- 1Department of Cancer Genetics with Cytogenetic Laboratory, Medical University of Lublin, Lublin, Poland
- 2Chair and Department of Hematooncology and Bone Marrow Transplantation, Medical University of Lublin, Lublin, Poland
- 3Department of Animals Physiology, The Jan Kochanowski University in Kielce, Kielce, Poland
- 4The Regional Blood Donors Center of Kielce, Kielce, Poland
Introduction: The insertion (I allele) deletion (D allele) polymorphism of ACE gene (rs4646994) may influence the etiopathogenesis of multiple myeloma (MM). ACE gene is expressed in bone marrow cells and encodes angiotensin converting enzyme (ACE). It converts angiotensin I to active peptide angiotensin II, which stimulates proliferation of hematopoietic stem cells. This suggests possible association of ACE I/D gene polymorphism with MM. The aim of our study was to check possible impact of this polymorphism on risk of development and outcome of MM, as well as, sensitivity to bortezomib in cell cultures derived from MM patients.
Objects and Methods: Genomic DNA from 98 newly diagnosed MM patients and 100 healthy blood donors were analyzed by PCR method. Chromosomal aberrations were detected by use of cIg-FISH. In a subgroup of 40 MM patients nucleated bone marrow cells were treated with bortezomib in vitro.
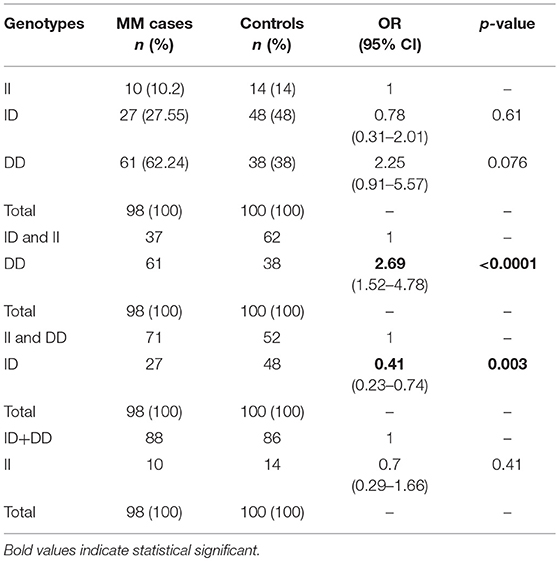
Results: The Hardy-Weinberg equilibrium test showed that genotypic frequencies diverged significantly from the equilibrium. The differences between I and D allele frequencies in control and study population were significant (p = 0.046). We observed the association between DD genotype and more than 2-fold risk of MM - OR = 2.69; p < 0.0001. We did not detect any significant differences among studied genotypes regarding clinical and laboratory parameters. Moreover, we did not observe the association between survival of MM patients and I/D genotypes. Bortezomib increased number of apoptotic and necrotic cells, but the only statistically significant differences were observed in the number of viable cells at 1 nM between ID and DD genotypes (p = 0.026).
Conclusion: Presented results confirmed the significant relationship between ACE (I/D) polymorphism and risk of MM development. We did not observe the association of ACE I/D polymorphism with disease outcome and bortezomib in vitro sensitivity.
Introduction
Multiple myeloma (MM) is characterized by the proliferation of malignant, clonal B-lymphocytic cells in bone marrow (1). The symptoms that appear in the course of disease include anemia, bone damage, hypercalcemia and also renal dysfunction (2, 3). Kidney disease is a common complication of MM, which occurs in 20–25% patients at diagnosis and in up to 50% patients during the course of disease (3, 4). In MM patients persistent kidney dysfunction is most commonly caused by tubular nephropathy due to secreted monoclonal immunoglobulin or monoclonal light chain (5).
Changes occurring in the bone marrow microenvironment lead to transformation of normal B lymphocytes into the malignant cells (6). Cancer cells are characterized by an increased proliferation and the ability to metastasize. These processes are regulated in part by the ubiquitin-proteasome system (UPS) (7). Bortezomib is a proteasome inhibitor approved for clinical use in MM patients and its function affects intracellular protein degradation. The inhibition of proteasome causes many effects, for example apoptosis of bone marrow cells (8, 9).
Bone marrow renin-angiotensin-aldosterone system (RAAS) influences the function of transcriptional factors and the response to growth factors released by microenvironment. A local bone marrow RAAS can affect proliferation of physiological and malignant cells (10). RAAS affects tumor growth and metastasis by modulating many processes such as proliferation of bone marrow cells (11). The RAAS includes the angiotensin I converting enzyme (ACE), which may be associated with increased cell proliferation (12). The main mechanism responsible for that function is cleavage by ACE enzyme of proteins, which show anti-proliferative effect on bone marrow cells (13).
Genetic factors play a major role in the pathogenesis of hematological malignancies including multiple myeloma. The ACE gene (17q23.3 locus) consists of 26 exons and 25 introns, and codes ACE enzyme (14). Functional polymorphism is present in intron 16 in the form of insertion (I allele) and/or deletion (D allele) of 289 bp Alu repeat sequence (rs4646994) (15). The I/D polymorphism may affect the expression of ACE gene and/or the function of angiotensin I converting enzyme (16). The DD genotype is associated with vessel wall thickness and higher blood pressure (17). The presence of D allele is associated with higher ACE enzyme activity and higher production of angiotensin II in comparison to I allele (18). Angiotensin II is known to activate several signaling pathways, including mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K)/AKT and protein kinase cAMP-dependent pathways, which play a role in regulation of cell growth, differentiation, reorganization of cytoplasmatic proteins and cell cycle progression (19). More and more data indicate that ACE gene product may be involved in cancer development (20). However, little is known on the biological and clinical significance of ACE I/D polymorphism in the context of MM.
The aim of our study was the analysis of correlation between ACE I/D polymorphism with the risk of development and the course of MM. Furthermore, we have analyzed whether this polymorphism predicts sensitivity to bortezomib in cell cultures derived from studied patients.
Materials and Methods
Patients and Samples
For the study, bone marrow aspirates and peripheral blood samples were collected from 98 newly-diagnosed patients with MM, who were hospitalized at the Chair and Department of Hematooncology and Bone Marrow Transplantation, Medical University of Lublin in years 2013–2018. The study was conducted after obtaining a positive opinion from the Bioethics Committee (no. KE-0254/165/2013 and no. KE-0254/337/2016), according with the ethical standards established by Helsinki Declaration. The research material was collected upon all patients and healthy blood donors provided written informed consent.
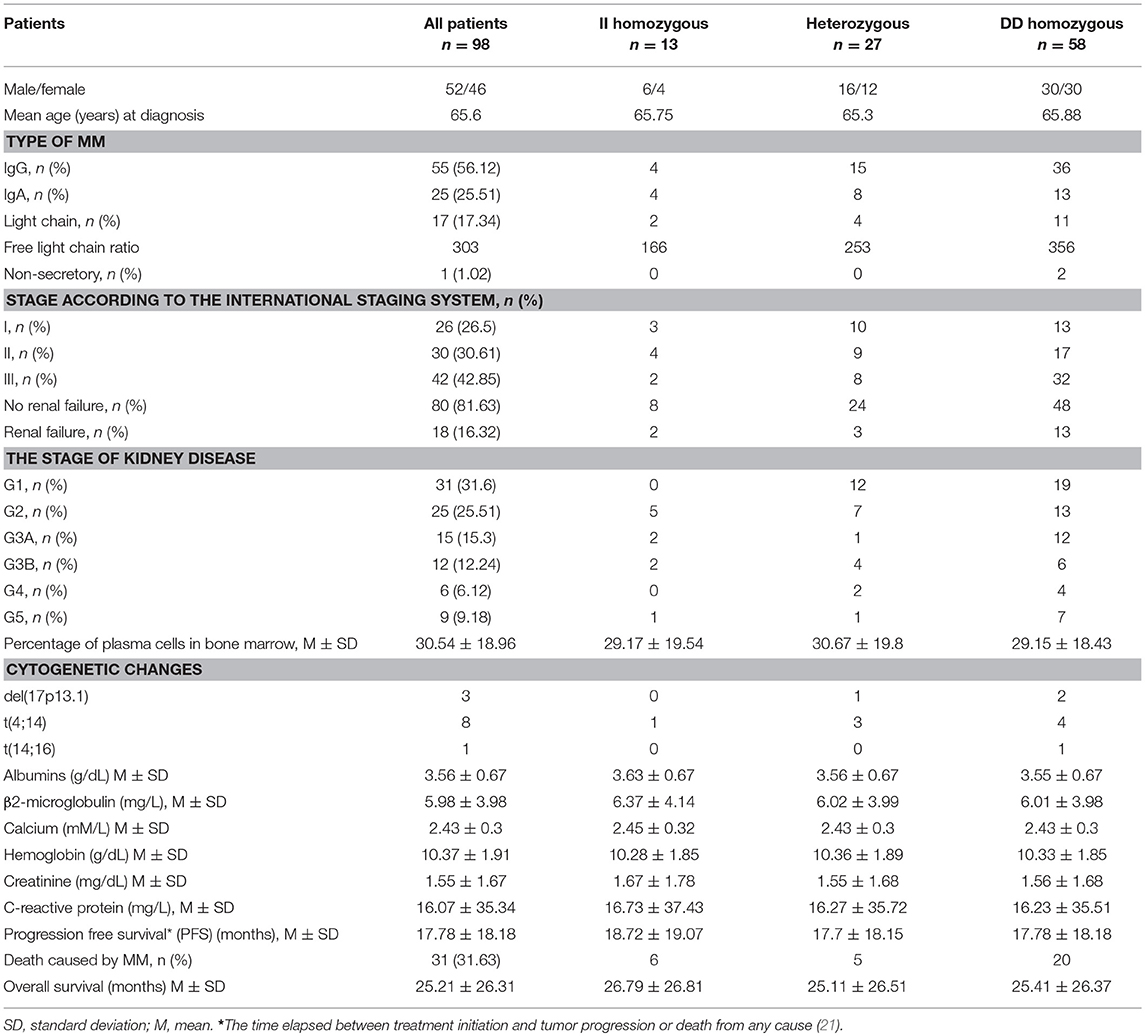
The characteristics of MM patients is shown in Table 1.
Therapeutic regimens consisted of thalidomide combined with steroids and/or chemotherapy followed or not by autologous hematopoietic stem cell transplant (auto-HSCT). Response was evaluated at the end of treatment using the International Myeloma Working Group guidelines, and classified as stringent complete remission (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), minimal response (MR), stable disease (SD) or progressive disease (PD) as described elsewhere (22, 23). Overall survival (OS) encompassed time from diagnosis until relapse, progression, death due to tumor effect or last follow-up, and time from diagnosis until death by any cause or last follow-up, respectively. The median follow-up time of MM patients enrolled in the study was 18 months (range 1–100 months).
Peripheral blood was used for DNA isolation and I/D polymorphism determination of ACE gene. From bone marrow aspirates, cell cultures were established to carry out the research associated with cIg-FISH (n = 98) and bortezomib treatment (n = 40).
Control samples were made of peripheral blood obtained from 100 healthy blood donors attending the Regional Blood Donation and Blood Treatment Center in Kielce.
DNA Isolation
DNA isolation from peripheral blood was performed using a commercial kit (Qiagen, Germany) according to manufacturer's procedure. The concentration and quality of DNA was checked using NanoDrop device (Thermo Fisher Scientific, USA).
ACE Gene Polymorphisms—Genotyping
The PCR method with two primers according to Yoshida (with modifications) was applied for analysis of ACE polymorphism (24). ACE gene intron 16 fragment length of 490 bp was amplified by using primers:
- forward 5′-CTG GAG ACC ACT CCC ATC CTT TCT-3′
-reverse 5′-GAT GTG GCC ATC ACA TTC GTC AGA T-3′
Each PCR mixture (25 μl) contained 150 ng genomic DNA and PCR buffer (Clontech Laboratories, USA), dNTPs mixture (0, 25 mM), HD polymerase (Clontech Laboratories, USA) and primers (10 μM of each). The mixture was heated 94°C for 5 min and underwent 35 cycles of amplification: denaturation 94°C for 30 s, annealing 58°C for 45 s, elongation 72°C for 30 s. The final elongation took 2 min at 72°C. The PCR reaction was performed in a Applied Biosystems 9700 Thermal Cycler.
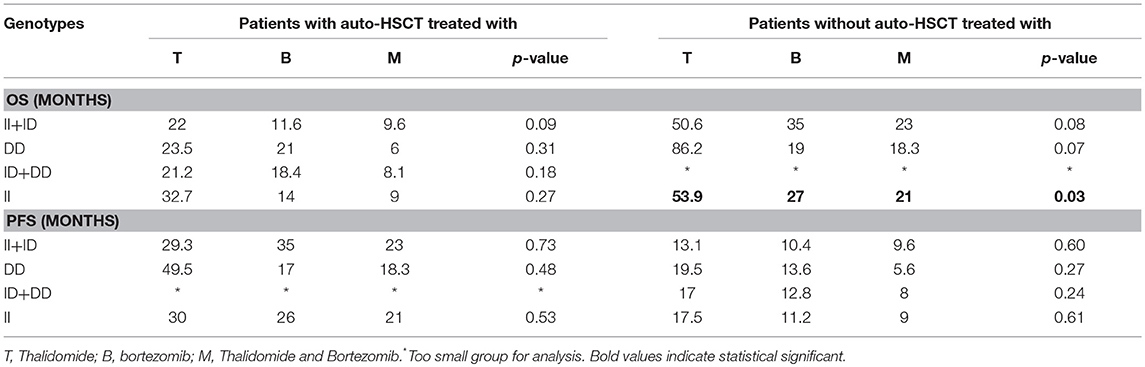
The PCR product was analyzed on 3% agarose gel and stained with SimplySafe (Eurx, Poland) and visualized in G:Box (Syngene, Great Britain) (Figure 1). D- or I-alleles were identified by the presence of 190 or 490 bp fragments, respectively. Heterozygous ID genotype shows the presence of two bands at 490 bp and one band at 190 bp. An independent PCR analysis was carried out for each sample.
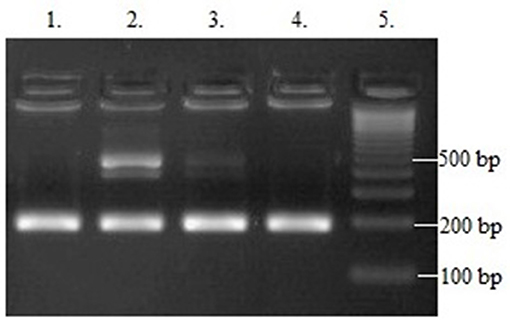
Figure 1. Results of ACE (I/D) gene polymorphism analysis: Line 1 and 4−190 bp band (DD homozygote); Line 2 and 3−190 and 490 bp bands (ID heterozygote); Line 5—leader marker (100 bp).
Cytogenetic Analyses
Abnormalities essential for MM, such as del(17p13.1) and IgHV gene rearrangements—t(4;14), t(14;16) were tested by cIg-FISH according to Ross et al. (25) recommendations (25). Cultured bone marrow malignant plasma cells from 98 patients were identified using simultaneous staining of cytoplasmic immunoglobulin and FISH (cIg-FISH) according to the previously described protocol with modifications (26, 27). The following probes, all from Abbott Molecular (Abbott Park, IL, USA), were used: Vysis TP53/CEP 17 FISH Probe Kit for detection of del(17p13.1), Vysis IGH/FGFR3 DF FISH Probe Kit for detection of t(4;14)(p16;q32), and Vysis IGH/MAF DF FISH Probe Kit for detection of t(14;16)(q32;q23) (Figure 2). Fluorescent microscopic analysis was performed by scoring 100 AMCA-positive plasma cells to determine the frequency of each aberration. Cut off level were 20% for deletion probes and 10% for dual fusion probes, according to the recommendations of the European Myeloma Network (25).
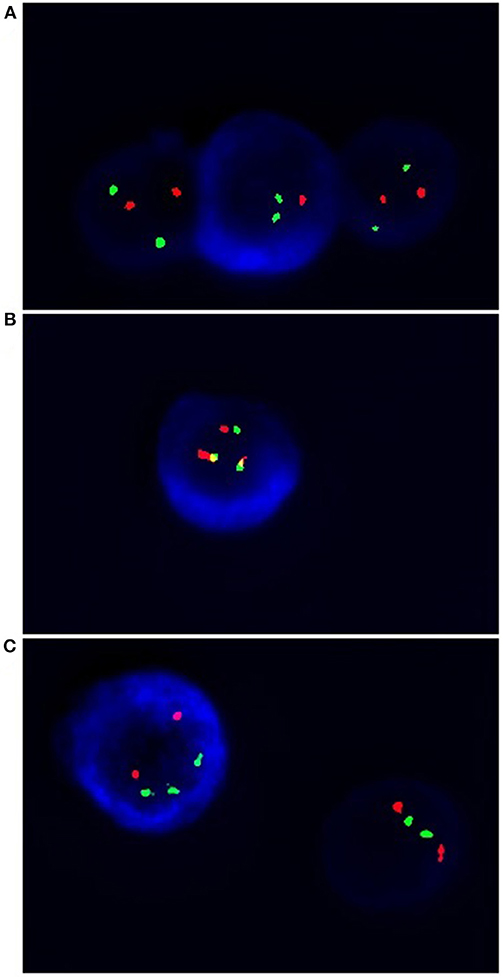
Figure 2. Simultaneous staining of cytoplasmic immunoglobin and FISH (cIg-FISH). The following probes were used: (A) Vysis TP53 (red signals)/CEP 17 (green signals) for detection of del(17p13.1), monoallelic deletion; (B) Vysis IGH (green signals)/FGFR3 (red signals) for detection of t(4;14)(p16;q32) (yellow signals); (C) Vysis IGH (green signals)/MAF (red signals) for detection of t(14;16)(q32;q23). Total magnification of 1.500 ×.
Cell Cultures, Apoptosis and Necrosis Detection
Bone marrow aspirates (n = 40) (mean number of plasma cells - 34% ± 17) were stratified on Lymphoprep (Axis-Shield PoC As, Norway) and lymphocyte fraction was used to established cell cultures, which were grown in 15 ml of culture medium—RPMI 1640 with L-glutamine (Biomed, Poland); 10% inactivated fetal calf serum (Biomed, Poland), 1% antibiotic antimycotic (A&E Scientific, Belgium), and different doses of bortezomib (LC Laboratories, USA, 200 mg/ml) - 1 nM/2 nM/4 nM. In the subgroup of 20 patients a higher bortezomib concentration (8 and 12 nM) was applied (data not shown) to estimate the 50%-cell growth inhibition. The dose 12 nM of bortezomib caused the above-mentioned inhibition. Bortezomib was dissolved in DMSO and stored at −80°C. The final DMSO concentration in culture medium was < 0.1%. As a control cell cultures without bortezomib (with 0.1% DMSO) were used. The amount of 1–1.5 ml of lymphocyte fraction (from each patient) was added, respectively, to the 15 ml of culture medium. The cultures were grown at 37°C in the atmosphere of 5% CO2 for 24 h (without G-CSF). The cell cultures were routinely terminated and cell suspensions were prepared to determine the number of apoptotic, necrotic and viable cells by means of Annexin V-Cy3 Apoptosis Detection Kit according to manufacturer's protocol (Sigma-Aldrich, USA). For fluorescence microscopy viable cells were stained with 6-CF (6-carboxyfluorescein)—green, necrotic cells were stained only with AnnCy3 (Annexin V Cy3.18). Cells starting apoptotic process were stained both with AnnCy3 (red) and 6-CF (green) (Figure 3). Mainly plasma cells (with diameter 9-12 μm) were analyzed using fluorescence microscope according to Carter's et al. (28).
Figure 3. Example of in vitro bortezomib activity (at 2 nM). Below are shown apoptotic cells, which are stained both AnnCy3 (red) and 6-CF (green). For statistical analysis of necrotic/apoptotic/viable cells plasmocytes with diameter 9–12 μm were counted. Cells were stained by immunofluorescent technique as described in Material and methods. Total magnification of 1.500 ×.
Statistical Analysis
Laboratory values of MM patients with polymorphism were compared using an independent t-test for continuous variables and Chi-square test for categorical variables. The association of ACE I/D polymorphism with prognostic factors was evaluated using Chi-square test or Fischer's exact test. The quantitative data was shown as frequency or percentage. Deviation of genotype frequencies in controls and cases from Hardy-Weinberg equilibrium (HWE) was assessed by Chi-squared test with one degree of freedom (df) using the Michael H. Court's (2005–2008) calculator (29). For 95% confidence interval (CI), we assumed p = 0.05 and χ2 = 3.84; therefore, if the χ2 = ≤ 3.84 and the corresponding p ≥ 0.05 then the population is in HWE. The Cox proportional hazard model was used for univariate and multivariate analysis of time-to-event and OS. The ANOVA test was used to analyze OS and PFS including different schemes of treatment and presence or absence of ASCT. The Kaplan-Meier method and the log-rank test were used for survival analysis. We assumed a 5% error of inference and the related level of significance p < 0.05 pointing to the existence of statistically significant differences. Statistical analyzes were performed using the Statistica ver. 12.5 (StatSoft).
Results
Fifty-Two males (53%) and 46 females (47%) among 98 MM patients were included in the study, with median age of 65.6 years. Detailed clinical characteristics at diagnosis are listed in Table 1. Genotyping was successful in all individuals investigated within the study. The HWE test confirmed that the genotypic frequencies for healthy individuals (controls) were balanced, while for MM patients diverged significantly from the equilibrium, thereby indicating possible disease association (Table 2). The differences between allelic frequencies in control and study groups were statistically significant. Allele frequencies in patient and control populations are summarized in Table 3. The frequency of D allele was higher, than the I allele, in both populations. Moreover, an association between MM and DD genotype was observed; OR = 2.69; p < 0.0001. The presence of I allele in heterozygotes did not elevate the MM risk - OR 0.41; p = 0.003. The associations between ACE I/D genotypes and MM risk are presented in Table 4.
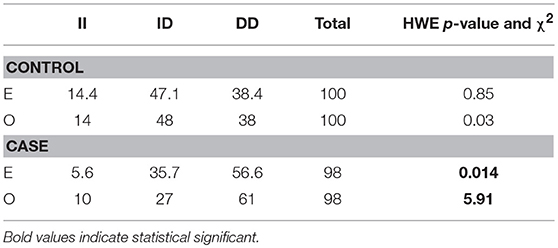
Table 2. Hardy-Weinberg equilibrium for ACE (I/D) polymorphism in case and control groups according to expected (E) and observed (O) values.
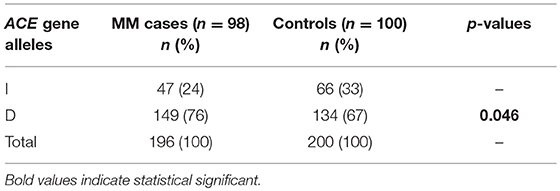
Table 3. The comparison of ACE I/D allele frequencies among Polish Caucasian MM patients and controls.
Furthermore, we analyzed potential relation between clinical and laboratory results at diagnosis of MM patients and I/D genotypes. We did not confirm any significant differences among studied genotypes regarding such parameters as sex, age of MM onset, ISS stage, cytogenetic changes, type of MM (secretory-, non-secretory MM, light chain disease), monoclonal protein class (IgG, IgA, etc.), light chain type (kappa, lambda), loss of body weight before treatment, baseline hemoglobin concentration, estimated glomerular filtration rate, the presence of renal insufficiency at diagnosis of MM, levels of albumin, β2-microglobulin, calcium, creatinine, and C-reactive protein.
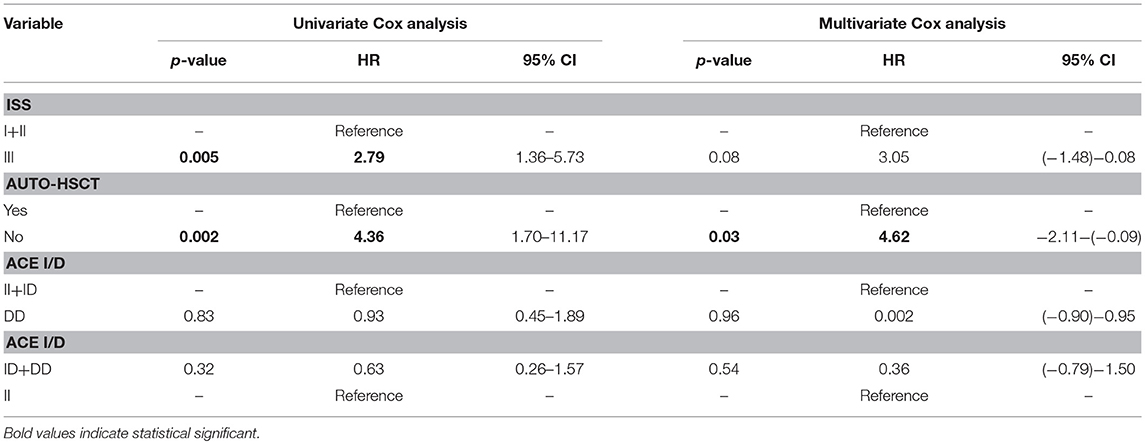
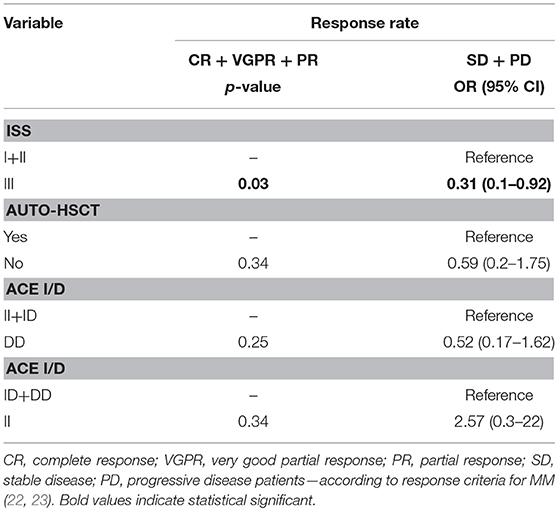
An univariate Cox analysis revealed that patients at stage III of ISS and patients who did not receive auto-HSCT had 2.79 and 4.36 increased risk of disease relapse or progression, respectively (Tables 5, 6). The multivariate regression analysis also confirmed that patients without auto-HSCT had statistically higher risk (H = 4.62) of disease progression (Table 5). We did not find the correlation between survival of MM patients and I/D genotypes (Figure 4). Time-to-event analysis, where the event was age at diagnosis, showed that DD, ID and II genotypes did not play a role for disease onset (p ≥ 0.35).
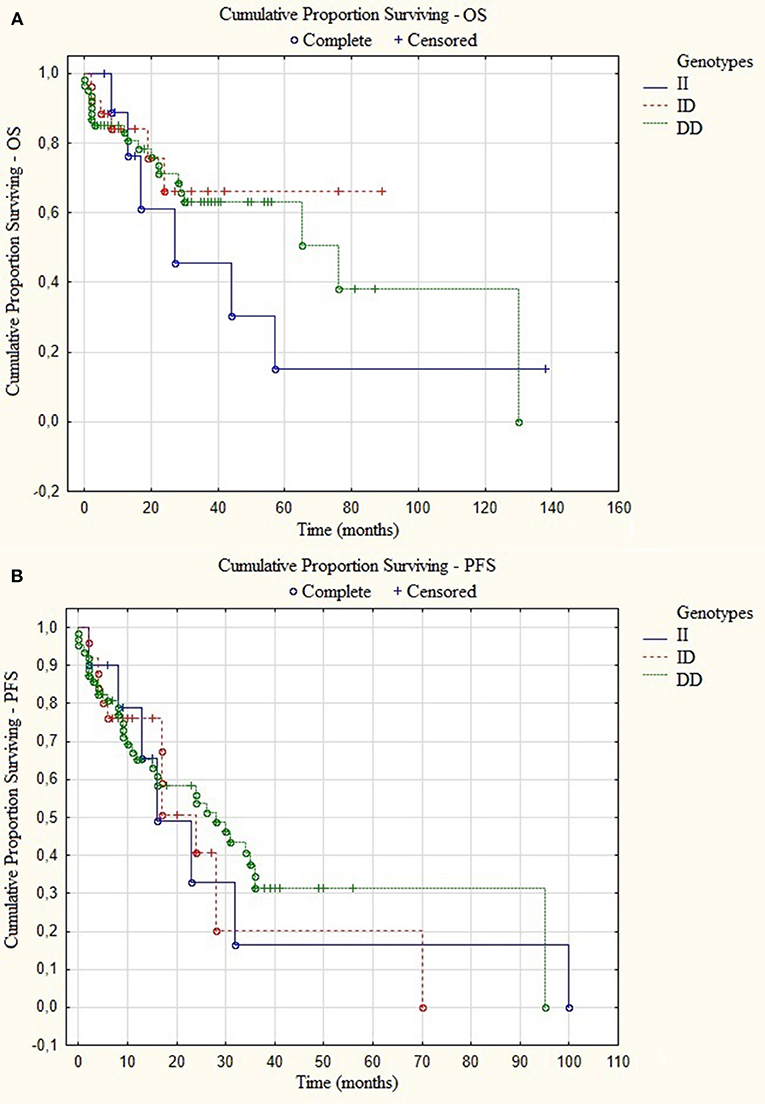
Figure 4. Kaplan-Meier analysis of (A) overall survival (OS) p = 0.94 and (B) progression-free survival (PFS) p = 0.99 in group of MM patients with different I/D genotypes.
The survival analysis showed that MM patients without ASCT and with II genotype treated with thalidomide have longer OS (54 months, p = 0.03) in comparison to MM patients treated with bortezomib (OS = 27 months) and both—thalidomide and bortezomib (OS = 21 months) (Table 7). In the case of PFS analysis the results were statistically insignificant.
Bortezomib increased number of apoptotic and necrotic cells in all studied genotypes. The only statistically significant difference was observed in the number of viable cells at 1 nM between ID and DD genotypes (p = 0.026; 82.15 vs. 74.74%). More detailed data are shown in Table 8. The dose of 12 nM (not shown) caused death of at least 50% of cells in all genotypes.
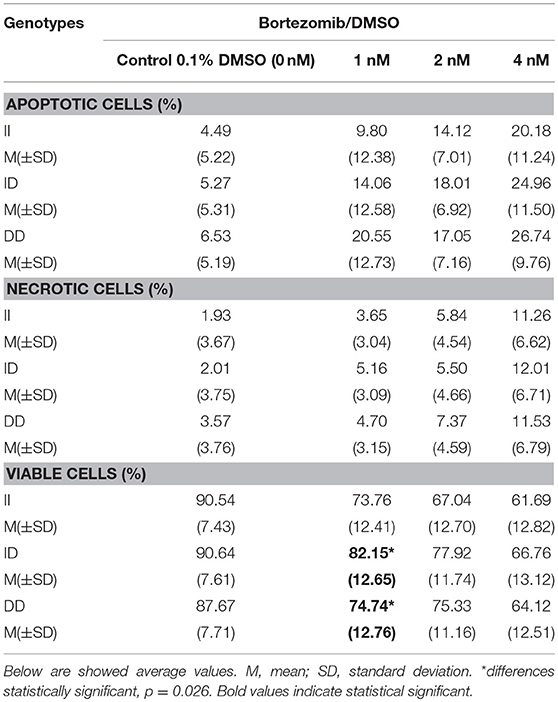
Table 8. The effect of different bortezomib doses on bone marrow cells apoptosis, viability and necrosis.
We have found the significant relationship between DD genotype and risk of MM development. We did not observe the association of ACE I/D polymorphism with disease outcome and bortezomib in vitro sensitivity.
Discussion
In this study, we have explored the association of ACE I/D polymorphism with MM in Caucasian population. To our knowledge, this is the first study elucidating the correlation between ACE (I/D) polymorphism and risk and outcome of MM, as well as, with response to bortezomib under in vitro conditions. Our findings provide the evidence implicating ACE (I/D) polymorphism in the development of MM.
In the present study, an association between ACE (I/D) polymorphism and MM risk was observed, where the DD genotype significantly conferred higher disease risk as compared to ID/II genotype. Many data indicate the association between D allele and cancer development, for example Liu et al. has highlighted the correlation between the presence of D allele and the development of moderate-sized adenocarcinomas and metastatic cancer (30). Similar findings were described by Ebert et al. in patients with gastric cancer in German population (31). Furthermore, Zha et al. found that the DD genotypes were associated with increased risk of hepatocellular carcinoma compared with these patients with II genotypes in Chinese Dai population (32).
In the case of hematological malignancies it is suggested, that II genotype can increase the patient's OS above the median depending on the type of leukemia (33). MM is a hematological disorder which arises from proliferation of monoclonal plasma cells derived from B-cells (33). Some leukemias, like CLL or B-ALL (B-cell acute lymphoblastic leukemia) affect the function of B cells. This suggests that in MM patients similar findings might be observed. We have noticed no association between studied genotypes and OS in MM. Moreover, we did not find correlation between ACE (I/D) polymorphism and clinical data/laboratory reports.
According to our knowledge, the relationship between ACE I/D polymorphism and the response to bortezomib under in vitro conditions was not investigated. We originally hypothesized that this polymorphism might interact with bortezomib treatment. Matsuki-Maramoto et al. found that bortezomib treatment might have suppressive effects on the immune and RAAS in NZB/W F1 mice (34). Beside direct effects of angiotensin II, like increasing the volume of fluid and blood pressure, it also regulates cell proliferation and growth. Qin et al. demonstrated that angiotensin II induced the expression of proteasome protein subunits (35). Bortezomib, as a proteasome inhibitor, leads to apoptosis (36). The introduction of bortezomib, as well as the immunomodulatory agents like thalidomide and lenalidomide influenced the survival of MM patients (6, 37). Bortezomib-based chemotherapy is preferred in MM patients with renal failure (22, 36).
Summarizing, we saw no consistent results of ACE polymorphism on apoptosis and necrosis in cell cultures derived from MM cases treated with bortezomib. The bone marrow plasma cells may contain somatic mutations in various DNA segments, which may interfere the analysis of ACE I/D polymorphism. We found spontaneous apoptosis and necrosis in cultures without borteozmib, which may be caused by laboratory conditions or changes in the microenvironment. Alternatively, myeloma cell lines could be used. However, in the case of studied polymorphism there are not available reference cell lines with all I/D genotypes, which may be used in in vitro study.
A limitation of our study is relatively small sample size in part due to the low incidence of the disease. Further analysis on larger cohort can help better understand the significance of ACE I/D polymorphism in the pathobiology of MM.
In conclusion, this study found that DD genotype is associated with more than 2-fold higher predisposition to MM. However, we did not confirm the association between studied ACE (I/D) polymorphism with disease outcome and bortezomib response under in vitro conditions. Our results may help to better understand the role of ACE (I/D) polymorphism in the diverse biology of MM.
Author Contributions
SZ, SP-M, MW-L, ML, SC, IK-P, and JJ carried out the experiment. SZ, SP-M, MW-L, IK-P, and JJ performed the molecular analysis of ACE polymorphism. SC performed cytogenetic analysis. SZ, ML, and SP-M performed in vitro study with bortezomib. GS-K, MH, and AF were involved in planning and supervised the work. SZ, A-S-S, MM, and WS processed the experimental data, performed the analysis. SZ, SP-M, and WS designed the figures. SZ, A-S-S, and WS wrote the manuscript with support from GS-K, MH, and AF. All authors discussed the results and commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the grant MNmb232. The equipment used was purchased with funds from The equipment of Innovative laboratories doing research on new medicines used in the therapy of civilization and neoplastic diseases Project, part of the Development of Eastern Poland Operational Program 2007–2013, Priority Axis and Modern Economy, Operations I.3 Innovation Promotion.
References
1. Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book (2016) 35:418–23. doi: 10.1200/EDBK_159009
2. Hameed A, Ali J, Munawar K, Arshad F, Badar F, Siddiqui N. Characteristics and outcomes of patients with multiple myeloma : data from a developing country. Med J Islam Repub Iran (2018) 1:1–5. doi: 10.14196/mjiri.32.1
3. Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma – a demographic study of 1353 patients. Eur J Haematol. (1994) 53:207–12. doi: 10.1111/j.1600-0609.1994.tb00190.x
4. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. (2003) 78:21–33. doi: 10.4065/78.1.21
5. Bridoux F, Fermand JP. Optimizing treatment strategies in myeloma cast nephropathy: rationale for a randomized prospective trial. Adv Chronic Kidney (2012) 19:333–41. doi: 10.1053/j.ackd.2012.07.003
6. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. (2011) 364:1046–60. doi: 10.1056/NEJMra1011442
7. Herrmann J, Ciechanover A, Lerman LO, Lerman A. The ubiquitin-proteasome system in cardiovascular diseases – a hypothesis extended. Cardiovasc Res. (2004) 61:11–21. doi: 10.1016/j.cardiores.2003.09.033
8. Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, et al. Linking of autophagy to ubiquitin-protesaome system is important for the regulation of endoplasmatic reticulum stress and cell viability. Am J Pathol. (2007) 171:513–24. doi: 10.2353/ajpath.2007.070188
9. Janen SB, Chaachouay H, Richter-Landsberg C. Autophagy is activated by proteasomal inhibition and involved in agresome clearance in cultured astrocytes. Glia (2010) 58:1766–74. doi: 10.1002/glia.21047
10. Akalin I, Koca E, Karabulut HG, Haznedaroglu IC, Cetier D, Hayran M, et al. Angiotensin converting enzyme insertion/deletion gene polymorphisms in leukemic hematopoiesis. UHOD (2011) 21:1–8. doi: 10.4999/uhod.09164
11. Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis (2008) 29:1675–84. doi: 10.1093/carcin/bgn171
12. Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. (2003) 35:881–900. doi: 10.1016/S1357-2725(02)00271-6
13. Hajek D, Kubesova H, Vyzula R, Mayer J, Druckmueller M, Tomiska M, et al. Bone marrow as multidimentional orbit oscillator after autologous bone marrow transplantation. Stem Cells (1999) 17:25–30. doi: 10.1002/stem.170025
14. Hubert CA, Houot P, Corvol P, Soubier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. (1991) 266:15377–83.
15. Lin C, Yang HY, Wu CC, Lee HS, Lin YF, Lu KC, et al. Angiotensin-converting enzyme insertion/deletion polymorphism contributes high risk for chronic kidney disease in Asian male with hypertension–a meta-regression analysis of 98 observational studies. PLoS ONE (2014) 9:e87604. doi: 10.1371/journal.pone.0087604
16. Castellon R, Hamdi HK. Demystifying the ACE polymorphism: from genetics to biology. Curr Pharm Des. (2007) 13:1191–8. doi: 10.2174/138161207780618902
17. Dietze GJ, Henriksen EJ. Angiotensin converting enzyme in skeletal muscle: sentinel of blood pressure control and glucose homeostasis. J Renin Angiotensin Aldosterone Syst. (2008) 9:75–88. doi: 10.3317/jraas.2008.011
18. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. (1990) 86:1343–6. doi: 10.1172/JCI114844
19. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. (2007) 292:82–97. doi: 10.1152/ajpcell.00287.2006
20. Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. (2005) 16:293–9. doi: 10.1016/j.tem.2005.07.009
21. Federal Drug Administration. Guidance for Industry: Clinical Trial Endpoints for the Approval of Non-Small Cell Lung Cancer Drugs Biologics. (2011). Available online at: https://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf (Accessed December 30, 2018).
22. Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and suppertive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. (2014) 32:587–600. doi: 10.1200/JCO.2013.48.7934
23. Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia (2007) 20:1467–73. doi: 10.1038/sj.leu.2404284
24. Yoshida H, Mitarai T, Kawamura T, Kitajima T, Miyazaki Y, Nagasawa R, et al. Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest. (1995) 96:2162–9. doi: 10.1172/JCI118270
25. Ross F, Avet-Loiseau H, Ameye G, Gutiérrez NC, Liebisch P, O'Connor S, et al. Report from the European myeloma network on interphase FISH in multiple myeloma and related disorders. Haematologica (2012) 97:1272–7. doi: 10.3324/haematol.2011.056176
26. Ahmann GJ, Jalal SM, Juneau AL, Christensen ER, Hanson CA, Dewald GW, et al. A novel three-color, clonespecific fluorescence in situ hybridization procedure for monoclonal gammopathies. Cancer Genet Cytogenet. (1998) 101:7–11. doi: 10.1016/S0165-4608(97)00058-7
27. Dmoszynska A, Chocholska S. Molecular biology methods in the diagnosis of multiple myeloma. In: Witt M, Dawidowska M, Szczepanski T, editors. Molecular Aspects of Hematologic Malignancies: Diagnostic Tools and Clinical Applications. Springer (2012). p. 443–9.
28. Carter A, Hocherman I, Linn S, Cohen Y, Tatarsky I. Prognostic significance of plasma cell morphology in multiple myeloma. Cancer (1987) 60:1060–5. doi: 10.1002/1097-0142(19870901)60:5<1060::AID-CNCR2820600522>3.0.CO;2-3
29. Court MH. MH. Court's (2005-2008) Online Calculator. Tuft University Web site, Medford and Somerville (2012).
30. Liu SY, Sima X, Wang CH, Gao M. The association between ACE polymorphism and risk of colorectal cancer in Chinese population. Clin Biochem. (2011) 44:1223–26. doi: 10.1016/j.clinbiochem.2011.07.016
31. Ebert MP, Lendeckel U, Westpal S, Dierkes J, Glas J, Folwaczny C, et al. The angiotensin I-converting enzyme gene insertion/deletion polymorphism is linked to early gastric cancer. Cancer Epidemiol Biomarkers Prev. (2005) 14:2987–9. doi: 10.1158/1055-9965.EPI-05-0411
32. Zha Y, Gan P, Liu Q, Tan J. Relationship between polymorphism of angiotensin-converting enzyme gene insertion/deletion and risk of hepatocellular carcinoma in a Chinese Dai population. J Renin Angiotensin Aldosterone Syst. (2015) 16:695–9. doi: 10.1177/1470320314539829
33. Hajek D, Tomiska M, Krahulcova E, Druckmuller M, Florianova M, Izakovicova-Holla L, et al. I/D ACE gene polymorphism in survival of leukemia patients – hypothesis and pilot study. Med Hypotheses (2003) 61:80–5. doi: 10.1016/S0306-9877(03)00122-1
34. Matsuki-Muramoto Y, Nozawa K, Uomori K, Sekigawa I, Takasaki Y. Bortezomib treatment prevents glomerulosclerosis associated with lupus nephritis in a murine model through suppressive effects on the immune and renin-angiotensin systems. Mod Rheumatol. (2017) 27:77–86. doi: 10.3109/14397595.2016.1170957
35. Qin XY, Zhang YL, Chi YF, Yan B, Zeng XJ, Li HH, et al. Angiotensin II regulates Th1 T cell differentiation through angiotensin II type 1 receptor-PKA-mediated activation of proteasome. Cell Physiol Biochem. (2018) 45:1366–76. doi: 10.1159/000487562
36. Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC. Bortezomib in multiple myeloma: systemic review and clinical considerations. Curr Oncol. (2014) 21:573–603. doi: 10.3747/co.21.1798
Keywords: ACE gene, I/D polymorphism, plasma cell myeloma, bortezomib, rs4646994
Citation: Zmorzynski S, Szudy-Szczyrek A, Popek-Marciniec S, Korszen-Pilecka I, Wojcierowska-Litwin M, Luterek M, Chocholska S, Styk W, Swiderska-Kołacz G, Januszewska J, Mielnik M, Hus M and Filip AA (2019) ACE Insertion/Deletion Polymorphism (rs4646994) Is Associated With the Increased Risk of Multiple Myeloma. Front. Oncol. 9:44. doi: 10.3389/fonc.2019.00044
Received: 25 September 2018; Accepted: 16 January 2019;
Published: 06 February 2019.
Edited by:
Pierluigi Porcu, Sidney Kimmel Cancer Center, United StatesReviewed by:
Antonello Di Paolo, University of Pisa, ItalySara Galimberti, University of Pisa, Italy
Copyright © 2019 Zmorzynski, Szudy-Szczyrek, Popek-Marciniec, Korszen-Pilecka, Wojcierowska-Litwin, Luterek, Chocholska, Styk, Swiderska-Kołacz, Januszewska, Mielnik, Hus and Filip. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Szymon Zmorzynski, cy56bW9yenluc2tpQGdtYWlsLmNvbQ==
 Szymon Zmorzynski
Szymon Zmorzynski Aneta Szudy-Szczyrek2
Aneta Szudy-Szczyrek2