Abstract
Hypoxia-inducible factor-2α (HIF-2α) plays an important role in tumor progression and metastasis. A number of studies have evaluated the correlation between HIF-2α overexpression and clinical outcome in cancer patients but yielded inconsistent results. To comprehensively and quantitatively summarize the evidence on the capability of HIF-2α to predict the prognosis of cancer patients with solid tumors, a meta-analysis was carried out. Renal cell carcinoma (CC-RCC) was separately analyzed due to an alternative mechanism of regulation. Systematic literature searches were performed in PubMed and Embase databases for relevant original articles until February 2018. Forty-nine studies with 6,052 patients were included in this study. The pooled hazard ratios (HRs) with corresponding confidence intervals were calculated to assess the prognostic value of HIF-2α protein expression in tumor cells. The meta-analysis revealed strong significant negative associations between HIF-2α expression and five endpoints: overall survival [HR = 1.69, 95% confidence interval (95% CI) 1.39–2.06], disease-free survival (HR = 1.87, 95% CI 1.2–2.92), disease-specific survival (HR = 1.57, 95% CI 1.06–2.34), metastasis-free survival (HR = 2.67, 95% CI 1.32–5.38), and progression-free survival (HR = 2.18, 95% CI 1.25–3.78). Subgroup analyses revealed similar associations in the majority of tumor sites. Overall, these data demonstrate a negative prognostic role of HIF-2α in patients suffering from different types of solid tumors.
Introduction
Hypoxia is a common feature of most of solid tumors resulting from an imbalance between oxygen supply and consumption by tumor cells. Hypoxic tumor areas are characterized by a disrupted vasculature causing inefficient oxygen and nutrient supply to neighboring cells (1). Hypoxia is one of the key factors in inducing the development of resistant cells with an aggressive phenotype (2), which leads to poor prognosis in patients and decreases the efficacy of chemoradiotherapy (3, 4). Accurate measurement of tumor hypoxia in patients together with the design of novel anti-hypoxia treatments has largely been a major goal in cancer research (5–8).
Hypoxia triggers important cellular stress responses allowing tumor cells to survive under extreme conditions, including the stabilization of the hypoxia-inducible factor (HIF) proteins (9, 10). Under normoxic conditions, prolyl-hydroxylation promotes HIF-α degradation via the von Hippel–Lindau (VHL) ubiquitin/proteasome pathway. Under hypoxia, this regulation is suppressed, leading to the stabilization of three independent HIF-α subunits (HIF-1α, HIF-2α, and HIF-3α) that dimerize with the constitutively expressed HIF-1β and activate the transcription of genes via hypoxia responsive elements in their promoter region. The HIF-2α protein, also named endothelial PAS domain protein-1, is equally oxygen regulated as its counterpart HIF-1α, and both present over 50% similarity in their amino acid sequence identity (11). In physiologic conditions, HIF-1α has a broad activity in several tissues which contain hypoxic regions, while HIF-2α is more restricted to specific cell types, e.g., kidney, lung, and heart (12). HIF proteins distinctly contribute to the upregulation of genes involved in proliferation, glucose metabolism, and angiogenesis and genes involved in invasion and metastasis in different types of cancer (13). HIF isoforms also differ in their ability to promote treatment resistance in cancer by playing highly divergent or even opposite roles, leading to distinct clinicopathologic features and prognosis (14). Specific activity of HIF-2α differently contributes to total HIF target gene expression among many types of cancers, which may influence the characteristics of these tumors and the outcome of patients (15). To date, much effort has been made to better understand the roles of HIF-2α in cancer and the consequences for patients suffering from high-HIF-2α expressing tumors. So far, there is clear evidence suggesting that HIF-2α is a crucial protein for the development and progression of many types of cancer. Indeed, HIF-2α seems to be crucial in regulating multiple aspects in cancer, including cell proliferation, apoptosis, epithelial-to-mesenchymal transition, cell metabolism, angiogenesis, and resistance to therapy (16, 17). Hypoxia-induced HIF-2α expression and its subsequent chain of events make this protein a relevant marker of tumor hypoxia and a promising target for anticancer therapies with novel inhibitors.
Several clinical studies describe the prognostic value of HIF-1α in cancer, including the elaboration of many meta-analyses for several kinds of cancer and different HIF-1α polymorphisms (18–20). For some of the clinical studies included in these meta-analyses, prognostic data on HIF-2α can be found which often differed from HIF-1α data (21). In addition, other studies specifically looked at the role of HIF-2α independently of HIF-1α expression. Nonetheless, many discrepancies are seen among the investigations that were performed during the last years, in which HIF-2α has been reported to be either a positive or negative prognostic factor in cancer. The purpose of this meta-analysis was to provide an updated comprehensive analysis regarding the prognostic value of HIF-2α expression in solid tumors.
Materials and Methods
Literature Search
The research question of this meta-analysis was defined as follows: “what is the prognostic value of tumoral HIF-2α expression in patients with solid tumors?” Meta-analysis included patients with solid tumors of different types (Table 1) independent of stage and/or grade or treatment modality. Various treatment outcomes (see below) were compared between patients with high and low tumoral HIF-2α expression. PubMed and Embase were used to identify studies that investigated the prognostic significance of HIF-2α in solid tumors to be included in this meta-analysis. The search for literature was performed including papers published until February 1, 2018. Three main key words were identified to address the research question of this meta-analysis, i.e., prognosis, cancer, and HIF2. Several combinations of the selected keywords (in any of the formulations or truncations) were tested as free text searches to identify potential articles to be included in this meta-analysis (Supplementary File S1 in Supplementary Material). A total of 636 papers were identified from both databases (Figure 1).
Table 1
| Organ site | Overall survival | Disease-free survival | Disease-specific survival | Locoregional control | Metastasis-free survival | Progression-free survival |
|---|---|---|---|---|---|---|
| Bladder | 0.51 (0.33–0.77)a | |||||
| Brain | 3.78 (1.64–8.75)a | |||||
| Breast | 1.18 (0.95–1.47) | 2.3 (1.3–4.1)a | 1.6 (1–2.4)a | |||
| Cartilage | 4.13 (1.47–11.58)a | |||||
| Cervix | 1.53 (1.14–2.03)a | |||||
| Colorectal | 1.46 (0.7–3.04) | 1.86 (0.89–3.92)a | 0.8 (0.61–1.05)a | 0.54 (0.25–1.17)a | ||
| Endometrium | 5.72 (1.51–21.6)a | |||||
| Head and neck | 1.55 (1.24–1.92) | 1.41 (0.99–2) | 1.45 (0.8–2.64) | 1.94 (1.43–2.63) | ||
| Kidney | 0.61 (0.27–1.35) | 1.21 (0.57–2.6) | 0.54 (0.11–2.57) | 0.08 (0.03–0.27)a | 0.75 (0.32–1.73) | |
| Liver | 1.06 (0.43–2.61) | 1.52 (0.82–2.84)a | 1.22 (0.87–1.83)a | |||
| Lung | 2.15 (1.65–2.81) | 8.47 (3.26–22.06)a | ||||
| Ovarium | 2.72 (1.27–5.83)a | |||||
| Pancreas | 2.11 (1.38–3.24) | 1.67 (1.26–2.21)a | ||||
| Prostate | 2.44 (1.07–5.57)a | 2.94 (1.99–4.36)a | ||||
| Salivary glands | 4.52 (1.06–19.3)a | 3.64 (0.72–18.42)a | 3.36 (0.89–12.66)a | |||
| Skin | 3.21 (1.15–8.97)a | 3.81 (1.66–8.78)a | ||||
| Soft tissues | 1.53 (1.03–2.28)a | |||||
| Stomach | 1.7 (1.25–2.32) | 1.6 (1–2.4)a |
Summary of results of subgroup meta-analyses of different organ sites.
Figure 1
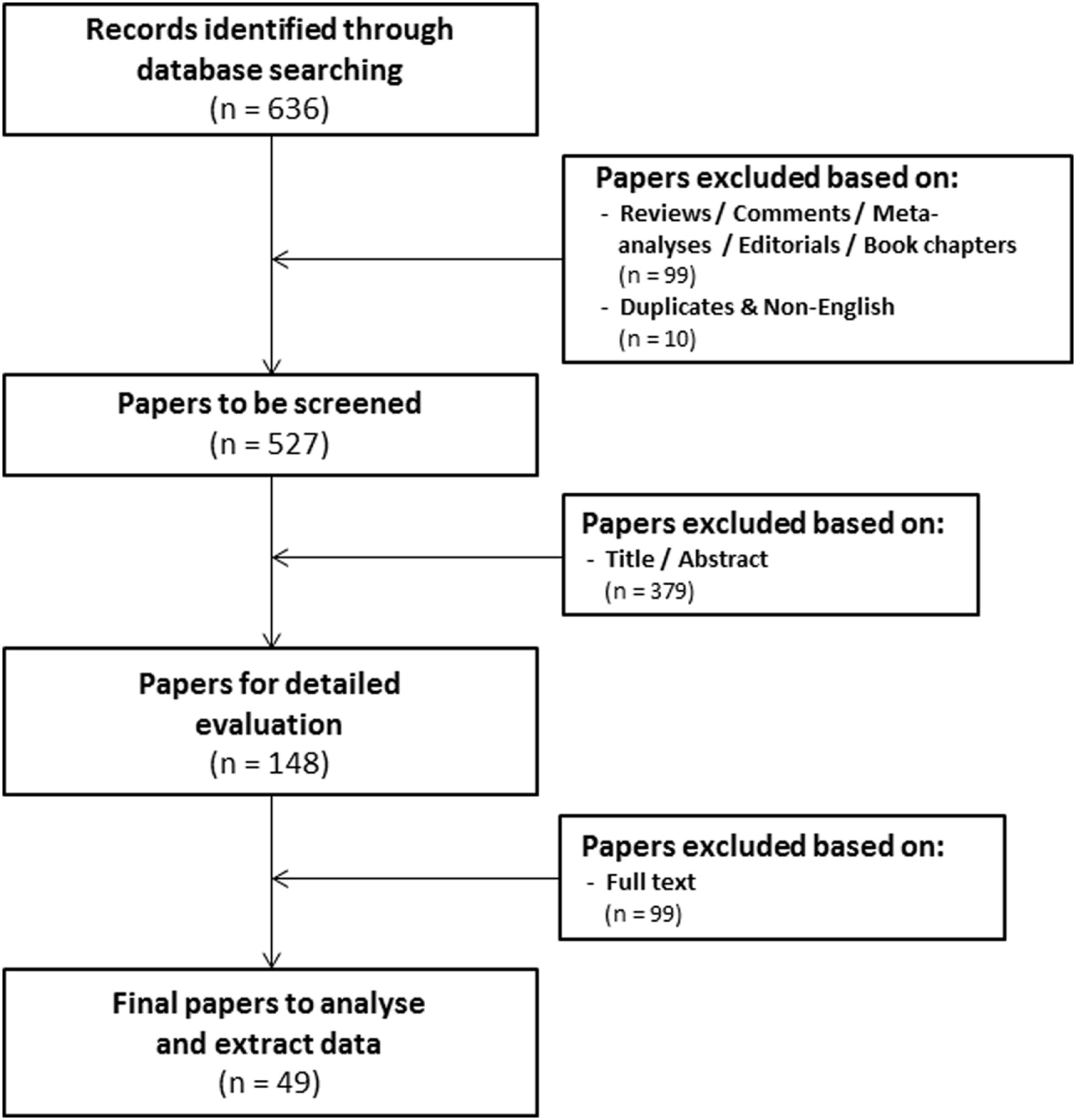
Flowchart of selecting articles describing the association between tumoral hypoxia-inducible factor-2α expression and prognosis.
Screening of Papers
Three independent reviewers assessed the eligibility of the studies. The first round of screening was based on the title and abstract (Eloy Moreno Roig and Arjan J. Groot). 99 review articles without original data, conference records, commentaries, meta-analyses, editorials, or book chapters were excluded, as were 10 overlapping or non-English papers. The total number of papers for further screening was thereby reduced to 527 (Figure 1). The second round consisted of a detailed evaluation of the full-text (Eloy Moreno Roig and Ala Yaromina).
In order to be included in the meta-analysis, a study had to fulfill predetermined inclusion/exclusion criteria: (1) only solid primary tumors of various types were included, (2) all endpoints were included with the minimal median follow-up of 1 year, (3) all treatments were included, (4) none of the patient populations were excluded by distinctive diagnosis, tumor grade, or tumor stage, and (5) pre-treatment protein expression by immunohistochemistry was the only technique for HIF-2α detection included. Discrepancies between the included papers by both reviewers were discussed and consensus was reached on all. A total of 49 papers were included in the meta-analysis (Figure 1) (21–69).
Data Extraction
Reported parameters were extracted from each paper, i.e., the number and origin of patients, treatment modalities, tumor organ, tumor stage, tumor type, group dichotomization, antibody supplier, expression pattern, cellular localization, positivity in macrophages, and outcome variables (see below). The univariate hazard ratio (HR) and 95% confidence interval (95% CI) were directly obtained from the information available in the text. If not reported, the method from Tierney et al. was used to calculate HR and thereby assess prognostic value of HIF-2α expression (70). Multivariate HR was only considered when the univariate HR could not be estimated. Authors were contacted to obtain additional data when not all the information was reported for estimating HR.
Kidney cancer was excluded from the main analysis because the loss of the VHL gene, a common mutation in CC-RCC, results in the stabilization of HIFs independent of oxygen, i.e., an alternative mechanism of HIF-2α activation in cancer absent in the majority of other solid tumors (71). Therefore, prognostic value of HIF-2α in this tumor type has been analyzed separately.
Quality Assessment
The methodological quality of the included papers was evaluated with an adjusted version of the Newcastle–Ottawa scale (NOS) to better suit the study design of the included papers (Supplementary File S2 in Supplementary Material) (72). The NOS was proposed in the 2011 version of the Cochrane Collaboration handbook being an easy method to evaluate the methodological quality of cohort studies (available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
Statistical Analysis and Sensitivity Analysis
Distribution and frequencies of the extracted data parameters were analyzed using SPSS (version 22). Meta-analysis was performed using R statistical software with the Metafor Library (version 2.0-0) (73). Fixed-effect modeling was performed when no statistical significant heterogeneity between studies was observed. When the heterogeneity between studies was statistically significant (p < 0.05), random-effects modeling was applied based on the DerSimonian and Laird method (74). The inverse variance of each study was used to assign an independent weight value. Six different endpoints were considered for the analysis: overall survival (OS), disease-free survival (DFS), locoregional control (LC), disease-specific survival (DSS), metastasis-free survival (MFS), and progression-free survival (PFS). Sensitivity analysis was performed by analyzing subgroups of studies based on organ site. We assessed the possibility of publication bias and heterogeneity among studies by generating and visually analyzing funnel plots. Asymmetric funnel plots and studies outside the pyramid suggest heterogeneity between them.
Results
This meta-analysis includes a total number of 6,052 patients across 49 independent studies (21–69). The median follow-up time ranged between 27 and 391 months and mostly included only a small number of patients (median 90, range 21–695). Selected papers were published between 2001 and 2017 of which 56% were published after 2010. Depending on the study, patients followed different treatment schedules. In most of the selected studies, patients were treated with surgery alone (56%), in combination with either chemotherapy (8%) or standard radiotherapy (10%), or a combination of all three modalities (14%). Other treatment alternatives such as hormonal therapy and tyrosine-kinase inhibitors were used in 8% of the studies. Overall, the majority of the patients (61%) were treated with surgery alone. Most studies report on head and neck and kidney cancer patients (both 18%) followed by colon, liver, pancreas, and lung cancer patients (all of them 8%). By contrast, cancers of the bladder, cartilage, cervix, endometrium, ovarian, and salivary glands were only described once.
Immunohistochemical staining of HIF-2α was most commonly performed using the EP190b Ab (38%) from Novus Biologicals. Other studies used anti-HIF2α antibodies obtained from other suppliers. Cytoplasmic expression of HIF-2α was described in 14% of the studies and 18% were positive in the nucleus. A combination of both positive cytoplasmic and nuclear staining was reported in 46% of the studies. HIF-2α expression was quantified using different methods and patient stratification into groups with low and high tumoral HIF-2α expression was performed using different thresholds. Taken together, 45% of the total tumors were classified as expressing high levels of HIF-2α. Also, 26% of these studies stated positive staining in macrophages.
Overall, patients suffering from tumors with high HIF-2α expression had a worse treatment outcome (Figure 2). This association was significant for OS (p < 0.0001), DFS (p = 0.0057), DSS (p = 0.0249), MFS (p = 0.0061), and PFS (p = 0.0058). No association was found between HIF-2α expression and LC (p = 0.1281). Subgroup analyses based on tumor type and treatment option were not performed due to the low number of studies. Studies on renal cell cancer were eliminated from the overall HR estimation as HIF-2α plays a different role in this cancer type (71). Funnel plots demonstrated systematic heterogeneity for almost all the endpoints. This can be due to publication bias, variation across the reports, or small number of studies (Figure S1 in Supplementary Material).
Figure 2
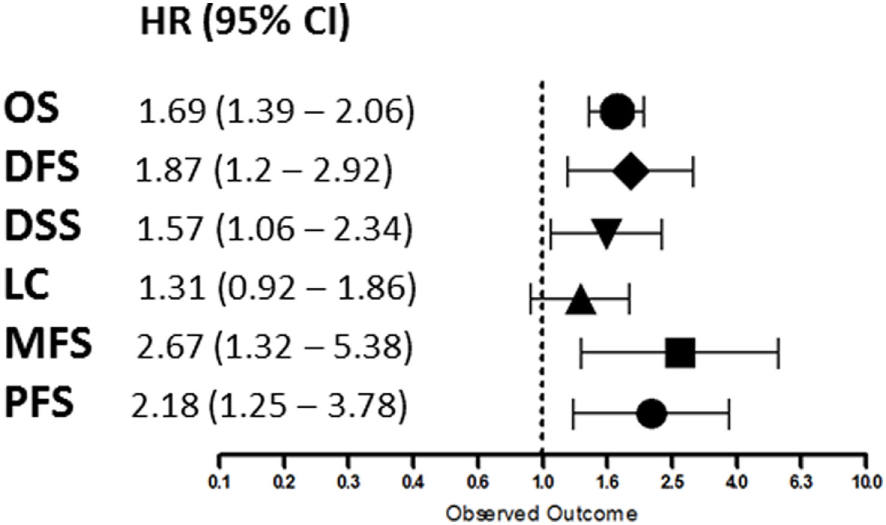
Summary of the overall hazard ratios (HRs) for different endpoints. Symbols represent the HR and horizontal bars the 95% confidence interval (95%CI) (21–69).
Overall Survival
A total of 38 from the selected 49 studies investigated the association between HIF-2α and OS. All the necessary information to estimate the HR could not be obtained from two papers and were therefore not included in the analysis (Table S1 in Supplementary Material) (21, 23, 24, 26–31, 33–35, 37–39, 41–43, 45, 46, 48, 49, 51, 52, 54–57, 59, 60, 62–69). Based on these studies, high HIF-2α expression was statistically significantly associated with a decreased OS (HR = 1.69, 95% CI 1.39–2.06, p < 0.0001, Figure 3). Subgroup analysis based on the different organ sites indicated a similar negative association between tumoral HIF-2α expression and OS: head and neck (HR = 1.55, 95% CI 1.24–1.92, p < 0.0001), lung (HR = 2.15, 95% CI 1.65–2.81, p < 0.0001), stomach (HR = 1.71, 95% CI 1.25–2.32, p = 0.0007), and pancreas (HR = 2.11, 95% CI 1.38–3.24, p = 0.0006). By contrast, no association between tumoral HIF-2α expression and OS was observed for breast (HR = 1.18, 95% CI 0.95–1.47, p = 0.1225), colon (HR = 1.46, 95% CI 0.7–3.07, p = 3121), liver (HR = 1.06, 95% CI 0.43–2.61, p = 0.89), and kidney (HR = 0.61, 95% CI 0.27–1.35, p = 0.2239) cancer (Table 1).
Figure 3
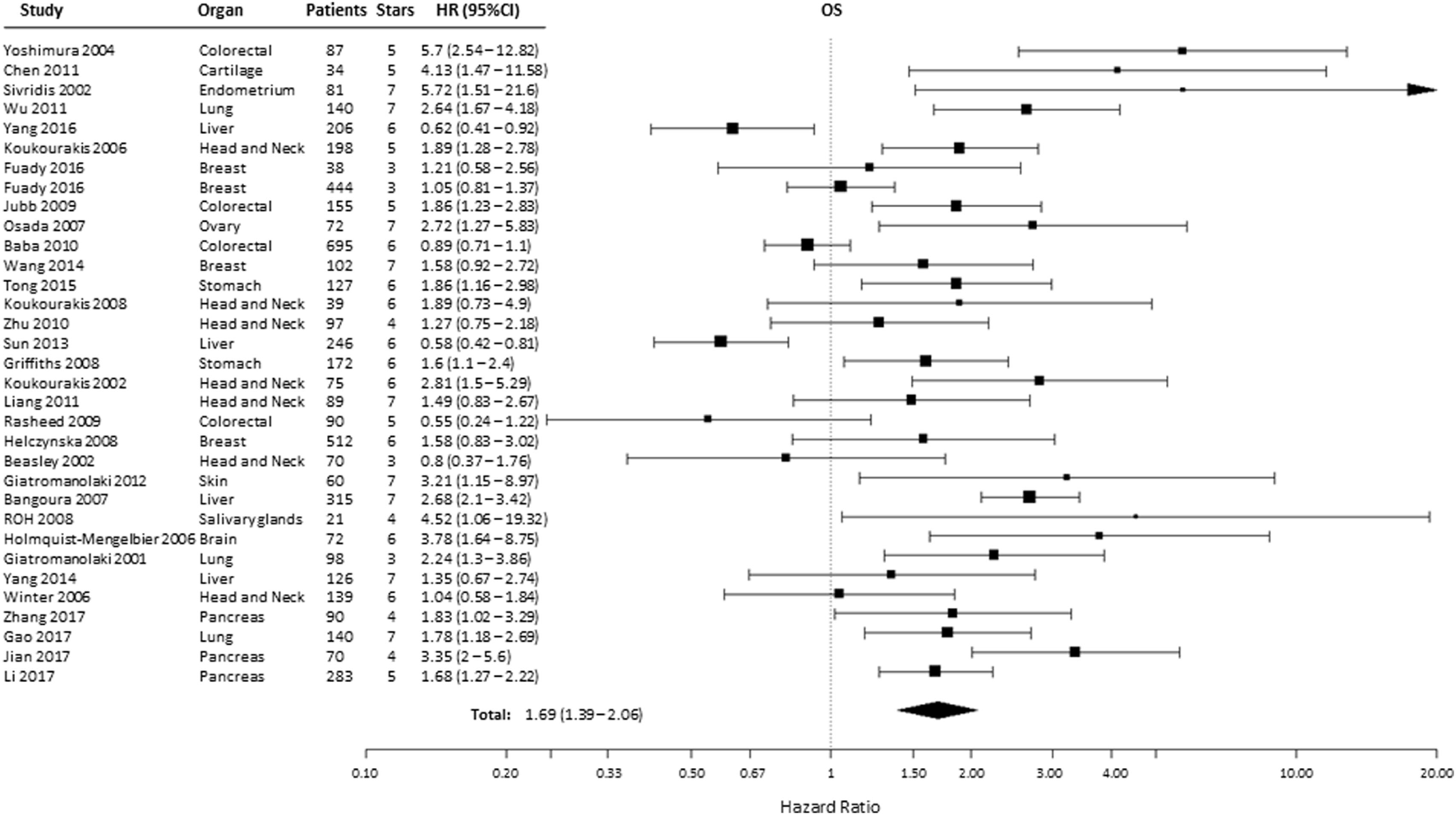
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association of hypoxia-inducible factor-2α expression and overall survival (OS). Symbol size represents the assigned weight of the study (21, 24, 26, 28, 30, 31, 33–35, 37, 38, 41–43, 45, 46, 48, 49, 52, 54–57, 59, 60, 62–64, 66–69).
Disease-Free Survival
Effect of pre-treatment expression of HIF-2α on DFS could be evaluated in six studies (36, 37, 54, 66, 67, 69). Overall, high HIF-2α expression was significantly associated with a decreased DFS (HR = 1.87, 95% CI 1.2–2.92, p = 0.0057, Figure 4). Subgroup analysis indicated that elevated HIF-2α levels were marginally significantly associated with DFS in head and neck cancer (HR = 1.41, 95% CI 0.99–2, p = 0.0577) (Table 1).
Figure 4
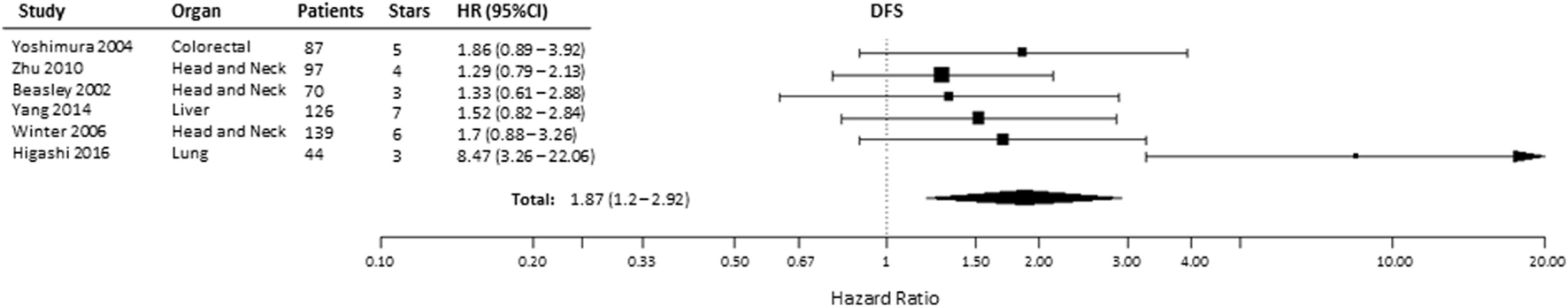
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association between hypoxia-inducible factor-2α expression and disease-free survival (DFS). Symbol size represents the assigned weight of the study (36, 37, 54, 66, 67, 69).
Disease-Specific Survival
A total of 12 studies evaluated the association of HIF-2α expression with DSS, of which 2 studies provided incomplete data to estimate the HR (Table S1 in Supplementary Material) and 3 were excluded for being CC-RCC. In the remaining seven studies, patients suffering from tumors with high HIF-2α had significantly shorter DSS (HR = 1.57, 95% CI 1.06–2.34, p = 0.0249, Figure 5) (24, 25, 32, 34, 35, 47, 49, 50, 53, 58, 61, 66). Subgroup analysis by organ site revealed not association between high HIF-2α expression and worse DSS in tumors of the head and neck (HR = 1.45, 95% CI 0.8–2.64, p = 0.2219) and kidney (HR = 1.21, 95% CI 0.57–2.6, p = 0.6138) cancer (Table 1).
Figure 5
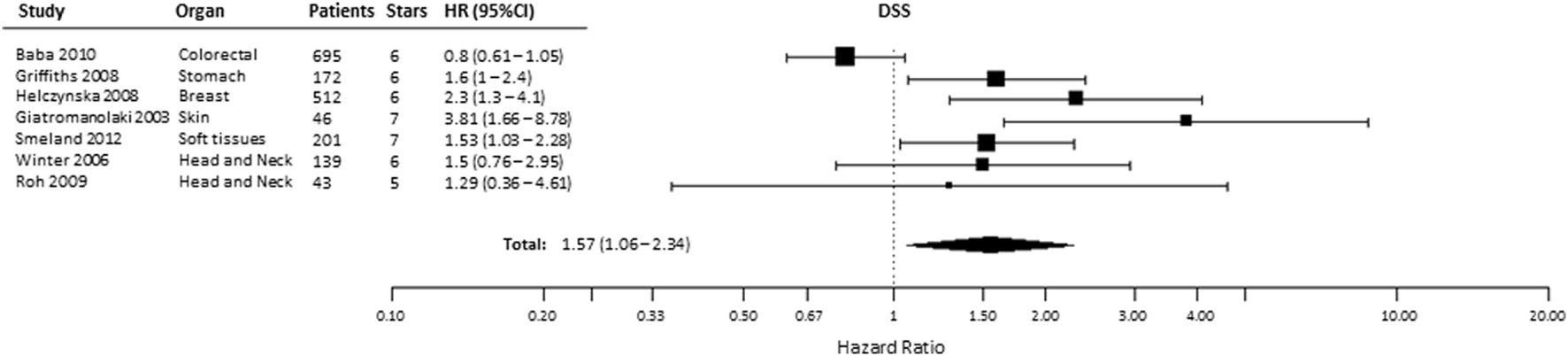
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association between hypoxia-inducible factor-2α expression and disease-specific survival (DSS). Symbol size represents the assigned weight of the study (24, 32, 34, 35, 58, 61, 66).
Locoregional Control
Ten studies were included to analyze the association of HIF-2α expression with risk of LC. High tumoral HIF-2α expression was not associated with a higher risk of locoregional recurrences compared with patients with low expression of HIF-2α in tumors (HR = 1.31, 95% CI 0.92–1.86, p = 0.1281, Figure 6) (35, 40, 44–46, 52, 56–59). However, subgroup analysis for the association between high HIF-2α expression in tumors and worse LC was significant in head and neck tumors (HR = 1.94, 95% CI 1.43–2.63, p < 0.0001). No significant association was observed in kidney tumors (HR = 0.54, 95% CI 0.11–2.57, p = 0.4409) (Table 1).
Figure 6
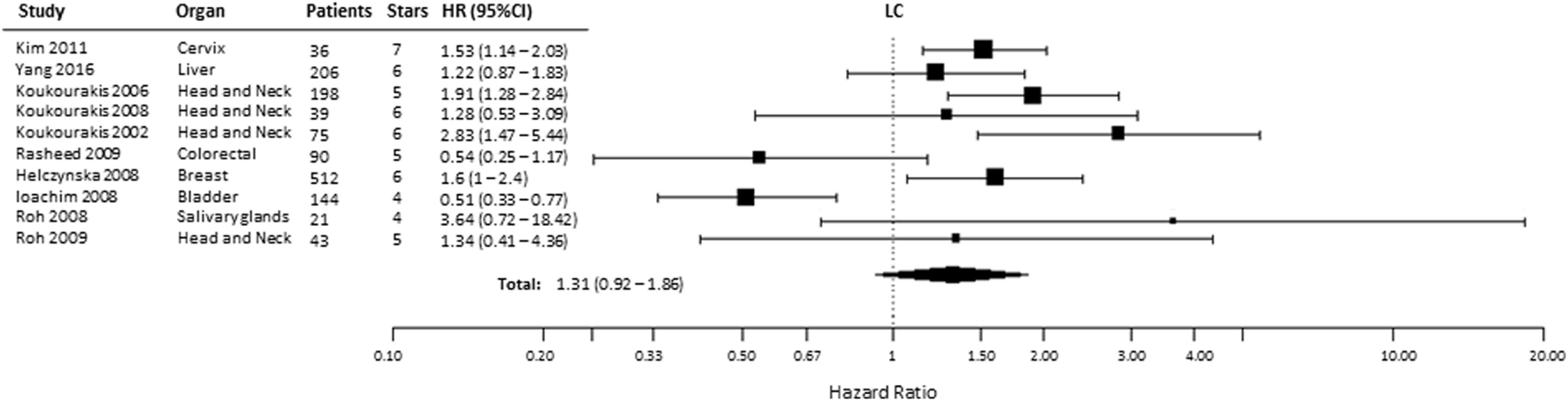
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association between hypoxia-inducible factor-2α expression and locoregional control (LC). Symbol size represents the assigned weight of the study (35, 40, 44–46, 52, 56–59).
Metastasis-Free Survival
Based on the available data reported in two studies, we analyzed the relationship of HIF-2α expression with MFS (53, 57). We found that the pooled HR for MFS was 2.67 (95% CI 1.32–5.38, p = 0.0061), indicating that HIF2α expression is a negative prognostic factor for MFS in patients with prostate and salivary gland cancer (Figure 7). In kidney cancer, one paper reported an inverse correlation between HIF-2α positivity and MFS (HR = 0.08, 95% CI 0.03–0.27, p < 0.001) (23).
Figure 7
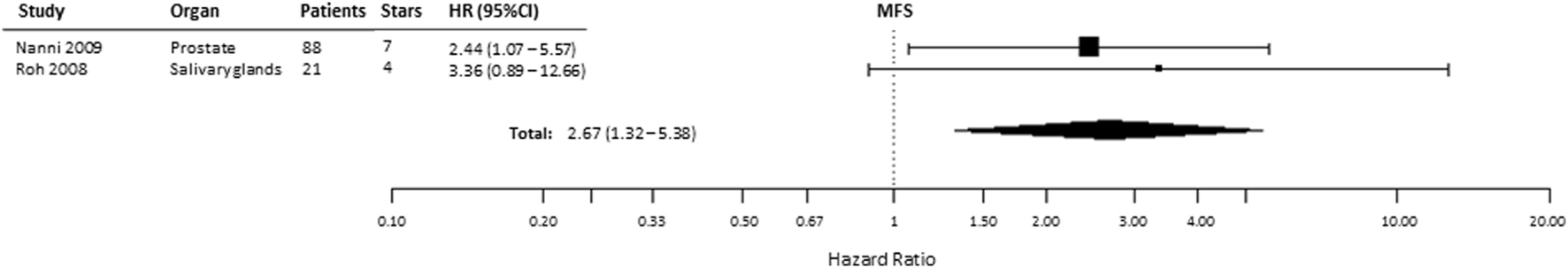
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association between hypoxia-inducible factor-2α expression and metastasis-free survival (MFS). Symbol size represents the assigned weight of the study (53, 57).
Progression-Free Survival
Progression-free survival was reported in 5 of 49 included studies, of which 1 study provided incomplete data (Table S1 in Supplementary Material) and 2 described patients with renal cell cancer (29, 39, 48, 51, 53). Similar to the other endpoints, PFS was significantly shorter (HR = 2.18, 95% CI 1.25–3.78, p = 0.0058) in patients with tumors expressing high levels of HIF-2α (Figure 8). In kidney cancers, these data showed no association between HIF-2α expression and PFS (HR = 0.75, 95% CI 0.32–1.72, p = 0.498) (Table 1).
Figure 8
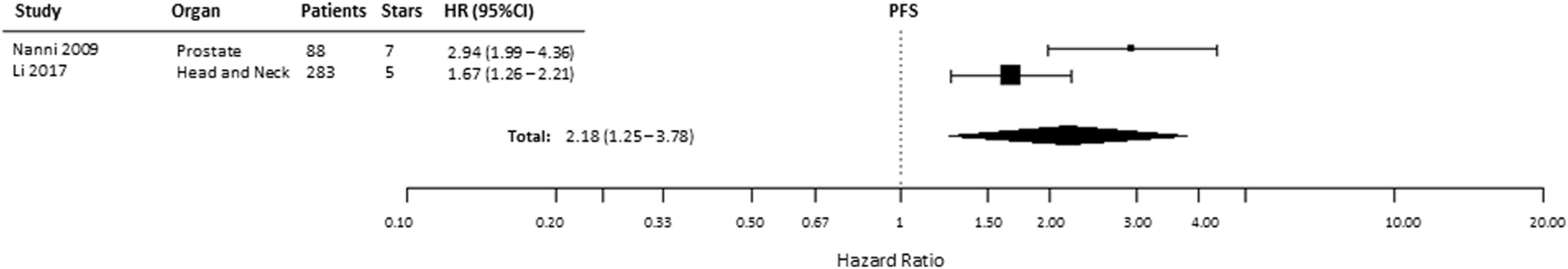
Forest plot of hazard ratios (HRs) with 95% confidence interval (95% CI) (horizontal bars) for the association between hypoxia-inducible factor-2α expression and progression-free survival (PFS). Symbol size represents the assigned weight of the study (48, 53).
High-Quality Papers
This meta-analysis used an adjusted version of the NOS to evaluate the quality of a study. The scores of this quality assessment ranged between 1 and 7 stars, i.e., the maximum, awarded per study. Approximately 78% of these studies were considered as high-quality studies, i.e., with a number of stars greater or equal to 5.
Discussion
There is growing evidence that overexpression of HIF-2α in cancer can contribute to differences in treatment outcome between patients. Although HIF-2α oncogenic activity has been proven, there is significant variability in its value as a biomarker for patient’s prognosis. In view of its role in regulating oncogenic processes triggered by hypoxia, the evaluation of its prognostic value in cancer is of great clinical importance, which may lead to a more accurate patient prognosis and the generation of targeted therapies in the future. This meta-analysis is the first complete overview to summarize all reported clinical studies investigating the impact of HIF-2α expression on treatment outcome in solid tumors.
Here, we show that high HIF-2α levels in cancer are correlated with worse prognosis for OS, DFS, DSS, MFS, and PFS. No association between HIF-2α expression and LC was found. These data suggest that HIF-2α might not be involved in treatment resistance directly but is indicative of more malignant phenotype with greater metastatic potential. Subgroup analyses were performed to explore the source of heterogeneity based on different organ sites. We found that this variable did not alter the prognostic value of HIF-2α for most of the endpoints assessed.
We excluded CC-RCCs from the main analysis due to a different regulatory mechanism of HIF-2α, which might affect the final outcome. A common mutation in CC-RCC is the loss of the VHL gene, which results in the stabilization of HIFs upon normoxia. This oncogenic process is specific for CC-RCC with an abundance of 80% in patients, resulting in an alternative mechanism of HIF-2α activation in cancer (71). However, the negative prognostic value of HIF-2α does not change when CC-RCC is included in the overall meta-analysis (data not shown). In CC-RCC, we found that HIF-2α is a positive prognostic biomarker for MFS only (Table 1). Subcellular localization might also affect the prognostic significance of HIF-2α in CC-RCC as previously noted (75). Their data show that high cytoplasmic expression of HIF-2α was significantly associated with poor DSS which is consistent with our findings. By contrast, high nuclear expression of HIF-2α is associated with better DSS in patients (Table S2 in Supplementary Material). Therefore, it might be that subcellular localization of HIF-2α is crucial in determining the prognostic value in CC-RCC patients, which requires further investigation. Another study demonstrated that HIF-2α can be used as a predictive biomarker related to drug selection for CC-RCC patients treated with sunitinib and sorafenib (51). Therefore, assessment of subgroup categories is necessary to better evaluate the prognostic and predictive value of HIF-2α in CC-RCC.
Previous studies have shown that increased infiltration of tumor-associated macrophages (TAMs) in cancer patients is associated with worse OS (76). In comparison with normal macrophages, TAMs express high levels of HIF-2α, which seems to be an indicator of poor prognosis in cancer patients (77). Using in vivo models of acute inflammation, it has been shown that HIF-2α expression in macrophages is essential for inflammatory responses by regulating proinflammatory cytokine expression (78). Together, these studies show that HIF-2α tightly regulates macrophage functions, which in turn may impact the patient prognosis. We excluded studies in which only macrophage data were reported, since the main goal of this meta-analysis is to determine the prognostic role of HIF-2α expression in tumor cells. To note, 26% of the studies included in this meta-analysis stated HIF-2α reactivity in macrophages together with tumor cells.
The papers included in this meta-analysis were all published between 2001 and 2018, which is likely attributed to the fact that HIF-2α was first identified by independent groups a few years earlier (79, 80). First studies showed the importance of HIF-2α on the transcription of hypoxia-regulated genes such as VEGF in endothelial cells, fibroblasts, and epithelial cells. In addition, researchers described the novel role of HIF-2α in comparison with the already studied HIF-1α, its counteractive protein, in hypoxia and tissue homeostasis (80–83). The discovery of this new hypoxia-activated transcription factor encouraged research to further evaluate the role of HIF-2α in vivo and during development in mice. Their data show that HIF-2α displays a specific pattern of developmental expression at different embryonic stages (84). HIF-2α was thereby defined as a novel bHLH-PAS protein involved in the response to hypoxia showing overlapping but also independent roles with HIF-1α. Basic HLH (helix–loop–helix)–PER–ARNT–SIM (bHLH-PAS) proteins are a family of transcription factors, which respond to environmental signals such as low oxygen levels. This family of proteins is involved in dimerization, DNA binding, and signal transduction (85). Future studies demonstrated that HIF-2α was expressed in a much larger number of cell types (86). Apart from its regulatory role in normal tissue homeostasis, HIF-2α was seen to be commonly upregulated in a broad range of cancers and to contribute to multiple aspects of tumorigenesis such as altered metabolism, angiogenesis, epithelial–mesenchymal transition, and metastasis. This is supported by data showing that HIF-2α is involved in promoting resistance of tumor cells to several treatment modalities and increased patient mortality (87). The important role of HIF-2α in cancer prognosis is also supported by the results of this meta-analysis, which shows that patients with high HIF-2α expression have shorter OS. HIF-1α protein, in turn, has been shown to induce more aggressive phenotype in tumors cells by regulating similar cellular mechanisms (88). Therefore, assessment of oxygen-sensing proteins in tumors prior and/or during therapy may represent a powerful prognostic and predictive biomarker as well as important targets for new anticancer treatments, which warrants further investigations.
Importantly, there is also the risk of encountering publication bias since positive results are more likely to be published than negative ones. This meta-analysis identified a total of 49 studies of which 4 could not be included in final analysis because the HR could not be estimated due to incomplete reporting. Three of four studies stated non-significant association between HIF-2α and outcome (Table S1 in Supplementary Material). Including these four papers in the analysis might therefore decrease the magnitude of the prognostic value of HIF-2α expression reported here. Nevertheless, since the prognostic value of HIF-2α expression is highly statistically significant and the number of excluded studies is very low, we believe that the possible effect of publication bias on this association is negligible.
There are also other limitations in this meta-analysis. The approach of extrapolating the HRs could potentially introduce the source of bias. First, when it was not possible to extract HR directly from the article, survival curves were used to extract data to estimate HR following the method of Tierney et al. (70). Second, significant heterogeneity was found for most of the endpoints tested, which might confirm the high variability among studies. Reduced variability could be achieved by better stratifying tumors into high and low expressing. Third, the use of different antibodies with varying dilutions to detect HIF-2α, different staining protocols, different scoring methods, subcellular localization, and cutoff values may contribute to heterogeneity. Finally, due to the lack of papers referring to each specific endpoint, treatment modality, and/or organ site, it is difficult to set a robust outcome and achieve significant data for different subgroups. Therefore, more high-quality, large-sample, prospectively designed studies are needed to strengthen the prognostic and predictive relevance of HIF-2α in solid cancers.
The results presented here clearly indicate that HIF-2α expression is associated with worse prognosis in a global patient population and in some tumor sites. Altogether, the results of this meta-analysis support the development of a clinical test to determine patient prognosis and/or predict treatment outcome based on HIF-2α expression, although standardized protocols remains to be developed and validated. While potent inhibitors targeting HIF-2α are being translated into clinical trials for renal cancer (89) such predictive tests would be crucial in advancing anti-HIF2 inhibitors not only in renal cancer but also in other solid cancers.
Statements
Author contributions
Study was conceived and designed by ER, AY, and MV. Screening of papers and data extraction was performed by ER, AY, and AG. Statistical analyses were performed by RH. Writing of the first draft of the manuscript was performed by ER. AY, RH, AG, LD, and MV contributed to the writing of the manuscript.
Funding
This work was supported by a H2020 grant from the European Research Council (ERC-CoG, 617060) and the Kankeronderzoekfonds Limburg from the Health Foundation Limburg.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fonc.2018.00224/full#supplementary-material.
References
1
VaupelPMayerA. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev (2007) 26(2):225–39.10.1007/s10555-007-9055-1
2
GortEHGrootAJvan der WallEvan DiestPJVooijsMA. Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr Mol Med (2008) 8(1):60–7.10.2174/156652408783565568
3
RockwellSDobruckiITKimEYMarrisonSTVuVT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med (2009) 9(4):442–58.10.2174/156652409788167087
4
WalshJCLebedevAAtenEMadsenKMarcianoLKolbHC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal (2014) 21(10):1516–54.10.1089/ars.2013.5378
5
DuboisLJNiemansRvan KuijkSJPanthKMParvathaneniNKPeetersSGet alNew ways to image and target tumour hypoxia and its molecular responses. Radiother Oncol (2015) 116(3):352–7.10.1016/j.radonc.2015.08.022
6
HammondEMAsselinMCForsterDO’ConnorJPSenraJMWilliamsKJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol) (2014) 26(5):277–88.10.1016/j.clon.2014.02.002
7
PettersenEOEbbesenPGielingRGWilliamsKJDuboisLLambinPet alTargeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem (2015) 30(5):689–721.10.3109/14756366.2014.966704
8
YarominaAGranzierMBiemansRLieuwesNvan ElmptWShakirinGet alA novel concept for tumour targeting with radiation: inverse dose-painting or targeting the “low drug uptake volume”. Radiother Oncol (2017) 124(3):513–20.10.1016/j.radonc.2017.04.020
9
EalesKLHollinsheadKETennantDA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis (2016) 5:e190.10.1038/oncsis.2015.50
10
WoutersBGKoritzinskyM. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer (2008) 8(11):851–64.10.1038/nrc2501
11
KeQCostaM. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol (2006) 70(5):1469–80.10.1124/mol.106.027029
12
AkenoNCzyzyk-KrzeskaMFGrossTSClemensTL. Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology (2001) 142(2):959–62.10.1210/endo.142.2.8112
13
CarrollVAAshcroftM. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res (2006) 66(12):6264–70.10.1158/0008-5472.CAN-05-2519
14
ShahTKrishnamacharyBWildesFMironchikYKakkadSMJacobDet alHIF isoforms have divergent effects on invasion, metastasis, metabolism and formation of lipid droplets. Oncotarget (2015) 6(29):28104–19.10.18632/oncotarget.4612
15
ImamuraTKikuchiHHerraizMTParkDYMizukamiYMino-KendusonMet alHIF-1alpha and HIF-2alpha have divergent roles in colon cancer. Int J Cancer (2009) 124(4):763–71.10.1002/ijc.24032
16
WigerupCPahlmanSBexellD. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther (2016) 164:152–69.10.1016/j.pharmthera.2016.04.009
17
BertoutJAMajmundarAJGordanJDLamJCDitsworthDKeithBet alHIF2α inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci U S A (2009) 106(34):14391–6.10.1073/pnas.0907357106
18
LiYLiCShiHLouLLiuP. The association between the rs11549465 polymorphism in the hif-1alpha gene and cancer risk: a meta-analysis. Int J Clin Exp Med (2015) 8(2):1561–74.
19
RenHYZhangYHLiHYXieTSunLLZhuTet alPrognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: a meta-analysis. Onco Targets Ther (2016) 9:1477–87.10.2147/OTT.S95490
20
ZhengSSChenXHYinXZhangBH. Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: a meta-analysis. PLoS One (2013) 8(6):e65753.10.1371/journal.pone.0065753
21
WuXHQianCYuanK. Correlations of hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J (2011) 124(1):11–8.
22
AbelEJBaumanTMWeikerMShiFDownsTMJarrardDFet alAnalysis and validation of tissue biomarkers for renal cell carcinoma using automated high-throughput evaluation of protein expression. Hum Pathol (2014) 45(5):1092–9.10.1016/j.humpath.2014.01.008
23
SzendrőiASzászAMKardosMTőkésAMIdanRSzűcsMet alOpposite prognostic roles of HIF1a and HIF2a expressions in bone metastatic clear cell renal cell cancer. Oncotarget (2016) 7(27):42086–98.10.18632/oncotarget.9669
24
BabaYNoshoKShimaKIraharaNChanATMeyerhardtJAet alHIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol (2010) 176(5):2292–301.10.2353/ajpath.2010.090972
25
BiswasSCharlesworthPJTurnerGDLeekRThambooPTCampoLet alCD31 angiogenesis and combined expression of HIF-1alpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIFalpha transcriptional products are not: implications for antiangiogenic trials and HIFalpha biomarker studies in primary CC-RCC. Carcinogenesis (2012) 33(9):1717–25.10.1093/carcin/bgs222
26
ChenCMaQMaXLiuZLiuX. Association of elevated HIF-2alpha levels with low Beclin 1 expression and poor prognosis in patients with chondrosarcoma. Ann Surg Oncol (2011) 18(8):2364–72.10.1245/s10434-011-1587-5
27
EbruTFulyaOPHakanAVuslatYCNecdetSNurayCet alAnalysis of various potential prognostic markers and survival data in clear cell renal cell carcinoma. Int Braz J Urol (2017) 43(3):440–54.10.1590/S1677-5538.IBJU.2015.0521
28
GaoZJWangYYuanWDYuanJQYuanK. HIF-2alpha not HIF-1alpha overexpression confers poor prognosis in non-small cell lung cancer. Tumour Biol (2017) 39(6):1010428317709637.10.1177/1010428317709637
29
Garcia-DonasJLeandro-GarciaLJGonzalez Del AlbaAMorenteMAlemanyIEstebanEet alProspective study assessing hypoxia-related proteins as markers for the outcome of treatment with sunitinib in advanced clear-cell renal cell carcinoma. Ann Oncol (2013) 24(9):2409–14.10.1093/annonc/mdt219
30
BangouraGLiuZSQianQJiangCQYangGFJingS. Prognostic significance of HIF-2alpha EPAS1 expression in hepatocellular carcinoma. World J Gastroenterol (2007) 13(23):3176–82.10.3748/wjg.v13.i23.3176
31
GiatromanolakiASivridisEBechrakisNEWillerdingGSt CharitoudisGFoersterMHet alPhosphorylated pVEGFR2/KDR receptor expression in uveal melanomas: relation with HIF2alpha and survival. Clin Exp Metastasis (2012) 29(1):11–7.10.1007/s10585-011-9424-6
32
GiatromanolakiASivridisEKouskoukisCGatterKCHarrisALKoukourakisMI. Hypoxia-inducible factors 1alpha and 2alpha are related to vascular endothelial growth factor expression and a poorer prognosis in nodular malignant melanomas of the skin. Melanoma Res (2003) 13(5):493–501.10.1097/00008390-200310000-00008
33
GiatromanolakiAKoukourakisMISivridisETurleyHTalksKPezzellaFet alRelation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer (2001) 85(6):881–90.10.1054/bjoc.2001.2018
34
GriffithsEAPritchardSAMcGrathSMValentineHRPricePMWelchIMet alHypoxia-associated markers in gastric carcinogenesis and HIF-2alpha in gastric and gastro-oesophageal cancer prognosis. Br J Cancer (2008) 98(5):965–73.10.1038/sj.bjc.6604210
35
HelczynskaKLarssonAMHolmquist-MengelbierLBridgesEFredlundEBorgquistSet alHypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res (2008) 68(22):9212–20.10.1158/0008-5472.CAN-08-1135
36
HigashiKYamagishiTUedaYIshigakiYShimasakiMNakamuraYet alCorrelation of HIF-1alpha/HIF-2alpha expression with FDG uptake in lung adenocarcinoma. Ann Nucl Med (2016) 30(10):708–15.10.1007/s12149-016-1116-5
37
YoshimuraHDharDKKohnoHKubotaHFujiiTUedaSet alPrognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res (2004) 10(24):8554–60.10.1158/1078-0432.CCR-0946-03
38
Holmquist-MengelbierLFredlundELofstedtTNogueraRNavarroSNilssonHet alRecruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell (2006) 10(5):413–23.10.1016/j.ccr.2006.08.026
39
HuiEPChanATPezzellaFTurleyHToKFPoonTCet alCoexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res (2002) 8(8):2595–604.
40
IoachimEMichaelMSalmasMMichaelMMStavropoulosNEMalamou-MitsiV. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha expression in bladder cancer and their associations with other angiogenesis-related proteins. Urol Int (2006) 77(3):255–63.10.1159/000094819
41
FuadyJHGutscheKSantambrogioSVargaZHoogewijsDWengerR. Estrogen-dependent downregulation of hypoxia-inducible factor (HIF)-2α in invasive breast cancer cells. Oncotarget (2016).10.18632/oncotarget.8866
42
YangJZhuDMZhouXGYinNZhangYZhangZXet alHIF-2α promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget (2017) 8(29):47801–15.10.18632/oncotarget.17999
43
JubbAMTurleyHMoellerHCSteersGHanCLiJLet alExpression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer (2009) 101(10):1749–57.10.1038/sj.bjc.6605368
44
KimMKKimTJSungCOChoiCHLeeJWBaeDSet alClinical significance of HIF-2alpha immunostaining area in radioresistant cervical cancer. J Gynecol Oncol (2011) 22(1):44–8.10.3802/jgo.2011.22.1.44
45
KoukourakisMIBentzenSMGiatromanolakiAWilsonGDDaleyFMSaundersMIet alEndogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol (2006) 24(5):727–35.10.1200/JCO.2005.02.7474
46
KoukourakisMIGiatromanolakiADanielidisVSivridisE. Hypoxia inducible factor (HIf1alpha and HIF2alpha) and carbonic anhydrase 9 (CA9) expression and response of head-neck cancer to hypofractionated and accelerated radiotherapy. Int J Radiat Biol (2008) 84(1):47–52.10.1080/09553000701616114
47
KroegerNSeligsonDBSignorettiSYuHMagyarCEHuangJet alPoor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of hypoxia inducible factor-2alpha. Eur J Cancer (2014) 50(8):1531–40.10.1016/j.ejca.2014.01.031
48
LiWChenCZhaoXYeHZhaoYFuZet alHIF-2alpha regulates non-canonical glutamine metabolism via activation of PI3K/mTORC2 pathway in human pancreatic ductal adenocarcinoma. J Cell Mol Med (2017) 21(11):2896–908.10.1111/jcmm.13202
49
LiangXZhengMJiangJZhuGYangJTangY. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol (2011) 47(2):92–7.10.1016/j.oraloncology.2010.11.014
50
LiuYTanXLiuWChenXHouXShenDet alFollistatin-like protein 1 plays a tumor suppressor role in clear-cell renal cell carcinoma. Chin J Cancer (2018) 37(1):2.10.1186/s40880-018-0267-2
51
MaXWangLLiHZhangYGaoYGuoGet alPredictive immunohistochemical markers related to drug selection for patients treated with sunitinib or sorafenib for metastatic renal cell cancer. Sci Rep (2016) 6:30886.10.1038/srep30886
52
KoukourakisMIGiatromanolakiASivridisESimopoulosCTurleyHTalksKet alHypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Clin Invest (2002) 53(5):1192–202.
53
NanniSBenvenutiVGrasselliAPrioloCAielloAMattiussiSet alEndothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest (2009) 119(5):1093–108.10.1172/JCI35079
54
BeasleyNJPLeekRAlamMTurleyHCoxGJGatterKet alHypoxia-inducible factors HIF1 and HIF2 in head and neck cancer. Cancer Res (2002) 59(1):67–71.
55
OsadaRHoriuchiAKikuchiNYoshidaJHayashiAOtaMet alExpression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum Pathol (2007) 38(9):1310–20.10.1016/j.humpath.2007.02.010
56
RasheedSHarrisALTekkisPPTurleyHSilverAMcDonaldPJet alHypoxia-inducible factor-1alpha and -2alpha are expressed in most rectal cancers but only hypoxia-inducible factor-1alpha is associated with prognosis. Br J Cancer (2009) 100(10):1666–73.10.1038/sj.bjc.6605026
57
RohJLChoKJKwonGYChoiSHNamSYKimSY. Prognostic values of pathologic findings and hypoxia markers in 21 patients with salivary duct carcinoma. J Surg Oncol (2008) 97(7):596–600.10.1002/jso.21045
58
RohJLChoKJKwonGYRyuCHChangHWChoiSHet alThe prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol (2009) 45(1):63–8.10.1016/j.oraloncology.2008.03.017
59
YangSLLiuLPNiuLSunYPYangXRFanJet alDownregulation and pro-apoptotic effect of hypoxia-inducible factor 2 alpha in hepatocellular carcinoma. Oncotarget (2016) 7(23):34571–81.10.18632/oncotarget.8952
60
SivridisEGiatromanolakiAGatterKCHarrisALKoukourakisMITumor and Angiogenesis Research Group. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer (2002) 95(5):1055–63.10.1002/cncr.10774
61
SmelandEKilvaerTKSorbyeSValkovAAndersenSBremnesRMet alPrognostic impacts of hypoxic markers in soft tissue sarcoma. Sarcoma (2012) 2012:541650.10.1155/2012/541650
62
SunHXXuYYangXRWangWMBaiHShiRYet alHypoxia inducible factor 2 alpha inhibits hepatocellular carcinoma growth through the transcription factor dimerization partner 3/E2F transcription factor 1-dependent apoptotic pathway. Hepatology (2013) 57(3):1088–97.10.1002/hep.26188
63
TongWWTongGHChenXXZhengHCWangYZ. HIF2alpha is associated with poor prognosis and affects the expression levels of survivin and cyclin D1 in gastric carcinoma. Int J Oncol (2015) 46(1):233–42.10.3892/ijo.2014.2719
64
WangHXQinCHanFYWangXHLiN. HIF-2alpha as a prognostic marker for breast cancer progression and patient survival. Genet Mol Res (2014) 13(2):2817–26.10.4238/2014.January.22.6
65
WangMChenMYGuoXJJiangJX. Expression and significance of HIF-1alpha and HIF-2alpha in pancreatic cancer. J Huazhong Univ Sci Technolog Med Sci (2015) 35(6):874–9.10.1007/s11596-015-1521-3
66
WinterSCShahKAHanCCampoLTurleyHLeekRet alThe relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer (2006) 107(4):757–66.10.1002/cncr.21983
67
YangSLLiuLPJiangJXXiongZFHeQJWuC. The correlation of expression levels of HIF-1alpha and HIF-2alpha in hepatocellular carcinoma with capsular invasion, portal vein tumor thrombi and patients’ clinical outcome. Jpn J Clin Oncol (2014) 44(2):159–67.10.1093/jjco/hyt194
68
ZhangQLouYZhangJFuQWeiTSunXet alHypoxia-inducible factor-2alpha promotes tumor progression and has crosstalk with Wnt/beta-catenin signaling in pancreatic cancer. Mol Cancer (2017) 16(1):119.10.1186/s12943-017-0689-5
69
ZhuGQTangYLLiLZhengMJiangJLiXYet alHypoxia inducible factor 1alpha and hypoxia inducible factor 2alpha play distinct and functionally overlapping roles in oral squamous cell carcinoma. Clin Cancer Res (2010) 16(19):4732–41.10.1158/1078-0432.CCR-10-1408
70
TierneyJFStewartLAGhersiDBurdettSSydesMR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16.10.1186/1745-6215-8-16
71
RazorenovaOVFingerECColavittiRChernikovaSBBoikoADChanCKet alVHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{delta}-driven migration. Proc Natl Acad Sci U S A (2011) 108(5):1931–6.10.1073/pnas.1011777108
72
van KuijkSJYarominaAHoubenRNiemansRLambinPDuboisLJ. Prognostic significance of carbonic anhydrase IX expression in cancer patients: a meta-analysis. Front Oncol (2016) 6:69.10.3389/fonc.2016.00069
73
ViechtbauerW. Conducting meta-analyses in R with the meta for package. J Stat Softw (2010) 36(3):1–48.10.18637/jss.v036.i03
74
DerSimonianRLairdN. Meta-analysis in clinical trials. Control Clin Trials (1986) 7(3):177–88.
75
FanYLiHMaXGaoYChenLLiXet alPrognostic significance of hypoxia-inducible factor expression in renal cell carcinoma: a PRISMA-compliant systematic review and meta-analysis. Medicine (2015) 94(38):e1646.10.1097/MD.0000000000001646
76
LeekRDLewisCEWhitehouseRGreenallMClarkeJHarrisAL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res (1996) 56(20):4625–9.
77
LeekRDTalksKLPezzellaFTurleyHCampoLBrownNSet alRelation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res (2002) 62(5):1326–9.
78
ImtiyazHZWilliamsEPHickeyMMPatelSADurhamACYuanLJet alHypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest (2010) 120(8):2699–714.10.1172/JCI39506
79
EmaMTayaSYokotaniNSogawaKMatsudaYFujii-KuriyamaY. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A (1997) 94(9):4273–8.10.1073/pnas.94.9.4273
80
TianHMcKnightSLRussellDW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev (1997) 11(1):72–82.10.1101/gad.11.1.72
81
FlammeIFrohlichTvon ReuternMKappelADamertARisauW. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev (1997) 63(1):51–60.10.1016/S0925-4773(97)00674-6
82
LadouxAFrelinC. Cardiac expressions of HIF-1 alpha and HLF/EPAS, two basic loop helix/PAS domain transcription factors involved in adaptative responses to hypoxic stresses. Biochem Biophys Res Commun (1997) 240(3):552–6.10.1006/bbrc.1997.7708
83
WiesenerMSTurleyHAllenWEWillamCEckardtKUTalksKLet alInduction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood (1998) 92(7):2260–8.
84
JainSMaltepeELuMMSimonCBradfieldCA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech Dev (1998) 73(1):117–23.10.1016/S0925-4773(98)00038-0
85
BerstenDCSullivanAEPeetDJWhitelawML. bHLH-PAS proteins in cancer. Nat Rev Cancer (2013) 13(12):827–41.10.1038/nrc3621
86
WiesenerMSJurgensenJSRosenbergerCScholzeCKHorstrupJHWarneckeCet alWidespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J (2003) 17(2):271–3.10.1096/fj.02-0445fje
87
KeithBJohnsonRSSimonMC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer (2011) 12(1):9–22.10.1038/nrc3183
88
LiaoDCorleCSeagrovesTNJohnsonRS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res (2007) 67(2):563–72.10.1158/0008-5472.CAN-06-2701
89
ChenWHillHChristieAKimMSHollomanEPavia-JimenezAet alTargeting renal cell carcinoma with a HIF-2 antagonist. Nature (2016) 539:112–7.10.1038/nature19796
Summary
Keywords
cancer, hypoxia-inducible factor-2, meta-analysis, prognosis, endothelial PAS domain protein-1
Citation
Moreno Roig E, Yaromina A, Houben R, Groot AJ, Dubois L and Vooijs M (2018) Prognostic Role of Hypoxia-Inducible Factor-2α Tumor Cell Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 8:224. doi: 10.3389/fonc.2018.00224
Received
20 March 2018
Accepted
30 May 2018
Published
11 June 2018
Volume
8 - 2018
Edited by
Massimo Bonora, Albert Einstein College of Medicine, United States
Reviewed by
Anca Maria Cimpean, University of Medicine and Pharmacy, Timisoara, Romania; Adriana De Siervi, Instituto de Biología y Medicina Experimental (IBYME), Argentina; Julie Valerie Decock, Qatar Biomedical Research Institute, Qatar
Updates
Copyright
© 2018 Moreno Roig, Yaromina, Houben, Groot, Dubois and Vooijs.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Vooijs, marc.vooijs@maastrichtuniversity.nl
Specialty section: This article was submitted to Molecular and Cellular Oncology, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.