- Department of Plastic and Hand Surgery, University Hospital of Erlangen, Friedrich-Alexander-University Erlangen-Nuernberg, Erlangen, Germany
Defects of the perineum may result from ablative procedures of different malignancies. The evolution of more radical excisional surgery techniques resulted in an increase in large defects of the perineum. The perineogenital region per se has many different functions for urination, bowel evacuation, sexuality, and reproduction. Up-to-date individual and interdisciplinary surgical treatment concepts are necessary to provide optimum oncological as well as quality of life outcome. Not only the reconstructive method but also the timing of the reconstruction is crucial. In cases of postresectional exposition of e.g., pelvic or femoral vessels or intrapelvic and intra-abdominal organs, simultaneous flap procedure is mandatory. In particular, the reconstructive armamentarium of the plastic surgeon should include not only pedicled flaps but also free microsurgical flaps so that no compromise in terms of the extent of the oncological resection has to be accepted. For intra-abdominally and/or pelvic tumors of the rectum, the anus, or the female reproductive system, which were resected through an abdominally and a sacrally surgical access, simultaneous vertical rectus abdominis myocutaneous (VRAM) flap reconstruction is recommendable. In terms of soft tissue sarcoma of the pelvic/caudal abdomen/proximal thigh region, two-stage reconstructions are possible. This review focuses on the treatment of perineum, genitals, and pelvic floor defects after resection of malignant tumors, giving a distinct overview of the different types of defects faced in this region and describing a number of reconstructive techniques, especially VRAM flap and pedicled flaps like antero-lateral thigh flap or free flaps. Finally, this review outlines some considerations concerning timing of the different operative steps.
Introduction
Defects of the perineum usually result from ablative procedures of different malignancies, such as gynecological (cervix, vagina, endometrial), urological (urinary bladder, prostate), and colorectal (anal and rectal carcinoma) tumors. The evolution of more radical excisional surgery techniques resulted in an increase in large defects of the perineum (1). The perineogenital region per se has many different functions for urination, bowel evacuation, sexuality, and reproduction (2), so extensive resection in this region results often in functional deficits.
Pelvic surgery is characterized by a complex anatomy, involvement of different organs and microbial environment of this region. Plastic-reconstructive measures like simultaneously used skin grafts, pedicled, or free flaps avoid different complications or reduce their incidence, such as chronic wound healing disorders and chronic secretion of intrapelvic or peritoneal wound cavities (3, 4). For locally advanced primary or recurrent rectal cancer invading the urine bladder or prostate, pelvic exenteration is often the only treatment, with is potentially curative (5–7). Radical surgery completely resects all malignant disease, often including the complete or at least large parts of pelvic viscera, vessels, muscles, ligaments, or pelvic bone. In modern concepts of advanced oncological surgery, survival is not the only consideration; quality of life has to be taken into account (8). Up-to-date individual and interdisciplinary surgical treatment concepts are necessary to provide optimum oncological as well as quality of life outcome (3, 9).
This review focuses on the treatment of perineum, genitals, and pelvic floor defects after resection of malignant tumors, giving a distinct overview of the different types of defects faced in this region and describing a number of reconstructive techniques. Finally, this review outlines some considerations concerning timing of the different operative steps.
Literature about pelvic reconstruction in particular is very rare and review articles or larger case series are absent, hence only case reports have been published. There is only scarce literature with sufficient evidence on this topic, apart from some single case reports and a few case series, this will be critically discussed in the following review.
Pelvic Region: Extra- and Intrapelvic Tumors
In the pelvic and the inguinal/proximal femoral region, there are essentially two various tumor entities to discuss. Their anatomical localization and extension require very different treatment concepts. In course of this, both initial multidisciplinary team approach and form of reconstructive measures have been adapted to it (3, 8). On the one hand, there are mainly intra-abdominal and pelvic tumors, which are mostly low rectal carcinomas and deep infiltrating anal carcinomas (10), as well as far advanced gynecological tumors [e.g., vulval cancer (11) or cervix cancer (3)]. On the other hand, there are soft tissue sarcomas of the pelvis, the caudal abdomen, and the proximal femoral region (12).
Despite this, there are many other indications, like congenital defects, infections, trauma, lymphedema, and other uncommon problems, e.g., transsexuality (2) requiring reconstructive surgery in the pelvic region, which are not subject of this review.
Different reconstruction methods are available for the above-mentioned malignancies. An overview of the most common pedicled (Table 1) and free flaps (Table 2) for reconstruction of the perigenital region is shown in Tables 1 and 2.
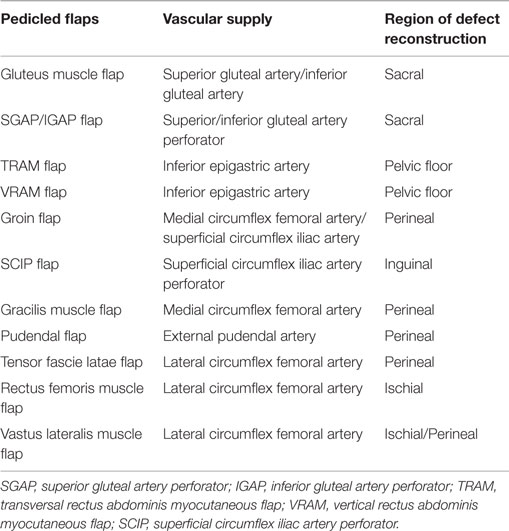
Table 1. The most useful pedicled flaps for defect reconstruction of the pelvic region [modification of Beier et al. (3) and Das Gupta et al. (13)].
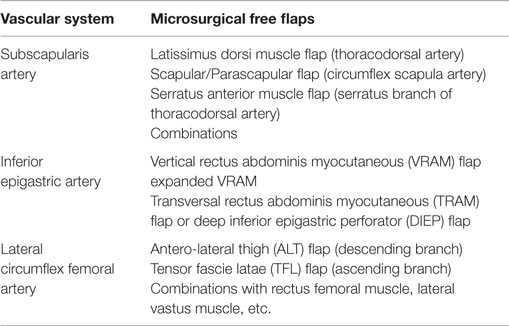
Table 2. The most useful microsurgical free flaps for defect reconstruction of the pelvic region [modification of Beier et al. (3) and Das Gupta et al. (13)].
In most of the cases, primary closure of perineal defects is not possible. Skin grafts are suboptimal in the perigenital area due high bacterial load in this region, frequently leading to graft loss, prolonged healing resulting in unsatisfactory scar quality and contractures that may affect urination or coitus (14). Over the past decades, flap reconstruction has replaced these techniques in the vast majority of cases. An ideal flap should provide soft-tissue volume to close dead space in pelvis and the skin island should replace resected perineal skin (15). These flaps will be described and discussed in detail for the two different groups of malignancies in the pelvic regions in the following paragraph.
Plastic-Reconstructive Measures of Rectal and Anal Carcinomas
Modern treatment of rectal and anal carcinomas includes a multimodal therapy concept. Preoperative neoadjuvant radio-chemotherapy has become standard treatment for rectal cancer and has been shown to downstage tumors (15, 16), before radical aggressive surgery is applied to achieve a lasting cure (8, 17). Despite the concept that multidisciplinary team approach is identically for both entities, the surgical therapy concept itself is very different, associated with the plastic-reconstructive possibilities (3).
For intra-abdominal and/or pelvic tumors of the rectum, the anus or the female reproductive system, which were resected through an abdominal and a perineal surgical access (18), simultaneous flap reconstruction is recommendable. The goal is not only a perineal and/or sacral defect reconstruction but also an intrapelvic sealing, as well as a vaginal partial reconstruction if necessary; both is facilitated by the vertical rectus abdominis myocutaneous (VRAM) flap (Figure 1) (8, 13, 19). To achieve an oncological safe situation, aggressive surgery must be implemented and performing pelvic exenteration with “en bloc” resection of multiple pelvic structures is necessary (8, 9, 18). After abdominoperineal extirpation, often a large intrapelvic cavity remains, perineal wound complications including wound dehiscence and longtime of secretion occur, even according to radiotherapy. Studies have shown that the VRAM flap is a reliable and safe method for pelvic reconstruction in patients with advanced disease requiring pelvic exenteration and radiation, with relatively low rate of donor and recipient site complications (20–22).
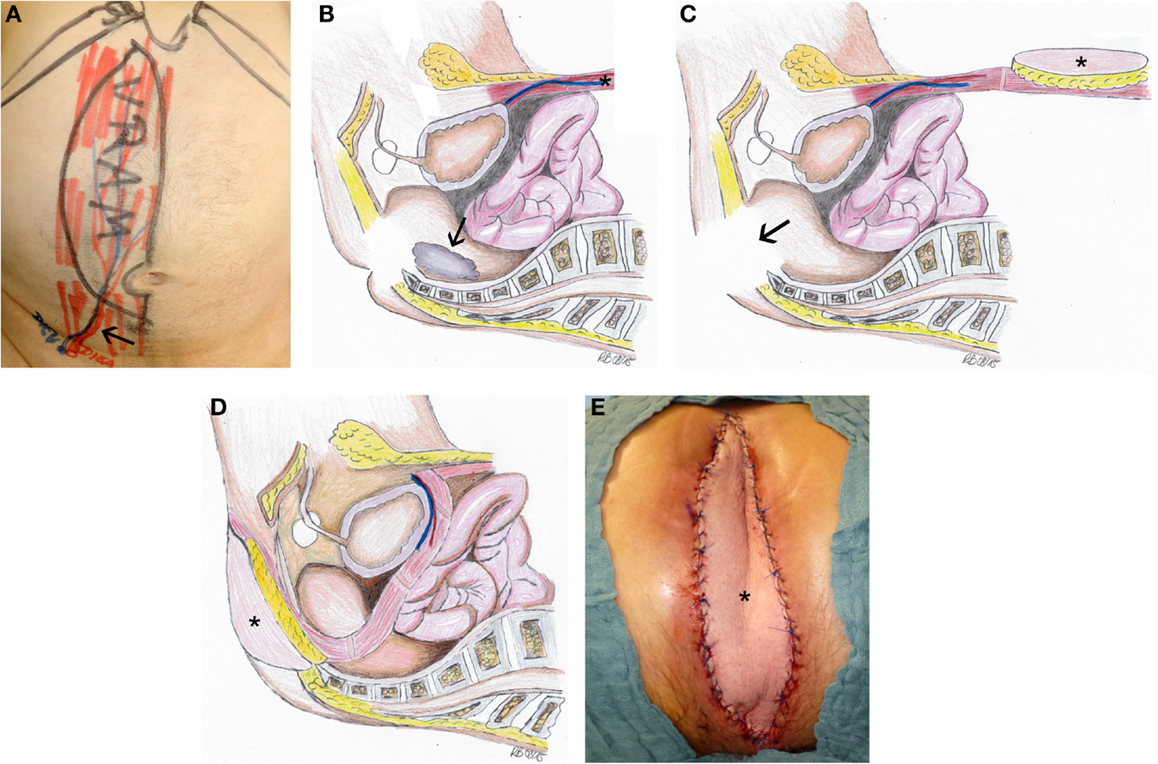
Figure 1. Defect reconstruction after resection of a rectal carcinoma using VRAM flap illustrated by intraoperative photographs and schematic drawings of the surgical technique. (A) Preoperative marking for VRAM-flap procedure with the planned skin paddle and location of the ostomy performed on the day before surgery. Black arrow marks the flap pedicle. (B) The operation involves a two-part procedure with an anterior abdominal dissection first, which is followed by a second step with perineal tumor excision (black arrow) in prone position. We first ensure the viability of the deep inferior epigastric vessels before we proceed with the flap raising. The design of the flap and the size of the skin paddle are then planned according to the prospective perineal and pelvic defect. The skin island is placed vertically over the rectus muscle. The rectus muscle is dissected cranially from the costal arch. In the prone phase, tumor excision (black arrow) had been completed (C). The flap (black asterisk) is then flipped and rotated at 180° into the pelvic cavity so that the skin paddle closes the defect (D). Intraoperative view with VRAM flap (black asterisk) inserted to reconstruct perineal defect (E).
During the last years, important advances in generation of vascularized tissue engineering have been achieved (23). However, until today flap surgery still remains the gold standard for plastic-reconstructive treatment of oncological defects (24). The immediately/simultaneously used transpelvic VRAM flap has several advantages: first of all, the VRAM flap is a very safe and robust flap and relatively easy to technical perform, when necessary plastic-reconstructive expertise exists. Furthermore, the vascular supply of the deep inferior epigastric vessel is constant. Using the VRAM flap as a transpelvic flap not only allows reconstruction of perineal and perigenital skin defects, but also enables obliteration of the sacral cavity (22, 25, 26). Principally, alloplastic and biological matrices have also been used to avoid a herniation of the small bowel, but these techniques are correlated with a significant risk for foreign body reaction, and are prone to infections and formation of chronic fistula, especially if non-absorbable matrices have been used in a radiated field (27, 28). Furthermore, a vascularized muscle flap can reliably fill dead space in the pelvis and can even help to cure local infection (29).
Vertical rectus abdominis myocutaneous flap can also be used for reconstruction of the vagina, when part of the vagina are infiltrated by the tumor and need to be excised. Therefore, the unilateral caudally pedicled VRAM flap can reconstruct half of circumference of the vagina (8). Moreover, vaginal fistula development can be avoided, because of the sealing effect and the well-vascularized tissue over the sutured vaginal stump (30).
A situation after multiple abdominal surgeries represents a major challenge for VRAM flap implementation. On the one hand, the deep inferior epigastric vascular supply may not be available anymore; on the other hand, significant scarring complicates the preparation. Careful preparation and experience of the surgeon permit the implementation of a VRAM flap even in these cases; furthermore, assessment of the deep inferior epigastric vessels through computer angiography is recommended.
Vertical rectus abdominis myocutaneous flaps can also be desepithelialized, which allows to obliterate larger dead space volumes and adjustment of the skin paddle to smaller skin defects, no bulky perineal skin surface and a shorter suture line is achieved. Vascularized dermis at the wound base seems to be associated with a rapidly healing, even in an irradiated field (31, 32).
The most common defects of tumors in this region are caused by rectal and anal carcinomas. In addition, excision of other tumors necessitates a resection of the neighboring skin-/soft tissue, which requires a reconstruction during the same operation. This includes, e.g., extensive gynecological and bladder carcinomas. In case of resection of pelvic bone, VRAM flap can be transferred anteriorly to the symphysis instead of the transpelvic course (33). Two-stage reconstruction has some disadvantages in such cases. Secondary reopening of the abdomen carries a lot of risks, like discrete intestinal loops that are easily being injured during dissection, e.g., interim negative wound pressure therapy is a possible option, but carries the risk for chronic fistulas through the continuous negative pressure (34), hence single-stage reconstruction is strongly recommended under these circumstances.
Summarizing, we suggest that VRAM flap is a particularly suitable method for pelvic reconstruction in patients with advanced colorectal cancer disease requiring pelvic exenteration (20–22).
Plastic-Surgical Measures of Sarcomas of the Pelvic Region
In terms of soft tissue sarcoma of the pelvic/caudal abdomen/proximal thigh region, two-stage reconstruction is possible. Until the final histopathological results, negative wound pressure therapy can be used after tumor resection. Additional resections can be performed until histopathological R0-margins are achieved. Nevertheless simultaneous tumor resection and defect reconstruction can be useful (35).
In general, perineal soft tissue tumors are rare, so optimizing their management and outcome of treatment are still subject to investigation (12). General guidelines have been published, although the histological types are very variable and the locations mostly very complex and various (36, 37). Soft tissue sarcoma requires individual treatments, because optimal local control prevents deaths, related to local progression (38). The soft tissue sarcoma tumor size is often very large, because these tumors grow often without symptoms in the ischioanal fossa (12). In case of chemo- or radiosensitive subtypes, neoadjuvant radio-chemotherapy should be discussed (36).
Treatment of arrosion hemorrhage and exposed osteosynthesis implants are urgent indications of simultaneous reconstructions (39). Another indication for simultaneous defect reconstruction is exposition of vulnerable structure like nerves or vessels, e.g., negative wound pressure therapy (possibly using an additional silicon membrane beneath the sponge for protection of underlying structures) may be applied in between, since harm of nerves by negative pressure therapy has not been reported so far. Nevertheless, the risk especially for infection of expanded defects even after radio-chemotherapy is very high; therefore, simultaneous defect reconstruction is recommended.
However, timing of reconstruction is very challenging. To achieve a long-lasting cure, thorough examination of the patient and his local findings, as well as the radiologic findings, has to be evaluated in a multidisciplinary approach. No compromise in terms of the extent of the oncological resection has to be accepted, and the extent of the defect decides the treatment regime as a simultaneous vs. a two-stage defect reconstruction (3).
As an example for two-stage defect reconstruction and using a pedicled antero-lateral thigh (ALT) flap, Figure 2 shows the case of a patient with dermatofibrosarcoma protuberans at the left groin. Negative wound pressure therapy was applied until the histopathological R0-result was confirmed. For defect reconstruction (Figure 2A), a caudally pedicled ALT flap was used (Figures 2B,C).
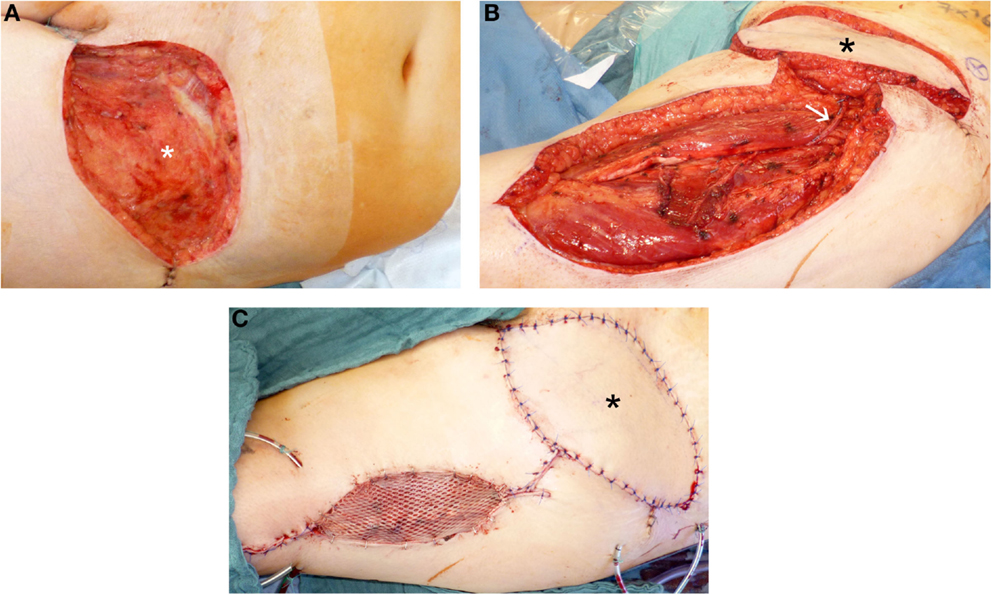
Figure 2. Defect reconstruction at groin after resection of a dermatofibrosarcoma protuberans using caudal pedicled ALT flap. (A) Extent of groin defect after resection (black asterisk) of a dermatofibrosarcoma protuberans. (B) Intraoperative view after dissection of ALT flap and rotation into the groin defect. For typical manner of harvesting ALT flap, ALT perforator is localized between the central to lower third of the ALT flap area after skin incision and initial preparation. White arrow shows the ALT perforator. (C) Intraoperative view at the end of the operation with ALT flap (black asterisk) and skin graft at donor site to reconstruct groin defect.
All in all, in cases of reconstructions after sarcoma resection in the pelvic region, we recommend a two-stage reconstruction where possible, i.e., when no vulnerable structures are exposed after tumor resection. Many factors like size and character of the defect have to receive attention, but often a pedicled flap like ALT flap or VRAM flap could be used (3, 40).
Combined Intra- and Extrapelvic Defects
Combined intrapelvic organ defects (e.g., chronic bladder fistulas) and concurrent abdominal skin defects (e.g., with abdominal skin fistulas and/or unstable scars or skin grafts) are very challenging. In particular, due to side effects of radiotherapy, skin grafts are prone to complications. Most times the defect may initially underestimated and skin grafts are applied, which need to be replaced during the further course by vascularized tissue/flaps.
As an example for such postoperative complication, a vesico-cutaneous fistula of the caudal rectus abdominis muscle after sarcoma resection and radiotherapy may result, which is very difficult to treat (41). For treatment of this rare entity, microsurgical free flap transplantation can become necessary, especially to avoid an additional weakening of the abdominal wall using the contralateral rectus abdominal muscle. A combined bipedicular latissimus dorsi/anterior serrate flap is capable of covering the intrapelvic defect (bladder vault using the part of serratus flap) as well as the abdominal wall defect cranial to the symphysis (using the part of latissimus dorsi flap) (42).
Reconstruction of combined intra- and extrapelvic defects is always very challenging. The literature describes no patent remedy, so we suggest for treatment of this rare entity microsurgical free flap transplantation (42, 43).
Secondary Treatment of Perigenital Defects
Sometimes patients present themselves, secondary or after complications have occured, like recurrent abscending or phlegmonous infections of the pelvis, persistent severe secretion out of chronic sacral cavities or fistulas in prostate/vaginal/urine bladder region. Treatment of such sequelae is technically very challenging and connected to a severe risk profile. A reopening of the abdominal access for VRAM flap is difficult, because of a high risk for injury of adherent small intestine or fibrosis as well as stenosis of the pelvic entry. Another problem is loss of both inferior epigastric vessels. In these cases, other pedicled regional flaps should be used for defect reconstruction (15).
To cover posterior defects, gluteal flaps are useful (1); for ventral defects, pudendal flaps (44) and groin flaps (45) should be mentioned. Similarly, the use of lateral vastus muscle flap (46) and ALT flap (15) for defect reconstruction after pelvic exenteration has been described. Myocutaneous gluteal flaps are mainly used as rotation – or as VY advancement – flaps (47), while the gracilis muscle flap is mainly used as proximally pedicled muscle flap (48). Complications result most from chronic lymphfistulas or lymphedema at femoral or groin region. Gluteal perforator flaps (SGAP and IGAP) do not affect the motor function and minimize the donor-side morbidity (49), thus, offering a technically more advanced solution with relatively low donor site morbidity.
A successful defect reconstruction including in particular a sufficient filling of presacral cavity requires an acceptable intrapelvic access using pedicled gluteal muscle flaps. Sometimes sacral or coccygeal bone has been resected (50).
Secondary sealing itself is very challenging, if chronic sacral cavities or recurrent pelvic infections are being observed. An access to and sufficient filling of presacral dead space is difficult to achieve in a secondary perineal approach. In such cases, sufficient three-dimensional defect reconstruction often is not possible without microsurgical free flaps. These could be anastomosed to local blood vessels, like gluteal vessels (51), femoral vessels, or iliac vessels after an arterio-venous loop (Figure 3).
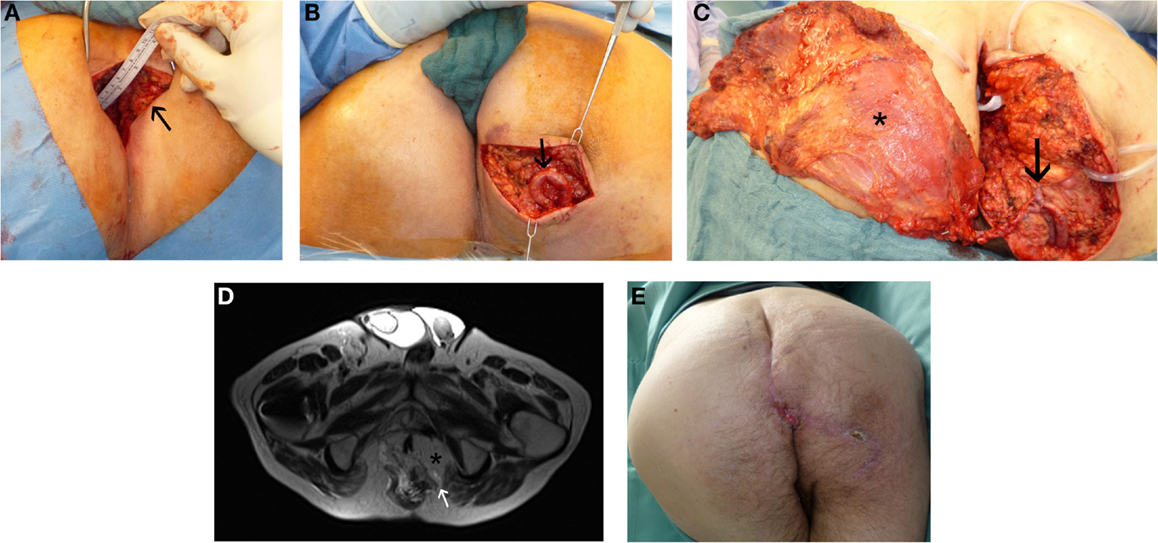
Figure 3. Perineal defect reconstruction after resection of a chronic sacral cavity with fistulas using a microsurgical free “buried” latissimus dorsi flap and an arterio-venous loop. In history, after radio-chemotherapy a rectal carcinoma had been resected. (A) Preoperative presentation of chronic sacral fistula (black arrow). (B) Situs after dissection of the arterio-venous loop (black arrow). (C) Intraoperative view at anastomosed latissimus dorsi flap (black asterisk) at the loop (black arrow). (D) MR-Angiography with microsurgical free “buried” latissimus dorsi flap (black asterisk) inserted to reconstruct perineal defect and imaging of the consistent arterio-venous loop (white arrow). (E) Result 1 month postoperatively.
After radiation, wound healing disorders are more frequent, therefore skin grafts are often not useful for defect reconstruction in such cases. Primary treatment with pedicled or microsurgical free flaps can be appropriate (52).
Conclusion
The evolution of more radical excisional surgery techniques resulted in an increase in large defects of the perineum. In most of the cases, primary closure of perineal defects is not possible. Skin grafts are suboptimal in the perigenital area. For intra-abdominally and/or pelvic tumors of the rectum, the anus or the female reproductive system, which were resected through an abdominally and a sacrally surgical access, simultaneous VRAM flap reconstruction is recommendable. We suggest the VRAM flap as a particularly suitable method for pelvic reconstruction in patients with advanced colorectal cancer disease requiring pelvic exenteration. In terms of soft tissue sarcoma of the pelvic/caudal abdomen/proximal thigh region, two-stage reconstructions are possible. Many factors, like size and character of the defect have to be considered, but often a pedicled flap, like ALT flap could be used in cases of sarcoma resection. Secondary sealing itself is very challenging, if chronic sacral cavities or recurrent pelvic infections are being observed. In such cases, sufficient three-dimensional defect reconstruction often is not possible without microsurgical free flaps (Figure 4).
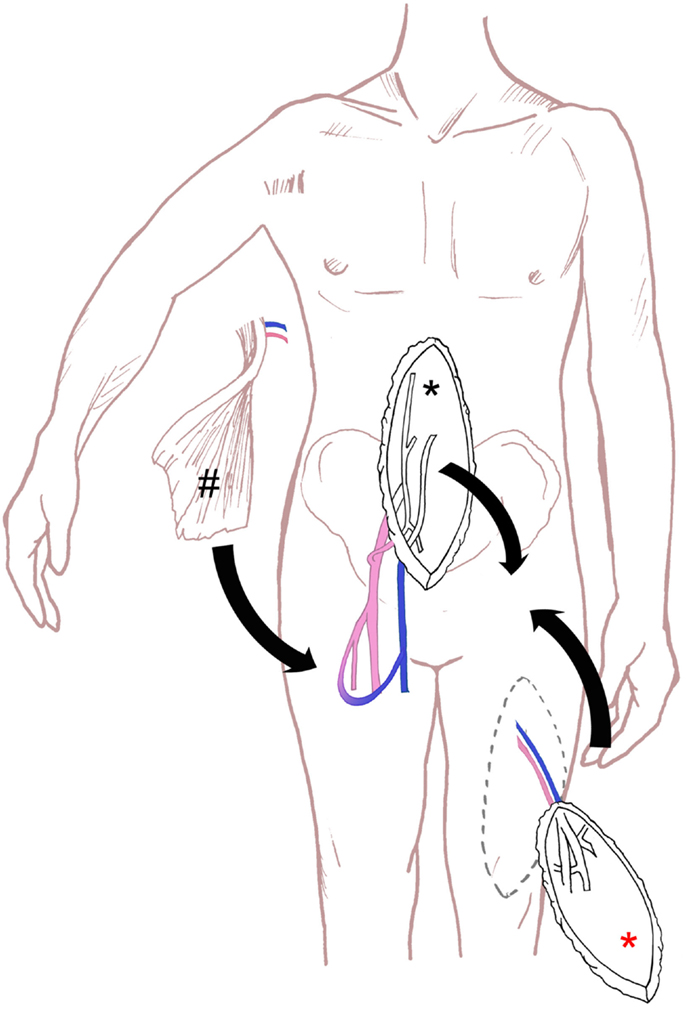
Figure 4. Schematic drawing of the surgical technique for perineal defect reconstruction, with schematic drawing of VRAM flap (black asterisk), ALT flap (red asterisk) and microsurgical free latissimus dorsi flap and an arterio-venous loop (hash).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Winterton RIS, Lambe GF, Ekwobi C, Oudit D, Mowatt D, Murphy JV, et al. Gluteal fold flaps for perineal reconstruction. J Plast Reconstr Aesthet Surg (2013) 66:397–405. doi:10.1016/j.bjps.2012.09.026
2. Kolehmainen M, Suominen S, Tukiainen E. Pelvic, perineal and genital reconstructions. Scand J Surg (2013) 102:25–31. doi:10.1177/145749691310200106
3. Beier JP, Croner RS, Lang W, Arkudas A, Schmitz M, Göhl J. Komplikationsvermeidung in der onkologischen Chirurgie der Becken-/Leistenregion. Der Chirurg (2015) 86:242–50. doi:10.1007/s00104-014-2835-6
4. Chessin B, Hartley J, Cohen AM, Mazumdar M, Cordeiro P, Disa J, et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: a cohort study. Ann Surg Oncol (2005) 12:104–10. doi:10.1245/ASO.2005.03.100
5. Nielsen MB, Rasmussen PC, Lindegaard JC, Laurberg S. A 10-year experience of total pelvic exenteration for primary advanced and locally recurrent rectal cancer based on a prospective database. Colorectal Dis (2012) 14:1076–83. doi:10.1111/j.1463-1318.2011.02893.x
6. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score. Ann Surg (2012) 255:922–8. doi:10.1097/SLA.0b013e31824f1c21
7. Christensen HK, Nerstrøm P, Tei T, Laurberg S. Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum (2011) 54:711–7. doi:10.1007/DCR.0b013e3182163c89
8. Horch RE, Hohenberger W, Eweida A, Kneser U, Weber K, Arkudas A, et al. A hundred patients with vertical rectus abdominis myocutaneous (VRAM) flap for pelvic reconstruction after total pelvic exenteration. Int J Colorectal Dis (2014) 29:813–23. doi:10.1007/s00384-014-1868-0
9. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med (2004) 351:1731–40. doi:10.1056/NEJMoa040694
10. Ferrand F, Malka D, Bourredjem A, Allonier C, Bouché O, Louafi S. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer (2013) 49:90–7. doi:10.1016/j.ejca.2012.07.006
11. Ghurani GB, Penalver MA. An update on vulvar cancer. Am J Obstet Gynecol (2001) 185:294–9. doi:10.1067/mob.2001.117401
12. Sourrouille I, Vilcot L, Honoré C, Coppola S, Terrier P, Le Cesne A. Algorithm for the surgical management of mesenchymal tumors of the perineum in adults. Dis Colon Rectum (2015) 58:304–13. doi:10.1097/DCR.0000000000000272
13. Das Gupta K, Busch K, Kall S, Vogt PM. Plastisch-rekonstruktive Therapie bei postonkologischen Defekten im Bereich der Beckenregion. Der Chirurg (2004) 75:1135–44. doi:10.1007/s00104-004-0964-z
14. Körlof B, Nylén B, Tillinger KG, Tjernberg B. Different methods of reconstruction after vulvectomies for cancer of the vulva. Acta Obstet Gynecol Scand (1975) 54:411–5. doi:10.3109/00016347509157103
15. Wong S, Garvey P, Skibber J, Yu P. Reconstruction of pelvic exenteration defects with anterolateral thigh-vastus lateralis muscle flaps. Plast Reconstr Surg (2009) 124:1177–85. doi:10.1097/PRS.0b013e3181b5a40f
16. Onaitis MW, Noone RB, Hartwig M, Hurwitz H, Morse M, Jowell P, et al. Neoadjuvant chemoradiation for rectal cancer: analysis of clinical outcomes from a 13-year institutional experience. Ann Surg (2001) 233:778–85. doi:10.1097/00000658-200106000-00007
17. Gerard JP, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol (2003) 4:158–66. doi:10.1016/S1470-2045(03)01020-9
18. Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J. Multivisceral resection for colon carcinoma. Dis Colon Rectum (2009) 52:1381–6. doi:10.1007/DCR.0b013e3181ab580b
19. Jain AK, DeFranzo AJ, Marks MW, Loggie BW, Lentz S. Reconstruction of pelvic exenterative wounds with transpelvic rectus abdominis flaps: a case series. Ann Plast Surg (1997) 38:115–22. doi:10.1097/00000637-199702000-00004
20. Horch RE, Gitsch G, Schultze-Seemann W. Bilateral pedicled myocutaneous vertical rectus abdominus muscle flaps to close vesicovaginal and pouch-vaginal fistulas with simultaneous vaginal and perineal reconstruction in irradiated pelvic wounds. Urology (2002) 60:502–7. doi:10.1016/S0090-4295(02)01823-X
21. Horch RE, Ulrich K, Weber K, Hohenberger W, Dragu A. The transpelvic vertical rectus abdominis flap: one interdisciplinary approach to reduce postoperative complications after surgery for rectal cancer. Ann Surg (2013) 257:e16. doi:10.1097/SLA.0b013e31828cbe3a
22. Nelson RA, Butler CE. Surgical outcomes of VRAM versus thigh flaps for immediate reconstruction of pelvic and perineal cancer resection defects. Plast Reconstr Surg (2009) 123:175–83. doi:10.1097/PRS.0b013e3181904df7
23. Horch RE, Beier JP, Kneser U, Arkudas A. Successful human long-term application of in situ bone tissue engineering. J Cell Mol Med (2014) 18:1478–85. doi:10.1111/jcmm.12296
24. Sun D, Yang Y, Wei Z, Xu Y, Zhang X, Hong B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med (2014) 18:434–43. doi:10.1111/jcmm.12157
25. Howell AM, Jarral OA, Faiz O, Ziprin P, Darzi A, Zacharakis E. How should perineal wounds be closed following abdominoperineal resection in patients post radiotherapy – primary closure or flap repair? Best evidence topic (BET). Int J Surg (2013) 11:514–7. doi:10.1016/j.ijsu.2013.05.004
26. Tei TM, Stolzenburg T, Buntzen S, Laurberg S, Kjeldsen H. Use of transpelvic rectus abdominis musculocutaneous flap for anal cancer salvage surgery. Br J Surg (2003) 90:575–80. doi:10.1002/bjs.4073
27. Foster JD, Pathak S, Smart NJ, Branagan G, Longman RJ, Thomas MG, et al. Reconstruction of the perineum following extralevator abdominoperineal excision for carcinoma of the lower rectum: a systematic review. Colorectal Dis (2012) 14:1052–9. doi:10.1111/j.1463-1318.2012.03169.x
28. Jensen KK, Rashid L, Pilsgaard B, Møller P, Wille-Jørgensen P. Pelvic floor reconstruction with a biological mesh after extralevator abdominoperineal excision leads to few perineal hernias and acceptable wound complication rates with minor movement limitations: single-centre experience including clinical examination and interview. Colorectal Dis (2014) 16:192–7. doi:10.1111/codi.12492
29. Anthony JP, Mathes SJ. The recalcitrant perineal wound after rectal extirpation. Applications of muscle flap closure. Arch Surg (1990) 125:1371–6. doi:10.1001/archsurg.1990.01410220155022
30. Huang JJ, Chang NJ, Chou HH, Wu CW, Abdelrahman M, Chen HY. Pedicle perforator flaps for vulvar reconstruction–new generation of less invasive vulvar reconstruction with favorable results. Gynecol Oncol (2015) 137:66–72. doi:10.1016/j.ygyno.2015.01.526
31. de Haas WG, Miller MJ, Temple WJ, Kroll SS, Schusterman MA, Reece GP, et al. Perineal wound closure with the rectus abdominis musculocutaneous flap after tumor ablation. Ann Surg Oncol (1995) 2:400–6. doi:10.1007/BF02306372
32. Campbell CA, Butler CE. Use of adjuvant techniques improves surgical outcomes of complex vertical rectus abdominis myocutaneous flap reconstructions of pelvic cancer defects. Plast Reconstr Surg (2011) 128:447–58. doi:10.1097/PRS.0b013e31821e6fd2
33. Ho K, Warrier S, Solomon MJ, Lee K. A prepelvic tunnel for the rectus abdominis myocutaneous flap in perineal reconstruction. J Plast Reconstr Aesthet Surg (2006) 59:1415–9. doi:10.1016/j.bjps.2006.01.050
34. Zhao JC, Xian CJ, Yu JA, Shi K. Reconstruction of infected and denuded scrotum and penis by combined application of negative pressure wound therapy and split-thickness skin grafting. Int Wound J (2013) 10:407–10. doi:10.1111/j.1742-481X.2012.00997.x
35. Steinau HU, Steinsträsser L, Hauser J, Tilkorn D, Stricker I, Daigeler A. Bösartige Weichgewebsgeschwülste: Resektion und plastische Wiederherstellung. Der Chirurg; Zeitschrift für alle Gebiete der operativen Medizen (2012) 83:673–84. doi:10.1007/s00104-011-2241-2
36. The ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2014) 25(Suppl 3):102–12. doi:10.1093/annonc/mdu254
37. von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU, DeLaney TF, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw (2012) 10:951–60.
38. Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol (2009) 27:31–7. doi:10.1200/JCO.2008.18.0802
39. Darouiche O. Treatment of infections associated with surgical implants. N Engl J Med (2004) 350:1422–9. doi:10.1056/NEJMra035415
40. Gravvanis AI, Tsoutsos DA, Karakitsos D, Panayotou P, Iconomou T, Zografos G, et al. Application of the pedicled anterolateral thigh flap to defects from the pelvis to the knee. Microsurgery (2006) 26:432–8. doi:10.1002/micr.20267
41. Hennessey DB, Bolton E, Thomas AZ, Lynch TH. Vesicocutaneous fistula following adjuvant radiotherapy for prostate cancer. BMJ Case Rep (2013) 2013 doi:10.1136/bcr-2013-008986
42. Ludolph I, Apel H, Horch RE, Beier JP. Treatment of a chronic vesicocutaneous fistula and abdominal wall defect after resection of a soft tissue sarcoma using a bipedicled latissimus dorsi and serratus anterior free flap. Int J Urol (2014) 21:1178–80. doi:10.1111/iju.12545
43. Fujioka M, Hayashida K, Morooka S, Saijo H, Nonaka T. Combined serratus anterior and latissimus dorsi myocutaneous flap for obliteration of an irradiated pelvic exenteration defect and simultaneous site for colostomy revision. World J Surg Oncol (2014) 12:319. doi:10.1186/1477-7819-12-319
44. Wee JT, Joseph VT. A new technique of vaginal reconstruction using neurovascular pudendal-thigh flaps: a preliminary report. Plast Reconstr Surg (1989) 83:701–9. doi:10.1097/00006534-198904000-00018
45. McGregor IA, Jackson IT. The groin flap. Br J Plast Surg (1972) 25:3–16. doi:10.1016/S0007-1226(72)80097-3
46. Waterhouse N, Healy C. Vastus lateralis myocutaneous flap for reconstruction of defects around the groin and pelvis. Br J Surg (1990) 77:1275–7. doi:10.1002/bjs.1800771125
47. Ay A, Aytekin O, Aytekin A. Interdigitating fasciocutaneous gluteal V-Y advancement flaps for reconstruction of sacral defects. Ann Plast Surg (2003) 50:636–8. doi:10.1097/01.SAP.0000054241.94281.D2
48. Becker DW, Massey FM, McCraw JB. Musculocutaneous flaps in reconstructive pelvic surgery. Obstet Gynecol (1979) 54:178–83.
49. Schmidt VJ, Horch RE, Dragu A, Weber K, Göhl J, Mehlhorn G, et al. Perineal and vaginal wall reconstruction using a combined inferior gluteal and pudendal artery perforator flap: a case report. J Plast Reconstr Aesthet Surg (2012) 65:1734–7. doi:10.1016/j.bjps.2012.04.055
50. Galandiuk S, Jorden J, Mahid S, McCafferty MH, Tobin G. The use of tissue flaps as an adjunct to pelvic surgery. Am J Surg (2005) 190:186–90. doi:10.1016/j.amjsurg.2005.05.009
51. Hung SJ, Chen HC, Wei FC. Free flaps for reconstruction of the lower back and sacral area. Microsurgery (2000) 20:72–6. doi:10.1002/(SICI)1098-2752(2000)20:2<72
Keywords: interdisciplinary surgery, exenteration, VRAM flap, perineal reconstruction, microsurgical free flap
Citation: Brodbeck R, Horch RE, Arkudas A and Beier JP (2015) Plastic and reconstructive surgery in the treatment of oncological perineal and genital defects. Front. Oncol. 5:212. doi: 10.3389/fonc.2015.00212
Received: 18 June 2015; Accepted: 17 September 2015;
Published: 08 October 2015
Edited by:
Björn Behr, Ruhr University Bochum, GermanyCopyright: © 2015 Brodbeck, Horch, Arkudas and Beier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justus P. Beier, Department of Plastic and Hand Surgery, University Hospital of Erlangen, Friedrich-Alexander-University Erlangen-Nuernberg, Erlangen, Germany,anVzdHVzLmJlaWVyQHVrLWVybGFuZ2VuLmRl
 Rebekka Brodbeck
Rebekka Brodbeck Raymund E. Horch
Raymund E. Horch Andreas Arkudas
Andreas Arkudas Justus P. Beier
Justus P. Beier