- 1Hematology, Oncology and Palliative Care, Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, United States
- 2Department of Pathology, School of Medicine, Virginia Commonwealth University, Richmond, VA, United States
- 3Fox Chase Cancer Center, Philadelphia, PA, United States
Gastrointestinal stromal tumors (GISTs) originate from interstitial cells of Cajal and account for over 5,000 newly diagnosed cases in the United States. The discovery of activated KIT and PDGFRA mutations and introduction of imatinib revolutionized the treatment strategy and opened up the new era of target therapy for solid tumors. Although surgery remains the primary modality of treatment for curative purpose, almost half of the patients experienced disease recurrence. Tailoring (neo)-adjuvant treatment with imatinib is ongoing to meet the need for an effective therapy. Currently, two drugs (sunitinib and regorafenib) have obtained Food and Drug Administration approval for GISTs after imatinib failure. However, most of the patients eventually progress due to primary or secondary resistance. Deeper understanding of the molecular mechanisms will guide us to develop personalized strategies in the future. Discussion in this review includes current standard management and the most recent advances and multiple ongoing clinical trials with different approaches. This review will provide further steps to be taken to conquer refractory disease.
Introduction
Gastrointestinal stromal tumors (GISTs) are known as the most common mesenchymal tumor of gastrointestinal (GI) system in the United States (1) with the incidence of 10–15 cases per million (2). Median age of diagnosis is around the mid-50s (3). Historically, unless it was completely resected, GIST was a devastating disease due to poor response to chemotherapy and radiotherapy (4). Smooth muscle cells were considered to be the cell type of origin, given the spindle cell morphology. A key finding for GIST classification was clarified due to its similarity to the interstitial cell of Cajal, a stromal cell that serves as the pacemaker for GI tract (5). In 1998, the discovery of activated KIT (CD117) mutation significantly reshaped the biological understanding (6) as well as the subsequently identified mutations in platelet derived growth factor receptor α (PDGFRA) (7). Mutations in both receptors drive downstream intracellular pathways and lead to tumorigenesis. Immunohistochemistry study of DOG1 is particularly helpful in diagnosing GISTs that do not express KIT (8, 9). The imatinib, a tyrosine kinase inhibitor (TKI), was originally evaluated in clinical trials for BCR/ABL positive chronic myelogenous leukemia with great success. The homologous structure between KIT, PDGFRA, and ABL kinases facilitated the introduction of imatinib to the therapy of GISTs in 2000 (10, 11) and the Food and Drug Administration (FDA) granted the approval in 2002. Nowadays, there is no doubt that the treatment for GIST set up a paradigm for use of targeted therapy in solid tumors. Approximately 85–90% of GISTs harboring KIT or PDGFRA mutations benefit from imatinib treatment before or after surgery and in the setting of unresectable/metastatic disease (12, 13), except specific mutation such as PDGFRA exon 18 D842V (14). The remaining 10–15% of GISTs without KIT or PDGFRA mutations are classified as wild-type (WT) GIST. They lack response to imatinib. In this group, several mutations have been identified including succinate dehydrogenase (SDH) complex subunits, neurofibromatosis type 1, BRAF, and other genes (15). These GISTs remain a great therapeutic challenge still. Further trials led to two more drugs approval by the FDA, sunitinib and regorafenib, which provide options after failure of imatinib. This study reviews the role of targeted therapies in non-metastatic and metastatic GIST, as well as future direction and ongoing clinical trials.
Risk Factors of Resectable GISTs
Macroscopic complete surgical resection (R0/R1) remains the major curative approach for GISTs. Any tumor more than 2 cm, symptomatic disease (e.g., bleeding and obstruction) or a tumor that is progressively increase in size should be considered for resection (16). For early stage disease, wedge resection with 1–2 cm margin or segmental resection is usually sufficient because primary GISTs generally displace rather than invade adjacent tissue (17). The risk of lymph node involvement is low, unless the tumor is SDH-deficient. Microscopic negative margins (R0 resection) is the goal of resection, though R1 resection with microscopic positive margins do not usually require re-excision (18). On the basis of American College of Surgeons Oncology Group (ACOSOG) Z9000 and Z9001 clinical trials, there was no difference in recurrence free survival (RFS) for patients undergoing R0 or R1 resection, regardless of adjuvant treatment (19, 20). Only tumor rupture, tumor size, mitotic index, and location are associated with increased risk of recurrence, which have been suggested by National Institutes of Health (NIH) consensus (21) and Miettinen’s classification system (16). This finding may indicate the primary prognostic factor is the inherent biological feature rather than resection status.
In the pre-imatinib era, the estimated 15-year RFS after surgery was 60% for all stages of operable GIST (22). An increased risk for recurrence was associated with an increased mitotic rate [>5/50 high power field (HPF)], large tumor size (>10 cm), and location (small bowel) (23). Further population-based cohort studies added tumor rupture as a new adverse prognostic factor (22). In addition, advances in surgical techniques brought better outcomes and shorter hospital stays. Novitsky et al. (24) and Otani et al. (25) reported the safety and low morbidity of utilizing laparoscopic resection for gastric GISTs (1–8.5 and 2–5 cm, respectively) with 100% negative resection margins compared with open resection. 3 years follow-up showed >90% patients remained disease free.
To date, although more and more gene mutations have been identified in GIST, none of them are incorporated into predictive models. Some evidence suggests that mitotic rate may be less predictive of biologic behavior in SDH-deficient GISTs (26) and depletions affecting codons 557–558 at KIT exon 11 have a greater risk of recurrence than other exon mutations (2, 27).
Neoadjuvant Treatment for Resectable GISTs
To downstage large advanced GISTs or achieve R0/R1 resection of poorly positioned tumors, neoadjuvant treatment, particularly imatinib, is considered before surgery. In 2006, first case report of using imatinib in the neoadjuvant setting obtained complete pathological response of a pelvic GIST patient (28). Subsequently, the Radiation Therapy Oncology Group 0132 trial was the first prospective phase II trial to evaluate the efficacy of imatinib before borderline resectable tumor (29). Thirty patients with primary GIST and twenty-two with recurrent GIST were administered imatinib 600 mg daily for 8–12 weeks preoperatively and extended for 2 years after surgery. Preoperative imatinib was well tolerated and did not affect surgical outcomes. The response rate at the time of surgery using RECIST criteria was 4.5 and 7% in recurrent and primary disease, respectively. Eventually 77% of the patients achieved R0/R1 resection. RFS at 2 years was 82.6%, and 2 years overall survival (OS) was 93% (29). Neoadjuvant treatment provides a chance to avoid morbid procedure. However, it is unclear whether the gain of RFS was attributing to 2 years adjuvant imatinib or not. In this study, the response rate to imatinib is low. Partly it is because of the limited duration of preoperative imatinib. In addition, RECIST criteria for evaluation of treatment response underestimate imatinib-induced cytoreduction (30). Although preoperative imatinib is useful to reduce tumor size, and there is no definitive evidence that it leads to increased OS. Long-term follow-up results from the same study showed 5 years RFS of 56% and 5 years OS of 77%. The prognosis was not correlated with surgical resection status (31). A phase II prospective APPOLLON trial evaluated overall tumor response in 41 patients with locally advanced KIT- and PDGFRA-positive GISTs. Imatinib 400 mg was taken daily for 6 months before the surgery. R0 resection was obtained 30/34 (88.2%) of patients; progression free survival (PFS) at 3 years was 85.2% (32); and no OS data were reported.
Newly published phase II study data from Asia further illustrate the strategy of utilizing neoadjuvant treatment. Specifically for large gastric GISTs (≥10 cm), patients received neoadjuvant imatinib (400 mg/day) for 6–9 months. In 53 patients who received neoadjuvant therapy, 3 patients refused surgery and 4 patients withdrew from the trial. 46 patients completed ≥6 months treatment, with response rate of 62% and R0 resection rate was 91%. 2-year OS was 89%. This study validated that the minimum duration of 6 months neoadjuvant imatinib therapy was required. Preoperative treatment was beneficial for patients who have large tumors (median size in this study was 12 cm) to achieve R0 resection and 94% of the patients avoided total gastrectomy. Most importantly, in this study, genotyping was carried out before neoadjuvant imatinib. One patient did not start neoadjuvant imatinib due to PDGFRA exon 18 D842V mutation. This study demonstrates that KIT exon 11 mutation tumors had high R0 resection rate post neoadjuvant imatinib. In addition, two patients with WT-GIST had successful R0 resection as well (33). This study demonstrated that mutational status testing is important to determine the potential benefit of neoadjuvant imatinib treatment. However, due to it is only a single-arm study and limited follow-up period, further studies are warranted to evaluate the survival benefit of preoperative treatment.
Adjuvant Treatment for Resectable GISTs
Historically, as many as 50% of patients died of recurrent disease despite complete resection of primary tumor with recurrence associated with tumor size (34). Therefore, given the known benefit of imatinib, the question of whether adjuvant imatinib would improve survival postoperatively was tested. The ACOSOG Z9000 Intergroup phase 2 trial was the first prospective study showing that 1 year of adjuvant imatinib did prolong RFS after complete resection in high-risk population compared with historical controls. RFS at 1, 3, and 5 years is 96, 60, and 40%, respectively (19). Subsequently, the ACOSOG Z9001 phase 3 trial clearly demonstrated that an increase RFS among patients with 1 year adjuvant imatinib after resection of 3 cm or larger tumors compared with placebo. Other risk features included mitoses greater than 5 per 50 HPF. The 1 year RFS was 98% with imatinib versus 83% with placebo, a statistically significant difference; and no survival improvement was observed (20, 35).
The next question that was tested was whether 1 year of adjuvant treatment is sufficient. In 2015, European Organization for Research and Treatment of Cancer (EORTC) 62024 trial reported on 2 years of imatinib 400 mg daily or observation only in patients with high- or intermediate-risk GISTs after R0/R1 surgery. Imatinib failure-free survival (IFFS) was a novel surrogate endpoint proposed in this trial, to avoid many years follow-up to determine the OS benefit. IFFS was not statistically different in the imatinib arm compared with the observation arm (87 versus 84%), as well as 5-year OS (100 versus 99%). RFS was significantly higher at 3 years (84 versus 66%) and 5 years (69 versus 63%) in the imatinib arm (36). For the intermediate-risk according to current classification, there was no improvement of IFFS. Half of the patients had non-gastric GISTs and mutational data were not available.
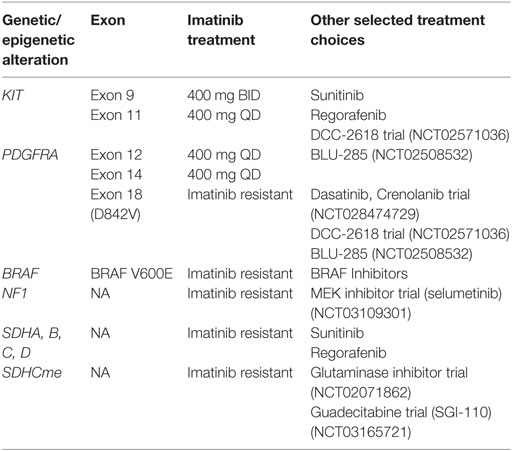
The Scandinavian/German SSG XVIII/AIO trial, a randomized, open-label phase 3 study, compared 1 year versus 3 years of postoperative imatinib with 400 mg daily after R0/R1 resection of high-risk primary or metastatic GIST (37). At a median follow-up of 4.5 years, both RFS and OS favored 3 years of therapy. The 5-year RFS in 3 years and 1 year treatment groups were 65 and 48%, respectively. 5-year survival was significantly longer in patients assigned to 3 years of imatinib (92 versus 82%, P = 0.02). A KIT or PDGFRA mutation was detected in 91% of tumors. However, tumors with KIT exon 9 mutation or WT-GISTs did not have significant clinical benefit (37). The result from EORTC and SSG trials further suggested the adjuvant imatinib treatment should be carefully applied to high-risk patients and genotype should also be taken into consideration. For example, in the advanced/metastatic setting, the PDGFRA exon 18 D842V mutated GISTs have no benefit from imatinib (14) and higher-dose of imatinib (800 mg daily) is recommended by some institutions for KIT exon 9-mutated GIST (38). Biologically, the results may be extrapolated to adjuvant treatment. In addition, in spite of frequent recommendation of 3-year adjuvant imatinib therapy based on SSG trial, both ACOSOG Z9001 and EORTC trials failed to show survival improvement (35, 36). The OS benefit was confirmed by Joensuu et al. (39) in the second planned analysis of the SSGXVIII/AIO trial after a median follow-up of 90 months. The 3-year group demonstrated improved RFS (71 versus 52%, P < 0.001) and survival benefit (92 versus 85%, P = 0.036). The most beneficial mutational subgroup was KIT exon 11 (39). Most recently, the same group published the final genotypic analysis data. Of the 341 patients from SSG XVIII/AIO trial, the study again found that KIT exon 11 deletion or insertion-deletion mutations involved codons 557 and/or 558 had better RFS following 3 years adjuvant imatinib treatment compared with 1 year, but not there was not a statistical difference in the in exon 11 substitution mutations, exon 9 mutations, PDGFRA mutation (including D842V mutation), or WT-GISTs; there were too few cases with other mutations to make conclusions (40). This pivotal study further elucidated 3-year adjuvant imatinib treatment helped the high recurrent risk group (> 10 mitoses/50 HPFs and KIT exon 11 mutation) the most (40). However, prolongation of treatment for more than 3 years is controversial and there is currently a lack of evidence. The NCCN guidelines currently recommend at least 3 years treatment. Two ongoing randomized trials (NCT02413736 and NCT02260505) are currently comparing 3 versus 5 or 6 years of adjuvant imatinib treatment would provide further benefits.
For low risk GISTs management, observation is still the standard of care after R0/R1 resection. If a patient underwent neoadjuvant imatinib treatment, it is recommended to continue it after surgery to accomplish cumulative 3-year course. The rationale for continuation of imatinib is because the mitotic count or biological markers from the postoperative samples is no longer reliable for assessing recurrence risk accurately. In patients who progress on the standard dose of imatinib (400 mg/day), dose escalation to 800 mg/day may be considered (17).
Treatment for Resectable WT-GISTs
WT-GIST was initially defined as the absence of both KIT and PDGFRA mutations. The unique feature of this subtype of GIST is it has poor response to TKIs, including imatinib (12). Recent studies have identified additional genetic mutations in this particular group that prompt us to revisit so-called WT-GISTs. Based upon the molecular features, a new classification for a subset of these tumors based upon the presence or absence of SDH activity, namely SDH-competent or SDH-deficient subgroups.
Due to the rarity of WT-GISTs, there has been a lack of definitive recommendations for this entity. The limited experience from subgroup analysis of ACOSOG Z9001 (32 WT-GIST patients) (20) and SSG XVIII/AIO trial (19 WT-GIST patients) (37) did not detect any benefit from postoperative imatinib treatment. A recent report from the NIH pediatric and WT-GIST clinic added valuable information to the overall picture (41). The WT-GIST clinic was established in 2008. It included patients who had undergone surgical resection of their tumor. With a median follow-up of 4.1 years, 5- and 10-year EFS in the patients seen at that clinic was 24 and 16%, respectively, showing a more indolent process than KIT/PDGFRA-mutated GISTs. The majority of lesions were located in the stomach (83%). The prognosis was related to mitotic index (>5 mitoses/50 HPF) and metastatic status; R0 resection, SDH mutation, or anatomic location were not prognostic. In the setting of hemorrhage, perforation, pain or obstruction, surgery was still the cornerstone of management (41). Although it provides the most comprehensive cohort study for WT-GISTs, the role of adjuvant TKI treatment was not reported in this study. Follow-up with this cohort regarding the evolution of targeted therapy will further help our understanding of adjuvant treatment in WT-GISTs (41).
Target Therapy for Advanced or Metastatic GISTs
Imatinib
Traditionally, GISTs were believed to be chemotherapy and radiotherapy resistant. Surgical resection was the only effective treatment option available before 2000. Median survival was about 10–20 months for unresectable or metastatic disease (42). In 1998, the breakthrough finding of activated KIT was a crucial diagnostic marker as well as potential therapeutic target for GIST, opening a new era for GIST therapy (6). Only 2 years later, imatinib was tested due to its potent antagonism of KIT in an in vitro cellular model (10). Subsequently a patient with metastatic GIST was treated with imatinib and demonstrated a significant response (11), further affirming targeting aberrant tyrosine kinase signaling can be therapeutic. This favorable outcome from a case report triggered the subsequent clinical trials. Demetri et al. reported total 147 patient cohort study randomized to receive imatinib 400 or 600 mg daily. The overall response rate was 54% and there was good tolerability (43). Long-term results from the same study validated identical efficacy of 400 and 600 mg daily dose. Nearly 50% of the patient with advanced GIST survived for more than 5 years with ORR of 68% and PFS of 24 months (44). The highest feasible dose of imatinib was identified as total 800 mg daily by the EORTC phase I and phase II studies (45, 46). Based on the successful outcome from the early phase clinical trials, two multi-center phase III studies tested two different daily doses, 400 mg daily versus 400 mg twice daily. The EORTC study recruited 946 patients and demonstrated that the 400 mg/day had a similar response rate compared with 800 mg/day. The high-dose imatinib treatment did lead to a significantly longer PFS at the expense of higher treatment interruption (64 versus 40%) or dose reductions (60 versus 16%) (47). The Southwest Oncology Group S0033 trial was conducted in 148 centers across United States and Canada enrolled 746 patients with metastatic or surgically unresectable GIST. Median OS was nearly 5 years (55 versus 51 months) in the 400 mg/daily and 800 mg/daily groups, respectively, and was not statistically different between the arms. Likewise, neither ORR nor PFS revealed any differences. As expected, the high-dose group had more grade 3–5 toxicities (63 versus 43%). Therefore, the study concluded that high-dose does not provide clinical advantage over standard dose (48). The meta-analysis evaluating these two randomized trials concluded the same result (38). Recently, 10-year follow-up results were updated from the EORTC international study. The median PFS was 1.7 and 2.0 years (P = 0.18) in the 400 and 800 mg arms, and median survival was 3.9 years in both arms. Only 10% of patients were progression free at 10 years. With longer follow-up, there is a lack of data to support a difference between the two dose levels (49).
Though mutational analysis was not mandatory to enroll on the protocols, subset of tumors was genotyped. KIT exon 9-mutated GIST was shown to benefit from high-dose imatinib for both PFS and OS, while WT-GIST more favored standard dose (49). Based on these data, imatinib 400 mg/daily is the standard of care; however, higher-dose (800 mg/daily) is a consideration for patients that have progressed on 400 mg/daily or for tumors that harbor KIT exon 9 mutations. In addition, PDGFRA D842V mutated tumors were resistant to therapy (50). With the greater insight of molecular profiles, we have better understanding of the potential for response to imatinib. Thus, the NCCN guideline strongly recommends mutation testing or genotyping for KIT and PDGFRA.
How long imatinib should be given if the disease is controlled was a question explored by the French Sarcoma Group. BFR14, a phase 3 trial, explored whether imatinib can be interrupted beyond 1 year of treatment in patients with advanced or unresectable disease. A considerably higher rate of disease progression was reported in interrupted group (81 versus 31%) without impairment of quality-of-life (51). The same group then tested interruption at 3 years. Similarly, after a median follow-up of 35 months, the 2-year PFS was 80 versus 16% in continuation group and interruption group, respectively (52). Likewise, cognate result was observed in 5 years interruption group (53). These data lead to the conclusion that ongoing imatinib maintenance is crucial for advanced/metastatic GISTs until there is evidence of disease progression or intolerance (51).
When patients progress after treatment with approved TKIs, including sunitinib, there are few treatment options left. A phase 3 RIGHT trial, tried to address this problem by reintroducing imatinib. Forty-one patients were assigned to rechallenge imatinib versus placebo after progression on sunitinib. PFS was 1.8 months with imatinib compared with 0.9 month (P = 0.005) with placebo (54). BFR14 trial also recapitulated the response of rechallenge imatinib if demonstrated progressive disease after discontinuation of imatinib (53). It provides a new strategy by continuous kinase inhibition by rechallenge with imatinib as an effective therapeutic approach if new investigational drugs are not readily available.
Sunitinib
The majority of patients develop resistance to treatment with imatinib, either due to primary or secondary resistance. Approximately 10% of patients with GISTs have primary resistance (progression within the first 6 months of starting imatinib) primarily because of the tumor mutational status (13). Secondary resistance is defined as disease progression after initial response to imatinib, largely due to acquired mutation in KIT or PDGFA. Therefore, there is a need for additional treatments with potent activity against KIT and PDGFRA. Sunitinib is approved worldwide for metastatic GISTs in patients with imatinib resistance or intolerance (55). In the pivotal phase 3 trial, 312 patients were enrolled to receive sunitinib or placebo after failure of imatinib with the dose of 50 mg daily, 4 weeks on and 2 weeks off. In spite of a very low response rate (only 7%), sunitinib demonstrated prolonged PFS (6.3 versus 1.5 months) and a fourfold higher TTP (27 versus 6 weeks), which were the designed primary endpoints. Numerically, OS was also better though it was not significant, as the study was unblinded and all patients on the placebo arm were crossed over to active therapy following the first interim analysis (56). To achieve better efficacy, the dosing of 37.5 mg sunitinib daily without interruption was also tested in an open-label phase 2 trial with similar outcomes. The response rate was about 13% and PFS was 34 weeks (57).
Although sunitinib has a wider spectrum of kinase inhibition, it is overall well tolerated. The most frequent adverse events are fatigue, diarrhea, hand-foot syndrome, or hypothyroidism, which can be managed by dose modification or interruption (56, 57). In a geriatric population, a report has suggested it might have a negative impact on cognitive function (58). Hypertension induced by sunitinib, associated with improved clinical outcomes, had a low incidence and was manageable (59).
Progression free survival and OS were significantly higher in KIT exon 9 mutation and WT-GIST subtypes with sunitinib, as well as KIT exon 11 mutations with secondary KIT exon 13 or 14 mutations (14), while secondary KIT exon 17 and 18 mutations involving the KIT activation loop had poor outcomes with sunitinib (60). 18F-fluorodeoxyglucose positron emission tomography was assessed as a predictive tool to individualize patient with sunitinib therapy in 4 weeks (61). Recently, a large real world study (Study 1036; NCT00094029) in which 1,124 sunitinib-treated patients were evaluated was reported. A significantly better PFS (median was 7.1 months) was observed in KIT exon 9 mutation compared with exon 11 mutation (hazard ratio = 0.59). Longer OS and ORR were reported as well (62). Combined with the existing evidence, sunitinib offered effectiveness as a post-imatinib therapy, regardless of mutational status.
Regorafenib
Regorafenib is another oral multi-targeted TKI with activity on oncogenic pathways (KIT, RET, PDGFR, FGFR, and BRAF) and angiogenesis pathways (VEGF1-3 and TIE2) (4). It has been approved by FDA for GIST patients previously treated with imatinib and sunitinib, as well as colorectal cancer and hepatocellular cancer. In 2011, Wilhem et al. first reported regorafenib suppressed growth of GIST in vitro as well as xenograft mouse model (63). Then a phase II study for treating GIST after failure of imatinib and sunitinib was reported in 2012, demonstrating that regorafenib at a dose of 160 mg daily for 3 weeks in a 4 weeks cycle had clinical benefit rate of 79% with median PFS of 10 months (64). On the basis of these promising data, a phase 3 trial (GRID trial) was performed in 199 patients who had progressed on previous imatinib and sunitinib therapy (65). Disease control rate was dramatically improved in regorafenib arm compared with placebo (52 versus 9%). Median PFS was 4.8 versus 0.9 months favoring regorafenib. No difference was observed in OS due to the crossover design. Grade III or higher toxicity was present in about 20% of patients (65). This evidence lead to FDA accelerated approval of regorafenib as the third-line agent in 2013.
Other New Target Therapies
Despite success of imatinb, sunitinib and regorafenib, eventually most patients develop resistant to these therapies, mainly due to acquired mutations. Several other TKIs have been evaluated in this setting, however, none of them have led to FDA approval to date.
Sorafenib, structurally closely related to regorafenib, targets multiple tyrosine kinases including KIT and PDGFRA. Currently it is approved for metastatic hepatocellular carcinoma, renal cell carcinoma, and differentiated thyroid cancer. A single-arm phase 2 trial had reported with 31 patients with GIST who failed both imatinib and sunitinib. The response rate was 13%. Median PFS and OS were 4.9 and 9.7 months (66).
Nilotinib is a second-generation TKI derived from imatinib. It has potency similar to that of imatinib against KIT and PDGFRA. In vitro activity of nilotinib suggested greater inhibitory effect against BCR-ABL and comparable KIT/PDGFRA effect (67). ENESTnd trial established its role in frontline therapy of newly diagnosed chronic myeloid leukemia with better efficacy than imatinib (68). Therefore, ENESTg1 trial was designed as a randomized phase 3 trial to assess the efficacy and safety of nilotinib versus imatinib as first-line therapy for patients with advanced GISTs (69). From 2009 to 2011, 647 patients were enrolled. The 2 years PFS was higher in the imatinib group than nilotinib group (59 versus 51%), primarily due to poorer disease control by nilotinib in the group with KIT exon 9-mutated GIST. In this regard, this trial was terminated early as the futility boundary was crossed at the interim analysis (69). In the third-line setting, nilotinib also failed to demonstrate significant activity in patients with prior imatinib and sunitinib treatment (70), though the best supportive care (BSC) group was allowed to continue imatinib or sunitinib. For the moment, nilotinib is not recommended for broad use for GIST. Nilotinib has adverse effect for KIT exon 9-mutated GIST and should be avoided. Future studies might identify some patient subsets might of clinical benefit.
Pazopanib, a multi-targeted angiogenesis inhibitor, has shown activity against non-GIST soft-tissue sarcoma in the PLAETTE trial (71) and achieved a favorable quality-of-life profile compared to sunitinib for metastatic renal cell carcinoma (72). A phase 2 PAZOGIST trial was performed to assess the activity of pazopanib in imatinib and sunitinib-resistant GIST or refractory to other therapies (73). The primary endpoint, 4-month PFS was higher in the pazopanib group than the BSC alone group (45 versus 17%). Median PFS was 3.4 months in pazopanib group and 2.3 months in BSC. OS was not significant (HR = 0.94) (73). Nevertheless, regorafenib which is approved for third-line therapy has median PFS of 4.8 months (65). Another phase 2 study of pazopanib conducted in USA did not show such high anti-tumor activity, with median PFS of only 1.9 months, though median lines of treatment was 3 (74). Accordingly, because of the low proportion of patients obtaining a response and limited evidence, pazopanib should be used in selected patients with GIST or those with no clinical trial options following progression with standard therapies. Interestingly, the phase 2 study suggested that the drug may be of benefit for SDH-deficient WT-GIST (74).
Masitinib is another highly selective TKI with inhibitory effect of KIT as well as WT-GIST in a phase 1 study (75). A pilot phase 2 study compared masitinib with imatinib in treatment-naive metastatic GIST patients (76). There was a similar safety and response profile to imatinib, with ORR of 53%, disease control rate of 97% and mPFS was 41.3% (76). Another phase 2 trial further evaluated masitinib in the second-line after failure of imatinib, using sunitinib as a comparative control. The mOS was significantly longer for patients receiving masitinib (HR = 0.27) with a 12.4 months survival advantage. Patients experienced less toxicity from masitinib than those receiving sunitinib (52 versus 91%) (77). This encouraging result is awaiting to be validated from an ongoing phase 3 trial (NCT01694277).
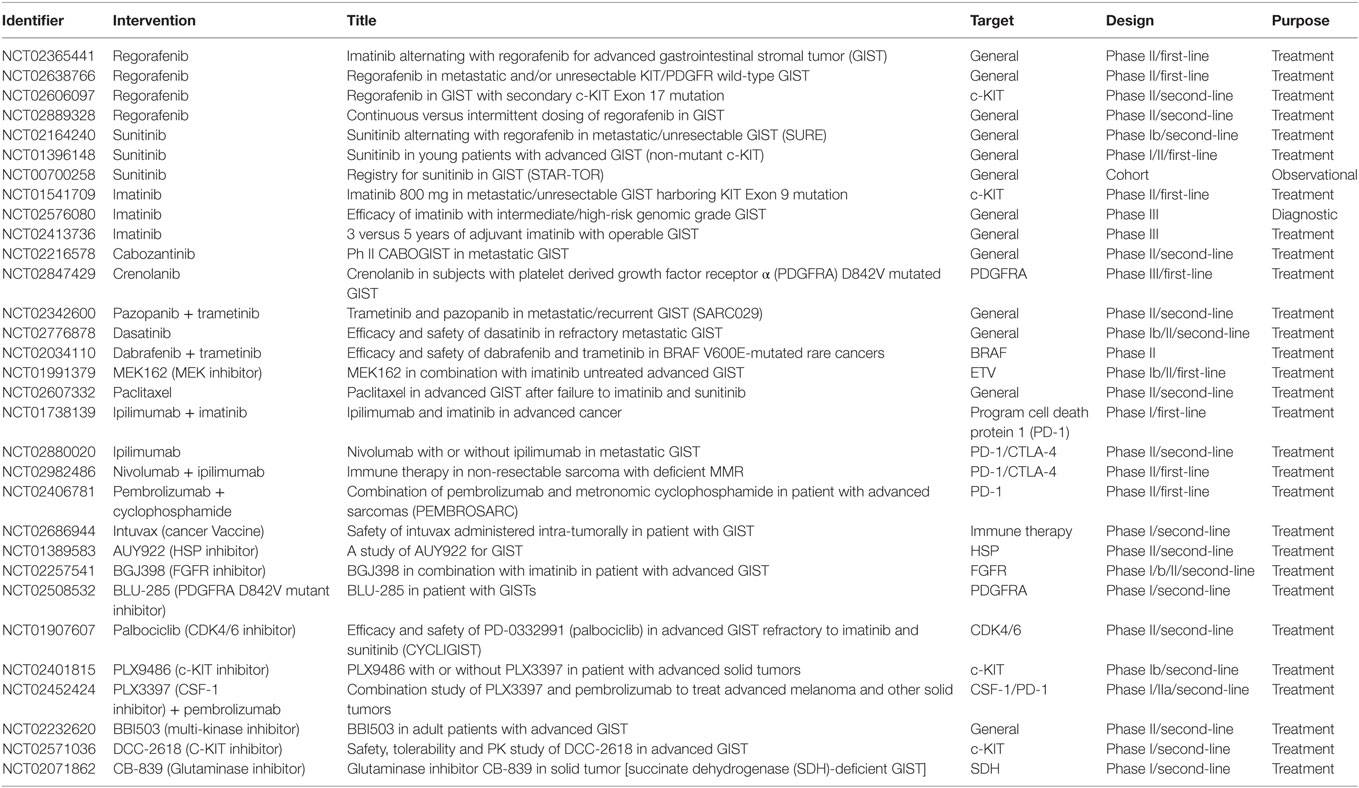
To date, drugs targeting KIT and PDGFRA have revolutionized GIST treatment. However, resistance to existing drugs and disease progression are not uncommon within a few years of treatment. Significant effort has been applied to find alternate agents with other mechanisms of action or combination therapy to circumvent the resistance without adding toxicity. Several studies with novel agents, such as BLU-285, crenolanib in patient harboring highly resistant mutation of PDGFRA D842V (78, 79), dabrafenib in BRAF-mutated GIST (80), are being tested with some early signs of benefit. ETV1 has emerged as a highly specific target in treatment of GISTs. Notably, MEK inhibitor had synergistic effect with imatinib (81). A phase 1 clinical trial (NCT01991379) is ongoing highlighting the rapid translation from bench work to bedside. Immunotherapy, which has revolutionized our concept of anti-tumor treatment in other tumor types, also maybe a new avenue for treating GISTs. Pre-clinical studies indicate that program cell death protein 1 (PD-1) signaling is correlated with clinical outcome and imatinib treatment (82). In a single-arm phase 2 study, Toulmonde et al. investigated the efficacy of pembrolizumab combined with metronomic cyclophosphamide in sarcoma including 10 GIST patients. The 6-month non-progression rate was observed in only 11.1% GIST patients (83). Hereby, due to the disappointing result, further strategies are warranted to assess the combination of anti-PD-1 with other approaches targeting tumor immune microenvironment. Current ongoing clinical trials in clinicaltrial.gov have been summarized in Tables 1 and 2.
Conclusion
The introduction of imatinib established a new paradigm for management of solid tumors in the era of targeted therapy. Although surgical resection remains the mainstay for cure, imatinib has demonstrated an important role in the neo/adjuvant setting. Currently, three drugs are available for advanced or metastatic GISTs, however, no further standard options left if patients fail to respond to regorafenib. The need to conquer drug resistance and develop new targeted agents motivates further basic research and clinical studies. Our recent published article summarized the clinical characteristics and treatment options according to genotype of GIST (84). Intensive research of molecular pathways and new knowledge of the pathophysiology of GIST will help us to guide the personalized treatment and development of new agents.
Author Contributions
LM and WD: drafting of work, analysis and interpretation of trials and literature, drafting of manuscript, and manuscript review. MI and MM: interpretation of trials and literature, drafting of manuscript, and manuscript review. SB: design, analysis and interpretation of trials and literature, drafting of manuscript, and manuscript review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Boikos SA, Stratakis CA. The genetic landscape of gastrointestinal stromal tumor lacking KIT and PDGFRA mutations. Endocrine (2014) 47(2):401–8. doi:10.1007/s12020-014-0346-3
2. von Mehren M. Management of gastrointestinal stromal tumors. Surg Clin North Am (2016) 96(5):1059–75. doi:10.1016/j.suc.2016.05.011
3. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol (2016) 40:39–46. doi:10.1016/j.canep.2015.10.031
4. Jakhetiya A, Garg PK, Prakash G, Sharma J, Pandey R, Pandey D. Targeted therapy of gastrointestinal stromal tumours. World J Gastrointest Surg (2016) 8(5):345–52. doi:10.4240/wjgs.v8.i5.345
5. Chetty R, Serra S. Molecular and morphological correlation in gastrointestinal stromal tumours (GISTs): an update and primer. J Clin Pathol (2016) 69(9):754–60. doi:10.1136/jclinpath-2016-203807
6. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (1998) 279(5350):577–80. doi:10.1126/science.279.5350.577
7. Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology (2003) 125(3):660–7. doi:10.1016/S0016-5085(03)01046-1
8. Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol (2009) 33(3):437–46. doi:10.1097/PAS.0b013e318186b158
9. West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol (2004) 165(1):107–13. doi:10.1016/S0002-9440(10)63279-8
10. Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood (2000) 96(3):925–32.
11. Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med (2001) 344(14):1052–6. doi:10.1056/NEJM200104053441404
12. Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol (2016) 2(7):922–8. doi:10.1001/jamaoncol.2016.0256
13. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer (2011) 11(12):865–78. doi:10.1038/nrc3143
14. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol (2003) 21(23):4342–9. doi:10.1200/JCO.2003.04.190
15. Gopie P, Mei L, Faber AC, Grossman SR, Smith SC, Boikos SA. Classification of gastrointestinal stromal tumor syndromes. Endocr Relat Cancer (2018) 25(2):R49–58. doi:10.1530/ERC-17-0329
16. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol (2006) 23(2):70–83. doi:10.1053/j.semdp.2006.09.001
17. Keung EZ, Raut CP. Management of gastrointestinal stromal tumors. Surg Clin North Am (2017) 97(2):437–52. doi:10.1016/j.suc.2016.12.001
18. Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw (2010) 8(Suppl 2):S1–41; quiz S42–4. doi:10.6004/jnccn.2010.0116
19. DeMatteo RP, Ballman KV, Antonescu CR, Corless C, Kolesnikova V, von Mehren M, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg (2013) 258(3):422–9. doi:10.1097/SLA.0b013e3182a15eb7
20. Corless CL, Ballman KV, Antonescu CR, Kolesnikova V, Maki RG, Pisters PW, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol (2014) 32(15):1563–70. doi:10.1200/JCO.2013.51.2046
21. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol (2002) 33(5):459–65. doi:10.1053/hupa.2002.123545
22. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol (2012) 13(3):265–74. doi:10.1016/S1470-2045(11)70299-6
23. Dematteo RP, Gold JS, Saran L, Gonen M, Liau KH, Maki RG, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer (2008) 112(3):608–15. doi:10.1002/cncr.23199
24. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg (2006) 243(6):738–45; discussion 745–7. doi:10.1097/01.sla.0000219739.11758.27
25. Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery (2006) 139(4):484–92. doi:10.1016/j.surg.2005.08.011
26. Nguyen SQ, Divino CM, Wang JL, Dikman SH. Laparoscopic management of gastrointestinal stromal tumors. Surg Endosc (2006) 20(5):713–6. doi:10.1007/s00464-005-0435-8
27. Martin J, Poveda A, Llombart-Bosch A, Ramos R, Lopez-Guerrero JA, Garcia del Muro J, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol (2005) 23(25):6190–8. doi:10.1200/JCO.2005.19.554
28. Salazar M, Barata A, Andre S, Venancio J, Francisco I, Cravo M, et al. First report of a complete pathological response of a pelvic GIST treated with imatinib as neoadjuvant therapy. Gut (2006) 55(4):585–6. doi:10.1136/gut.2005.086744
29. Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol (2009) 99(1):42–7. doi:10.1002/jso.21160
30. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol (2007) 25(13):1753–9. doi:10.1200/JCO.2006.07.3049
31. Wang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol (2012) 19(4):1074–80. doi:10.1245/s10434-011-2190-5
32. Hohenberger P, Langer C, Wendtner CM, Hohenberger W, Pustowka A, Wardelmann E, et al. Neoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J Clin Oncol (2012) 30(15_suppl):10031. doi:10.1200/jco.2012.30.15_suppl.10031
33. Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer (2017) 117(1):25–32. doi:10.1038/bjc.2017.144
34. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg (2000) 231(1):51–8. doi:10.1097/00000658-200001000-00008
35. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet (2009) 373(9669):1097–104. doi:10.1016/S0140-6736(09)60500-6
36. Casali PG, Le Cesne A, Poveda Velasco A, Kotasek D, Rutkowski P, Hohenberger P, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on sarcomas. J Clin Oncol (2015) 33(36):4276–83. doi:10.1200/JCO.2015.62.4304
37. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA (2012) 307(12):1265–72. doi:10.1001/jama.2012.347
38. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol (2010) 28(7):1247–53. doi:10.1200/JCO.2009.24.2099
39. Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, et al. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol (2016) 34(3):244–50. doi:10.1200/JCO.2015.62.9170
40. Joensuu H, Wardelmann E, Sihto H, Eriksson M, Sundby Hall K, Reichardt A, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol (2017) 3(5):602–9. doi:10.1001/jamaoncol.2016.5751
41. Weldon CB, Madenci AL, Boikos SA, Janeway KA, George S, von Mehren M, et al. Surgical management of wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Pediatric and Wildtype GIST clinic. J Clin Oncol (2016) 35(5):523–8. doi:10.1200/JCO.2016.68.6733
42. Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg (2003) 90(3):332–9. doi:10.1002/bjs.4046
43. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med (2002) 347(7):472–80. doi:10.1056/NEJMoa020461
44. Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol (2008) 26(4):620–5. doi:10.1200/JCO.2007.13.4403
45. Verweij J, van Oosterom A, Blay JY, Judson I, Rodenhuis S, van der Graaf W, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer (2003) 39(14):2006–11.
46. van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet (2001) 358(9291):1421–3. doi:10.1016/S0140-6736(01)06535-7
47. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet (2004) 364(9440):1127–34. doi:10.1016/S0140-6736(04)17098-0
48. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol (2008) 26(4):626–32. doi:10.1200/JCO.2007.13.4452
49. Casali PG, Zalcberg J, Le Cesne A, Reichardt P, Blay JY, Lindner LH, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on imatinib at two dose levels. J Clin Oncol (2017) 35(15):1713–20. doi:10.1200/JCO.2016.71.0228
50. Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol (2005) 23(23):5357–64. doi:10.1200/JCO.2005.14.068
51. Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol (2007) 25(9):1107–13. doi:10.1200/JCO.2006.09.0183
52. Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol (2010) 11(10):942–9. doi:10.1016/S1470-2045(10)70222-9
53. Patrikidou A, Chabaud S, Ray-Coquard I, Bui BN, Adenis A, Rios M, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol (2013) 24(4):1087–93. doi:10.1093/annonc/mds587
54. Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2013) 14(12):1175–82. doi:10.1016/S1470-2045(13)70453-4
55. Serrano C, George S. Recent advances in the treatment of gastrointestinal stromal tumors. Ther Adv Med Oncol (2014) 6(3):115–27. doi:10.1177/1758834014522491
56. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet (2006) 368(9544):1329–38. doi:10.1016/S0140-6736(06)69446-4
57. George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer (2009) 45(11):1959–68. doi:10.1016/j.ejca.2009.02.011
58. Mulder SF, Bertens D, Desar IM, Vissers KC, Mulders PF, Punt CJ, et al. Impairment of cognitive functioning during sunitinib or sorafenib treatment in cancer patients: a cross sectional study. BMC Cancer (2014) 14:219. doi:10.1186/1471-2407-14-219
59. George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol (2012) 23(12):3180–7. doi:10.1093/annonc/mds179
60. Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol (2008) 26(33):5352–9. doi:10.1200/JCO.2007.15.7461
61. Prior JO, Montemurro M, Orcurto MV, Michielin O, Luthi F, Benhattar J, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol (2009) 27(3):439–45. doi:10.1200/JCO.2008.17.2742
62. Reichardt P, Demetri GD, Gelderblom H, Rutkowski P, Im SA, Gupta S, et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer (2016) 16:22. doi:10.1186/s12885-016-2051-5
63. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer (2011) 129(1):245–55. doi:10.1002/ijc.25864
64. George S, Wang Q, Heinrich MC, Corless CL, Zhu M, Butrynski JE, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol (2012) 30(19):2401–7. doi:10.1200/JCO.2011.39.9394
65. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (2013) 381(9863):295–302. doi:10.1016/S0140-6736(12)61857-1
66. Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs (2012) 30(6):2377–83. doi:10.1007/s10637-012-9795-9
67. Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell (2005) 7(2):129–41. doi:10.1016/j.ccr.2005.03.026
68. Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol (2011) 12(9):841–51. doi:10.1016/S1470-2045(11)70201-7
69. Blay JY, Shen L, Kang YK, Rutkowski P, Qin S, Nosov D, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol (2015) 16(5):550–60. doi:10.1016/S1470-2045(15)70105-1
70. Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol (2012) 23(7):1680–7. doi:10.1093/annonc/mdr598
71. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2012) 379(9829):1879–86. doi:10.1016/S0140-6736(12)60651-5
72. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med (2013) 369(8):722–31. doi:10.1056/NEJMoa1303989
73. Mir O, Cropet C, Toulmonde M, Cesne AL, Molimard M, Bompas E, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol (2016) 17(5):632–41. doi:10.1016/S1470-2045(16)00075-9
74. Ganjoo KN, Villalobos VM, Kamaya A, Fisher GA, Butrynski JE, Morgan JA, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol (2014) 25(1):236–40. doi:10.1093/annonc/mdt484
75. Soria JC, Massard C, Magne N, Bader T, Mansfield CD, Blay JY, et al. Phase 1 dose-escalation study of oral tyrosine kinase inhibitor masitinib in advanced and/or metastatic solid cancers. Eur J Cancer (2009) 45(13):2333–41. doi:10.1016/j.ejca.2009.05.010
76. Le Cesne A, Blay JY, Bui BN, Bouche O, Adenis A, Domont J, et al. Phase II study of oral masitinib mesilate in imatinib-naive patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Eur J Cancer (2010) 46(8):1344–51. doi:10.1016/j.ejca.2010.02.014
77. Adenis A, Blay JY, Bui-Nguyen B, Bouche O, Bertucci F, Isambert N, et al. Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: a randomized controlled open-label trial. Ann Oncol (2014) 25(9):1762–9. doi:10.1093/annonc/mdu237
78. Hayashi Y, Bardsley MR, Toyomasu Y, Milosavljevic S, Gajdos GB, Choi KM, et al. Platelet-derived growth factor receptor-alpha regulates proliferation of gastrointestinal stromal tumor cells with mutations in KIT by stabilizing ETV1. Gastroenterology (2015) 149(2):420–32.e16. doi:10.1053/j.gastro.2015.04.006
79. Heinrich MC, Griffith D, McKinley A, Patterson J, Presnell A, Ramachandran A, et al. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res (2012) 18(16):4375–84. doi:10.1158/1078-0432.CCR-12-0625
80. Falchook GS, Trent JC, Heinrich MC, Beadling C, Patterson J, Bastida CC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget (2013) 4(2):310–5. doi:10.18632/oncotarget.864
81. Ran L, Sirota I, Cao Z, Murphy D, Chen Y, Shukla S, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov (2015) 5(3):304–15. doi:10.1158/2159-8290.CD-14-0985
82. Tan Y, Trent JC, Wilky BA, Kerr DA, Rosenberg AE. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther (2017) 24(3):130–3. doi:10.1038/cgt.2016.58
83. Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol (2018) 4(1):93–7. doi:10.1001/jamaoncol.2017.1617
Keywords: GIST, imatinib, KIT, PDGFRA, SDH, NF1, SDHCme
Citation: Mei L, Du W, Idowu M, von Mehren M and Boikos SA (2018) Advances and Challenges on Management of Gastrointestinal Stromal Tumors. Front. Oncol. 8:135. doi: 10.3389/fonc.2018.00135
Received: 29 December 2017; Accepted: 13 April 2018;
Published: 07 May 2018
Edited by:
Motohiro Kojima, National Cancer Center Hospital East, JapanReviewed by:
Savio George Barreto, Medanta The Medicity, IndiaMirko Omejc, University of Ljubljana, Slovenia
Johan Nicolay Wiig, Oslo University Hospital, Norway
Copyright: © 2018 Mei, Du, Idowu, von Mehren and Boikos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sosipatros A. Boikos, c2Fib2lrb3NAdmN1LmVkdQ==
 Lin Mei1
Lin Mei1 Margaret von Mehren
Margaret von Mehren Sosipatros A. Boikos
Sosipatros A. Boikos