- 1Department of Radiation Oncology, Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
- 2State Key Laboratory of Oncology in South China, Department of Radiation Oncology, Collaborative Innovation Center of Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
Introduction: Metaplastic breast cancer (MBC) is a rare and aggressive form of breast cancer. The present study aimed to assess the effect of post-mastectomy radiotherapy (PMRT) in MBC patients with intermediate-risk (T1-2N1M0 and T3N0M0) and high-risk (T1-4N2-3M0 and T4N0-1M0) disease.
Methods: The Surveillance, Epidemiology and End Results database was used to analyze patients with MBC between 2000 and 2014. Kaplan–Meier analysis, log-rank tests, and the multivariate Cox proportional model were used for statistical analysis.
Results: We identified 460 patients with a median follow-up time of 31 months (range, 2–178 months). Five-year breast cancer specific survival (BCSS) for all patients was 57.5%. In the entire group, multivariate analysis showed that PMRT was associated with better BCSS (hazard ratio (HR) 0.500, 95% confidence interval (CI) 0.366–0.683, P < 0.001). The 5-year BCSS in PMRT and non-PMRT groups were 62.3 and 50.3%, respectively (P = 0.001). When stratified the patients into intermediate-risk and high-risk groups, PMRT could improve BCSS compared with that in non-PMRT patients in both the intermediate- and high-risk groups. For the intermediate-risk group, the 5-year BCSS was 74.3 and 64.7% in PMRT and non-PMRT groups (P = 0.042), respectively, and was 52.1 and 28.8% in high-risk patients treated with PMRT and non-PMRT, respectively (P < 0.001).
Conclusion: PMRT could improve the BCSS of MBC patients with intermediate- and high-risk disease.
Background
Metaplastic breast cancer (MBC) was identified as a unique pathological type of breast cancer by the World Health Organization in 2000, and the rate of MBC diagnosis has increased ever since. MBC is a rare disease with aggressive biological behavior, accounting for 0.25–1% of all breast cancers (1, 2), and is characterized by either a homogeneous population or mixtures of squamous cell carcinoma, adenocarcinoma, epithelial, and mesenchymal components (3–8). In addition, patients with MBC have distinct histopathological and molecular signatures, including larger tumor size; less frequently with axillary nodal metastases; triple-negative disease; and higher Ki-67, p53, CK5/6, and EGFR expression levels (9–11). Several previous studies have indicated that the survival of patients with MBC was significantly lower than in those with invasive ductal carcinomas (IDC) (11–14).
The optimal management of MBC remains controversial due to the rarity of this disease. In the current practice, most patients were treated with mastectomy because of the larger tumor size associated with this disease (15). The role of post-mastectomy radiotherapy (PMRT) might be important in this patient subset because of about 28–46% of patients may develop locoregional recurrence (LRR) after surgery (10, 12, 16). However, the effect of PMRT in MBC is a matter of debate. Several studies showed better outcomes in PMRT groups (15, 17–20), while other studies indicated that the receipt of PMRT was not associated with better outcomes (2, 21, 22). The main reasons for above conflicting results might be the difference in sample sizes and treatment patterns of the study populations. In addition, the recommendation to use of PMRT is also controversial in patients with intermediate-risk disease (T1-2N1M0 and T3N0M0) (2). Consequently, the present study was aimed to assess the role of PMRT in MBC, especially in patients with intermediate-risk, using a real-world population-based database (Surveillance, Epidemiology, and End Results, SEER).
Materials and Methods
SEER Database and Patients
Data were obtained from the SEER database of the National Cancer Institute, which is an open access resource with 18 population-based cancer registries of patients in the United States for cancer-based epidemiology and survival. Women with intermediate-risk and high-risk MBC (23) treated with mastectomy and chemotherapy from 2000 to 2014 were identified. The code for MBC is 8,575 in the SEER database, according to the third edition of International Classification of Diseases for Oncology. The intermediate-risk group included patients with stage T1-2N1M0 and T3N0M0 disease (23), and the high-risk group included patients with stage T1-4N2-3M0 and T4N0-1M0 disease (24). Patients with available race/ethnicity, tumor (T) stage, nodal (N) stage, and having records on whether they received PMRT were included. We excluded patients without a positive histology diagnosis and receipt of non-beam irradiation. Using the SEER data is exempt from the approval process of Institutional Review Board.
Variables
We included the following patient demographic and clinicopathological variables: age ( ≤ 50 years, > 50 years), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, and Other), T stage (T1, T2, T3, and T4), N stage (N0, N1, N2, and N3), risk stratification (intermediate-risk, high-risk), estrogen receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor-2 (HER2) status. The definition of TNM (T-tumor, N-node, M-metastasis) stage was according to the six edition of the Union for International Cancer Control /American Joint Committee on Cancer pathologic staging system. We only included the status of HER-2 after 2010 because SEER only recorded these data after 2010. The primary end point of this study was breast cancer specific survival (BCSS), which was calculated as the date from the diagnosis of MBC to death from breast cancer.
Statistical Analysis
The χ2 test was used to analyze the differences between PMRT and non-PMRT groups. Kaplan–Meier analysis and log-rank testing were used to compare BCSS curves. The risk factors for BCSS were assessed using the Cox proportional hazards model. All calculations were performed using SPSS statistical software (version 22.0; IBM Corporation, Armonk, NY, USA), and P < 0.05 was considered statistically significant.
Results
Patient Characteristics
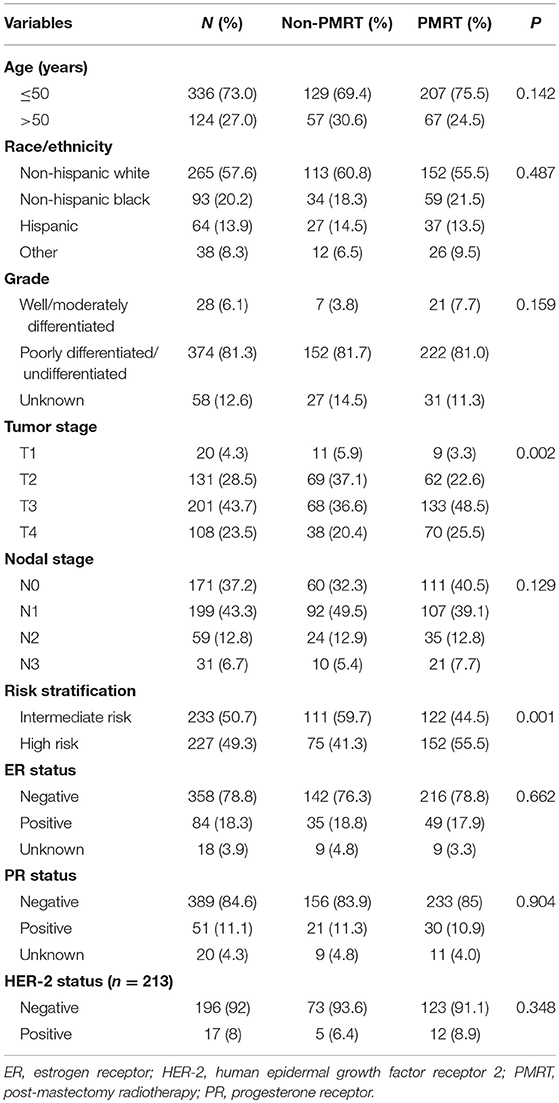
A total of 460 patients were identified. The characteristics of the patients in the study population are presented in Table 1. The surgical procedures of the 460 patients are listed in Table 2. Among all the patients, 73% of them were aged <50 years, with a median age of 57 years (range, 27–88 years). Most of them were Non-Hispanic White (n = 265, 57.6%), poor differentiation/undifferentiated (n = 374, 81.3%), ER negative (n = 358, 78.8%), PR negative (n = 389, 84.6%), and HER2 negative (n = 196, 92% in HER2 available patients). In patients with available breast cancer subtype information, 65.3% were triple-negative patients. A total of 20 (4.3%), 131 (28.5%), 201 (43.7%), and 108 (23.5%) of the patients had T1, T2, T3, and T4 stage disease, respectively. In addition, 171 (37.2%), 199 (43.3%), 59 (12.8%), and 31 (6.7%) patients had N0, N1, N2, and N3 stage disease, respectively.
Among the 460 patients, 59.6% (n = 274) of them received PMRT, and patients with advanced T stage (P = 0.002) and high-risk group (P = 0.001) were more likely to receive PMRT.
Survival
The median follow-up time for the entire cohort was 31 months (range, 2–178 months), and were 33.0 months (range, 5–178 months) and 29.5 months (range, 2–176 months) in PMRT group and non-PMRT group, respectively. A total of 250 deaths were observed, including 174 breast cancer related-deaths. The 5-year BCSS for all patients was 57.5%. The 5-year BCSS in the PMRT and non-PMRT groups were 62.3 and 50.3%, respectively (P = 0.001) (Figure 1). When stratified the patients into intermediate-risk and high-risk groups, PMRT could improve BCSS compared with that for patients in the non-PMRT group, in both the intermediate- and high-risk groups. For the intermediate-risk group, the 5-year BCSS were 74.3 and 64.7% in the PMRT and non-PMRT groups (P = 0.042) (Figure 2A), respectively, and were 52.1 and 28.8% in high-risk patients treated with PMRT and non-PMRT (P < 0.001), respectively (Figure 2B).
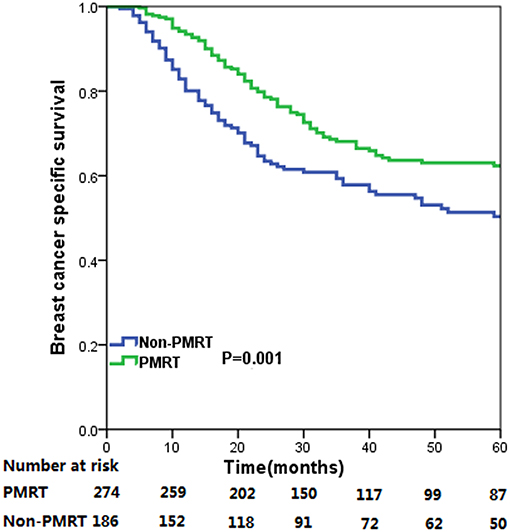
Figure 1. Breast cancer specific survival curves for all cases of metaplastic breast carcinoma with and without post-mastectomy radiotherapy.
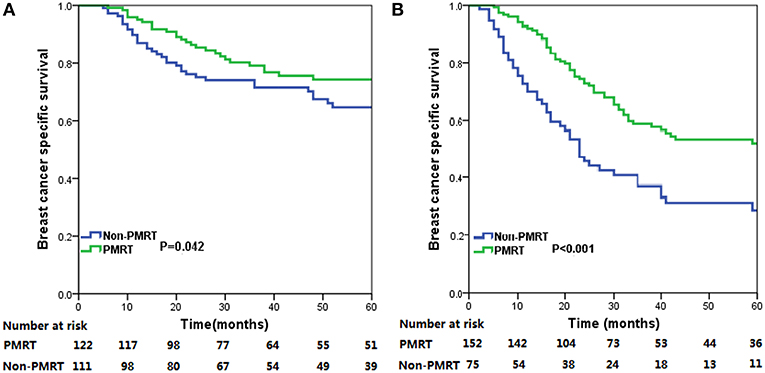
Figure 2. Breast cancer specific survival (BCSS) curves for all patients with metaplastic breast cancer (MBC) with and without post-mastectomy radiotherapy (PMRT) in the intermediate-risk (A) and high-risk (B) groups.
Analysis of Prognostic Factors
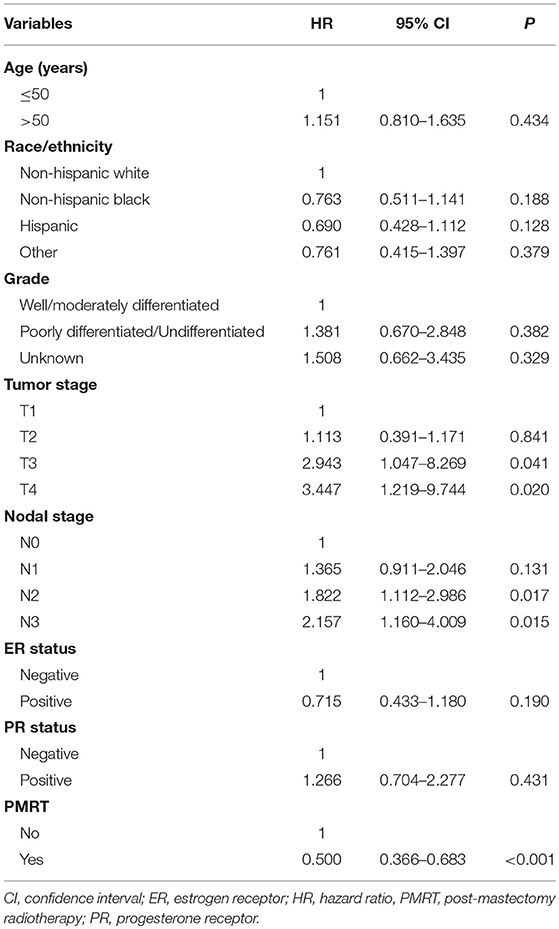
We further analyzed the independent prognostic factors related to BCSS for MBC using the Cox proportional hazards model. In the entire group, multivariate analysis showed that PMRT was an independent prognostic factor related to better BCSS [hazard ratio [HR] 0.500, 95% confidence interval [CI] 0.366–0.683, P < 0.001] (Table 3). In addition, tumor stage and nodal stage were also independent indicators for BCSS. However, age, race/ethnicity, grade, ER status, and PR status were not related to BCSS.
When stratified by the risk groups, PMRT was also an independent prognostic factor for BCSS in both the intermediate- and high-risk groups. Patients who received PMRT were associated with better BCSS compared with those in non-PMRT group in the intermediate-risk (HR = 0.466, 95% CI 0.280–0.774, P = 0.003) and high-risk (HR = 0.450 95% CI 0.301–0.675, P < 0.001) groups.
Discussion
In the present study, we assessed the effect of PMRT in MBC, and our results found that receipt of PMRT was associated better BCSS in patients with intermediate-risk (T1-2N1M0 and T3N0M0) and high-risk (T1-4N2-3M0 and T4N0-1M0) disease.
We recognized homogeneity and heterogeneity in our study population. The results of our study were consistent with previous studies, which indicated that most patients with MBC patients presented with larger tumors, triple-negative disease, and higher tumor grade (9, 11, 16). In addition, we only included patients with intermediate- and high-risk cohorts, 19.5% of patients had stage N2 and N3 disease, and 80.5% of patients had N0 and N1 disease. Moreover, Pizza et al. reported that N0 stage was 78.1 vs. 65.7%, and N1 or above was 21.9 vs. 34.3% in MBC and IDC patients, respectively (P < 0.001) (1), which indicated that a high nodal involvement burden was less common in patients with MBC. Similar to previous study (25), we found that the advanced nodal stage was related to poor outcome in MBC, which was similar to the results for IDC (26).
The recurrence pattern of MBC has not been well-delineated in this rare disease. We were unable to assess the patterns of disease recurrence because of the lack of recurrence data in the SEER database. A previous study from the MD Anderson Cancer Center that included 47 patients with MBC showed that 28% of the patients developed LRR, with a median follow-up time of 30 months. However, the study included a limited number of patients, and this cohort may have received insufficient treatment (12). Another study from Korea that included 35 patients also showed that MBC had a higher recurrence rate of 46.8% compared with 9.3% in IDC patients, and MBC was a poor prognostic factor for disease recurrence (HR 3.89, 95% CI 1.36–11.14, P = 0.01) (10). Moreover, several previous studies found that the MBC subtype was more resistant to chemotherapy (27–29). Therefore, PMRT may play an important role in the management of MBC.
In the National Comprehensive Cancer Network breast cancer guidelines, PMRT is strongly recommended in T1-2N1 stage disease and is routinely used in patients with N2 stage disease (30). However, the guidelines were not stratified by histological subtypes. The percentage of PMRT receipt was only 58.1% in our study, and the percentage of PMRT receipt was markedly different in previous studies, ranging from 38.6 to 72% (2, 15, 17–20, 31), which indicated that the role of PMRT in patients with MBC is unclear, despite high recurrence.
The effect of PMRT in MBC remains controversial. Although some investigators have reported favorable prognosis in the PMRT group (15, 17–20), others have observed that adjuvant PMRT had no association with survival (21, 22). Haque et al. examined the effect of PMRT with MBC using the National Cancer Database and showed that 45.2% of the patients received PMRT, and that PMRT could lead to higher overall survival (OS) in patients with pT3–4/N+ disease (p < 0.001), but not in patients with pT1–2N0 disease (17).However, their observed end point was OS, which was affected by variety of uncontrollable factors and could not accurately reflect death from breast cancer. In addition, in a study from Tseng et al., which included 1,501 patients with stage I-IV MBC, the results of univariate analysis showed that post-operative radiotherapy was associated with better BCSS (P < 0.01) and OS (P = 0.003) in patients who received breast conserving surgery, whereas it was not related to better outcomes in patients who received PMRT. However, the results of multivariate analysis indicated that post-operative radiotherapy provided an OS benefit but not a BCSS benefit to patients receiving breast conserving surgery and mastectomy (2). The study by Tseng et al. also included patients with stage T1-2N0 and stage IV patients, which are not indications for PMRT in current clinical practice. Moreover, the information on chemotherapy receipt was not recorded in this study. Our study included patients with node-positive disease or larger tumor size (>5 cm) who received chemotherapy, and our findings suggested that PMRT could significantly improve BCSS in this patient subset.
The value of PMRT in patients with intermediate-risk invasive breast cancer has been controversial. A meta-analysis from the Early Breast Cancer Trialists Collaborative Group further supported the view that PMRT could reduce LRR and improve BCSS in patients with 1–3 positive lymph nodes (32). However, a recent study showed that survival in patients not receiving PMRT was comparable to that in patients receiving PMRT in the era of modern taxane-based chemotherapy (33). Whether the current chemotherapy practice could further determine the survival benefit of PMRT in patients with MBC remains controversial. We further analyzed whether the patients with MBC in the intermediate-risk group could benefit from PMRT, and found that PMRT was also associated with better BCSS in this population. However, because of the low incidence of MBC, it is difficult to conduct a large-scale randomized controlled trial. SUPREMO, the largest prospective trial to assess the value of PMRT in intermediate-risk groups, is ongoing, and MBC is not listed as an exclusion criterion. We anticipate the results of the stratified analysis to assess the value of PMRT in patients with intermediate-risk MBC in the era of systemic therapy.
There were several limitations in our study. First, it was a retrospective study with the observational nature and the possibility of selection bias, and patients were not randomly assigned to PMRT group and non-PMRT group. Second, the number of cycles and specific agents of chemotherapy, the sequence of chemotherapy and surgery, endocrine therapy, technique, dose, and target volume of PMRT as well as compliance to therapy were not included in the SEER database. Third, the patient baseline characteristics including performance status, comorbidities, and socioeconomic environments were also lacking in the SEER program. In addition, with a higher risk of death in MBC, the median follow-up time of our study was only 31 months, longer-term and prospective results are needed to draw definitive conclusions on the utilization of PMRT in MBC. However, the low incidence of MBC limits the possibility to conduct prospective clinical trials with large cohorts. Moreover, the patterns of LRR and distant recurrence were also missing from the SEER database. Finally, the percentage of PMRT receipt was under-reported in the SEER database (34). Therefore, our study may not be better than single institution retrospective data. However, the results of our study will contribute to the current knowledge of the role of PMRT in MBC.
Conclusion
In conclusion, our study suggests that PMRT could improve BCSS in MBC patients with the intermediate- and high-risk groups. More prospective studies are required to confirm our results and to identify the optimal protocols for the use of PMRT in the management of MBC.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: www.seer.cancer.gov.
Ethics Statement
This study was exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is de-identified.
Author Contributions
JW, Z-YH, S-GW, and W-WZ are lead authors who participated in data collection, manuscript drafting, table/figure creation, and manuscript revision. C-LL and J-YS are senior authors who aided in drafting the manuscript and manuscript revision. Z-YH and S-GW are the corresponding authors who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by the National Natural Science Foundation of China (81872459, 81803050), Natural Science Foundation of Fujian Province (No. 2016J01635), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pizza CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. (2007) 14:166–73. doi: 10.1245/s10434-006-9124-7
2. Tseng WH, Martinez SR. Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol. (2011) 18:94–103. doi: 10.1245/s10434-010-1198-6
3. Oberman HA. Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Pathol. (1987) 11:918–29. doi: 10.1097/00000478-198712000-00002
4. Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Hum Pathol. (1989) 20:628–35. doi: 10.1016/0046-8177(89)90149-4
5. Wargotz ES, Deos PH, Norris HJ. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol. (1989) 20:732–40. doi: 10.1016/0046-8177(89)90065-8
6. Lakhani SR, Ellise IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC (2012).
7. Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer. (1990) 65:272–6. doi: 10.1002/1097-0142(19900115)65:2<272::AID-CNCR2820650215>3.0.CO;2-6
8. Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer. (1989) 64:1490–9. doi: 10.1002/1097-0142(19891001)64:7<1490::AID-CNCR2820640722>3.0.CO;2-L
9. Nelson RA, Guye ML, Luu T, Lai LL. Survival outcomes of metaplastic breast cancer patients: results from a US population based analysis. Ann Surg Oncol. (2015) 22:24–31. doi: 10.1245/s10434-014-3890-4
10. Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. (2010) 120:627–37. doi: 10.1007/s10549-010-0780-8
11. Yu JI, Choi DH, Huh SJ, Ahn SJ, Lee JS, Shin KH, et al. Unique characteristics and failure patterns of metaplastic breast cancer in contrast to invasive ductal carcinoma: a retrospective multicenter case-control study (KROG 13-07). Clin Breast Cancer. (2015) 15:e105–15. doi: 10.1016/j.clbc.2014.10.002
12. Lester TR, Hunt KK, Nayeemuddin KM, Bassett RL Jr, Gonzalez-Angulo AM, Feig BW, et al. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res Treat. (2012) 131:41–8. doi: 10.1007/s10549-011-1393-6
13. El Zein D, Hughes M, Kumar S, Peng X, Oyasiji T, Jabbour H, et al. Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: a study from a single institution and review of literature. Clin Breast Cancer. (2017) 17:382–91. doi: 10.1016/j.clbc.2017.04.009
14. Altaf FJ, Mokhtar GA, Emam E, Bokhary RY, Mahfouz NB, Al Amoudi S, et al. Metaplastic carcinoma of the breast: an immunohistochemical study. Diagn Pathol. (2014) 9:139. doi: 10.1186/1746-1596-9-139
15. Ong CT, Campbell BM, Thomas SM, Greenup RA, Plichta JK, Rosenberger LH, et al. Metaplastic breast cancer treatment and outcomes in 2500 patients: a retrospective analysis of a national oncology database. Ann Surg Oncol. (2018) 25:2249–60. doi: 10.1245/s10434-018-6533-3
16. Song Y, Liu X, Zhang G, Song H, Ren Y, He X, et al. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. (2013) 11:129. doi: 10.1186/1477-7819-11-129
17. Haque W, Verma V, Naik N, Butler EB, Teh BS. Metaplastic breast cancer: practice patterns, outcomes, and the role of radiotherapy. Ann Surg Oncol. (2018) 25:928–36. doi: 10.1245/s10434-017-6316-2
18. Haque W, Verma V, Butler EB, Teh BS. Omission of radiotherapy in elderly women with early stage metaplastic breast cancer. Breast. (2018) 38:154–9. doi: 10.1016/j.breast.2018.01.005
19. Nowara E, Drosik A, Samborska-Plewicka M, Nowara EM, Stanek-Widera A. Metaplastic breast carcinomas – analysis of prognostic factors in a case series. Contemp Oncol. (2014) 18:116–9. doi: 10.5114/wo.2014.41392
20. He X, Ji J, Dong R, Liu H, Dai X, Wang C, et al. Prognosis in different subtypes of metaplastic breast cancer: a population-based analysis. Breast Cancer Res Treat. (2019) 173:329–41. doi: 10.1007/s10549-018-5005-6
21. Leo F, Bartels S, Mägel L, Framke T, Büsche G, Jonigk D, et al. Prognostic factors in the myoepithelial-like spindle cell type of metaplastic breast cancer. Virchows Arch. (2016) 469:191–201. doi: 10.1007/s00428-016-1950-9
22. Rakha EA, Tan PH, Varga Z, Tse GM, Shaaban AM, Climent F, et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Br J Cancer. (2015) 112:283–9. doi: 10.1038/bjc.2014.592
23. Velikova G, Williams LJ, Willis S, Dixon JM, Loncaster J, Hatton M, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2- year follow-up results of a randomised controlled trial. Lancet Oncol. (2018) 19:1516–29. doi: 10.1016/S1470-2045(18)30515-1
24. Wang SL, Fang H, Song YW, Wang WH, Hu C, Liu YP, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. (2019) 20:352–60. doi: 10.1016/S1470-2045(18)30813-1
25. Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. (2011) 126:471–8. doi: 10.1007/s10549-011-1359-8
26. Wu SG, Zhang WW, Sun JY, He ZY. Prognostic value of ductal carcinoma in situ component in invasive ductal carcinoma of the breast: a Surveillance, Epidemiology, and End Results database analysis. Cancer Manag Res. (2018) 10:527–34. doi: 10.2147/CMAR.S154656
27. Kaufman MW, Marti JR, Gallager HS. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer. (1984) 53:1908–17. doi: 10.1002/1097-0142(19840501)53:9<1908::AID-CNCR2820530917>3.0.CO;2-F
28. Beatty JD, Atwood M, Tickman R. Metaplastic breast cancer: Clinical significance. Am J Surg. (2006) 191:657–64. doi: 10.1016/j.amjsurg.2006.01.038
29. Chao TC, Wang CS, Chen SC, Chen MF. Metaplastic carcinomas of the breast. J Surg Oncol. (1999) 71:220–5. doi: 10.1002/(SICI)1096-9098(199908)71:4<220::AID-JSO3>3.0.CO;2-L
30. NCCN. NCCN Clinical Practice Guidelines in Oncology V.2.2018. Breast Cancer. (2018) Available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed October 21, 2018).
31. Leyrer CM, Berriochoa CA, Agrawal S, Donaldson A, Calhoun BC, Shah C, et al. Predictive factors on outcomes in metaplastic breast cancer. Breast Cancer Res Treat. (2017) 165:499–504. doi: 10.1007/s10549-017-4367-5
32. EBCTCG (Early Breast Cancer Trialists Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
33. Zeidan YH, Habib JG, Ameye L, Paesmans M, de Azambuja E, Gelber RD, et al. Postmastectomy radiation therapy in women with T1-T2 tumors and 1 to 3 positive lymph nodes: analysis of the breast international group 02-98 trial. Int J Radiat Oncol Biol Phys. (2018) 101:316–24. doi: 10.1016/j.ijrobp.2018.01.105
Keywords: breast neoplasms, radiotherapy, mastectomy, lymph node metastasis, survival, SEER
Citation: Wang J, Zhang W-W, Lian C-L, Sun J-Y, He Z-Y and Wu S-G (2019) The Effect of Post-mastectomy Radiotherapy in Patients With Metaplastic Breast Cancer: An Analysis of SEER Database. Front. Oncol. 9:747. doi: 10.3389/fonc.2019.00747
Received: 01 May 2019; Accepted: 25 July 2019;
Published: 12 August 2019.
Edited by:
Drexell Hunter Boggs, University of Alabama at Birmingham, United StatesReviewed by:
Michael S. Rutenberg, University of Florida, United StatesCaleb Dulaney, University of Alabama at Birmingham, United States
Copyright © 2019 Wang, Zhang, Lian, Sun, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==; San-Gang Wu, dW5vd3UxMjM0NUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Jun Wang
Jun Wang Wen-Wen Zhang
Wen-Wen Zhang Chen-Lu Lian
Chen-Lu Lian Jia-Yuan Sun2
Jia-Yuan Sun2 Zhen-Yu He
Zhen-Yu He San-Gang Wu
San-Gang Wu