- 1Office of Strategic Information and Research, Institute of Human Virology, Abuja, Nigeria
- 2Department of Oncology, National Hospital, Abuja, Nigeria
- 3Department of Hematology, University of Abuja Teaching Hospital, Gwagwalada, Nigeria
- 4Department of Surgery, University of Nigeria Teaching Hospital Enugu, Enugu, Nigeria
- 5Federal Ministry of Health, Abuja, Nigeria
- 6International Research Center of Excellence, Institute of Human Virology, Abuja, Nigeria
- 7Division of Cancer Epidemiology, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, United States
- 8University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland School of Medicine, Baltimore, MD, United States
- 9Department of Research, Center for Research and Bioethics, Ibadan, Nigeria
Background: Overweight and obesity are known risk factors for chronic diseases including cancers. In this study, we evaluated the age standardized incidence rates (ASR) and proportion of cancers attributable to overweight and obesity in Nigeria.
Methods: We obtained incidence data from the databases of two population-based cancer registries (PBCRs) in Nigeria (Abuja and Enugu cancer registries), on cancer site for which there is established evidence of an association with overweight or obesity based on the International Agency for Research on Cancer (IARC) and the World Cancer Research Fund (WCRF) classification. We analyzed the data using population attributable fraction (PAF) for overweight or obesity associated cancers calculated using prevalence data and relative risk estimates in previous studies.
Results: The two PBCRs reported 4,336 new cancer cases (ASR 113.9 per 100,000) from 2012 to 2014. Some 21% of these cancers were associated with overweight and obesity. The ASR for overweight and obesity associated cancers was 24.5 per 100,000; 40.7 per 100,000 in women and 8.2 per 100,000 in men. Overall, only 1.4% of incident cancers were attributable to overweight and obesity. The ASR of cancers attributable to overweight and obesity was 2.0 per 100,000. Postmenopausal breast cancer was the most common cancer attributable to overweight and obesity (n = 25; ASR 1.2 per 100,000).
Conclusion: Our results suggest that a small proportion of incident cancer cases in Nigeria are potentially preventable by maintaining normal body weight. The burden of cancer attributed to overweight and obesity in Nigeria is relatively small, but it may increase in future.
Introduction
The incidence of overweight (body-mass index [BMI] ≥ 25 kg/m2–<30 kg/m2) and obesity (BMI ≥ 30 kg/m2) is rising globally. In 2015, an estimated 603.7 million adults were overweight and obese worldwide (1). Overweight and obesity alone contributed to 4.0 million deaths and 120 million disability-adjusted life-years in 2015, representing 7.1 and 4.9%, respectively, of death from any cause among adults globally (1). Globally, the prevalence of obesity has increased during the past three decades. However, both the trend and magnitude of BMI and BMI-related disease burden vary widely across countries (1).
Overweight and obesity are known risk factors for chronic diseases including certain cancers, cardiovascular and metabolic diseases (2–5). In 2012, it was estimated that approximately 481,000 or 3·6% of all new cancer cases in adults, aged 30 years and older after a 10-year lag period, were attributable to overweight and obesity worldwide (6). The burden of cancers attributable to overweight and obesity was higher in high-income countries (HIC) with population attributable fraction (PAF) of 5·3% compared to PAF of 1·6% in middle-income countries (MIC) and 1·0% in low-income countries (LIC) (6). Over the past few decades, the prevalence of overweight and obesity has increased markedly in Nigeria. Data from the Global Burden of Diseases (GBD) in 2015 reported an increase in the prevalence of overweight and obesity in Nigeria from 20% in 1980 to 74% in 2015 (1). In 2011, we found that the prevalence of overweight and obesity among urbanized adult Nigerians was 64%, with a higher prevalence in women (74%) than in men (57%) (5).
The International Agency for Research on Cancer (IARC) and the World Cancer Research Fund (WCRF) identified esophageal, colon, rectal, kidney, pancreatic, gallbladder (women only), postmenopausal breast, endometrial, and ovarian cancers as overweight and obesity associated cancers (7–13). There are country-specific estimates of cancers attributable to overweight and obesity for the United States of America (6.0%), United Kingdom (5.5%), Canada (5.1%), Australia (3.4%), and China (0.7%) (14–18). However, there are no country-specific estimates for sub-Saharan African countries including Nigeria, where the prevalence of overweight and obesity is increasing (19) and two-thirds of the urbanized adults are either overweight or obese (5). Given the high prevalence of adults with high BMI in Nigeria, estimating the proportion of cancers attributable to overweight and obesity would be informative to research programs and policy makers.
In this study, we used data from population-based cancer registries (PBCRs) to evaluate the incidence and proportion of cancers attributable to overweight and obesity from 2012 to 2014 in Nigeria.
Methods
Data Sources
We retrieved data on cancer incidence from Abuja and Enugu PBCRs in Nigeria, from 2012 to 2014. The details of the registries have been described elsewhere (20, 21). The Abuja Cancer Registry (ABCR) began in 2005 as a hospital-based cancer registry (HBCR). In 2009, the Nigerian National Systems of Cancer Registries (NSCR) provided the logistic support for the registry's upgrade to a PBCR. ABCR has a catchment area that covers the entire Federal Capital Territory with a population of 1,406,239 people, according to the national population census (22). The Enugu Cancer Registry (ECR) began in 1988 as a HBCR and was developed into a PBCR by the NSCR in 2012. It is domiciled in the Oncology Department of the University of Nigeria Teaching Hospital Ituku Ozalla, Enugu, Nigeria. ECR covers an area around the greater Enugu city metropolis with a population of 1,103,153 people (22). ABCR and ECR utilize the International Classification of Diseases for Oncology, 3rd Edition (ICD-O3) for coding and classification of cancers. ABCR uses CanReg4, while the ECR uses CanReg5 software for storing and processing data. Approval for cancer registration activities and research was obtained from the National Health and Research Ethics Committee of Nigeria (NHREC).
Data Handling and Statistical Analysis
We obtained de-identified data in accordance with international best practices from both cancer registries. We cleaned the data to remove duplicates and carried out quality control checks using the in-built internal consistency checks in the IARC CanReg 5 software. We generated crude incidence and age-standardized incidence rates (ASR) using the direct method based on WHO World Standard Population. We extracted data on the cancers that have been shown to be associated with overweight and obesity. These cancers and their International Classification of Diseases for Oncology (ICD-O) codes are esophagus (C15), colon (C18), rectum (C19–20), kidney (C64), pancreas (C25), gallbladder (C23–24), postmenopausal breast (C50), endometrium (C54), and ovary (C56). Because there were no previous studies on cancers associated with overweight and obesity in Nigeria, we were unable to estimate the PAF for the study population. Thus, we used the PAF for overweight and obesity for SSA, derived in the Global burden of cancer attributable to high body-mass index by Arnold et al. (6), where PAF was estimated as the proportional reduction in population disease that would occur if exposure to a risk factor were reduced to an alternative ideal exposure scenario, as described by the WHO (23). We calculated the numbers of cancer cases attributable to overweight and obesity (ACalc) for each sex by multiplying the PAF of each cancer site with the overall numbers of cancer cases reported per cancer site (24). We carried out sensitivity analyses for cancers attributable to overweight and obesity, using the GLOBOCAN 2012 database. GLOBOCAN is a data source developed by IARC and provides estimates on cancer incidence, mortality, and prevalence for 184 countries of the world including Nigeria (25).
Results
A total of 4,336 new cancer cases were reported by the PBCRs within the study period, 2012–2014 (ASR 113.9 per 100,000). Among these, 2,709 (62.5%, ASR 145.9 per 100,000) were reported in women and 1,627 (37.5%, ASR 82.0 per 100,000) in men. Of the total cancers in both sexes, 21.0% (n = 907) were associated with overweight and obesity, with 81% (n=734) of these occurring in women and 19% (n = 173) in men (Figure 1). The ASR for overweight and obesity associated cancer was 24.5 per 100,000 in both sexes, 40.7 per 100,000 in women and 8.2 per 100,000 in men (Table 1).
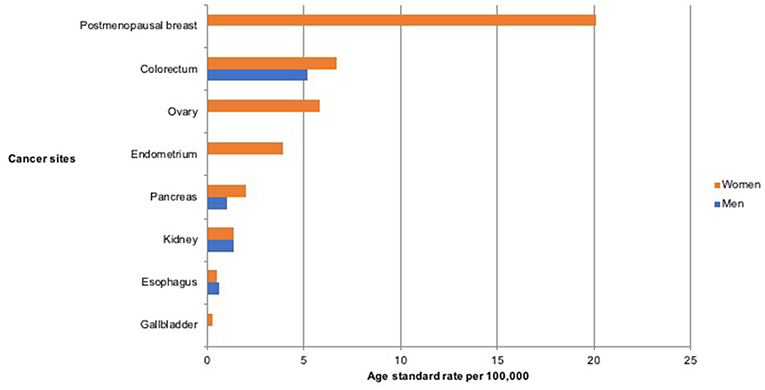
Figure 1. Age standardized incidence rate per 100,000 of cancers associated with overweight and obesity in Nigeria from 2012 to 2014.
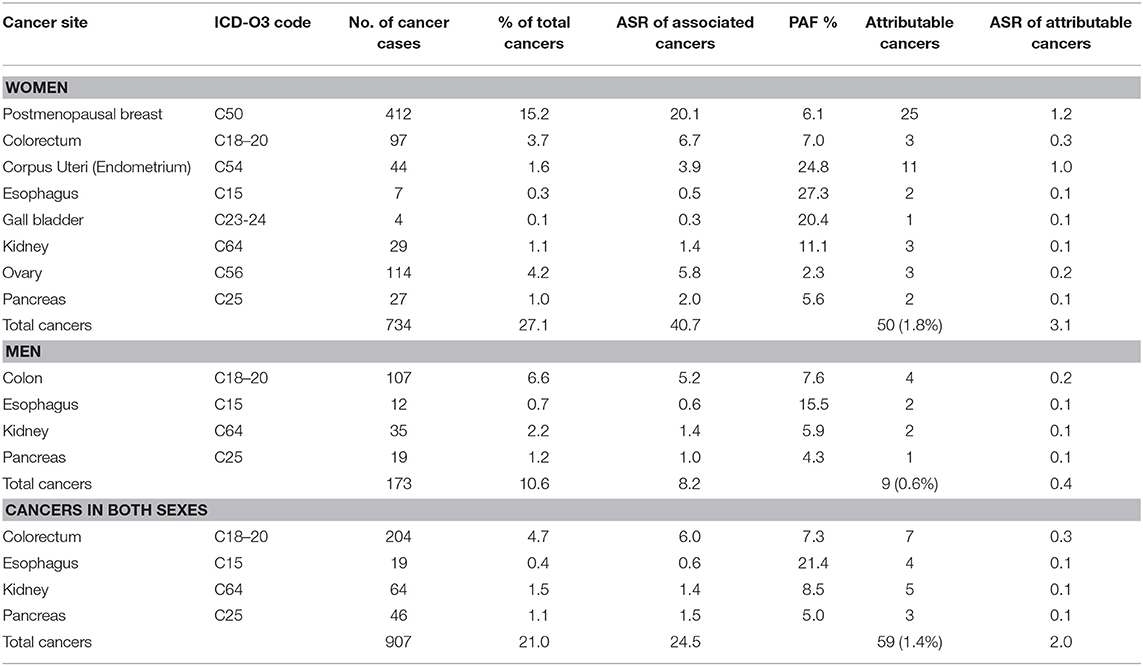
Table 1. Population attributable fraction (PAF) and estimated numbers of cancers attributable to overweight and obesity in Nigeria from 2012 to 2014.
Some 1.4% (59 of 4,336) of the incident cancers in this population were attributable to overweight and obesity giving an ASR 2.0 per 100,000. Of these overweight and obesity attributable cancers, 84.7% (50 of 59) occurred in women giving an ASR of 3.1 per 100,000 while 15.3% (9 of 59) occurred in men giving an ASR 0.4 per 100,000.
Postmenopausal Breast Cancer
Postmenopausal breast cancer was the commonest cancer associated with overweight and obesity in our study. The two registries reported 412 postmenopausal breast cancer cases from 2012 to 2014 (ASR 20.1 per 100,000). Of these, 6.1% (25 of 412) were attributable to overweight and obesity yielding an ASR 1.2 per 100,000 (6). Postmenopausal breast cancer was the most common cancer attributable to overweight and obesity in the study population.
Colorectal Cancer
There were 204 cases of colorectal cancers reported by the two registries during the study period with an ASR of 6.0 per 100,000. About half (47.5%, n = 97, ASR 6.7 per 100,000) of this was in women and 52.5% (n = 107, ASR 5.2 per 100,000) was in men. Some 3.4% (7 of 204, ASR 0.3 per 100,000) of these cancers were attributable to overweight and obesity based on a PAF estimate of 7.0% in women and 7.6% in men (6). The ASR for colorectal cancers attributable to overweight and obesity was 0.3 per 100,000 in women and 0.2 per 100,000 in men.
Ovarian Cancer
Ovarian cancer was the second most common cancer associated with overweight and obesity in women after postmenopausal breast cancer. There were 114 ovarian cancers reported by the registries (ASR 5.8 per 100,000). Of these, only 2.6% (n = 3, ASR 0.2 per 100,000) were attributable to overweight and obesity, using a PAF estimate of 2.3% (6).
Kidney Cancer
There were 64 kidney cancer cases reported by the registries (ASR 1.4 per 100,000), with 45.3% (n = 29, ASR 1.4 per 100,000) in women and 54.7% (n = 35, ASR 1.4 per 100,000) in men. With a PAF estimate of 11.1% in men and 5.9% in women (6), we computed that 7.8% (n = 5, ASR 0.1 per 100,000) kidney cancer cases were attributable to overweight and obesity. The ASR for cases attributable to overweight and obesity was same in women and men, respectively (ASR 0.1 per 100,000).
Pancreatic Cancer
Some 46 pancreatic cancer cases were reported within the study period (ASR 1.5 per 100,000), of which 58.7% (n = 27, ASR 2.0 per 100,000) were seen in women and 41.3% (n = 19, ASR 1.0 per 100,000) were seen in men. Using a PAF estimate of 5.6% in women and 4.3% in men (6), we estimated that 6.5% (n = 3, ASR 0.1 per 100,000) cases were attributable to overweight and obesity. The ASR for pancreatic cancer cases attributable to overweight and obesity was 0.1 per 100,000 in each of the sexes.
Endometrial Cancer
Some 44 endometrial cancer cases were reported in women within the study period (ASR 3.9 per 100,000). Of these, we estimated that 25.0% (11 of 44, ASR 1.0 per 100,000) cases were attributable to overweight and obesity, using a PAF estimate of 24.8% (6).
Esophageal Cancer
We found 19 esophageal cancer cases (ASR 0.6 per 100,000), with 36.8% (n = 7, ASR 0.5 per 100,000) in women and 63.2% (n = 12, ASR 0.6 per 100,000) in men. We computed that 21.1% (4 of 19, ASR 0.1 per 100,000) esophageal cancer cases were attributable to overweight and obesity, using a PAF estimate of 27.3% in women and 15.5% in men (6). The ASR for esophageal cancers attributable to overweight and obesity in women was 0.1 per 100,000 in both sexes.
Gall Bladder Cancer (Women Only)
The registries reported 4 gall bladder cases in women (ASR 0.3 per 100,000). Of these, 25.0% (n = 1, ASR 0.3 per 100,000) was attributable to overweight and obesity using a PAF estimate of 27.3% (6).
Premenopausal Breast Cancer
There were 805 cases of premenopausal breast cancers reported in women by the PBCRs from 2012 to 2014. The ASR for premenopausal breast cancer was 30.0 per 100,000. To determine how much more premenopausal breast cancer there may have been if the women were not overweight or obese, we calculated the association of overweight and obesity, and the reduced risk of premenopausal breast cancer using a relative risk of 0.95 from meta-analyses (26). We estimated that 847 premenopausal breast cancer cases (ASR 32.0 per 100,000), would have occurred if the premenopausal women were not overweight or obese.
Sensitivity Analyses
GLOBOCAN estimated that 102,079 cancer cases occurred in Nigeria in 2012, 64,709 (63.3%) in women and 37,370 (36.6%) in men. Of these, some 22.1% (n = 22,477, ASR 21.5 per 100,000) were associated with overweight and obesity, 83.7% (18,820 of 22,477) in women and 16.3% (3,657 of 22,567) in men (Table 2). Of the overweight and obesity associated cancers reported by GLOBOCAN, 1.7% (1,707 of 102,079, ASR 2.0 per 100,000) were attributable to overweight and obesity, with 1,457 (2.9%, ASR 2.9 per 100,000) in women and 250 (0.6%, ASR 0.6 per 100,000) in men (Table 2).
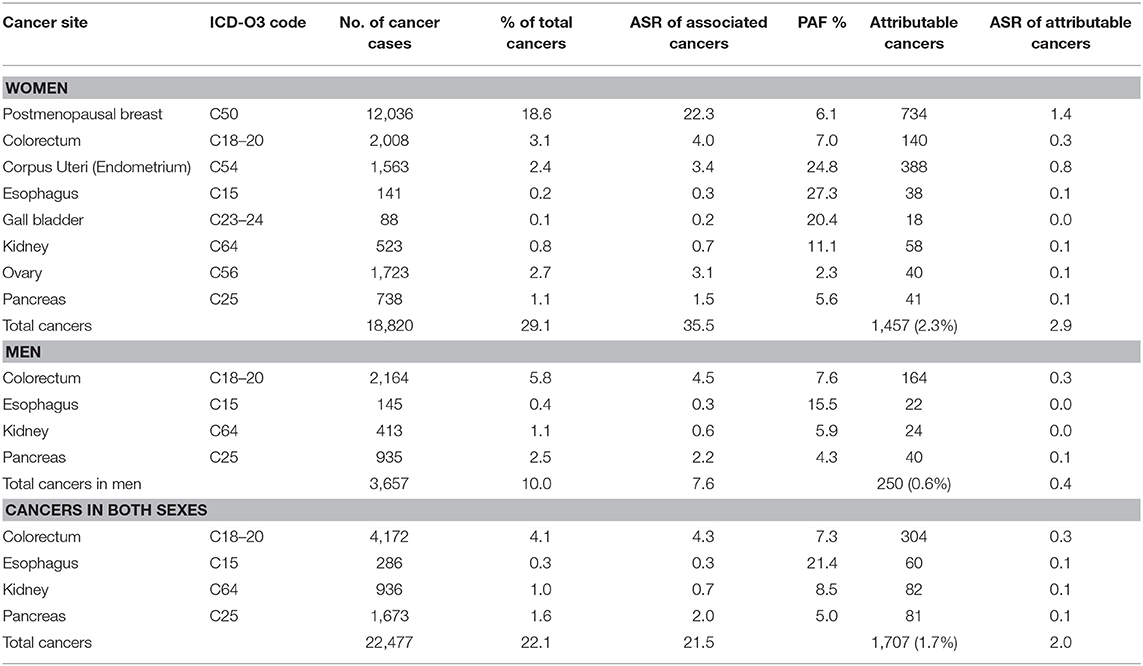
Table 2. Sensitivity analyses of cancers attributable to overweight and obesity in Nigeria from GLOBOCAN 2012 database.
Postmenopausal breast cancer was the commonest cancer attributable to overweight and obesity in Nigeria as evident from the two independent data sources. This cancer represents 1.2% (25 of 4,336) in data from Nigerian PBCRs and 1.4% (734 of 102,079) in data from GLOBOCAN 2012.
Discussion
This study showed that 1.4% of incident cancer cases in Nigeria were attributable to overweight and obesity. In our study, a higher proportion of cancers were attributable to overweight and obesity in women (1.8%), than men (0.6%). The most common cancers attributable to overweight and obesity in women were postmenopausal breast cancers (ASR 1.2 per 100,000) and endometrial cancers (ASR 1.0 per 100,000). These cancers contributed about three-quarters (72.0%) of attributable cancers in women and more than half (61.0%) of attributable cancers in both sexes. Colon, kidney and esophageal cancers with ASR 0.1 per 100,000 each, were the most common cancers attributable to overweight and obesity in men. These cancers accounted for 77.8% of total attributable cancers in men.
Our findings of incident cancer cases attributable to overweight and obesity are similar to findings from other developing countries, but lower than global estimates (6) and findings from developed countries such as United States (6.0%), United Kingdom (5.5%), and Australia (3.4%), where there is a higher prevalence of overweight and obesity (14, 15, 17).
In Nigeria, the burden of overweight and obesity is high and increasing (1, 5). As the Nigerian economy continues to improve and food becomes more widely available and affordable, individuals' dietary calorie intake will increase and contribute to the rising prevalence of overweight and obesity (5). Other factors shown to be associated with overweight and obesity among Nigerian adults include age, female gender, marital status, high socio-economic status, urban residence, and sedentary lifestyle (5, 27, 28). The proportion of people who engage in recreational physical activity in Nigeria is low. In a previous study, we found that up to two-thirds of our study population in urban Nigeria, did not engage in significant recreational physical activity (29). Our findings from this study showed that the burden of cancers attributable to overweight and obesity is higher in women, and these are women who are also likely to be obese and less likely to engage in recreational physical activities in this population.
Postmenopausal breast cancer was the most common cancer attributable to overweight and obesity in our study. This is similar to findings from other countries in sub-Saharan Africa (SSA) (6). Postmenopausal breast cancer contributed to half of cancers attributable to overweight and obesity in women and 42.4% of all attributable cancers in both sexes. The association between obesity and the risk of breast cancer is modified by menopausal status, with obesity directly associated with increased risk of breast cancer in postmenopausal women and reduced risk in premenopausal women (30). The increased risk of postmenopausal breast cancer in obese women has been suggested to be due to the high estrogen production, resulting in increased endogenous exposure to estrogen and increased risk of breast cancer (31–33). Conversely, some studies have shown that women who were overweight or obese during premenopausal ages may have a reduced risk of breast cancer compared to women of normal body weight (26, 34, 35). A potential biological mechanism for this inverse association is that among premenopausal women, overweight and obesity is associated with anovulation and lower levels of circulating estrogen levels (36).This is in contrast to overweight and obese postmenopausal women that have elevated circulating estrogen levels (26).
Colorectal cancer was the second most common cancer associated with overweight and obesity in both sexes in Nigeria. The incidence of colorectal cancers attributable to overweight and obesity in our study was higher in females than males, similar to findings from SSA countries (6). The rising incidence of colorectal cancers is associated with excessive high-fat and low-fiber diet, consumption of refined products, lack of physical activity, and obesity (31, 32). The relationship between obesity and colorectal cancer development can be attributed to metabolic syndrome and expression of various adipokines (leptin and adiponectin) which drive colorectal cancer development (33, 37, 38).
Endometrial cancer is the second most common cancer attributable to overweight and obesity in Nigerian women after breast cancer. Approximately 22% of endometrial cancer cases in Nigeria were attributable to overweight and obesity in our study. This estimate was lower than that reported in the US (34%) and US (47%). The higher prevalence of endometrial cancer attributable to overweight and obesity in developed countries may be due to the high prevalence of overweight and obesity, increased cancer awareness, opportunities for wellness checks and more resources for diagnosis in those countries. The proportion of endometrial cancers attributable to overweight and obesity in Nigeria may increase, as the country continues to develop. Other risk factors for endometrial cancers include older age, early menarche, late menopause, family history of endometrial cancer, and long-term use of estrogen for hormone replacement therapy (39). Obesity is associated with pro-inflammatory adipokines stimulation, estrogen and progesterone imbalance as well as dysregulation of insulin and insulin-like growth factor activity, which collectively contribute to endometrial proliferation and carcinogenesis (40). Although lifestyle and diet modification have been considered to reduce the risk of developing endometrial cancer, these findings were inconclusive (41–43).
Gallbladder cancer is uncommon globally and in the Nigerian population, except in areas with high prevalence of Clonorchis sinensis which is associated with cholangiocarcinoma such as the Koreas. The incidence of gallbladder cancer in Nigeria (ASR 0.2 per 100,000) is similar to findings from other SSA countries, but lower than the findings from Europe and American regions (44). This may be due to the fact that gallbladder cancer is underreported in SSA countries (45, 46). Gallstones, alcohol consumption, smoking, diabetes mellitus, genetic susceptibility, and obesity are major risk factors for gall bladder cancer (47–51). The risk of overweight associated gall bladder cancers has been reported to be more significant in women than men, a finding that is consistent with our study (6, 52, 53). Studies has shown that obesity increases the risk of gall bladder cancer by altering lipid and endogenous hormone metabolism, promoting formation of gallstones, and elevating blood glucose level (50, 54).
Ovarian cancer is the third most common gynecological cancer among Nigerian women after breast and cervical cancer (55). The incidence of ovarian cancer attributable to overweight and obesity in our study is consistent with findings from other SSA countries (6). The lack of knowledge of the premalignant stage of ovarian cancer and non-availability of a screening tool makes early diagnosis of this cancer difficult (56). This results in the late presentation and poor prognosis associated with ovarian cancer in Nigeria (57, 58). Studies suggest that the binding of leptin to its receptor (OB-Rb), activates the signaling pathways for ovarian cancer progression, particularly in obese women, resulting in a poor survival rate (59, 60).
Kidney cancer is responsible for 2.4% of the total cancer burden worldwide and 1.0% of total cancers reported in Nigeria in 2012 (44). Result from prospective studies showed that overweight and obese individuals at baseline were found to have an elevated subsequent risk of kidney cancers in a dose-response manner (61–63). In our study, approximately 8.5% of incident kidney cancer cases were attributable to overweight and obesity, which is lower than findings from developed countries (6). The higher incidence of kidney cancer in developed countries can be attributable to early diagnosis, availability of better diagnostic tools and the high prevalence of risk factors which include smoking, obesity, physical inactivity, high blood pressure and occupational exposure to trichloroethylene, cadmium and asbestos (64, 65).
The incidence of pancreatic cancer varies greatly across different populations and regions of the world with higher rates in developed countries, compared to developing countries (6). Like many other developing countries, pancreatic cancer diagnosis in Nigeria is associated with late presentation resulting in poor survival (66). In our study, pancreatic cancer accounted for 5.1% of cancers attributable to overweight and obesity. Other risk factors for pancreatic cancer include cigarette smoking, positive family history and genetics, heavy alcohol use, diabetes mellitus, dietary factors and physical inactivity (67, 68).
Our study has several limitations. We included data from two out of six PBCRs in Nigeria because the other PBCRs were newly established and did not have data covering the time period reviewed. Nonetheless, the two PBCRs used in our study represent distinct regions of Nigeria (North-Central and South-East) with nationally representative socio-demographic characteristics. Given the dearth of data, we were unable to estimate Nigerian-specific PAF. Thus, we used the estimates from the GBD, which may not be a true representation of the Nigerian population. Also, a 10-year lag period between obesity and incident cancer was assumed in the study where our PAF was obtained. We could not account for time lived with overweight or obesity in our analyses. Lastly, we cannot rule out the possibility of the incompleteness of cancer data and under-reporting from the two PBCRs considered in this study.
However, to our knowledge, this study is the first to evaluate country-specific incidence of cancers attributable to overweight and obesity in Africa. Our findings on the incidence of cancers attributable to overweight and obesity in this study (1.4%, ASR 2.0 per 100,000) are further strengthened by the results of the sensitivity analysis, which were similar to the results reported in GLOBOCAN 2012 for Nigeria (1.7%, ASR 2.0 per 100,000).
Conclusion
Our results suggest that 1.4–1.7% of incident cancer cases in Nigeria are potentially preventable by maintaining normal body weight. The burden of cancer attributed to overweight and obesity is still relatively small in Nigeria, but it is likely to increase in future. Findings from our study may help guide decision making on control and prevention of overweight and obesity, which may reduce the burden of these cancers in future.
Author Contributions
MO and SA contributed to data collection, data analyses, and drafting the manuscript. TO, FI, TIO, EE, RH, and EJ-A contributed to the data collection and data quality. SA guided all aspects of the manuscript and provided critical revisions. All authors contributed to the manuscript and approved the final version.
Funding
This work was supported by the UM-Capacity Development for Research in AIDS Associated Malignancy Grant (NIH/NCI 1D43CA153792-01) and African Collaborative Center for Microbiome and Genomics Research Grant (NIH/NHGRI 1U54HG006947).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, or the National Institutes of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EAO declared a past co-authorship with several of the authors MO, FI, TIO, EE, RH, EJ-A and SA to the handling editor.
Acknowledgments
We acknowledge Prof. Clement Adebamowo who obtained funding and reviewed the manuscript, the staff of Abuja and Enugu population-based cancer registries, including, Gloria Harrisson-Osagie, Chinyere Chuks, Henry Kumai, and Ann Okoroafor for their work in data collection and entry.
References
1. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, GBD 2015 Obesity Collaborators, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
2. World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research (2007).
3. Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. (2008) 168:928–35. doi: 10.1001/archinte.168.9.928
4. Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. (2000) 8:605–19. doi: 10.1038/oby.2000.79
5. Akarolo-Anthony SN, Willett WC, Spiegelman D, Adebamowo CA. Obesity epidemic has emerged among Nigerians. BMC Public Health. (2014) 14:455. doi: 10.1186/1471-2458-14-455
6. Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. (2015) 16:36–46. doi: 10.1016/S1470-2045(14)71123-4
7. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
8. World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR (2007).
9. World Cancer Research Fund. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer. Washington, DC: American Institute for Cancer Research (2010).
10. World Cancer Research Fund. Continuous Update Project Report. Food, Nutrition, Physical Activity and the Prevention of Colorectal Cancer. Washington, DC: American Institute for Cancer Research (2011).
11. World Cancer Research Fund. Continuous Update Project Summary. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer. Washington, DC: American Institute for Cancer Research (2012).
12. World Cancer Research Fund. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Endometrial Cancer. Washington, DC: American Institute for Cancer Research (2013).
13. World Cancer Research Fund. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Ovarian Cancer. Washington, DC: American Institute for Cancer Research (2014).
14. Polednak AP. Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev. (2008) 32:190–9. doi: 10.1016/j.cdp.2008.08.004
15. Parkin DM, Boyd L. 8. Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer. (2011) 105 (Suppl. 2):S34–7. doi: 10.1038/bjc.2011.481
16. Krueger H, Andres EN, Koot JM, Reilly BD. The economic burden of cancers attributable to tobacco smoking, excess weight, alcohol use, and physical inactivity in Canada. Curr Oncol. (2016) 23:241–9. doi: 10.3747/co.23.2952
17. Kendall BJ, Wilson LF, Olsen CM, Webb PM, Neale RE, Bain CJ, et al. Cancers in Australia in 2010 attributable to overweight and obesity. Aust N Z J Public Health. (2015) 39:452–7. doi: 10.1111/1753-6405.12458
18. Wang D, Zheng W, Wang SM, Wang JB, Wei WQ, Liang H, et al. Estimation of cancer incidence and mortality attributable to overweight, obesity, and physical inactivity in China. Nutr Cancer. (2012) 64:48–56. doi: 10.1080/01635581.2012.630166
19. Ono T, Guthold R, Strong K. WHO Global Comparable Estimates: Global Infobase Data for Saving Lives 2005. (2012). Available online at: https://apps.who.int/infobase/Index.aspx (accessed July, 2018).
20. Odutola M, Jedy-Agba EE, Dareng EO, Oga EA, Igbinoba F, Otu T, et al. Burden of cancers attributable to infectious agents in Nigeria: 2012–2014. Front Oncol. (2016) 6:216. doi: 10.3389/fonc.2016.00216
21. Akarolo-Anthony SN, Maso LD, Igbinoba F, Mbulaiteye SM, Adebamowo CA. Cancer burden among HIV-positive persons in Nigeria: preliminary findings from the Nigerian AIDS-cancer match study. Infect Agent Cancer. (2014) 9:1. doi: 10.1186/1750-9378-9-1
22. Odutola MK, Jedy-Agba EE, Dareng EO, Adebamowo SN, Oga EA, Igbinoba F, et al. Cancers attributable to alcohol consumption in Nigeria: 2012–2014. Front Oncol. (2017) 7:183. doi: 10.3389/fonc.2017.00183
23. WHO. World Health Organization (WHO) Health Statistics and Information System. Quantifying the Contribution of Risk Factors to the Burden of Diseases. Available online at: http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/ (accessed July, 2018).
24. Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J Cancer. (2006) 119:884–7. doi: 10.1002/ijc.21903
25. International Agency for Research on Cancer. GLOBOCAN 2012. Lyon: IARC Cancer Base. (2016). Available online at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed July, 2018).
26. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. (2014) 36:114–36. doi: 10.1093/epirev/mxt010
27. Olatunbosun ST, Kaufman JS, Bella AF. Prevalence of obesity and overweight in urban adult Nigerians. Obes Rev. (2011) 12:233–41. doi: 10.1111/j.1467-789X.2010.00801.x
28. Oyeyemi AL, Adegoke BO, Oyeyemi AY, Deforche B, De Bourdeaudhuij I, Sallis JF. Environmental factors associated with overweight among adults in Nigeria. Int J Behav Nutr Phys Act. (2012) 9:32. doi: 10.1186/1479-5868-9-32
29. Akarolo-Anthony SN, Adebamowo CA. Prevalence and correlates of leisure-time physical activity among Nigerians. BMC Public Health. (2014) 14:529. doi: 10.1186/1471-2458-14-529
30. Ogundiran TO, Huo D, Adenipekun A, Campbell O, Oyesegun R, Akang E, et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control. (2012) 23:565–74. doi: 10.1007/s10552-012-9916-y
31. Pietrzyk L, Torres A, Maciejewski R, Torres K. Obesity and obese-related chronic low-grade inflammation in promotion of colorectal cancer development. Asian Pac J Cancer Prev. (2015) 16:4161–8. doi: 10.7314/APJCP.2015.16.10.4161
32. Birmingham JM, Busik JV, Hansen-Smith FM, Fenton JI. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis. (2009) 30:690–7. doi: 10.1093/carcin/bgp041
33. Gialamas SP, Sergentanis TN, Antonopoulos CN, Dessypris N, Chrousos GP, Petridou ET. Circulating leptin levels and risk of colorectal cancer and adenoma: a case-control study and meta-analysis. Cancer Causes Control. (2013) 24:2129–41. doi: 10.1007/s10552-013-0290-1
34. Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res. (2012) 5:515–21. doi: 10.1158/1940-6207.CAPR-12-0091
35. Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS ONE. (2012) 7:e51446. doi: 10.1371/journal.pone.0051446
36. Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. (1996) 88:756–8. doi: 10.1093/jnci/88.11.756
37. Erkasap N, Ozkurt M, Erkasap S, Yasar F, Uzuner K, Ihtiyar E, et al. Leptin receptor (Ob-R) mRNA expression and serum leptin concentration in patients with colorectal and metastatic colorectal cancer. Braz J Med Biol Res. (2013) 46:306–10. doi: 10.1590/1414-431X20122559
38. Healy LA, Howard JM, Ryan AM, Beddy P, Mehigan B, Stephens R, et al. Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis. (2012) 14:157–65. doi: 10.1111/j.1463-1318.2011.02562.x
40. Carlson MJ, Thiel KW, Yang S, Leslie KK. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discov Med. (2012) 14:215–22.
41. Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. (2016) 34:4225–30. doi: 10.1200/JCO.2016.69.4638
42. Nagle CM, Olsen CM, Ibiebele TI, Spurdle AB, Webb PM, Australian National Endometrial Cancer Study Group, et al. Glycemic index, glycemic load and endometrial cancer risk: results from the Australian National Endometrial Cancer study and an updated systematic review and meta-analysis. Eur J Nutr. (2013) 52:705–15. doi: 10.1007/s00394-012-0376-7
43. George SM, Ballard R, Shikany JM, Crane TE, Neuhouser ML. A prospective analysis of diet quality and endometrial cancer among 84,415 postmenopausal women in the Women's Health Initiative. Ann Epidemiol. (2015) 25:788–93. doi: 10.1016/j.annepidem.2015.05.009
44. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
45. Chianakwana GU, Okafor PI, Anyanwu SN. Carcinoma of the gallbladder at the nnamdi azikwe university teaching hospital- a 5 year retrospective study. Niger J Clin Pract. (2005) 8:10–13.
46. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. (2014) 6:99–109.
47. Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. (2003) 4:167–76. doi: 10.1016/S1470-2045(03)01021-0
48. Hou L, Xu J, Gao YT, Rashid A, Zheng SL, Sakoda LC, et al. CYP17 MspA1 polymorphism and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Int J Cancer. (2006) 118:2847–53. doi: 10.1002/ijc.21708
49. Moerman CJ, Bueno de Mesquita HB, Runia S. Smoking, alcohol consumption and the risk of cancer of the biliary tract; a population-based case-control study in The Netherlands. Eur J Cancer Prev. (1994) 3:427–36. doi: 10.1097/00008469-199409000-00007
50. Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. (2013) 132:645–57. doi: 10.1002/ijc.27645
51. Schlesinger S, Aleksandrova K, Pischon T, Jenab M, Fedirko V, Trepo E, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. (2013) 24:2449–55. doi: 10.1093/annonc/mdt204
52. Tan W, Gao M, Liu N, Zhang G, Xu T, Cui W. Body mass index and risk of gallbladder cancer: systematic review and meta-analysis of observational studies. Nutrients. (2015) 7:8321–34. doi: 10.3390/nu7105387
53. Liu H, Zhang Y, Ai M, Wang J, Jin B, Teng Z, et al. Body mass index can increase the risk of gallbladder cancer: a meta-analysis of 14 cohort studies. Med Sci Monit Basic Res. (2016) 22:146–55. doi: 10.12659/MSMBR.901651
54. Hsing AW, Bai Y, Andreotti G, Rashid A, Deng J, Chen J, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. (2007) 121:832–8. doi: 10.1002/ijc.22756
55. Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. (2012) 36:e271–8. doi: 10.1016/j.canep.2012.04.007
56. Zayyan MS, Ahmed SA, Oguntayo AO, Kolawole AO, Olasinde TA. Epidemiology of ovarian cancers in Zaria, Northern Nigeria: a 10-year study. Int J Womens Health. (2017) 9:855–60. doi: 10.2147/IJWH.S130340
57. Iyoke CA, Ugwu GO, Ezugwu EC, Ezugwu FO, Lawani OL, Onyebuchi AK. Challenges associated with the management of gynecological cancers in a tertiary hospital in South East Nigeria. Int J Womens Health. (2014) 6:123–30. doi: 10.2147/IJWH.S55797
58. Bassey G, Nyengidiki T, Inimgba NM, Otoide A. Clinical and histopathological patterns of ovarian malignancy in the University of Port Harcourt teaching hospital. Port Harcourt Med J. (2016) 10:14–20. doi: 10.4103/0795-3038.179443
59. Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. (2013) 42:1113–9. doi: 10.3892/ijo.2013.1789
60. Kato S, Abarzua-Catalan L, Trigo C, Delpiano A, Sanhueza C, García K, et al. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget. (2015) 6:21100–19. doi: 10.18632/oncotarget.4228
61. Pischon T, Lahmann PH, Boeing H, Tjønneland A, Halkjaer J, Overvad K, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. (2006) 118:728–38. doi: 10.1002/ijc.21398
62. Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. (2008) 168:268–77. doi: 10.1093/aje/kwn122
63. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
64. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. (2008) 34:193–205. doi: 10.1016/j.ctrv.2007.12.001
65. Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969–2013. JAMA. (2015) 314:1731–9. doi: 10.1001/jama.2015.12319
66. Alatise OI, Lawal O, Ojo OT. Challenges of pancreatic cancer management in a resource scarce setting. East Central Afr J Surg. (2010) 15:52–8.
67. Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. (2011) 105 (Suppl. 2):S77–81. doi: 10.1038/bjc.2011.489
Keywords: overweight, obesity, cancer, incidence, cancer registry, Nigeria
Citation: Odutola MK, Olukomogbon T, Igbinoba F, Otu TI, Ezeome E, Hassan R, Jedy-Agba E and Adebamowo SN (2019) Cancers Attributable to Overweight and Obesity From 2012 to 2014 in Nigeria: A Population-Based Cancer Registry Study. Front. Oncol. 9:460. doi: 10.3389/fonc.2019.00460
Received: 03 September 2018; Accepted: 14 May 2019;
Published: 11 June 2019.
Edited by:
Maria Paula Curado, International Prevention Research Institute, FranceReviewed by:
Kun Chen, Zhejiang University, ChinaEmmanuel Aja Oga, University of Maryland, United States
Alireza Sadjadi, Tehran University of Medical Sciences, Iran
Copyright © 2019 Odutola, Olukomogbon, Igbinoba, Otu, Ezeome, Hassan, Jedy-Agba and Adebamowo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sally N. Adebamowo, c2FkZWJhbW93b0Bzb20udW1hcnlsYW5kLmVkdQ==
 Michael K. Odutola
Michael K. Odutola Temitope Olukomogbon
Temitope Olukomogbon Festus Igbinoba
Festus Igbinoba Theresa I. Otu
Theresa I. Otu Emmanuel Ezeome4
Emmanuel Ezeome4 Ramatu Hassan
Ramatu Hassan Elima Jedy-Agba
Elima Jedy-Agba Sally N. Adebamowo
Sally N. Adebamowo