- 1Department of Otolaryngology, Children's Hospital of Hebei Province, Shijiazhuang, China
- 2Department of Otorhinolaryngology Head and Neck Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Neurothoracic Surgery, Children's Hospital of Hebei Province, Shijiazhuang, China
Background: The prognostic value of programmed cell death ligand-1 (PD-L1) in patients with nasopharyngeal carcinoma (NPC) remains controversial. Therefore, we conducted this meta-analysis to understand the role of PD-L1 in NPC.
Method: We searched PubMed, Embase, Web of Science, and Cochrane Library up to April 2019. We determined the pooled hazard ratio (HR) and 95% confidence intervals (CIs) to assess the relationship between PD-L1 and various survival outcomes. Begg's funnel plot was used to assess any publication bias.
Results: Eleven studies involving 1,315 patients were included in this meta-analysis. For overall survival (OS), the HR was 1.48 and 95% CI was 1.00–2.18 (p = 0.049). For disease-free survival (DFS), the HR was 1.51 and 95% CI was 0.85–2.69 (p = 0.162). For distant metastasis-free survival (DMFS), the HR was 1.75 and 95% CI was 0.64–4.79 (p = 0.277). For local recurrence-free survival (LRFS), the HR was 0.67 and 95% CI was 0.06–8.16 (p = 0.756). The results of prognosis of PD-L1 and OS were more significant after sensitivity analysis. The pooled odds ratio indicated that PD-L1 expression was not associated with T stage, N stage, M stage, overall stage, sex, age, smoking, or alcohol intake. No publication bias was found.
Conclusion: Our meta-analysis showed that PD-L1 overexpression in NPC was associated with a poor OS and may be useful as a novel prognostic factor for NPC.
Introduction
Nasopharyngeal carcinoma (NPC) originates from the nasopharynx epithelium. NPC is rare compared with other types of cancer; moreover, its geographical distribution presents a unique pattern (1): it is a highly common type of head and neck cancer in the eastern and southeastern parts of Asia. NPC tends to metastasize to distant sites in the head and neck, and about 70% of the patients with NPC present with stage III or IV disease at the time of initial diagnosis (2). Radiotherapy (RT) is primarily used to treat NPC; however, concurrent chemoradiotherapy (CCRT) is recommended as the standard treatment for locoregionally advanced NPC (3). Although the 5-year survival rate of patients with NPC is ~60–70%, NPC management remains challenging because of locoregional failure, recurrence, and distant metastasis after primary treatment (4). Currently, several prognostic parameters are used for NPC management, including age and sex (5). However, these parameters lack sensitivity or specificity in some patients with NPC, and are, therefore, insufficient for predicting survival outcomes. Thus, the identification of novel and efficient prognostic markers is highly important in NPC treatment.
Programmed cell death ligand-1 (PD-L1), also known as B7-H1 or CD274, was cloned in 1999 (6). PD-L1 belongs to the B7/CD28 co-stimulator superfamily and is highly expressed in tumor-associated antigen-presenting cells (APCs), dendritic cells (7), macrophages (8), T cells (8), and various types of cancer cells (9, 10). PD-L1 along with programmed cell death 1 (PD-1; CD279), which is an inhibitory receptor expressed by tumor-infiltrating lymphocytes, can induce T cells apoptosis and inhibit the proliferation of immune cells (11): PD-1 and PD-L1 constitute the immune checkpoint that promotes tumor immune evasion (12). Using immunohistochemistry (IHC), PD-L1 overexpression was shown to be associated with a poor prognosis across multiple tumor types (10, 13), including non-small cell lung cancer (14), hepatocellular carcinoma (15), colorectal cancer (16), and renal cell carcinoma (17). Several studies also demonstrated the relationship between PD-L1 expression and prognosis of patients with NPC; however, results were controversial (18–23). Therefore, we conducted a meta-analysis to assess the impact of PD-L1 on the prognosis of patients with NPC.
Materials and Methods
Literature Search
Eligible studies were identified by searching PubMed, Embase, Web of Science, and Cochrane Library up to April 2019. The following search terms were used: “Programmed Cell Death Ligand 1” or “Programmed Death Ligand 1” or “PDL1” or “B7-H1” or “CD274” or “Programmed Cell Death 1” or “Programmed Death 1” or “PD-1” or “CD279” and “nasopharyngeal carcinoma” or “nasopharyngeal cancer” or “NPC.” References in the retrieved articles were also manually searched for additional studies.
Inclusion and Exclusion Criteria
Eligible studies were identified according to the following inclusion criteria: (1) the NPC cases were pathologically confirmed; (2) PD-L1 expression was detected in NPC by using IHC; (3) studies provided the association between PD-L1 and survival outcomes and/or clinical characteristics; (4) studies provided the hazard ratio (HR) and 95% confidence interval (CI) for survival outcomes or sufficient information to calculate the HR and 95% CI in accordance with the methods by Parmar (24) and Tierney (25); (5) for studies (22, 26, 27) detected the expression of PD-L1 in both tumor cells (TCs) and immune cells (ICs), we only extracted the data of PD-L1 expression on TCs for analysis; and (6) original articles were published in English. The following were the exclusion criteria: (1) meeting abstracts, case reports, reviews, or letters; (2) duplicate studies; and (3) non-human studies.
Data Extraction and Quality Assessment
Two authors extracted relevant data independently, and disagreements were settled by discussion. The following data were extracted: the name of the first author; the year of publication; country/region; sample size; age; tumor stage; treatment modality; study design; study duration; and HR and 95% CI related to PD-L1 expression. The quality of eligible studies was evaluated according to the Newcastle-Ottawa Scale (NOS) (28). The scale includes three categories: selection, comparability, and outcome assessment. The top score is 9 points, and studies with a score >6 are regarded as high-quality studies.
Statistical Analysis
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (29). Pooled HR and 95% CI were determined to assess the relationship between the PD-L1 and various survival outcomes. The pooled odds ratio (OR) and 95% CI were determined to study the correlation between PD-L1 and clinicopathological features. Statistical heterogeneity among all studies was assessed using the I2 and Chi-squared test. Heterogeneity was considered statistically significant when the I2 > 50% or P < 0.10. A random-effects model was used when significant heterogeneity was present; alternatively, a fixed-effects model was used. Sensitivity analysis and subgroup analysis were adopted to assess the heterogeneity and stability of the results. Begg's funnel plot was used to assess any publication bias (30). All statistical analyses were conducted with Stata version 12.0 statistical software (Stata Corporation, College Station, TX, US). A P < 0.05 was considered statistically significant.
Results
Literature Search
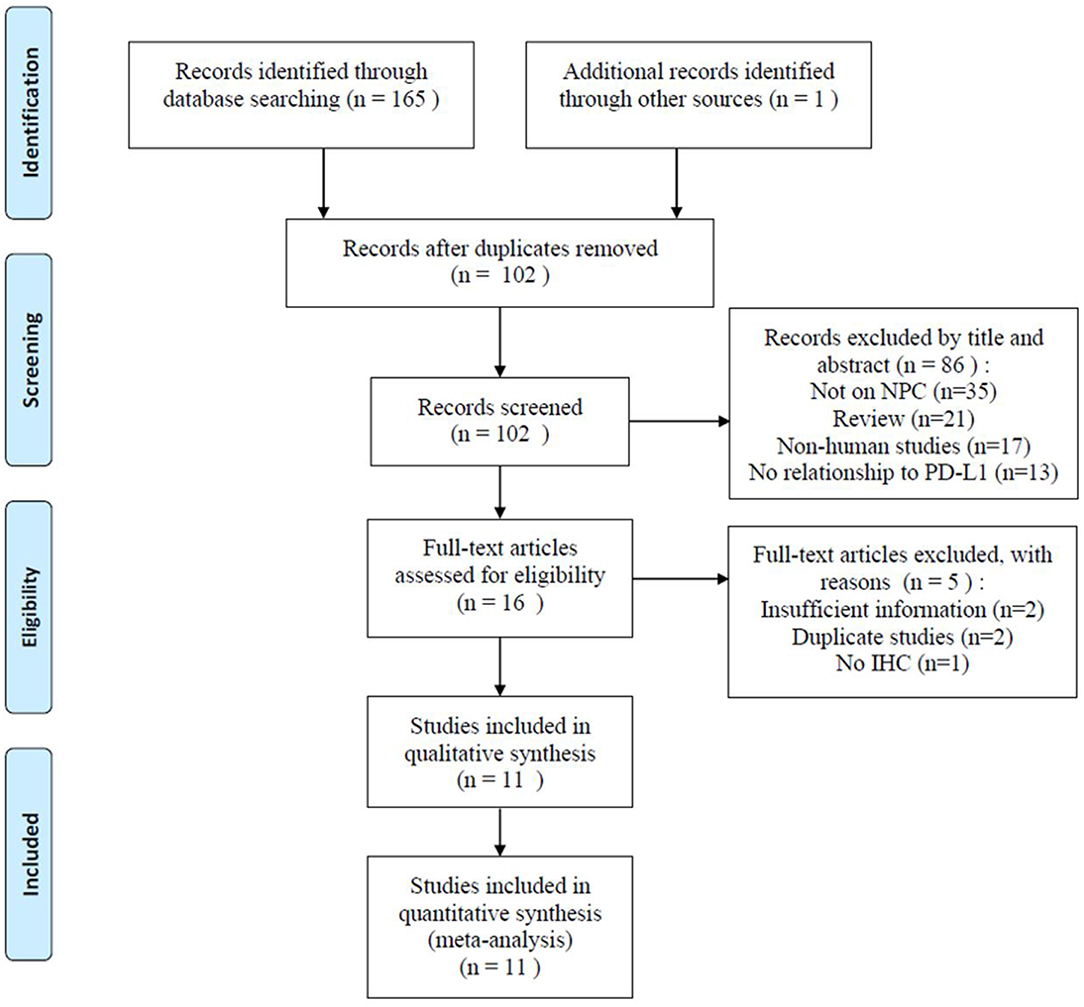
A total of 166 related studies were initially retrieved. As shown in Figure 1, following the exclusion of 64 duplicate studies, 102 studies were screened. Eighty-six studies were excluded after title and/or abstract screening for the following reasons: not based on NPC (n = 35), reviews (n = 21), non-human studies (n = 17), and not related to PD-L1 (n = 13). Thereafter, the full text of 16 studies was assessed; five studies were excluded because of insufficient data (n = 2), duplicate studies from the same research group (n = 2), and no use of IHC (n = 1). Finally, 11 studies were included in this meta-analysis (18–23, 26, 27, 31–33).
Study Characteristics
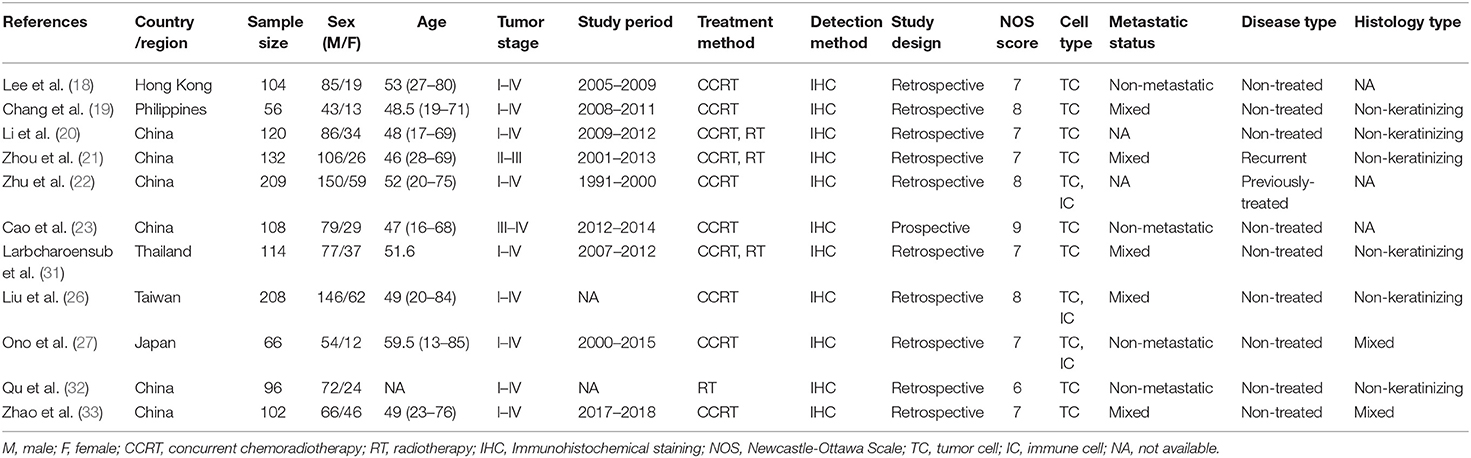
Baseline characteristics of the eligible studies are summarized in Table 1. The studies were published from 2016 to 2019 and were all conducted in Asia. Six studies were conducted in China (20–23, 32, 33), and one each were in Hong Kong (18), Philippines (19), Thailand (31), Taiwan (26), and Japan (27), respectively. Eight studies detected the expression of PD-L1 in TCs (18–23, 31–33) and 3 studies detected PD-L1 expression in both TCs and ICs (22, 26, 27). The data of PD-L1 expression of TCs were used for meta-analysis. Five studies recruited patients with both non-metastatic and metastatic NPC (19, 21, 26, 31, 33), 4 studies recruited patients with non-metastatic NPC (18, 23, 27, 32), and 2 studies did not provide the information on metastatic status (20, 22). Nine studies included non-treated NPC patients (18–20, 23, 26, 27, 31–33), one study recruited recurrent patients (21), and one study included previously-treated patients (22). In addition, 6 studies recruited patients with non-keratinizing histology type (WHO II) (19–21, 26, 31, 32), 2 studies included patients with both keratinizing and non-keratinizing histology types (27, 33), and 3 studies did not provide relevant data (18, 22, 23). The sample size ranged from 56 to 209 and the total number of patients was 1,315. There was one prospective study (23) and the remaining 10 studies were retrospective. The NOS scores ranged from 6 to 9 and the mean was 7.36; this indicated that all included studies were of high quality.
Relationship Between PD-L1 Expression and Prognosis of NPC
Nine studies (18–23, 26, 27, 31) with 1,117 patients reported the prognostic value of PD-L1 regarding overall survival (OS). The results revealed that PD-L1 overexpression was associated with significantly poorer OS compared with PD-L1-negative tumors (HR = 1.48, 95% CI = 1.00–2.18, p = 0.049; Table 2 and Figure 2A). The pooled HR and 95% CI from six studies (18, 20, 22, 23, 26, 27) indicated that PD-L1 overexpression was not correlated with poor disease-free survival (DFS; HR = 1.51, 95% CI = 0.85–2.69, p = 0.162; Table 2 and Figure 2B). As shown in Table 2 and Figure 2, the pooled data also demonstrated that PD-L1 overexpression was not correlated with distant metastasis-free survival (DMFS; HR = 1.75, 95% CI = 0.64–4.79, p = 0.277) or local recurrence-free survival (LRFS; HR = 0.67, 95% CI = 0.06–8.16, p = 0.756).
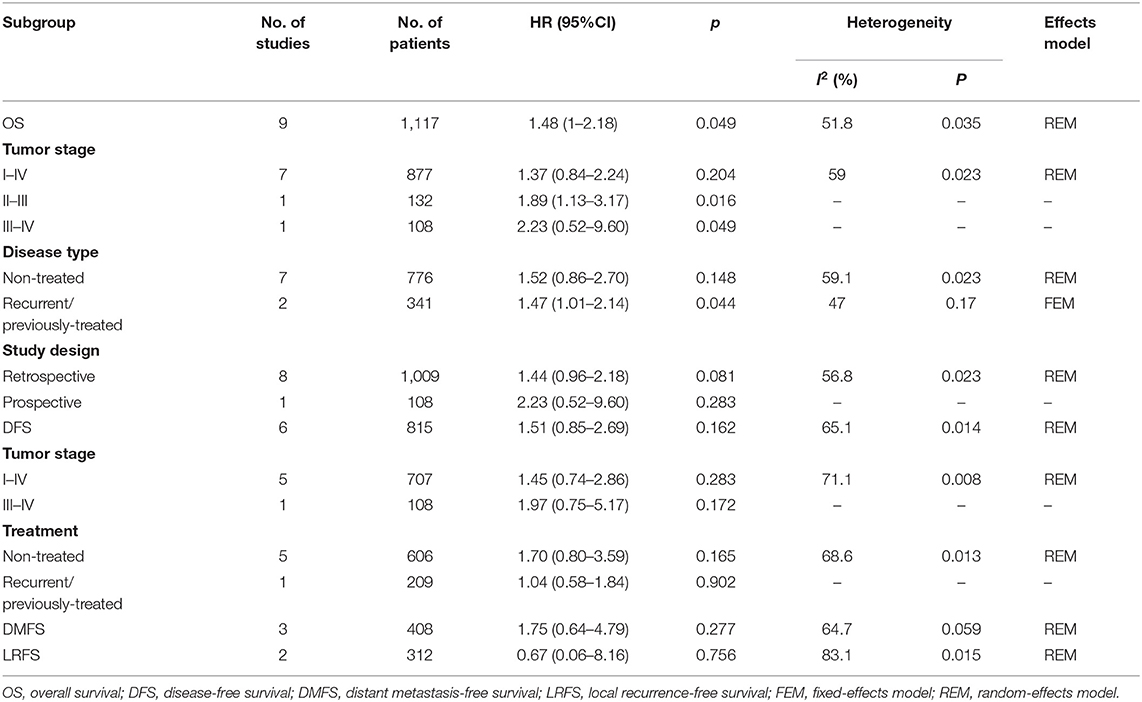
Table 2. Meta-analysis of association between PD-L1 and OS, DFS, DMFS, LRFS in nasopharyngeal carcinoma.
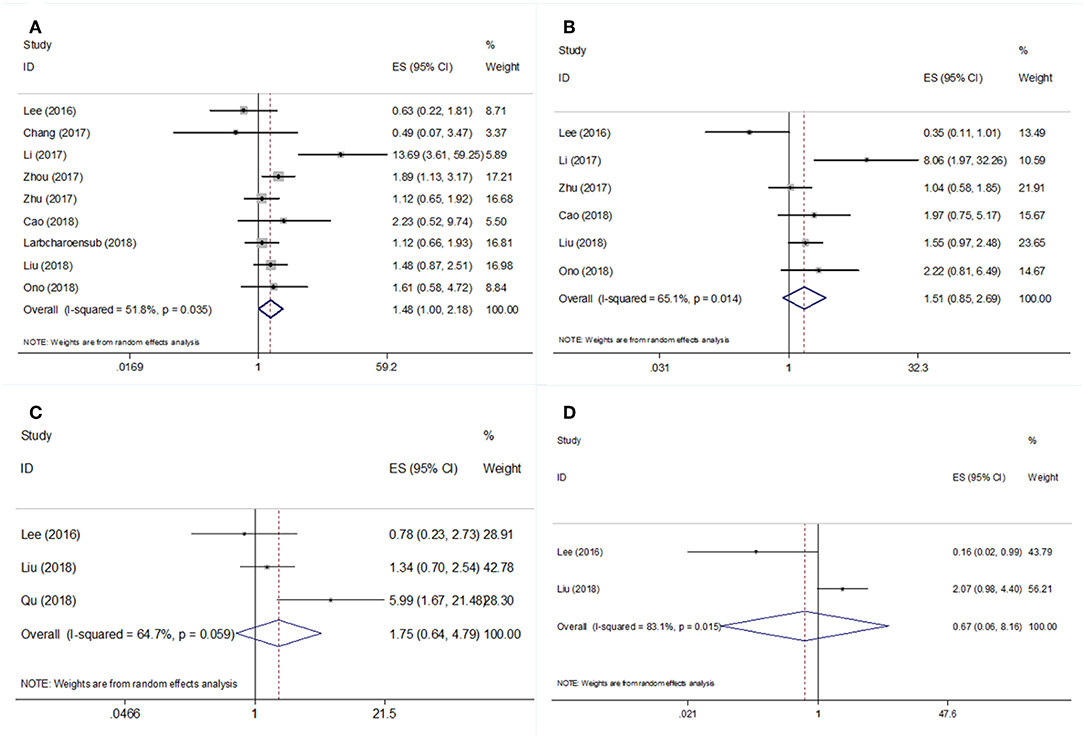
Figure 2. Forest plot diagrams of hazard ratios for correlations between PD-L1 expression and (A) OS, (B) DFS, (C) DMFS, and (D) LRFS.
The Association of PD-L1 Expression With Clinicopathological Features
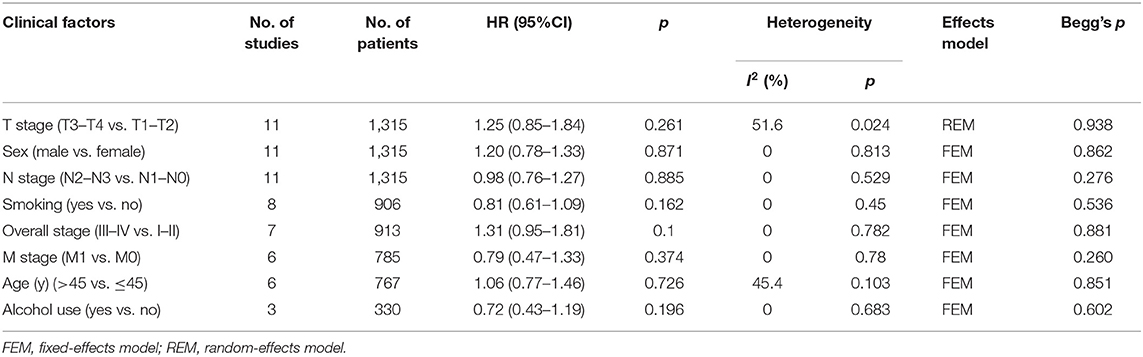
To explore the correlation between PD-L1 expression and clinicopathological characteristics of NPC, the pooled OR and 95% CI were determined. As shown in Table 3, the pooled data indicated that PD-L1 overexpression was not associated with T stage (OR = 1.25, 95% CI = 0.85–1.84, p = 0.261; random-effects model), N stage (OR = 0.98, 95% CI = 0.76–1.27, p = 0.885; fixed-effects model), M stage (OR = 0.79, 95% CI = 0.47–1.33, p = 0.374; fixed-effects model), overall stage (OR = 1.31, 95% CI = 0.95–1.81, p = 0.1; fixed-effects model), sex (OR = 1.20, 95% CI = 0.78–1.33, p = 0.871; fixed-effects model), age (OR = 1.06, 95% CI = 0.77–1.46, p = 0.726; fixed-effects model), smoking (OR = 0.81, 95% CI = 0.61–1.09, p = 0.162; fixed-effects model), or alcohol intake (OR = 0.72, 95% CI = 0.43–1.19, p = 0.196; fixed-effects model).
Subgroup Analysis and Sensitivity Analysis
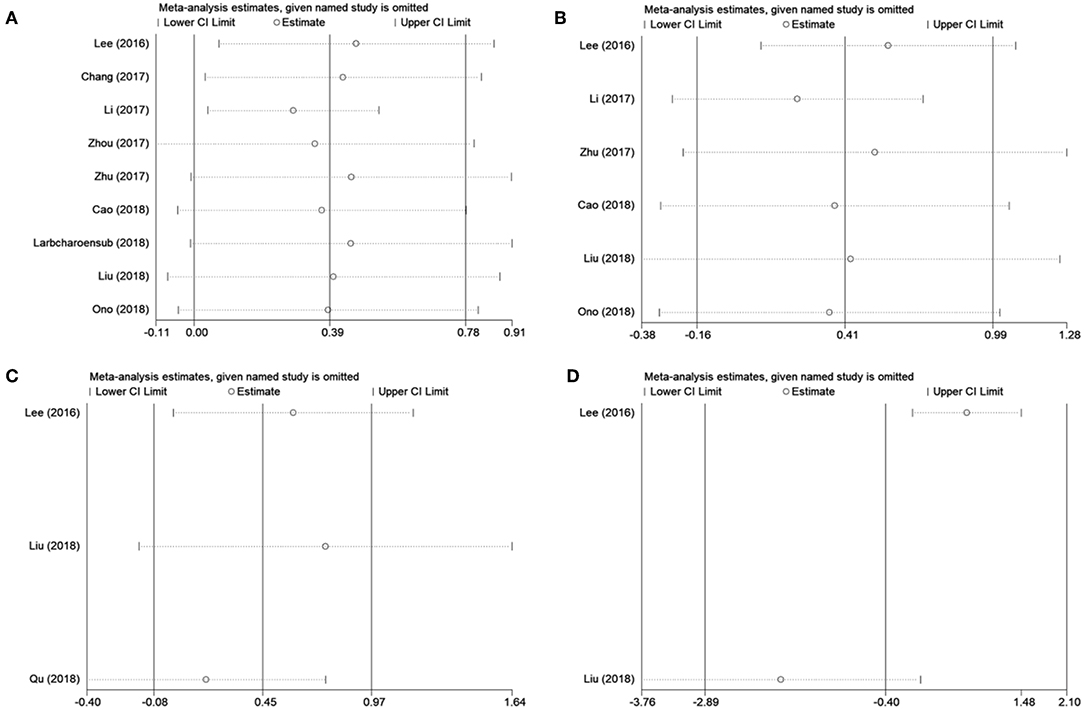
Because several baseline characteristics varied among included studies, which may cause heterogeneity in meta-analysis. Therefore, subgroup analysis was conducted for OS and DFS. As shown in Table 2, PD-L1 expression was associated with poor OS in patients with stage II-III (n = 1, p = 0.016), stage III-IV (n = 1, p = 0.049), and recurrent/ previously-treated patients (n = 2, p = 0.044). However, PD-L1 was not significantly correlated to OS in both retrospective and prospective studies. Subgroup analysis also showed that PD-L1 remained a non-significant prognostic marker for DFS irrespective of tumor stage and disease type. Subgroup analysis was not performed for DMFS and LRFS because of the limited number of studies and uniformity among these studies. Sensitivity analysis by omitting one study in each turn was conducted to test the credibility of the prognostic value of PD-L1. As shown in Figure 3A, the results for OS were significantly altered when Li' s study (20) was excluded. Therefore, we re-analyzed the prognostic value of PD-L1 for OS after exclusion of Li's study (20), the results were HR = 1.33, 95% CI = 1.04–1.70, p = 0.022; I2 = 0, P = 0.513 (Figure S1). The heterogeneity in the analysis of PD-L1 and OS was significantly reduced after deletion of Li's study (I2 = 0, P = 0.513). No other individual study influenced the results for DFS, DMFS, and LRFS (Figure 3). The results of prognosis of PD-L1 and OS were more significant after sensitivity analysis, which suggested that PD-L1 could be a prognostic factor for OS in NPC patients.
Publication Bias
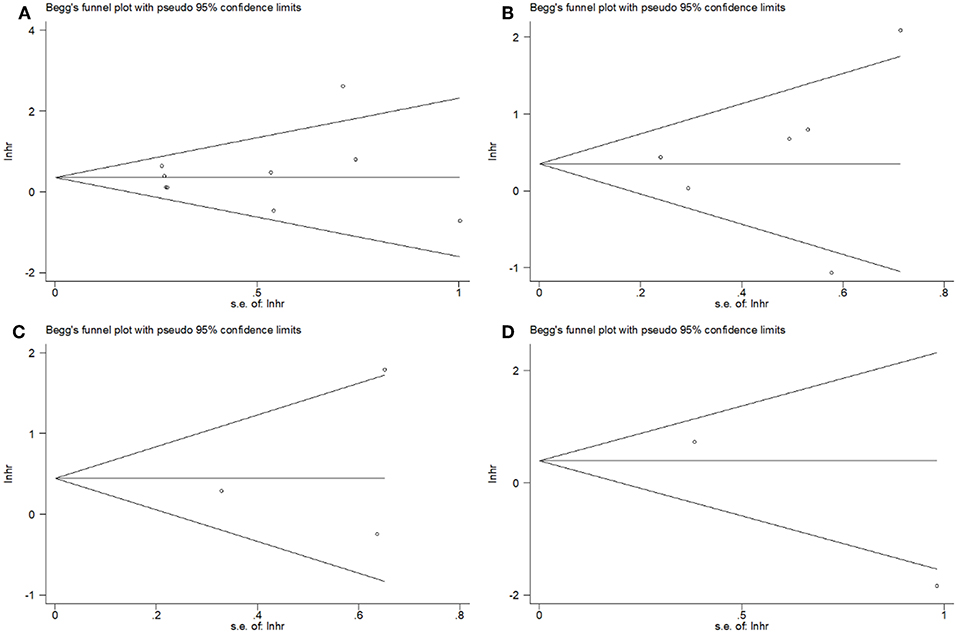
Begg's tests showed that there was no publication bias in the eligible studies involving PD-L1 and OS (p = 0.602), DFS (p = 0.452), DMFS (p = 1), and LRFS (p = 0.317) (Figure 4). In addition, the funnel plots showed no publication bias for clinicopathological factors (Table 3).
Discussion
Many studies demonstrated that PD-L1 plays an important role in tumor immune evasion (34, 35). The upregulation of PD-L1 inhibits T cell function and triggers immune evasion in cancer (36). In the tumor microenvironment, tumor-associated PD-L1 increases the apoptosis of T cells and leads to the growth of immunogenic tumors (36). PD-L1 is mainly expressed on the surface of tumor cells and tumor-associated APCs in various types of cancer including pancreatic cancer (37), ovarian cancer (38), thymoma (39), and colorectal cancer (40). PD-L1 and its receptor PD-1 constitute the PD-1/PD-L1 immune checkpoint signaling pathway (41). Checkpoint inhibition targets regulatory pathways in T cells to promote anti-tumor immune responses (12, 42). A recent phase II clinical trial including 44 patients revealed a promising activity of the PD-L1 antibody in NPC and a favorable 1-year OS rate (43). This study also suggested an association of PD-L1 expression with a higher response rate in patients with NPC (43).
To the best of our knowledge, this is the first meta-analysis to investigate the prognostic and clinicopathologic significance of PD-L1 expression in NPC. Survival data of 1,315 patients from 11 eligible studies were systematically analyzed. We found that PD-L1 overexpression was a significant prognostic factor for poor OS, whereas PD-L1 expression did not predict worse DFS, DMFS, or LRFS. Furthermore, PD-L1 expression was independent of T stage, N stage, M stage, overall stage, sex, smoking, age, or alcohol intake. Overall, this meta-analysis highlighted the potential of PD-L1 as a prognostic biomarker for poor OS in patients with NPC.
Previous meta-analyses exploring the prognostic value of PD-L1 in solid malignant tumors also indicated the unfavorable impact of PD-L1 on survival outcomes (10, 44, 45). A meta-analysis involving 13 studies showed that a high PD-L1 expression could predict a shorter OS (HR = 1.57, 95% CI = 1.09–2.27, P < 0.00001) and poorer DFS (HR = 2.07, 95% CI = 1.20–3.58, P = 0.009) in hepatocellular carcinoma (15). Another study also demonstrated a significant association of PD-L1 expression with a poor biochemical recurrence-free survival (BCR-FS) (HR = 1.78; 95% CI = 1.39 to 2.27; p < 0.00001) in prostate cancer (46). Wang et al. showed an association of PD-L1 expression with a poor OS in RCC (47). In addition, PD-L1 expression was found to be significantly associated with tumor stage, regional lymph node involvement, distant metastases, nuclear grade, and histologic tumor necrosis in RCC (47). The current meta-analysis showed the positive correlation of PD-L1 with OS, but not with DFS, DMFS, or LRFS. This may due to the limited sample size and the relatively short follow-up duration. Furthermore, we did not detect any association of PD-L1 with clinical factors in NPC. This requires further verification in future prospective studies.
There are several limitations in this study. First, all the eligible studies were conducted in Asia, which may reflect the high incidence of NPC in Asia. However, the results of this meta-analysis might be applied to patients in Asia. Second, although we selected eligible studies using uniform criteria, inter-study heterogeneity still exists in this meta-analysis. Therefore, subgroup analysis was performed to detect the source of heterogeneity. Third, the majority of the eligible studies were retrospective in design, which may compromise the validity of this study.
Conclusions
In summary, this meta-analysis showed that PD-L1 overexpression in NPC was associated with poor OS and may be useful as a novel prognostic factor. Nevertheless, because of the aforementioned limitations, well-designed, large-scale, prospective clinical trials are required to verify the findings of this meta-analysis.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
YS designed the study, performed the literature search and screening, performed the data analyses, and wrote the manuscript. XL and YS designed the study, retrieved the literature and data, analyzed the retrieved data, and participated in the writing of manuscript. CS and JD assisted in the designing of the study, performed the literature search and screening, assisted in the data analyses, and participated in the writing of manuscript. JD designed the study and supervised the study. Every author approved the final version of this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JM and the handling editor declared their shared affiliation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01111/full#supplementary-material
Figure S1. Meta-analysis of PD-L1 overexpression was associated with worse OS after deletion of Li's study.
References
1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. (2016) 387:1012–24. doi: 10.1016/S0140-6736(15)00055-0
2. Chiesa F, De Paoli F. Distant metastases from nasopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. (2001) 63:214–6. doi: 10.1159/000055743
3. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2012) 13:163–71. doi: 10.1016/S1470-2045(11)70320-5
4. Ma BBY, Chan ATC. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. (2005) 103:22–31. doi: 10.1002/cncr.20768
5. Sun R, Qiu HZ, Mai HQ, Zhang Q, Hong MH, Li YX, et al. Prognostic value and differences of the sixth and seventh editions of the UICC/AJCC staging systems in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. (2013) 139:307–14. doi: 10.1007/s00432-012-1333-9
6. Dong HD, Zhu GF, Tamada K, Chen LP. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. (1999) 5:1365–9. doi: 10.1038/70932
7. Curiel TJ, Wei S, Dong HD, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. (2003) 9:562–7. doi: 10.1038/nm863
8. Kuang DM, Zhao QY, Peng C, Xu J, Zhang JP, Wu CY, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. (2009) 206:1327–37. doi: 10.1084/jem.20082173
9. Pyo JS, Kang G, Kim JY. Prognostic role of PD-L1 in malignant solid tumors: a meta-analysis. Int J Biol Markers. (2017) 32:E68–74. doi: 10.5301/jbm.5000225
10. Wang QQ, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine 96:e6369. doi: 10.1097/MD.0000000000006369
11. Karwacz K, Bricogne C, Macdonald D, Arce F, Bennett CL, Collins M, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. (2011) 3:581–92. doi: 10.1002/emmm.201100165
12. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. (2016) 27:409–16. doi: 10.1093/annonc/mdv615
13. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14:847–56. doi: 10.1158/1535-7163.MCT-14-0983
14. Hu XY, Zhang W, Hu Y, Zhang Y, Gong R, Liang JY, et al. A meta-analysis reveals prognostic role of programmed death ligand-1 in Asian patients with non-small cell lung cancer. J Huazhong Univ Sci Technol Med Sci. (2016) 36:313–20. doi: 10.1007/s11596-016-1585-8
15. Li JH, Ma WJ, Wang GG, Jiang X, Chen X, Wu L, et al. Clinicopathologic significance and prognostic value of programmed cell death ligand 1 (PD-L1) in patients with hepatocellular carcinoma: a meta-analysis. Front Immunol. (2018) 9:2077. doi: 10.3389/fimmu.2018.02077
16. Shen Z, Gu L, Mao D, Chen M, Jin R. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. (2019) 17:4. doi: 10.1186/s12957-018-1544-x
17. Iacovelli R, Nole F, Verri E, Renne G, Paglino C, Santoni M, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. (2016) 11:143–8. doi: 10.1007/s11523-015-0392-7
18. Lee VHF, Lo AWI, Leung CY, Shek WH, Kwong DLW, Lam KO, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS ONE. (2016) 11:e0157969. doi: 10.1371/journal.pone.0157969
19. Chang AMV, Chiosea SI, Altman A, Pagdanganan HA, Ma CQ. Programmed death-ligand 1 expression, microsatellite instability, epstein-barr virus, and human papillomavirus in nasopharyngeal carcinomas of patients from the Philippines. Head Neck Pathol. (2017) 11:203–11. doi: 10.1007/s12105-016-0765-y
20. Li YF, Ding JW, Liao LM, Zhang ZL, Liao SS, Wu Y, et al. Expression of programmed death ligand-1 predicts poor outcome in nasopharyngeal carcinoma. Mol Clin Oncol. (2017) 7:378–82. doi: 10.3892/mco.2017.1318
21. Zhou Y, Miao J, Wu H, Tang H, Kuang J, Zhou X, et al. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget. (2017) 8:51210–23. doi: 10.18632/oncotarget.17214
22. Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology. (2017) 6:e1312240. doi: 10.1080/2162402X.2017.1312240
23. Cao C, Wei Q, Tang X, Jia Y, Sun X, Li W, et al. PD-1 and PD-L1 in locoregionally advanced nasopharyngeal carcinoma: a substudy of a randomized phase III trial. Head Neck. (2018) 41:1427–33. doi: 10.1002/hed.25601
24. Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
25. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
26. Liu YJ, Tsang NM, Hsueh C, Yeh CJ, Ueng SH, Wang TH, et al. Low PD-L1 expression strongly correlates with local recurrence in epstein-barr virus-positive nasopharyngeal carcinoma after radiation-based therapy. Cancers. (2018) 10:E374. doi: 10.3390/cancers10100374
27. Ono T, Azuma K, Kawahara A, Sasada T, Matsuo N, Kakuma T, et al. Prognostic stratification of patients with nasopharyngeal carcinoma based on tumor immune microenvironment. Head Neck. (2018) 40:2007–19. doi: 10.1002/hed.25189
28. Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2009). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
31. Larbcharoensub N, Mahaprom K, Jiarpinitnun C, Trachu N, Tubthong N, Pattaranutaporn P, et al. Characterization of PD-L1 and PD-1 expression and CD8+tumor-infiltrating lymphocyte in Epstein-Barr virus-associated nasopharyngeal carcinoma. Am J Clin Oncol. (2018) 41:1204–10. doi: 10.1097/COC.0000000000000449
32. Qu Y, Wang D, Yang L, Liu HY, Cui W, Che YQ. Expression and clinical significance of programmed death ligand 1 in nasopharyngeal carcinoma. Mol Clin Oncol. (2018) 9:75–81. doi: 10.3892/mco.2018.1633
33. Zhao L, Liao XY, Hong GJ, Zhuang YZ, Fu KL, Chen PQ, et al. Mismatch repair status and high expression of PD-L1 in nasopharyngeal carcinoma. Cancer Manag Res. (2019) 11:1631–40. doi: 10.2147/CMAR.S193878
34. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
35. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. (2014) 515:568–71. doi: 10.1038/nature13954
36. Dong HD, Chen LP. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. (2003) 81:281–7. doi: 10.1007/s00109-003-0430-2
37. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. (2007) 13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746
38. Huang LJ, Deng XF, Chang F, Wu XL, Wu Y, Diao QZ. Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma A systematic review and meta-analysis. Medicine. (2018) 97:e128858. doi: 10.1097/MD.0000000000012858
39. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe SI, Tsuta K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. (2015) 88:154–9. doi: 10.1016/j.lungcan.2015.03.003
40. Ni X, Sun X, Wang D, Chen Y, Zhang Y, Li W, et al. The clinicopathological and prognostic value of programmed death-ligand 1 in colorectal cancer: a meta-analysis. Clin Transl Oncol. (2018) 21:674–86. doi: 10.1007/s12094-018-1970-9
41. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. (2017) 8:561. doi: 10.3389/fphar.2017.00561
42. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. (2015) 348:56–61. doi: 10.1126/science.aaa8172
43. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol. (2018) 36, 1412–8. doi: 10.1200/JCO.2017.77.0388
44. Wu P, Wu D, Li LJ, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS ONE. (2015) 10:e0131403. doi: 10.1371/journal.pone.0131403
45. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. (2018) 362:k3529. doi: 10.1136/bmj.k3529
46. Li Y, Huang Q, Zhou Y, He M, Chen J, Gao Y, et al. The clinicopathologic and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with prostate cancer: a systematic review and meta-analysis. Front Pharmacol. (2018) 9:1494. doi: 10.3389/fphar.2018.01494
Keywords: PD-L1, meta-analysis, prognosis, nasopharyngeal carcinoma, clinical use
Citation: Liu X, Shan C, Song Y and Du J (2019) Prognostic Value of Programmed Cell Death Ligand-1 Expression in Nasopharyngeal Carcinoma: A Meta-Analysis of 1,315 Patients. Front. Oncol. 9:1111. doi: 10.3389/fonc.2019.01111
Received: 15 July 2019; Accepted: 07 October 2019;
Published: 25 October 2019.
Edited by:
Yu-Pei Chen, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Edgar K. Selzer, Medical University of Vienna, AustriaJun Ma, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2019 Liu, Shan, Song and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingluan Song, MzMxOTAzMTY4QHFxLmNvbQ==
 Xiaofeng Liu1
Xiaofeng Liu1 Yingluan Song
Yingluan Song