- 1Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States
- 2Department of Pathology, University of Michigan, Ann Arbor, MI, United States
- 3Biostatistics Department, School of Public Health, University of Michigan, Ann Arbor, MI, United States
- 4Department of Radiation Oncology, University of Michigan, Ann Arbor, MI, United States
- 5Department of Orthopedic Surgery, University of Michigan, Ann Arbor, MI, United States
Background: Radiation-associated osteosarcoma (RAO) is a rare, life-threatening complication from radiation. Many physicians presume RAO has a worse prognosis than sporadic osteosarcoma (SO), although limited objective data exist. We conducted a retrospective study comparing these entities.
Methods: We identified adults treated at our institution with osteosarcoma (1990–2016) and categorized tumors as SO or RAO based on location within a prior radiation field. We extracted data on demographics, treatment and primary malignancy and examined available tumor samples for MTA-1 and ezrin using immunohistochemistry (IHC).
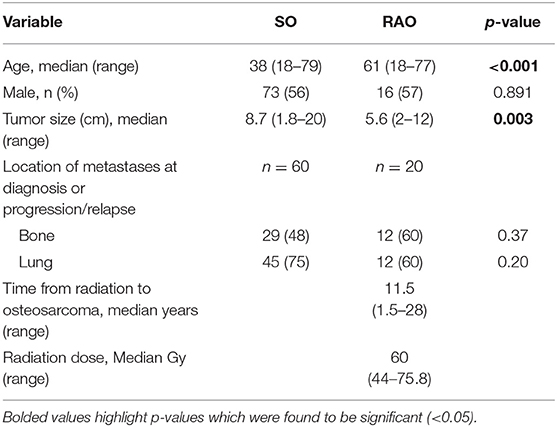
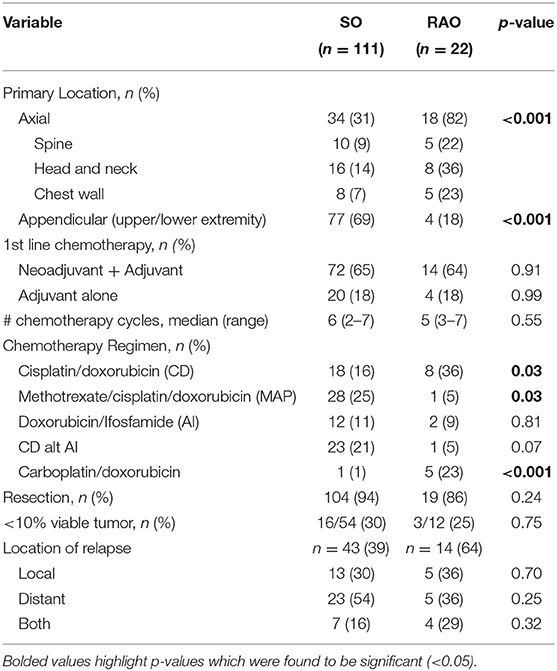
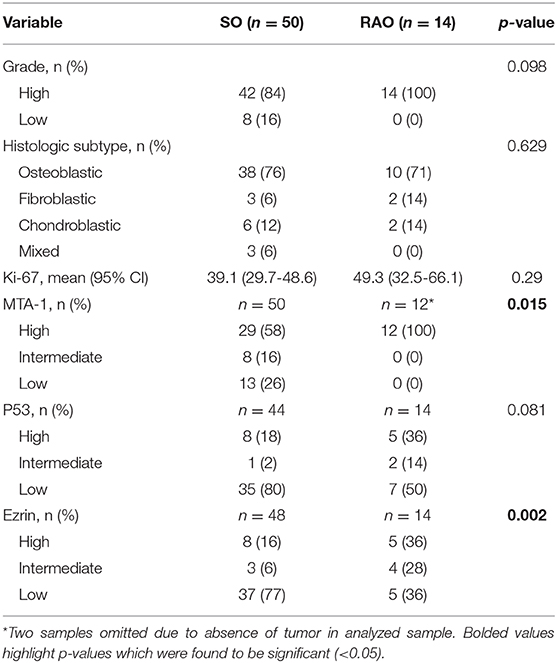
Results: Of 159 identified patients, 28 had RAO, diagnosed at a median interval from radiation of 11.5 years (1.5–28 years). Median follow-up was 2.8 years (0.1–19.6 years). Median progression free survival (PFS) and overall survival (OS) were not significantly different in the small population of patients with metastases, SO (n = 20) vs. RAO (n = 6): PFS 10.3 months vs. 4.8 months (p = 0.45) and OS 15.6 months vs. 6.1 months (p = 0.96), respectively. For the larger group with localized disease, median relapse-free survival (RFS) and OS were significantly different, NR vs. 12.2 months (p < 0.001) and NR vs. 27.6 months (p = 0.001) in SO (n = 111) vs. RAO (n = 22), respectively. On IHC, there were significant differences in distribution of high, intermediate or low MTA-1 (p = 0.015) and ezrin (p = 0.002) between RAO and SO tumors.
Conclusions: Patients with metastases at diagnosis fared poorly irrespective of prior radiation. RAO patients with localized disease had worse outcomes without detectable differences in therapy rendered or treatment effect in resected specimens. Higher expression of MTA-1 in RAO patients may suggest an underlying difference in tumor biology to explain differences in outcomes.
Introduction
Osteosarcoma is a rare, primary bone tumor that has a bimodal distribution of incidence with a peak in children and adolescents and a second, smaller peak in the elderly. The relationship between radiation therapy and subsequent development of osteosarcoma was first recognized in 1922 in patients who received external beam radiation therapy for tuberculous arthritis (1). As the use of radiation therapy for the management of cancer has increased, so have reports of radiation-associated osteosarcoma (RAO). The latency period between radiation therapy and diagnosis of RAO is highly variable, often 10–15 years after radiation therapy (2, 3).
Historically, RAO has been thought to carry a poor prognosis based on retrospective analyses, with a reported disease free survival as low as 17% (2). Known risk factors contributing to a worse prognosis include metastatic disease at diagnosis, incomplete or no resection and tumor size >5 cm (4). In addition, it is postulated that prior chemotherapies, elderly status of functional status, may limit optimal treatment for patients with RAO. While there have been several series describing treatment and outcomes of RAO, there is little objective data directly comparing demographics, treatment and outcomes of RAO to sporadic osteosarcoma (SO). McHugh et al. reported on the difference between primary vs. radiation-associated craniofacial osteosarcoma (5). Craniofacial osteosarcoma carries an overall favorable prognosis compared to its appendicular counterpart. In this series, 47% of de-novo craniofacial OS were high-grade with 80% of patients alive without disease. Alternatively, all radiation-associated tumors were high grade, all patients experienced recurrent disease and half of the patients died of their disease.
Several factors associated with more aggressive tumor biology have been described in osteosarcoma. Ezrin, a cytoskeleton linker protein involved in regulation of growth, has been associated with metastatic potential and poor prognosis in mouse models of osteosarcoma (6).
Metastatic tumor antigen-1 (MTA-1) promotes migration, invasion and survival of human keratinocytes (7). Elevated levels of MTA-1 in breast cancer enhances metastasis, increases motility and potentiates growth (8). In a series of 53 osteosarcoma specimens, MTA-1 was expressed in 81% of high-grade tumor samples, but in none of the low grade tumors (9). P53 participates in regulation of cell cycle and apoptosis and plays a role in cancer pathogenesis (10). Multiple series evaluating p53 expression in craniofacial osteosarcomas noted increased expression in high-grade tumors (11–13). Furthermore, there is some suggestion that TP53 gene mutations, which are often accompanied by p53 overexpression, play a role in post-radiation osteosarcoma (14). Ki67 serves as a maker of cell proliferation and is used as a prognostic factor in multiple cancer types. McHugh et al. noted higher Ki67, p53, and ezrin expression in radiation-associated craniofacial osteosarcoma compared to sporadic tumors (5).
We conducted a retrospective study comparing demographics, therapy and outcomes of SO to RAO at our institution with the aim of better understanding the differences in natural history and treatments rendered. We conducted immunohistochemistry (IHC) studies to evaluate differences in markers of aggressiveness to identify differences in biology and behavior of these entities.
Materials and Methods
Patient Identification
The University of Michigan Electronic Medical Record Search Engine (EMERSE) (15) was searched using the term “osteosarcoma” to identify patients with a diagnosis of osteosarcoma treated at our institution between 1990 and 2016. Patients under age 18 were excluded given concern for potential differences in biology of adult vs. pediatric sporadic osteosarcoma. Furthermore, given the latency between radiation and development of osteosarcoma, there were unlikely to be pediatric patients in the RAO cohort. Patient medical records were reviewed, and tumors were characterized as sporadic or radiation-associated based on a history of prior radiation within the field of osteosarcoma. Details regarding demographics, clinical presentation, pathologic features, treatment protocols, outcomes, and primary malignancy in the setting of radiation-associated tumors were extracted from clinical records. All research was approved by the University of Michigan Institutional Review Board (HUM00068553).
Pathology
Available representative tumor samples were obtained and reviewed by a sarcoma pathologist to confirm the diagnosis and assess tumor grade. Immunohistochemical staining for Ki67, MTA-1, p53, and ezrin were conducted. Immunohistochemical staining was performed on the DAKO Autostainer (DAKO, Carpinteria, CA) using Envision+ or liquid streptavidin-biotin and diaminobenzadine (DAB) as the chromogen. De-paraffinized sections were labeled with the antibodies for 30 min at ambient temperature. Microwave 10 mM citrate, pH6 epitope retrieval was used prior to staining for both antibodies. Appropriate negative (no primary antibody) and positive controls were stained in parallel with each set of slides studied. Ki67 was reported as percentage of tumor nuclei positive. All other results are given as a modified Allred intensity score (0, 1 = weak; 2 = moderate, and 3 = strong).
Statistical Analysis
Descriptive statistics, such as median and range, were calculated for continuous variables; frequencies were presented for categorical variables. To compare two categorical variables, a frequency table was created and analyzed using the Chi-square test of independence or Fisher's exact test. Continuous variables were compared using t-tests. Tumor size was log-transformed for statistical testing. Progression-free survival (PFS) and overall survival (OS) from date of diagnosis was estimated by the Kaplan-Meier method and compared using the Log-rank test. Cox proportional hazards regression models were created for RAO association, adjusting for patient age at diagnosis, metastatic status, tumor size on the logarithmic scale, and an interaction effect for metastatic status and RAO association. Statistical significance was defined as a two-sided P < 0.05. All analyses were conducted using SAS (version 9.4, SAS Institute, Cary, NC).
Results
We identified 159 patients with osteosarcoma, of which 28 had RAO (Table 1). Median follow-up for all patients was 2.8 years (range 0.1–19.6 years). Median follow-up for survivors was 5.3 years (range 1–19.6). RAO patients were older (p < 0.001) and had smaller tumors (p = 0.003) at diagnosis than SO patients. The most common location for metastatic disease at diagnosis or at progression was bone and lung. There were no differences in the location of metastases between the two groups. The most common primary cancers for which patients received radiation therapy were breast cancer, prostate cancer and lymphoma, as well as retinoblastoma in two patients. The median radiation dose was 60 Gy and a median latency period of 11.5 years.
Localized Disease
Twenty-two RAO and 111 SO patients had localized disease at diagnosis (Table 2). RAO tumors were more prevalent in the axial skeleton while SO tumors were more prevalent in the appendicular skeleton (p < 0.001). Nineteen (86.4%) RAO patients and 104 (93.7%) SO patients underwent resection (p = 0.24). RAO and SO patients received a similar number of chemotherapy cycles, with a median of 5 and 6 cycles, respectively (p = 0.55). While patients in both groups received doxorubicin-based regimens, SO patients received more cisplatin and methotrexate (p = 0.03) while RAO patients received more carboplatin (p < 0.001). There was no difference in patients achieving <10% viable tumor from pre-operative chemotherapy in resected specimens (p = 0.75).
Median relapse-free survival (RFS) was significantly different for localized SO and RAO patients at not reached and 12.2 months, respectively (p < 0.001, Figure 1A). Median OS for SO patients was not reached, while median OS for RAO patients was 27.6 months (p = 0.001, Figure 1B). Adjusting for age and tumor size, RAO portended a worse PFS (HR 2.92, 95% CI 1.51–5.69) and OS (HR 2.46, 95% CI 1.20–5.07).
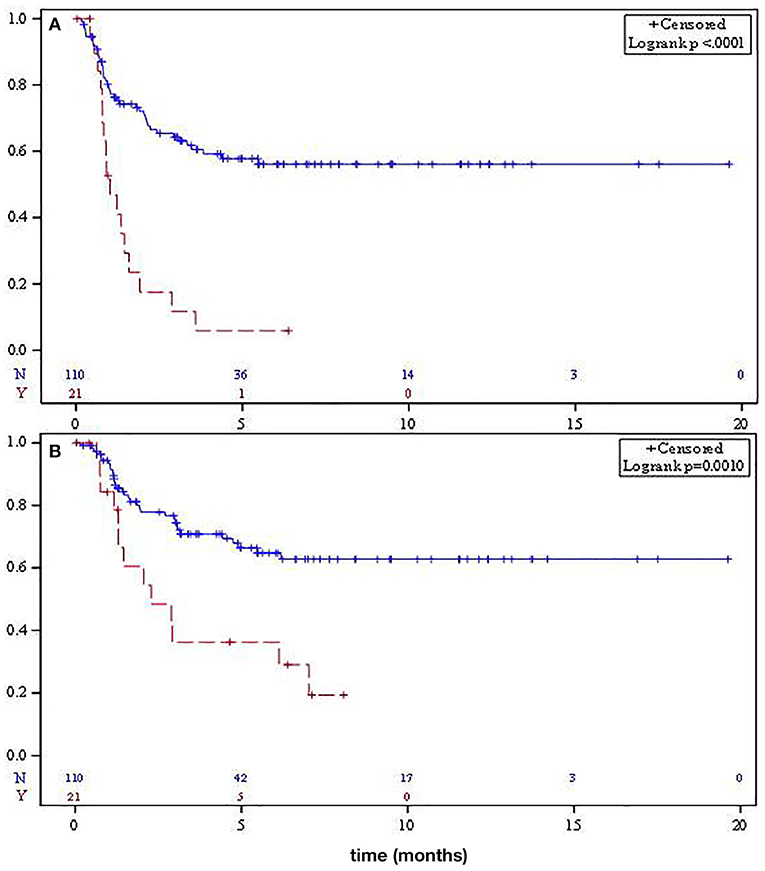
Figure 1. Median relapse-free survival (A) and overall survival (B) for localized sporadic osteosarcoma (solid) and radiation-associated osteosarcoma (dashed).
Metastatic Disease
Twenty patients with SO and 6 patients with RAO had metastatic disease at diagnosis. Resection of the primary tumor was performed in 11 (55%) SO patients and 2 (33.3%) RAO patients (p = 0.35). Metastasectomy was completed in 7 (35%) SO patients and 2 (33.3%) RAO patients (p = 0.94). Patients with SO received a median of 2 lines of chemotherapy (range 0–6) compared to 1.5 lines (range 0–4) in RAO patients (p = 0.46).
There was no statistical difference in median PFS for SO and RAO disease, 10.3 and 4.8 months, respectively (p = 0.95, Figure 2A). Median OS was not significantly different between the two groups (15.6 vs. 6.1 months, respectively, p = 0.45, Figure 2B).
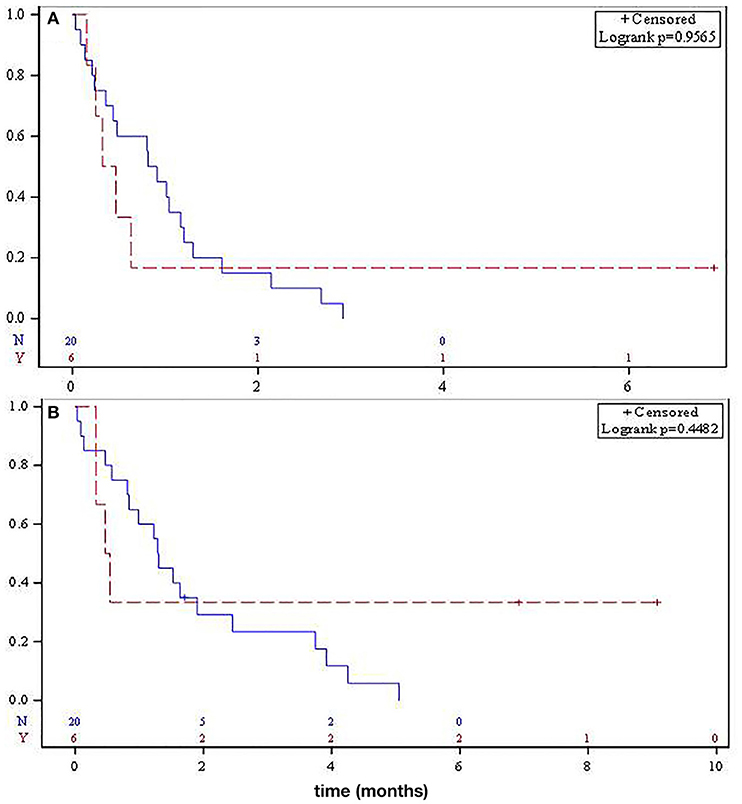
Figure 2. Median progression-free survival (A) and overall survival (B) for metastatic sporadic osteosarcoma (solid) and radiation-associated osteosarcoma (dashed).
Pathology
Fifty SO and 14 RAO tumor samples were available for pathology review. Pathology characteristics are shown in Table 3. There were no differences in the grade, histologic subtype of osteosarcoma (osteoblastic, chondroblastic, or fibroblastic) or p53 IHC between the two groups. Interestingly, significant differences between SO and RAO were detected in tumor expression of MTA-1 (p = 0.015, Figure 3) and ezrin (0.002) with all RAO samples having high MTA-1 and most SO samples having low ezrin expression.
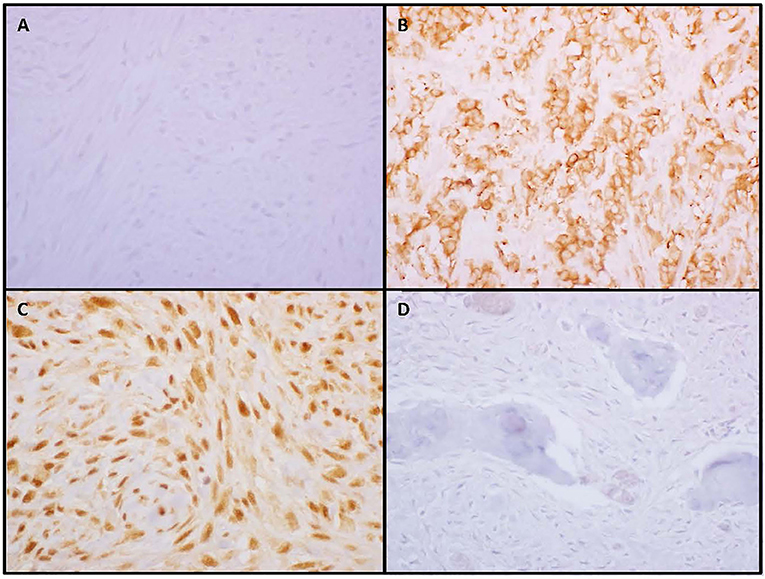
Figure 3. Low or negative ezrin expression (A) was more common in sporadic osteosarcoma while high membranous expression (B) was more common in radiation-associated tumors. All radiation-associated osteosarcomas exhibited high nuclear metastatic tumor antigen-1 staining (C) while a subset of sporadic tumors were negative (D).
Discussion
Osteosarcoma is a rare but significant complication of radiation therapy that has historically been associated with a worse prognosis compared to its sporadic counterpart. It is important to understand the natural history and prognosis of this rare entity to appropriately manage our patients and choose therapies. In our retrospective analysis, patients with metastatic osteosarcoma at diagnosis, irrespective of prior radiation therapy, had poor median progression-free and overall survival rates. Worse outcomes for metastatic osteosarcoma compared to localized tumors has been reported in the literature, including the EURAMOS trial in which 3-year event-free survival for patients with metastatic disease was significantly worse (16). These data highlight an unmet medical need to develop more effective drug therapy in this patient population. While prior radiation did not impact outcomes in metastatic disease, the sample size was relatively small with only six patients with metastatic RAO, making conclusions about metastatic population inconclusive. Patients with localized SO in our series had significantly improved RFS and OS rates compared to RAO.
RAO patients were significantly older than SO patients. This is not surprising given the median latency period between radiation therapy and the development of osteosarcoma being 10 years or greater in multiple studies including ours (2, 3). Older patients are more likely to have comorbidities that may impact chemotherapy choice. While doxorubicin administration was similar, RAO patients received more carboplatin and less cisplatin and methotrexate. Despite these differences, similar numbers of chemotherapy cycles were administered and examination of available tumors noted no difference in treatment effect as indicated by the number of patients with <10% viable tumor.
Interestingly, patients with RAO had smaller primary tumors than SO patients. RAO patients also have a history of prior cancer, and may undergo more routine imaging and physical examination, may be more apt to notice changes in a tumor bed and report symptoms earlier than younger patients with little past medical history.
RAO tumors were more commonly located in the axial skeleton while SO tumors were more commonly located in the appendicular skeleton. The difference in location of RAO vs. SO tumors is expected given the primary diagnoses for which patients received radiation therapy (breast cancer, prostate cancer, and lymphoma). In patients with localized disease who relapsed, there was no difference in the location of relapse (distant vs. local) between the two groups.
The obvious difference in outcomes of localized disease despite overall similarities in therapies rendered suggests an underlying difference in the biology of these tumors. Multiple markers of tumor aggressiveness have been identified in exploratory studies, and while not prospectively validated may provide insight into the biology of these tumors. In osteosarcoma mouse models, ezrin was associated with increased metastatic potential and poorer prognosis while high ezrin expression correlated with chemotherapy resistance (6, 17). Over-expression of MTA-1 has been noted in high-grade osteosarcoma tumor samples with little to no expression in low-grade samples (9). Mardanpour et al. evaluated tumor samples from 56 osteosarcoma patients and noted a correlation between increased p53 and Ki67 with worse PFS and OS (18). In 47 resected osteosarcomas following neoadjuvant chemotherapy, increased p53 expression correlated with worse overall survival while expression in biopsy samples was not predictive of chemotherapy response or survival (19). On IHC evaluation of p53, Ki67, and ezrin in RAO vs. SO craniofacial osteosarcoma, there was higher expression of all three in the radiation-associated tumors (5).
In our series, the majority of patients had high-grade tumors and there was no difference in p53 expression. However, the distribution of high, intermediate and low expression of MTA-1 and ezrin was significantly different between the two groups. A higher proportion of RAO patients had high MTA-1 while a higher proportion of SO patients had low ezrin expression. These data, while limited by the small number of tumor samples available for review, suggest there may be an underlying difference in the aggressiveness of RAO tumors, which may account for the significant difference in outcomes in localized disease despite similar treatment.
Metastatic osteosarcoma portends an overall poor prognosis irrespective of sporadic or radiation-associated disease, with the limited sample size in this study limiting conclusions in outcomes. Patients with localized SO have significantly better PFS and OS compared to patients with RAO despite similar therapies. Differences in outcomes and expression of biomarkers (such as MTA-1 and ezrin) suggest an underlying difference in the biology of radiation-associated tumors, as well as the necessity for alternative therapeutic strategies.
Data Availability Statement
The datasets for this article are not publicly available. Requests to access the datasets should be directed to the corresponding author, Rashmi Chugh (cmFzaG1pbUBtZWQudW1pY2guZWR1).
Ethics Statement
The studies involving human participants were reviewed and approved by University of Michigan Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
BS and RC involved with the study concept and design, data acquisition, analysis, and interpretation as well as manuscript preparation. JM conducted the pathology review as well as immunohistochemistry testing and interpretation. DT assisted with IHC testing and interpretation. ER and LZ performed statistical analysis. DO, LB, JB, and SS assisted with manuscript editing and review.
Funding
Research reported in this publication was supported by the National Cancer Institutes of Health under Award Number P30CA046592 by the use of the following Cancer Center Shared Resource(s): Biostatistics, Tissue pathology.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BVT declared a past co-authorship with several of the authors LB, SS, and RC to the handling Editor.
References
1. Beck A. Zur frage des Rontgensarkoms, zugleich ein Beitrag zur pathogenese des Sarkoms. Muench Med Wochenschr. (1929) 69:623–5.
2. Huvos AG, Woodard HQ, Cahan WG, Higinbotham NL, Stewart FW, Butler A, et al. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer. (1985) 55:1244–55. doi: 10.1002/1097-0142(19850315)55:6<1244::AID-CNCR2820550617>3.0.CO;2-1
3. Wiklund TA, Blomqvist CP, Raty J, Elomaa I, Rissanen P, Miettinen M. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. (1991) 68:524–31. doi: 10.1002/1097-0142(19910801)68:3<524::AID-CNCR2820680313>3.0.CO;2-E
4. Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. (1992) 127:1379–85. doi: 10.1001/archsurg.1992.01420120013002
5. McHugh JB, Thomas DG, Herman JM, Ray ME, Baker LH, Adsay NV, et al. Primary versus radiation-associated craniofacial osteosarcoma: biologic and clinicopathologic comparisons. Cancer. (2006) 107:554–62. doi: 10.1002/cncr.22019
6. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. (2004) 10:182–6. doi: 10.1038/nm982
7. Mahoney MG, Simpson A, Jost M, Noe M, Kari C, Pepe D, et al. Metastasis-associated protein (MTA)1 enhances migration, invasion, and anchorage-independent survival of immortalized human keratinocytes. Oncogene. (2002) 21:2161–70. doi: 10.1038/sj.onc.1205277
8. Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. (1994) 269:22958–63.
9. Park HR, Jung WW, Kim HS, Bacchini P, Bertoni F, Park YK. Overexpression of metastatic tumor antigen in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Cancer Res Treat. (2005) 37:360–4. doi: 10.4143/crt.2005.37.6.360
10. Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. (1989) 342:705–8. doi: 10.1038/342705a0
11. Lopes MA, Nikitakis NG, Ord RA, Sauk J Jr. Amplification and protein expression of chromosome 12q13-15 genes in osteosarcomas of the jaws. Oral Oncol. (2001) 37:566–71. doi: 10.1016/S1368-8375(00)00130-5
12. Oliveira P, Nogueira M, Pinto A, Almeida MO. Analysis of p53 expression in osteosarcoma of the jaw: correlation with clinicopathologic and DNA ploidy findings. Hum Pathol. (1997) 28:1361–5. doi: 10.1016/S0046-8177(97)90224-0
13. Junior AT, de Abreu Alves F, Pinto CA, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. (2003) 39:521–30. doi: 10.1016/S1368-8375(03)00017-4
14. Nakanishi H, Tomita Y, Myoui A, Yoshikawa H, Sakai K, Kato Y, et al. Mutation of the p53 gene in postradiation sarcoma. Lab Invest. (1998) 78:727–33.
15. Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan's nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform. (2015) 55:290–300.
16. Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. (2016) 17:1396–408. doi: 10.1016/S1470-2045(16)30214-5
17. Liu Q, Xu B, Zhou W. Correlation between chemotherapy resistance in osteosarcoma patients and PAK5 and Ezrin gene expression. Oncol Lett. (2018) 15:879–84. doi: 10.3892/ol.2017.7355
18. Mardanpour K, Rahbar M, Mardanpour S. Coexistence of HER2, Ki67, and p53 in Osteosarcoma: a strong prognostic factor. N Am J Med Sci. (2016) 8:210–4. doi: 10.4103/1947-2714.183013
Keywords: radiation-induced neoplasms, sporadic osteosarcoma, radiation-associated osteosarcoma, secondary malignancy, metastatic tumor antigen-1 (MTA-1), ezrin
Citation: Siontis BL, McHugh JB, Roberts E, Zhao L, Thomas DG, Owen D, Baker LH, Biermann JS, Schuetze SM and Chugh R (2020) Differential Outcomes and Biologic Markers of Radiation-Associated vs. Sporadic Osteosarcoma: A Single-Institution Experience. Front. Oncol. 9:1523. doi: 10.3389/fonc.2019.01523
Received: 24 June 2019; Accepted: 18 December 2019;
Published: 22 January 2020.
Edited by:
Christina Tsien, Washington University in St. Louis, United StatesReviewed by:
David Roberge, Université de Montréal, CanadaJames William Snider, University of Maryland, Baltimore, United States
Copyright © 2020 Siontis, McHugh, Roberts, Zhao, Thomas, Owen, Baker, Biermann, Schuetze and Chugh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rashmi Chugh, cmFzaG1pbUBtZWQudW1pY2guZWR1
†Present address: Brittany L. Siontis, Division of Medical Oncology, Mayo Clinic, Rochester, MN, United States
Dawn Owen, Department of Radiation Oncology, Mayo Clinic, Rochester, MN, United States
 Brittany L. Siontis
Brittany L. Siontis Jonathan B. McHugh2
Jonathan B. McHugh2 Emily Roberts
Emily Roberts Dafydd G. Thomas
Dafydd G. Thomas Rashmi Chugh
Rashmi Chugh