- 1Department of Medical Oncology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 2Department of Medical Oncology, Hôpital Riviera-Chablais, Vevey, Switzerland
- 3Department of Otorhinolaryngology, Head and Neck Surgery, Geneva University Hospital, Geneva, Switzerland
- 4Department of Otorhinolaryngology, Head and Neck Surgery, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 5Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Zurich, Zurich, Switzerland
- 6Department of Radiation Oncology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 7Department of Radiation Oncology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 8Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital of Lausanne, Lausanne, Switzerland
- 9Department of Radiation Oncology, Cantonal Hospital Graubünden, Chur, Switzerland
- 10Department of Radiation Oncology, Cantonal Hospital of Winterthur, Winterthur, Switzerland
- 11Department of Radiation Oncology, Clinica Luganese SA, Lugano, Switzerland
- 12Department of Radiation Oncology, Cantonal Hospital Lucerne, Lucerne, Switzerland
- 13Department of Otorhinolaryngology, Head and Neck Surgery, Inselspital, Bern University Hospital, Bern, Switzerland
- 14Department of Medical Oncology, University Hospital of Basel, Basel, Switzerland
- 15Department of Otorhinolaryngology, Lindenhofspital, Bern, Switzerland
Background: The Head and Neck Cancer Working Group of Swiss Group for Clinical Cancer Research (SAKK) has investigated the level of consensus (LOC) and discrepancy in everyday practice of diagnosis and treatment in head and neck cancer.
Materials and Methods: An online survey was iteratively generated with 10 Swiss university and teaching hospitals. LOC below 50% was defined as no agreement, while higher LOC were arbitrarily categorized as low (51–74%), moderate (75–84%), and high (≥85%).
Results: Any LOC was achieved in 62% of topics (n = 60). High, moderate, and low LOC were found in 18, 20, and 23%, respectively. Regarding Head and Neck Surgery, Radiation Oncology, Medical Oncology, and biomarkers, LOC was achieved in 50, 57, 83, and 43%, respectively.
Conclusions: Consensus on clinical topics is rather low for surgeons and radiation oncologists. The questions discussed might highlight discrepancies, stimulate standardization of practice, and prioritize topics for future clinical research.
Introduction
This is the third part of the article “A Review of Controversial Issues in the Management of Head and Neck Cancer: A Swiss Multidisciplinary and Multi-Institutional Patterns of Care Study,” providing the results for the items concerning medical oncology discipline, each followed by a short discussion if deemed relevant. The details of the methodology is presented in the first part of this series.
Results and Discussion
Medical Oncology
This section contains some overlapping topics with the previous sections regarding concurrent CRT and induction chemotherapy. The focus remains on the medical oncologists' point of view.
Concurrent Chemoradiotherapy
➢ Cetuximab is preferred in combination with definitive radiotherapy in loco-regionally advanced HNSCC for cisplatin-ineligible patients: moderate LOC (80%).
An important question remains which approach is preferred in cases where cisplatin cannot be applied due to contraindications or patient related factors precluding its application (age, performance status, hearing loss etc.). For this situation, cetuximab (1) as alternative choice is favored in 8/10 centers. One center prefers carboplatin, whereas in another center a combination regimen with 5-fluorouracil (5-FU) and mitomycin C (2, 3) vs. Cetuximab is discussed on patient basis.
Different systemic modalities for concurrent treatment were investigated during the last decades. Cisplatin given every 3 weeks remains the standard of care (4, 5). A minimal dose of ≥200 mg/m2 cisplatin has to be administered to achieve optimal outcome (6). Nevertheless, only 61% of patients tolerate the standard dose of 100 mg/m2 times three (7). Therefore, different alternatives are investigated. Among them, the well-tolerated platinum alternative carboplatin, alone, or in combination with 5-FU was the combination used by the GORTEC group (8). Cetuximab, based on high level evidence (1), was the preferred choice within our survey, despite the lack of randomized comparison to cisplatin at the time of the survey. Recently, two phase III randomized trials showed that cetuximab is associated with inferior overall survival compared to cisplatin even in the low and intermediate risk HPV-associated OPSCC (9, 10). For mitomycin C in combination with 5-FU, one randomized trial showed superiority of CRT in terms of locoregional control and survival to a dose escalated hyperfractionated accelerated radiation therapy schedule without systemic therapy (11, 12). For mitomycin C, as monotherapy or in combination, no randomized phase III data is available, in comparison to standard of care cisplatin or cetuximab.
➢ No agreement in the radiosensitizer indication in post-operative setting for cisplatin-ineligible patients: no consensus.
The same question in the adjuvant CRT setting yielded a different pattern: cetuximab was the preferred choice in 4, carboplatin in 5 centers, In the remaining center, the radiation oncologist would prefer 5-fluorouracil with mitomycin c, whereas the medical oncologist would opt for cetuximab, or carboplatin instead.
In the adjuvant setting, no high-level evidence is available for cetuximab. Despite this fact, almost half the centers adopt the data from non-operated locally advanced disease (1) and prescribe cetuximab. Carboplatin is the preferred agent as monotherapy. For mitomycin C as monotherapy or in combination with dicumarol, an improvement was shown but not regarding overall survival (13). For the combination of 5-FU an extrapolation from the existing data from non-operated locally advanced disease is assumed.
➢ The cisplatin regimen in terms of dose and cycle frequency concomitant with radiotherapy is quite heterogeneous: no consensus.
Platinum-based regimens are administered weekly in 4/10, every 3 weeks in 5 centers, and every 3 weeks but distributed over 5 days every 3 weeks in 1 center.
Shortly after our survey was completed, data presented at the annual congress of clinical oncology ASCO 2017 was presented and later on published, showing superiority of the 3-weeks application of cisplatin vs. a weekly application (14). Probably, from the four centers applying cisplatin weekly, some would consider changing their opinion.
➢ All centers prefer to continue the treatment with another systemic agent in patients who cannot complete the planned number of cycles of cisplatin: high LOC (100%).
If a patient was not able to continue with cisplatin after ≥1 cycle, systemic treatment is switched to another regimen in 10/10 centers. In one center, treatment is switched to 5-FU and mitomycin c or carboplatin alone. All other centers prefer cetuximab or carboplatin.
We are not aware of any solid data confirming the benefit of any switch strategy, and with which combination, if there is any value at all. Of note, one of the participating centers recently published a hypothesis-generating retrospective study indicating a higher incidence in second primary cancers, when cetuximab was administered after the discontinuation of platinum-based chemotherapy, compared to pure cetuximab, or platinum-based therapy (15).
➢ Age is not considered as a strict factor regarding the decision whether to administer concomitant chemotherapy: high LOC (100%).
There was total consensus (10/10) about administering chemotherapy concomitant with radiotherapy to selected, medically fit patients even older than 70 years.
Even if there is no randomized prospective data confirming the efficacy of a concomitant strategy in this patient group, all centers apply the same regimen as in their younger counterparts. Some analyses show similar outcomes for these patients despite the higher age (16). Biological age seems to be of importance more than chronological age.
➢ ECE is a well-established high-risk factor for post-operative concomitant CRT indication: high LOC (100%).
➢ In most centers, positive resection margin is considered a high-risk factor for post-operative concomitant CRT indication: high LOC (90%).
Risk factors warranting adjuvant concomitant chemotherapy to radiotherapy vary between centers and are elucidated in Table 1.
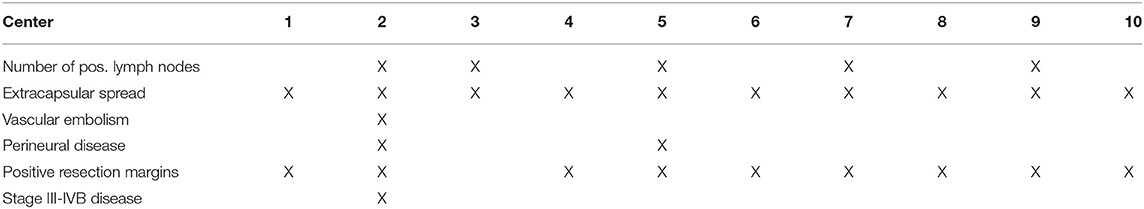
Table 1. Depending on the following risk factors the centers administer concurrent chemotherapy together with adjuvant radiotherapy.
Induction Chemotherapy
➢ The use of induction chemotherapy is not part of the routine: low LOC (60%).
The use of induction chemotherapy with the intention of increasing oncological outcome is used in 4/10 centers. The other centers either never administer induction chemotherapy, or only do so in rare cases in presence of bulky disease, in which performing an up-front curative CRT with full-dose is not realistically applicable or feasible. An exact specification of the induction regimen was not pointed out [classic TPF regimen (docetaxel, cisplatin, 5-fluorouracil) (17, 18), adapted TPF, other combination chemotherapy].
Induction chemotherapy is a controversial topic in HNSCC. Nevertheless, during the last decade one regimen, applied “classically” or “adapted” showed level I evidence for having better survival compared to radiotherapy alone in selected patients (17, 18). With the standard of care approach of concurrent radiotherapy and cisplatin, trials comparing these two approaches were eagerly awaited. From five randomized phase III trials, only two compared standard concurrent treatment vs. induction with TPF followed by the same treatment (19, 20). All the other trials were underpowered or did not reach their recruitment goal. Moreover, inadequate systemic agents were applied concurrently to radiotherapy. The trial by Hitt et al. showed a trend toward an improvement of overall survival, but was formally negative (19). A trial with an “adapted” TPF regimen also called “Italian” TPF was able to show a marked and impressive overall survival benefit of more than 20 months (20). The trial is controversial for its design, but the main question, whether an induction approach irrespective of the following concurrent treatment (cisplatin and 5-FU or cetuximab), defined after a second randomization, improved outcome was clearly answered. Concerns about a lower rate of completion of radiotherapy and a higher mortality rate were raised, but could in part be refuted by recent trials. Despite these arguments, induction chemotherapy reduces distant metastases rates more prominently than concurrent CRT alone (21). In the particular case of locally advanced laryngeal cancer, value of induction chemotherapy is higher, due to available data and long-term outcome of pivotal trials, showing better outcome with higher larynx-preservation rate (22–24).
Whether to administer induction chemotherapy in nasopharynx cancer or not is an ongoing discussion. The most recently published study by Sun et al. (25) is a well-designed and conducted study, whose results indicate a favorable progression-free survival with the addition of TPF administered before CRT. However, it is important to note the eligibility criteria and the patient collective of this study. Only cN+ patients younger than 60 years old were allowed. Moreover, the distribution of WHO histological subtypes are neither reported nor mentioned in the published article. Considering the dramatic geographic differences of the histology, a direct implementation of the results of a study from China to European and American patients, especially those with non-EBV tumors, is questionable. Nevertheless, for those who find the study results convincing enough to change their practice, the investigators of the same study created a helpful nomogram based on the trial database to predict the extent of potential gain via induction chemotherapy for a given patient (26).
➢ The use of induction/neoadjuvant chemotherapy for optimal decision-making in locally advanced laryngeal cancer is preferred: low LOC (70%).
However, 7/10 centers favor the use of induction/neoadjuvant (the term “neoadjuvant” is rather used, if a surgery is planned afterwards) chemotherapy for decision making purposes concerning larynx preservation (22, 27).
Nasopharyngeal, Nasal, and Paranasal Sinus Tumors
➢ Administration of chemotherapy before the primary treatment of sino-nasal tumors is preferred due to various reasons: low LOC (60%).
For the treatment of clinically aggressive, highly proliferating nasal cavity and paranasal sinus tumors, induction/neoadjuvant chemotherapy is considered in 6/10 centers, especially in case of bulky tumors, and/or presence of symptoms to avoid disease progression until start of radiotherapy (5/6), further to achieve clear surgical margins (1/6).
Due to the relatively low incidence and variety of histological subtypes of nasal cavity and paranasal sinus tumors, there is no convincing level of evidence for or against the use of chemotherapy before, during, or after the primary treatment. Nevertheless, it is interesting to see a low but presence LOC among participating centers.
➢ Concomitant CRT is preferred for the treatment of sino-nasal tumors: moderate LOC (70%).
For the treatment of loco-regionally advanced nasal and paranasal sinus tumors, concurrent chemotherapy is regularly administered in 7/10 centers. In 2 centers, it is administered only in selected cases based on tumor board discussion. One center never performs radiotherapy with concomitant chemotherapy.
There is moderate consensus, that locally advanced disease needs multimodality treatment. This according to almost all guidelines available (NCCN, ESMO, etc.). One center seems to diverge from this approach, probably due to toxicity concerns.
➢ Concerning the indication of adjuvant chemotherapy for nasopharynx cancer, no standard approach was observed: no consensus.
Among participating centers, adjuvant chemotherapy for nasopharynx cancer is omitted in three out of ten centers; performed in all cases in three centers; in selected cases at four centers. However, when asked, the definition of “selected cases” was not further specified in three centers. In one center selection was based on treatment response and EBV titer if applicable.
Treatment of nasopharyngeal cancer is a field of controversy. Stages > I need multimodality treatment, where CRT is established as the standard of care (28, 29). Further adjuvant chemotherapy, traditionally proposed for years is based on a pivotal Intergroup 0099 study (30), which had its caveats, raising concerns about the quality of the radiotherapy in the trial and highlighting the importance of patient selection. Despite the co-existence of negative trials showing the futility of adjuvant chemotherapy after radiotherapy alone (31, 32) or CRT (33, 34), an added benefit of adjuvant treatment was confirmed by meta-analyses, one published in 2015 of 19 trials with a total of 4,806 patients, showing the most favorable overall survival (HR 0.65; 95% CI, 0.56–0.76) compared to CRT without adjuvant chemotherapy (HR, 0.80; 95% CI, 0.70–0.93) (35). The other meta-analysis including 20 trials and 5,144 patients, showed that the addition of adjuvant chemotherapy to CRT was associated with better PFS compared to CRT only (HR 0.81; 95% CI, 0.66–0.98) (36). On the other hand, the most recently published phase III trial showed no benefit of adjuvant chemotherapy when added to CRT, even though the study only included high-risk patients with detectable post-CRT plasma EBV DNA (37). Moreover, a majority of patients do not tolerate full adjuvant treatment. Therefore, induction treatment was studied within phase III trials and showed differing results. Nevertheless, two phase 3 trials (25, 38) and a meta-analysis (36) were positive for the primary endpoint overall survival.
Supportive Measures and Oligometastatic Disease
➢ Prophylactic use of colony stimulating factors is not preferred during CRT: moderate LOC (80%).
In 2/10 centers, prophylactic use of colony-stimulating factors during CRT was reported.
Cautious application of colony-stimulating factors is probably due to reports finding adverse outcome during chemo-radiation (39) and pre-clinical data suggesting tumor proliferation (40) with such agents. Additionally, the efforts of reducing treatment-related mucositis were futile (41, 42). Although not belonging to the same category of agents, it is also worth to note that the use of erythropoiesis-stimulating agents to overcome anemia and hypoxia was shown to cause an unexpected negative outcome (43).
➢ Induction/neoadjuvant chemotherapy for subsequent decision-making is preferred in oligometastatic HNSCC: low LOC (60%).
For the treatment of oligometastatic (defined as up to 3 metastases) cases at the initial diagnosis, 6/10 centers consider administering induction/neoadjuvant chemotherapy, and decide thereafter based on response the final treatment concept (curative vs. palliative). Three centers never pursue this strategy. One center directly treats the locoregional and distant disease with curative intent.
Compared to other tumor entities (e.g., breast, colorectal, prostate, non-small lung cancer, malignant melanoma), the concept of oligometastatic disease and its treatment in HNSCC were not extensively investigated. Retrospective series demonstrate 5-years survival rates of 20% and higher after local ablation by means of surgery or SBRT of oligometastatic disease (44, 45). However, a high level of evidence is still lacking. Moreover, the optimal strategy for the synchronous presentation of the oligometastases at the time of initial diagnosis poses a more specific question, which still remains unanswered. The heterogeneity in the patterns of treatment among our 10 centers seems to reflect this ambiguity.
Systemic Treatments for Recurrent/Metastatic Disease
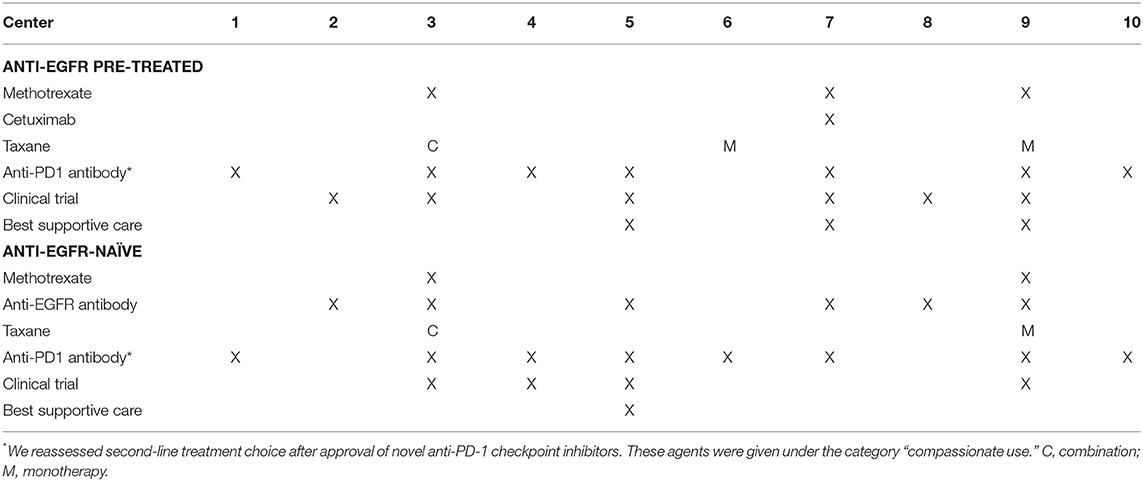
➢ In first line, EXTREME is the preferred systemic treatment regimen for recurrent/metastatic disease (R/M): low LOC (60%).
➢ The use of 2nd line anti-PD1 checkpoint inhibitors are preferred in anti-EGFR pre-treated and not pre-treated R/M: moderate LOC (70–80%, respectively).
➢ Anti-EGFR pre-treated patients would be encouraged to participate in clinical trials for ≥2nd line treatment: low LOC (60%).
➢ Anti-EGFR-naïve patients are considered for anti-EGFR treatment as ≥2nd line: low LOC (60%).
The EXTREME regimen containing a platinum compound with 5-fluorouracil and cetuximab is considered for patients with R/M and an ECOG performance status 0–2 in 6/10 centers. The remaining four centers do not necessarily consider systemic treatment according to the pivotal EXTREME trial especially for patients with higher ECOG performance status (46). Second-line systemic treatment choice was mostly based on whether or not previous treatment contained cetuximab (Table 2). There was a moderate LOC (70–80%) among the centers about the application of nivolumab in this setting (47). Nevertheless, the general heterogeneity in the R/M setting among participating centers is not to be overlooked.
Conclusion
The findings of our survey indicate a low LOC among head and neck oncologists working in academic and multidisciplinary setting in 10 Swiss institutions. Regarding the results and the discussion concerning the specialties other than medical oncology, the reader is advised to read the corresponding parts of this article. The highest LOC was achieved among medical oncologists, whereas the lowest was observed among head and neck surgeons. On the other hand, this level of disagreement may also depend on the topics chosen for the survey, and not necessarily the heterogeneity within the disciplines. It is also interesting to witness a low LOC regarding topics, where a high level of evidence actually does exist, and vice versa. This article is expected to serve the head and neck oncologists to be aware of their discrepancies and to stimulate discussion toward standardization of practice and prioritize topics of future clinical research.
Data Availability Statement
All datasets generated for this study are included in the manuscript/supplementary files.
Author Contributions
GH, MB, OE, PD, and PP: conception and design. OE and PP: collection of data. All co-authors: generation of the initial and final versions of the questions, drafting of the manuscript, and approval of the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank each of our colleagues working with the local coordinators for filling out the part of the questionnaire corresponding to their area of expertise in their institution.
References
1. Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. (2010) 11:21–8. doi: 10.1016/S1470-2045(09)70311-0
2. Jeremic B, Shibamoto Y, Stanisavljevic B, Milojevic L, Milicic B, Nikolic N. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol. (1997) 43:29–37. doi: 10.1016/S0167-8140(97)00048-0
3. Budach W, Hehr T, Budach V, Belka C, Dietz K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer. (2006) 6:28. doi: 10.1186/1471-2407-6-28
4. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Altered fractionation radiotherapy combined with concurrent low-dose or high-dose cisplatin in head and neck cancer: a systematic review of literature and meta-analysis. Oral Oncol. (2018) 76:52–60. doi: 10.1016/j.oraloncology.2017.11.025
5. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. (2017) 22:1056–66. doi: 10.1634/theoncologist.2017-0015
6. Strojan P, Vermorken JB, Beitler JJ, Saba NF, Haigentz M, Bossi P, et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: a systematic review. Head Neck. (2016) 38 (Suppl. 1):E2151–8. doi: 10.1002/hed.24026
7. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. (2004) 350:1937–44. doi: 10.1056/NEJMoa032646
8. Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. (2004) 22:69–76. doi: 10.1200/JCO.2004.08.021
9. Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. (2019) 393:51–60. doi: 10.1016/S0140-6736(18)32752-1
10. Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. (2019) 393:40–50. doi: 10.1016/S0140-6736(18)32779-X
11. Budach V, Stromberger C, Poettgen C, Baumann M, Budach W, Grabenbauer G, et al. Hyperfractionated accelerated radiation therapy (HART) of 70.6 Gy with concurrent 5-FU/Mitomycin C is superior to HART of 77.6 Gy alone in locally advanced head and neck cancer: long-term results of the ARO 95-06 randomized phase III trial. Int J Radiat Oncol Biol Phys. (2015) 91:916–24. doi: 10.1016/j.ijrobp.2014.12.034
12. Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy coo. J Clin Oncol. (2005) 23:1125–35. doi: 10.1200/JCO.2005.07.010
13. Rewari AN, Haffty BG, Wilson LD, Son YH, Joe JK, Ross DA, et al. Postoperative concurrent chemoradiotherapy with mitomycin in advanced squamous cell carcinoma of the head and neck: results from three prospective randomized trials. Cancer J. (2006) 12:123–9.
14. Noronha V, Joshi A, Patil VM, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. (2018) 36:1064–72. doi: 10.1200/JCO.2017.74.9457
15. Elicin O, Sermaxhaj B, Bojaxhiu B, Shelan M, Giger R, Rauch D, et al. Incidence of second primary cancers after radiotherapy combined with platinum and/or cetuximab in head and neck cancer patients. Strahlenther Onkol. (2018) 195:468–74. doi: 10.1007/s00066-018-1400-5
16. Amini A, Jones BL, McDermott JD, Serracino HS, Jimeno A, Raben D, et al. Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. (2016) 122:1533–43. doi: 10.1002/cncr.29956
17. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. (2007) 357:1695–704. doi: 10.1056/NEJMoa071028
18. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. (2007) 357:1705–15. doi: 10.1056/NEJMoa070956
19. Hitt R, Grau JJ, López-Pousa A, Berrocal A, García-Girón C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. (2014) 25:216–25. doi: 10.1093/annonc/mdt461
20. Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codecà C, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol Off J Eur Soc Med Oncol. (2017) 28:2206–12. doi: 10.1093/annonc/mdx299
21. Pignon J-P, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. (2009) 92:4–14. doi: 10.1016/j.radonc.2009.04.014
22. Lefebvre J-LL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. (1996) 88:890–9. doi: 10.1093/jnci/88.13.890
23. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. (2013) 31:845–52. doi: 10.1200/JCO.2012.43.6097
24. Licitra L, Bonomo P, Sanguineti G, Bacigalupo A, Baldi GG, Valerini S, et al. Different view on larynx preservation evidence-based treatment recommendations. J Clin Oncol. (2018) 36:1376–7. doi: 10.1200/JCO.2018.77.8001
25. Sun Y, Li W-F, Chen N-Y, Zhang N, Hu G-Q, Xie F-Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
26. Zhang Y, Li W-F, Liu X, Chen L, Sun R, Sun Y, et al. Nomogram to predict the benefit of additional induction chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: analysis of a multicenter, phase III randomized trial. Radiother Oncol. (2017) 129:8–12. doi: 10.1016/j.radonc.2017.12.002
27. Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. (1991) 324:1685–90. doi: 10.1056/NEJM199106133242402
28. Lin J-C, Jan J-S, Hsu C-Y, Liang W-M, Jiang R-S, Wang W-Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. (2003) 21:631–7. doi: 10.1200/JCO.2003.06.158
29. Chan ATC, Leung SF, Ngan RKC, Teo PML, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2005) 97:536–9. doi: 10.1093/jnci/dji084
30. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
31. Rossi A, Molinari R, Boracchi P, Del Vecchio M, Marubini E, Nava M, et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4-year multicenter randomized study. J Clin Oncol. (1988) 6:1401–10. doi: 10.1200/JCO.1988.6.9.1401
32. Chi K-H, Chang Y-C, Guo W-Y, Leung M-J, Shiau C-Y, Chen S-Y, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. (2002) 52:1238–44. doi: 10.1016/S0360-3016(01)02781-X
33. Chen L, Hu C-S, Chen X-Z, Hu G-Q, Cheng Z-B, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2012) 13:163–71. doi: 10.1016/S1470-2045(11)70320-5
34. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer. (2017) 75:150–8. doi: 10.1016/j.ejca.2017.01.002
35. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. (2015) 16:645–55. doi: 10.1016/S1470-2045(15)70126-9
36. Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. (2016) 35:JCO2016674119. doi: 10.1200/JCO.2016.67.4119
37. Chan ATC, Hui EP, Ngan RKC, Tung SY, Cheng ACK, Ng WT, et al. Analysis of plasma epstein-barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol. (2018) JCO2018777847. doi: 10.1200/JCO.2018.77.7847. [Epub ahead of print].
38. Lee AWM, Ngan RKC, Tung SY, Cheng A, Kwong DLW, Lu TX, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fr. Cancer. (2015) 121:1328–38. doi: 10.1002/cncr.29208
39. Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy - results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. (2001) 50:1161–71. doi: 10.1016/S0360-3016(01)01544-9
40. Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. (2006) 66:8026–36. doi: 10.1158/0008-5472.CAN-06-0158
41. Ryu JK, Swann S, LeVeque F, Scarantino CW, Johnson D, Chen A, et al. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective Phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys. (2007) 67:643–50. doi: 10.1016/j.ijrobp.2006.09.043
42. Hoffman KE, Pugh SL, James JL, Scarantino C, Movsas B, Valicenti RK, et al. The impact of concurrent granulocyte-macrophage colony-stimulating factor on quality of life in head and neck cancer patients: results of the randomized, placebo-controlled Radiation Therapy Oncology Group 9901 trial. Qual Life Res. (2014) 1841–58. doi: 10.1007/s11136-014-0628-5
43. Overgaard J, Alsner J. Effect of ESA as a modifier of radiotherapy in curative intended treatment of squamous cell carcinoma of the head and neck (HNSCC). Radiother Oncol. (2018) 130:14–5. doi: 10.1016/j.radonc.2018.08.014
44. Florescu C, Thariat J. Local ablative treatments of oligometastases from head and neck carcinomas. Crit Rev Oncol Hematol. (2014) 91:47–63. doi: 10.1016/j.critrevonc.2014.01.004
45. Sun XS, Michel C, Babin E, De Raucourt D, Péchery A, Gherga E, et al. Approach to oligometastatic disease in head and neck cancer, on behalf of the GORTEC. Future Oncol. (2018) 14:877–89. doi: 10.2217/fon-2017-0468
46. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
Keywords: consensus, head and neck cancer, patterns of care, practice patterns, survey
Citation: Siano M, Dulguerov P, Broglie MA, Henke G, Putora PM, Simon C, Zwahlen D, Huber GF, Ballerini G, Beffa L, Giger R, Rothschild S, Negri SV and Elicin O (2019) A Review of Controversial Issues in the Management of Head and Neck Cancer: A Swiss Multidisciplinary and Multi-Institutional Patterns of Care Study—Part 3 (Medical Oncology). Front. Oncol. 9:1127. doi: 10.3389/fonc.2019.01127
Received: 26 April 2019; Accepted: 09 October 2019;
Published: 24 October 2019.
Edited by:
Thorsten Fuereder, Medical University of Vienna, AustriaReviewed by:
Konrad Klinghammer, Charité Medical University of Berlin, GermanyThomas Melchardt, Paracelsus Medical University, Austria
Copyright © 2019 Siano, Dulguerov, Broglie, Henke, Putora, Simon, Zwahlen, Huber, Ballerini, Beffa, Giger, Rothschild, Negri and Elicin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olgun Elicin, b2xndW4uZWxpY2luQGluc2VsLmNo
 Marco Siano1,2
Marco Siano1,2 Pavel Dulguerov
Pavel Dulguerov Martina A. Broglie
Martina A. Broglie Guido Henke
Guido Henke Christian Simon
Christian Simon Daniel Zwahlen
Daniel Zwahlen Roland Giger
Roland Giger Sandro V. Negri
Sandro V. Negri Olgun Elicin
Olgun Elicin