- Gastrointestinal Malignancy Section, Medical Oncology Branch, National Cancer Institute, Bethesda, MD, USA
For patients with locally advanced esophageal cancer no clear standard of care exists. Notwithstanding several negative phase III studies the data provide support for so-called trimodality treatment and this is probably the most common approach. Even the role of surgery has been questioned. These alternative approaches are set against a changing epidemiological background whereby adenocarcinoma has become the predominant tumor type, at least in the western world. In recent times an emphasis has been placed on the better selection of patients, predominantly based on data that shows a markedly improved survival in those who exhibit a response to neo-adjuvant therapy. In this article we review the major studies and discuss new approaches to the management of patients with locally advanced cancer of the esophagus.
Introduction
Cancer of the esophagus is a disease in transition, at least in terms of its epidemiology. The “fundamentals” of esophageal cancer – in terms of its epidemiology and anatomical distribution – have changed in the recent past (at least in the “western” world). These “fundamentals” have by contrast remained relatively static for other solid tumors. Over the past 20 years esophageal cancer has changed from being a so-called “blue collar” disease (Bollschweiler et al., 2000) of predominantly squamous pathology affecting the mid and upper esophagus to being a disease of the lower esophagus of glandular pathology affecting an increasing number of females. Any review of this disease must be placed in the context of this changing epidemiology.
Epidemiology
Esophageal cancer is diagnosed in approximately half a million people worldwide annually and is the eighth leading cause of cancer death worldwide (Kamangar et al., 2006). Its generally poor outlook is reflected in a high mortality-to-incidence rate ratio of 0.83 (compared to 0.51–0.52 in colorectal cancer for example). In the last decade, there has been only a modest improvement in the 5-year survival rate (Siegel et al., 2011). Historically, esophageal cancer was predominantly of squamous pathology. In the 1970s adenocarcinoma was reported as accounting for as low as 4% of the disease burden (Bosch et al., 1979). For the US and other “western” countries this has changed over the past 20 years such that in the US and some northern European countries adenocarcinoma represents the predominant histological subtype (Blot and McLaughlin, 1999; Bosetti et al., 2008; Umar and Fleischer, 2008). The greater part of this increase has occurred in white males however a significant increase has also been seen in females and across racial categories (Bollschweiler et al., 2001; Brown et al., 2008). This is not a global phenomenon since in Asian countries squamous cell carcinoma (SCC) of the esophagus remains predominant (Shibata et al., 2008). There are also marked ethnic variations within geographic areas (Trivers et al., 2008).
The histologic categorization (squamous vs. adenocarcinoma) does not in itself dictate the therapeutic choice at the present time. However, as will be discussed the relative importance of surgery and chemoradiation (CRT) may be different dependent on pathology. At a minimum the epidemiological trend has impacted indirectly the field in that it has been paralleled by changes in anatomical location. In contrast to SCC, which arises from the upper esophagus, adenocarcinoma tends to arise from Barrett’s dysplasia in the lower esophagus or gastro-esophageal (GE) junction. The mode of presentation may be different also in that fewer than half of those with adenocarcinoma present with the “classic” symptomatology of dysphagia and weight loss (Gibbs et al., 2007). From a public health standpoint both entities have overlapping but different risk factor profiles which would require different preventive strategies (Trivers et al., 2008). For example, obesity appears to be a risk factor for adenocarcinoma but not squamous cancer (Lagergren et al., 1999), whereas there has been a conflicting data regarding the role of high risk HPV infection in the development of SCC (Dillner et al., 1995; Kato et al., 2011).
Staging
Recently, several changes were adopted in the seventh edition of the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging system in esophageal cancer (Edge and Compton, 2010). The three major changes are: (1) The inclusion of tumors at the esophagogastric junction and proximal 5 cm of the stomach that extend into the EGJ or esophagus as esophageal cancers. (2) The development of separate stage grouping based on the histology of the tumor (SCC vs. adenocarcinoma). (3) The classification of T4 disease based on the resectability of the tumor and its relation to the adjacent organs. Accordingly, in tumors that invade adjacent structures, such as pleura, pericardium, or diaphragm (T4a) surgery may still have a role. In contrast, T4b tumors that involve the trachea, aorta, or vertebral body are considered unresectable. Locally advanced cancer of the esophagus, defined as stage IIb to IIIc, includes tumors that invade regional lymph nodes (N1-3) or local structures (T4 disease). Patients within this category may be resectable, inoperable for medical reasons, or technically unresectable for reasons of local tumor extension. As will be discussed a loco-regional approach (i.e., chemoradiation) with curative intent is still indicated for these latter two patient groups. For disease that is not loco-regionally confined (i.e., stage IV, metastatic disease) systemic chemotherapy forms the major part of therapy and local measures directed toward the esophagus are palliative in intent.
Role of PET
A recurring theme encountered in the neo-adjuvant studies mentioned above is that responders have a superior outcome to non-responders. Identifying non-responders early in the course of their neo-adjuvant therapy has the potential to allow them to go directly to surgery (or alternatively to consider different chemotherapy). In addition to its potential role as a “biomarker” in the early identification of non-responders PET scanning at baseline will uncover metastatic disease in ∼15% (Downey et al., 2003). Although it’s not clear what relevance the initial absolute SUV value has (Shenfine et al., 2009), decreases in SUV in response to neo-adjuvant therapy appear to predict for improved survival outcomes (Downey et al., 2003). An elevated SUV at baseline may also predict for likelihood of response (Rizk et al., 2009).
The feasibility of so-called PET-directed therapy was evaluated by Lordick et al. (2007) in a phase II (“MUNICON”) study where 119 patients with adenocarcinoma of the esophago-gastric junction underwent PET scanning 2 weeks into their neo-adjuvant chemotherapy to evaluate metabolic response (defined as a reduction of SUV by ≥35%). Non-responders were taken directly to surgery and had a median survival of 25.8 months. Approximately half the patients responded and continued on chemotherapy, 96% of whom underwent R0 resection (compared to 74% in the non-responders). The median survival for the PET-responders was not reached at the time of report. A refinement of this approach was the MUNICON-2 study by the same group of investigators, again in adenocarcinoma of the esophago-gastric junction, where non-responders (identified by PET at 2 weeks) received “salvage” chemoradiation (Lordick et al., 2008). Unfortunately the majority of these non-responders (>80%) showed no evidence of response to this chemoradiation at a subsequent PET scan and only 69% underwent an R0 resection with a median survival of 13.8 months. While it appears therefore that PET-directed therapy may prevent patients from receiving a full course of neo-adjuvant therapy that is not benefiting them, it is not clear whether it will steer them toward therapy that will alter their inferior prognosis. Furthermore, a recent retrospective study questioned if post chemoradiotherapy PET scan response, characterized as post treatment (SUV) of ≤3, can predict the need for surgery. In this study 105 patients with stage I-IVA esophageal cancer, mainly adenocarcinoma, received trimodality therapy (55 patients) or chemoradiation therapy only (50 patients). The PET-responders on the chemoradiation cohort had longer 2-year overall survival (71 vs. 11%, P < 0.01) compared to the non-responders and 2-year freedom from local failure was also longer (75 vs. 28%, P < 0.01). On the other hand, no difference in outcome was noted between responders and non-responders in patients who underwent surgery possibly because FDG-PET residual disease was resected (Monjazeb et al., 2010).
Treatment of Locally Advanced Esophageal Cancer
Surgery has been the historical mainstay for resectable, localized esophageal cancer, and surgical resection remains the major therapeutic modality today. However, unlike most other solid tumors, it is not the only curative modality. In addition, median survival times for patients in surgery only arms of randomized studies have ranged from 11 to 18.6 months only and 5-year survival rates of 16–32% (Walsh et al., 1996; Bosset et al., 1997; Kelsen et al., 1998, 2007; Medical Research Council Oesophageal Cancer Working Group, 2002; Tepper et al., 2008). The necessity for surgery to be performed in high-throughput centers has been well documented. In addition, the technical refinements and advances in postoperative care that have occurred in recent years have resulted in concrete improvements in patient outcome (Altorki and Skinner, 2001; Whooley et al., 2001; Wouters et al., 2008). Notwithstanding these real advances there are limits going forward to what surgical resection alone can achieve. These limits primarily relate to disease biology and extent, i.e., the presence or absence of subclinical micrometastatic disease. Over the course of the last two decades the priority has been to establish a role for additional modalities in the management of localized esophageal cancer. In this context even the role of surgery itself has been a matter of debate.
Neo-adjuvant Chemotherapy
The rationale for the addition of chemotherapy to surgery is primarily the eradication of presumptive micrometastatic disease. The proof of principle for this strategy has been demonstrated in several solid tumor types. Administering this chemotherapy in advance of surgery has a number of potential advantages including the following: earlier treatment against micrometastatic disease; potentially increased resectability; and proportion of R0 or margin-negative resections; and better tolerability compared to the postoperative period.
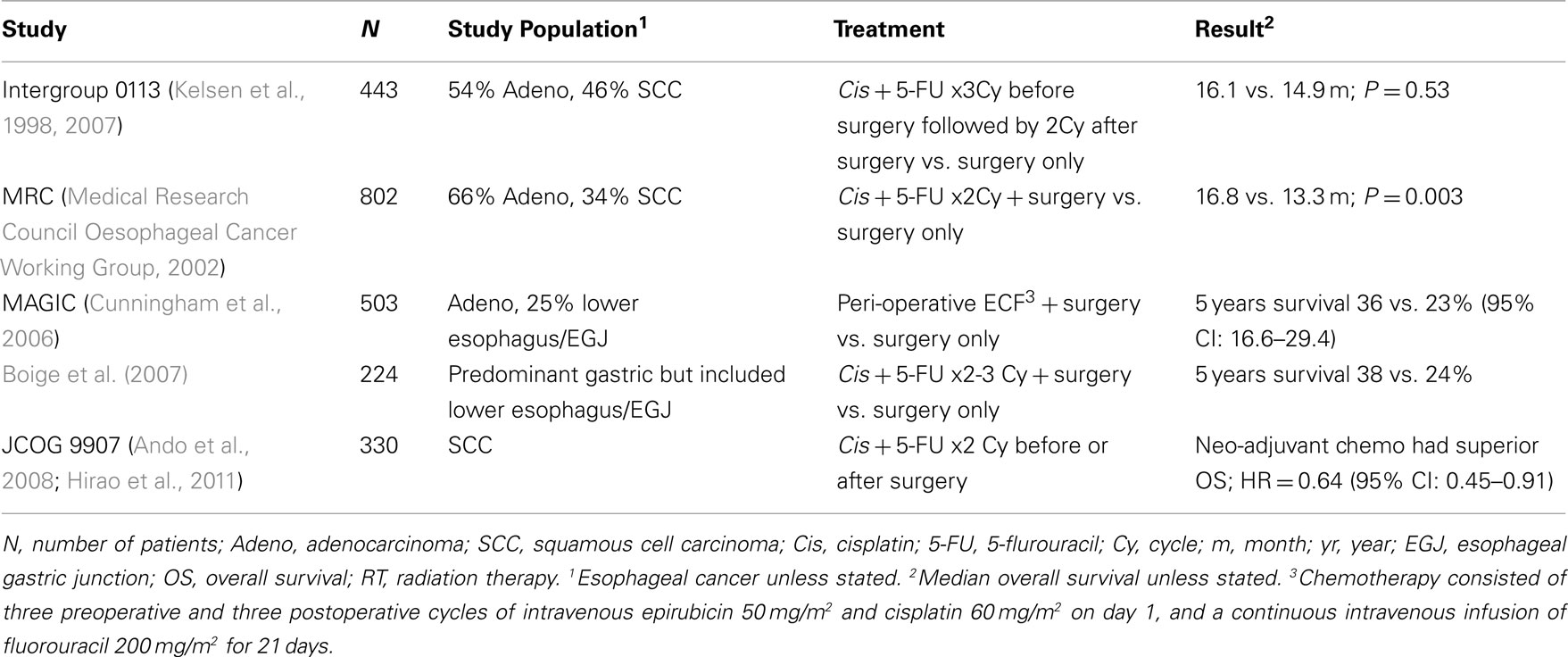
The Intergroup 0113 study (N = 443) compared immediate surgery with surgery that was preceded by three cycles of chemotherapy (cisplatin and 5-fluorouracil). In the chemotherapy arm two additional cycles were given after surgery. Disappointingly there was no difference in median survival between the two groups (16.1 vs. 14.9 months; P = 0.53) nor in 2- and 5-year survival rates (Kelsen et al., 1998, 2007). This study confirmed the poor prognosis of patients who had an incomplete, R1 or R2 resection. Only patients who underwent R0 resection had a substantial chance of long-term disease-free survival, although a small proportion of patients (N = 9) who had an R1 resection appeared to be “salvaged” by postoperative chemoradiation. Chemotherapy did appear to decrease the R1 resection rate however. Probably the most important observation from this study was that patients who responded to chemotherapy had a superior outcome (median survival 3 years) compared to those who did not respond, for whom the median survival was not different to the surgery only arm (1.1 and 1.3 years, respectively). Likewise a phase II study of preoperative chemotherapy in patients with resectable disease (mostly adenocarcinoma) revealed a similar disparity in survival in favor of those who showed clinical evidence of response to treatment (median survival 63.4 vs. 21.5 months in favor of responders, P = 0.005; Pennathur et al., 2008).
A similar but larger (N = 802) study by the Medical Research Council (MRC) in the UK provided a slightly different result than the Intergroup trial. This study compared two cycles of neo-adjuvant chemotherapy (again cisplatin and 5-fluorouracil) plus surgery with surgery alone in patients with localized disease (Medical Research Council Oesophageal Cancer Working Group, 2002). Patients who received chemotherapy had a higher complete resection rate (78 vs. 70%; P < 0.001) and here there was an overall survival benefit for patients receiving chemotherapy (HR, 0.78; P = 0.003), which translated into an absolute benefit in 5-year survival of 6% (23 vs. 17.1%). How does one reconcile the ostensibly different outcomes in both the Intergroup and the MRC studies? As mentioned above, it is likely that the benefit of preoperative chemotherapy is confined to a select group of patients, i.e., those who manifest a response. This was inferred by the updated results of the Intergroup study but perhaps needed the larger numbers of the MRC study to translate into a survival benefit for the overall chemotherapy cohort and result in a statistically positive study.
The other major applicable study evaluating the role of preoperative chemotherapy was the Medical Research Council Adjuvant Gastric Cancer Infusional Chemotherapy (MAGIC) trial (N = 503; Cunningham et al., 2006). Although primarily a study of gastric cancer ∼25% had adenocarcinoma of the lower esophagus or esophago-gastric junction and therefore this study is relevant to this discussion. Chemotherapy consisted of cisplatin, fluorouracil, and epirubicin and was administered in a peri-operative fashion (three cycles before and three cycles after surgery). Patients in the control arm underwent surgery alone. The difficulty in administering postoperative treatment in this population was underlined by the fact that only 42% of those assigned to the chemotherapy arm completed postoperative treatment. In the chemotherapy group the median diameter of resected tumors was smaller, there were relatively more T1 and T2 tumors and there was a trend toward less of a nodal burden. This extended into a survival advantage (HR for death = 0.75; 95% CI: 0.6–0.93; 5-year survival 36.3 vs. 23%, P = 0.009). These results apply to the study population as a whole. As determined in the subgroup analysis there was no evidence of heterogeneity of treatment effect according to the site of the primary tumor (gastric vs. esophageal tumor). A similar result was reported in a French study (N = 224) which evaluated preoperative chemotherapy (cisplatin and 5-fluorouracil) in patients with adenocarcinoma of the stomach or lower esophagus (Boige et al., 2007). Of the patients who were randomized to the chemotherapy arm ∼50% of them also received postoperative chemotherapy. Preoperative chemotherapy was associated with an increased R0 rate (84 vs. 73%, P = 0.04) and improvements in 5-year disease-free (34 vs. 21%) and overall survival (38 vs. 24%) in favor of the chemotherapy arm.
It is unclear whether the relative importance of chemotherapy differs according to histology. The MAGIC study was exclusively in patients with adenocarcinoma and in the MRC study 66% had adenocarcinoma. However the authors of the latter study point out that the hazard ratios for treatment effect were the same for both squamous and adenocarcinoma pathology (0.78) albeit with different confidence intervals.
The optimal timing for the peri-operative chemotherapy administration was evaluated in a large prospective phase III trial (N = 330) conducted by the Japan Clinical Oncology Group (JCOG 9907). This trial was terminated early after it showed an advantage benefit of neo-adjuvant chemotherapy with cisplatin plus 5-fluorouracil (CF) compared to adjuvant CF for stage II/III esophageal SCC. Patients who received neo-adjuvant chemotherapy had a longer overall survival (OS; HR = 0.64, 95% CI: 0.45–0.91, two-sided P = 0.014) with no increase in the risk of complications or hospital mortality after surgery (Ando et al., 2008; Hirao et al., 2011). Phase III trials that used neo-adjuvant chemotherapy are summarized in Table 1.
Chemoradiation as Primary Modality or before Surgery
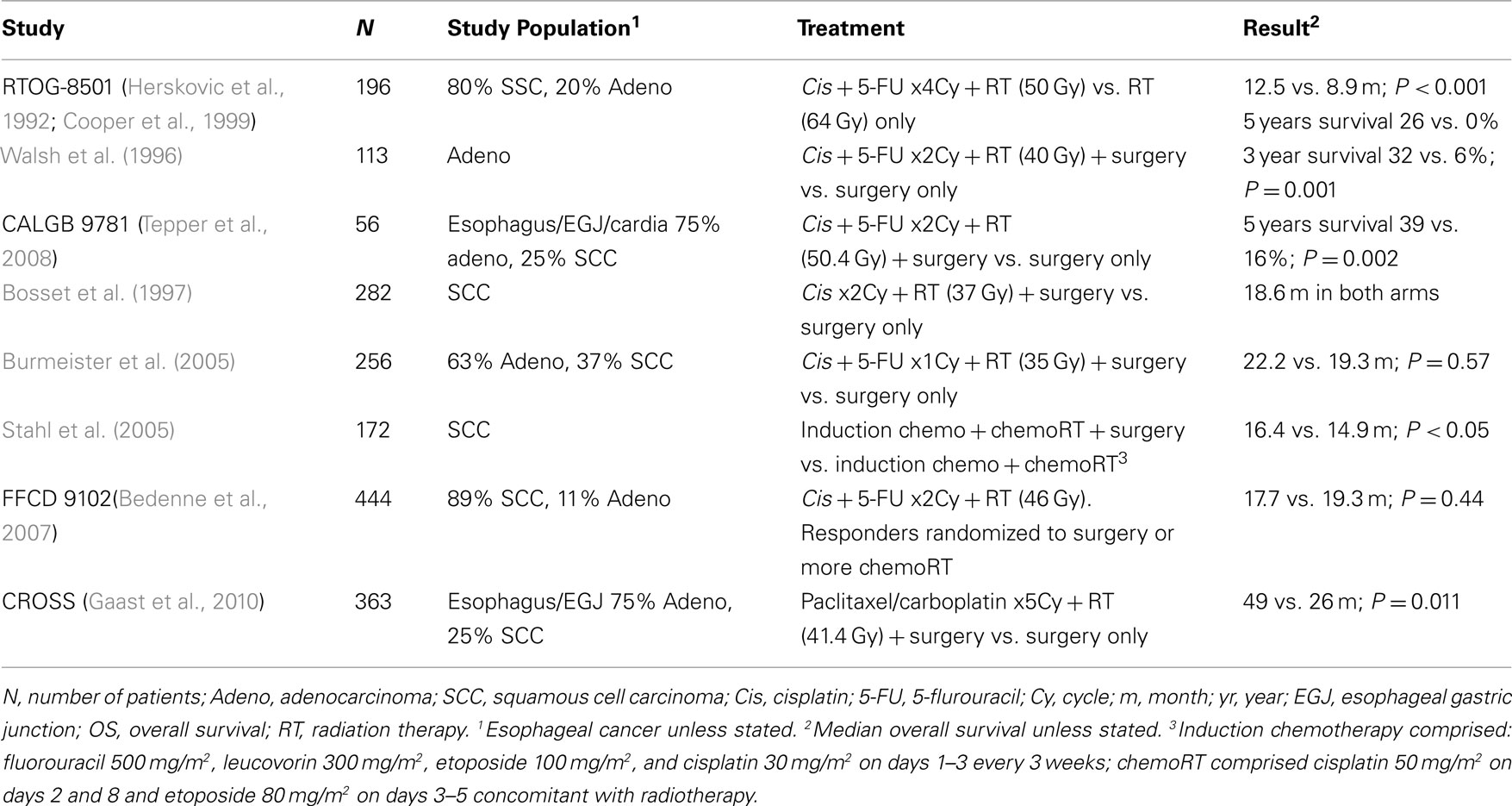
Combined chemoradiation became established in esophageal cancer as a result of the RTOG 85-01 study, which compared radiation alone to chemotherapy plus radiation (Herskovic et al., 1992). In this landmark study chemoradiation comprised four cycles of combined 5-fluorouracil (1000 mg/m2 daily for 4 days) and cisplatin (75 mg/m2 on day 1) with 50 Gy of radiation therapy, as compared with a higher dose of radiation therapy (64 Gy) alone. The majority of patients (∼80%) had squamous cancer of the thoracic esophagus. Patients with gastric, mediastinal, or supraclavicular lymph nodes involvement were excluded. Median survival was better for the chemoradiation group (12.5 vs. 8.9 months) and longer follow-up revealed the potential for long-term survival in this disease even in the absence of surgical resection (5-year survival 26 vs. 0%; Cooper et al., 1999). A subsequent study addressed the question of higher doses of radiation but this was not found to be effective (Minsky et al., 2002). A major argument for the incorporation of radiation therapy – as opposed to neo-adjuvant chemotherapy alone as discussed in the previous section – is the higher pathological complete response (pCR) rate that is achieved and the correlation of this with survival (Berger et al., 2005; Donahue et al., 2009). Chemoradiation has been associated with a pCR rate of 20–30% but with chemotherapy alone this is extremely low (<5%; MacGuill et al., 2006).
A logical extension from RTOG 85-01 was to see if the addition of chemoradiation to surgery provided an additional benefit compared to surgery alone. Two meta-analyses have suggested a benefit for chemoradiation and surgery (so-called trimodality therapy) compared to surgery alone (Urschel and Vasan, 2003; Gebski et al., 2007). Individual phase III studies however have been conflicting with several randomized studies showing no benefit (Bosset et al., 1997; Urba et al., 2001; Burmeister et al., 2005) and three phase III trials demonstrating a benefit from trimodality therapy. The first positive study was reported by Walsh et al. and has been criticized due to the poor survival of the surgery alone group (Walsh et al., 1996; Ku and Ilson, 2008). The second positive study was the CALGB 9781, which attempted to address the question in a definitive fashion by randomizing patients to trimodality therapy or surgery alone. Unfortunately, only 56 of a planned 475 patients were enrolled (Tepper et al., 2008). Nevertheless the study was well-designed, well-conducted, and showed a 5-year survival rate of 39% (95% CI: 21–57%) vs. 16% (95% CI: 5–33%) in favor of trimodality therapy. The last study to show a benefit from trimodality therapy compared to surgery alone was the CROSS trial (N = 363). Patients on the CRT arm had higher R0 resection rate (92.3 vs. 64.9%) and median overall survival [49 vs. 26 months, P = 0.011, HR 0.67 (95% CI: 0.50–0.92)] with no difference in the postoperative mortality rate (Gaast et al., 2010). Noteworthy, patients on CRT arm received lower dose of RT compared to CALGB 9781 trial (41.4 vs. 50.4 Gy) administered concurrently with weekly paclitaxel 50 mg/m2 and carboplatin AUC = 2 for 5 weeks. Another logical question deriving from RTOG 85-01 was to question the added value of surgery given the potential and ostensibly equivalent long-term survival rate with chemoradiation alone. Stahl and colleagues addressed this question in an interesting study in patients with squamous pathology. In this study all patients received induction chemotherapy and chemoradiation, the experimental question being the added role of surgery (Stahl et al., 2005). Those who were randomized to the non-surgery arm received a higher dose of radiation (65 vs. 40 Gy). The results were not clear-cut in that those who underwent surgery had better local control but a higher treatment-related mortality. There was no difference in overall survival.
Similarly Bedenne et al. (2007) compared surgery to additional chemoradiation in a population of patients with predominantly squamous cancer, all of whom received initial chemoradiation. The essential feature in the design was that only patients who showed evidence of clinical response to chemoradiation were randomized to either surgery or continued chemoradiation. The results were consistent with the Stahl study. 58% of patients responded to chemoradiation and were randomized. There was no difference in survival between the two arms. The 3-month mortality was less in the chemoradiation arm (0.8 vs. 9.3%, P = 0.002) at the apparent expense of less local control (loco-regional recurrence 43 vs. 34%, respectively).
A consistent feature of studies in this disease indication has been the relatively slow pace of clinical accrual. As seen in CALGB 9781 this results in well-designed but underpowered studies and is a significant barrier to advancement in the field, particularly in the era of targeted therapy. As an example FFCD9102 and Stahl et al. (2005) studies required 8 and 9 years, respectively, to accrue patients (Bedenne et al., 2007). Phase III trials that used neo-adjuvant chemoradiation therapy are summarized in Table 2.
Neo-adjuvant Chemoradiation vs. Neo-adjuvant Chemotherapy
Stahl et al. (2009) attempted to compare the two competing neo-adjuvant approaches in a phase III randomized study of chemotherapy (consisting of cisplatin and fluorouracil) vs. chemoradiation, followed by surgery. Chemoradiation comprised a lower dose (30 Gy) of radiation than usual given over a shorter period (3 weeks) in association with cisplatin and etoposide. Unfortunately accrual was slow and the study was closed early with 119 patients treated. Nevertheless the results are an important addition to the field. Patients who received chemoradiation had a significant higher probability achieving a pCR (15.6 vs. 2%) and proportionally higher N0 rate (64.4 vs. 37.7%) at resection. In addition, preoperative chemoradiation therapy was associated with an improved 3-year survival rate (47.4 vs. 27.7%, log-rank P = 0.07), although this was not statistically significant. The other feature of this study was the homogeneity of the study population confined as it was to patients with adenocarcinoma of the esophago-gastric junction.
Newer Chemo Regimens
The MUNICON-2 study illustrates the need for more effective treatments given the dismal outcome of patients who received salvage treatment. In the vast majority of neo-adjuvant studies cisplatin and fluorouracil have been the agents used either alone or with radiation. Oxaliplatin is another platinum agent that was investigated in a phase II study by the Southwestern Oncology Group (SWOG) in combination with infusional fluorouracil revealing an impressive pCR rate of 33% (Leichman et al., 2009). Moreover, several phase II studies have demonstrated the feasibility and efficacy of various schedules of paclitaxel in combination with platinum agents showing impressive pCR rates (Blanke et al., 1999; Meluch et al., 2003; Urba et al., 2003; Brenner et al., 2004; van Meerten et al., 2006; van de Schoot et al., 2008). Likewise the addition of docetaxel to cisplatin and 5-FU (DCF) was found to be well tolerated with a pCR rate of 47%, a 3 year survival of 81% (Pasini et al., 2009), and an overall response of 60% (Hara et al., 2011). Adding docetaxel to cisplatin and capecitabine (DCX) also resulted in tumor downstaging in 56% of patients (Fonseca et al., 2011) and successful R0 resection in 90% of patients (Thuss-Patience et al., 2011). Furthermore, many trials had compared the efficacy of different chemotherapy combinations. Recently the regimen of FOLFOX4 was compared to cisplatin and 5-FU, both combined with radiation therapy. FOLOFOX4 was found to have a higher response rate (45 vs. 29%), which translated to a longer overall survival (22.7 vs. 15.1 months). This regimen is now being tested in a phase III trial (Conroy et al., 2010). Another randomized phase II study (ECOG 1201) compared paclitaxel/cisplatin-based and irinotecan/cisplatin-based chemoradiation. Pathological CR rates were reasonable, 16 and 14%, respectively (Kleinberg et al., 2007). Survival was similar for both treatment arms and did not appear to be better compared to the historical experience e with cisplatin/5-FU (Kleinberg et al., 2008).
Targeted Therapy
Many targeted therapies have been recently investigated in advanced esophageal cancer showing an encouraging efficacy that still needs to be validated. Approximately 50–70% of esophageal cancers have an overexpression of the EGFR protein, however based on the experience of other malignancies, this is not necessarily predictive of response. Moreover, the incidence of KRAS mutations in junctional or gastric adenocarcinoma (6–21%) and in esophageal squamous cancer (16% in one report) appears to be less than in colon cancer where these mutations are predictive of resistance to cetuximab (Sommerer et al., 2004; Lyronis et al., 2008). Accordingly, the role of cetuximab – a monoclonal antibody directed against EGFR – needs to be clarified. The use of cetuximab in the neo-adjuvant setting with radiation in esophageal cancer appears to be feasible and to provide good overall response rate (66.6–80%) and pCR rate (36%; Agarwala et al., 2009; Ruhstaller et al., 2009; Dahan et al., 2011; Sunpaweravong et al., 2011). In addition, the combination of FOLFIRI and cetuximab showed an impressive survival of 16 months (Pinto et al., 2007) in patients with untreated advanced gastric or gastro-esophageal junction (GEJ) adenocarcinoma. Currently, the RTOG is evaluating cetuximab in combination with cisplatin, paclitaxel, and radiation in esophageal cancer. Another EGFR targeted therapy, panitumumab, is currently being assessed in the REAL3 trial in combination with chemotherapy (epirubicin, oxaliplatin, and capecitabine).
HER-2 overexpression as detected by immunohistochemistry ranges from 19 to 43% but in contrast to breast cancer, HER-2-positive status in esophageal adenocarcinoma is not an independent prognostic factor (Shah et al., 2011a; Yoon et al., 2011). The efficacy of trastuzumab, a monoclonal antibody directed against HER-2, in advanced gastric cancer, and GEJ cancer was recently reported in the ToGA trial. Patients (N = 298) treated with trastuzumab in combination with capecitabine plus cisplatin had a longer OS of 13.8 months compared to 11.1 months in patients (N = 296) treated with the same chemotherapy regimen alone (P = 0.0046; Bang et al., 2010).
Blocking the tumor angiogenesis is another strategy that has been extensively investigated in cancer including esophageal cancer. Bevacizumab, a humanized monoclonal antibody that inhibits the vascular endothelial growth factor A (VEGF-A), has been shown to be active in some studies in esophageal cancer. A phase II study of bevacizumab in combination with irinotecan and cisplatin in patients with advanced gastric or junctional adenocarcinoma demonstrated the safety of this approach and an encouraging efficacy (Shah et al., 2006; Ilson et al., 2009). Likewise the addition of Bevacizumab to docetaxel, cisplatin, and 5-FU appeared to be tolerable with promising response rate of 85% in GEJ tumors (Shah et al., 2011b). Although the addition of Bevacizumab to chemotherapy with capecitabine or 5-FU and cisplatin in the AVAGAST trial (N = 774) resulted in a significant improvement in overall response rate (37.4–46%) and PFS (5.3–6.7 months) in patients with advanced gastric or GEJ adenocarcinoma, this trial failed to meet its primary endpoint of prolonging OS (12.1 vs. 10.1 months, HR 0.87; P = 0.1002; Ohtsu et al., 2011). However, the authors argued that American patients demonstrated significantly improved OS (HR 0.63), while Asian region patients did not (HR 0.97; Shah et al., 2012). This regional variation in benefit is still to be further studied in future trials. Nonetheless, the applicability of bevacizumab to esophageal cancer will be at least partially answered by the MAGIC-B study in the UK evaluating this agent in combination with epirubicin/cisplatin/capecitabine (ECX) chemotherapy in junctional or gastric adenocarcinoma. Other targeted agents such as sunitinib and sorafenib are currently being studied in esophageal cancer (Ilson et al., 2011; Knox et al., 2011)
Conclusion/Future Perspectives
Amidst the confusion of the phase III studies in locally advanced esophageal cancer over the past decade – and from which no clear standard of care has emerged – one theme is recurrent: patients who respond to neo-adjuvant therapy have a far superior survival than those who do not.
Whilst the so-called targeted agents have yet to be evaluated in the phase III setting it is unlikely that this central problem will have changed, i.e., trying to prospectively identify those who will benefit from neo-adjuvant therapy and those who will not. Although we have an increasing number of active agents in this disease we remain in the dark ages in terms of our ability to predict what will work and what will not. In esophageal cancer we do not have a single predictive marker that is of use to the clinician. Even the chief differentiating factor – squamous vs. adenocarcinoma – is of little help in the clinic in dictating therapy choices. Differential gene expression profiling has the potential to better categorize esophageal cancer in terms of its biology and response to therapy (Hammoud et al., 2009). In correlative studies as part of the ECOG 1201 study mentioned above, analysis of single nucleotide polymorphisms in DNA repair pathways to seek predictors of response identified an allele associated with a lower likelihood of pCR. It is hoped that the accumulation of this type of information will lead us to molecularly directed therapy for individual patients. However the MUNICON-2 study underlines the fact that whilst predictive biomarkers may help us to better direct care and provide us with a better indicator of prognosis, newer more effective therapies are ultimately required to capitalize on this knowledge.
One of the difficulties with interpreting the data in esophageal cancer has been the heterogeneity of diseases included. Eligibility criteria have tended to cross histological boundaries (squamous vs. adenocarcinoma). Even where accrual is confined to adenocarcinoma, as in the MAGIC study, tumors from different geographical locations have been mixed together. There is a considerable difference between adenocarcinoma of the stomach, lower esophagus, and esophago-gastric junction in terms of molecular characteristics, pattern of spread and pre-disposing risk factors (Tepper and O’Neil, 2009). Even within junctional tumors there is a marked difference in terms of behavior and these tumors have been classified accordingly (Siewert and Stein, 1998). Nonetheless, the majority of the clinical trials conducted in locally advanced esophageal cancer were underpowered due to poor accrual, which remains a challenge facing the ongoing and future trials.
Conflict of Interest Statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
Agarwala, A. K., Hanna, N., Mccollum, A., Bechar, N., Dimaio, M., Yu, M., Tong, Y., Becerra, C. R., and Choy, H. (2009). Preoperative cetuximab and radiation (XRT) for patients (PTS) with surgically resectable esophageal and gastroesophageal junction (GEJ) carcinomas: a pilot study from the Hoosier Oncology Group and the University of Texas Southwestern. J. Clin. Oncol. 27, 4557.
Altorki, N., and Skinner, D. (2001). Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann. Surg. 234, 581–587.
Ando, N., Kato, H., Shinoda, M., Ozawa, S., Shimizu, H., Nakamura, T., Yabuzaki, Y., Aoyama, N., Kurita, A., and Fukuda, H. (2008). “A randomized trial of postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus neoadjuvant chemotherapy for localized squamous cell carcinoma of the thoracic esophagus (JCOG 9907),” in Gastrointestinal Cancers Symposium 2008, Orlando.
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., Lordick, F., Ohtsu, A., Omuro, Y., Satoh, T., Aprile, G., Kulikov, E., Hill, J., Lehle, M., Ruschoff, J., and Kang, Y. K. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697.
Bedenne, L., Michel, P., Bouche, O., Milan, C., Mariette, C., Conroy, T., Pezet, D., Roullet, B., Seitz, J. F., Herr, J. P., Paillot, B., Arveux, P., Bonnetain, F., and Binquet, C. (2007). Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 25, 1160–1168.
Berger, A. C., Farma, J., Scott, W. J., Freedman, G., Weiner, L., Cheng, J. D., Wang, H., and Goldberg, M. (2005). Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J. Clin. Oncol. 23, 4330–4337.
Blanke, C. D., Choy, H., Teng, M., Beauchamp, R. D., Leach, S., Roberts, J., Washington, K., and Johnson, D. H. (1999). Concurrent paclitaxel and thoracic irradiation for locally advanced esophageal cancer. Semin. Radiat. Oncol. 9, 43–52.
Blot, W. J., and McLaughlin, J. K. (1999). The changing epidemiology of esophageal cancer. Semin. Oncol. 26, 2–8.
Boige, V., Pignon, J., Saint-Aubert, B., Lasser, P., Conroy, T., Bouche, O., Segol, P., Bedenne, L., Rougier, P., and Ychou, M. (2007). “Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial,” in ASCO Meeting Abstracts 25, 4510, Chicago.
Bollschweiler, E., Schroder, W., Holscher, A. H., and Siewert, J. R. (2000). Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br. J. Surg. 87, 1106–1110.
Bollschweiler, E., Wolfgarten, E., Gutschow, C., and Holscher, A. H. (2001). Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 92, 549–555.
Bosch, A., Frias, Z., and Caldwell, W. L. (1979). Adenocarcinoma of the esophagus. Cancer 43, 1557–1561.
Bosetti, C., Levi, F., Ferlay, J., Garavello, W., Lucchini, F., Bertuccio, P., Negri, E., and La Vecchia, C. (2008). Trends in oesophageal cancer incidence and mortality in Europe. Int. J. Cancer 122, 1118–1129.
Bosset, J. F., Gignoux, M., Triboulet, J. P., Tiret, E., Mantion, G., Elias, D., Lozach, P., Ollier, J. C., Pavy, J. J., Mercier, M., and Sahmoud, T. (1997). Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N. Engl. J. Med. 337, 161–167.
Brenner, B., Ilson, D. H., Minsky, B. D., Bains, M. S., Tong, W., Gonen, M., and Kelsen, D. P. (2004). Phase I trial of combined-modality therapy for localized esophageal cancer: escalating doses of continuous-infusion paclitaxel with cisplatin and concurrent radiation therapy. J. Clin. Oncol. 22, 45–52.
Brown, L. M., Devesa, S. S., and Chow, W. H. (2008). Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J. Natl. Cancer Inst. 100, 1184–1187.
Burmeister, B. H., Smithers, B. M., Gebski, V., Fitzgerald, L., Simes, R. J., Devitt, P., Ackland, S., Gotley, D. C., Joseph, D., Millar, J., North, J., Walpole, E. T., and Denham, J. W. (2005). Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 6, 659–668.
Conroy, T., Yataghene, Y., Etienne, P. L., Michel, P., Senellart, H., Raoul, J. L., Mineur, L., Rives, M., Mirabel, X., Lamezec, B., Rio, E., Le Prise, E., Peiffert, D., and Adenis, A. (2010). Phase II randomised trial of chemoradiotherapy with FOLFOX4 or cisplatin plus fluorouracil in oesophageal cancer. Br. J. Cancer 103, 1349–1355.
Cooper, J. S., Guo, M. D., Herskovic, A., Macdonald, J. S., Martenson, J. A. Jr., Al-Sarraf, M., Byhardt, R., Russell, A. H., Beitler, J. J., Spencer, S., Asbell, S. O., Graham, M. V., and Leichman, L. L. (1999). Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 281, 1623–1627.
Cunningham, D., Allum, W. H., Stenning, S. P., Thompson, J. N., Van De Velde, C. J., Nicolson, M., Scarffe, J. H., Lofts, F. J., Falk, S. J., Iveson, T. J., Smith, D. B., Langley, R. E., Verma, M., Weeden, S., Chua, Y. J., and Participants, M. T. (2006). Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20.
Dahan, L., Chibaudel, B., Di Fiore, F., Artru, P., Mineur, L., Galais, M., Dupuis, O., Blondin, V., Abdiche, S., Attia, M., De Gramont, A., and Lledo, G. (2011). “Chemoradiation with Folfox plus cetuximab in locally advanced cardia or esophageal cancer: final results of a Gercor phase II trial (ERaFOX),” in ASCO Annual Meeting, Chicago.
Dillner, J., Knekt, P., Schiller, J. T., and Hakulinen, T. (1995). Prospective seroepidemiological evidence that human papillomavirus type 16 infection is a risk factor for oesophageal squamous cell carcinoma. BMJ 311, 1346.
Donahue, J. M., Nichols, F. C., Li, Z., Schomas, D. A., Allen, M. S., Cassivi, S. D., Jatoi, A., Miller, R. C., Wigle, D. A., Shen, K. R., and Deschamps, C. (2009). Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann. Thorac. Surg. 87, 392–398; discussion 398–399.
Downey, R. J., Akhurst, T., Ilson, D., Ginsberg, R., Bains, M. S., Gonen, M., Koong, H., Gollub, M., Minsky, B. D., Zakowski, M., Turnbull, A., Larson, S. M., and Rusch, V. (2003). Whole body 18FDG-Pet and the response of esophageal cancer to induction therapy: results of a prospective trial. J. Clin. Oncol. 21, 428–432.
Edge, S. B., and Compton, C. C. (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474.
Fonseca, P. J., Vieitez, J. M., Turienzo, E., Sanz, L., Perez, G., Izquierdo, M., Pardo-Coto, P., Frunza, M., Gutierrez Restrepo, E., and Lacave, A. J. (2011). “A phase II study with perioperative modified docetaxel, cisplatin, and capecitabine (mDCX) in resectable gastroesophageal adenocarcinoma,” in ASCO Annual Meeting 2011, Chicago.
Gaast, A. V., Van Hagen, P., Hulshof, M., Richel, D., Van Berge Henegouwen, M. I., Nieuwenhuijzen, G. A., Plukker, J. T., Bonenkamp, J. J., Steyerberg, E. W., Tilanus, H. W., and Cross Study Group. (2010). “Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: results from a multicenter randomized phase III study,” in ASCO Annual Meeting, Chicago.
Gebski, V., Burmeister, B., Smithers, B. M., Foo, K., Zalcberg, J., and Simes, J. (2007). Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 8, 226–234.
Gibbs, J. F., Rajput, A., Chadha, K. S., Douglas, W. G., Hill, H., Nwogu, C., Nava, H. R., and Sabel, M. S. (2007). The changing profile of esophageal cancer presentation and its implication for diagnosis. J. Natl. Med. Assoc. 99, 620–626.
Hammoud, Z. T., Badve, S., Zhao, Q., Li, L., Saxena, R., Thorat, M. A., Morimiya, A., Rieger, K. M., and Kesler, K. A. (2009). Differential gene expression profiling of esophageal adenocarcinoma. J. Thorac. Cardiovasc. Surg. 137, 829–834.
Hara, H., Daiko, H., Kato, K., Igaki, H., Kadowaki, S., Tanaka, Y., Hamamoto, Y., Matsushita, H., Nagase, M., Hosoya, Y., and Tahara, M. (2011). “Final results of feasibility study of neoadjuvant chemotherapy with docetaxel, cisplatin, and fluorouracil (DCF) for clinical stage II/III esophageal squamous cell carcinoma,” in ASCO Annual Meeting, Chicago.
Herskovic, A., Martz, K., Al-Sarraf, M., Leichman, L., Brindle, J., Vaitkevicius, V., Cooper, J., Byhardt, R., Davis, L., and Emami, B. (1992). Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 326, 1593–1598.
Hirao, M., Ando, N., Tsujinaka, T., Udagawa, H., Yano, M., Yamana, H., Nagai, K., Mizusawa, J., and Nakamura, K. (2011). Influence of preoperative chemotherapy for advanced thoracic oesophageal squamous cell carcinoma on perioperative complications. Br. J. Surg. 98, 1735–1741.
Ilson, D., Bains, M., Rizk, N., Rusch, V., Flores, R., Park, B., Shah, M., Kelsen, D., Miron, B., and Goodman, K. (2009). Phase II trial of preoperative bevacizumab (BEV), irinotecan (I), cisplatin (C), and radiation (RT) in esophageal adenocarcinoma: preliminary safety analysis. J. Clin. Oncol. 27, 4573.
Ilson, D., Y, Y. J., Shah, M. A., Kelsen, D. P., Tang, L. H., Campbell, J., Fuqua, L., and Capanu, M. (2011). “Phase II trial of sorafenib in esophageal (E) and gastroesophageal junction (GEJ) cancer: response and protracted stable disease observed in adenocarcinoma,” in ASCO Annual Meeting, Chicago.
Kamangar, F., Dores, G. M., and Anderson, W. F. (2006). Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 24, 2137–2150.
Kato, S., Pu, M., and Read, W. L. (2011). Association of human papillomavirus and squamous cell carcinoma of esophagus: a Seer database analysis in ASCO Annual Meeting, 2011, Chicago.
Kelsen, D. P., Ginsberg, R., Pajak, T. F., Sheahan, D. G., Gunderson, L., Mortimer, J., Estes, N., Haller, D. G., Ajani, J., Kocha, W., Minsky, B. D., and Roth, J. A. (1998). Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 339, 1979–1984.
Kelsen, D. P., Winter, K. A., Gunderson, L. L., Mortimer, J., Estes, N. C., Haller, D. G., Ajani, J. A., Kocha, W., Minsky, B. D., Roth, J. A., and Willett, C. G. (2007). Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J. Clin. Oncol. 25, 3719–3725.
Kleinberg, L., Powell, M. E., Forastiere, A., Keller, S., Anne, P., and Benson, A. B. (2007). E1201: an Eastern Cooperative Oncology Group (ECOG) randomized phase II trial of neoadjuvant preoperative paclitaxel/cisplatin/RT or irinotecan/cisplatin/RT in endoscopy with ultrasound (EUS) staged adenocarcinoma of the esophagus. J. Clin. Oncol. 25, 4533.
Kleinberg, L., Powell, M. E., Forastiere, A. A., Keller, S., Anne, P., and Benson, A. B. (2008). “Survival outcome of E1201: an Eastern Cooperative Oncology Group (ECOG) randomized phase II trial of neoadjuvant preoperative paclitaxel/cisplatin/radiotherapy (RT) or irinotecan/cisplatin/RT in endoscopy with ultrasound (EUS) staged esophageal adenocarcinoma,” in ASCO Meeting Abstracts 26, 4532, Chicago.
Knox, J. J., Wong, R., Darling, G. E., Lister, J., Guindi, M., Liu, G., Xu, W., Kim, J. J., Jonker, D. J., Wells, J., Kendal, W., Mackay, H., Visbal, A., Dinniwell, R. E., Pierre, A., Feld, R., Sundaresan, S., Bayley, A., Shargall, Y., and Horgan, A. M. (2011). “Adjuvant sunitinib (SU) for locally advanced esophageal cancer (LAEC): results of a phase II trial,” in ASCO Annual Meeting, Chicago.
Ku, G. Y., and Ilson, D. H. (2008). Preoperative therapy in esophageal cancer. Clin. Adv. Hematol. Oncol. 6, 371–379.
Lagergren, J., Bergstrom, R., and Nyren, O. (1999). Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann. Intern. Med. 130, 883–890.
Leichman, L., Goldman, B. H., Benedetti, J. K., Billingsley, K. G., Thomas, C. R., Iqbal, S., Lenz, H., Blanke, C., Gold, P. J., and Corless, C. L. (2009). “Oxaliplatin (OXP) plus protracted infusion 5-fluorouracil (PIFU) and external beam radiation (EBRT) prior to surgery (S) for potentially curable esophageal adenocarcinoma (EA): a Southwest Oncology Group (SWOG) phase II trial with molecular correlates (S0356),” in 2009 ASCO Annual Meeting, Orlando.
Lordick, F., Ott, K., Krause, B. J., Herrmann, K., Schuster, T., Geinitz, H., Molls, M., Peschel, C., Schwaiger, M., and Siewert, J. R. (2008). “Salvage radiochemotherapy in locally advanced gastroesophageal junction tumors that are metabolically resistant to induction chemotherapy: the MUNICON-2 trial,” in Gastrointestinal Cancer Symposium, Orlando.
Lordick, F., Ott, K., Krause, B. J., Weber, W. A., Becker, K., Stein, H. J., Lorenzen, S., Schuster, T., Wieder, H., Herrmann, K., Bredenkamp, R., Hofler, H., Fink, U., Peschel, C., Schwaiger, M., and Siewert, J. R. (2007). Pet to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 8, 797–805.
Lyronis, I. D., Baritaki, S., Bizakis, I., Krambovitis, E., and Spandidos, D. A. (2008). K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathol. Oncol. Res. 14, 267–273.
MacGuill, M., Mulligan, E., Ravi, N., Rowley, S., Byrne, P. J., Hollywood, D., Kennedy, J., Keeling, P. N., and Reynolds, J. V. (2006). Clinicopathologic factors predicting complete pathological response to neoadjuvant chemoradiotherapy in esophageal cancer. Dis. Esophagus 19, 273–276.
Medical Research Council Oesophageal Cancer Working Group. (2002). Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359, 1727–1733.
Meluch, A. A., Greco, F. A., Gray, J. R., Thomas, M., Sutton, V. M., Davis, J. L., Kalman, L. A., Shaffer, D. W., Yost, K., Rinaldi, D. A., and Hainsworth, J. D. (2003). Preoperative therapy with concurrent paclitaxel/carboplatin/infusional 5-FU and radiation therapy in locoregional esophageal cancer: final results of a Minnie Pearl Cancer Research Network phase II trial. Cancer J. 9, 251–260.
Minsky, B. D., Pajak, T. F., Ginsberg, R. J., Pisansky, T. M., Martenson, J., Komaki, R., Okawara, G., Rosenthal, S. A., and Kelsen, D. P. (2002). Int 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J. Clin. Oncol. 20, 1167–1174.
Monjazeb, A. M., Riedlinger, G., Aklilu, M., Geisinger, K. R., Mishra, G., Isom, S., Clark, P., Levine, E. A., and Blackstock, A. W. (2010). Outcomes of patients with esophageal cancer staged with [(1)F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J. Clin. Oncol. 28, 4714–4721.
Ohtsu, A., Shah, M. A., Van Cutsem, E., Rha, S. Y., Sawaki, A., Park, S. R., Lim, H. Y., Yamada, Y., Wu, J., Langer, B., Starnawski, M., and Kang, Y. K. (2011). Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976.
Pasini, F. Sr., De Manzoni, G., Stievano, L., Grandinetti, A., Maluta, S., Capirci, C., Durante, E., Bonetti, A., Zanoni, A., and Cordiano, C. (2009). Effect of neoadjuvant combined modality therapy with weekly docetaxel (D) and cisplatin (P), 5-fluorouracil (5-FU) continuous infusion (c.i.), and concurrent radiotherapy (RT) on pathological response rate in esophageal cancers (EC): a phase II study. J. Clin. Oncol. 27, 4548.
Pennathur, A., Luketich, J. D., Landreneau, R. J., Ward, J., Christie, N. A., Gibson, M. K., Schuchert, M., Cooper, K., Land, S. R., and Belani, C. P. (2008). Long-term results of a phase II trial of neoadjuvant chemotherapy followed by esophagectomy for locally advanced esophageal neoplasm. Ann. Thorac. Surg. 85, 1930–1936; discussion 1936–1937.
Pinto, C., Di Fabio, F., Siena, S., Cascinu, S., Rojas Llimpe, F. L., Ceccarelli, C., Mutri, V., Giannetta, L., Giaquinta, S., Funaioli, C., Berardi, R., Longobardi, C., Piana, E., and Martoni, A. A. (2007). Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann. Oncol. 18, 510–517.
Rizk, N. P., Tang, L., Adusumilli, P. S., Bains, M. S., Akhurst, T. J., Ilson, D., Goodman, K., and Rusch, V. W. (2009). Predictive value of initial pet-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J. Thorac. Oncol. 4, 875–879.
Ruhstaller, T., Pless, M., Schuller, J. C., Kranzbuhler, H., Von Moos, R., Moosmann, P., Rauch, D., Montemurro, M., Schneider, P. M., and Hess, V. (2009). “Cetuximab in combination with chemoradiotherapy prior to surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase lb-ll trial of the Swiss Group for Clinical Cancer Research (SAKK 75/06),” in ASCO Meeting Abstracts 27, 4570, Orlando.
Shah, M. A., Janjigian, Y. Y., Pauligk, C., Werner, D., Kelsen, D. P., Jaeger, E., Altmannsberger, H., Robinson, E., Tang, L. H., Barbashina, V. V., and Al-Batran, S. (2011a). “Prognostic significance of human epidermal growth factor-2 (HER2) in advanced gastric cancer: a U.S. and European international collaborative analysis,” in ASCO Annual Meeting, Chicago.
Shah, M. A., Jhawer, M., Ilson, D. H., Lefkowitz, R. A., Robinson, E., Capanu, M., and Kelsen, D. P. (2011b). Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J. Clin. Oncol. 29, 868–874.
Shah, M. A., Ramanathan, R. K., Ilson, D. H., Levnor, A., D’Adamo, D., O’Reilly, E., Tse, A., Trocola, R., Schwartz, L., Capanu, M., Schwartz, G. K., and Kelsen, D. P. (2006). Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J. Clin. Oncol. 24, 5201–5206.
Shah, M. A., Van Cutsem, E., Kang, Y.-K., Dakhil, S. R., Satoh, T., Chin, K., Bang, Y.-J., Bu, L., Bilic, G., and Ohtsu, A. (2012). “Survival analysis according to disease subtype in AVAGAST: first-line capecitabine and cisplatin plus bevacizumab (BEV) or placebo in patients (PTS) with advanced gastric cancer,” in 2012 Gastrointestinal Cancers Symposium, San Francisco.
Shenfine, J., Barbour, A. P., Wong, D., Thomas, J., Martin, I., Gotley, D. C., and Smithers, B. M. (2009). Prognostic value of maximum standardized uptake values from preoperative positron emission tomography in resectable adenocarcinoma of the esophagus treated by surgery alone. Dis. Esophagus. 22, 668–675.
Shibata, A., Matsuda, T., Ajiki, W., and Sobue, T. (2008). Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993-2001. Jpn. J. Clin. Oncol. 38, 464–468.
Siegel, R., Ward, E., Brawley, O., and Jemal, A. (2011). Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 61, 212–236.
Siewert, J. R., and Stein, H. J. (1998). Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 85, 1457–1459.
Sommerer, F., Vieth, M., Markwarth, A., Rohrich, K., Vomschloss, S., May, A., Ell, C., Stolte, M., Hengge, U. R., Wittekind, C., and Tannapfel, A. (2004). Mutations of BRAF and KRAS2 in the development of Barrett’s adenocarcinoma. Oncogene 23, 554–558.
Stahl, M., Stuschke, M., Lehmann, N., Meyer, H. J., Walz, M. K., Seeber, S., Klump, B., Budach, W., Teichmann, R., Schmitt, M., Schmitt, G., Franke, C., and Wilke, H. (2005). Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 23, 2310–2317.
Stahl, M., Walz, M. K., Stuschke, M., Lehmann, N., Meyer, H. J., Riera-Knorrenschild, J., Langer, P., Engenhart-Cabillic, R., Bitzer, M., Konigsrainer, A., Budach, W., and Wilke, H. (2009). Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J. Clin. Oncol. 27, 851–856.
Sunpaweravong, P., Sunpaweravong, S., Sangthawan, D., Pinaikul, S., Attasaranya, S., Dechaphunkul, A., Mitarnun, W., and Fungthammasarn, T. (2011). “Cetuximab plus chemoradiation with cisplatin and 5-fluorouracil (5-FU) in locally advanced unresectable esophageal squamous cell carcinoma,” in ASCO Annual Meeting 2011, Chicago.
Tepper, J., Krasna, M. J., Niedzwiecki, D., Hollis, D., Reed, C. E., Goldberg, R., Kiel, K., Willett, C., Sugarbaker, D., and Mayer, R. (2008). Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092.
Tepper, J. E., and O’Neil, B. (2009). Transition in biology and philosophy in the treatment of gastroesophageal junction adenocarcinoma. J. Clin. Oncol. 27, 836–837.
Thuss-Patience, P. C., R, H., Arnold, D., Florschütz, A., Daum, S., Kretzschmar, A., Mantovani-Löffler, L., Bichev, D., Gahn, B., Schumacher, G., and Kneba, M. (2011). “Perioperative chemotherapy with docetaxel, cisplatin, and capecitabine (DCX) in gastroesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO),” in 2011 ASCO Annual Meeting, Chicago.
Trivers, K. F., Sabatino, S. A., and Stewart, S. L. (2008). Trends in esophageal cancer incidence by histology, United States, 1998–2003. Int. J. Cancer 123, 1422–1428.
Umar, S. B., and Fleischer, D. E. (2008). Esophageal cancer: epidemiology, pathogenesis and prevention. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 517–526.
Urba, S. G., Orringer, M. B., Ianettonni, M., Hayman, J. A., and Satoru, H. (2003). Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer 98, 2177–2183.
Urba, S. G., Orringer, M. B., Turrisi, A., Iannettoni, M., Forastiere, A., and Strawderman, M. (2001). Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J. Clin. Oncol. 19, 305–313.
Urschel, J. D., and Vasan, H. (2003). A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am. J. Surg. 185, 538–543.
van de Schoot, L., Romme, E. A., Van Der Sangen, M. J., Creemers, G. J., Van Lijnschoten, G., Van Driel, O. J., Rutten, H. J., and Nieuwenhuijzen, G. A. (2008). A highly active and tolerable neoadjuvant regimen combining paclitaxel, carboplatin, 5-FU, and radiation therapy in patients with stage II and III esophageal cancer. Ann. Surg. Oncol. 15, 88–95.
van Meerten, E., Muller, K., Tilanus, H. W., Siersema, P. D., Eijkenboom, W. M., Van Dekken, H., Tran, T. C., and Van Der Gaast, A. (2006). Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br. J. Cancer 94, 1389–1394.
Walsh, T. N., Noonan, N., Hollywood, D., Kelly, A., Keeling, N., and Hennessy, T. P. (1996). A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N. Engl. J. Med. 335, 462–467.
Whooley, B. P., Law, S., Murthy, S. C., Alexandrou, A., and Wong, J. (2001). Analysis of reduced death and complication rates after esophageal resection. Ann. Surg. 233, 338–344.
Wouters, M. W., Wijnhoven, B. P., Karim-Kos, H. E., Blaauwgeers, H. G., Stassen, L. P., Steup, W. H., Tilanus, H. W., and Tollenaar, R. A. (2008). High-volume versus low-volume for esophageal resections for cancer: the essential role of case-mix adjustments based on clinical data. Ann. Surg. Oncol. 15, 80–87.
Yoon, H. H., Shi, Q., Sukov, W. R., Wiktor, A. E., Khan, M., Sattler, C. A., Grothey, A., Wu, T., Diasio, R. B., Jenkins, R. B., and Sinicrope, F. (2011). “HER2 expression/amplification: frequency, clinicopathologic features, and prognosis in 713 patients with esophageal adenocarcinoma (EAC),” in ASCO Annual Meeting, Chicago.
Keywords: esophageal, cancer, advanced, treatment, update, review
Citation: Rahma OE, Greten TF and Duffy A (2012) Locally advanced cancer of the esophagus, current treatment strategies, and future directions. Front. Oncol. 2:52. doi: 10.3389/fonc.2012.00052
Received: 01 March 2012; Accepted: 04 May 2012;
Published online: 24 May 2012.
Edited by:
Mauro Risio, Institute for Cancer Research and Treatment, ItalyReviewed by:
Alessio Giuseppe Morganti, Università Cattolica del S. Cuore, ItalyAlberto Righi, Institute for Cancer Research and Treatment, Italy
Copyright: © 2012 Rahma, Greten and Duffy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Austin Duffy, Gastrointestinal Malignancy Section, Medical Oncology Branch, National Cancer Institute, Room 10/12N228, 10 Center Drive, Bethesda, MD 20892, USA. e-mail:ZHVmZnlhQG1haWwubmloLmdvdg==