- Division of Dermatology, University of Washington, Seattle, WA, United States
Skin directed therapies (SDTs) serve important roles in the treatment of early stage cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF), as well as managing symptoms and improving quality of life of all stages. There are now numerous options for topical therapies that demonstrate high response rates, particularly in early/limited MF. Phototherapy retains an important role in treating MF, with increasing data supporting efficacy and long-term safety of both UVB and PUVA as well as some newer/targeted methodologies. Radiation therapy, including localized radiation and total skin electron beam therapy, continues to be a cornerstone of therapy for all stages of MF.
Introduction
Skin directed therapies (SDTs) in cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF) serve important roles in treating disease, but also in treating symptoms. Although SDTs can be used to cure CTCL in some patients with limited or early stage MF (stage 1A, 1B), they are most often used with palliative intent at all stages (1), with adjunct roles for both treatment and symptom management in more advanced MF, particularly managing pruritus and maintaining the skin barrier. The current National Comprehensive Cancer Network (NCCN) guidelines (1) recommend a general list of SDTs, but do not dictate the order in which they should be selected, allowing flexibility for selection based on both practitioner and patient factors.
One of the most important considerations when selecting a SDT is the extent of skin involvement (T stage). Although most SDT are appropriate for patients with any stage MF, topical preparations may be most practical (and therefore utilized with the highest compliance and best response) for those with limited skin body surface area involvement compared to patients with generalized skin involvement. In addition, a patient's ability to use a treatment as prescribed must be considered; a patient who lives alone may not be able to apply a topical therapy to their back, and someone who cannot stand without assistance may not be able to safely comply with phototherapy. Lastly, there are regional differences in preference for particular SDTs, and not all therapies are available in all regions worldwide.
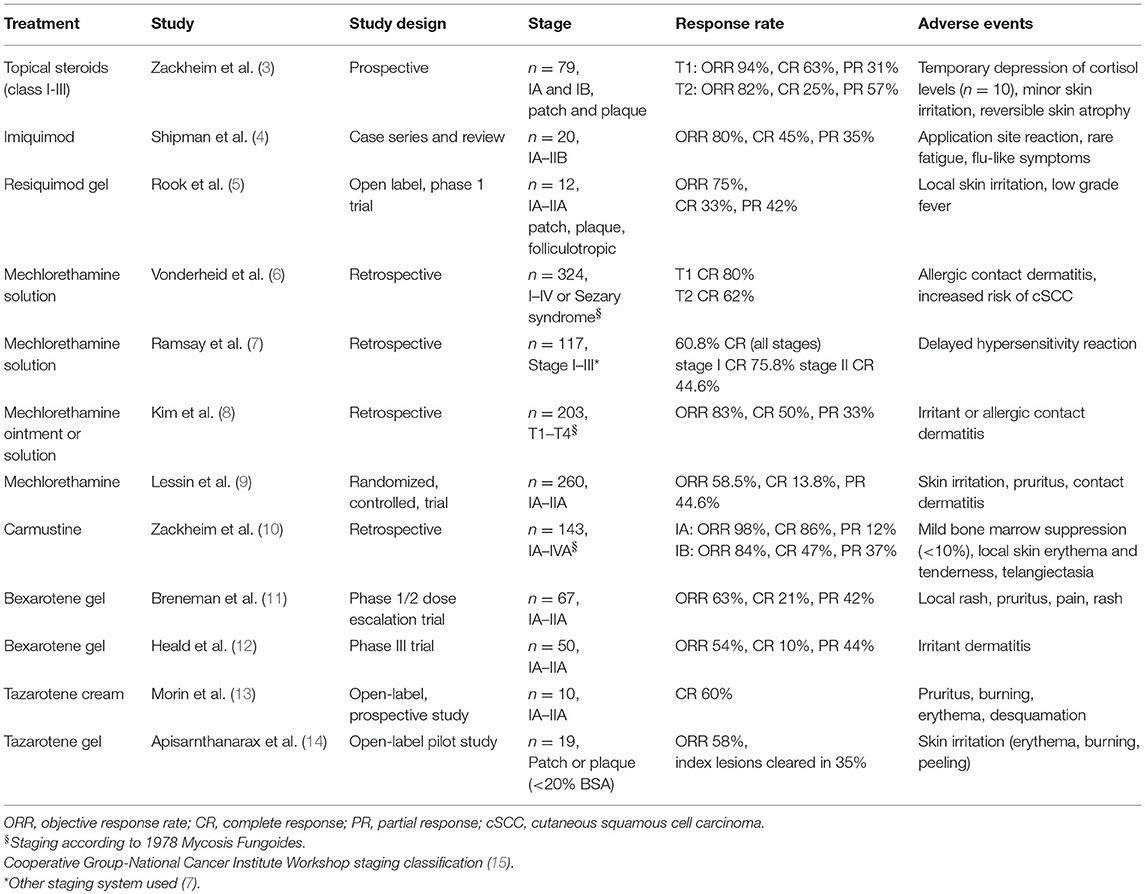
Many of the studies evaluating SDTs were performed before standard definitions of clinical end points and response criteria were defined for CTCL/MF (2). As such, the difficulty comparing efficacy rates of different therapies should be considered when evaluating treatment options. A summary of the significant studies supporting the use of the various SDTs reviewed in this article is included in Table 1.
Topical Therapies
Topical Corticosteroids
Topical corticosteroids have been reported in CTCL since the 1960s (16). A prospective study of 79 patients demonstrated overall response rates (ORR) of 94% for T1 (IA) disease and 82% for T2 (IB) disease, with complete response (CR) rates of 63% and 25%, respectively (3). In an updated report, 200 patients with early MF were treated with class I corticosteroids, with ORR of 80–90% (17). Less potent topical steroids may also be effective when used under occlusion (18).
Cutaneous side effects to topical corticosteroids in CTCL are common. In Zackheim et al.'s series, 10–20% of patients using class I steroids for ≥3 months developed irritant dermatitis or purpura, and some developed atrophy and striae (17). Reversible suppression of cortisol levels can also occur with topical steroids in CTCL (3).
Imidazoquinolines (Imiquimod and Resiquimod)
Imiquimod is a toll-like receptor-7 (TLR) agonist with antiviral and antitumor properties. Topical imiquimod leads to local production of interferon (IFN)-α, tumor necrosis factor-α, interleukin (IL)–-1, and IL-6, (19, 21) and induces direct tumor cell death and apoptosis (19, 22).
Published data on imiquimod in CTCL is limited. In a case series of 20 patients with IA-IIB mycosis fungoides (MF), treatment with imiquimod 5% yielded an ORR of 80%, with 45% CR and 35% partial response (PR). Twenty percent of patients did not respond (4). Side effects of imiquimod are typically skin-limited, including pain, redness, local irritation, ulceration, and pruritus (4). There are rare reports of patients experiencing flu like symptoms and fatigue (23, 24). Most adverse events resolve after the first few weeks of treatment, or with short treatment interruption (25, 26).
Resiquimod is a potent agonist of TLR-7 and TLR-8, leading to production of IFN-α, IL-12, and IL-15 (21). In a Phase I trial of 12 patients with IA-IIA CTCL, treatment with 0.03 and 0.06% topical resiquimod gel resulted in clinical improvement in 75% of treated lesions. Three patients had responses in untreated lesions, suggesting systemic activity. Adverse events were mostly skin limited, but 2 patients developed fever. Ninety percent of patients had a decrease in malignant T cell clones in treated lesions (5).
Mechlorethamine Hydrochloride (Nitrogen Mustard)
Mechlorethamine hydrochloride [nitrogen mustard (NM)] is one of the most studied treatments for CTCL, with studies reporting on the topical use of NM for CTCL from the late 1950s onward (27, 28). NM is a cytotoxic alkylating agent thought to act directly on malignant cells by causing apoptosis, though the exact mechanism of action of topical NM in CTCL is unknown (22, 29).
Topical NM is a first line treatment for localized and generalized MF (1). NM can be used as a compounded solution or ointment, or a commercial gel formulation. Data on efficacy of NM vary by study and preparation, but all topical preparations have high response rates in MF, including significant rates of CR. For NM solution, 50–60% CR rates are reported (6–8). Responses are similar with aqueous solution and ointment (8). Median time to CR with NM is on the order of 6–12 months (7, 8).
The phase 2 trial leading to FDA approval of topical mechlorethamine gel (Valchlor) included 260 stage IA to IIA MF patients treated with 0.02% gel daily for up to 12 months. The ORR (CR + PR) using Composite Assessment of Index Lesion Severity (CAILS) was 58.5%, with 13.8% CR. Responses were durable; 85.5% had ongoing responses at 12 months (9).
Overall, topical NM is well tolerated, with side effects minor and skin limited (8, 9, 30). Common side effects include contact dermatitis, which is most frequent with the aqueous solution (64.7% in one series) (31). The rate of allergic contact dermatitis (ACD) with ointment preparations is significantly lower (<10%), though irritant reactions still occur in about 25% (particularly on the face and intertriginous areas). Decreasing frequency or concentration of NM may improve tolerability (8, 29). The rate of ACD with commercial NM gel was 16.4%, though 25% experienced skin irritation (9). The use of topical steroids and NM together may improve tolerability and allow for less frequent NM application (30).
The risk of secondary malignancies in patients treated with topical NM is conflicting and difficult to assess, as many patients also receive concurrent treatments (29). There are reports of MF patients with no significant risk factors developing skin cancer after topical NM (6, 32), while at least one study has showed no significant increase in secondary skin cancers with long duration of NM therapy (33).
Carmustine
Carmustine (bis-chloroethyl-nitrosourea; BCNU) is an alkylating agent that cross-links DNA, causing apoptosis (34, 35). Zackheim reported 143 patients with CTCL treated with topical carmustine solution at variable dosing (from 2 mg/mL local up to 60 mg total body daily) (10). The ORR in patients with T1 (IA) disease was 98%, with 86% CR and 12% PR. In patients with T2 (IB) disease, ORR was 84%, with 47% CR and 37% PR. The median time to CR for all patients was 11.5 weeks (range 3–104 weeks) (10).
Cutaneous adverse events with BCNU are common, with frequent erythema, particularly in the intertriginous skin. Contact dermatitis can also occur. Telangiectasias can develop in areas of prior erythema, and may be permanent. Mild bone marrow suppression (leukopenia and anemia) can occur with widespread BCNU therapy; monthly monitoring of complete blood counts is recommended for these patients (10).
Topical Retinoids
Bexarotene
Bexarotene is a retinoid X receptor agonist. Topical bexarotene 1% gel is FDA approved for the treatment of stage IA and IB persistent or refractory CTCL. The mode of action of bexarotene in CTCL is unclear, but it has been reported to cause apoptosis in CTCL cell lines (36, 37).
Topical bexarotene demonstrates benefit for early MF, however, adverse events and intolerance are common. The Phase 1/2 trial of bexarotene gel enrolled 67 patients with stage IA-IIA CTCL/MF. ORR was 63%, with CR in 21% and PR in 42%. Local adverse events occurred in 87% of patients, and included rash, pruritus, pain, and vesiculobullous rash (11). A phase III trial of topical bexarotene 1% gel showed similar findings, with an ORR of 54%, clinical CR in 10%, and frequent dose related irritant dermatitis (12).
Tazarotene
Tazarotenic acid binds retinoic acid receptors (RAR)-β and RAR-γ, exerting anti-proliferative and anti-inflammatory affects in the skin (38, 39). The first study of topical tazarotene as monotherapy in CTCL was published in 2016. Ten patients with early stage CTCL/MF were treated with tazarotene 0.1% cream to index lesions every other day for 2 weeks, then once daily for 6 months. Sixty percent of patients had a CR, with mean time to CR 3.8 months. Seventy percent of patients reported grade I or II side effects including pruritus, burning, erythema, and desquamation, and two patients withdrew from study because adverse events (13). Topical tazarotene 0.1% gel has also been evaluated in 19 patients as adjuvant therapy, with an ORR of 58% with once daily application for 24 weeks. Local skin irritation was reported in 84% (14).
Phototherapy
Several groups have reviewed the existing studies and published guidelines for the use of phototherapy in CTCL/MF (40, 41). The United States Cutaneous Lymphoma Consortium (USCLC) recommends phototherapy as monotherapy for patients with early (stages IA–IIA) CTCL/MF, and in combination with systemic therapies for refractory early disease or advanced disease (41). The choice of nbUVB vs. PUVA as the initial therapy may be dictated by patient preference or by access issues. UVA has better skin penetration than UVB, and patients with thicker plaques or folliculotropic disease (41) or darker skin (42) may get more benefit from PUVA.
The USCLC offers expert consensus recommendations for phototherapy treatment protocols (41). In general, phototherapy for CTCL includes clearance, consolidation, and maintenance phases. The USCLC defines the goal of the clearance phase as 100% clearance (41). The clearance phase for CTCL may take longer than for other skin diseases that are treated with phototherapy. Patients with CTCL may also benefit from a 1–3 month long “consolidation phase” between clearance and maintenance phases, in which the frequency and dose of treatments is held constant (41). The consolidation phase may maximize the potential of histologic and molecular clearance (including loss of the dominant T-cell clone), which can lag behind clinical clearance (43, 44). Inclusion of a prolonged maintenance phase after clearance of MF may prolong the time to relapse (45) and reduce relapse rates (46), but remains controversial given the potential for increased UV exposure and a lack of significant data supporting a decrease in relapse (40).
Contraindications and Side Effects of Phototherapy
General contraindications to phototherapy include photosensitive disorders, including xeroderma pigmentosa, lupus erythematosus, and porphyrias. PUVA should not be used in pregnant or lactating women, or those with a history of melanoma or multiple non-melanoma skin cancers. Relative contraindications to phototherapy in general include chronic actinic dermatitis and claustrophobia. Phototherapy should be used cautiously in those who are immunosuppressed, in children, or those who take photosensitizing medications (41).
Common side effects during phototherapy for CTCL include erythema and pruritus. Pruritus can be a particularly bothersome issue after starting phototherapy, particularly during PUVA (“PUVA itch”) (47). Photodamage is also a common side effect of phototherapy, particularly with PUVA, in which at least 27% of CTCL/MF patients show signs of photodamage (48).
One of the most serious potential side effects of phototherapy is secondary skin cancer. There are meta-analyses and large studies of patients assessing the risk of skin cancer with psoriasis and other skin disorders treated with UVB. In general, the risk of skin cancer overall does not appear to be significantly increased with UVB phototherapy alone (49, 50), though there may be an increase in basal cell carcinomas (BCCs) and genital skin cancers in patients who have received both UVB and PUVA (50). Patients treated with PUVA do have an increased risk of skin cancer, particularly squamous cell carcinoma (SCC) and BCC (51). In a long term study of a cohort of CTCL patients that received PUVA, the incidence of skin cancer was 26% (48).
Ultraviolet radiation induces UV-signature DNA mutations, and there is a theoretical concern that phototherapy itself could induce progression of CTCL. Hoot et al analyzed their cohort of 345 MF patients, and found that patients treated with phototherapy had a longer time to tumor progression (3.5 years) compared to those who did not receive phototherapy (1.2 years), arguing that phototherapy does not appear to increase the risk of tumor progression (52).
Psoralen Plus UVA (PUVA)
PUVA was the first type of phototherapy used to treat CTCL (53), and is still the initial phototherapy choice preferred by many CTCL experts (54). PUVA is effective for early MF, with estimated response rates of 85% for stage IA, and 65% for stage IB (41). Patients with phototypes I or II may respond better to PUVA compared to patients with skin of color (45). Time to CR with PUVA therapy is reported in the 2–4 month range when patients are treated 2–3 times weekly (48). Those with thicker or infiltrated plaques may require longer times to clearance compared to thin plaques or patches, and PUVA is not as effective for tumor stage MF (55). Patients with hand and/or foot lesions can be treated with hand/foot bath PUVA alone or in addition to whole body treatment.
Evidence supports the use of maintenance therapy with PUVA after attaining complete clearance. Inclusion of a maintenance period is associated with longer time until relapse (45), though doesn't appear to impact overall relapse rates or survival (48).
In addition to the general contraindications and side effects of phototherapy listed above, 8-methoxypsoralen (8-MOP; methoxsalen, Oxsoralen Ultra), the most commonly used psoralen in the United States, can cause nausea and abdominal pain. 5-methoxypsoralen (5-MOP) shows similar efficacy to 8-MOP and with fewer side effects (56), but is not currently available in the United States. Psoralens can accumulate in the lens of the eye and theoretically increase the likelihood of cataracts. When eye protection is used at the current recommendation of 12–24 h after ingestion of psoralens, the risk of cataracts with PUVA therapy does not appear to be significantly increased (57).
UVB
Broadband UVB (bbUVB) was historically used to treat CTCL/MF, with high clearance rates (71% for patients with stage IA and 44% of patients with IB), but frequent (70%) relapses (46). Among lymphoma experts, bbUVB has largely been replaced by nbUVB (54).
Narrowband UVB (nbUVB) has complete response rates in the 54–90% range (41), with patches responding better than plaques (44). Among CTCL experts, nbUVB is the initial phototherapy treatment of choice for patients with stage IA disease and fair (phototypes I and II) skin (45). Patients with the hypopigmented variant of MF may not respond as well compared to other variants of MF (42).
Whether maintenance therapy with nbUVB delays relapse is unclear. The USCLC recommends maintenance therapy given that there does seem to be decrease in relapse rate when patients undergo maintenance after complete clearance with nbUVB compared to those who do not receive maintenance (41).
In general, nbUVB is better tolerated than PUVA, with fewer reported side effects (45).
Other Types of Phototherapy
Excimer (MEL)
There are several reports supporting the use of the monochromatic excimer light (308 nm) (“MEL”) for patch MF. Two series reported high response rates to MEL, with one study reporting 4/4 patients with complete clinical and histologic response (58) and another with complete clinical response in 4/5 patients (59). Follow up times were short. MEL may have a particularly useful role for sanctuary sites or sites that are not as easily accessible by phototherapy or topical preparations, such as acral surfaces (60) or intertriginous areas.
UVA1
Longwave or narrowband UVA (UVA1) penetrates the skin at the level of the dermis, and does not require adjunct psoralen, resulting in lower phototoxicity compared to PUVA (61). Several small series have reported on the use of UVA1 for early (stage IA–IIA) MF. The largest included 19 patients, with CR in 63% and PR in 37% with a treatment protocol of 30 J/cm2 5 times a week for 5 weeks. Relapses occurred in 58% within 3 months of completing therapy (62). Prior reports have suggested higher response rates, but with low clarity on the definition of response and no follow up interval reported (63). Given low toxicity, UVA1 therapy may be a safe alternative or adjunct to other skin directed therapies.
Photodynamic Therapy
Photodynamic therapy (PDT) uses visible light to activate a topically applied photosensitizing agent, leading to the generation of reactive oxygen species and cell death in the affected cells. PDT demonstrates activity treating actinic keratosis and non-melanoma skin cancers (64). There are several reports supporting the use of PDT for MF. The most commonly reported photosensitizer used in MF is 5-aminolevulinic acid (ALA). In one series, plaque and tumor lesions from 10 patients were treated. Seven out of nine treated plaques had complete clinical response; tumors did not respond. A commonly reported side effect of PDT is pain; in this series, one patient dropped out because of pain, and most patients reported erythema and local edema (65).
Radiation Therapy
MF is highly radiosensitive, and radiation therapy is effective in most stages of MF. Radiotherapy can be used with curative intent in patients with single (unilesional) early stage MF, and with palliative intent in all stages, with the goal of improving symptoms and cosmesis (66).
Electron beam (e-beam) is the preferred type of radiation therapy for MF, except in certain instances such as exophytic tumors or complex surfaces, in which X-rays or photons may be superior (67). The International Lymphoma Radiation Oncology Group (ILROG) recommends guidelines for radiation therapy for MF (67). When patients with single lesions of MF are treated with the intent to cure, CR rates can be as high as 100%, with no recurrences at treated sites (68). Radiation therapy is utilized most frequently as a palliative treatment in MF. Palliative doses of 8–12 Gy are recommended, given CR rates of >90% at doses ≥8 Gy, and higher non-response rates at lower doses (1, 69). Higher doses may be required for tumor stage or large cell transformed MF (69). For patients traveling long distances or with difficulty accessing radiation therapy, treatment can occur in a single fraction, while retaining a high CR rate (94.4%) and at significant cost savings (70). Localized radiotherapy can be especially helpful for otherwise difficult to treat sites, such as the eyelids, and the hands and feet (71).
Side effects of local radiation therapy are dose dependent, and at very low doses (2 Gy) radiotherapy to the skin can have essentially no side effects (69). Even at higher doses, side effects can be minimal (72); commonly reported side effects include erythema, desquamation, atrophy, and skin dryness (68).
Total skin electron beam (TSEBT) is a technique of delivering electron beam radiation to the entire skin surface, usually by positioning patients and exposing to multiple intersecting beams (73). TSEBT has been described as the single most effective SDT for MF (74). Traditionally, doses as high as 36 Gy (“conventional dose”) have been used, with complete response rates in the 75–95% range (73). Relapses are frequent after TSEBT, occurring in the majority of patients. Common side effects of conventional dosing TSEBT include erythema, desquamation, alopecia, nail changes, and lower extremity edema (73). Higher dose TSEBT is also associated with irreversible alopecia and low sperm counts in men (73).
Hoppe et al. published pooled data on the use of low dose (12 Gy) TSEBT for CTCL. Potential advantages to the use of low dose TSEBT over conventional dose TSEBT include lower toxicity and the potential for repeat treatment courses. ORR with low dose TSEBT was 88%, with 27% CR. Side effects were mild, reversible and less severe than reported with 36 Gy dosing. Of note, 12% of patients developed skin infections with Staphylococcus aureus during treatment (74); coverage with anti-staphylococcal antibiotics during TSEBT may be warranted.
The use of adjunct treatments to “consolidate” TSEBT may improve the durability. Data from the Stanford group suggest that following a TSEBT-induced CR with topical nitrogen mustard allows for longer freedom from relapse (but did not impact survival) (75). The addition of extracoroporeal photopheresis (ECP) concurrently or immediately after TSEBT has also been shown to be helpful, improving survival for patients with erythrodermic MF (76).
The literature evaluating the risk of secondary skin malignancy in CTCL is limited, but some reports suggest a possible increase in both melanoma non-melanoma skin cancer in CTCL patients with prior TSEBT (77).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
MS has served as principle investigator for Actelion and Soligenix.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Primary, Cutaneous Lymphomas (2018). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf (Accessed November 21, 2018).
2. Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and sezary syndrome: a consensus statement of the international society for cutaneous lymphomas, the united states cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the european organisation for research and treatment of cancer. J Clin Oncol. (2011) 29:2598–607. doi: 10.1200/JCO.2010.32.0630
3. Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. (1998) 134:949–54. doi: 10.1001/archderm.134.8.949
4. Shipman AR, Scarisbrick J. New treatment options for mycosis fungoides. Indian J Dermatol. (2016) 61:119. doi: 10.4103/0019-5154.174085
5. Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. (2015) 126:1452–61. doi: 10.1182/blood-2015-02-630335
6. Vonderheid EC, Tan ET, Kantor AF, Shrager L, Micaily B, Van Scott EJ. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol. (1989) 20:416–28. doi: 10.1016/S0190-9622(89)70051-7
7. Ramsay DL, Halperin PS, Zeleniuch-Jacquotte A. Topical mechlorethamine therapy for early stage mycosis fungoides. J Am Acad Dermatol. (1988) 19:684–91. doi: 10.1016/S0190-9622(88)70223-6
8. Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. (2003) 139:165–73. doi: 10.1001/archderm.139.2.165
9. Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. (2013) 149:25–32. doi: 10.1001/2013.jamadermatol.541
10. Zackheim HS, Epstein EH, Jr, Crain WR. Topical carmustine (BCNU) for cutaneous T cell lymphoma: a 15-year experience in 143 patients. J Am Acad Dermatol. (1990) 22(5 Pt 1):802–10. doi: 10.1016/0190-9622(90)70112-U
11. Breneman D, Duvic M, Kuzel T, Yocum R, Truglia J, Stevens VJ. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol. (2002) 138:325–32. doi: 10.1001/archderm.138.3.325
12. Heald P, Mehlmauer M, Martin AG, Crowley CA, Yocum RC, Reich SD. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. (2003) 49:801–15. doi: 10.1016/S0190-9622(03)01475-0
13. Besner Morin C, Roberge D, Turchin I, Petrogiannis-Haliotis T, Popradi G, Pehr K. Tazarotene 0.1% cream as monotherapy for early-stage cutaneous T-cell lymphoma. J Cutan Med Surgery. (2016) 20:244–8. doi: 10.1177/1203475415626686
14. Apisarnthanarax N, Talpur R, Ward S, Ni X, Kim HW, Duvic M. Tazarotene 0.1% gel for refractory mycosis fungoides lesions: an open-label pilot study. J Am Acad Dermatol. (2004) 50:600–7. doi: 10.1016/j.jaad.2003.09.005
15. Bunn PA, Jr, Lamberg SI. Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep. (1979) 63:725–8.
16. Farber EM, Cox AJ, Steinberg J, McClintock RP. Therapy of mycosis fungoides with topically applied fluocinolone acetonide under occlusive dressing. Cancer. (1966) 19:237–45.
17. Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. (2003) 16:283–7. doi: 10.1111/j.1396-0296.2003.01639.x
18. Farber EM, Zackheim HS, McClintock RP, Cox AJ, Jr. Treatment of mycosis fungoides with various strengths of fluocinolone acetonide cream. Arch Dermatol. (1968) 97:165–72. doi: 10.1001/archderm.1968.01610080069013
19. Schon MP, Schon M. Immune modulation and apoptosis induction: two sides of the antitumoral activity of imiquimod. Apoptosis. (2004) 9:291–8. doi: 10.1023/B:APPT.0000025805.55340.c3
20. Dummer R, Urosevic M, Kempf W, Hoek K, Hafner J, Burg G. Imiquimod in basal cell carcinoma: how does it work? Br J Dermatol. (2003) 149(Suppl 66):57–8. doi: 10.1046/j.0366-077X.2003.05630.x
21. Huen AO, Rook AH. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr Opin Oncol. (2014) 26:237–44. doi: 10.1097/CCO.0000000000000048
22. Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. (2005) 115:798–812. doi: 10.1172/JCI24826
23. Lewis DJ, Byekova YA, Emge DA, Duvic M. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatol Treat. (2017) 28:567–9. doi: 10.1080/09546634.2017.1294728
24. Deeths MJ, Chapman JT, Dellavalle RP, Zeng C, Aeling JL. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. (2005) 52:275–80. doi: 10.1016/j.jaad.2004.04.049
25. Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. (2006) 16:391–3.
26. Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-Cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. (2002) 138:1137–9. doi: 10.1001/archderm.138.9.1137
27. Remission of lesions in mycosis fungoides following topical application of nitrogen mustard. By John R. Haserick, Joseph H. Richardson, and Douglas J. Grant. Cleve Clin Q. (1983) 50:91–5.
28. Watson JI, Wilkinson RD, Craig GE. Topical nitrogen mustard in cutaneous lymphosarcoma (mycosis fungoides). Can Med Assoc J. (1962) 87:1284–5.
29. Kim YH. Management with topical nitrogen mustard in mycosis fungoides. Dermatol Ther. (2003) 16:288–98. doi: 10.1111/j.1396-0296.2003.01640.x
30. de Quatrebarbes J, Esteve E, Bagot M, Bernard P, Beylot-Barry M, Delaunay M, et al. Treatment of early-stage mycosis fungoides with twice-weekly applications of mechlorethamine and topical corticosteroids: a prospective study. Arch Dermatol. (2005) 141:1117–20. doi: 10.1001/archderm.141.9.1117
31. Lindahl LM, Fenger-Gron M, Iversen L. Topical nitrogen mustard therapy in patients with mycosis fungoides or parapsoriasis. J Eur Acad Dermatol Venereol. (2013) 27:163–8. doi: 10.1111/j.1468-3083.2011.04433.x
32. Lee LA, Fritz KA, Golitz L, Fritz TJ, Weston WL. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. (1982) 7:590–8. doi: 10.1016/S0190-9622(82)70138-0
33. Lindahl LM, Fenger-Gron M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. (2014) 170:699–704. doi: 10.1111/bjd.12620
34. Tong WP, Kirk MC, Ludlum DB. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N'-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. (1982) 42:3102–5.
35. Kohn KW. Interstrand cross-linking of DNA by 1,3-bis(2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. (1977) 37:1450–4.
36. Zhang C, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res. (2002) 8:1234–40.
38. Talpur R, Cox K, Duvic M. Efficacy and safety of topical tazarotene: a review. Exp Opin Drug Metabol Toxicol. (2009) 5:195–210. doi: 10.1517/17425250902721250
39. Chandraratna RA. Tazarotene–first of a new generation of receptor-selective retinoids. Br J Dermatol. (1996) 135(Suppl 49):18–25. doi: 10.1111/j.1365-2133.1996.tb15662.x
40. Grandi V, Fava P, Rupoli S, Alberti Violetti S, Canafoglia L, Quaglino P, et al. Standardization of regimens in Narrowband UVB and PUVA in early stage mycosis fungoides: position paper from the Italian task force for cutaneous lymphomas. J Eur Acad Dermatol Venereol. (2018) 32:683–91. doi: 10.1111/jdv.14668
41. Olsen EA, Hodak E, Anderson T, Carter JB, Henderson M, Cooper K, et al. Guidelines for phototherapy of mycosis fungoides and Sezary syndrome: a consensus statement of the United States cutaneous lymphoma consortium. J Am Acad Dermatol. (2016) 74:27–58. doi: 10.1016/j.jaad.2015.09.033
42. Gathers RC, Scherschun L, Malick F, Fivenson DP, Lim HW. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. (2002) 47:191–7. doi: 10.1067/mjd.2002.120911
43. Dereure O, Picot E, Comte C, Bessis D, Guillot B. Treatment of early stages of mycosis fungoides with narrowband ultraviolet B. A clinical, histological and molecular evaluation of results. Dermatology. (2009) 218:1–6. doi: 10.1159/000161114
44. Gokdemir G, Barutcuoglu B, Sakiz D, Koslu A. Narrowband UVB phototherapy for early-stage mycosis fungoides: evaluation of clinical and histopathological changes. J Eur Acad Dermatol Venereol. (2006) 20:804–9. doi: 10.1111/j.1468-3083.2006.01635.x
45. Nikolaou V, Sachlas A, Papadavid E, Economidi A, Karambidou K, Marinos L, et al. Phototherapy as a first-line treatment for early-stage mycosis fungoides: the results of a large retrospective analysis. Photodermatol Photoimmunol Photomed. (2018) 34:307–13. doi: 10.1111/phpp.12383
46. Pavlotsky F, Barzilai A, Kasem R, Shpiro D, Trau H. UVB in the management of early stage mycosis fungoides. J Eur Acad Dermatol Venereol. (2006) 20:565–72. doi: 10.1111/j.1468-3083.2006.01557.x
47. Roelandts R, Stevens A. PUVA-induced itching and skin pain. Photodermatol Photoimmunol Photomed. (1990) 7:141–2.
48. Querfeld C, Rosen ST, Kuzel TM, Kirby KA, Roenigk HH, Jr, Prinz BM, et al. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Arch Dermatol. (2005) 141:305–11. doi: 10.1001/archderm.141.3.305
49. Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. (2008) 159:931–5. doi: 10.1111/j.1365-2133.2008.08776.x
50. Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. (2005) 44:355–60. doi: 10.1111/j.1365-4632.2004.02186.x
51. Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. (2012) 26(Suppl 3):22–31. doi: 10.1111/j.1468-3083.2012.04520.x
52. Hoot JW, Wang L, Kho T, Akilov OE. The effect of phototherapy on progression to tumors in patients with patch and plaque stage of mycosis fungoides. J Dermatol Treat. (2018) 29:272–6. doi: 10.1080/09546634.2017.1365113
53. Gilchrest BA, Parrish JA, Tanenbaum L, Haynes HA, Fitzpatrick TB. Oral methoxsalen photochemotherapy of mycosis fungoides. Cancer. (1976) 38:683–9.
54. Carter J, Zug KA. Phototherapy for cutaneous T-cell lymphoma: online survey and literature review. J Am Acad Dermatol. (2009) 60:39–50. doi: 10.1016/j.jaad.2008.08.043
55. Briffa DV, Warin AP, Harrington CI, Bleehen SS. Photochemotherapy in mycosis fungoides. A study of 73 patients. Lancet. (1980) 2:49–53. doi: 10.1016/S0140-6736(80)92937-2
56. Wackernagel A, Hofer A, Legat F, Kerl H, Wolf P. Efficacy of 8-methoxypsoralen vs. 5-methoxypsoralen plus ultraviolet A therapy in patients with mycosis fungoides. Br J Dermatol. (2006) 154:519–23. doi: 10.1111/j.1365-2133.2005.07008.x
57. Malanos D, Stern RS. Psoralen plus ultraviolet a does not increase the risk of cataracts: a 25-year prospective study. J Am Acad Dermatol. (2007) 57:231–7. doi: 10.1016/j.jaad.2007.04.027
58. Mori M, Campolmi P, Mavilia L, Rossi R, Cappugi P, Pimpinelli N. Monochromatic excimer light (308 nm) in patch-stage IA mycosis fungoides. J Am Acad Dermatol. (2004) 50:943–5. doi: 10.1016/j.jaad.2004.01.047
59. Passeron T, Zakaria W, Ostovari N, Perrin C, Larrouy JC, Lacour JP, et al. Efficacy of the 308-nm excimer laser in the treatment of mycosis fungoides. Arch Dermatol. (2004) 140:1291–3. doi: 10.1001/archderm.140.10.1291
60. Jin SP, Jeon YK, Cho KH, Chung JH. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. (2010) 163:651–3. doi: 10.1111/j.1365-2133.2010.09793.x
61. Krutmann J, Morita A. Mechanisms of ultraviolet (UV) B and UVA phototherapy. J Invest Dermatol Symp Proc. (1999) 4:70–2. doi: 10.1038/sj.jidsp.5640185
62. Adisen E, Tektas V, Erduran F, Erdem O, Gurer MA. Ultraviolet A1 phototherapy in the treatment of early mycosis fungoides. Dermatology. (2017) 233:192–8. doi: 10.1159/000458149
63. Olek-Hrab K, Silny W, Danczak-Pazdrowska A, Osmola-Mankowska A, Sadowska PA, Polanska A, et al. Ultraviolet A1 phototherapy for mycosis fungoides. Clin Exp Dermatol. (2013) 38:126–30. doi: 10.1111/ced.12001
64. Ross K, Cherpelis B, Lien M, Fenske N. Spotlighting the role of photodynamic therapy in cutaneous malignancy: an update and expansion. Dermatol Surgery. (2013) 39:1733–44. doi: 10.1111/dsu.12319
65. Edstrom DW, Porwit A, Ros AM. Photodynamic therapy with topical 5-aminolevulinic acid for mycosis fungoides: clinical and histological response. Acta Dermato Venereol. (2001) 81:184–8. doi: 10.1080/000155501750376276
66. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. (2009) 114:4337–53. doi: 10.1182/blood-2009-07-202895
67. Specht L, Dabaja B, Illidge T, Wilson LD, Hoppe RT. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma radiation oncology group. Int J Rad Oncol Biol Phys. (2015) 92:32–9. doi: 10.1016/j.ijrobp.2015.01.008
68. Micaily B, Miyamoto C, Kantor G, Lessin S, Rook A, Brady L, et al. Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncol Biol Phys. (1998) 42:361–4. doi: 10.1016/S0360-3016(98)00218-1
69. Neelis KJ, Schimmel EC, Vermeer MH, Senff NJ, Willemze R, Noordijk EM. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys. (2009) 74:154–8. doi: 10.1016/j.ijrobp.2008.06.1918
70. Thomas TO, Agrawal P, Guitart J, Rosen ST, Rademaker AW, Querfeld C, et al. Outcome of patients treated with a single-fraction dose of palliative radiation for cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys. (2013) 85:747–53. doi: 10.1016/j.ijrobp.2012.05.034
71. Goddard AL, Vleugels RA, LeBoeuf NR, O'Farrell DA, Cormack RA, Hansen JL, et al. Palliative therapy for recalcitrant cutaneous T-cell lymphoma of the hands and feet with low-dose, high dose-rate brachytherapy. JAMA Dermatol. (2015) 151:1354–7. doi: 10.1001/jamadermatol.2015.3028
72. Piccinno R, Caccialanza M, Cuka E, Recalcati S. Localized conventional radiotherapy in the treatment of mycosis fungoides: our experience in 100 patients. J Eur Acad Dermatol Venereol. (2014) 28:1040–4. doi: 10.1111/jdv.12254
73. Jones GW, Kacinski BM, Wilson LD, Willemze R, Spittle M, Hohenberg G, et al. Total skin electron radiation in the management of mycosis fungoides: consensus of the European Organization for Research and Treatment of Cancer (EORTC) cutaneous lymphoma project group. J Am Acad Dermatol. (2002) 47:364–70. doi: 10.1067/mjd.2002.123482
74. Hoppe RT, Harrison C, Tavallaee M, Bashey S, Sundram U, Li S, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. (2015) 72:286–92. doi: 10.1016/j.jaad.2014.10.014
75. Chinn DM, Chow S, Kim YH, Hoppe RT. Total skin electron beam therapy with or without adjuvant topical nitrogen mustard or nitrogen mustard alone as initial treatment of T2 and T3 mycosis fungoides. Int J Radiation Oncol Biol Phys. (1999) 43:951–8. doi: 10.1016/S0360-3016(98)00517-3
76. Wilson LD, Jones GW, Kim D, Rosenthal D, Christensen IR, Edelson RL, et al. Experience with total skin electron beam therapy in combination with extracorporeal photopheresis in the management of patients with erythrodermic (T4) mycosis fungoides. J Am Acad Dermatol. (2000) 43(1 Pt 1):54–60. doi: 10.1067/mjd.2000.105510
77. Licata AG, Wilson LD, Braverman IM, Feldman AM, Kacinski BM. Malignant melanoma and other second cutaneous malignancies in cutaneous T-cell lymphoma. The influence of additional therapy after total skin electron beam radiation. Arch Dermatol. (1995) 131:432–5. doi: 10.1001/archderm.1995.01690160060009
Keywords: cutaneous T-cell lymphoma, mycosis fungoides, skin-directed therapy, nitrogen mustard, phototherapy
Citation: Tarabadkar ES and Shinohara MM (2019) Skin Directed Therapy in Cutaneous T-Cell Lymphoma. Front. Oncol. 9:260. doi: 10.3389/fonc.2019.00260
Received: 15 December 2018; Accepted: 22 March 2019;
Published: 11 April 2019.
Edited by:
Catherine Grace Chung, The Ohio State University, United StatesReviewed by:
Alberto Fabbri, Azienda Ospedaliera Universitaria Senese, ItalySabarish Ayyappan, The Ohio State University, United States
Copyright © 2019 Tarabadkar and Shinohara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michi M. Shinohara, bXNoaW5vaGFAdXcuZWR1
†These authors have contributed equally to this work
 Erica S. Tarabadkar
Erica S. Tarabadkar Michi M. Shinohara
Michi M. Shinohara