune surveillance, and the elimination of drug-resistant cancer cells. It is also involved in pathological processes, including inflammation, neurodegenerative diseases, and ischemia–reperfusion injury. Its regulatory network encompasses redox homeostasis, iron metabolism, lipid metabolism, and mitochondrial activity (Jiang et al., 2021; Sun et al., 2020; Wu et al., 2021; Chen et al., 2021).
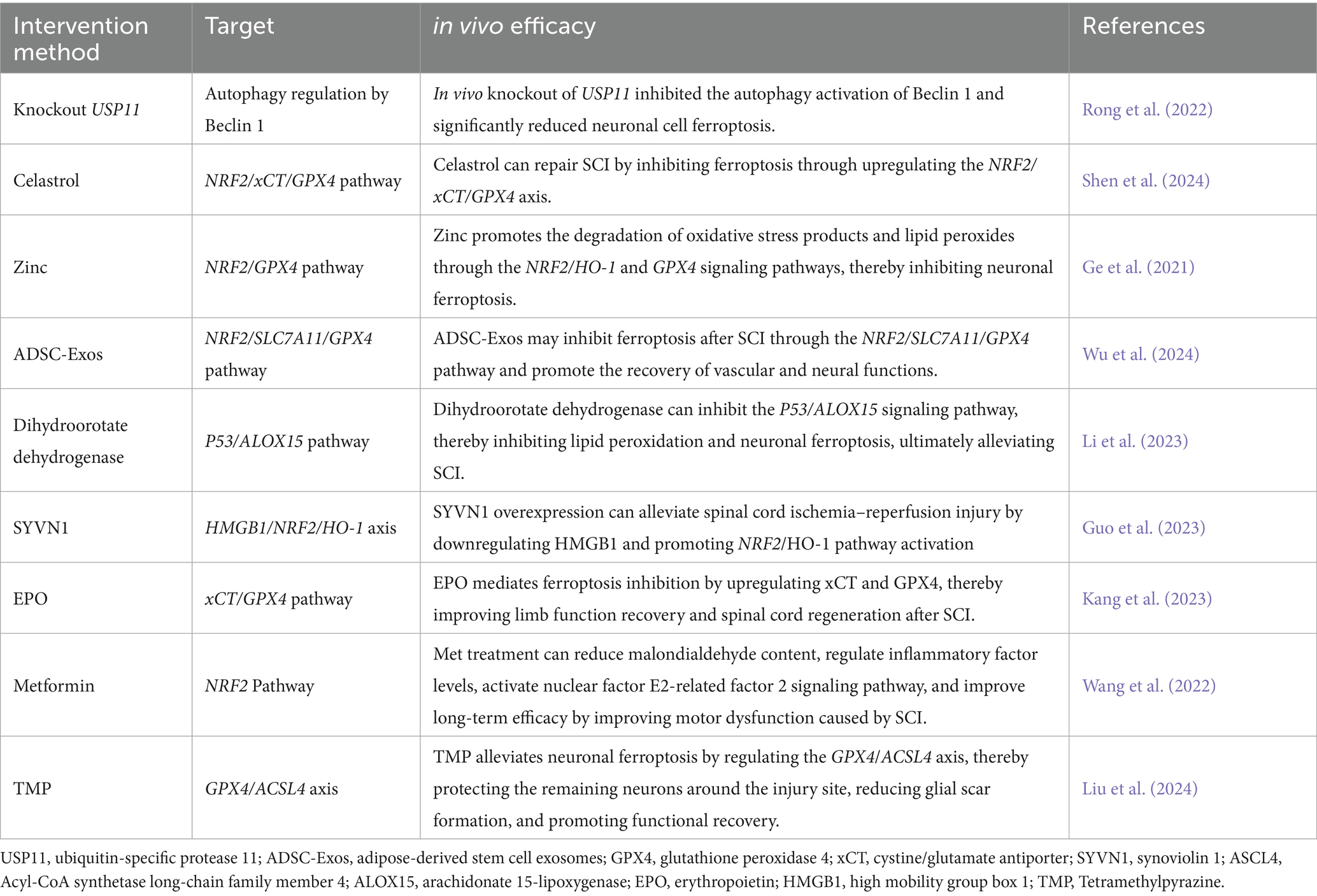
In the pathological process of SCI, ferroptosis exacerbates secondary neural injury through a multi-step mechanism. Local bleeding from the primary injury leads to red blood cell rupture and the release of heme, which is degraded to generate free iron (Fe3+). This iron enters the cell through transferrin receptor (TFRC) endocytosis and is reduced to Fe2+ (Ohgami et al., 2005), forming an unstable labile iron pool (Breuer et al., 2008). Fe2+ catalyzes the generation of hydroxyl radicals (·OH) through the Fenton reaction, triggering lipid peroxidation of polyunsaturated fatty acid phospholipids (PUFA-PLs). This generates lipid peroxides (PLOOHs), which disrupt cell membrane integrity and release damage-associated molecular patterns (DAMPs), further activating inflammatory responses (Reis and Spickett, 2012; Valko et al., 2005). Simultaneously, dysfunction of System Xc− leads to insufficient glutathione (GSH) synthesis, resulting in the loss of GSH-dependent GPX4 activity and an inability to reduce PLOOHs (Zhang et al., 2021; Zheng and Conrad, 2020). The insufficient compensation of the Ferroptosis inhibitory protein 1-ubiquinone pathway further exacerbates the lipid free radical chain reaction. In the inflammatory microenvironment, microglia release nitric oxide (NO) and proinflammatory factors (e.g., TNF-α), upregulate iron uptake-related proteins, and inhibit iron export, establishing a vicious cycle of “iron overload-oxidative stress-inflammation” (Feng et al., 2021). Oligodendrocytes, which are rich in PUFA-PLs, are highly sensitive to ferroptosis. Their death leads to myelin disintegration and white matter damage, hindering nerve conduction. Experimental studies have shown that iron chelators (e.g., deferoxamine) inhibit the Fenton reaction by reducing free iron levels. Additionally, zinc enhances antioxidant defenses by activating the NRF2 pathway, and targeting Acyl-CoA synthetase long-chain family member 4 (ACSL4) or supplementing monounsaturated fatty acids can regulate lipid metabolism. These strategies significantly improve neurological function recovery in animal models and provide potential targets for clinical intervention (Li et al., 2022; Figure 2).
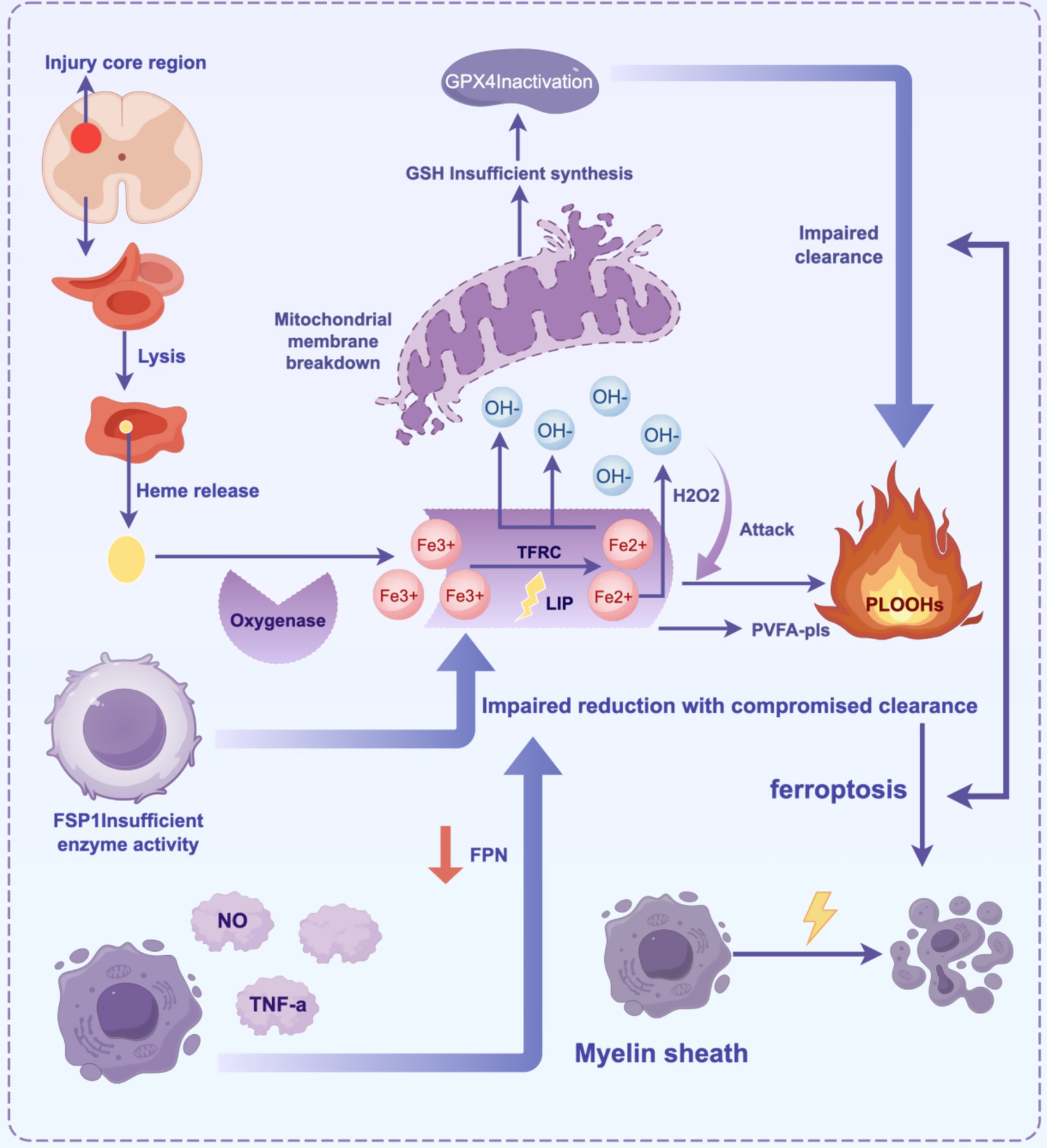
Figure 2. The core mechanism of ferroptosis in SCI involves the cascade of “iron overload-lipid peroxidation-antioxidant imbalance-inflammatory cycle”.
Primary injury leads to bleeding, and red blood cells release heme, which degrades into Fe3+. This iron is endocytosed and reduced to Fe2+ by TFRC, forming an unstable LIP. Fe2+ generates OḤ through the Fenton reaction, which attacks membrane PUFA-PLs, triggering lipid peroxidation and generating PLOOHs. This destroys membrane structure and releases DAMPs, which activate inflammation. The collapse of the antioxidant system occurs when System Xc− dysfunction reduces GSH synthesis, and GPX4 inactivation prevents the clearance of PLOOHs. The FSP1-CoQ10 pathway provides insufficient compensation, aggravating oxidative damage. Activated microglia in the inflammatory microenvironment release NO/TNF-α, upregulate TFRC, and inhibit FPN, forming a cycle of “iron accumulation → oxidative stress → inflammation.” Oligodendrocytes are sensitive to ferroptosis due to the high concentration of PUFA-PLs in myelin. Their death triggers demyelination and white matter damage (Table 1).
4 Application of stem cell therapy targeting ferroptosis in SCI
The previous article described the mechanism of ferroptosis after spinal cord injury. Concurrently, studies have shown that ferroptosis is an important pathological process of neuronal death after spinal cord injury. Once iron overload, ROS accumulation, lipid peroxidation or characteristic mitochondrial changes occur in cells, ferroptosis will occur rapidly (Berndt et al., 2024; Zheng et al., 2024). Therefore, inhibiting lipid peroxidation and iron overload can be an important way to treat spinal cord injury by targeted control of ferroptosis. Stem cells can interfere with ferroptosis through complex molecular networks, including RNA regulation, mitochondrial repair, and activation of key signaling pathways, inhibiting lipid peroxidation and iron overload, thereby inhibiting ferroptosis and promoting spinal cord injury repair. The following article will describe the specific mechanism of stem cell targeted control of ferroptosis to treat spinal cord injury.
Recent studies have demonstrated that stem cell-derived exosomes play a multi-target role in inhibiting ferroptosis after SCI by delivering non-coding RNA or regulating key signaling pathways (Li et al., 2023). For example, long non-coding RNA lncGm36569, enriched in mesenchymal stem cell exosomes (MSC-exos), binds to miR-5627-5p, relieving its transcriptional inhibition of FSP1 and enhancing the antioxidant capacity of neurons (Shao et al., 2022). In microRNA-mediated mechanisms, miR-219-5p delivered by bone marrow mesenchymal stem cell exosomes (BMSC-exos) inhibits the ubiquitination degradation of NRF2 by targeting the ubiquitin-binding enzyme UBE2Z. This stabilizes NRF2 protein expression and activates the downstream SLC7A11/GPX4 axis, reducing lipid peroxidation (Dong et al., 2024). Another study showed that miR-26a-5p delivered by BMSC-exos inhibited the expression of Enhancer of Zeste Homolog 2, promoting the activation of the BDNF/TrkB signaling pathway and the phosphorylation of cAMP response element-binding protein. Additionally, miR-26a-5p upregulated the K+-Cl− co-transporter 2, which significantly improved Lipopolysaccharide-induced Pheochromocytoma Cell Line 12 cell injury and promoted spinal cord function recovery (Chen et al., 2024). At the circular RNA level, Shao et al. generated hypoxia-induced adipose-derived stem cell-derived exosomes (ADSC-exo) by pre-treating ADSCs under hypoxic conditions (93% N₂, 5% CO₂, 2% O₂). These exosomes specifically carried circ-Wdfy3, which acted as a molecular sponge, adsorbing miR-423-3p and preventing its degradation of GPX4 mRNA. This intervention significantly reduced ROS accumulation and inflammatory factors (Shao et al., 2024). In terms of signaling pathway regulation, Wen et al. demonstrated that BMSC-exos inhibited the IL-17 pathway, reduced levels of Fe2+, malondialdehyde, and ROS in the SCI model, upregulated the expression of GSH, GPX4, and cystine/glutamate antiporter, and downregulated the long-chain family member 4 of acyl-CoA synthetase. This combination of actions synergistically inhibited ferroptosis and promoted functional recovery (Tang et al., 2024).
This non-coding RNA and pathway-based regulatory network is highly specific in terms of spatiotemporal control, enabling intervention at key points in the ferroptosis process. It provides a promising strategy for the precise treatment of SCI.
Mitochondria, crucial for regulating energy metabolism in eukaryotic cells, also play an indispensable role in cell proliferation, differentiation, immune responses, and redox balance (Nunnari and Suomalainen, 2012; Ni et al., 2015). In response to various physiological signals or external stimuli, mitochondria have developed a complex set of mitochondrial quality control (MQC) mechanisms, encompassing mitochondrial biogenesis, dynamics, and autophagy (Liu et al., 2024; Ashrafi and Schwarz, 2013). MQC is essential for cells to cope with internal and external stresses, maintaining mitochondrial function and homeostasis. This process involves multiple regulatory levels, including mitochondrial-nuclear communication (mitochondrial retrograde signaling), changes in mitochondrial morphology (mitochondrial dynamics), and selective removal of damaged mitochondria (mitochondrial autophagy) (Al Amir Dache and Thierry, 2023). Through these mechanisms, cells can sense and respond to stress, reshaping the mitochondrial network and removing dysfunctional mitochondria, thereby ensuring systemic mitochondrial function and intracellular homeostasis (Roca-Portoles and Tait, 2021; Rahman and Quadrilatero, 2021).
MSCs transfer functional mitochondria to injured neurons through intercellular tunneling nanotubes (TNTs), directly intervening in MQC imbalance. This process is crucial for maintaining mitochondrial homeostasis and promoting neuronal survival (Hsu et al., 2016). After SCI, neurons experience ferroptosis due to excessive mitochondrial fission and abnormal mitochondrial autophagy. Single-cell transcriptome analysis revealed significant upregulation of neuronal ferroptosis markers, such as TFRC and 4-Hydroxynonenal, while mitochondrial morphology showed shrinkage and cristae destruction. MSC-derived mitochondria restored mitochondrial dynamic balance by fusing with the neuronal mitochondrial network, inhibiting excessive autophagy mediated by PINK1/Parkin. In vitro experiments demonstrated that MSC co-culture significantly reduced mitochondrial ROS in neurons, restored membrane potential and ATP levels, and decreased lipid peroxidation products (MDA) and free iron content. In vivo, the transplantation of MSCs improved neuronal mitochondrial morphology and promoted motor function recovery through TNT-dependent mitochondrial transfer. The TNT inhibitor cytochalasin D completely reversed this effect. This mechanism reveals a novel way for MSCs to inhibit neuronal ferroptosis at the sub-organelle level by repairing mitochondrial function and reducing the expression of ferroptosis-related markers, offering an experimental basis for neuroprotective strategies targeting mitochondrial metabolism (Yao et al., 2023).
ADSC-Exos activated NRF2 nuclear translocation, upregulated System Xc− expression, promoted GSH synthesis, and enhanced GPX4 activity to clear lipid peroxides. In the oxygen–glucose deprivation/reperfusion-induced endothelial cell injury model, ADSC-Exos treatment reduced intracellular ROS, increased GSH levels, and reversed the abnormal accumulation of MDA, a marker of ferroptosis. Immunofluorescence and Western blot analysis revealed that ADSC-Exos significantly upregulated NRF2 nuclear expression and the downstream SLC7A11/GPX4 pathway. NRF2 inhibition with ML385 blocked these effects, confirming the critical role of this pathway in inhibiting ferroptosis. In vivo, ADSC-Exos promoted angiogenesis in the SCI area and improved motor function. This mechanism synergistically inhibited ferroptosis at the molecular level by enhancing cystine uptake, GSH metabolism, and lipid peroxidation repair, providing a new strategy for targeted vascular-neuron repair (Wu et al., 2024). BMSC-exos reduce ACSL4 expression and ROS levels by inhibiting the IL-17 signaling pathway. Studies have shown that IL-17 pathway activation after SCI promotes ACSL4-mediated lipid peroxidation, leading to neuronal ferroptosis. Following BMSC-exo intervention, the expression of IL-17 downstream inflammatory factors (such as IL-17A, IL-17RA, Act1) decreased, as did ACSL4 protein levels and ROS generation. In vitro experiments further demonstrated that IL-17 neutralizing antibodies could mimic the effects of BMSC-exos (Tang et al., 2024).
5 Biomaterials synergy
While many studies have confirmed that stem cells can intervene in ferroptosis and promote SCI neuron repair, their effectiveness is often limited by the local microenvironment. Therefore, understanding the stem cell microenvironment and developing a biomaterial synergy with stem cell engineering delivery systems are critical for improving the success rate of SCI repair treatments (Pennings et al., 2018; Papa et al., 2020; Kubinová, 2020).
The shear-thinning terephthalic acid (TPA)@Laponite hydrogel, loaded with dental pulp stem cells (DPSCs), removes ROS and regulates synaptic balance through both physical and chemical effects (Ying et al., 2023). The hydrogel demonstrates excellent physical properties, allowing it to remain in the body for an extended period. Its shear-thinning properties enable it to adapt to the mechanical microenvironment of the spinal cord, remove lipid ROS in the injury area, inhibit lipid peroxidation, and regulate the balance between inhibitory and excitatory neurons. This is achieved through the secretion of neurotrophic factors by DPSCs, promoting SCI recovery. While TPA@Laponite hydrogel can reduce the entanglement of blood vessels and fibrous scars, it cannot significantly promote vascular function recovery. In contrast, DPSCs can reduce fibrous scar formation, differentiate into neural and vascular cells, and aid in vascular function restoration. In vivo experiments revealed that combined treatment reduced muscle spasms caused by excessive excitation and promoted motor function recovery.
Small extracellular vesicles-loaded N-acryloylglycinamide/gelatin methacrylate/laponite/tannic acid hydrogel (sEVs-NGL/T) integrates the antioxidant and anti-inflammatory properties of tannic acid with the mechanical support function of laponite to construct a three-dimensional scaffold that offers sustained release and bioactivity. The hydrogel is characterized by a porous network structure, good degradation stability, and a low initial degradation rate, providing long-lasting mechanical support. Its moderate swelling properties prevent compression of surrounding tissues after implantation. In vitro experiments demonstrated that sEVs-NGL/T hydrogel exhibited significant antioxidant capacity in a simulated peroxidative microenvironment, effectively scavenging DPPH free radicals and reducing H₂O₂ concentration. Additionally, it enhanced the ROS protection of PC12 cells through the continuous release of sEVs derived from mesenchymal stem cells. In a rat model of spinal cord complete transection, the implantation of sEVs-NGL/T significantly improved motor function recovery, reduced cystic cavity formation at the injury site, and promoted neural tissue repair by inhibiting excessive astrocyte proliferation and encouraging nerve fiber regeneration. Furthermore, sEVs-NGL/T synergistically alleviated the inflammatory response by regulating the ROS microenvironment, reducing the levels of lipid peroxidation products (4-Hydroxynonenal and 8-Hydroxy-2′-deoxyguanosine), and inhibiting the release of proinflammatory cytokines (TNF-α, IL-6, IL-1β). The sustained release of sEVs enhanced the local anti-inflammatory and antioxidant effects (Liu et al., 2022). Studies have shown that human umbilical mesenchymal stem cells (Huc-MSCs) can improve the inflammatory microenvironment of SCI and promote nerve regeneration. However, due to their lack of inherent targeting ability and rapid clearance by immune cells, their efficacy is limited by the local SCI microenvironment (Wu et al., 2019; Bonilla et al., 2021). Therefore, the researchers developed a synergistic Huc-MSCs and ferroptosis inhibitor nanoparticle sustained-release system. The ROS-sensitive nanosystem, mPEG-b-Lys-BECI-TCO, anchors Huc-MSCs through a CD44 targeting sequence (Tz-A6 peptide) and loads a novel ferroptosis inhibitor, Feb-1. In the SCI microenvironment, high ROS concentrations trigger the degradation of nanocarriers and the release of Feb-1, in combination with Huc-MSC transplantation therapy. Experimental results showed that this combined therapy significantly promoted the expression of GPX4 and xCT signaling pathways, reduced neuronal loss, and improved motor function recovery in rats by inhibiting ferroptosis and inflammatory responses. Furthermore, Western blot analysis revealed that the expression of proinflammatory factors (iNOS and IL-1β) was significantly reduced in the combined treatment group, and immunofluorescence staining confirmed increased survival of neurons in the injury area (Hua et al., 2024).
6 Clinical trial cases
6.1 Clinical trial cases and efficacy differences
A phase I study for subacute spinal cord injury (Akhlaghpasand et al., 2024) showed that 9 patients received intrathecal injection of human umbilical cord mesenchymal stem cells (HUC-MSCs) exosomes and no treatment-related adverse events occurred. The efficacy evaluation showed that the American Spinal Injury Association (ASIA) scale score improved 6 months (37.89 ± 20.65, p = 0.066) and 12 months (38.22 ± 20.95, p = 0.066) after injection compared with the baseline (36.22 ± 20.92), but due to the small sample size, the efficacy conclusion still needs to be interpreted with caution. Among patients with chronic spinal cord injury, 3 patients received combined transplantation treatment (Zamani et al., 2022). No motor function recovery was observed in the two-year follow-up, which only confirmed the safety of the treatment. The efficacy of a phase III trial (Oh et al., 2016) showed that only 2 of the 16 patients followed up had improved upper limb motor grade, and the remaining 14 showed no improvement in the 6-month follow-up, accounting for only 12.5% of the patients. This shows that autologous MSCs transplantation has limited improvement in functional recovery after spinal cord injury, further highlighting the efficacy bottleneck of single therapy. Current clinical trials generally have problems such as small sample size and inconsistent efficacy evaluation tools, resulting in the effectiveness of stem cell therapy has not been fully verified. In the future, larger-scale controlled clinical trials are needed to verify the effectiveness (Jiang et al., 2013).
6.2 Key barriers to clinical translatio
Most of the current research conclusions are quite different from those of animal experiments. The reason is that some studies believe that the period, site, and dose of stem cell transplantation have a great impact on prognosis. For example, acute application will expose stem cells to cytotoxic environments such as excitatory transmitters, reactive oxygen species, and inflammatory molecules, which will affect the survival rate of stem cells. Some injured parts can be injected with stem cells, but they are not suitable for stem cell survival due to low blood perfusion, while the normal proximal spinal cord above the injured part is at risk of re-injury due to high tissue pressure (Oh et al., 2016). However, this is only a conjecture and speculation stage, which needs to be further verified by control experiments, and is also one of the directions for future exploration of stem cell transplantation therapy. In addition, allogeneic stem cells may trigger immune rejection reactions, and the long-term tumorigenic risk (such as teratoma) still needs more follow-up data support.
7 Outlook and conclusion
The stem cell-targeted ferroptosis strategy has opened a new avenue for SCI treatment. By intervening in the key pathways of ferroptosis through multiple targets, this approach demonstrates significant neuroprotective and regenerative potential. When combined with a biomaterial delivery system, it can further enhance stem cell survival and targeting efficiency, overcoming the limitations of the local microenvironment and enabling precise, time- controlled treatments. In addition, exosome-mediated RNA regulation and mitochondrial transfer mechanisms offer innovative strategies for developing cell-free therapies, which may help circumvent the ethical and safety concerns associated with traditional stem cell transplantation.
Although preclinical studies have confirmed the therapeutic potential of stem cell-targeted ferroptosis strategies in animal models, clinical translation still faces substantial challenges, including a lack of clinical trial data and insufficient evidence of efficacy (Oh et al., 2016; Akhlaghpasand et al., 2024; Zamani et al., 2022; Shin et al., 2015). Due to the physiological and anatomical differences between animal models and human SCI, the immune responses also vary. These differences encompass factors such as SCI level, severity, timing, stem cell heterogeneity, low survival rates post-transplantation, inhibitory effects from the local microenvironment, and long-term tumorigenic risks. These issues have yet to be systematically addressed, creating a significant gap between basic research and clinical application (Lukovic et al., 2014; Lukovic et al., 2012).
Future research should focus on the following areas: (1) deepening the study of ferroptosis regulation and stem cell mechanisms; (2) clarifying the spatiotemporal expression patterns and synergistic effects of key molecules; (3) optimizing stem cell engineering strategies to enhance their antioxidant, anti-inflammatory, and exosome secretion capabilities through gene editing; (4) improving survival rates; (5) promoting multimodal treatments, such as stem cell-material-electrical stimulation synergy, while integrating microenvironment regulation and functional reconstruction (Jiang et al., 2013; Li et al., 2024); (6) For ethical optimization of alternative cell sources, it is recommended to give priority to adult stem cells with less ethical controversy to reduce dependence on embryonic stem cells (Lo and Parham, 2009).
Clinical translation faces several challenges: (1) stem cell heterogeneity leads to fluctuations in efficacy, and a standardized quality control system must be established; (2) differences between animal models and human pathology could undermine treatment efficacy, requiring verification through organoids or non-human primate models; and (3) long-term safety concerns (e.g., tumorigenicity and immune rejection) and large-scale preparation processes need further improvement (Shin et al., 2015). (4) The standardization of the ethical review framework and the ethical transparency of clinical transformation require the establishment of an interdisciplinary ethical review mechanism covering the legitimacy of stem cell sources and safety assessment of gene editing technology (Barker et al., 2018); emphasis is placed on improving the transparency and social acceptance of stem cell therapy and reducing ethical resistance in technology promotion through the disclosure of clinical trial data, optimization of the patient informed consent process, and public scientific education (King and Perrin, 2014; McCaughey et al., 2016). Despite these obstacles, with the continued integration of precision medicine and regenerative technologies, the stem cell-targeted ferroptosis strategy holds great promise for bridging the gap from laboratory research to clinical application, offering breakthrough treatment options for SCI patients in the future.
Author contributions
QS: Conceptualization, Writing – review & editing, Writing – original draft. LW: Writing – review & editing, Writing – original draft. CC: Writing – review & editing, Funding acquisition. BL: Writing – review & editing, Writing – original draft. FY: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by Medical Scientific Research Foundation of Zhejiang Province (2020KY841) and Project of National Key Clinical Specialty (Department of Medical Imaging), grant no. 2024017 and NINGBO Leading Medical&Health Discipline (No. 2022-S02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akhlaghpasand, M., Tavanaei, R., Hosseinpoor, M., Yazdani, K. O., Soleimani, A., Zoshk, M. Y., et al. (2024). Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res Ther 15:264. doi: 10.1186/s13287-024-03868-0
Al Amir Dache, Z., and Thierry, A. R. (2023). Mitochondria-derived cell-to-cell communication. Cell Rep. 42:112728. doi: 10.1016/j.celrep.2023.112728
Anjum, A., Yazid, M. D., Fauzi Daud, M., Idris, J., Athi Kumar, R. K., and Lokanathan, Y. (2020). Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 21:7533. doi: 10.3390/ijms21207533
Ankrum, J. A., Ong, J. F., and Karp, J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260. doi: 10.1038/nbt.2816
Ashrafi, G., and Schwarz, T. L. (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20, 31–42. doi: 10.1038/cdd.2012.81
Barker, R. A., Carpenter, M. K., Forbes, S., Goldman, S. A., Jamieson, C., Murry, C. E., et al. (2018). The challenges of first-in-human stem cell clinical trials: what does this mean for ethics and institutional review boards? Stem Cell Reports. 10, 1429–1431. doi: 10.1016/j.stemcr.2018.04.010
Berndt, C., Alborzinia, H., Amen, V. S., Ayton, S., Barayeu, U., Bartelt, A., et al. (2024). Ferroptosis in health and disease. Redox Biol. 75:103211. doi: 10.1016/j.redox.2024.103211
Bonilla, P., Hernandez, J., Giraldo, E., González-Pérez, M. A., Alastrue-Agudo, A., Elkhenany, H., et al. (2021). Human-induced neural and mesenchymal stem cell therapy combined with a curcumin Nanoconjugate as a spinal cord injury treatment. IJMS. 22:5966. doi: 10.3390/ijms22115966
Breuer, W., Shvartsman, M., and Cabantchik, Z. I. (2008). Intracellular labile iron. Int. J. Biochem. Cell Biol. 40, 350–354. doi: 10.1016/j.biocel.2007.03.010
Bydon, M., Qu, W., Moinuddin, F. M., Hunt, C. L., Garlanger, K. L., Reeves, R. K., et al. (2024). Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: phase I trial. Nat. Commun. 15:2201. doi: 10.1038/s41467-024-46259-y
Chen, X., Kang, R., Kroemer, G., and Tang, D. (2021). Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18, 280–296. doi: 10.1038/s41571-020-00462-0
Chen, M., Lin, Y., Guo, W., and Chen, L. (2024). BMSC-derived exosomes carrying miR-26a-5p ameliorate spinal cord injury via negatively regulating EZH2 and activating the BDNF-TrkB-CREB signaling. Mol. Neurobiol. 61, 8156–8174. doi: 10.1007/s12035-024-04082-y
Cofano, F., Boido, M., Monticelli, M., Zenga, F., Ducati, A., Vercelli, A., et al. (2019). Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. IJMS. 20:2698. doi: 10.3390/ijms20112698
Dong, J., Gong, Z., Bi, H., Yang, J., Wang, B., du, K., et al. (2024). BMSC-derived exosomal miR-219-5p alleviates ferroptosis in neuronal cells caused by spinal cord injury via the UBE2Z/NRF2 pathway. Neuroscience 556, 73–85. doi: 10.1016/j.neuroscience.2024.06.011
Eli, I., Lerner, D. P., and Ghogawala, Z. (2021). Acute traumatic spinal cord injury. Neurol. Clin. 39, 471–488. doi: 10.1016/j.ncl.2021.02.004
Evans, M. J., and Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156.
Fan, B., Wei, Z., Yao, X., Shi, G., Cheng, X., Zhou, X., et al. (2018). Microenvironment imbalance of spinal cord injury. Cell Transplant. 27, 853–866. doi: 10.1177/0963689718755778
Farzaneh, M., Anbiyaiee, A., and Khoshnam, S. E. (2020). Human pluripotent stem cells for spinal cord injury. CSCR. 15, 135–143. doi: 10.2174/1574362414666191018121658
Fehlings, M. G., Tetreault, L. A., Wilson, J. R., Kwon, B. K., Burns, A. S., Martin, A. R., et al. (2017). A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale, and scope. Glob. Spine J. 7, 84S–94S. doi: 10.1177/2192568217703387
Feng, Z., Min, L., Chen, H., Deng, W., Tan, M., Liu, H., et al. (2021). Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 43:101984. doi: 10.1016/j.redox.2021.101984
Gao, L., Peng, Y., and Xu, W. (2020). Progress in stem cell therapy for spinal cord injury. Petrenko, Y. Stem Cells Int. 2020, 1–16. doi: 10.1155/2020/2853650
Ge, M., Tian, H., Mao, L., Li, D. Y., Lin, J. Q., Hu, H. S., et al. (2021). Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci. Ther. 27, 1023–1040. doi: 10.1111/cns.13657
Glaser, T., Opitz, T., Kischlat, T., Konang, R., Sasse, P., Fleischmann, B. K., et al. (2008). Adult germ line stem cells as a source of functional neurons and glia. Stem Cells 26, 2434–2443. doi: 10.1634/stemcells.2008-0163
Guo, L., Zhang, D., Ren, X., and Liu, D. (2023). SYVN1 attenuates ferroptosis and alleviates spinal cord ischemia–reperfusion injury in rats by regulating the HMGB1/NRF2/HO-1 axis. Int. Immunopharmacol. 123:110802. doi: 10.1016/j.intimp.2023.110802
He, W., Jiang, C., Zhou, P., Hu, X., Gu, X., and Zhang, S. (2024). Role of tendon-derived stem cells in tendon and ligament repair: focus on tissue engineer. Front. Bioeng. Biotechnol. 12:1357696. doi: 10.3389/fbioe.2024.1357696
He, W., Li, Z. q., Gu, H. y., Pan, Q. l., and Lin, F. x. (2024). Targeted therapy of spinal cord injury: inhibition of apoptosis is a promising therapeutic strategy. Mol. Neurobiol. 61, 4222–4239. doi: 10.1007/s12035-023-03814-w
Hsu, Y. C., Wu, Y. T., Yu, T. H., and Wei, Y. H. (2016). Mitochondria in mesenchymal stem cell biology and cell therapy: from cellular differentiation to mitochondrial transfer. Semin. Cell Dev. Biol. 52, 119–131. doi: 10.1016/j.semcdb.2016.02.011
Hu, C., Wu, Z., and Li, L. (2020). Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 16, 893–903. doi: 10.7150/ijbs.39725
Hua, R., Zhao, C., Xu, Z., Liu, D., Shen, W., Yuan, W., et al. (2024). ROS-responsive nanoparticle delivery of ferroptosis inhibitor prodrug to facilitate mesenchymal stem cell-mediated spinal cord injury repair. Bioact Mater. 38, 438–454. doi: 10.1016/j.bioactmat.2024.05.015
Huang, L., Fu, C., Xiong, F., He, C., and Wei, Q. (2021). Stem cell therapy for spinal cord injury. Cell Transplant. 30:0963689721989266. doi: 10.1177/0963689721989266
Hughes, J. T. (1988). The Edwin smith surgical papyrus: an analysis of the first case reports of spinal cord injuries. Paraplegia 26, 71–82.
Jendelova, P. (2018). Therapeutic strategies for spinal cord injury. IJMS. 19:3200. doi: 10.3390/ijms19103200
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282. doi: 10.1038/s41580-020-00324-8
Jiang, P. C., Xiong, W. P., and Wang, G. (2013). A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 6, 140–146. doi: 10.3892/etm.2013.1083
Kang, Y., Zhu, R., Li, S., Qin, K. P., Tang, H., Shan, W. S., et al. (2023). Erythropoietin inhibits ferroptosis and ameliorates neurological function after spinal cord injury. Neural Regen. Res. 18, 881–888. doi: 10.4103/1673-5374.353496
Khorasanizadeh, M., Yousefifard, M., Eskian, M., Lu, Y., Chalangari, M., Harrop, J. S., et al. (2019). Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J. Neurosurg. Spine 30, 683–699. doi: 10.3171/2018.10.SPINE18802
King, N. M., and Perrin, J. (2014). Ethical issues in stem cell research and therapy. Stem Cell Res Ther 5:85. doi: 10.1186/scrt474
Kolios, G., and Moodley, Y. (2013). Introduction to stem cells and regenerative medicine. Respiration 85, 3–10. doi: 10.1159/000345615
Kubinová, Š. (2020). Biomaterials and magnetic stem cell delivery in the treatment of spinal cord injury. Neurochem. Res. 45, 171–179. doi: 10.1007/s11064-019-02808-2
Li, J., Cao, F., Yin, H. L., Huang, Z. J., Lin, Z. T., Mao, N., et al. (2020). Ferroptosis: past, present and future. Cell Death Dis. 11:88. doi: 10.1038/s41419-020-2298-2
Li, J. Z., Fan, B. Y., Sun, T., Wang, X. X., Li, J. J., Zhang, J. P., et al. (2023). Bioinformatics analysis of ferroptosis in spinal cord injury. Neural Regen. Res. 18, 626–633. doi: 10.4103/1673-5374.350209
Li, D., Lu, X., Xu, G., Liu, S., Gong, Z., Lu, F., et al. (2023). Dihydroorotate dehydrogenase regulates ferroptosis in neurons after spinal cord injury via the P53-ALOX15 signaling pathway. CNS Neurosci. Ther. 29, 1923–1939. doi: 10.1111/cns.14150
Li, C., Luo, Y., and Li, S. (2024). The roles of neural stem cells in myelin regeneration and repair therapy after spinal cord injury. Stem Cell Res Ther 15:204. doi: 10.1186/s13287-024-03825-x
Li, B., Meng, X., and Zhang, L. (2019). microRNAs and cardiac stem cells in heart development and disease. Drug Discov. Today 24, 233–240. doi: 10.1016/j.drudis.2018.05.032
Li, F., Wang, H., Chen, H., Guo, J., Dang, X., Ru, Y., et al. (2022). Mechanism of Ferroptosis and its role in spinal cord injury. Front. Neurol. 13:926780. doi: 10.3389/fneur.2022.926780
Liu, G., Deng, B., Huo, L., Fan, X., Bai, H., Zhao, Y., et al. (2024). Tetramethylpyrazine alleviates ferroptosis and promotes functional recovery in spinal cord injury by regulating GPX4/ACSL4. Eur. J. Pharmacol. 977:176710. doi: 10.1016/j.ejphar.2024.176710
Liu, Z., Guo, S., Dong, L., Wu, P., Li, K., Li, X., et al. (2022). A tannic acid doped hydrogel with small extracellular vesicles derived from mesenchymal stem cells promotes spinal cord repair by regulating reactive oxygen species microenvironment. Mater Today, Bio. 16:100425. doi: 10.1016/j.mtbio.2022.100425
Liu, J., Kang, R., and Tang, D. (2022). Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 289, 7038–7050. doi: 10.1111/febs.16059
Liu, W., Ma, Z., Li, J., and Kang, X. (2021). Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther 12:102. doi: 10.1186/s13287-021-02153-8
Liu, B. H., Xu, C. Z., Liu, Y., Lu, Z. L., Fu, T. L., Li, G. R., et al. (2024). Mitochondrial quality control in human health and disease. Mil. Med. Res. 11:32. doi: 10.1186/s40779-024-00536-5
Lo, B., and Parham, L. (2009). Ethical issues in stem cell research. Endocr. Rev. 30, 204–213. doi: 10.1210/er.2008-0031
Lukovic, D., Moreno Manzano, V., Stojkovic, M., Bhattacharya, S. S., and Erceg, S. (2012). Concise review: human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells 30, 1787–1792. doi: 10.1002/stem.1159
Lukovic, D., Stojkovic, M., Moreno-Manzano, V., Bhattacharya, S. S., and Erceg, S. (2014). Perspectives and future directions of human pluripotent stem cell-based therapies: lessons from geron’s clinical trial for spinal cord injury. Stem Cells Dev. 23, 1–4. doi: 10.1089/scd.2013.0266
Martin-Lopez, M., Fernandez-Muñoz, B., and Canovas, S. (2021). Pluripotent stem cells for spinal cord injury repair. Cells 10:3334. doi: 10.3390/cells10123334
McCaughey, T., Chen, C. Y., and De Smit, E. (2016). Participant understanding and recall of informed consent for induced pluripotent stem cell biobanking. Cell Tissue Bank. 17, 449–456. doi: 10.1007/s10561-016-9563-8
Mikłosz, A., and Chabowski, A. (2023). Adipose-derived mesenchymal stem cells therapy as a new treatment option for diabetes mellitus. J. Clin. Endocrinol. Metab. 108, 1889–1897. doi: 10.1210/clinem/dgad142
Nagoshi, N., Okano, H., and Nakamura, M. (2020). Regenerative therapy for spinal cord injury using iPSC technology. Inflamm Regener. 40:40. doi: 10.1186/s41232-020-00149-0
Ni, H. M., Williams, J. A., and Ding, W. X. (2015). Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6–13. doi: 10.1016/j.redox.2014.11.006
Nombela-Arrieta, C., Ritz, J., and Silberstein, L. E. (2011). The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 12, 126–131. doi: 10.1038/nrm3049
Nunnari, J., and Suomalainen, A. (2012). Mitochondria: in sickness and in health. Cell 148, 1145–1159. doi: 10.1016/j.cell.2012.02.035
Oh, S. K., Choi, K. H., Yoo, J. Y., Kim, D. Y., Kim, S. J., and Jeon, S. R. (2016). A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 78, 436–447. doi: 10.1227/NEU.0000000000001056
Ohgami, R. S., Campagna, D. R., Greer, E. L., Antiochos, B., McDonald, A., Chen, J., et al. (2005). Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 37, 1264–1269. doi: 10.1038/ng1658
Papa, S., Pizzetti, F., Perale, G., Veglianese, P., and Rossi, F. (2020). Regenerative medicine for spinal cord injury: focus on stem cells and biomaterials. Expert. Opin. Biol. Ther. 20, 1203–1213. doi: 10.1080/14712598.2020.1770725
Pennings, S., Liu, K. J., and Qian, H. (2018). The stem cell niche: interactions between stem cells and their environment. Stem Cells Int. 2018, 1–3. doi: 10.1155/2018/4879379
Rahman, F. A., and Quadrilatero, J. (2021). Mitochondrial network remodeling: an important feature of myogenesis and skeletal muscle regeneration. Cell. Mol. Life Sci. 78, 4653–4675. doi: 10.1007/s00018-021-03807-9
Reis, A., and Spickett, C. M. (2012). Chemistry of phospholipid oxidation. Biochim. Biophys. Acta Biomembr. 1818, 2374–2387. doi: 10.1016/j.bbamem.2012.02.002
Roca-Portoles, A., and Tait, S. W. G. (2021). Mitochondrial quality control: from molecule to organelle. Cell. Mol. Life Sci. 78, 3853–3866. doi: 10.1007/s00018-021-03775-0
Rong, Y., Fan, J., Ji, C., Wang, Z., Ge, X., Wang, J., et al. (2022). USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia-reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ. 29, 1164–1175. doi: 10.1038/s41418-021-00907-8
Shao, C., Chen, Y., Yang, T., Zhao, H., and Li, D. (2022). Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Rev. Rep. 18, 1127–1142. doi: 10.1007/s12015-022-10327-x
Shao, A., Tu, S., Lu, J., and Zhang, J. (2019). Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther 10:238. doi: 10.1186/s13287-019-1357-z
Shao, M., Ye, S., Chen, Y., Yu, C., and Zhu, W. (2024). Exosomes from hypoxic ADSCs ameliorate neuronal damage post spinal cord injury through circ-Wdfy3 delivery and inhibition of ferroptosis. Neurochem. Int. 177:105759. doi: 10.1016/j.neuint.2024.105759
Shen, W., Li, C., Liu, Q., Cai, J., Wang, Z., Pang, Y., et al. (2024). Celastrol inhibits oligodendrocyte and neuron ferroptosis to promote spinal cord injury recovery. Phytomedicine 128:155380. doi: 10.1016/j.phymed.2024.155380
Shi, Z., Yuan, S., Shi, L., Li, J., Ning, G., Kong, X., et al. (2021). Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 54:e12992. doi: 10.1111/cpr.12992
Shin, J. C., Kim, K. N., Yoo, J., Kim, I. S., Yun, S., Lee, H., et al. (2015). Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015:630932. doi: 10.1155/2015/630932
Song, Q. F., Cui, Q., Wang, Y. S., and Zhang, L. X. (2023). Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury. Neural Regen. Res. 1:838. doi: 10.4103/1673-5374.367838
Sun, Y., Chen, P., Zhai, B., Zhang, M., Xiang, Y., Fang, J., et al. (2020). The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 127:110108. doi: 10.1016/j.biopha.2020.110108
Tang, D., Chen, X., Kang, R., and Kroemer, G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125. doi: 10.1038/s41422-020-00441-1
Tang, W., Zhao, K., Li, X., Zhou, X., and Liao, P. (2024). Bone marrow mesenchymal stem cell-derived exosomes promote the recovery of spinal cord injury and inhibit Ferroptosis by inactivating IL-17 pathway. J. Mol. Neurosci. 74:33. doi: 10.1007/s12031-024-02209-3
Tian, Z., Yu, T., Liu, J., Wang, T., and Higuchi, A. (2023). Introduction to stem cells. Prog. Mol. Biol. Transl. Sci. 199, 3–32. doi: 10.1016/bs.pmbts.2023.02.012
Valko, M., Morris, H., and Cronin, M. (2005). Metals, toxicity and oxidative stress. CMC 12, 1161–1208. doi: 10.2174/0929867053764635
Wang, Z., Wu, Z., Xie, Z., Zhou, W., and Li, M. (2022). Metformin attenuates Ferroptosis and promotes functional recovery of spinal cord injury. World Neurosurg. 167, e929–e939. doi: 10.1016/j.wneu.2022.08.121
Wu, S., Chen, Z., Wu, Y., Shi, Q., Yang, E., Zhang, B., et al. (2024). ADSC-exos enhance functional recovery after spinal cord injury by inhibiting ferroptosis and promoting the survival and function of endothelial cells through the NRF2/SLC7A11/GPX4 pathway. Biomed. Pharmacother. 172:116225. doi: 10.1016/j.biopha.2024.116225
Wu, X., Li, Y., Zhang, S., and Zhou, X. (2021). Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11, 3052–3059. doi: 10.7150/thno.54113
Wu, H. H., Zhou, Y., Tabata, Y., and Gao, J. Q. (2019). Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J. Control. Release 294, 102–113. doi: 10.1016/j.jconrel.2018.12.019
Yamanaka, S. (2020). Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell 27, 523–531. doi: 10.1016/j.stem.2020.09.014
Yamazaki, K., Kawabori, M., Seki, T., and Houkin, K. (2020). Clinical trials of stem cell treatment for spinal cord injury. IJMS. 21:3994. doi: 10.3390/ijms21113994
Yao, S., Pang, M., Wang, Y., Wang, X., Lin, Y., Lv, Y., et al. (2023). Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol. 67:102871. doi: 10.1016/j.redox.2023.102871
Ying, Y., Huang, Z., Tu, Y., Wu, Q., Li, Z., Zhang, Y., et al. (2023). A shear-thinning, ROS-scavenging hydrogel combined with dental pulp stem cells promotes spinal cord repair by inhibiting ferroptosis. Bioactive Materials. 22, 274–290. doi: 10.1016/j.bioactmat.2022.09.019
Yu, H., Huang, Y., and Yang, L. (2022). Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 80:101684. doi: 10.1016/j.arr.2022.101684
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M., and Rybak, Z. (2019). Stem cells: past, present, and future. Stem Cell Res Ther 10:68. doi: 10.1186/s13287-019-1165-5
Zamani, H., Soufizomorrod, M., Oraee-Yazdani, S., Naviafar, D., Akhlaghpasand, M., Seddighi, A., et al. (2022). Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord 60, 63–70. doi: 10.1038/s41393-021-00687-5
Zhang, Y., Mignone, J., and MacLellan, W. R. (2015). Cardiac regeneration and stem cells. Physiol. Rev. 95, 1189–1204. doi: 10.1152/physrev.00021.2014
Zhang, S., Shang, J., Gu, Z., Gu, X., Wang, F., Hu, X., et al. (2023). Global research trends and hotspots on tendon-derived stem cell: a bibliometric visualization study. Front. Bioeng. Biotechnol. 11:1327027. doi: 10.3389/fbioe.2023.1327027
Zhang, Y., Swanda, R. V., Nie, L., Liu, X., Wang, C., Lee, H., et al. (2021). mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12:1589. doi: 10.1038/s41467-021-21841-w
Zheng, J., and Conrad, M. (2020). The metabolic underpinnings of Ferroptosis. Cell Metab. 32, 920–937. doi: 10.1016/j.cmet.2020.10.011
Zheng, Q., Wang, D., Lin, R., and Xu, W. (2024). Pyroptosis, ferroptosis, and autophagy in spinal cord injury: regulatory mechanisms and therapeutic targets. Neural Regen. Res. 20:2787. doi: 10.4103/NRR.NRR-D-24-00112
Zou, Y., Zhao, Y., Xiao, Z., Chen, B., Ma, D., Shen, H., et al. (2020). Comparison of regenerative effects of transplanting three-dimensional longitudinal scaffold loaded-human mesenchymal stem cells and human neural stem cells on spinal cord completely transected rats. ACS Biomater Sci. Eng. 6, 1671–1680. doi: 10.1021/acsbiomaterials.9b01790
Keywords: spinal cord injury, ferroptosis, stem cell therapy, programmed cell death, neural repair
Citation: Shen Q, Wu L, Cai C, Li B and Yu F (2025) Targeting ferroptosis in spinal cord injury through stem cell therapy: mechanisms and therapeutic prospects. Front. Neurosci. 19:1622787. doi: 10.3389/fnins.2025.1622787
Edited by:
Wenqing Liang, Zhoushan Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, ChinaReviewed by:
YiPing Luo, Tongji University School of Medicine, ChinaYining Xu, Sir Run Run Shaw Hospital, China
Copyright © 2025 Shen, Wu, Cai, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Yu, c3N4MTEyMjA1MDRAMTYzLmNvbQ==
 Qiqin Shen
Qiqin Shen Lingdi Wu1
Lingdi Wu1 Bingbing Li
Bingbing Li