- 1Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Stomatology, Sun Yat-sen University, Guangzhou, China
- 3Division of Rheumatology, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, United States
Head and neck cancer is the 6th most common malignancy worldwide and urgently requires novel therapy methods to change the situation of low 5-years survival rate and poor prognosis. Targeted therapy provides more precision, higher efficiency while lower adverse effects than traditional treatments like surgery, radiotherapy, and chemotherapy. Blockade of PD-1 pathway with antibodies against PD-1 or PD-L1 is such a typical targeted therapy which reconstitutes anti-tumor activity of T cell in treatments of cancers, especially those highly expressing PD-L1, including head and neck cancers. There are many clinical trials all over the world and FDA has approved anti-PD-1/PD-L1 drugs for head and neck cancers. However, with the time going, the dark side of this therapy has emerged, including some serious side effects and drug resistance. Novel materials like nanoparticles and combination therapy have been developed to improve the efficacy. At the same time, standards for evaluation of activity and safety are to be established for this new therapy. Here we provide a systematic review with comprehensive depth on the application of anti-PD1/PD-L1 antibodies in head and neck cancer treatment: mechanism, drugs, clinical studies, influencing factors, adverse effects and managements, and the potential future developments.
Introduction of Head and Neck Cancers
Head and neck cancers are composed of various kinds of epithelial malignant tumors, including oral cancers, maxillofacial cancers, larynx cancers, and many others, almost all of which are head and neck squamous cell carcinoma (HNSCC). Although, there are other pathological types such as verrucous carcinoma, basaloid squamous cell carcinoma, papillary squamous cell carcinoma, they only make up a small percentage (1). HNSCC is the 6th most common malignancy worldwide, with number of 650,000 new cases a year and 350,000 deaths (2). Around 2/3 of patients present with advanced disease, often with regional lymph node involvement, while 10% present with distant metastases (3). According to epidemiological survey, the 5-years survival rate of HNSCC in all stages was about 60%, and the survival rate was even worse for specific primary sites such as hypopharynx. The main causes of head and neck cancers are tobacco and alcohol consumption (1, 4–8). Chewing betel quid is also well-recognized as a risk factor for the cancer of oral cavity (9). And human papillomavirus (HPV) and p53 mutation are related to certain subsets of head and neck cancers (10–12). About 25% of HNSCC contain HPV genomic DNA (13). However, HPV positivity is a favorable prognostic factor in HNSCC (14). Patients with HPV+ HNSCC show better responsiveness to radiation, chemotherapy, or both, and might be more susceptive to immunosurveillance of tumor-specific antigens (14).
Common Treatment Strategies for Head and Neck Cancers
The location of the cancers makes it necessary to take the spiritual and plastic factors into consideration. Primary tumor site, stage, and resectability are also treatment concerns as well as the patient factors such as swallowing, airway, organ preservation, and comorbid illnesses. For plan making, doctors are needed and organized from different departments which include head and neck surgeons, plastic surgeons, medical oncologists, radiation oncologists, radiologists, and dentists (2).
Common treatment strategies for head and neck cancers include surgery, radiotherapy, and chemotherapy. At present, surgery is still the standard therapy for HNSCC. However, surgical operations are limited, owing to the complexity of structures and the need for organ preservation. Most surgeons agree that the carotid artery, the base of the skull, and the invasion of the pre-vertebral muscle tissue are unresectable (2). Moreover, when the tumor is too extensive or there are multiple distant metastases, patients are generally not suitable for surgical treatment. Radiotherapy alone can improve the cure rate of early glottis, tongue, and tonsil cancers (15). However, prolonged interruption of radiotherapy or delayed post-operative radiotherapy may impair the patient's prognosis, which may be due to the proliferation of cancer cells (16). Delivery of radiation remains to be improved with continuous technological progress, and customization of radiation dose and volume (17). Chemotherapy is the core component of local advanced HNSCC treatment (18). Platinum compounds Cisplatin is a standard reagent for combination with radiotherapy or other drugs. Huperzine compounds are active and have been tested in locally advanced HNSCC chemotherapy (19, 20). Concurrent chemotherapy with normo-fractionated radiotherapy (2 Gy/day, 5 days/week, for 5–7 weeks) is used most in current practice (21).
Traditional therapy can result in serious complications, from pain to malnutrition, risk of infection, and psychological distress (21). In order to ameliorate these drawbacks, comprehensive treatments are currently preferred for the advanced tumors. Comprehensive treatments must be well-designed and planned according to the patient's general condition and the stage of tumor development. At present, the treatment of oral and maxillofacial malignant tumors emphasizes the comprehensive treatment based on surgery, especially the triple therapy, which combines surgery with radiotherapy and chemotherapy.
Modern research has been keen on identifying specific molecular targets involved in the occurrence and progression of head and neck cancers. EGFR and VEGF are two main targets which are overexpressed in majority of both precancerous oral lesions and HNSCC (22–24). EGFR can bind to and be activated by different ligands, including the epidermal growth factor (EGF) and transforming growth factor-α (TGF-α) (25). EGFR activation initiates subsequent signaling pathways, eventually resulting in tumor cell resistance to apoptosis and promoting angiogenesis, tumor cell migration, and tumor cell proliferation (Figure 1) (25, 26). Current EGFR-targeted therapies include monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs). Antibodies target the extracellular domain of EGFR while TKIs hinder downstream signaling pathways by binding to the cytoplasmic region of EGFR (27). To date, Cetuximab remains the only FDA-approved EGFR-targeted mAb for the treatment of recurrent/metastatic (R/M) HNSCC. Cetuximab in combination with radiotherapy is a standard treatment option for locally or regionally advanced HNSCC (28). VEGF, is a key regulator of physiological angiogenesis during embryogenesis, skeletal growth, and reproductive functions (29). The biological effects of VEGF, mediated by two receptor tyrosine kinases (RTKs), VEGFR-1 and VEGFR-2, cause receptor TK activation and downstream signaling to stimulate endothelial cell proliferation, vessel permeability, and migration (27). Bevacizumab, a humanized monoclonal antibody targeting VEGF-A, was approved by the FDA for treatment of advanced cancer types. Bevacizumab could increase the sensitivity of HNSCC to radiotherapy in preclinical trials. Bevacizumab was evaluated in phase I and II clinical trials in combination with Erlotinib, an EGFR inhibitor, in patients with R/M HNSCC (30, 31) and the combined treatments increased the complete response rate by ~15% and median survival by 7.1 months (30). The phase II trial on the combination of Bevacizumab with chemotherapy, radiotherapy or EGFR inhibitors are ongoing.
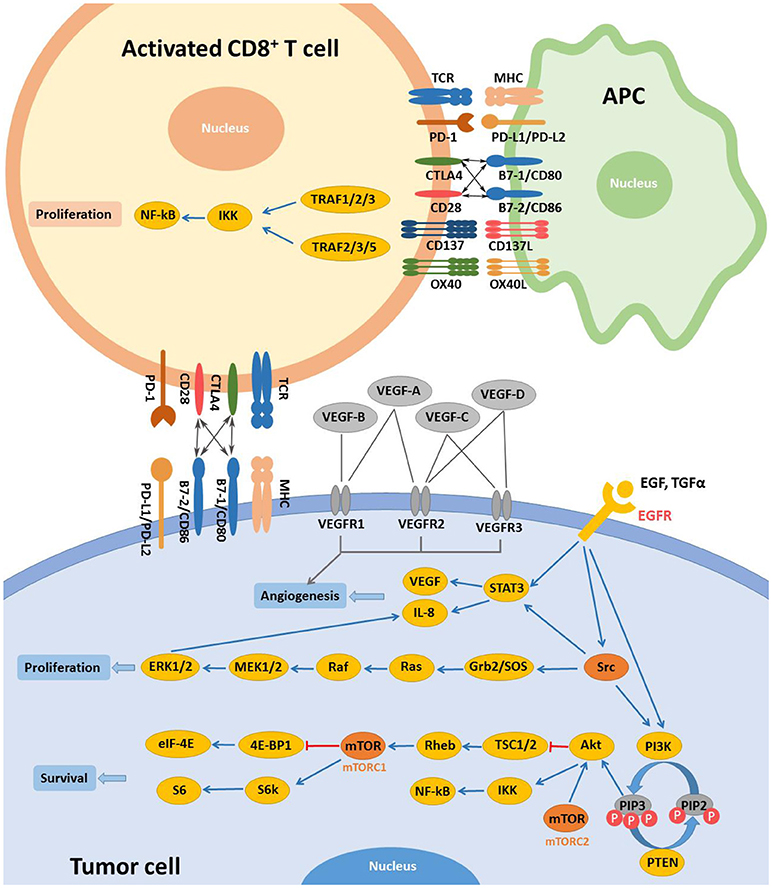
Figure 1. Main targets and related signaling pathways involved in the targeted therapy for R/M HNSCC. Activation of EGFR by extracellular ligands initiates activation of Src, STAT3, and PI3K. Activated Src promotes cell proliferation mainly via RAS/RAF/MAPK pathway. In the PI3K/Akt pathway, phosphorylation of PIP2 is mediated by PI3K while dephosphorylation of PIP3 is controlled by PTEN. Akt could be activated independently by mTORC2 activation. Activation of Akt and mTORC1 inhibit TSC1/2/Rheb and 4E-BP1/eIF-4E downstream signaling, respectively while IKK/NF-kB and S6/S6k pathways are initiated, promoting tumor cell survival. Once activated, other targets, including VEGFR and c-MET, expressed on tumor cells share similar downstream signaling with EGFR. CD137L and OX40L activate CD137 and OX40, respectively. And proliferation of activated T cells is achieved via TRAF/IKK/NF-κB downstream signaling. CTLA-4 and its ligands are also demonstrated. Some pathways were simplified for clearer demonstration.
Immunological Targeted Therapy
Immunotherapies stimulate host antitumor immune system and can elicit endurable responses in subsets of patients across different types of tumors (Figure 1) (32). Immune checkpoints, like cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death-1 (PD-1), work as inhibitory pathways, playing an important role in self-tolerance under healthy conditions. Checkpoint inhibitors are part of immunotherapies that enhance antitumor T cell activity by hindering initiation of suppressive signaling pathways of activated T cells. The 2018 Nobel Prize in Physiology or Medicine was recently given to James P. Allison and Tasuku Honjo for their discovery and contribution in cancer immunotherapy correlated with CTLA-4 and PD-1. Other targets such as CD137 and OX40, unlike CTLA-4 and PD-1, work as immune activators and are as well under active investigation for cancer therapy (Table 1) (37, 38).
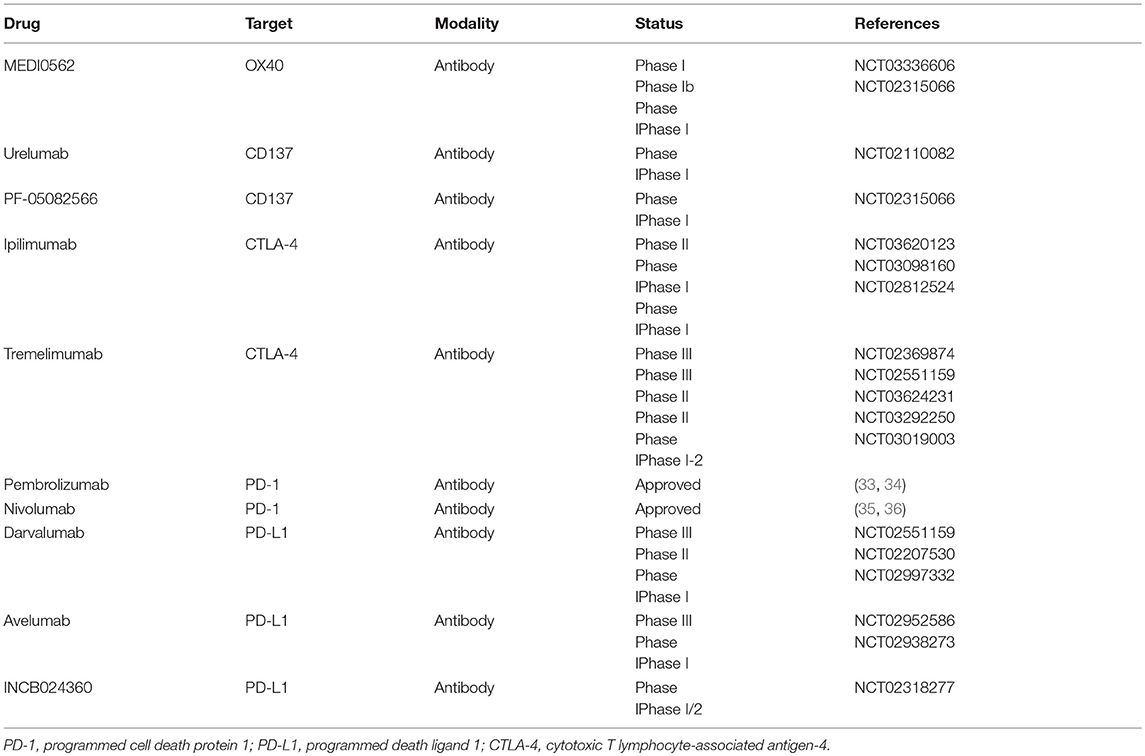
Table 1. Immunological targeted therapies approved or under investigation for the treatment of head and neck cancers.
CTLA-4
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4; also known as CD152) is the first clinically targeted immune checkpoint receptor. CTLA-4, expressed on activated CD8+ effector T cells, mainly regulates the early stage of T cell activation, enhances the activity of effector CD4+ T cell, and inhibits Treg cell-dependent immunosuppression (39, 40). CD28 and CTLA-4 have the same ligands B7-1 (also known as CD80) and B7-2 (also known as CD86); and CTLA-4, compared to CD28, has a much higher affinity for B7-1 (41). CTLA-4 has been proved to be a negative regulator of T cell activation in an effort to prevent autoimmunity, antagonizing the CD28-B7 co-stimulatory signals. Research showed that the blockade of CTLA-4 results in enhanced antitumor immunity (42). Clinical studies using anti-CTLA-4 antibodies demonstrated activity in melanoma. Ipilimumab, an anti-CTLA-4 antibody, was the first targeted immunotherapy to prove a survival advantage for patients with metastatic melanoma. Hence, it was approved by FDA for the treatment of advanced melanoma in 2010 (43). In HNSCC, Yu et al. showed that CTLA4 was upregulated in the tumor-infiltrating lymphocyte (TIL) of HNSCC and the high CD8+/CTLA4 ratio was associated with improved prognosis (44). Further, Jie et al. found that intratumoral Tregs, compared to circulating Tregs, induced higher expression of CTLA-4 in HNSCC (45). Currently, clinical trials of Ipilimumab (NCT02551159, NCT03212469), alone or in combination with other treatments, for HNSCC are in progress (40).
CD137
CD137, a member of TNF receptor superfamily, is widely induced on activated CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and DC. The engagement of CD137 could promote the proliferation of T cells. The introduction of Urelumab, the fully human CD137-agonist mAb, has enabled modulation of CD137 function in immune-oncology, including application in combination with tumor targeting mAb (46). Srivastava et al. (38) confirmed that Cetuximab combined with CD137 agonist was effective in the treatment of HNC. CD137 has provided a new mechanism for the enhancement of Cetuximab (38).
OX40
OX40 is a member of the TNF receptor family and mediates an effective co-stimulation pathway which can enhance T cell memory, proliferation, and antitumor activity in patients with metastatic cancers (47, 48). Overexpression of OX40 in the TIL of patients with HNSCC has been identified (49). Furthermore, Montler et al. have noted co-expression of OX40 with PD-1 and CTLA-4 in a majority of tumor specimens, especially within the Treg population (49). The preclinical model showed the synergistic effects of anti-OX40 and anti-PD1, anti-OX40 and anti-CTLA-4, as well as anti-OX40 and anti-PDL1 (49). Anti-OX40 is currently being tested in early clinical trials of HNSCC, both as monotherapy and in combination with other immunotherapies (37).
Anti-PD-1/PD-L1 Therapy
T cells express the inhibitory receptor known as PD-1 on their surfaces to guard our body (50). When bound by its ligands PD-L1 or PD-L2, PD-1 transduces a signal into T cells to attenuate downstream signaling through the PI3K and PKCθ pathways (50, 51), which results in inhibition of T cell activation and proliferation. This protective mechanism is also utilized by tumor cells to escape immune attack through expressing high abundance of PD-L1 ligands on their surfaces.
Anti-PD-1/PD-L1 therapy has been a routine treatment to patients with PD-L1 highly expressing tumor (52). This kind of immunotherapy could target tumors more precisely. Meanwhile, as anti-PD-1/PD-L1 therapy has been applied to more and more patients, the side effects and the factors hindering the therapeutic effects have been noticed. Thus, combined treatments and better administrating methods have been raised to improve the treatment.
Mechanism of PD-1/PD-L1 Inhibitors
Tumor infiltrating lymphocytes, especially CD8+ T cells, exhibit high levels of PD-1 in HPV+ HNSCC (12). When PD-1 binds to PD-L1 on tumor cells, T cell proliferation is suppressed and tumor cells are able to evade immune attack more effectively in the tumor microenvironment (12). Since tumors expressing PD-L1, compared to PD-L1–negative tumors, showed improved response to Nivolumab (a PD-1 inhibitor) (53), it is important to investigate the level of PD-L1 expression in tumor microenvironment. One study suggested that patients with HPV− HNSCC expressed high levels of PD-1 in T cells and PD-L1 in a majority of tumor cells (54). Despite primary tumor sites, PD-L1 has been spotted on metastatic lesions (55). In summary, more than 29% of HPV− and around 70% of HPV+ HNSCC express PD-L1, suggesting that the majority of these cancers have potential for responding to PD-1 inhibitors (56). PD-L1 and PD-1 interaction is among the signals beneficial for tumor cells, which also include EGFR signaling, CD 28 stimulation and many others. And there are plenty of downstream pathways as well, which are composed of SHP2, RAS, ZAP70, P13K, and so on (Figure 2).
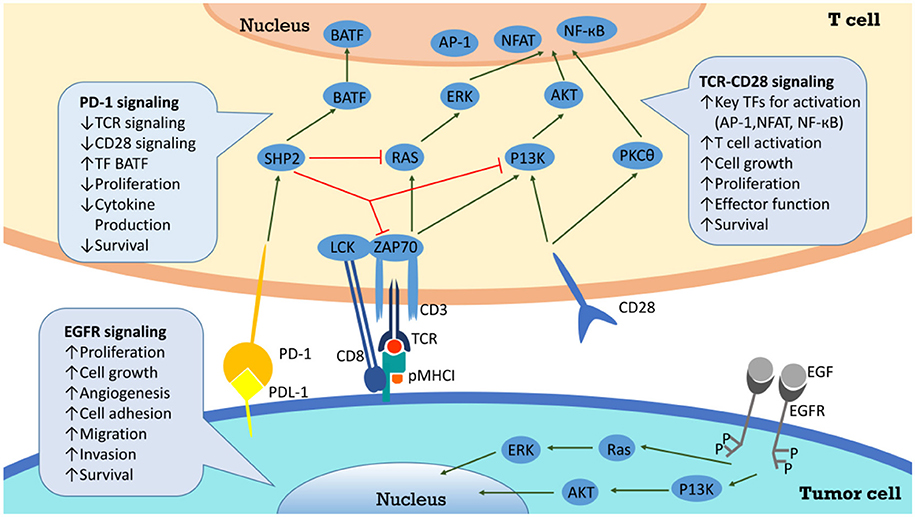
Figure 2. PD-L1/PD-1 signaling pathway and the correlated network. Interaction between PD-L1 and PD-1 on T cells results in inhibition of Zap70 phosphorylation and PI3K activation, and finally attenuates TCR signaling, CD28 mediated co-stimulation, NF-κB, and AP-1 activation, and IL2 production. Through inhibition of T cell via overexpression of PD-L1, cancer cells evade the host immune system.
When bond by PD-1 ligands, PD-1 is able to recruit phosphatases including SHP2 toinhibit T cell functions by countering the positive signaling events mediated by the T cell receptors (TCR) and CD28 (50). For instance, they restrain ZAP70 and PI3K–AKT and RAS signaling pathways (50). In conclusion, this lowers down the activation of transcription factors such as AP-1, NFAT, and NF-κB, which are important for T cell activation, proliferation, growth, and survival. Besides, PD-1 is able to inhibit T cell functions by improving the expression of BATF transcription factor to inhibit the effector transcriptional programs. EGFR is an important target for mediating tumor metastasis and adhesion. After combining with epidermal growth factor (EGF), EGFR can deliver positive signaling events downstream. For example, it activates PI3K–AKT and RAS signaling pathways to promote tumor cells proliferation and migration (50). Successful anti-PD-1/PD-L1 therapy requires adequate amount of specific T cells in tumor microenvironment and competent ability of T cells to get enough nutrients (57). Studies have shown aerobic glycolysis is essential for T cells to secrete IFN-γand attack tumor cells. PD-1/PD-L1 inhibitors may help T cells compete for glucose in tumor microenvironment, promoting T cell glycolysis and IFN-γ secretion (57, 58).
Daste et al. reported a case that a 64-years-old patient with HNSCC developed local tumor flare-up under immunotherapy, and a dramatic response was achieved in the following chemotherapy (59). Owing to the “loco-regional phenomena” described in their case study, they suggested that although clinical efficacy was not achieved in this case, immunotherapy might enhance response sensitivity to chemotherapy in patients with HNSCC (59).
Overview of FDA-Approved PD-1 Inhibitors for Head and Neck Cancers
Pembrolizumab
Pembrolizumab was the first anti-PD-1 antibody approved by FDA to treat patients with unresectable or metastatic melanoma who progress after Ipilimumab treatment. It is also approved for the treatment for melanoma patients harboring a BRAF V600E mutation, following treatment with a BRAF inhibitor. Pembrolizumab has also been legal for the treatment of non-small-cell lung cancer (NSCLC) without EGFR mutation and ALK rearrangement but with disease progression or following platinum-based chemotherapy (60). In August 2016, FDA approved the use of Pembrolizumab in R/M HNSCC that has progressed on or after platinum-containing chemotherapy (33, 34).
Nivolumab
Nivolumab, a PD-1 inhibitor, has been approved by FDA to treat Hodgkin lymphoma, renal cell carcinoma, NSCLC, and melanoma. Recent breakthrough in the application of Nivolumab in patients with processed HNSCC during chemotherapy or R/M HNSCC after chemotherapy with platinum-based drugs has made Nivolumab second to the Pembrolizumab approved by FDA in HNSCC treatment (35, 36).
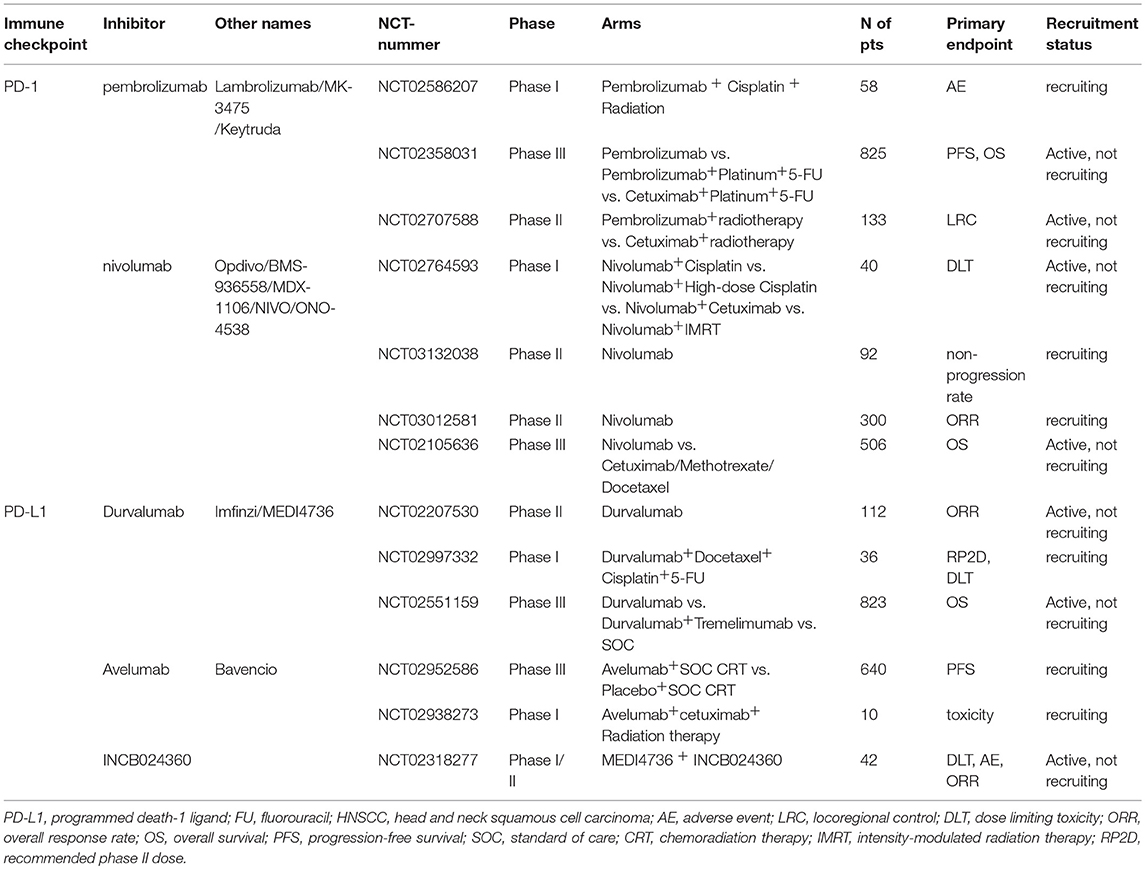
Clinical Studies of PD-1/PD- L1 Inhibitors
Inhibiting either PD-1 or PD-L1 function can block the PD-1 pathway. A number of PD-1/PD-L1 inhibitors are being investigated clinically and described in more details below (Table 2).
PD-1
Pembrolizumab (MK-3475, Previously Known as Lambrolizumab)
Preclinical anti-tumor effects were demonstrated in animals bearing multiple tumors. The first phase I clinical trial was carried out in patients with advanced solid tumors (61). Results suggested that Pembrolizumab was well-tolerated and associated with durable antitumor activity in multiple solid tumors (61). Two mg/kg per 3 weeks is considered a safe and effective minimum dose of antitumor activity (61). KEYNOTE-012 trial was a multicenter, open-label, phase Ib trial that included patients with R/M HNSCC in one of the cohorts. The objective response rate (ORR) was ~20% and overall survival (OS) was better in HPV+ patients (33). Then a larger HNSCC expansion cohort of KEYNOTE-012 reported an ORR of 18.2%, and response rates were similar in HPV+ and HPV− patients (62). In a recent single-arm, phase II KEYNOTE-055 study conducted in patients with R/M HNSCC, ORR was 16% and response rates were similar in HPV+ and HPV− patients, providing rationale for treatment with Pembrolizumab (NCT02255097) (63).
Monotherapy with Pembrolizumab is being carried out in patients with NSCLC (NCT01840579), advanced solid tumors (NCT01295827) and hematologic malignancies (NCT01953692). Clinical trials of Pembrolizumab focusing on HNSCC are ongoing in comparison to chemotherapy (NCT02358031), in combination with radiotherapy (NCT02707588), and in combination with cisplatin and radiation (NCT02586207).
Nivolumab (MDX-1106, BMS-936558, ONO-4538)
The first phase I clinical trial was conducted in patients with treatment-refractory solid tumors such as advanced metastatic melanoma, colorectal cancer, castrate-resistant prostate cancer, NSCLC, and renal cell carcinoma (64). The study exhibited good tolerance and meaningful antitumor activity of PD-1 inhibitors, and the early results from a follow-up trial (NCT00730639) further confirmed this. It appeared that the PD-1 antibody was well-tolerated and demonstrated anti-tumor activity in many patients whose previous treatment failed (65). In a recent randomized, open-label, phase III clinical trial conducted in patients with R/M HNSCC, the ORR was 26.1% for Nivolumab, demonstrating a survival advantage compared with conventional treatments with ORR of 0% for investigators' choices of therapy (NCT02105636) (66). Ongoing clinical trials focusing on HNSCC include comparison to Cetuximab, Methotrexate or Docetaxel (NCT02105636), combination with Cisplatin, Cetuximab, or IMRT (NCT02764593), and monotherapy (NCT03132038, NCT03012581).
PD-L1
Durvalumab (MEDI4736)
In a phase I/II clinical trial that included a group of HNSCC patients, ORR was 17%, especially higher (25%) in PD-L1high patients. The disease control rate in PD-L1 high subgroup was 44.9%, much greater than that in PD-L1 low or negative subgroup (21.5%) (67). These data support continued clinical development of Durvalumab in HNSCC. Durvalumab is being tested as monotherapy (NCT02207530), in combination with Docetaxel plus Displatin and 5-FU (NCT02997332), and in comparison to Durvalumab plus Tremelimumab (NCT02551159).
Avelumab
Avelumab is an anti-PD-L1 antibody. Studies of Avelumab targeting HNSCC has been scarce. It's currently assessed in combination with Cetuximab and radiotherapy in a phase I trial (NCT02938273), and in combination with standard care in a phase III trial (NCT02952586).
Factors Influencing Anti-PD-1/PD-L1 Therapy
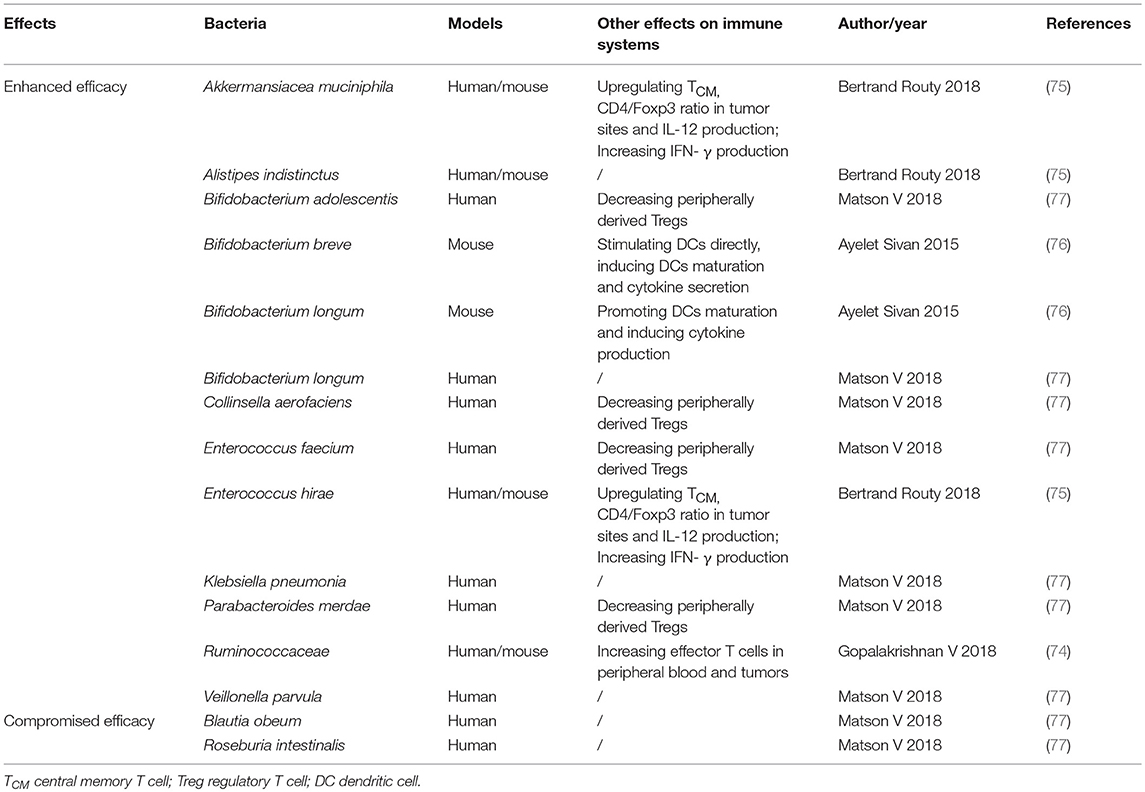
Gut Microbiota
It has been lately reported that gut microbiome plays important roles in many diseases, including influenza (68), multiple sclerosis (69, 70), diabetes (71), colorectal cancer (68, 72), and many others in various preclinical models, among which gut microbiome may modulate PD-1/PD-L1-based immunotherapy (73–76). Many kinds of bacteria have been proved to facilitate PD-1/PD-L1 blockades, meanwhile, there are bacteria that hamper the treatment (Table 3). It is reported that oral gavage of Bifidobacterium could achieve the same effects as anti-PD-L1 treatment, and combinational therapy almost eliminated tumor outgrowth, in which enhanced dendritic cell function led to more priming and accumulation of CD8+ T cells in the tumor microenvironment (76). On one hand, Akkermansia muciniphila was screened out to affect the anti-PD-1-based therapy in epithelial tumors in an IL-12 dependent fashion by enhancing the recruitment of CCR9+CXCR3+CD4+ T cells (75). Further study in patients also revealed that responding patients had more diverse and abundant bacteria of the Ruminococcaceae family, enhanced systemic and antitumor immunity, functioning better in anabolic pathways as well (74). On the other hand, the recent study by Matson V reported Blautia obeum and Roseburia intestinalis with compromised efficacy of PD-1 blockade (77). These results provide important information for cancer therapy with immune checkpoint inhibitors.
Molecules Regulating PD-1/PD- L1
Some tumors respond more sensitively to anti-PD-1/PD-L1 therapy, while others do not. The mechanisms regulating anti-PD-1/PD-L1 therapy sensitivity have arisen wide attention. Recently, two molecules, CMTM6 and CMTM4, have been reported as PD-L1 protein regulators. CMTM6 could prevent the degradation of PD-L1, maintaining the stability of PD-L1 and facilitating the immune escape of tumors. Interfering either CMTM6 or CMTM4 would hamper the expression of PD-L1. They function through reducing the ubiquitination of PD-L1, prolonging its half-life period. This provides a new target for immunotherapy to enhance the anti-PD-1/PD-L1 treatment (78, 79).
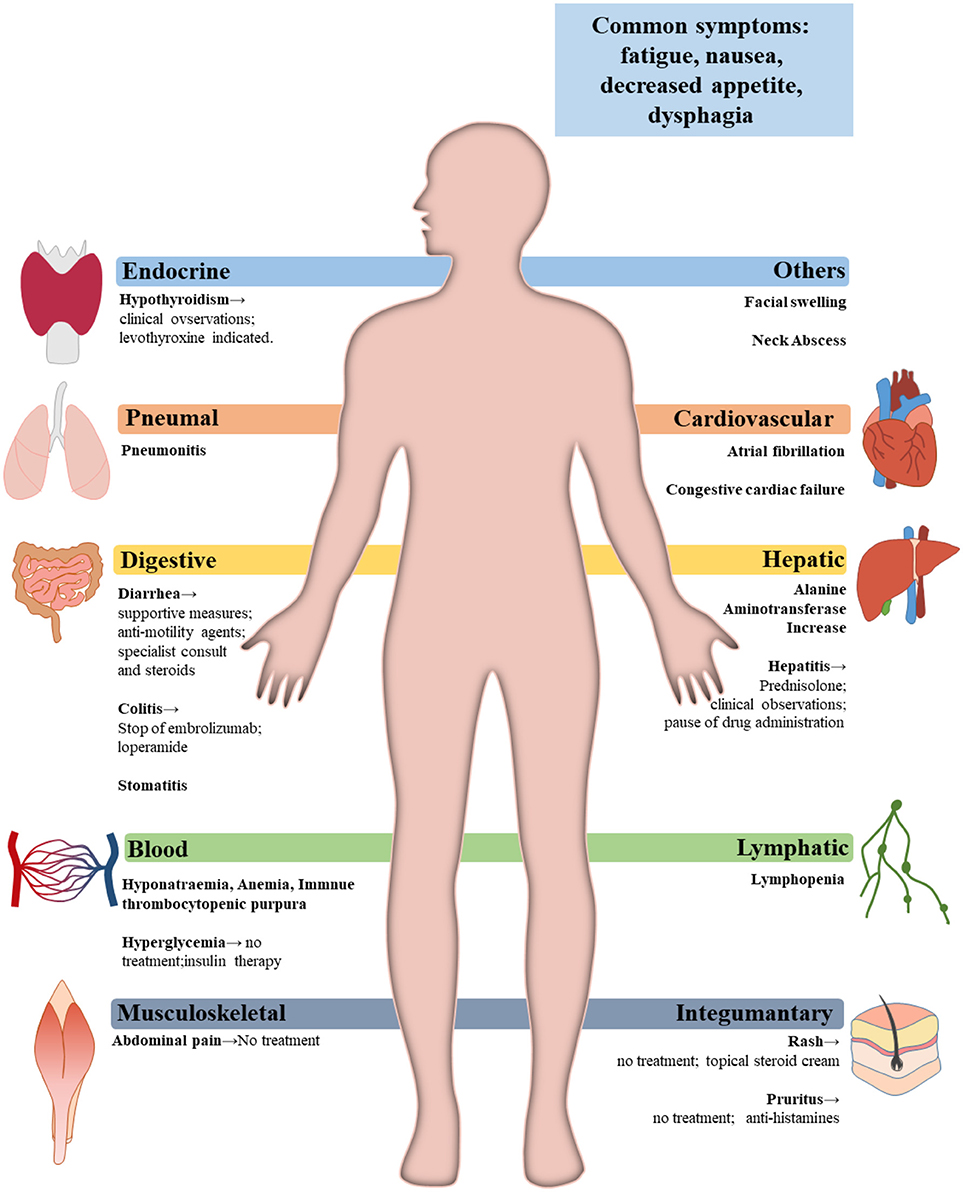
Adverse Events of FDA-Approved PD-1 Inhibitors and the Relevant Managements for Head and Neck Cancers
The fact that PD-1/PD-L1 axis contributes to the maintenance of self-tolerance implies that immune checkpoint blockade might disturb the balance of immune systems, resulting in treatment-related adverse events (trAEs) (80) (Table 4). TrAEs are frequent and occur in up to 80% of patients treated with an PD-1/PD-L1 antibody (81, 82). In the KEYNOTE-012 trial and the KEYNOTE-055 trial, trAEs occured in 63%-65% HNSCC patients treated with Pembrolizumab (33, 63). The most commonly observed trAEs were fatigue, decreased appetite, rash, hypothyroidism, nausea and diarrhea (63). Grade 3–4 trAEs occurred in around 9–14% of patients who had PD-1 inhibitors treatment. Three deaths were reported due to pulmonary toxicity (53, 82).
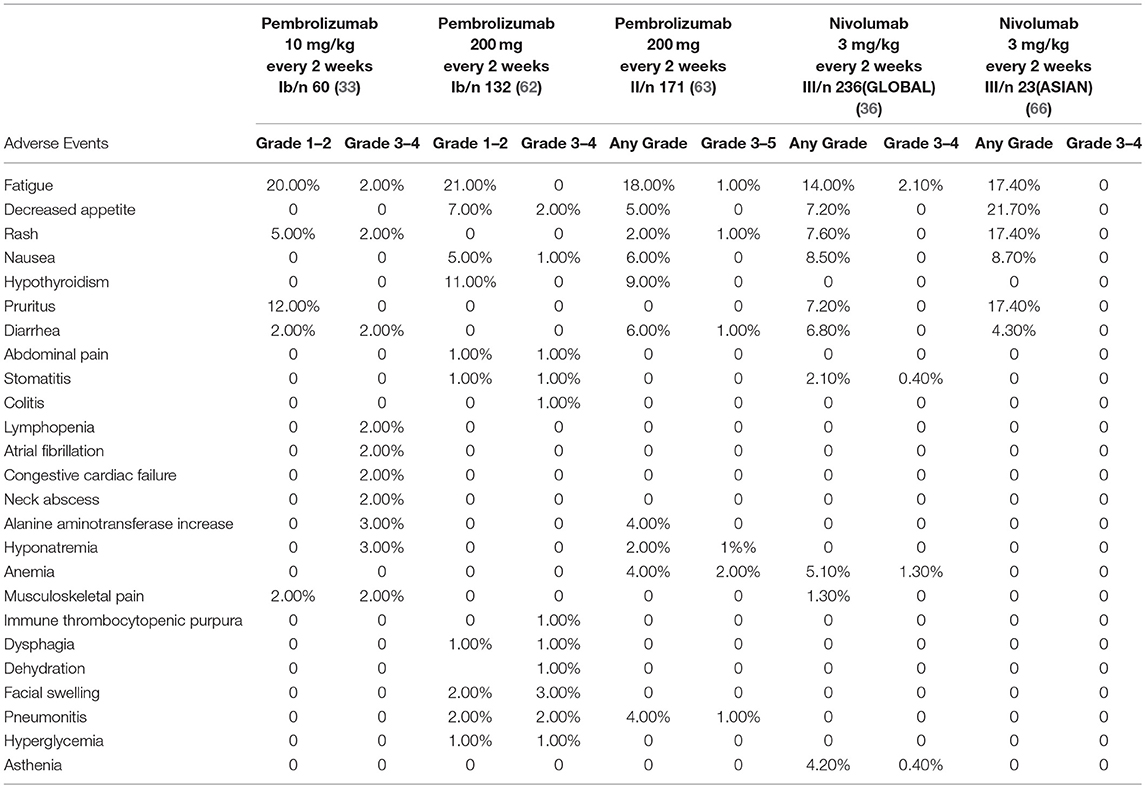
Table 4. Incidents of treatment-related adverse events occurring in patients with head and neck cancers.
By comparing the various organs involved, grade 1–2 trAEs mainly influence the skin and the gut, while grade 3–4 events mainly affect the digestive tract. Data suggest that trAEs usually occur within 3–6 months after the PD-1/PD-L1 blockade treatment (83). Accumulative toxic effects with prolonged treatment of anti-PD-1 were not observed (65).
For T cell tumors, like T-cell non-Hodgkin's lymphoma (T-NHL), anti-PD-1/PD-L1 therapy could render the tumors better proliferative. The reason is in this kind of tumors, T cells don't play the role to attack the tumors, instead, they are the major part of the tumor. It highlights a dangerous possible adverse event of anti-PD-1 treatment (84).
Nivolumab
A randomized, open-label, phase III study was designed to investigate efficacy and safety of Nivolumab for patients with recurrent HNSCC that progressed within 6 months post platinum-based chemotherapy (36). In this trial, the primary end point was OS. Although rates of trAEs of any grade were similar between two groups, fewer events of grade 3 or 4 were observed in the Nivolumab treatment group when treated with Nivolumab than the standard therapy group. Fatigue, nausea, rash, decreased appetite, and pruritus were the most commonly reported trAEs of any grade in patients receiving Nivolumab. Two treatment-related deaths owing to pneumonitis and hypercalcemia were reported in the Nivolumab treatment group (36). Daste et al. (59) reported a case of a patient with HNSCC developed tumor flare-up after therapy with Nivolumab (59).
Pembrolizumab
TrAEs of any grade occurred within an average of 9 weeks after the initiation of Pembrolizumab (85, 86). In the KEYNOTE-012 trial, trAEs of any grade were observed in 63% of patients. The most frequently observed trAEs were fatigue, pruritus, nausea, decreased appetite and rash. Grade 3–4 trAEs were reported in 10 of 60 patients (17%), including increased ALT and AST, hyponatremia, atrial fibrillation and congestive heart failure (33). In the expansion cohort, 62% of patients had trAEs of any grade. The most common trAEs were fatigue, hypothyroidism and decreased appetite. Grade 3–4 trAEs were observed in around 9% of patients, including lowered appetite, facial swelling and pneumonitis (62). In the KEYNOTE-055 trial, around 64% of patients exhibited trAEs. Grade 3–5 trAEs were reported in 15% of patients. One death owing to treatment-related pneumonitis was reported (63).
Severe Immune-Related Adverse Events in Crucial Organs
Myocarditis
Accounting for <0.3% of patients, myocarditis is a rare but severe immune-related adverse event that frequently results in rapid dyspnea and acute heart failure (87). More and more cases of patients with anti-PD-1/PD-L1 treatment-related heart diseases have been reported in recent 3 years (88). Semper et al. (89) reported a case of a patient, diagnosed with squamous cell carcinoma of the lung, developing Nivolumab-induced myocarditis. Three days post the 9th cycle of Nivolumab therapy, the patient with tumor remission developed acute chest pain and severe dyspnea, which was later confirmed to be immunotherapy-related (89). Johnson et al. (87) reported two more cases of patients, diagnosed with metastatic melanoma, developing lethal myocarditis induced by Nivolumab and Ipilimumab combined (87). Läubli et al. (90) reported a case of Pembrolizumab-induced myocarditis. A 73-years-old female patient with metastatic uveal melanoma developed severe Pembrolizumab-induced myocarditis which resulted in potentially life-threatening acute heart failure (90). In 2018, Frigeri et al. (91) reported the patients achieved complete remission of recurrent metastatic pulmonary adenocarcinoma after 7 cycles of Nivolumab administration. Unfortunately, she experienced rapid cardiogenic shock afterwards (91). A fatal case was reported by Matson et al. (92). One patient with NSCLC receiving Nivolumab developed acute heart failure (92). Moslehi et al. (88) have identified altogether 101 cases of severe immune checkpoint inhibitors-induced (ICIs-induced) myocarditis, 46% of which resulted in patients' deaths (88). A more conclusive mechanism of anti-PD-1-induced myocarditis is under investigation (87). Studies revealed that PD-L1 could be found on endothelium. Interaction between PD-1 and its ligands on endothelium is important in limiting T cell responses in the heart and thus controlling immune-mediated cardiac injury (93, 94). One suspected mechanism is that PD-L1 is expressed on the surface of various types of cells and tissues, including tumor cells and cardiac muscle cells. When patients receive anti-PD-1/PD-L1 treatment, owing to the distribution of drugs, T cell responses in cardiac muscles might be disturbed and enhanced, leading to the occurrence of lethal immune-related myocarditis (87, 95).
Pneumonitis
Incidence of pneumonitis of all grades during anti-PD-1 therapy was 2.7% and the incidence of pneumonitis for grade 3 or higher was around 0.8% (96). Patients diagnosed with lung cancers, compared to patients with other types of cancers had higher incidence of treatment-related pneumonitis, with incidence of grade 3 or higher being 1.8% and incidence of deaths being 0.4% (96, 97). In a randomized, open-label, phase II/III study on efficacy and safety of Pembrolizumab for patients with advanced NSCLC, three cases of deaths resulting from treatment-related pneumonitis were reported (85). As in clinical trial of PD-1 blockade treating HNSCC, two treatment-related deaths owing to pneumonitis and hypercalcemia were reported in the Nivolumab group of a randomized, open-label, phase III trial (NCT02105636) (36). In a phase II study, Bauml et al. evaluated efficacy of Pembrolizumab in patients with previously treated refractory head and neck cancers (KEYNOTE-055) and one death owing to immune-related pneumonitis was observed (63).
One patient with NSCLC, after receiving 2 cycles of anti–PD-1 therapy, developed symptoms of pneumonitis and received proper treatment. However, symptoms relapsed; treatments with corticosteroids displayed less efficacy and the patient died. Another case of a female patient with small-cell lung cancer (SCLC), treated with an anti-CTLA-4/PD-1 combination therapy, was reported. The patient showed responsiveness to corticosteroid treatment; with discontinuation of current immunotherapy, the patient recovered from pneumonitis and started next line of anti-tumor therapy (98).
Hepatitis
The incidence of immune-related hepatitis of all grades was around 3.1% and the incidence of grade 3 or higher was 0.5–0.6% (99). For a clinical trial with Pembrolizumab in patients with previously treated NSCLC (KEYNOTE-010), three cases of immune-related hepatitis were reported (97).
Management of Adverse Events
Before confirming the occurrence of immune-related adverse events, specialist should rule out all other possible diagnoses, including but not limited to infection and tumor progression (83). Figure 3 gives a glimpse of main adverse events in patients receiving anti-PD-1/PD-L1 therapy. The general principle for managing trAEs are suggested as followed: patients with grade 1 adverse events are provided with supportive care; patients with grade 2 events are advised on treatment with topical or systemic steroids (0.5–1 mg/kg/day); patients with grade 3 or 4 events require hospitalization, treatment of steroids, 1–2 mg/kg/day, or discontinuation of the current immunotherapy, depending on specialists' assessments (97, 100). Table 5 shows the management of some commonly observed trAEs. Most trAEs are manageable with steroids, which should be provided at a sufficient dose and gradually withdrawn. But there are some cases where trAEs may be permanent, and in those scenarios, adverse events can be treated with hormone instead (83, 100).
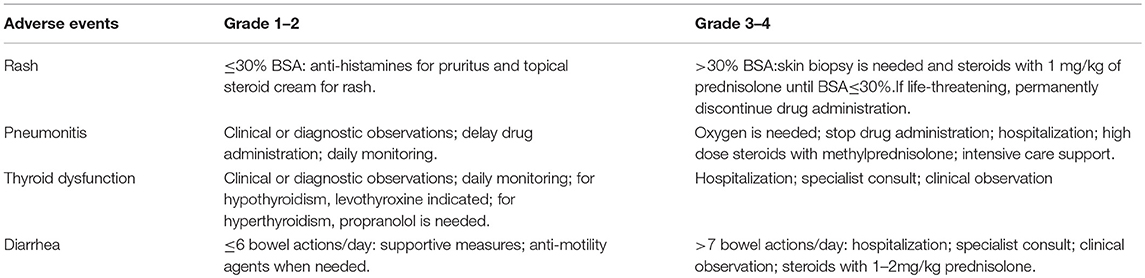
Table 5. Management of treatment-related rash, pneumonitis, thyroid dysfunction and diarrhea (100).
The Perspectives of Anti-PD-1/PD-L1 Therapy in Head and Neck Cancers
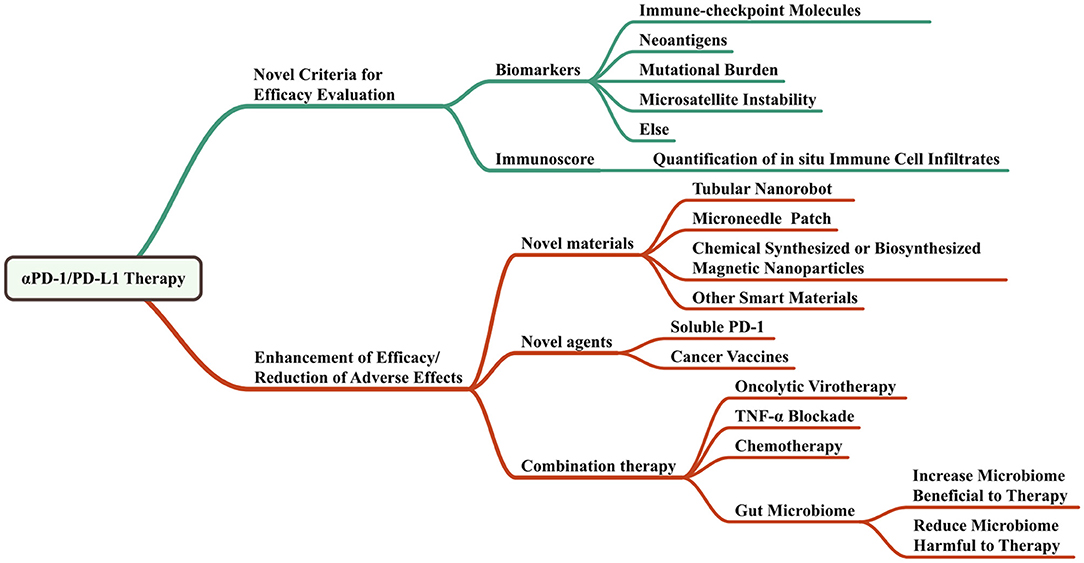
Figure 4 shows the perspectives of anti-PD-1/PD-L1 therapy.
Criteria to Monitor the Immune-Checkpoint Blockade
Scientists brought up the importance of monitoring immune-checkpoint blockade. As it is a novel therapy for cancers, the response evaluation and biomarkers should be different. Immune-related response criteria is an important concept to evaluate the immunotherapy and is the first step of precision immunotherapy (101). There are many biomarkers of immunotherapy response including PD-L1, other immune-checkpoint molecules, tumor-infiltrating lymphocytes (TILs), IFN-γ (102–104), mutational burden, neoantigens, microsatellite instability, serum markers, radiographic markers, and the “immunoscore” (105) which evaluates the distribution of TILs in the core and in the invasive margin of tumors. A recent study showed that the frequency of CD14+CD16−HLA−DRhi monocytes had strong correlation with progression-free and OS in response to therapy with anti-PD-1. The researchers used single-cell mass cytometry to analyze the immune cell subpopulations in the peripheral blood of patients with stage IV melanoma before and after anti-PD-1 therapy. It is an effective predictive biomarkers of a clinical response (106). Similarly, more predictive biomarkers are expected to be found and used in the near future.
Novel Materials Advancing the Effect
Nanoscale materials have potential as drug delivery systems that assist or advance the treatment in cancers. Some could even respond intelligently to molecular triggers (107, 108). A recent research reported that an autonomous DNA robot was programmed to transport blood coagulation protease thrombin within tubular nanorobot while DNA outside of the nanorobot as both a targeting domain and a molecular trigger. It could target the nucleolin specifically expressed in tumor blood vessels and caused tumor necrosis. Animal experiments with this DNA robot showed promising results (109). As it could carry the blood coagulation protease thrombin that is a type of protein, it would also be able to transport the anti-PD-1/PD-L1 antibody to specific areas with certain DNA targeting domains.
A microneedle, made by hyaluronic acid and pH-sensitive dextran nanoparticles, is developed to encapsulate anti-PD-1 antibody and glucose oxidase. Glucose oxidase can turn blood glucose into gluconic acid and generate an acidic environment in tumors to drive the self-dissociation of nanoparticles and finally substantially release anti-PD-1 antibodies. This newly developed tool with immunotherapy induced more robust immune response in melanoma. And the microneedle could carry more than one antitumor therapeutics like combination of anti-PD-1 and anti-CTLA-4 antibodies to enhance the treatment effect (110).
Years ago, Sun et al. utilized bacterial magnetosomes as drug carriers transporting doxorubicin to treat hepatocellular carcinoma and got a better result compared with the sole doxorubicin group (111). Immobilization of anti-PD-1/PD-L1 antibodies on magnetic nanoparticles may also provide an efficient local delivery strategy of the drugs for malignant solid tumors. Local magnetic delivery of these immobilized antibodies would increase local concentration while reduce the administration times, total usage and peripheral distribution of the antibodies, reducing the adverse effects. It would be very easy to immobilize antibodies on either biosynthesized or chemical synthesized magnetic nanoparticles since there are a lot of linking methods available (112).
Novel Agents Providing Similar Blockade Effects of Anti-PD-1/PD-L1 Antibodies
Despite the anti-PD-1/PD-L1 antibodies, soluble PD-1 (sPD-1) peptides may provide similar inhibition effect of PD-1 pathway by competitively binding to PD-L1 expressed on tumor cells. The plasmids expressing sPD-1 peptides could also be developed as gene therapy drugs which turn tumor cells as producers of sPD-1.
Soluble Immune Checkpoint Molecules
In addition to membrane bound form, there are sPD-1 and soluble PD-L1 (sPD-L1). Currently, sPD-1 is thought to be the translational product of the PD-1Δex3 mRNA transcript, and sPD-L1 may be derived from the cleavage of membrane bound PD-L1 by matrix metalloproteinases.
sPD-1 and sPD-L1 can also bind to ligands, thus blocking the PD-1/PD-L1 signaling pathway, resulting in potent peripheral T-cell anti-tumor responses. It's reported that the PD-1 extracellular domain was transfected into tumors by adenoviral vectors and could antagonize the negative regulation of T cells by PD-1/PD-L1 pathway, thus inhibiting tumor growth and prolong survival of mice (113).
Compared with membranous molecules, soluble molecules can not only affect neighboring cells in the tumor microenvironment, but also affect the body farther through the blood circulation, having a wider range of biological effects.
The production and function of the sPD-1 and sPD-L1 require further investigation. sPD-1 and sPD-L1 can be used in immunomodulatory therapy in combination with other antitumor therapy, such as HSP70 vaccine, to enhance the anti-tumor efficacy of tumor vaccine (114). In addition, the soluble forms may be used as an additional biomarker to the membrane bound forms, helping more accurately determine the patient's immune status and predict efficacy (115).
Cancer Vaccines
Up to now, preclinical and recent clinical studies have indicated that combining PD-1 or PD-L1 checkpoint inhibitors with cancer vaccines improves antitumor activity compared with anti-PD-1 or PD-L1 antibody monotherapy alone (116). However, satisfactory results about vaccines targeting PD-1 or PD-L1 checkpoint molecular are few. The DNA vaccines under active study work well but safety is hard to guarantee. In contrast, protein vaccines are low in cost and high in safety. It provides a promising research direction for the future development of cancer treatment. A study using genetic engineering to prepare a Cholera Toxin B based vaccine that targets both mouse MUC1 and mouse PD-1 showed that this fused protein vaccine can produce a stronger immune response (117).
Combination Therapy
Luo et al. (118) developed a nano-vaccine by simply mixing an antigen with a synthesized polymeric nanoparticle, PC7A NP. It delivered tumor antigens to APCs in draining lymph nodes, increasing surface presentation and simultaneously activating type I interferon-triggered genes through STING pathway. Combination of PC7A nano-vaccine with anti-PD-1 antibodies demonstrated increased survival rate in animal tumor models. Tumor growth was completely inhibited when these vaccinated animals were rechallenged with tumor cells, suggesting generation of antitumor memory (118). Researchers found that exploiting the individual tumor mutations as neo-epitopes and utilizing them as vaccines could enhance the immune response to tumors. Some patients even completely responsed to vaccination during combinational therapy with anti-PD-1 (119, 120).
Oncolytic virotherapy has demonstrated promise, however, it only had efficacy in a small fraction of tumor patients. As the virus could upregulate PD-L1 expression on tumor cells, combination of oncolytic virus, and anti-PD-1/PD-L1 therapy could synergistically promote the treatment of cancers. This was tested in colon and ovarian cancer models, but was believed to own wider indications (121).
Recent study revealed that TNF-α blockade prevents death of tumor infiltrating T lymphocyte induced by anti-PD-1 as well as PD-L1 and TIM-3 expression. It is strongly rationalized to develop a combinational therapy with anti-PD-1/PD-L1 and anti-TNF-α in cancer patients (122).
Chemotherapy drug gemcitabine (GEM) and anti-PD-L1 antibodies could be released locally when an engineered reactive oxygen species (ROS)-degradable hydrogel was injected and formed in tumor microenvironment, which contained abundant ROS. Anti-PD-L1-GEM scaffold promoted an tumor regression in the tumor-bearing mice and prevention of tumor recurrence after primary resection (123). In this research, a novel material together with the combination therapy reinforced the effect and reduced side effects of the treatment.
The trends of anti-PD-1/PD-L1 therapy are to enhance the therapy effects while reduce the side effects. It would benefit from the combination of anti-PD-1/PD-L1 antibodies with other checkpoint inhibitors, other suppressor inhibitors, cytokine inhibitors or chemotherapy drugs. Emerging novel materials and delivery strategies like nanorobots, microneedle patches, and magnetic immobilization could help the therapeutics work better in the way of localizing them in the cancer sites or carrying other biomarkers like DNAs or proteins to target better.
Author Contributions
BY, TL, YQ, and HL summarized the literature, wrote the manuscript, and prepared figures. SZ and BC provided critical comments and wrote part of the manuscript. JS supervised all the work and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Natural Science Foundation of Guangdong Province (2018A030313563) to JS; Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S252) to JS; Science and Technology Program of Guangzhou (201704020063) to BC; and Special Grant for Precision Medicine from Sun Yat-sen University to BC.
Abbreviations
APC, antigen presenting cell; ATF, activating transcription factor; CRC, colorectal cancer; GEM, chemotherapy drug gemcitabine; GOx, glucose oxidase; HNSCC, head and neck squamous cell carcinoma; IGF, insulin-like growth factor; NFAT, nuclear factor of activated T cells; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PIP, phosphatidylinositol; PLGF, placental growth factor; RCC, renal cell carcinoma; ROS, reactive oxygen species; RTK, receptor tyrosine kinases; SAEs, severe adverse events; sPD-1/sPD-L1, soluble PD-1/ soluble PD-L1; TCR, T cell receptors; TGF, transforming growth factor; TILs, tumor-infiltrating lymphocytes; TKIs, tyrosine kinase inhibitors; TNF, tumor necrosis factor; T-NHL, T-cell non-Hodgkin's lymphoma; trAEs, treatment-related adverse events.
References
1. Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res. (2003) 114:15–60. doi: 10.1007/0-306-48060-3_2
2. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet (2008) 371:1695–709. doi: 10.1016/S0140-6736(08)60728-X
3. Albers AE, Strauss L, Liao T, Hoffmann TK, Kaufmann AM. T cell-tumor interaction directs the development of immunotherapies in head and neck cancer. Clin Dev Immunol. (2010) 2010:236378. doi: 10.1155/2010/236378
4. Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. (2004) 96:99–106. doi: 10.1093/jnci/djh014
5. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. (1988) 48:3282–87.
6. Tuyns AJ, Esteve J, Raymond L, Berrino F, Benhamou E, Blanchet F, et al., Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int J Cancer (1988) 41:483–91. doi: 10.1002/ijc.2910410403
7. Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. (2006) 15:696–703. doi: 10.1158/1055-9965.EPI-05-0710
8. Sturgis EM, Wei Q. Genetic susceptibility–molecular epidemiology of head and neck cancer. Curr Opin Oncol. (2002) 14:310–17. doi: 10.1097/00001622-200205000-00010
9. Warnakulasuriya S. Areca nut use following migration and its consequences. Addict Biol. (2002) 7:127–32. doi: 10.1080/13556210120091491
10. D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. (2007) 356:1944–56. doi: 10.1056/NEJMoa065497
11. Chan PK, Chor JS, Vlantis AC, Chow TL, Fung SC, Lau CH, et al. Smoking, human papillomavirus infection, and p53 mutation as risk factors in oropharyngeal cancer: a case-control study. Hong Kong Med J. (2017) 23 (Suppl. 5):12–16.
12. Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. (2013) 73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384
13. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. (2005) 14:467–75. doi: 10.1158/1055-9965.EPI-04-0551
14. Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. (2006) 24:5630–6. doi: 10.1200/JCO.2005.04.6136
15. Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. (2004) 59:28–42. doi: 10.1016/j.ijrobp.2003.10.032
16. Bentzen SM. Repopulation in radiation oncology: perspectives of clinical research. Int J Radiation Biol. (2003) 79:581–5. doi: 10.1080/09553000310001597002
17. Lin A. Radiation therapy for oral cavity and oropharyngeal cancers. Dent Clin N Am. (2018) 62:99–109. doi: 10.1016/j.cden.2017.08.007
18. Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. (2004) 22:1743–52. doi: 10.1200/JCO.2004.06.147
19. Argiris A. Induction chemotherapy for head and neck cancer: will history repeat itself? J Natl Comprehen Cancer Netw. (2005) 3:393–403. doi: 10.6004/jnccn.2005.0020
20. De Andres L, Brunet J, Lopez-Pousa A, Burgues J, Vega M, Tabernero JM, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin Oncol. (1995) 13:1493–500. doi: 10.1200/JCO.1995.13.6.1493
21. Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. (2012) 62:400–22. doi: 10.3322/caac.21157
22. Young M, Neville B, Chi AC, Lathers D, Boyd-Gillespie M, Day T. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol Immunother. (2007) 56:1077–86. doi: 10.1007/s00262-006-0242-7
23. Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. (1993) 53:3579.
24. Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Investig. (1994) 71:374–9.
25. Pancari P, Mehra R. Systemic therapy for squamous cell carcinoma of the head and neck. Surg Oncol Clin N Am. (2015) 24:437–54. doi: 10.1016/j.soc.2015.03.004
26. Rubin GJ, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. (1998) 90:824.
27. Ferreira MB, Lima JP, Cohen EE. Novel targeted therapies in head and neck cancer. Expert Opin Investig Drugs (2012) 21:281–95. doi: 10.1517/13543784.2012.651455
28. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Eng J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
29. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature (2000) 407:242–8. doi: 10.1038/35025215
30. Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. (2009) 10:247–57. doi: 10.1016/S1470-2045(09)70002-6
31. Burtness B. Commentary: bevacizumab and erlotinib with chemoradiation for head and neck cancer. Cancer J. (2011) 17:273–5. doi: 10.1097/PPO.0b013e3182326944
32. Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. (2016) 37:462–76. doi: 10.1016/j.it.2016.04.010
33. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase Iphase Ib trial. Lancet Oncol. (2016) 17:956–65. doi: 10.1016/S1470-2045(16)30066-3
34. Mulvey A. “FDA Approves Pembrolizumab (Keytruda®), a PD-1 Antibody, for Head And Neck Cancer”. Cancer Research Institute (2016). Available online at: https://www.cancerresearch.org/blog/august-2016/fda-approves-pembrolizumab-keytruda-pd-1-antibody
35. Staff N. “FDA Approves Nivolumab for Head and Neck Cancer was originally published by the National Cancer Institute”. National Cancer Institute (2016). Available online at: https://www.cancer.gov/news-events/cancer-currents-blog/2016/fda-nivolumab-scchn
36. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Eng J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
37. Bell RB, Leidner RS, Crittenden MR, Curti BD, Feng Z, Montler R, et al. OX40 signaling in head and neck squamous cell carcinoma: overcoming immunosuppression in the tumor microenvironment. Oral Oncol. (2016) 52:1–10. doi: 10.1016/j.oraloncology.2015.11.009
38. Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, et al. CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin Cancer Res. (2017) 23:707–16. doi: 10.1158/1078-0432.CCR-16-0879
39. Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell (1992) 71:1065–8. doi: 10.1016/S0092-8674(05)80055-8
40. Deng WW, Wu L, Sun ZJ. Co-inhibitory immune checkpoints in head and neck squamous cell carcinoma. Oral Dis. (2018) 24:120–3. doi: 10.1111/odi.12746
41. Collins AV, Brodie DWR, Gilbert JC, Iaboni A, Manso-Sancho R, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity (2002) 17:201–10. doi: 10.1016/S1074-7613(02)00362-X
42. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (1996) 271:1734–36.
43. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12:252–64. doi: 10.1038/nrc3239
44. Yu GT, Bu LL, Zhao YY, Mao L, Deng WW, Wu TF, et al. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology (2016) 5:e1151594. doi: 10.1080/2162402X.2016.1151594
45. Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer (2013) 109:2629–35. doi: 10.1038/bjc.2013.645
46. Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. (2013) 19:1009–20. doi: 10.1158/1078-0432.CCR-12-2982
47. Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. (2001) 167:6669–77. doi: 10.4049/jimmunol.167.11.6669
48. Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. (2008) 68:5206–15. doi: 10.1158/0008-5472.CAN-07-6484
49. Montler R, Bell RB, Thalhofer C, Leidner R, Feng Z, Fox BA, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunol. (2016) 5:e70. doi: 10.1038/cti.2016.16
50. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway, Nature reviews. Immunology (2018) 18:153–67. doi: 10.1038/nri.2017.108
51. Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. (2014) 15:808–14. doi: 10.1038/ni.2941
52. Ludin A, Zon LI. Cancer immunotherapy: the dark side of PD-1 receptor inhibition. Nature (2017) 552:41–42. doi: 10.1038/nature24759
53. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
54. Malm IJ, Bruno TC, Fu J, Zeng Q, Taube JM, Westra W, et al. Expression profile and in vitro blockade of programmed death-1 in human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck (2015) 37:1088–95. doi: 10.1002/hed.23706
55. Brahmer J, Reckamp KL, Baas P, Crinò LW, Eberhardt EE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Eng J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
56. Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer-review of current data. Oral Oncol. (2015) 51:12–15. doi: 10.1016/j.oraloncology.2014.10.010
57. Sukumar M, Kishton RJ, Restifo NP. Metabolic reprograming of anti-tumor immunity. Curr Opin Immunol. (2017) 46:14–22. doi: 10.1016/j.coi.2017.03.011
58. Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Investig. (2015) 125:194–207. doi: 10.1172/JCI76012
59. Daste A, de Mones E, Digue L, Francois L, Domblides C, Dupin C, et al. Immunotherapy in head and neck cancer: need for a new strategy? Rapid progression with nivolumab then unexpected response with next treatment. Oral Oncol. (2017) 64:e1–3. doi: 10.1016/j.oraloncology.2016.10.020
60. Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer (2015) 3:36. doi: 10.1186/s40425-015-0078-9
61. Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in patients with advanced solid tumors. Clin Cancer Res. (2015) 21:4286–93. doi: 10.1158/1078-0432.CCR-14-2607
62. Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. (2016) 34:3838–45. doi: 10.1200/JCO.2016.68.1478
63. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase ii study. J Clin Oncol. (2017) 35:1542–9. doi: 10.1200/JCO.2016.70.1524
64. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. (2010) 28:3167–75. doi: 10.1200/JCO.2009.26.7609
65. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. (2014) 32:1020–30. doi: 10.1200/JCO.2013.53.0105
66. Kiyota N, Hasegawa Y, Takahashi S, Yokota T, Yen CJ, Iwae S, et al. A randomized, open-label, Phase III clinical trial of nivolumab vs. therapy of investigator's choice in recurrent squamous cell carcinoma of the head and neck: a subanalysis of Asian patients versus the global population in checkmate 141. Oral Oncol. (2017) 73:138–46. doi: 10.1016/j.oraloncology.2017.07.023
67. Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a Phase I Phase I/2 open-label study. JAMA Oncol. (2017) 3:e172411. doi: 10.1001/jamaoncol.2017.2411
68. Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell (2017) 171:1015–28 e1013. doi: 10.1016/j.cell.2017.09.016
69. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. (2017) 114:10713–18. doi: 10.1073/pnas.1711235114
70. Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. (2017) 114:10719–24. doi: 10.1073/pnas.1711233114
71. Yu H, Gagliani N, Ishigame H, Huber S, Zhu S, Esplugues E, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc Natl Acad Sci USA. (2017) 114:10443–8. doi: 10.1073/pnas.1705599114
72. Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology (2017) 153:1621–33 e1626. doi: 10.1053/j.gastro.2017.08.022
73. Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. (2018) 11:47. doi: 10.1186/s13045-018-0592-6
74. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359:97–103. doi: 10.1126/science.aan4236
75. Routy B, Le Chatelier E, Derosa LC, Duong PM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359:91–97. doi: 10.1126/science.aan3706
76. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (2015) 350:1084–9. doi: 10.1126/science.aac4255
77. Matson V, Fessler J, Bao R, Chongsuwat T, Zha YY, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science (2018) 359:104–8. doi: 10.1126/science.aao3290
78. Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature (2017) 549:106–10. doi: 10.1038/nature23669
79. Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature (2017) 549:101–5. doi: 10.1038/nature23643
80. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Eng J Med. (2016) 375:1767–78. doi: 10.1056/NEJMra1514296
81. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
82. Brahmer JR, Tykodi SS, Chow QM, Hwu J, Topalian SL, Hwu P, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Eng J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
83. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
84. Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Ollinger R, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature (2017) 552:121–5. doi: 10.1038/nature24649
85. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
86. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Eng J Med. (2015) 372:2521–2. doi: 10.1056/NEJMoa1503093
87. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Eng J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
88. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6
89. Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohe C. Drug-induced myocarditis after nivolumab treatment in a patient with PDL1- negative squamous cell carcinoma of the lung. Lung Cancer (2016) 99:117–19. doi: 10.1016/j.lungcan.2016.06.025
90. Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer (2015) 3:11. doi: 10.1186/s40425-015-0057-1
91. Frigeri M, Meyer P, Banfi C, Giraud R, Hachulla AL, Spoerl D, et al. Immune checkpoint inhibitor-associated myocarditis: a new challenge for cardiologists. Can J Cardiol. (2018) 34:92.e91–2.e3. doi: 10.1016/j.cjca.2017.09.025
92. Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. J Foren Sci. (2017) 63:954–7. doi: 10.1111/1556-4029.13633
93. Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. (2012) 188:4876–84. doi: 10.4049/jimmunol.1200389
94. Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation (2007) 116:2062–71. doi: 10.1161/CIRCULATIONAHA.107.709360
95. Cheng F, Loscalzo J. Autoimmune cardiotoxicity of cancer immunotherapy. Trends Immunol. (2017) 38:77–8. doi: 10.1016/j.it.2016.11.007
96. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
97. O'Kane GM, Labbe C, Doherty MK, Young K, Albaba H, Leighl NB. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist (2017) 22:70–80. doi: 10.1634/theoncologist.2016-0164
98. Balaji A, Verde F, Suresh K, Naidoo J. Pneumonitis from anti-PD-1/ PD-L1 therapy. Oncology (2017) 31 739–46.
99. Zhang X, Ran Y, Wang K, Zhu Y, Li J. Incidence and risk of hepatic toxicities with PD-1 inhibitors in cancer patients: a meta-analysis. Drug Design Dev Ther. (2016) 10:3153–61. doi: 10.2147/DDDT.S115493
100. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
101. Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. (2017) 14:655–68. doi: 10.1038/nrclinonc.2017.88
102. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell (2016) 167:397–404 e399. doi: 10.1016/j.cell.2016.08.069
103. Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma drives treg fragility to promote anti-tumor immunity. Cell (2017) 169:1130–41 e1111. doi: 10.1016/j.cell.2017.05.005
104. Bifulco CB, Urba WJ. Unmasking PD-1 resistance by next-generation sequencing. N Eng J Med. (2016) 375:888–9. doi: 10.1056/NEJMe1606042
105. Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. (2014) 232:199–209. doi: 10.1002/path.4287
106. Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. (2018) 24:144–53. doi: 10.1038/nm.4466
107. Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science (2012) 335:831–4. doi: 10.1126/science.1214081
108. Modi S, Nizak C, Surana S, Halder S, Krishnan Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. Nat Nanotechnol. (2013) 8:459–67. doi: 10.1038/nnano.2013.92
109. Li S, Jiang Q, Liu S, Zhang Y, Tian Y, Song C, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. (2018) 36:258–64. doi: 10.1038/nbt.4071
110. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. (2016) 16:2334–40. doi: 10.1021/acs.nanolett.5b05030
111. Sun JB, Duan JH, Dai SL, Ren J, Zhang YD, Tian JS, et al. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bio-nanoparticles as drug carriers. Cancer Lett. (2007) 258:109–17. doi: 10.1016/j.canlet.2007.08.018
112. Sun J, Li Y, Liang XJ, Wang PC. Bacterial magnetosome: a novel biogenetic magnetic targeted drug carrier with potential multifunctions. J Nanomater. (2011) 2011:469031–43. doi: 10.1155/2011/469031
113. Elhag OA, Hu XJ, Wen-Ying Z, Li X, Yuan YZ, Deng LF, et al. Reconstructed adeno-associated virus with the extracellular domain of murine PD-1 induces antitumor immunity. Asian Pacific J Cancer Prev. (2012) 13:4031–6. doi: 10.7314/APJCP.2012.13.8.4031
114. Wang XH, Zhang GM, He YF, Zhang H, Feng ZH. [Soluble PD-1 can augment anti-tumor immunity induced by HSP70-peptide complex in tumor-bearing mice]. Chin J Cell Mol Immunol. (2004) 20:655–8. doi: 10.1007/s11670-004-0048-0
115. Liu C, Jiang J, Gao L, Wang X, Hu X, Wu M, et al. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther. (2015) 17:340. doi: 10.1186/s13075-015-0859-z
116. Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T cell infiltration into pancreatic tumors. J Immunother. (2015) 38:1–11. doi: 10.1097/CJI.0000000000000062
117. Qiu L, Lu W, Lin Z, Cai H, Li R. Construction and Humoral Immunological analysis of a fusion protein vaccine that targets MUC1 and PD-1. Genom Appl Biol. (2016) 35:513–9. doi: 10.13417/j.gab.035.000513
118. Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. (2017) 12:648–54. doi: 10.1038/nnano.2017.52
119. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature (2017) 547:222–26. doi: 10.1038/nature23003
120. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature (2017) 547:217–21. doi: 10.1038/nature22991
121. Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. (2017) 8:14754. doi: 10.1038/ncomms14754
122. Bertrand F, Montfort A, Marcheteau E, Imbert C, Gilhodes J, Filleron T, et al. TNF alpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. (2017) 8:2256. doi: 10.1038/s41467-017-02358-7
Keywords: PD-1, PD-L1, immune checkpoint inhibitor, head and neck cancer, immunotherapy, adverse effects
Citation: Yang B, Liu T, Qu Y, Liu H, Zheng SG, Cheng B and Sun J (2018) Progresses and Perspectives of Anti-PD-1/PD-L1 Antibody Therapy in Head and Neck Cancers. Front. Oncol. 8:563. doi: 10.3389/fonc.2018.00563
Received: 23 August 2018; Accepted: 12 November 2018;
Published: 28 November 2018.
Edited by:
Jian-ye Zhang, Guangzhou Medical University, ChinaReviewed by:
Alexandre Corthay, Department of Pathology, Oslo University Hospital, NorwayKarishma Rajani, Mayo Clinic, United States
Copyright © 2018 Yang, Liu, Qu, Liu, Zheng, Cheng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Cheng, Y2hlbmdiaW5AbWFpbC5zeXN1LmVkdS5jbg==
Jianbo Sun, c3VuamIzQG1haWwuc3lzdS5lZHUuY24=
 Bo Yang
Bo Yang Tingjun Liu
Tingjun Liu Yang Qu
Yang Qu Hangbo Liu
Hangbo Liu Song Guo Zheng3
Song Guo Zheng3 Bin Cheng
Bin Cheng Jianbo Sun
Jianbo Sun