- Department of Radiation Oncology, Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center, Cleveland Clinic, Cleveland, OH, United States
Brain metastases remain the most common neurologic complication of cancer. With improvement in surveillance and systemic therapy, patients with limited CNS disease are living longer after diagnosis, thus influencing the importance of optimal radiation treatment in order to maximize local control and minimize morbidity. In patients with a limited number of brain metastases, stereotactic radiosurgery is more recently seen as an appropriate sole modality for management with excellent local control. As newer systemic therapies emerge and with the advent of immunotherapies and targeted therapies for metastatic CNS disease, further research is needed in the optimal timing and sequencing of these modalities.
Introduction
Up to 30% of cancer patients develop brain metastases during their lifetime making it the most common neurological complication of cancer (1, 2). The most common primary cancers that metastasize to the brain include lung cancer, breast cancer, kidney cancer and melanoma (3, 4). Incidence has increased due to more routine surveillance, detection of smaller lesions with MRI, as well as improved systemic therapies and thus improved length of survival. Given the available treatment options and strong proponents of various treatment options, optimal treatment has been controversial given the historically poor outcomes (5). While overall prognosis after development of brain metastases remains poor, a subset of patients can live several years after diagnosis, especially those with limited CNS disease (6). Given potential for long term survival, stereotactic radiosurgery (SRS), with or without whole-brain radiation (WBRT), has become an increasingly recognized standard of care in order to minimize morbidity. More recently, SRS alone has been supported as a sole modality for the management of1 to 4 brain metastases.
Historical Standards
The early randomized trials by Patchell et al. (7) answered initial questions about the best management strategy for single brain metastasis. In his initial study, patients with a single brain metastasis were randomized to surgery plus WBRT or biopsy plus WBRT which showed an overall survival (OS) benefit to surgical resection (40 vs. 15 weeks, p < 0.01) and local control improvement. Therefore, a subsequent study by Patchell et al. (8) was designed in which patients with a single brain metastasis had complete surgical resection and then randomized to WBRT or observation. Post-operative WBRT reduced intracranial failure from 70 to 18% (p < 0.001) and local recurrence (LR) from 46 to 10% (p < 0.001). Consequently, the optimal treatment of single brain metastasis was resection followed by WBRT. With the advent of SRS, future investigations focused on the addition of SRS to WBRT in order to improve local control (LC).
WBRT + SRS
One of the earliest uses of SRS for brain metastases was as an adjunct to WBRT. At that time, the maximum number of brain metastases able to be treated was up to 3 or 4 due to technical limitations of the treatment machines. An initial study by Kondziolka et al. (9) randomized 27 patients with 2 to 4 brain metastases, all <2.5 cm, to WBRT vs. WBRT plus SRS boost. WBRT dose was 30 Gy in 12 fractions with an SRS boost of 16 Gy in a single fraction. Patients who received WBRT alone, had local failure rates of 100% vs. only 8% in patients who received SRS boost. Survival was 11 months in patients receiving SRS and 7.5 months in patients receiving WBRT alone (p = 0.22), which was expected given the small sample size that was underpowered to detect a survival difference. This data suggested that given poor LC rates with WBRT, SRS boost should be considered in patients with an otherwise reasonable survival expectation.
A subsequent larger randomized study (RTOG 95-08) (1) sought to further investigate the role of SRS boost. Three hundred, 33 patients with 1–3 brain metastases were randomized to WBRT vs. WBRT plus SRS boost. LC at 1 year improved from 71 to 82% with the addition of SRS (p = 0.01), though <50% of patients had adequate follow up imaging at 3 months. Overall, there was no difference in survival between the arms. In the subset of patients with single brain metastasis or recursive partition analysis (RPA) Class I, there was improved survival with SRS boost from 4.9 to 6.5 months (p = 0.39) and 9.6 to 11.6 months (p = 0.045), respectively. On secondary analysis (10), patients were classified by Graded Prognostic Assessment (GPA) score, a more modern prognostic scoring system compared to the RPA initially used. Patients with a high GPA (3.5–4) had improved survival regardless of number of brain metastases. This study further supported the observation that SRS boost improves LC and OS, particularly in patients with good performance status.
A Cochrane Database review updated in 2017 (11) synthesized available data regarding the benefit of SRS boost after WBRT. This review included three randomized trials which included a total of 358 patients. There was decreased local failure in the WBRT plus SRS group (HR 0.27 95% CI 0.14–0.52) as well as an improvement in performance status scores and decreased steroid use (RR 0.64 CI 0.42–0.97). There was no difference in OS in either group, though in participants with single brain metastasis had significantly longer median survival in the WBRT plus SRS group (p = 0.04).
SRS Alone
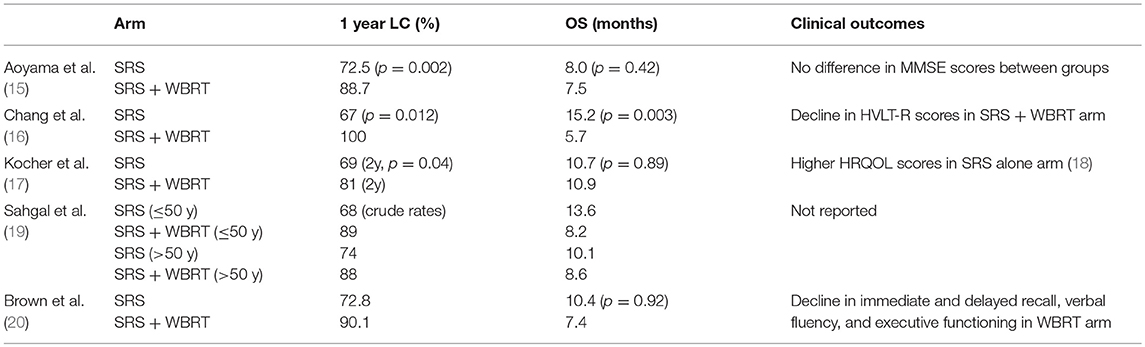
Subsequent data indicated there may be an association between WBRT and neurocognitive decline as well as an increased risk of dementia, though data was conflicting and some argued that progressive CNS disease caused more deleterious side effects than those related to WBRT (12–14). Thus, future studies focused on maximizing control, while further investigating effects of progressive brain metastases and treatment on neurocognition and quality of life. The debate surrounding the need for upfront WBRT in patients with a limited number of brain metastases was the subject of multiple future investigations. There have been four randomized trials investigating SRS alone vs. SRS plus WBRT (Table 1), which overall, have indicated that SRS alone allows for reduced effects on neurocognition, while still effectively managing brain metastases.
Aoyama et al. (15) published the first prospective study exploring this topic. In this phase III randomized control trial (RCT), 132 patients with 4 or less brain metastases <3 cm in size were randomized to SRS plus WBRT vs. SRS alone. The study was underpowered to detect an OS difference, and the primary endpoint was brain tumor recurrence. At 1 year, brain tumor recurrence decreased from 76 to 47% with the addition of WBRT (p < 0.001). WBRT also improved 1-year freedom from new brain metastases from 41.5% in SRS group to 64% (p = 0.003), and subsequently, there was more salvage treatment in the SRS alone group. There were no noted differences in toxicities between the groups. A subset of 28 patients had neurocognitive testing with Mini-Mental Status Examination (MMSE) at baseline and at least once at follow up. This group showed there was no difference after treatment between the two arms. Conflicting conclusions were drawn by various groups from this data, with the authors concluding that WBRT could be omitted safely, while others felt that WBRT improved LC and brain tumor recurrence and should be delivered routinely. In a secondary analysis of the data, published 9 years later, in the subset of patients with non-small cell lung cancer (NSCLC) with GPA score of 2.5–4, there was an improvement in OS from 10.6 to 16.7 months (p = 0.04) in patients receiving SRS plus WBRT (21). As expected, this group of patients had a lower rate of brain metastases recurrence (p < 0.01) which may contribute to improved OS. There was no improvement in survival for patients with lower GPA scores. This small sub-study of 47 patients is suggestive of benefit, though with small number of patients with 12 months follow up (n = 24), it may be considered hypothesis generating that maximal intracranial control is ideal for patients with potential for long survival.
More modern data have been acquired to further determine the neurocognitive impact of WBRT. Another phase III RCT compared SRS plus WBRT to SRS alone in patients with 1–3 brain metastasis and followed neurocognitive outcomes with the Hopkins Verbal Learning Test Revised (HVLT-R) (16). Fifty-eight patients were enrolled, and at interim analysis, there was a 96% probability that the SRS plus WBRT arm would show a decline in neurocognition, and the trial was ended early. As previously seen, there was a higher rate of CNS recurrence in SRS-only group compared to SRS plus WBRT, 73 vs. 27%, respectively (p = 0.0003). Median OS was surprisingly improved in SRS alone group at 15.2 vs. 5.7 months in the SRS plus WBRT group (p = 0.003). It was speculated that there was perhaps more surgical salvage and/or earlier start to systemic therapy in SRS alone group, or higher burden of systemic disease in those assigned to SRS plus WBRT. Given improved neurocognitive scores as well as potential for OS benefit, the authors concluded the SRS alone was preferred over SRS plus WBRT provided patients undergo close and careful follow up.
The European Organization for Research and Treatment of Cancer (EORTC) conducted a phase III trial in patients with 1–3 brain metastases who underwent SRS or surgery, then randomized patients to WBRT or observation (17). As expected, WBRT decreased the risk of intracranial relapse, however, there was no difference in OS between the groups. Interestingly, there was no difference in functional improvement between the two groups, indicating that while WBRT reduced the risk of recurrence, there was no clinical improvement in functional independence. Follow up publication by Soffietti et al. (18) focused on health-related quality of life (HRQOL) parameters in these patients. Patients in the observation arm had higher HRQOL scores in global health at 9 months (p = 0.148), as well as improved physical function and fatigue at 8 weeks, and cognitive functioning at 12 months compared to those in WBRT arm.
An individual patient-level meta-analysis of the above three studies was done to further characterize these findings. This showed that patients younger than 50 years old had improved survival with SRS alone when compared to SRS plus WBRT (10 vs. 8.2 months, p = 0.04). This patient group also had no difference in distant brain metastasis rate. It was concluded from this data set that the side effect profile of WBRT coupled with no improvement in distant brain metastasis rate may lead to the survival advantage seen in younger patients receiving SRS alone (19).
The most recent study investigating SRS vs. SRS plus WBRT was the results of the North Central Cancer Treatment Group (NCCTG) N0574 phase III study randomizing patients with 1–3 brain metastases to SRS vs. SRS plus WBRT (20). Two hundred eight patients were enrolled and the primary endpoint was neurocognitive function as defined as decline of >1 standard deviation from baseline in any of 7 cognitive domains at 3 months follow up. 91.7% of patients in the SRS plus WBRT arm had cognitive decline vs. 63.5% in SRS alone group (p < 0.001). Particular cognitive domains that were most affected by the addition of WBRT included immediate recall, delayed recall, and verbal fluency. In patients living 12 months or more, there was more frequent cognitive decline with the addition of WBRT, most notably in executive functioning (p = 0.05). However, there was improvement in 12 months intracranial control with addition of WBRT (84.6%) vs. SRS alone (50.5%). There was a numerical, though not statistically significant, improvement in median OS for SRS alone of 10.4 vs. 7.4 months (p = 0.92), though the study was not powered to detect OS differences. This larger study confirmed previous results (16), with a larger patient population, that in patients with 1–3 brain metastases, SRS alone may be preferred treatment modality.
From these four trials, we are able to glean several important points regarding the preferred treatment of patients with 1–4 brain metastases which were outlined by Arvold et al. (22). First, there is no negative impact on OS by eliminating WBRT in this patient population. Next, there is additive benefit in terms of LC with SRS plus WBRT, though SRS alone has similarly high rates of LC. Determining LC can be complicated by radiographic findings of pseudoprogression and radiation necrosis. Thirdly, when WBRT is withheld, there is increased rate of new distant brain metastases which leads to more frequent salvage treatment, and about a quarter of patients will ultimately require WBRT. Finally, the risk of neurocognitive decline is lower with SRS alone. Additionally, a Cochrane Database analysis of RCTs comparing of SRS or surgery alone vs. SRS or surgery plus whole brain further highlight the important data points (23). At 1 year, adding WBRT to SRS decreased relative risk of intracranial disease progression by 53%. However, there is no clear evidence of OS differences and subgroup analyses show similar OS regardless of therapy used, number of brain metastases as well as dose and sequence of WBRT.
With growing data as outlined above, ASTRO consensus guidelines were updated recommending against the routine use of WBRT in addition to SRS in patients with limited brain metastases. In addition, multiple other groups through editorials as well as groups such as The National Comprehensive Cancer Network (24), Deutsche Gesellschaft fur Radioonkologie (25), and International Stereotactic Radiosurgery Society (ISRS) (26) have voiced that SRS alone is favored in patients with limited brain metastasis burden and WBRT to be reserved for salvage options (27). Further studies have begun investigating the utility of SRS alone in >4 brain metastases. Yamamoto et al. reported their prospective observational study of SRS alone for treatment of 5–10 brain metastases compared to treated of two to four brain metastases (28). They found that overall survival was similar between patients with 2–4 metastases as compared to 5–10 metastases with no difference in acute toxicities. Future study is necessary to optimize appropriate settings for SRS alone.
Optimal Timing of SRS and Systemic Therapy
The typical approach for management of systemic disease with brain metastases is treatment of CNS disease first, followed by initiation of systemic therapy. A recent randomized trial out of Korea, specifically evaluated timing of SRS relative to the start of chemotherapy in patients with limited number of asymptomatic brain metastases (29). Patients with NSCLC were randomized to upfront SRS prior to chemotherapy initiation vs. initiation of chemotherapy without treatment of CNS disease. Median OS was equivalent between the groups, though there was a trend toward longer CNS progression free survival, lower symptomatic brain progression rate and lower CNS salvage rates in the upfront SRS group. It appears from this data, that upfront SRS may be preferable, though in cases that urgent chemotherapy is needed, delaying CNS treatment is likely safe.
New emerging data suggests that systemic therapy may be safely given concurrently with SRS. In retrospective studies, there does not appear to be an association between timing of systemic therapy and increased rates of myelosuppression. A retrospective review from Johns Hopkins showed that in patients receiving concurrent systemic therapy with SRS, only 4% of patients developed grade 3 or 4 neurotoxicity (30). There was an association between higher grade of neurotoxicity with concurrent use of immune therapy as well as lower use of steroids with concurrent targeted therapy. There was no difference in rates of radiation necrosis, grade of neurotoxicity, or steroid use based on timing of systemic therapies. Interestingly, in newly diagnosed cancer patients found to have brain metastases, treatment with concurrent systemic therapy and SRS had improved survival compared to SRS alone (41.6 vs. 21.5 months, p < 0.05). In a larger retrospective review of 1,650 patients with 27% of patients receiving concurrent systemic therapy, similar results were found. In patients who received SRS plus WBRT, there was a higher rate of radiation necrosis, compared to SRS alone when patients received concurrent vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs; 14.3 vs. 6.6%, p = 0.04) or epidermal growth factor receptor TKIs (15.6 vs. 6% p = 0.04). There was no association between other systemic therapies, including hormonal therapy, cytotoxic chemotherapy or other targeted agents, and risk of radiation necrosis when given concurrently with SRS (31). Similar results were seen in secondary analysis of patients enrolled on RTOG 0320 and concurrent use of temozolomide or erlotinib with concurrent SRS or SRS plus WBRT. This analysis showed that patients had more toxicity and worse survival when receiving either systemic agent in combination with WBRT plus SRS vs. WBRT or SRS alone (32).
In the era of new targeted therapies, the indications and timing of SRS is not always clear, and in some cases radiation may be deferred for immediate targeted therapy start. A recent multi-institutional retrospective review evaluated 351 patients with EGFR-mutant NSCLC with new brain metastases who were TKI naïve (33). Patients were treated with SRS or WBRT followed by TKI therapy or TKI therapy alone with radiation reserved at time of progression. Outcomes showed that delaying radiation, WBRT or SRS alone, is associated with significantly worse OS in this patient population. Patients treated with SRS followed by TKI had the longest median OS at 46 months, compared to 30 months with WBRT + TKI and 25 months with TKI alone (P < 0.001 for each group). Further randomized data is needed to better define the optimal timing and sequencing of radiation and systemic therapy, particularly in the setting of new targeted therapies.
Conclusion
Historically, WBRT was used in conjunction with SRS in order to improve intracranial control, with major disadvantage being neurocognitive decline with the addition of WBRT. In the era of improved surveillance with MRI imaging, better systemic therapy, and improved patient survival, goals have transformed to limit late toxicity, particularly in favorable patient populations with limited CNS disease. Multiple studies have shown that SRS alone for 1–4 brain metastases has acceptable local control with reduced neurocognitive decline as compared to WBRT, and thus, is the favored treatment modality in this patient population (15–17, 20, 27). SRS alone may be appropriate for patients with >4 brain metastases, though further study is necessary to clarify optimal patient selection.
Author Contributions
SS and SC: development, writing, and editing; JS, EM, and JY: writing and editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor and reviewer AS declared their involvement as co-editors in the Research Topic, and confirm the absence of any other collaboration.
References
1. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (2004) 363:1665–72. doi: 10.1016/S0140-6736(04)16250-8
2. Wen PY, Loeffler JS. Management of brain metastases. Oncology (1999) 13:941–54, 957–61; discussion: 961–2.
3. Le Chevalier T, Smith FP, Caille P, Constans JP, Rouesse JG. Sites of primary malignancies in patients presenting with cerebral metastases. a review of 120 cases. Cancer (1985) 56:880–82. doi: 10.1002/1097-0142(19850815)56:4<880::AID-CNCR2820560430>3.0.CO;2-I
4. Suh JHCS, Peereboom DP, Barnett GH. Metastatic cancer to brain. In: DeVita VT, Lawrence TS, Rosenberg S, editiors. Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 10th ed. Philadelphia, PA: Wolters Kluwer (2015). p. 1832–1844.
5. Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. (2010) 362:1119–27. doi: 10.1056/NEJMct0806951
6. Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. (2016) 34:123–9. doi: 10.1200/JCO.2015.62.0138
7. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. (1990) 322:494–500. doi: 10.1056/NEJM199002223220802
8. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA (1998) 280:1485–9. doi: 10.1001/jama.280.17.1485
9. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. (1999) 45:427–34. doi: 10.1016/S0360-3016(99)00198-4
10. Sperduto PW, Shanley R, Luo X, Andrews D, Werner-Wasik M, Valicenti R, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys. (2014) 90:526–31. doi: 10.1016/j.ijrobp.2014.07.002
11. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochr Database System Rev. (2017) 9:Cd006121. doi: 10.1002/14651858.CD006121.pub4
12. Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. (2004) 22:157–65. doi: 10.1200/JCO.2004.05.128
13. DeAngelis LM, Mandell LR, Thaler HT, Kimmel DW, Galicich JH, Fuks Z, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery (1989) 24:798–805. doi: 10.1227/00006123-198906000-00002
14. Regine WF, Scott C, Murray K, Curran W. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys. (2001) 51:711–7. doi: 10.1016/S0360-3016(01)01676-5
15. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA (2006) 295:2483–91. doi: 10.1001/jama.295.21.2483
16. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009) 10:1037–44. doi: 10.1016/S1470-2045(09)70263-3
17. Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. (2011) 29:134–41. doi: 10.1200/JCO.2010.30.1655
18. Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. (2013) 31:65–72. doi: 10.1200/JCO.2011.41.0639
19. Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. (2015) 91:710–7. doi: 10.1016/j.ijrobp.2014.10.024
20. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs. radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA (2016) 316:401–9. doi: 10.1001/jama.2016.9839
21. Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. (2015) 1:457–64. doi: 10.1001/jamaoncol.2015.1145
22. Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro Oncol. (2016) 18:1043–65. doi: 10.1093/neuonc/now127
23. Soon YY, Tham IW, Lim KH, Koh WY, Lu JJ. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochr Database System Rev. (2014) CD009454. doi: 10.1002/14651858.CD009454.pub2
24. National Comprehensive Cancer Network: Central Nervous System Cancers. Available online at: https://www.nccn.org/professionals/physician_gls/default.aspx (Accessed May 10, 2018).
25. Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO working group on stereotactic radiotherapy. Strahlenther Onkol. (2014) 190:521–32. doi: 10.1007/s00066-014-0648-7
26. Chao ST, De Salles A, Hayashi M, Levivier M, Ma L, Martinez R, et al. Stereotactic radiosurgery in the management of limited (1-4) brain metasteses: systematic review and international stereotactic radiosurgery society practice guideline. Neurosurgery (2017) 83:345–53. doi: 10.1093/neuros/nyx522
27. Sahgal A, Larson D, Knisely J. Stereotactic radiosurgery alone for brain metastases. Lancet Oncol. (2015) 16:249–50. doi: 10.1016/S1470-2045(14)71106-4
28. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. (2014) 15:387–95. doi: 10.1016/S1470-2045(14)70061-0
29. Lim SH, Lee JY, Lee MY, Kim HS, Lee J, Sun JM, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol. (2015) 26:762–8. doi: 10.1093/annonc/mdu584
30. Shen CJ, Kummerlowe MN, Redmond KJ, Rigamonti D, Lim MK, Kleinberg LR. Stereotactic radiosurgery: treatment of brain metastasis without interruption of systemic therapy. Int J Radiat Oncol Biol Phys. (2016) 95:735–42. doi: 10.1016/j.ijrobp.2016.01.054
31. Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro Oncol. (2017) 133:357–68. doi: 10.1007/s11060-017-2442-8
32. Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. (2013) 85:1312–8. doi: 10.1016/j.ijrobp.2012.11.042
33. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. (2017) 35:1070–77. doi: 10.1200/JCO.2016.69.7144
Keywords: brain metastases (BM), stereotactic radiosurgery (SRS), radiation therapy (XRT), systemic therapy, review (article)
Citation: Sittenfeld SMC, Suh JH, Murphy ES, Yu JS and Chao ST (2018) Contemporary Management of 1–4 Brain Metastases. Front. Oncol. 8:385. doi: 10.3389/fonc.2018.00385
Received: 27 June 2018; Accepted: 28 August 2018;
Published: 24 September 2018.
Edited by:
Sunit Das, St. Michael's Hospital, CanadaReviewed by:
Arjun Sahgal, University of Toronto, CanadaBrigitta G. Baumert, Maastricht University Medical Centre, Netherlands
Grant Hunter, Intermountain Healthcare, United States
Copyright © 2018 Sittenfeld, Suh, Murphy, Yu and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel T. Chao, Y2hhb3NAY2NmLm9yZw==
 Sarah M. C. Sittenfeld
Sarah M. C. Sittenfeld John H. Suh
John H. Suh Erin S. Murphy
Erin S. Murphy Jennifer S. Yu
Jennifer S. Yu Samuel T. Chao
Samuel T. Chao