- 1Department of Obstetrics and Gynaecology, University Medical Centre Mannheim, Heidelberg University, Mannheim, Germany
- 2Department of Medical Statistics and Biomathematics, University Medical Centre Mannheim, Heidelberg University, Mannheim, Germany
- 3Private Oncology Center Fuxius/Karcher, Heidelberg, Germany
- 4Department of Gynaecology and Obstetrics, St. Hedwig-Klinik, Mannheim, Germany
Background: The purpose of this investigation was to explore patient perception regarding the importance of efficacy, toxicity, and logistics in the choice of regimen of taxane-based chemotherapy (CHT) for patients with metastatic breast cancer (MBC).
Methods: This dual-center study analyzed data of 100 women diagnosed with MBC, who were asked for their preferences regarding chemotherapy by means of conjoint analysis. Included attributes were progression free survival (PFS), application form, time and frequency, need of premedication, risk of alopecia, fatigue, febrile neutropenia, and neuropathy. Furthermore, participants completed a questionnaire about their personal and medical history. Regression analyses were performed to identify factors that influence patient preference in terms of specific treatment choice.
Results: Of 8 attributes, severe neutropenia was top priority for the majority of patients, followed by alopecia, neuropathy and PFS. When combining these patient preferences and the results of the questionnaire, patients' age as, well as, relationship status had significant impact on the importance of PFS. Moreover, longer travel time to the treatment center was significantly associated with preferences regarding PFS. Ranking by combination of respective part-worth values demonstrated nab-paclitaxel to be favored over paclitaxel and docetaxel.
Conclusion: Side effects of CHT and PFS prove to be critical factors for patients affecting choice of treatment in MBC with severe neutropenia being top priority, followed by alopecia, neuropathy, and PFS. Age, commute time, and relationship status were identified as significant determinants of patient preference. Total utility calculation by combination of part-worth values ranked nab-paclitaxel as the most preferable taxane.
Introduction
Both curative and palliative treatment of breast cancer nowadays are characterized by a multimodal therapeutic approach and have led to significant improvements in survival (1, 2). At the time of initial diagnosis almost 5% of women with breast cancer already have metastatic disease (3) necessitating a palliative regimen with chemotherapy (CHT) as the central element of treatment. Taxane-based CHT may improve time to progression and survival (4, 5), but associated side effects constitute an important consideration when evaluating the best treatment concept for a patient (6). With regard to the latter, aspects of patient comfort and preference are gaining more attention in oncologic treatment of metastatic breast cancer (MBC). Perhaps more so than in other malignancies, the concept of shared decision-making is now well-established in the treatment of patients with breast cancer.
Risk of recurrence and overall survival have been elucidated as key parameters that guide patient preferences in regard to different therapeutic approaches (7). However, a critical goal of treatment in the palliative setting is to optimize quality of life (QoL), as therapy of MBC can seriously impair QoL (8, 9). The study of Lindley et al. demonstrates that patients who had severe disruptions in QoL are less willing to receive additional treatment for an extension of life compared with patients who experienced only little disruption to normal life (10).
The purpose of this investigation was to further explore and quantify patient preferences in terms of palliative chemotherapy by using the tool of conjoint analysis comparing common taxanes (Docetaxel, Paclitaxel, and nab-Paclitaxel) administered for MBC. Moreover, we attempted to identify different subgroups of patients, whose therapeutic choices—as evaluated in our conjoint analysis—are associated with sociodemographic factors, lifestyle and general mindset assessed in a separate questionnaire.
Methods
Over 15 months a total of 111 patients with MBC were initially included in this dual-center study. These patients with an indication for a palliative treatment regimen were recruited at the University Medical Center Mannheim (UMM) and at the Oncology Center (OC), Outpatient Clinic Fuxius/Karcher in Heidelberg. All patients provided informed consent. The ethical approval for this study was obtained from the Ethics Committee II of Heidelberg University, Medical Faculty Mannheim (2014-536N-MA). Eleven patients had to be excluded because they did not understand the type of questioning and/or did not complete the conjoint analysis, so that full data sets were only available for 100 women.
Questionnaire on Socioeconomic/-Demographic Factors and General Mindset
Participants obtained a questionnaire about their personal, professional, and medical history, as well as, their general mindset. In particular, women were asked to provide details about age, age at first diagnosis of BC, past medical history, distance to treatment facility, relationship status, level of education and working situation. In detail, questions covered, if their health had changed in the past year, if they had children and if they had to take care of somebody else. Finally, they were questioned about any prior personal experience with chemotherapies and possible side effects and if they had previous experience with glucocorticoids.
Conjoint Analysis
The tool of conjoint analysis was implemented to assess different attributes that drive individual patient preferences in regard to taxane-based chemotherapies. Generally, the technique of conjoint analysis is widely used in the medical and non-medical field for assessment of preferences and has been demonstrated to offer a valuable tool to elicit patient preferences or utilities for specific treatments (11–14). In regard to medical treatment, conjoint analysis has proven to be useful for preference elicitation mainly in cancer therapy (11, 13, 15, 16). By having participants evaluate alternatives and letting them choose between different combinations of attributes, the relative importance of each attribute can be deducted (17).
Attributes relevant to respective taxane-regimens assessed in our study were time to progression, application form and time, application frequency, need of premedication, alopecia, fatigue, febrile neutropenia, and polyneuropathy. While the attribute of alopecia does not have relevance for the differentiation between currently used taxanes because they do not actually differ in their high risk of alopecia (18–20), the attribute was nevertheless included in this study to allow for transfer of our results to potential future substances (such as cabazitaxel) that might differ in this respect.
In a second step, levels for each attribute were selected based on current literature (21–24) (Table 1). Attributes were described in non-medical terminology to optimize comprehension. Respective scenarios were created with Sawtooth software according to the manufacturers guidelines and current literature (25). As implementation of 8 attributes, each with 3 or 4 levels, gives rise to 300 possible combinations, each patient was confronted with a total of 20 randomly assigned conjoint sets.
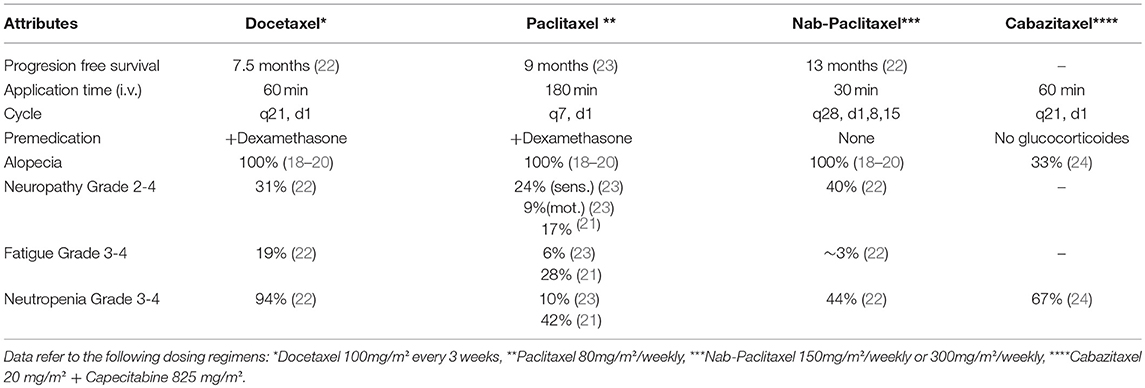
Table 1. Characteristics of taxanes in treatment of metastatic breast cancer (MBC) used for conjoint analysis.
In practice, after completing the questionnaire each patient sat with the investigator to be educated about the technique of conjoint analysis in general and the survey conduction at the notebook in particular. In this regard, they were provided with a couple of examples compatible to the ones used in the survey. At this point, all attributes and levels were explained in non-medical terminology. Specifically, each attribute itself was explained in detail, and (if applicable) grading, duration and severity of side effects were elaborated on. For the attribute of neuropathy, grade II-IV were presented as relevant side effects for patient's therapy. Patients were informed that grade III and IV would have impact on their activities of daily living and were likely to affect them for a longer period of time. However, moderate potential for rehabilitation was mentioned. Regarding the attribute of neutropenia, patients received information that chemotherapeutic agents generally confer leukopenia but that a relevant medical side effect would only arise from concurrent infection. Risk of infection was explained to be associated with severity of leukopenia. Patients were educated that only in moderate to severe infection in-hospital treatment with intravenous antibiotics and adjuvants would be required. Alopecia was described as hair loss that generally occurs shortly after the first course of CHT, while it usually resolves after completion of the last cycle of CHT.
Patients had to choose between 2 treatment options for each conjoint question (please refer to Supplemental Figure 1 for respective examples). Importantly, to reduce potential bias the conjoint survey did not include any drug names at all, but was only labeled with neutral terms (Option 1 and Option 2) from which patients chose their preferred alternative. Moreover, the investigator remained at the patient's side to answer any questions that would arise during conduction of the survey or help in case of technical difficulties. Sawtooth software was used again to analyse acquired data, yielding respective part-worth utilities and relative importance scores (RIS) for each attribute. These RIS for each attribute were then compared allowing for individual ranking of various attributes.
Finally, on the basis of the examined 8 attributes total utility of currently used taxanes was calculated by adding the respective part-worth utilities of each attribute, which most closely resembled those values published in recent literature for each respective taxane. This ranking allows for matching preferences of our study cohort with taxanes currently available for therapy of MBC.
Univariate Analyses of Determinants
Integrating both the conjoint analysis and the results from the personal questionnaire, we sought to identify socioeconomic and—demographic factors, as well as, general attitudes in our cohort that had influenced patient preference toward any of the chemotherapeutics agents. Specifically, mean RIS values for each attribute and subgroup of patients were compared.
Statistical assessment was performed using SPSS (Version 22; SPSS Inc., USA) in cooperation with the Department of Statistics and Bioinformatics of the Medical Faculty Mannheim, Heidelberg University. Data are presented as mean ± standard deviation, respectively. The RIS values for each attribute and subgroup of patients were analyzed using univariate significance testing (t-test and ANOVA where applicable). A p-value below 0.05 was considered statistically significant.
Results
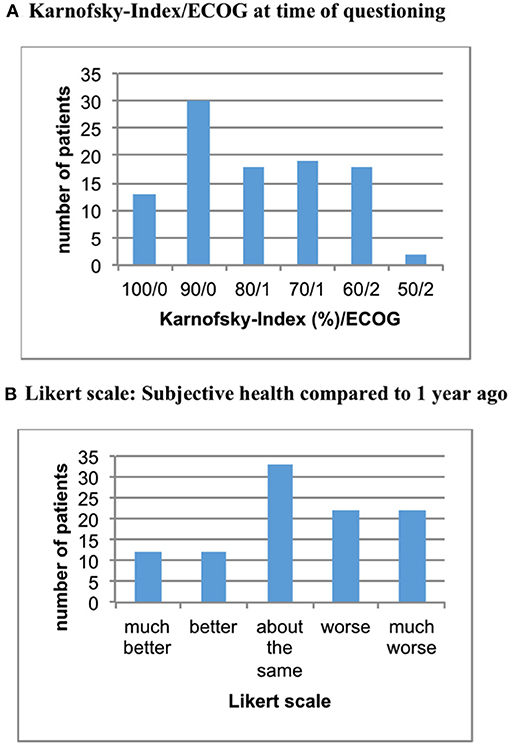
This dual-center study analyzed data of 100 patients. The age of women included ranged from 32 to 87 years (mean = 64 years). At time of initial diagnosis of breast cancer mean age was 55 years, with 29% of women having metastatic disease at this point. Mean duration of illness in our study cohort was 10.5 years. In terms of QoL, approximately 80% of women showed a Karnofsky-index of 70% or more (ECOG < 2) with the remaining women classified as Karnofsky 50–60% (= ECOG 2). About 4 in 10 patients reported progression of symptoms during the past year (see Figure 1).
Lifestyle, Mindset and Sociodemographic Factors
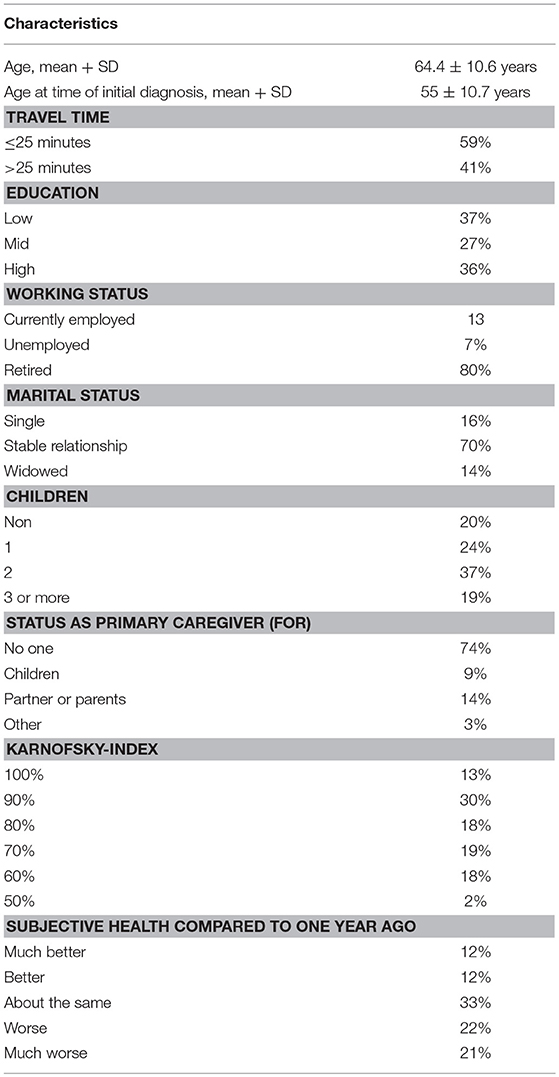
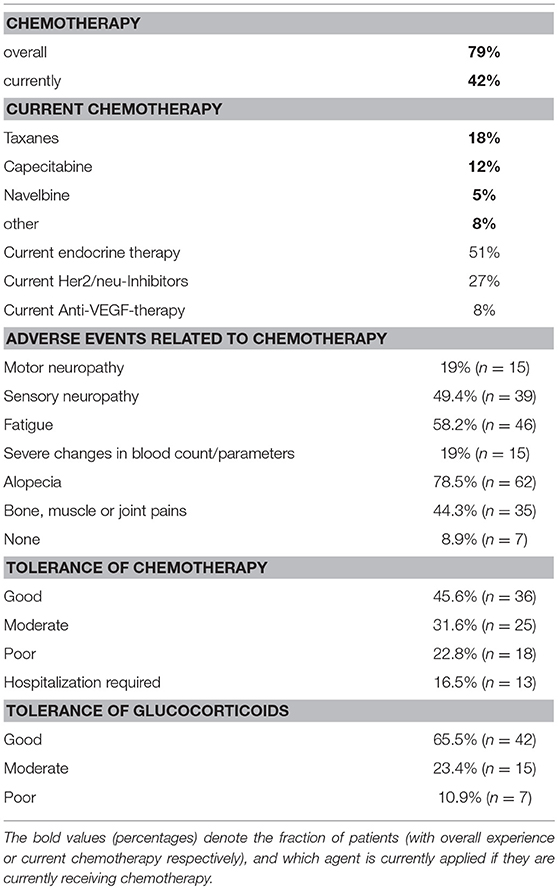
Detailed patient characteristics and sociodemographic factors, as well as, treatment experience are presented in Tables 2, 3.
In terms of marital status, the majority (70%) of women were in a stable relationship, 14% of patients were widowed and the rest were single/divorced (16%). With regard to family, 80% of patients had children. However, only 26% of the women had to take care of somebody else in the family at the time of questioning.
The majority of patients had low or medium education (64%), while only 36% of women had a higher level of education.
Most of the women (80%) were retired at the time of questioning with only 13% of women still working. Pertaining to the dual-center character of our study, 51% of patients received medical treatment at the UMM, whereas 49% were patients at the OC Fuxius/Karcher. In terms of travel time, 59% of women stated that it took < 25 min commuting to the institution, while 41% of patients had to travel more than 25 min.
Breast Cancer Experience
At the time of survey 42% of all patients were currently receiving CHT for MBC. Overall, 79% of the study population had already received CHT as part of their BC-treatment regimen in the past. The most common chemotherapeutics mentioned as part of the current chemotherapy were taxanes and capecitabine (Table 3). When asked about tolerance of previous treatment regimens, the majority (45.6%) had tolerated CHT well, whereas 22.8% of patients described poor tolerance. Hospitalization due to therapy occurred in 16.5% of cases. As part of their supportive therapy, 64% of women had taken oral glucocorticoids to reduce side effects, with 90% of these patients reporting moderate or good tolerance of this adjuvant.
Conjoint Analysis
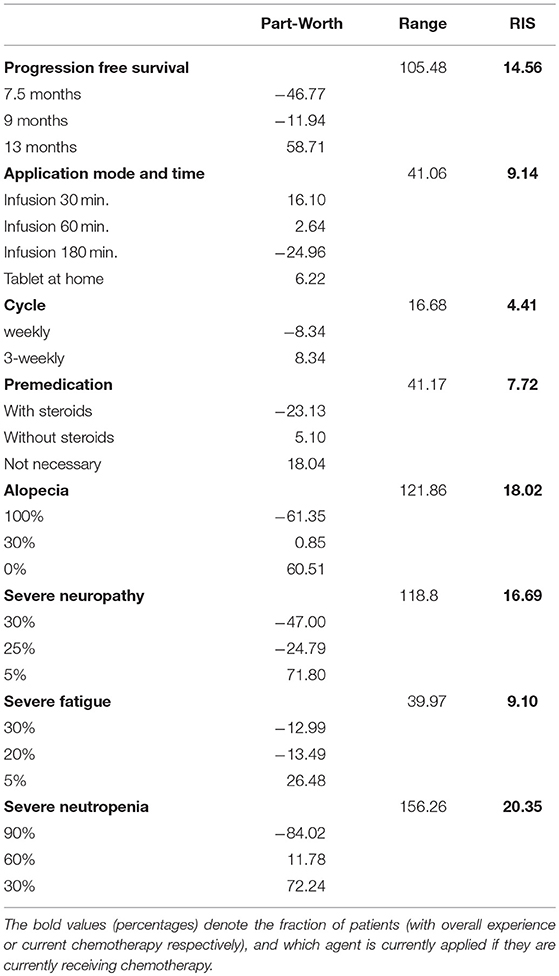
Using Sawtooth Software, preference weights (part-worth utilities) and relative importance scores (RIS) were calculated. On this basis, the attributes with the highest utility to patients were concerned with relevant side effects of therapy: avoidance of clinically significant neutropenia as top priority for the majority of patients (RIS = 20.35), followed by avoidance of alopecia (RIS = 18.02) and severe neuropathy (RIS = 16.79). Progression free survival was also crucial for patients, but only ranked fourth in this preference elicitation (RIS = 14.56). Severe fatigue (RIS = 9.10), application time (RIS = 9.14), necessity of premedication (RIS = 7.72) and number of application cycles (RIS = 4.41) represented considerably less relevant determinants of patient preference (Figure 2). A detailed list of RIS and respective part-worth values of the Conjoint Analysis are presented in Table 4.
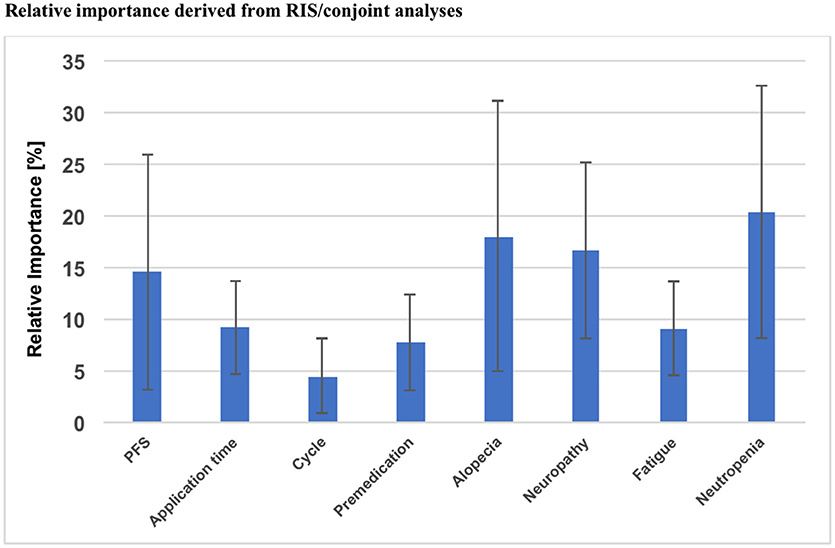
Figure 2. Overall results of the Conjoint Analysis: relative importance of each attribute, PFS, Progression free survival; RIS, Relative important score.
Association of Individual Patient Data and Treatment Preference
Another aim of this study was to determine whether these treatment preferences and RIS values were associated with any of the items assessed in the questionnaire on social and personal aspects of the patients' lives.
Using univariate regression, a significant association was observed for age and importance of PFS in that the RIS of PFS was lower when the patient was older (ß = −0.348, p = 0.001). On the other hand, age did not have a significant influence on decisions regarding severe side effects of chemotherapy, when tested in a univariate regression model (Figure 3).
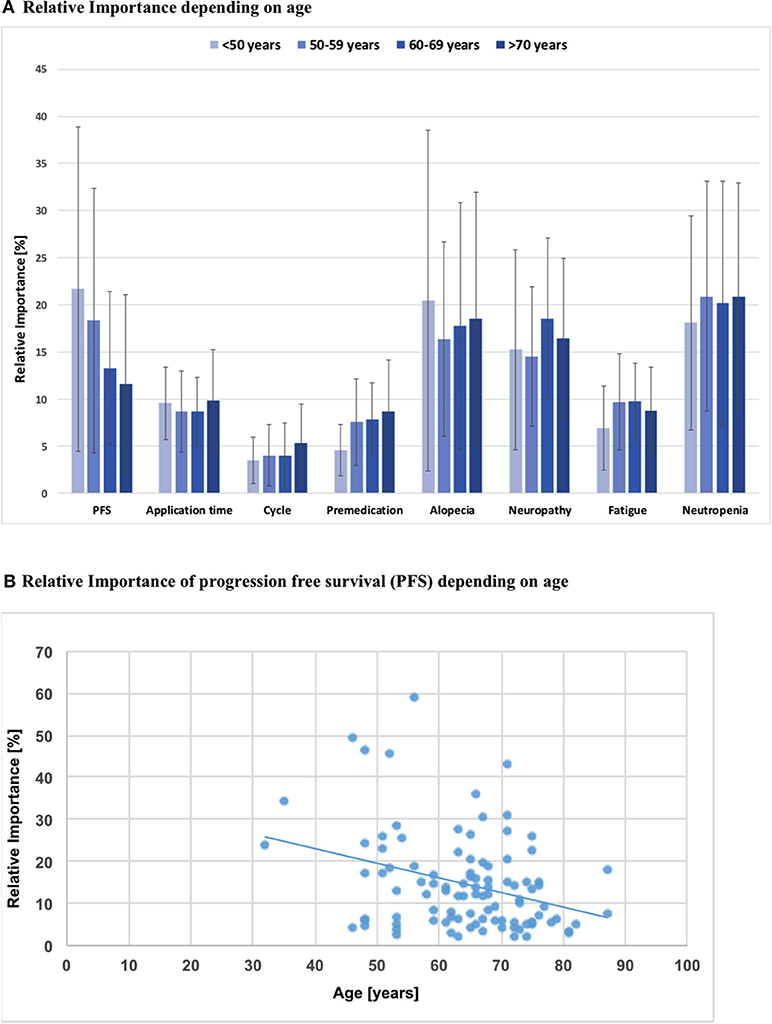
Figure 3. (A) Relative importance of each attribute depending on patients'age; PFS, Progression free survival; Intra-attribute RIS comparison does not show significant differences when stratified by age groups (p > 0.05, all n.s.). (B) Simple analysis of regression: Relative importance of PFS depending on patients' age; b = −0.35; p = 0.001.
Moreover, a significant association between travel time to treatment facility and PFS, application time and alopecia was observed (Figure 4A): Women with a commute time of more than 25 min showed a higher RIS for PFS than patients with less travel time (RIS 17.5 vs. 12.5; p < 0.01). For these patients with a longer distance to their respective treatment facility, time for application was not so important as for the other subgroup (RIS 7.8 vs. 10.1; p < 0.05). In addition, women with a longer travel time rather accepted alopecia than patients with a shorter distance to the treatment facility (RIS 14.7 vs. 20.3; p < 0.05).
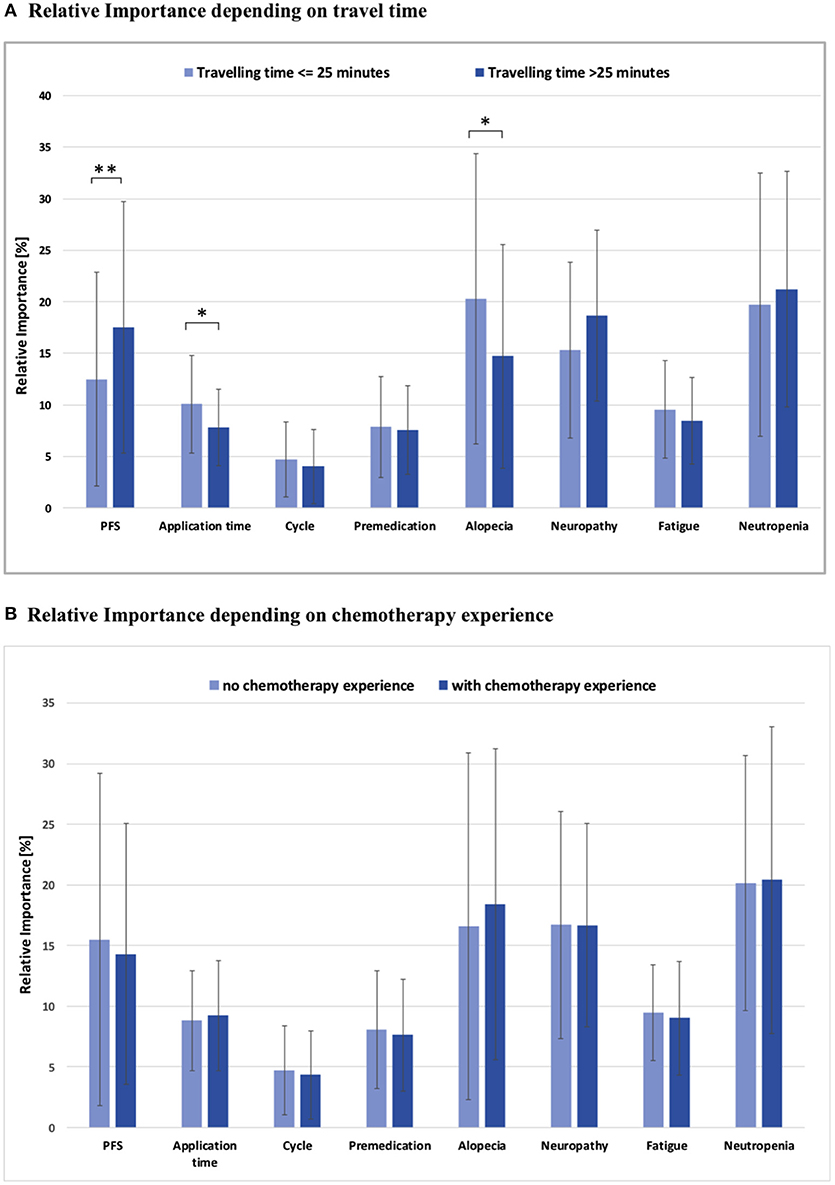
Figure 4. (A) Relative importance of each attribute depending on travel time to the medical center, *p < 0.05, **p < 0.01; all other comparisons not significant (p > 0.05). (B) Relative importance of each attribute depending on chemotherapy experience; PFS, Progression free survival; all comparisons not significant (p > 0.05).
Analysis of relationship status yielded a significant difference in terms of PFS, in that women who lived with a partner had a significantly higher RIS for PFS (RIS 16.1 vs. 10.9; p < 0.05).
Patient preference was remarkably consistent and did not vary significantly when stratified by previous treatment experience, as no substantial differences in the RIS of toxicities and CHT aspects were observed (Figure 4B).
In subgroup analyses no collective showed a higher RIS for alopecia than the group of patients who did not have prior experience with alopecia (RIS = 22.7). Comparing the RIS of patients with or without CHT in the past, there were no significant differences.
Regarding mode of application of CHT, women demonstrated preference for a short infusion at the treatment facility as compared to longer infusion times or regular domestic oral medication (Figure 4A). Moreover, they preferred longer cycles without premedication.
Comparison of Different Taxanes
As the synopsis of this study, the data collected on patient preferences was used to rank the three most widely used taxanes (Docetaxel, Paclitaxel and nab-Paclitaxel). This calculation of total utility for each taxane on the basis of the investigated 8 attributes demonstrated nab-Paclitaxel as top preference with the highest total utility (14.4), followed by Paclitaxel (−95.3). Docetaxel had a (much) lower preference with a total utility of −264.8.
Discussion
Breast cancer treatment has seen an impressive evolution in the past decades as therapeutic regimens have been augmented by addition and combination of new drugs, while at the same time the concepts of patient preference, shared decision-making and individualized therapy have steadily gained attention. From an economic perspective patient-centered approaches might also confer significant benefits, as estimations attribute about half of healthcare costs to decisions primarily driven by doctors and hospital supply rather than patient need and demand (26, 27). Therefore, focusing on patient preferences and elucidating reasons for individual choices in regard to certain therapies may strengthen economical but still effective approaches. Conjoint analysis and other questionnaires have been successfully used to determine these patient preferences in (breast) cancer therapy (6, 9). Importantly, implementation of conjoint analysis offers means to individualize a therapeutic strategy in order to carefully weigh risks and benefits on an individual level rather than extrapolate with population level data from clinical trials. Using conjoint analysis techniques may help in identification of trends in general patient preferences over time and detection of relevant shifts in culture. Interestingly, among other recent studies an excellent study by Ballinger et al. found patients to tend to favor toxicity concerns over treatment benefit (11, 13), while older studies often found survival benefit to be of supreme importance (16, 28–30). Clinical trials on population level generally focus on survival benefit as the primary endpoint and most critical aspect of therapy.
The results of our study show that patient preference in terms of favoring a chemotherapeutic regimen was mainly driven by avoidance of adverse effects: neutropenia as top priority (RIS 20.4), followed by alopecia (RIS 18.0) and neuropathy (RIS 16.8). Progression free survival—also considered crucial by patients—only ranked fourth in this preference elicitation (RIS 14.6). This is in congruence with data from previous studies (28–30), which could also show that the most influential factors in driving patient preferences for treatment were improved survival, risk of neutropenia and other side effects (28–30). Another important study by Smith et al. was published in 2014 and demonstrated treatment benefits to outweigh toxicity aspects (16). In contrast to our study, these above mentioned studies demonstrated that even a small incremental survival advantage had the highest relative importance seconded by side effects. This difference could be explained by the fact that the mean age of our study cohort was about 10 years higher as compared to the other studies. Furthermore, these younger patients often had a role as primary caregiver for children younger than 18 years of age (>35% in Smith's cohort vs. 9% in our cohort). This could possibly serve as an explanation, why (survival) benefits from treatment were shown to be critical in other studies (16). Accordingly, we could show that age was a significant determinant of treatment preferences in that the RIS of PFS was lower when patients were older (ß = −0.348, p = 0.001). Therefore, a decrease in age by 10 years led to a rise of the RIS of PFS by 3.5%. In addition, our study collective of MBC patients only will at least in part account for observed differences, as our study cohort of palliative patients differs from Beusterien's and Kuchuk's studies which included patients with all tumor stages (28, 30). As expected, women with an adjuvant therapy showed a higher RIS for PFS and accepted more side effects in these trials (28, 30).
A significant association between commute time to treatment facility and PFS, application time, as well as, alopecia was observed (Figure 4A): Women with a commute time of more than 25 min showed a higher RIS for PFS, while duration of each CHT session was not so important but significant. Furthermore, these patients rather accepted alopecia. It is tempting to speculate that these patients with greater distance to the treatment facility accepted more side effects and attached less importance to the application time because they consciously decided to go to a specialized center to optimize their treatment with focus on longer overall survival (31).
Patients living in a stable relationship also had a higher RIS for PFS. This might implicate that these patients weigh the risks and benefits differently, in that they appear to accept more side effects as the price for being able to spend as much time as possible with their partners. Other groups also found, that they would accept more side effects for longer overall survival (32–34).
Patient preference was remarkably consistent independent of prior experiences and did not vary significantly when stratified by previous treatment experience, as no substantial differences in the RIS of toxicities and CHT aspects were observed (Figure 4B). This is in congruence with data from previous studies, which could also show that determinants driving patient preference for treatment were generally independent of previous experience with CHT (28, 29). The only aspect that showed a trend toward differential preferences and ranking of potential side effects pertains to alopecia, which represented the overall second top priority for the majority of patients (RIS 18.0) in our study. The comparison of RIS values of patients with or without CHT in the past demonstrated that patients with prior CHT-based hair loss exhibit a lower RIS for this particular side effect. No subgroup showed a higher RIS for alopecia than the one without any prior experience of hair loss (RIS 22.7). A review of the literature shows markedly heterogenous reports concerning patient preference and alopecia. A study of DiBonaventura (29) demonstrated a distinct preference for treatment effectiveness and avoidance of alopecia. In contrast, investigations of Beusterien and Kuchuk (28, 30) suggested that alopecia does not have a significant impact. Another study by Al Batran and colleagues (35) also concluded that alopecia had no significant influence on QoL, but it deserves mentioning that most patients in this trial (73%) were men.
In unison, authors agree that the importance patients attribute to alopecia remains a very personal and still mostly unpredictable factor that should be addressed distinctly and individually when planning chemotherapy regimens (36). Furthermore, side effects that medical personnel might consider less relevant seem to have high priority for patients (37) and the question remains to which degree medical personnel should guide or sway these choices based on their expertise and experience. This may especially be true for putting alopecia into perspective, as patients that had never experienced hair loss before attribute a higher RIS to alopecia as compared to patients with a history of alopecia.
The literature offers a heterogeneous picture in terms of perceived severity of side effects of CHT and thus attributed RIS/ranks. In our study “severe fatigue” was considered the least relevant potential side effect for choosing a specific treatment, while Beusterien and DiBonaventura (28, 29) show that fatigue was more important than neutropenia to their study population. Consistent with our study, in the trial of Lloyd and colleagues neutropenia was also top priority for the majority of patients when asked about CHT and QoL.
At first glance, these observed differences in ranking the importance of side effects of chemotherapy seem surprising. However, they probably only reflect differences in study conduction, survey technique and patient education, as Lalla could adeptly show for MBC therapy in the past. In essence, Lalla et al. (6) investigated avoidance of side effects by primarily employing the “willingness-to-pay” technique. In principle, they could show that they acquired different results depending on the type of survey (6).
Another reason for differences in RIS/ranking could be derived from the level-effect: This effect can be developed if various attributes do not have the same number of values. Generally, the level effect will cause an attribute to show a higher RIS if the values are divided into more levels (38).
Consistent with previous studies, logistic aspects of therapy such as application time (RIS 9.1), necessity of premedication (RIS 7.7) and number of application cycles (RIS 4.4) had only minor influence on patient preferences and are much less controversially discussed (16, 28, 29).
As the synopsis of this study, total utility of each of the three most relevant taxane chemotherapeutics currently used (Docetaxel, Paclitaxel and nab-Paclitaxel) was calculated by combining RIS values of the 8 attributes examined in this study. This ranking of therapeutics by combined RIS values ranked nab-Paclitaxel top of the list with the highest total utility (14.4), followed by Paclitaxel (−95.3) and Docetaxel (−264.8),
Total utility calculation by addition of part-worth values must be interpreted very carefully due to the known limitations from this approach: only 8 attributes were examined in our study and these do not comprehensively characterize each chemotherapeutic agent. Furthermore, absolute differences in total utility do not reflect the dimension/degree by which one agent is considered superior over the other. Total utility calculation only allows for general ranking of substances. Moreover, this preference elicitation may only be used as guidance in a population that closely resembles the one from our study.
However, conjoint analyses and calculation of single or combined part-worth values offer a potential means to optimize the mosaic of the shared decision-making process by more closely monitoring and assessing patient preferences for given therapeutic interventions. Conjoint analysis also helps to weigh risks and benefits on an individualized level as compared to clinical trials that only yield population level data.
Study Limitations
Our study was not without limitations. First, the levels for the attributes were selected based on current literature and may not be reflective of individual experience. In addition, the participants of our study did not represent the full range of breast cancer patients (only MBC patients were included). Therefore, our preference elicitation must be interpreted with caution when applied to the general population of BC patients. Furthermore, our patient collective was rather old (mean age 64.4) as compared to other studies.
Moreover, patient information and explanation of attributes were not conducted according to a written standardized protocol. This might potentially cause a bias due to implicit connotations of the treating physician's explanations. Survey introduction, explanation of attributes and—maybe most importantly—the resulting discussion with the physician present at the time of survey may significantly impact a patient's preferences and the resulting effect cannot be dissected in this study. Future studies should therefore address this aspect carefully by optimisation of neutrality. These future studies should also systematically investigate this bias, as discussion of treatment options, risks and benefits with treating physicians itself is a cornerstone of shared decision-making.
Conclusion
Our study on patient preferences regarding the most common taxanes (Docetaxel, Paclitaxel, and nab-Paclitaxel) validates that side effects of CHT and PFS are critical factors affecting choice of treatment in most MBC patients. For the majority, avoidance of neutropenia was the top priority, followed by alopecia, neuropathy, and PFS. However, treatment preferences significantly depended on age, as the RIS of PFS was highest among younger patients, causing it to be the top priority for choice of CHT agents in the group aged 50 or younger.
Apart from age as a determinant, our data show, that women in a stable relationship exhibited a higher RIS for PFS. Patients with a commute time of more than 25 min showed a higher RIS for PFS, while rendering application time and alopecia less important.
Total utility calculation by combination of part-worth values ranked nab-Paclitaxel as the most preferable CHT agent, followed by Paclitaxel and Docetaxel.
In essence, our data stress the notion that a critical goal of treatment in the palliative setting is to optimize quality of life (8, 9). Here, conjoint analysis may prove a very useful tool to individualize the therapeutic strategy by carefully weighing risks and benefits on an individual level rather than extrapolating with population level data from clinical trials.
Author Contributions
JK, SS, MS, and AG: Conception and design. MS and AG: Administrative support. JK, SS, AG, SF, and MS: Provision of study materials or patients. JK and SF: Collection and assembly of data. JK, SS, AG, SH, and MS: Data analysis and interpretation. SS, JK, and MS: Manuscript writing. SS, JK, SH, SF, AG, and MS: Final approval of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00535/full#supplementary-material
Abbreviations
BC, breast cancer; BMI, body mass index; CHT, chemotherapy; MBC, metastatic breast cancer; RIS, relative importance score; PFS, progression free survival; QoL, quality of life.
References
1. Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. (2007) 110:973–9. doi: 10.1002/cncr.22867
2. Pal SK, Dehaven M, Nelson RA, Onami S, Hsu J, Waliany S, et al. Impact of modern chemotherapy on the survival of women presenting with de novo metastatic breast cancer. BMC Cancer (2012) 12:435. doi: 10.1186/1471-2407-12-435
3. Howlader N, Noone AM, Yu M, Cronin KA. Use of imputed population-based cancer registry data as a method of accounting for missing information: application to estrogen receptor status for breast cancer. Am J Epidemiol. (2012) 176:347–56. doi: 10.1093/aje/kwr512
4. Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, Group EGW. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2012) 23 (Suppl. 7):vii11–9. doi: 10.1093/annonc/mds232
5. Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: the epothilones. Oncologist. (2007) 12:271–80. doi: 10.1634/theoncologist.12-3-271
6. Lalla D, Carlton R, Santos E, Bramley T, D'Souza A. Willingness to pay to avoid metastatic breast cancer treatment side effects: results from a conjoint analysis. SpringerPlus (2014) 3:350. doi: 10.1186/2193-1801-3-350
7. Alvarado MD, Conolly J, Park C, Sakata T, Mohan AJ, Harrison BL, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res.Treat. (2014) 143:135–40. doi: 10.1007/s10549-013-2782-9
8. Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. NE J Med. (2001) 344:1997–2008. doi: 10.1056/NEJM200106283442607
9. Beusterien K, Grinspan J, Kuchuk I, Mazzarello S, Dent S, Gertler S, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist. (2014) 19:127–34. doi: 10.1634/theoncologist.2013-0359
10. Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. (1998) 16:1380–7. doi: 10.1200/JCO.1998.16.4.1380
11. Ballinger TJ, Kassem N, Shen F, Jiang G, Smith ML, Railey E, et al. Discerning the clinical relevance of biomarkers in early stage breast cancer. Breast Cancer Res. Treat. (2017) 164:89–97. doi: 10.1007/s10549-017-4238-0
12. Green PES, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Mark. (1990) 54:3–19. doi: 10.2307/1251756
13. Hofheinz R, Clouth J, Borchardt-Wagner J, Wagner U, Weidling E, Jen MH, et al. Patient preferences for palliative treatment of locally advanced or metastatic gastric cancer and adenocarcinoma of the gastroesophageal junction: a choice-based conjoint analysis study from Germany. BMC Cancer. (2016) 16:937. doi: 10.1186/s12885-016-2975-9
14. Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ (2000) 320:1530–3. doi: 10.1136/bmj.320.7248.1530
15. Johnson DC, Mueller DE, Deal AM, Dunn MW, Smith AB, Woods ME, et al. Integrating patient preference into treatment decisions for men with prostate cancer at the point of care. J Urol. (2016) 196:1640–4. doi: 10.1016/j.juro.2016.06.082
16. Smith ML, White CB, Railey E, Sledge GWJr. Examining and predicting drug preferences of patients with metastatic breast cancer: using conjoint analysis to examine attributes of paclitaxel and capecitabine. Breast Cancer Res Treat. (2014) 145:83–9. doi: 10.1007/s10549-014-2909-7
17. Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. (2002) 37:1681–705. doi: 10.1111/1475-6773.01115
19. Trueb RM. Chemotherapy-induced alopecia. Semi Cutaneous Med Surg. (2009) 28:11–4. doi: 10.1016/j.sder.2008.12.001
20. Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, et al. Dermatological adverse events with taxane chemotherapy. Eur J Dermatol. (2016) 26:427–43. doi: 10.1684/ejd.2016.2833
21. Aigner J, Marme F, Smetanay K, Schuetz F, Jaeger D, Schneeweiss A. Nab-paclitaxel monotherapy as a treatment of patients with metastatic breast cancer in routine clinical practice. Anticancer Res. (2013) 33:3407–13.
22. Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. (2009) 27:3611–9. doi: 10.1200/JCO.2008.18.5397
23. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. (2008) 26:1642–9. doi: 10.1200/JCO.2007.11.6699
24. Villanueva C, Awada A, Campone M, Machiels JP, Besse T, Magherini E, et al. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: a phase I/II study. Eur J Cancer. (2011) 47:1037–45. doi: 10.1016/j.ejca.2011.01.001
25. Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. (2011) 14:403–13. doi: 10.1016/j.jval.2010.11.013
26. Herzlinger R. A bold new consumer-driven health care system. The laws and their legislators. Managed Care (2007) 16:34–6.
27. Herzlinger RE, Falit BP. Consumer-driven health care. J Am Med Assoc. (2009) 301:2093–4. doi: 10.1001/jama.2009.699
28. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Women's Health (2012) 4:279–87. doi: 10.2147/IJWH.S31331
29. daCosta DiBonaventura M, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits (2014) 7:386–96.
30. Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. (2013) 142:101–7. doi: 10.1007/s10549-013-2727-3
31. Vetterlein MW, Loppenberg B, Karabon P, Dalela D, Jindal T, Sood A, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer (2017) 123:3241–52. doi: 10.1002/cncr.30744
32. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
33. Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. (2015) 6:7339–47. doi: 10.18632/oncotarget.3129
34. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. (2005) 93:41–7. doi: 10.1007/s10549-005-3702-4
35. Al-Batran SE, Hozaeel W, Tauchert FK, Hofheinz RD, Hinke A, Windemuth-Kieselbach C, et al. The impact of docetaxel-related toxicities on health-related quality of life in patients with metastatic cancer (QoliTax). Ann Oncol. (2015) 26:1244–8. doi: 10.1093/annonc/mdv129
36. Dua P, Heiland MF, Kracen AC, Deshields TL. Cancer-related hair loss: a selective review of the alopecia research literature. Psychooncology (2017) 26:438–43. doi: 10.1002/pon.4039
37. Blinman P, Hughes B, Crombie C, Christmas T, Hudson M, Veillard AS, et al. Patients' and doctors' preferences for adjuvant chemotherapy in resected non-small-cell lung cancer: what makes it worthwhile? Eur J Cancer (2015) 51:1529–37. doi: 10.1016/j.ejca.2015.05.022
Keywords: metastatic breast cancer, patient preference, shared decision-making, chemotherapy, conjoint analysis, taxane
Citation: Spaich S, Kinder J, Hetjens S, Fuxius S, Gerhardt A and Sütterlin M (2018) Patient Preferences Regarding Chemotherapy in Metastatic Breast Cancer—A Conjoint Analysis for Common Taxanes. Front. Oncol. 8:535. doi: 10.3389/fonc.2018.00535
Received: 03 August 2018; Accepted: 31 October 2018;
Published: 21 November 2018.
Edited by:
José Bines, Instituto Nacional de Câncer (INCA), BrazilReviewed by:
Tarah Ballinger, Indiana University, Purdue University Indianapolis, United StatesConnie Irene Diakos, Kolling Institute, Australia
Copyright © 2018 Spaich, Kinder, Hetjens, Fuxius, Gerhardt and Sütterlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saskia Spaich, c2Fza2lhLnNwYWljaEB1bW0uZGU=
†These authors have contributed equally to this work
 Saskia Spaich
Saskia Spaich Johanna Kinder
Johanna Kinder Svetlana Hetjens2
Svetlana Hetjens2