- 1 Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
- 2 Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Over the past two decades, management of newly diagnosed glioblastoma has undergone significant evolution. While surgery has long been a mainstay of management for this disease, and while radiotherapy has a proven survival role, initial efforts at radiotherapy dose escalation, use of radiosurgery, brachytherapy, and altered fractionation did not improve patient survival. Recently, multiple modality therapy integrating maximal safe resection, postoperative radiation, and new systemic therapies have resulted in improved patient outcomes compared with older regimens utilizing surgery and postoperative radiation alone. Numerous trials are currently underway investigating the combination of surgery, radiation, and systemic therapy with targeted agents to find ways to further improve outcomes for adults with glioblastoma.
Introduction
Glioblastoma (GBM) remains a highly lethal and aggressive tumor with dismal prognosis. Malignant astrocytomas constitute around 80% of all gliomas, with WHO grade IV glioma or glioblastoma representing the vast majority of high-grade gliomas (Jukich et al., 2001; Wrensch et al., 2002; Black and Loeffler, 2005). Until recently, long-term survivors of glioblastoma were exceedingly rare, with 5-year survival of 5% or less (Chandler et al., 1993).
Level 1 evidence supporting a categorical role for complete surgical resection does not exist, in part due to the impossibility of performing a trial in which patients could prospectively be randomized to gross total resection versus lesser resection. Therefore, this issue remains controversial. Indirect evidence in support of more complete versus less complete resection comes from trials such as the one conducted by Vuorinen et al., which showed a survival benefit to tumor resection over biopsy alone in elderly patients, albeit with no difference in time to deterioration between the two groups. We recognize that this does not constitute definitive evidence in support of more complete resection yielding improved survival; however the conventional practice approach is to perform as complete a resection as safely possible (Vuorinen et al., 2003). Immediate postoperative contrast-enhanced MRI following resection of glioblastoma, generally performed within 72 h or less (to avoid the confounding postoperative changes that start soon after surgery) often demonstrates residual enhancement surrounding the resection cavity, an area along with postoperative edema becomes an important target for radiotherapy. A recent phase III study using 5-aminolevulinic acid (5-ALA) for fluorescence-guided resection showed an almost 20% improvement in 6-month progression-free survival compared with tumors resected under white light alone, underscoring the importance of complete resection in these highly infiltrative tumors whose borders are difficult to discern (Stummer et al., 2006). By using 5-ALA, contrast enhancing tumor was completely resected in 65% of patients versus in 36% of control patients. As alluded to earlier, there are insufficient level 1 data to conclude that complete resection imparts a survival benefit; however, in a large single institutional analysis of all GBM patients undergoing resection, with each patient having comprehensive prospective data storage, when the extent of resection exceeded 98% of all enhancing tumor, a survival benefit of approximately 5 months started to emerge (Lacroix et al., 2001).
Role of Postoperative Radiotherapy
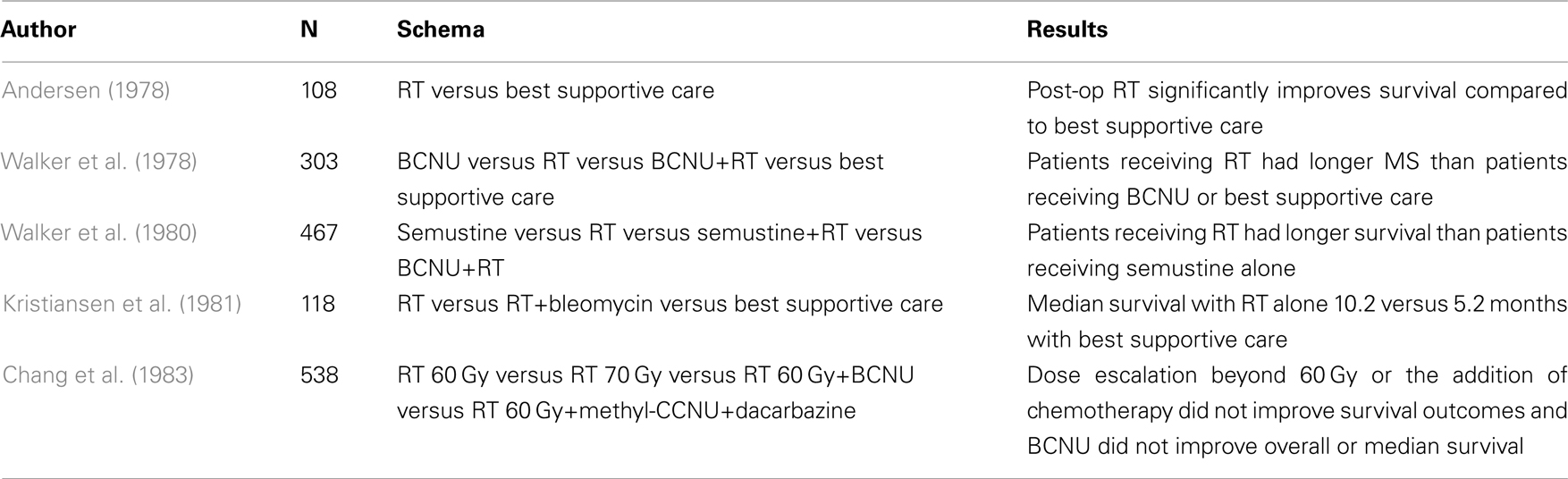
The Brain Tumor Study Group trial was one of the earliest randomized studies to show a survival advantage with postoperative radiotherapy versus best supportive care in patients with anaplastic gliomas (90% of whom had glioblastoma; Table 1; Walker et al., 1978). Study arms included BCNU alone, radiation alone, combined BCNU and radiation, and supportive care. Patients who received postoperative radiation had a median survival of 37.5 versus 17 weeks for supportive care. The combination of BCNU and radiation resulted in median survival of 40.5 weeks, not statistically different from radiotherapy alone. All arms were superior to supportive care. Of note, in this and other early studies, fractionated radiation was delivered to the whole brain in doses of over 50 Gy. A randomized study of radiotherapy versus best supportive care by Andersen (1978) showed a 6-month survival rate of 64% in the irradiated group versus 28% without radiation. Another study by Walker et al. (1980) randomized patients with malignant glioma to either semustine alone, radiation alone, semustine and radiation, or BCNU and radiation. Patients receiving radiotherapy had significantly longer survival than patients who received semustine alone. Kristiansen et al. (1981) randomized 118 patients with grade 3 and 4 astrocytoma to radiation, radiation and bleomycin, or supportive care and found a median survival of 10.2 months with radiation alone compared to 5.2 months with supportive care.
Although no randomized data are available directly comparing whole brain to partial brain radiation, one intergroup study changed the field set-up from whole brain to whole brain plus a partial brain boost during the study period (Shapiro et al., 1989). Patients were randomized to one of three BCNU-containing chemotherapy regimens, and all patients received radiation. Of 571 enrolled patients, eighty percent had glioblastoma. Those enrolled in 1980 or 1981 received 60.2 Gy radiation to the whole brain, and those enrolled later received 43 Gy to the whole brain followed by a 17.2-Gy tumor volume boost. No statistically significant difference in survival was observed between the two radiotherapy regimens. Consequently, an approach of treating a larger volume encompassing the “edema” which putatively also includes microscopic extension, and is best visualized on MR FLAIR or T2 sequences to approximately 46 Gy followed by a boost to the enhancing residual disease and surgical cavity to 60 Gy has become a widely used “standard.” More recently, several institutions have adopted further margin modifications but without formal randomized comparisons (Chang et al., 2007).
Several studies have examined dose escalation in an attempt to improve local control and survival. A pooled analysis of three randomized trials from the Brain Tumor Study Group showed improved survival as dose was increased from 45 to 60 Gy (Walker et al., 1979). Further dose escalation beyond 60 Gy was attempted in a joint RTOG/ECOG four-arm randomized trial (Chang et al., 1983; Nelson et al., 1988). Patients received 60 Gy alone in the control arm, 60 Gy with one of two nitrosourea regimens in two combined modality arms, or 60 Gy with the addition of a 10-Gy boost in the final arm. No significant difference in survival was noted for any of the experimental arms over the control arm. The University of Michigan conducted a phase I dose escalation trial, and at the top dose of 90 Gy, treated 34 malignant glioma patients with 3-D conformal intensity modulation (Chan et al., 2002). Despite the higher dose, median survival was only 11.7 months, and failures were primarily local. Of the patients who recurred, 9% experienced marginal or distant recurrences. In a recent paper by Nieder et al., the authors suggest revisiting dose escalation based on the hypothesis that the success of temozolomide concurrently with radiotherapy may provide the increased radiosensitivity and improvement in local control of microscopic disease required to observe a treatment effect from dose escalation. Furthermore, the authors suggest that identifying molecular signatures of radioresistant tumors as well as utilization of targeted agents may reveal a population of patients who could benefit from dose escalation strategies (Nieder and Mehta, 2011).
Altered fractionation has also been studied in several randomized studies. Prados et al. (2001) studied an accelerated hyperfractionation schedule of 70.4 Gy in 1.6 Gy fractions twice daily compared with 59.4 Gy at 1.8 Gy per day. Patients were randomized to either of these radiation techniques and to treatment with the radiosensitizer difluoromethylornithine. Neither the sensitizer, nor the hyperfractionation regimen demonstrated an overall survival or progression-free survival benefit. RTOG 90-06 similarly found no survival difference between 72 Gy at 1.2 Gy twice daily and 60 Gy conventionally fractionated, both given concurrently with carmustine (Scott et al., 1998). A meta-analysis of altered fractionation by Nieder et al. (2004) showed that although treatment time was decreased, no survival benefit was observed. As a result of these and similar studies, 60 Gy has become an established dose for conventional external beam radiotherapy in postoperative treatment of newly diagnosed glioblastoma.
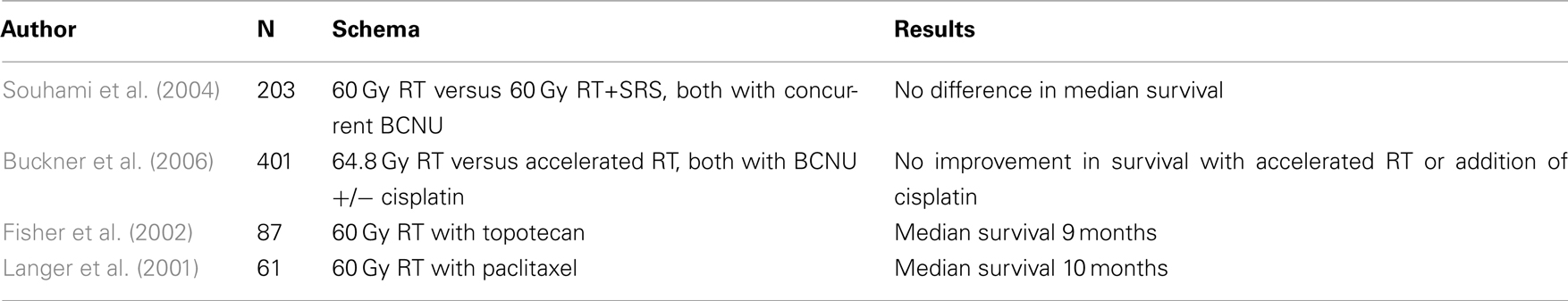
Radiosurgery has also been studied as a way to deliver a boost dose in conjunction with a course of conventional chemoradiation. In a randomized RTOG study, 203 patients with newly diagnosed glioblastoma less than or equal to 4 cm in size were randomized after surgery to an up-front radiosurgery boost or not, with all patients receiving 60 Gy partial brain radiation with concurrent BCNU (Souhami et al., 2004). Radiosurgery doses varied from 15 to 24 Gy depending on the target volume. Median survival in the radiosurgery arm was 13.5 months compared to 13.6 months in the conventional arm, and no differences in patterns of failure between the two arms were observed.
Finally, brachytherapy has also been employed in an attempt to decrease local failures, both with permanent and temporary implants. Two randomized trials have been conducted for newly diagnosed malignant gliomas. The Princess Margaret Hospital randomized patients with malignant astrocytomas to 50 Gy external beam radiation or the same dose followed by 60 Gy boost via an I-125 implant (Laperriere et al., 1998). Tumors were less than or equal to 6 cm in size and not crossing midline. No significant difference in survival was observed between treatment groups. A second randomized trial reported by Selker et al. (2002) was also negative. A new device, the GliaSite system, implants an intracavitary balloon into the tumor cavity which is infused percutaneously with an I-125 solution allowing delivery of 40–60 Gy over several days, after which the isotope is removed (Wernicke et al., 2010).
Radiation Sensitizers and Modulators
One strategy for increasing the efficacy of radiation without increasing physical dose involves the use of radiation sensitizers or modulators. The halogenated pyrimidines, including bromodeoxyuridine (BUdR), and iododeoxyuridine (IUdR) are thymidine analogs which become incorporated in DNA during synthesis and function as S-phase radiosensitizers. RTOG 94-04 was a randomized study of external beam radiation with procarbazine, lomustine, and vincristine (PCV) with or without BUdR for anaplastic gliomas (Prados et al., 2004). The study showed no survival benefit for BUdR. IUdR has been studied in several GBM trials, with no convincing evidence for superior efficacy. Hypoxic sensitizers such as nitroimidazoles and tirapazamine have failed to show efficacy. The Medical Research Council randomized patients who to misonidazole or placebo with 45 Gy radiotherapy and found no difference in median survival between groups Anonymous (1983). Tirapazamine was administered with 60 Gy of partial brain radiation in RTOG 94-17, and comparison with RPA class controls from RTOG again failed to demonstrate improved survival (Del Rowe et al., 2000).
Initially applied to the treatment of brain metastases, motexafin gadolinium (MGd) has also been studied in malignant gliomas. MGd oxidizes intracellular redox metabolites necessary for DNA damage repair thereby impairing strand-break repair, and it also generates reactive oxygen species which are selectively concentrated in tumor cells, promoting apoptosis. An additional benefit of MGd is that cells which selectively uptake the compound can be visualized by MRI since gadolinium is paramagnetic. A phase I dose escalation trial investigating MGd demonstrated median survival of 17.6 months, leading to a phase II trial which has not yet reported final results (Ford et al., 2007). RSR13 is a novel hypoxic sensitizer that increases oxygen unloading in hypoxic tissue through allosteric hemoglobin modification (Kleinberg et al., 2002). This agent was administered in a phase II trial of 50 newly diagnosed glioblastoma patients, and median survival was 12.3 months with a favorable toxicity profile.
Cytotoxic Chemotherapy
Although numerous agents including topoisomerase inhibitors, platinoids, and taxanes have been used both with and without radiation, the most effective agent is temozolomide, FDA approved for newly diagnosed GBM in 2005. Previously, alkylating agents were frequently employed in the treatment of malignant gliomas. Taken individually, each of these trials was negative. Fine et al. undertook a meta-analysis of 16 randomized trials that included over 3000 patients and found a 10.1% increase in survival at 1 year and 8.6% increase at 2 years with combination chemotherapy and radiation over radiation alone. Median overall survival increased from 9.4 to 12 months (Fine et al., 1993). An overview of other recent trials with chemotherapy in the pre-temozolomide era is presented in Table 2.
Polymer wafers impregnated with BCNU were developed to increase exposure of tumor cells in the perioperative bed to localized chemotherapy doses, bypass the blood–brain barrier, and minimize systemic toxicities. A randomized trial of BCNU versus placebo which included not only glioblastoma, but also anaplastic gliomas demonstrated a significant increase in median overall survival of 13.9 months with the BCNU wafers over 11.6 months in the placebo arm (Westphal et al., 2003). However, the wafers have never been directly compared to conventional systemic chemotherapy.
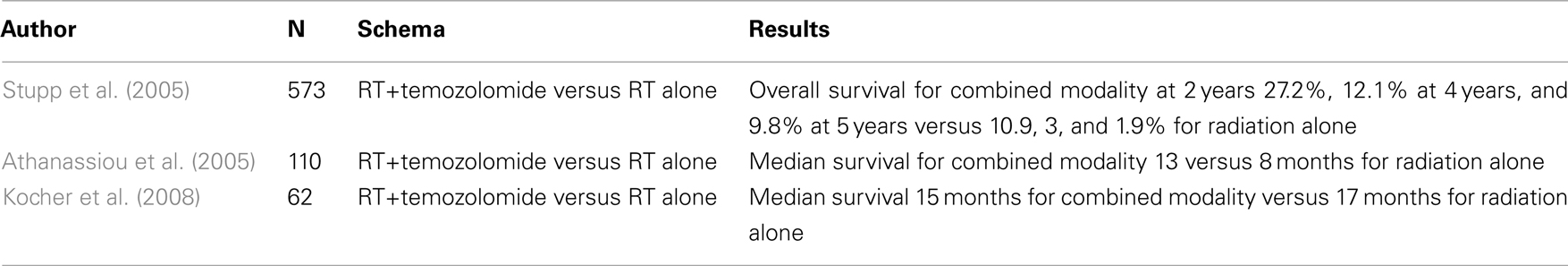
Temozolomide, a pro-drug, is an alkylating agent able to cross the blood–brain barriers (Hegi et al., 2004). After the oral pro-drug is converted to its active form at physiologic pH, the drug methylates DNA at multiple sites, including guanine at the O-6 position (a methylation event that occurs about 6 times out of every 100 DNA methylation events). Unless the methylation in this specific location is repaired by a process involving the enzyme methylguanine methyltransferase (MGMT), the active drug leads to double strand breaks. The MGMT gene promoter itself can be hypermethylated which results in epigenetic gene silencing and enzyme inactivation with consequent increased sensitivity to temozolomide (Hegi et al., 2005, 2009). The EORTC phase III randomized trial of concurrent temozolomide and radiation versus radiation alone showed a survival benefit to the addition of temozolomide, which was robustly sustained with long-term follow-up (Table 3; Stupp et al., 2005). Patients with newly diagnosed glioblastoma were randomized to 75 mg/m2 of temozolomide given 7 days a week, concurrent with radiation which was given 5 days a week. Patients in the drug arm then received 6 months of adjuvant therapy for five out of every 28 days at a dose of 150–200 mg/m2/d. Median overall survival for temozolomide and radiation was 14.6 versus 12.1 months for radiation alone. In a 2009 update, overall survival at 2, 4, and 5 years with temozolomide and radiation was reported to be 27.2, 12.1, and 9.8%, respectively, compared to 10.9, 3, and 1.9% with radiation alone, and the benefit of treatment with drug was noted in all prognostic subgroups (Stupp et al., 2009). Methylation status of the MGMT gene promoter was the strongest predictor of response and outcome. A retrospective analysis of tissue samples generated data to support the role of MGMT in determining resistance to chemotherapy and radiotherapy. Among the 92 assessable cases with evidence of MGMT promoter methylation (i.e., transcriptionally inactive and not producing the DNA-repair enzyme MGMT), a statistically significant improvement in survival was observed in patients receiving temozolomide in combination with radiotherapy compared with radiotherapy alone (21.7 versus 15.3 months, p = 0.007). Approximately 60% of patients in the control arm received temozolomide at recurrence, and survival among these patients with promoter methylation was significantly better than for patients with an unmethylated promoter (overall survival 15.3 versus 11.8 months, respectively). This trial provided the first convincing evidence of survival benefit from the addition of chemotherapy to radiotherapy for patients with GBM. Similar evidence for this trial comes from a Greek phase II randomized trial (Athanassiou et al., 2005). Median time to progression with radiation alone was 5.2 months, similar to the EORTC/NCIC study, compared to 10.8 months after combined therapy, longer than the 6.9-months in the EORTC/NCIC study.
The use of temozolomide has raised several unique issues and questions. It has now been recognized that treatment with temozolomide and radiotherapy may result in an increased frequency of pseudoprogression which manifests on MR imaging as an increase in contrast enhancement, possibly from alterations in the blood–brain barrier, falsely suggesting tumor progression. Typically, response to therapy utilizes two dimensional size measurements from CT and MRI imaging along with clinical response and steroid use (Wen et al., 2010). However, recent awareness has grown of limitations in focusing on contrast enhancement to evaluate disease extent, particularly with the use of antiangiogenic agents and recognition that enhancement is sometimes non-specific. In one study, MGMT methylation was a significant predictor of increased likelihood of pseudoprogression (Brandes et al., 2008). This may be a concern because patients with pseudoprogression are sometimes deemed to have true progression resulting in discontinuation of a possibly effective therapy, whereas in reality patients with pseudoprogression may in fact have longer survival compared to patients with no imaging changes.
A major unanswered question is the value of adjuvant temozolomide; pre-clinical experiments suggest that the benefit is derived from the concomitant use with radiotherapy (Chakravarti et al., 2006). Although the question has not been investigated in a randomized trial, Combs et al. conducted a single agent trial of temozolomide at 50 mg/m2/day of during radiotherapy, without the use of temozolomide in the adjuvant phase. Median overall survival was 19 months, with 1 and 2 year survival rates of 72 and 29%, comparable to the EORTC–NCIC trial results (Combs et al., 2005).
Another randomized trial attempted to address the importance of concurrent temozolomide without adjuvant drug (Kocher et al., 2008). This German study randomized patients after gross total resection to radiotherapy alone or radiotherapy with concurrent but no adjuvant temozolomide. Progression-free survival in the radiation alone arm was 7 months compared to 6 months with radiation and temozolomide. No difference in overall survival was observed with chemoradiation over radiation alone, with median overall survival of 15 and 17 months, respectively. The study was stopped early after the results of the EORTC study were released. The authors argued the negative result may have been a consequence of the small sample size, although the possibility that the simultaneous portion of temzolomide therapy had less impact on survival than the adjuvant component could not be excluded. A significant proportion of patients received the drug later at time of progression which may explain the absence of a survival difference; also, this trial did not report whether or not the arms were balanced by MGMT methylation.
Given the significance of MGMT in determining outcome, MGMT-depleting strategies are clearly attractive; in this context, specific agents to inhibit MGMT have been developed, such as Patrin2, O-6BG, and methoxyamine, but, when used in combination with alkylating agents result in inordinate toxicity such as myelosuppression, requiring considerable temozolomide dose reductions (Liu and Gerson, 2004; Warren et al., 2005; Sabharwal and Middleton, 2006; Woolford et al., 2006). MGMT may also be depleted through enzymatic supply exhaustion since every repair event consumes a molecule of MGMT and irreversibly methylates it. RTOG 0525 compared conventional adjuvant temozolomide with dose-intensive therapy in patients with newly diagnosed glioblastoma, with the hypothesis that the continuous dose-adjuvant approach in the adjuvant setting would lead to MGMT depletion and superior outcomes (Gilbert et al., 2011). In addition the study prospectively looked at the question of whether methylation of the MGMT promoter leads to improved outcome with temozolomide treatment. No benefit was observed for intensified temozolomide regardless of methylation status, although the importance of MGMT methylation as a prognostic factor in GBM was confirmed.
Targeted Therapy
Significant progress has been made recently in molecular characterization of glioblastoma. Historically, two subtypes have been defined based on molecular and genetic characteristics (Wen and Kesari, 2008; Anonymous, 2008). Primary glioblastomas typically present in patients over 50 years, have loss of heterozygosity of chromosome 10q, EGFR amplification, and deletion of PTEN and p16. The Cancer Genome Atlas consortium work on glioblastoma also provided preliminary evidence that primary glioblastoma could be divided into four subtypes: classical, mesenchymal, neural, and proneural. The classical subtype demonstrates response to radiation and chemotherapy, putatively a response consequential to intact p53 pathways. This subtype also demonstrates increased expression in Notch and Sonic Hedgehog signaling pathways. The second type is associated with mesenchyme and angiogenesis, has frequent inactivation of p53, PTEN, and NF1, responds to aggressive chemoradiation, and may respond to Ras, PI3K, and angiogenesis inhibitors. The proneural subtype has superior survival compared to the other three types yet shows the least response to classical treatments. The neural subtype is the least defined and has gene expression signatures similar to that in normal brain. Secondary glioblastomas are typically transformations of lower grade gliomas in younger patients and possess p53 mutations, overexpression of PDGFR, abnormalities in the p16 and pRb pathways, and loss of heterozygosity of 10q.
Approximately half of patients with primary glioblastoma and EGFR amplification express EGFRvIII, a mutated form of EGFR that has a severely truncated extra-cellular ligand-binding domain. Downstream pathways activated by EGFR signaling include the PI3K–Akt–mTOR pathway, involved in cell growth and death. The tumor suppressor gene PTEN is an inhibitor of the PI3K pathway and is inactivated in 40 to 50% of glioblastomas. These pathways, when active, may in turn lead to upregulation of vascular endothelial growth factor (VEGF) and angiogenesis.
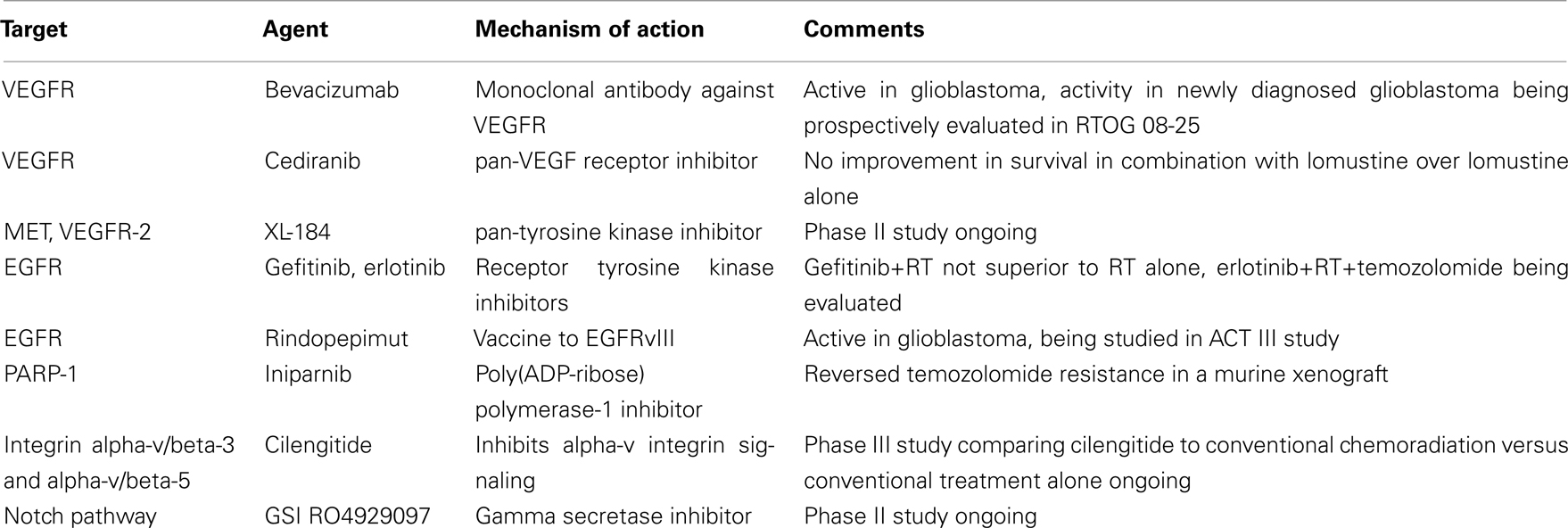
Single agent targeted therapies typically result in response rates below 20% with no improvement in 6-month progression-free survival. Phase II testing of erlotinib in newly diagnosed glioblastoma concurrently with temozolomide was not effective and yielded unacceptable toxicity (Table 4; Peereboom et al., 2010). In a study by Mellinghoff, patients with recurrent malignant gliomas who received EGFR tyrosine kinase inhibitors were analyzed (Mellinghoff et al., 2005). The authors noted that clinical response to the kinase inhibitors was associated with co-expression of EGFRvIII and wild-type PTEN, highlighting a possible route to genetically characterize a subgroup of patients who would benefit from targeted agents. Recently, a vaccine against EGFRvIII, rindopepimut, has been evaluated in three small phase I/II studies (http://www.celldextherapeutics.com/wt/page/cdx_110., f). The ACT III trial studied rindopepimut in patients with newly diagnosed glioblastoma following gross total resection and treatment with temozolomide and radiation. This built on the study of rindopepimut vaccine alone (ACTIVATE) and rindopepimut with temozolomide (ACT II), with preliminary results demonstrating increased time to progression and overall survival compared to historical controls. Initial results of ACT III showed significant improvement in progression-free rate over a predetermined estimate, irrespective of MGMT expression status.
Malignant gliomas are highly vascular tumors, and studies with older antiangiogenic agents such as thalidomide did not show significant activity. Bevacizumab is a humanized monoclonal antibody against VEGF that prevents endothelial cell proliferation and migration. In the recurrent glioblastoma, bevacizumab combined with irinotecan showed 6-month progression-free survival of 46% and overall survival of 77% with only moderate toxicity (Vredenburgh et al., 2007). In AVF3708g, a phase II trial for recurrent glioblastoma, Friedman et al. (2009) studied bevacizumab alone and in combination with irinotecan. The authors showed a 6-month progression-free survival of 42.6% with single agent bevacizumab and 50.3% for combination therapy, and median overall survival times were an impressive 9.2 and 8.7 months, respectively. The single-arm, single-site NCI 06-C-0064E study of single agent bevacizumab in recurrent disease also showed a durable median response of 3.9 months (Kreisl et al., 2009). The FDA recently approved use of bevacizumab as monotherapy in recurrent glioblastoma on the basis of the favorable responses to single agent bevacizumab in these studies.
RTOG 08-25, and AVAGLIO are two ongoing phase III trials comparing concurrent radiation and temozolomide with and without bevacizumab. A recent phase II study of bevacizumab and temozolomide during and after radiation for newly diagnosed glioblastoma found a 13.6-month progression-free survival and 19.6 month overall survival, an improvement in progression-free survival over the UCLA/KPLA control cohort (6.9 months) but not in overall survival. The authors suggested that since many patients in the control cohort received bevacizumab at recurrence that bevacizumab at progression may provide the same survival benefit as first-line treatment (Lai et al., 2011). Until mature results of phase III trials become available, bevacizumab should not be considered as having a proven role in the up-front treatment setting.
Unlike bevacizumab, which is a humanized monoclonal antibody against all isoforms of VEGF, cediranib is a pan-VEGFR receptor tyrosine kinase inhibitor. A phase II trial of cediranib showed greater than a 50% radiographic response rate (Batchelor et al., 2010). However, the randomized REGAL study of cediranib alone or in combination with lomustine failed to show improved progression-free or overall survival compared to lomustine alone for patients with recurrent GBM; a phase II randomized trial of cediranib, temozolomide and radiotherapy for newly diagnosed GBM is currently being conducted by the RTOG (Stupp et al., 2010). XL-184 is a pan-tyrosine kinase inhibitor whose principal targets are VEGFR-2 and MET; NCT00704288, a phase II study of this drug in progressive or recurrent glioblastoma has just been completed (Zhang et al., 2010).
With recent evidence that endothelial integrins interact with extra-cellular ligands to promote angiogenesis, a class of inhibitors has been developed to target alpha-v/beta-3 and alpha-v/beta-5 integrins (Silvestre et al., 2005). Cilengitide is a novel compound selective for alpha-v integrins under investigation as an antiangiogenic agent. The combination of cilengitide with conventional chemoradiation versus chemoradiation alone for newly diagnosed GBM is being investigated in the phase III CENTRIC trial.
PARP inhibitors are a novel class of compounds which have demonstrated activity in solid tumors. Alkylation by temozolomide more often targets the N-7 guanine and N-3 adenine over the O-6 guanine, and the former two events are repaired by enzymes in the base excision pathway which can be inhibited by PARP inhibitors. In a mouse xenograft model, PARP-1 inhibition reversed temozolomide resistance, suggesting that PARP inhibitors may improve efficacy of temozolomide particularly in tumors with mismatch repair defects (Cheng et al., 2005). NCT00687765 is a phase I clinical trial studying a poly (ADP-ribose) polymerase-1 (PARP-1) inhibitor, BSI-201, also known as iniparnib. Patients with newly diagnosed glioblastoma will first receive conventional radiotherapy and temozolomide, which will be followed by adjuvant temozolomide and iniparnib. The NCCTG, RTOG, and ABTC are also evaluating PARP inhibitors in GBM.
Another signaling pathway that may be a malignant glioma treatment target is Notch, which is involved in stem cell differentiation (Lino et al., 2010). New data suggest that tumor stem cells may be important as progenitors of malignant gliomas and may constitute radio- and possibly chemo-resistant clones that contribute to resistance of malignant gliomas to conventional treatments (Vescovi et al., 2006; Dirks, 2008). Notch pathway inhibition with gamma secretase inhibitors (GSIs) reduces glial stem cell proliferation and increased apoptosis associated with decreased Akt and Stat3 phosphorylation. A new phase II clinical trial, NCT01122901, is studying GSI RO4929097 in recurrent or progressive glioblastoma.
Conclusion
Despite median survival in patients with newly diagnosed glioblastoma of around 1 year, significant progress in the treatment of this malignancy over the past few decades has been made. Refined surgical techniques have improved extent of resection and decreased surgical morbidity. A shift from whole brain to partial brain radiation with a boost focused on the tumor bed did not compromise rates of local and marginal tumor control. Still, dose escalation beyond 60 Gy using radiosurgery, brachytherapy, or fractionated external beam approaches with nitrosoureas, has not demonstrated improved survival or reduced rates of local failure. With the advent of temozolomide, new opportunities for improved outcome have emerged. Molecular characterization of glioblastoma is allowing definition of subgroups of patients most likely to benefit from particular therapies. Numerous targeted agents aimed at a broad array of intra- and extra-cellular targets are currently in clinical trials with the hope that new and even more effective therapies will be discovered.
Conflict of Interest Statement
Dr. Mehta has served as a consultant to Adnexus, Bayer, Genentech, Merck, Schering Plough, and Tomotherapy; he serves on the Board of Directors of Pharmacyclics, and as an advisor to Stemina, and is on the DSMB for Apogenix. He holds stock options in Colby, Procertus, Pharmacyclics, and Tomotherapy. Dr. Raizer has served as a consultant for Genentech and Speakers Bureau for Genentech and Merck/Schering Plough.
References
Anonymous. (1983). A study of the effect of misonidazole in conjunction with radiotherapy for the treatment of grades 3 and 4 astrocytomas. A report from the MRC Working Party on misonidazole in gliomas. Br. J. Radiol. 56, 673–682.
Anonymous. (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068.
Andersen, A. P. (1978). Postoperative irradiation of glioblastomas. Results in a randomized series. Acta Radiol. Oncol. Radiat. Phys. Biol. 17, 475–484.
Athanassiou, H., Synodinou, M., Maragoudakis, E., Paraskevaidis, M., Verigos, C., Misailidou, D., Antonadou, D., Saris, G., Beroukas, K., and Karageorgis, P. (2005). Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 23, 2372–2377.
Batchelor, T. T., Duda, D. G., di Tomaso, E., Ancukiewicz, M., Plotkin, S. R., Gerstner, E., Eichler, A. F., Drappatz, J., Hochberg, F. H., Benner, T., Louis, D. N., Cohen, K. S., Chea, H., Exarhopoulos, A., Loeffler, J. S., Moses, M. A., Ivy, P., Sorensen, A. G., Wen, P. Y., and Jain, R. K. (2010). Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 28, 2817–2823.
Black, P. M., and Loeffler, J. S. (2005). Cancer of the nervous system, 2nd Edn. Philadelphia: Lippincott Williams and Wilkins, 891.
Brandes, A. A., Franceschi, E., Tosoni, A., Blatt, V., Pession, A., Tallini, G., Bertorelle, R., Bartolini, S., Calbucci, F., Andreoli, A., Frezza, G., Leonardi, M., Spagnolli, F., and Ermani, M. (2008). MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 26, 2192–2197.
Buckner, J. C., Ballman, K. V., Michalak, J. C., Burton, G. V., Cascino, T. L., Schomberg, P. J., Hawkins, R. B., Scheithauer, B. W., Sandler, H. M., Marks, R. S., O’Fallon, J. R., North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. (2006). Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. J. Clin. Oncol. 24, 3871–3879.
Chakravarti, A., Erkkinen, M. G., Nestler, U., Stupp, R., Mehta, M., Aldape, K., Gilbert, M. R., Black, P. M., and Loeffler, J. S. (2006). Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin. Cancer Res. 12, 4738–4746.
Chan, J. L., Lee, S. W., Fraass, B. A., Normolle, D. P., Greenberg, H. S., Junck, L. R., Gebarski, S. S., and Sandler, H. M. (2002). Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J. Clin. Oncol. 20, 1635–1642.
Chandler, K. L., Prados, M. D., Malec, M., and Wilson, C. B. (1993). Long-term survival in patients with glioblastoma multiforme. Neurosurgery 32, 716–720; discussion 720.
Chang, C. H., Horton, J., Schoenfeld, D., Salazer, O., Perez-Tamayo, R., Kramer, S., Weinstein, A., Nelson, J. S., and Tsukada, Y. (1983). Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer 52, 997–1007.
Chang, E. L., Akyurek, S., Avalos, T., Rebueno, N., Spicer, C., Garcia, J., Famiglietti, R., Allen, P. K., Chao, K. S., Mahajan, A., Woo, S. Y., and Maor, M. H. (2007). Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 68, 144–150.
Cheng, C. L., Johnson, S. P., Keir, S. T., Quinn, J. A., Ali-Osman, F., Szabo, C., Li, H., Salzman, A. L., Dolan, M. E., Modrich, P., Bigner, D. D., and Friedman, H. S. (2005). Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol. Cancer Ther. 4, 1364–1368.
Combs, S. E., Gutwein, S., Schulz-Ertner, D., van Kampen, M., Thilmann, C., Edler, L., Wannenmacher, M. M., and Debus, J. (2005). Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Phase I/II study. Strahlenther. Onkol. 181, 372–377.
Del Rowe, J., Scott, C., Werner-Wasik, M., Bahary, J. P., Curran, W. J., Urtasun, R. C., and Fisher, B. (2000). Single-arm, open-label phase II study of intravenously administered tirapazamine and radiation therapy for glioblastoma multiforme. J. Clin. Oncol. 18, 1254–1259.
Dirks, P. B. (2008). Brain tumor stem cells: bringing order to the chaos of brain cancer. J. Clin. Oncol. 26, 2916–2924.
Fine, H. A., Dear, K. B., Loeffler, J. S., Black, P. M., and Canellos, G. P. (1993). Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 71, 2585–2597.
Fisher, B., Won, M., Macdonald, D., Johnson, D. W., and Roa, W. (2002). Phase II study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group 9513. Int. J. Radiat. Oncol. Biol. Phys. 53, 980–986.
Ford, J. M., Seiferheld, W., Alger, J. R., Wu, G., Endicott, T. J., Mehta, M., Curran, W., and Phan, S. C. (2007). Results of the phase I dose-escalating study of motexafin gadolinium with standard radiotherapy in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 69, 831–838.
Friedman, H. S., Prados, M. D., Wen, P. Y., Mikkelsen, T., Schiff, D., Abrey, L. E., Yung, W. K., Paleologos, N., Nicholas, M. K., Jensen, R., Vredenburgh, J., Huang, J., Zheng, M., and Cloughesy, T. (2009). Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740.
Gilbert, M., Wang, M., Aldape, K., Stupp, R., Hegi, M., Jaeckle, K., Armstrong, T., Wefel, J., Won, M., Blumenthal, D., Mahajan, A., Schultz, C., Erridge, S., Brown, P., Chakravarti, A., Curran, W., and Mehta, M. (2011). RTOG 0525: a randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM). J. Clin. Oncol. 29.
Hegi, M. E., Diserens, A. C., Godard, S., Dietrich, P. Y., Regli, L., Ostermann, S., Otten, P., Van Melle, G., de Tribolet, N., and Stupp, R. (2004). Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 10, 1871–1874.
Hegi, M. E., Diserens, A. C., Gorlia, T., Hamou, M. F., de Tribolet, N., Weller, M., Kros, J. M., Hainfellner, J. A., Mason, W., Mariani, L., Bromberg, J. E., Hau, P., Mirimanoff, R. O., Cairncross, J. G., Janzer, R. C., and Stupp, R. (2005). MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003.
Hegi, M. E., Sciuscio, D., Murat, A., Levivier, M., and Stupp, R. (2009). Epigenetic deregulation of DNA repair and its potential for therapy. Clin. Cancer Res. 15, 5026–5031.
Jukich, P. J., McCarthy, B. J., Surawicz, T. S., Freels, S., and Davis, F. G. (2001). Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-oncology. 3, 141–151.
Kleinberg, L., Grossman, S. A., Carson, K., Lesser, G., O’Neill, A., Pearlman, J., Phillips, P., Herman, T., and Gerber, M. (2002). Survival of patients with newly diagnosed glioblastoma multiforme treated with RSR13 and radiotherapy: results of a phase II new approaches to brain tumor therapy CNS consortium safety and efficacy study. J. Clin. Oncol. 20, 3149–3155.
Kocher, M., Frommolt, P., Borberg, S. K., Rühl, U., Steingräber, M., Niewald, M., Staar, S., Stuschke, M., Becker, G., Fischedick, A. R., Herfarth, K., Grauthoff, H., and Müller, R. P. (2008). Randomized study of postoperative radiotherapy and simultaneous temozolomide without adjuvant chemotherapy for glioblastoma. Strahlenther. Onkol. 184, 572–579.
Kreisl, T. N., Kim, L., Moore, K., Duic, P., Royce, C., Stroud, I., Garren, N., Mackey, M., Butman, J. A., Camphausen, K., Park, J., Albert, P. S., and Fine, H. A. (2009). Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27, 740–745.
Kristiansen, K., Hagen, S., Kollevold, T., Torvik, A., Holme, I., Nesbakken, R., Hatlevoll, R., Lindgren, M., Brun, A., Lindgren, S., Notter, G., Andersen, A. P., and Elgen, K. (1981). Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer 47, 649–652.
Lacroix, M., Abi-Said, D., Fourney, D. R., Gokaslan, Z. L., Shi, W., DeMonte, F., Lang, F. F., McCutcheon, I. E., Hassenbusch, S. J., Holland, E., Hess, K., Michael, C., Miller, D., and Sawaya, R. (2001). A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 95, 190–198.
Lai, A., Tran, A., Nghiemphu, P. L., Pope, W. B., Solis, O. E., Selch, M., Filka, E., Yong, W. H., Mischel, P. S., Liau, L. M., Phuphanich, S., Black, K., Peak, S., Green, R. M., Spier, C. E., Kolevska, T., Polikoff, J., Fehrenbacher, L., Elashoff, R., and Cloughesy, T. (2011). Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 29, 142–148.
Langer, C. J., Ruffer, J., Rhodes, H., Paulus, R., Murray, K., Movsas, B., and Curran, W.. (2001). Phase II radiation therapy oncology group trial of weekly paclitaxel and conventional external beam radiation therapy for supratentorial glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 51, 113–119.
Laperriere, N. J., Leung, P. M., McKenzie, S., Milosevic, M., Wong, S., Glen, J., Pintilie, M., and Bernstein, M. (1998). Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int. J. Radiat. Oncol. Biol. Phys.41, 1005–1011.
Lino, M. M., Merlo, A., and Boulay, J. L. (2010). Notch signaling in glioblastoma: a developmental drug target? BMC Med. 8, 72. doi:10.1186/1741-7015-8-72
Liu, L., and Gerson, S. L. (2004). Therapeutic impact of methoxyamine: blocking repair of abasic sites in the base excision repair pathway. Curr. Opin. Investig. Drugs 5, 623–627.
Mellinghoff, I. K., Wang, M. Y., Vivanco, I., Haas-Kogan, D. A., Zhu, S., Dia, E. Q., Lu, K. V., Yoshimoto, K., Huang, J. H., Chute, D. J., Riggs, B. L., Horvath, S., Liau, L. M., Cavenee, W. K., Rao, P. N., Beroukhim, R., Peck, T. C., Lee, J. C., Sellers, W. R., Stokoe, D., Prados, M., Cloughesy, T. F., Sawyers, C. L., and Mischel, P. S. (2005). Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024.
Nelson, D. F., Diener-West, M., Horton, J., Chang, C. H., Schoenfeld, D., and Nelson, J. S. (1988). Combined modality approach to treatment of malignant gliomas – re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 6, 279–284.
Nieder, C., Andratschke, N., Wiedenmann, N., Busch, R., Grosu, A. L., and Molls, M. (2004). Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther. Onkol. 180, 401–407.
Nieder, C., and Mehta, M. P. (2011). Advances in translational research provide a rationale for clinical re-evaluation of high-dose radiotherapy for glioblastoma. Med. Hypotheses 76, 410–413.
Peereboom, D. M., Shepard, D. R., Ahluwalia, M. S., Brewer, C. J., Agarwal, N., Stevens, G. H., Suh, J. H., Toms, S. A., Vogelbaum, M. A., Weil, R. J., Elson, P., and Barnett, G. H. (2010). Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 98, 93–99.
Prados, M. D., Seiferheld, W., Sandler, H. M., Buckner, J. C., Phillips, T., Schultz, C., Urtasun, R., Davis, R., Gutin, P., Cascino, T. L., Greenberg, H. S., and Curran, W. J. Jr. (2004). Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int. J. Radiat. Oncol. Biol. Phys. 58, 147–1152.
Prados, M. D., Wara, W. M., Sneed, P. K., McDermott, M., Chang, S. M., Rabbitt, J., Page, M., Malec, M., Davis, R. L., Gutin, P. H., Lamborn, K., Wilson, C. B., Phillips, T. L., and Larson, D. A. (2001). Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 49, 71–77.
Sabharwal, A., and Middleton, M. R. (2006). Exploiting the role of O6-methylguanine-DNA-methyltransferase (MGMT) in cancer therapy. Curr. Opin. Pharmacol. 6, 355–363.
Scott, C. B., Curran, W. J. J., Yung, W. K., Scarantino, C., Urtasun, R., Movsas, B., Jones, C., Simpson, J. R., Fischbach, A. J., Petito, C., and Nelson, J. (1998). “Long term results of RTOG-9006: a randomized trial of hyperfractionated radiotherapy to 72 Gy and carmustine vs. standard RT and carmustine for malignant glioma patients with emphasis on anaplastic astrocytoma patients,” in Proceedings of American Society of Clinical Oncology, Los Angeles, CA, 1546.
Selker, R. G., Shapiro, W. R., Burger, P., Blackwood, M. S., Arena, V. C., Gilder, J. C., Malkin, M. G., Mealey, J. J. Jr., Neal, J. H., Olson, J., Robertson, J. T., Barnett, G. H., Bloomfield, S., Albright, R., Hochberg, F. H., Hiesiger, E., Green, S., and Brain Tumor Cooperative Group. (2002). The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 51, 343–355; discussion 355–357.
Shapiro, W. R., Green, S. B., Burger, P. C., Mahaley, M. S. Jr., Selker, R. G., VanGilder, J. C., Robertson, J. T., Ransohoff, J., Mealey, J. Jr., and Strike, T. A. (1989). Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J. Neurosurg. 71, 1–9.
Silvestre, J. S., Théry, C., Hamard, G., Boddaert, J., Aguilar, B., Delcayre, A., Houbron, C., Tamarat, R., Blanc-Brude, O., Heeneman, S., Clergue, M., Duriez, M., Merval, R., Lévy, B., Tedgui, A., Amigorena, S., and Mallat, Z. (2005). Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. 11, 499–506.
Souhami, L., Seiferheld, W., Brachman, D., Podgorsak, E. B., Werner-Wasik, M., Lustig, R., Schultz, C. J., Sause, W., Okunieff, P., Buckner, J., Zamorano, L., Mehta, M. P., and Curran, W. J. Jr. (2004). Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int. J. Radiat. Oncol. Biol. Phys. 60, 853–860.
Stummer, W., Pichlmeier, U., Meinel, T., Wiestler, O. D., Zanella, F., Reulen, H. J., and ALA-Glioma Study Group. (2006). Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401.
Stupp, R., Hegi, M., and Weller, M. (2010). Neuro-oncology, a decade of temozolomide and beyond. Expert Rev. Anticancer Ther. 10, 1675–1677.
Stupp, R., Hegi, M. E., Mason, W. P., van den Bent, M. J., Taphoorn, M. J., Janzer, R. C., Ludwin, S. K., Allgeier, A., Fisher, B., Belanger, K., Hau, P., Brandes, A. A., Gijtenbeek, J., Marosi, C., Vecht, C. J., Mokhtari, K., Wesseling, P., Villa, S., Eisenhauer, E., Gorlia, T., Weller, M., Lacombe, D., Cairncross, J. G., Mirimanoff, R. O., European Organisation for Research Treatment of Cancer Brain Tumour and Radiation Oncology Groups and National Cancer Institute of Canada Clinical Trials Group. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466.
Stupp, R., van den Bent, M. J., and Hegi, M. E. (2005). Optimal role of temozolomide in the treatment of malignant gliomas. Curr. Neurol. Neurosci. Rep. 5, 198–206.
Vescovi, A. L., Galli, R., and Reynolds, B. A. (2006). Brain tumour stem cells. Nat. Rev. Cancer 6, 425–436.
Vredenburgh, J. J., Desjardins, A., Herndon, J. E. II, >Marcello, J., Reardon, D. A., Quinn, J. A., Rich, J. N., Sathornsumetee, S., Gururangan, S., Sampson, J., Wagner, M., Bailey, L., Bigner, D. D., Friedman, A. H., and Friedman, H. S. (2007). Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 25, 4722–4729.
Vuorinen, V., Hinkka, S., Färkkilä, M., and Jääskeläinen, J. (2003). Debulking or biopsy of malignant glioma in elderly people – a randomised study. Acta Neurochir. (Wien) 145, 5–10.
Walker, M. D., Alexander, E. Jr., Hunt, W. E., MacCarty, C. S., Mahaley, M. S. Jr., Mealey, J. Jr., Norrell, H. A., Owens, G., Ransohoff, J., Wilson, C. B., Gehan, E. A., and Strike, T. A. (1978). Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 49, 333–343.
Walker, M. D., Green, S. B., Byar, D. P., Alexander, E. Jr., Batzdorf, U., Brooks, W. H., Hunt, W. E., MacCarty, C. S., Mahaley, M. S. Jr., Mealey, J. Jr., Owens, G., Ransohoff, J. II, Robertson, J. T., Shapiro, W. R., Smith, K. R. Jr., Wilson, C. B., and Strike, T. A. (1980). Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 303, 1323–1329.
Walker, M. D., Strike, T. A., and Sheline, G. E. (1979). An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 5, 1725–1731.
Warren, K. E., Aikin, A. A., Libucha, M., Widemann, B. C., Fox, E., Packer, R. J., and Balis, F. M. (2005). Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J. Clin. Oncol. 23, 7646–7653.
Wen, P. Y., Macdonald, D. R., Reardon, D. A., Cloughesy, T. F., Sorensen, A. G., Galanis, E., Degroot, J., Wick, W., Gilbert, M. R., Lassman, A. B., Tsien, C., Mikkelsen, T., Wong, E. T., Chamberlain, M. C., Stupp, R., Lamborn, K. R., Vogelbaum, M. A., van den Bent, M. J., and Chang, S. M. (2010). Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972.
Wernicke, A. G., Sherr, D. L., Schwartz, T. H., Pannullo, S. C., Stieg, P. E., Boockvar, J. A., Ivanidze, J., Moliterno, J. A., Parashar, B., Trichter, S., Sabbas, A. M., and Nori, D. (2010). Feasibility and safety of GliaSite brachytherapy in treatment of CNS tumors following neurosurgical resection. J. Cancer Res. Ther. 6, 65–74.
Westphal, M., Hilt, D. C., Bortey, E., Delavault, P., Olivares, R., Warnke, P. C., Whittle, I. R., Jääskeläinen, J., and Ram, Z. (2003). A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology 5, 79–88.
Woolford, L. B., Southgate, T. D., Margison, G. P., Milsom, M. D., and Fairbairn, L. J. (2006). The P140K mutant of human O(6)-methylguanine-DNA-methyltransferase (MGMT) confers resistance in vitro and in vivo to temozolomide in combination with the novel MGMT inactivator O(6)-(4-bromothenyl)guanine. J. Gene Med. 8, 29–34.
Wrensch, M., Minn, Y., Chew, T., Bondy, M., and Berger, M. S. (2002). Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncology 4, 278–299.
Keywords: glioblastoma, radiotherapy, chemotherapy
Citation: Shirazi HA, Grimm S, Raizer J and Mehta MP (2011) Combined modality approaches in the management of adult glioblastoma. Front. Oncol. 1:36. doi: 10.3389/fonc.2011.00036
Received: 02 June 2011;
Paper pending published: 21 June 2011;
Accepted: 29 September 2011;
Published online: 28 October 2011.
Edited by:
Jann Sarkaria, Mayo Clinic, USACopyright: © 2011 Shirazi, Grimm, Raizer and Mehta. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Minesh P. Mehta, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, 251 E. Huron Street, LC-178, Chicago, IL 60611, USA. e-mail:bW1laHRhQG5tZmYub3Jn

 Sean Grimm2
Sean Grimm2