- 1Department of Ultrasound, China–Japan Friendship Hospital, Beijing, China
- 2Department of Ultrasound, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Ultrasound, Affiliated Hospital of Qingdao University, Qingdao, China
- 4Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
According to the 2015 American Thyroid Association (ATA), referred risk stratification and thyroid nodules with intermediate- and low-suspicion patterns are difficult to diagnose. The objective of this study is to evaluate the diagnostic performance of contrast-enhanced ultrasonography (CEUS) and elastosonography (ES) for the differentiation of these thyroid nodules. From November 2011 to June 2016, a total of 163 thyroid nodules with intermediate- and low-suspicion patterns in 150 consecutive patients at our hospital were studied before surgery. With surgical pathology as the standard, the diagnostic value of CEUS and ES was analyzed. There were 29 (17.8%) malignant lesions and 134 (82.2%) benign lesions. The enhancement patterns of CEUS, the echogenicity, and the elastography were significantly different between malignant and benign lesions (P < 0.05). Heterogenous enhancement was more common in malignant nodules, and the sensitivity, specificity, positive predictive value, negative predictive value, and odds ratio were 51.7, 88.1, 48.4, 89.4, and 10.1%, respectively. The diagnostic accuracy of CEUS was better than the conventional ultrasound [area under the curve (AUC), 0.729 vs. 0.616, P = 0.021]. The enhancement patterns of CEUS were helpful in the differential diagnosis of thyroid nodules with intermediate and low suspicion.
Introduction
The 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer (1) (2015 ATA guidelines) proposed a risk stratification of thyroid nodules based on a series of studies on the ultrasonographic features of thyroid nodules. The guide classifies the sonographic appearance of the vast majority of thyroid nodules into the following categories of ultrasound patterns: high suspicion, intermediate suspicion, low suspicion, very low suspicion, and benign. Intermediate suspicion (malignancy risk 10–20%) nodules are hypoechoic solid nodules with smooth regular margins and without malignant features such as microcalcifications, extrathyroidal extension, or a taller-than-wide shape. Low-suspicion (malignancy risk, 5–10%) nodules are isoechoic or hyperechoic solid nodules or partially cystic nodules with eccentric uniformly solid areas without malignant features. These nodules have similar ultrasound features as nodules suspicious of follicular lesions in the “British Thyroid Association Guidelines for the Management of Thyroid Cancer” (2) (BTA guidelines). Despite the fact that the malignancy risk is not relatively high, differentiating malignant from benign lesions by conventional ultrasonography (CUS) is challenging.
Contrast-enhanced ultrasonography (CEUS) has been widely used to evaluate the microvessel perfusion of thyroid nodules. Elastosonography (ES) quantifies the firmness of the tissue and displays it as a color map on which the hardness of the tissues can be reflected. Many studies have concluded that the accuracy of the diagnosis of thyroid nodules can be increased by combining CEUS and ES with CUS (3–5). However, the value of CEUS and ES in evaluating nodules with intermediate- and low-suspicion patterns is unclear.
Methods
This study was approved by the ethics committee of Peking Union Medical College Hospital (PUMCH), and informed consent was obtained from all patients before CEUS and ES.
Patients
A total of 604 patients with thyroid nodules admitted to PUMCH from November 2011 to June 2016 who met the following criteria were included in this study: (a) CUS indicated that the thyroid nodule was of intermediate or low suspicion according to the 2015 ATA guidelines for referred risk stratification, and (b) the largest diameter of the nodule was larger than 5 mm in size. The exclusion criteria were as follows: (a) patients who did not undergo surgery, (b) patients younger than 18 years of age, (c) women during pregnancy or lactation, and (d) patients who could not tolerate the intravenous injection of contrast agents because of heart, lung, kidney, or other vital organ dysfunction or severe allergies. A total of 163 thyroid nodules in 150 consecutive patients (mean age, 47.9; range, 18–83 years) were included. Two nodules in eight patients each and three nodules in three patients each were included. In six of those eight patients, the two nodules were located in different lobes. In two other patients, the two nodules were not near each other, although they were in the same lobe. In three patients, two of the three nodules were located in the same lobe, and the other was located in a different lobe. CUS, CEUS, and ES were performed in all patients, and the ultrasound-guided fine needle aspiration (FNA) was performed in nine patients before surgery.
Ultrasound Examination
All ultrasound (US) examinations were performed with a 5- to 12-MHz liner probe (iU22; Philips Medical System, Bothell, WA, United States). The CUS examination was performed with the standard equipment settings for thyroid glands. Standard machine settings for CEUS were used, and the contrast medium used was SonoVue (Bracco Imaging, Milan, Italy). With a 20- or 22-gauge peripheral intravenous cannula, SonoVue was injected intravenously as a bolus at a dose of 1.2 ml, followed by 5 ml of normal saline as a flush. The timer on the US machine was then started, and each contrast imaging acquisition lasted more than 2 min after the bolus injection; the imaging was digitally stored. During real-time elastography (RTE), the probe was positioned perpendicular to the skin, and no compression was applied at the skin above the targeted thyroid nodule. When there were two nodules in a patient, CEUS was performed on the second nodule after the first injected contrast agent was completely cleaned in the whole gland. CUS, RTE, and CEUS images and cine clips were analyzed by two radiologists with more than 5 years of experience in thyroid US. They were blinded to the patients' clinical data and pathological results. In cases of discrepancies between the two readers, a consensus was reached after discussion.
The CUS features of all thyroid nodules were recorded according to the halo, echogenicity, internal component, echotexture (homogenous or heterogenous), calcifications, and vascularity. Halos were classified as absent, regularly thin, or irregular (including thick halos, incomplete halos, and halos of different widths). Echogenicity was classified as hypoechogenicity, isoechogenicity, hyperechogenicity, or marked hyperechogenicity relative to the surrounding normal parenchyma. The internal component of a nodule was classified as solid (defined as composed entirely or nearly entirely of soft tissue, with only a few tiny cystic spaces) (6), predominantly solid, and predominantly cystic. Calcifications, when present, were categorized as microcalcifications, disrupted rim calcifications, and other types of calcifications. Microcalcifications were defined as calcifications that were ≤1 mm in diameter and visualized as tiny hyperechoic foci. Disrupted rim calcifications were defined as interrupted peripheral calcifications in association with a soft tissue rim outside the calcification. When a nodule had both microcalcifications and other types of calcifications, it was classified as having microcalcifications. The vascularity of nodules was classified into five types by Frates (7) as follows: 0 for no visible flow, 1 for minimal internal flow without a peripheral ring, 2 for a peripheral ring of flow (defined as >25% of the nodule's circumference) with minimal or no internal flow, 3 for a peripheral ring of flow with a small to moderate amount of internal flow, and 4 for extensive internal flow with or without a peripheral ring. We regarded type 4 as increased intranodular vascularity. CEUS characteristics included peak intensity (categorized as low, equal, or high) and enhanced pattern (categorized as homogenous, heterogenous, or ring enhancing). Elastography score elasticity was classified in five different patterns, adding elastography score (ESS) 0 to the version of Asteria criteria (8): ESS 0, red and blue, or blue and green, or red and green are layered distribution in cystic nodules or predominantly cystic nodules; ESS 1, homogenously in green (soft); ESS 2, predominantly in green with few blue areas/spots; ESS 3, predominantly in blue with a few green areas/spots; and ESS 4, completely in blue (hard).
Review of Histopathology
An experienced pathologist who was blinded to the clinical information reviewed the histopathology of all the specimens. The papillary thyroid carcinomas (PTCs) were divided into classical PTC and follicular variants of the PTC (FVPTC).
Statistical Analysis
The statistical analysis was performed with the SPSS statistical package (Version 19.0, SPSS Chicago, IL, United States) and MedCalc 11.4.2.0 software (MedCalc Software, Ostend, Belgium). The quantitative data were expressed as the mean ± standard deviation. The Student's t-test was used for comparing the groups. The χ2 test or Fisher's exact test was used to compare categorical data. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated through a comparison with the pathological findings. A receiver operating characteristic (ROC) curve analysis was used to compare CEUS, ES, and CUS. A P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
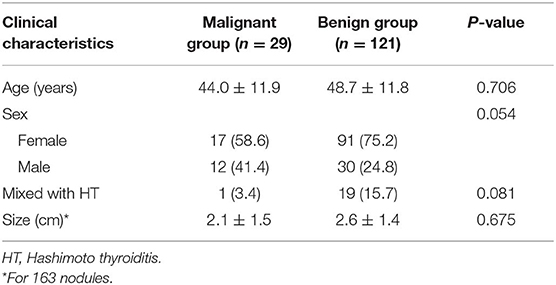
The clinical characteristics are summarized in Table 1. The age, gender, nodule size, and incidence of coexistence with Hashimoto thyroiditis (HT) did not significantly differ between the benign and malignant groups (P > 0.05). The results of the cytopathology and histopathology of the nine nodules that underwent FNA are summarized in Table 2. Histological pathology demonstrated that 29 lesions (17.8%) were malignant, and 134 lesions (82.2%) were benign. One hundred one nodular goiters (75.4%) constituted the majority of benign lesions, 18 of the 134 lesions were adenomatous nodules (ANs). The remainder of the benign lesions consisted of six follicular adenomas (FAs, 25.4%) and nine HTs (6.7%). Of the malignant lesions, 21 cases were PTCs, with 17 classical variant and 4 follicular variants, 7 cases were follicular carcinomas (FCs), and 1 case was medullary carcinoma (MC). Cervical lymph node metastasis was confirmed in one FC and six PTCs. Metastatic lesions were found in cervical striated muscles and fibrous connective tissues in another FC.
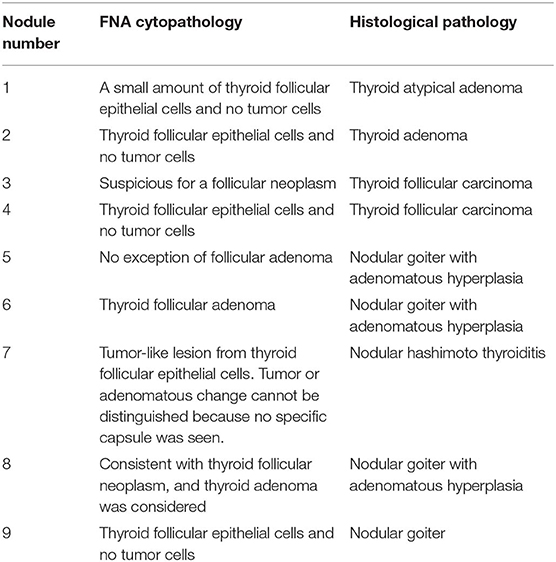
Table 2. Comparison of fine needle aspiration (FNA) cytopathology and histological pathology of nine nodules.
US Characteristics
The CUS characteristics are summarized in Table 3. No significant difference was observed in the US features of absent or irregular halos (a solid internal component, a heterogenous echo texture, and microcalcification or disrupted rim calcification between benign and malignant lesions). Moderate or abundant blood flow (Frates type 3 or 4) was detected in the majority of the nodules with intermediate- and low-suspicion patterns. The difference in increased intranodular vascularity (Frates type 4) between benign and malignant lesions was not statistically significant (P > 0.05). Of the malignant nodules, two PTCs showed peripheral vascularity without intranodular vascularity, and four PTCs showed partial hypervascularity with perforating vessels into the nodule. Regular peripheral and intranodular vascularity was found in the other 15 malignant lesions.
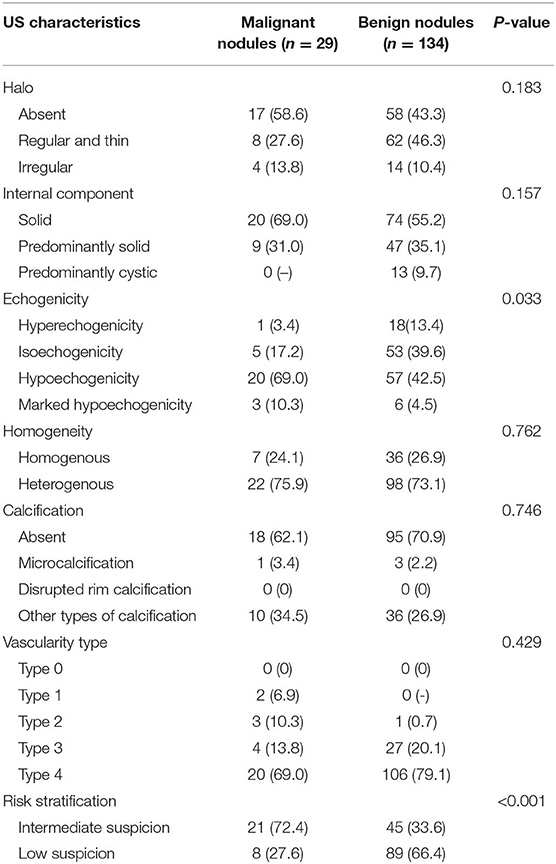
Table 3. Ultrasound (US) characteristics of benign and malignant thyroid nodules with intermediate and low suspicion.
The CEUS characteristics and ES of the nodules are shown in Table 4 and Figures 1–4. Heterogenous enhancement was significantly (P < 0.001) associated with a malignant outcome with a 10.1 odds ratio [OR; 95% confidence interval (CI) ranging from 3.9 to 25.7]. The sensitivity, specificity, positive predictive value, and negative predictive value were 51.7, 88.1, 48.4, and 89.4%, respectively. The enhancement patterns of 12 PTCs and 2 FCs and 1 MC were heterogenous, 2 PTCs were homogenous, 1 PTC had no enhancement, and the remaining 6 PTCs and 5 FCs exhibited a ring-enhancing pattern. Among the benign nodules, 89 cases (66.4%) showed a ring-enhancing pattern; 24 cases (17.9%) showed homogenous enhancement, including 18 nodular goiters and 3 HT; and 16 cases (11.9%) showed heterogenous enhancement, including 10 nodular goiter, 2 FAs, and 4 HTs. The intensity of enhancement was not significantly different (P = 0.289). Most of the nodules showed soft texture in elasticity imaging, and the difference in the ES between the benign and malignant groups was significant (P = 0.015).
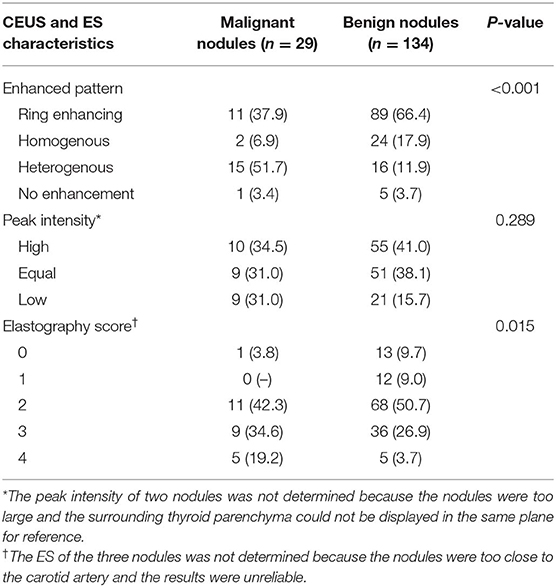
Table 4. Contrast-enhanced ultrasonography (CEUS) and elastosonography (ES) characteristics of malignant and benign thyroid nodules.
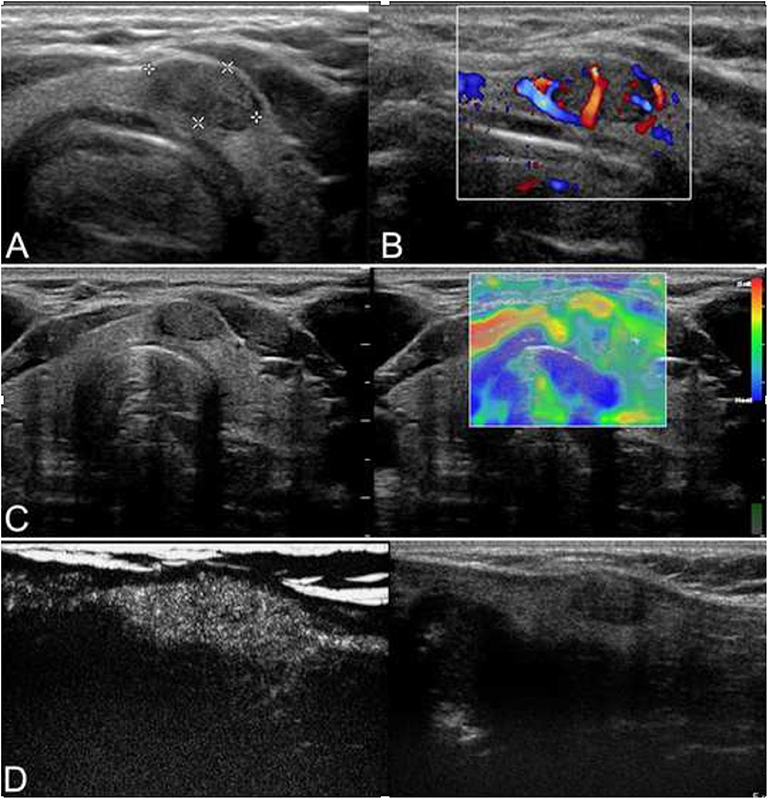
Figure 1. Ultrasound of a 42-year-old man who incidentally detected a thyroid nodule is shown. (A) Conventional ultrasonography (CUS) showed that there was a solid hypoechoic nodule with a regular margin in the isthmus of the thyroid. The nodule was of intermediate suspicion. (B) Color Doppler showed intranodular and peripheral vascularity. (C) The elastography score was 2, indicating that the nodule was soft. (D) Contrast-enhanced ultrasonography (CEUS) revealed heterogenous enhancement. The nodule was a papillary thyroid carcinoma (PTC) confirmed by histological pathology.
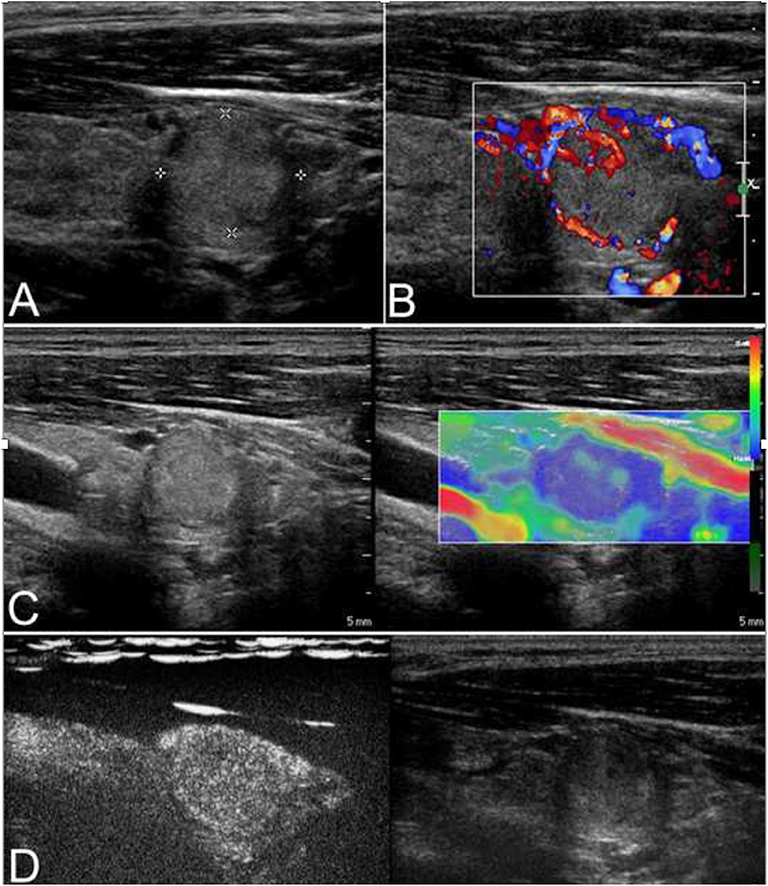
Figure 2. This case is a 27-year-old man with an follicular carcinoma (FC) nodule. (A) Conventional ultrasonography (CUS) showed a solid hyperechoic nodule in the left lobe that was associated with a regular thin halo and an inside area that was hypoechoic, which is indicative of low suspicion. (B) Abundant intranodular and peripheral vascularity was detected. (C) The elastography score was 2, indicating a soft stiffness. (D) Contrast-enhanced ultrasonography (CEUS) revealed heterogenous enhancement.
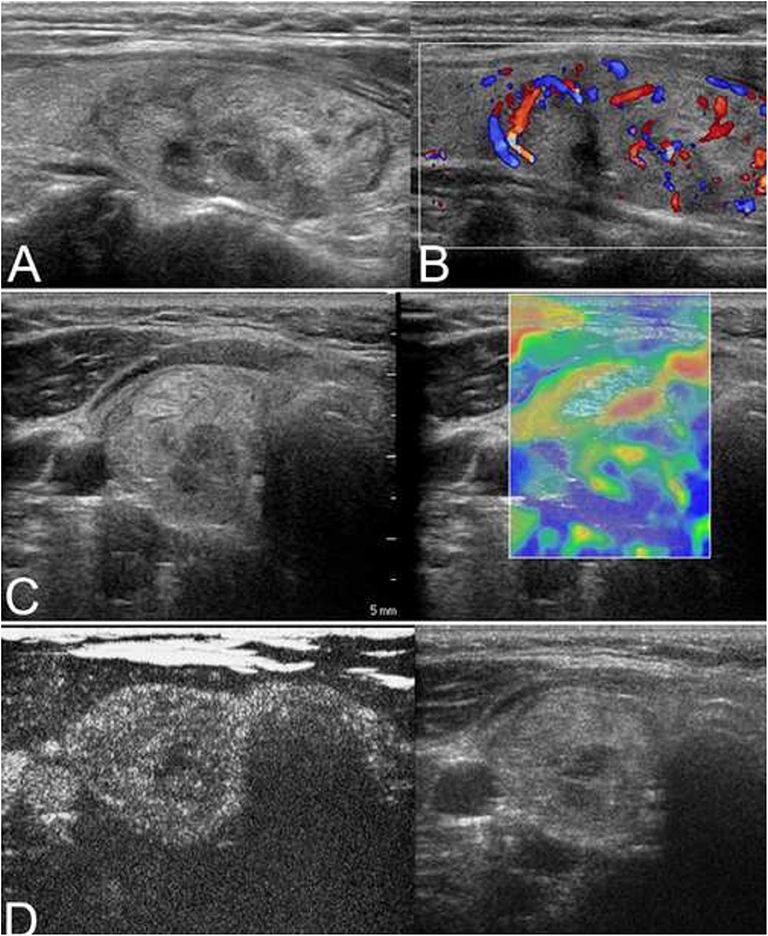
Figure 3. (A) The isoechoic solid nodule with a regular thin halo was evaluated as low suspicion by conventional ultrasonography (CUS) in a 38-year-old man. (B) Color Doppler showed intranodular and peripheral vascularity. (C) The elastography score was 3, indicating a hard stiffness. (D) Contrast-enhanced ultrasonography (CEUS) revealed ring enhancement. The nodule was a follicular adenoma.
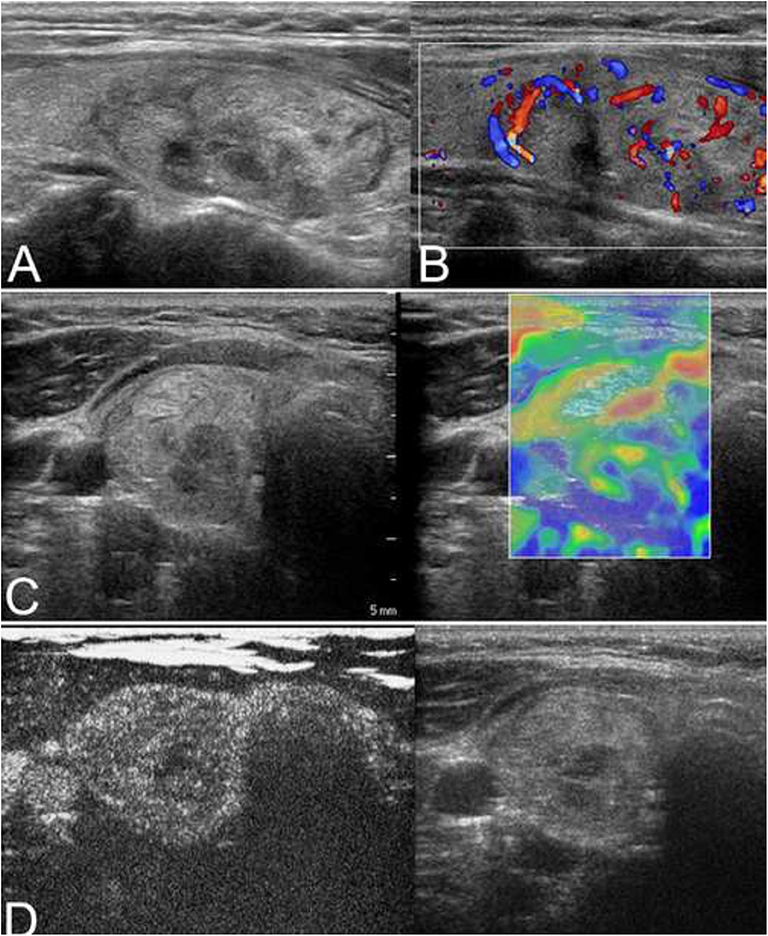
Figure 4. A nodular goiter with adenomatous hyperplasia in a 59-year-old woman is shown. (A) Conventional ultrasonography (CUS) showed that the solid hyperechoic nodule in the right lobe was heterogenous in echotexture and had regular margins, indicative of low suspicion. (B) Color Doppler showed intranodular and peripheral vascularity. (C) The elastography score was 2, indicating a soft stiffness. (D) Contrast-enhanced ultrasonography (CEUS) revealed ring-enhancement.
Diagnostic Performance of the CEUS and ES
Sensitivity, specificity, PPV, NPV, accuracy, and area under the curve (AUC) of CUS were 69.0, 57.5, 26.0, 89.5, 59.5, and 0.61% (95% CI, 0.53–0.69), respectively. Nodules with the CEUS patterns of heterogenous enhancement was considered malignant. The sensitivity, specificity, PPV, NPV, accuracy, and AUC for CEUS were 51.7, 88.1, 48.4, 89.4, 81.6, and 0.73% (95% CI, 0.65–0.80), respectively. The ROC curves demonstrated that the best cutoff value for ES was 3. The sensitivity, specificity, PPV, NPV, accuracy, and AUC for ES were 53.8, 69.4, 25.5, 88.6, 66.9, and 0.62% (95% CI, 0.54–0.69), respectively (Table 5). The CEUS showed a higher AUC than CEUS and ES (0.729 vs. 0.616, P = 0.021; 0.729 vs. 0.608, P = 0.046). We added this in Result and Abstract and emphasized in red color (lines 37 and 88–99).
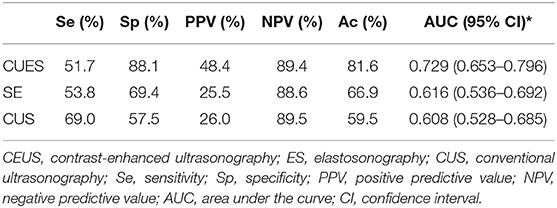
Table 5. Diagnostic efficiency of the contrast-enhanced ultrasonography (CEUS), elastosonography (SE) and conventional ultrasonography (CUS).
Discussion
High-resolution neck ultrasonography is the most important method for the evaluation of a thyroid nodule. The latest ATA guidelines have proposed criteria for risk stratification that categorizes the thyroid nodules and stratify the risk of malignancy. However, the differential diagnosis of nodules with intermediate and low suspicion is difficult. In the “British Thyroid Association Guidelines for the Management of Thyroid Cancer” (2), these nodules are defined as suspicious of follicular lesions on ultrasound and classified as indeterminate/equivocal. A total of 71.4% of the nodules were thyroid tumors with a follicular growth pattern. Our study showed that the enhancement pattern was a significant factor for differentiating benign and malignant nodules with intermediate and low suspicion. Some researchers have revealed that age, sex, and tumor size are predictive parameters of malignancy in follicular neoplasms of the thyroid (diagnosed by intraoperative frozen section or FNA) (9, 10). The male gender, ages <45 years, and nodules larger than 4 cm were risk factors for malignancy. In our study, 15.7% of women and 28.6% of men had malignant nodules. Sixteen patients (10.7%) younger than 45 years of age and 13 patients (8.7%) older than 45 years of age were diagnosed with malignant lesions, with no statistically significant differences observed. Of the 29 malignant nodules, only 3 (10.3%) were larger than 4 cm. Therefore, using sex, age, and nodule size as the predictive malignant parameters may result in missed diagnoses and misdiagnoses.
Conventional ultrasound features such as a solid internal component, hypoechogenicity, irregular margins, a taller-than-wide shape, and microcalcification are predictive of thyroid carcinoma (11, 12). However, these features are classical malignant characteristics for non-follicular neoplasms. Koike et al. (13) found that the sensitivity of preoperative US diagnosis was 18.2% for follicular neoplasms. Multiple logistic regression analyses revealed that conventional ultrasound could not identify FC and FA (13). The results of this study were similar to these previous results.
CEUS can reflect the state of microcirculation perfusion of nodules. Three meta-analyses showed that both the sensitivity and specificity of CEUS were more than 85% (14–16). Most research have suggested that a heterogenous enhancement pattern is a reliable index for predicting malignancy and that a ring-enhancement pattern is a feature of benign nodules. Our study has revealed that the predictivity of malignancy of ATA risk stratification is improved by adding CEUS. The malignancy risk of the intermediate and low suspicious nodules is 5–20%. The risk can increase to 38.5% when the nodule shows heterogenous enhancement. Despite the low sensitivity (51.7%), it has a very high specificity and OR for malignancy. Of the 29 malignant nodules, 12 PTCs and 2 FCs and MC presented with heterogenous enhancement, which might be due to the more heterogenous growth of malignant nodules and the more imbalanced blood distribution, with the coexistence of areas of rich and poor microvasculature. In addition, malignant nodules contain regions of complicated morphological collagen degeneration, which have either no small vessels or vessels whose caliber is so small that microbubbles cannot enter, resulting in heterogenous enhancement. However, the other six PTCs and five FCs presented with ring enhancement. Two FVPTC nodules were 3.8 and 4.0 cm in ultrasound images, but the pathological size of the cancer area was only 0.4 and 0.5 cm, respectively, with the surrounding tissue presenting with nodular goiter. This finding may result in a benign enhancement pattern. The reason that malignant nodules showed ring enhancement may be that the nodules were surrounded by vessels or that vessels existed in the capsule of the nodule, which is consistent with the color Doppler features.
Elastosonography provided an estimation of tissue stiffness. In our study, only 14 malignant nodules were hard, with 3–4 elastography scores. The other 15 malignant nodules were relatively soft. This result was different from most studies, which reported that malignant thyroid nodules were often stiff. The most likely reason is that the constitution of the cases was vastly different. In this study, 24.1% of the malignant nodules were FCs, and 13.8% of the nodules were FVPTCs. Pathological changes in these nodules showed that follicular cells containing colloid were abundant, making the texture soft. Therefore, real-time elastography was not able to distinguish benign and malignant nodules with intermediate and low suspicion.
FNA is the best preoperative diagnostic method for thyroid nodules but is indeterminate in 15–20% of the cases especially when follicular neoplasms are involved. When a follicular neoplasm is suspected, the histological possibilities include an AN, FA, or FC. FVPTC will also sometimes fall into this category when the nuclear features are more subtle. These features cannot be distinguished by the use of cytology alone because the evaluation of morphology is subjective (17, 18) and the sample is too limited to represent the entire heterogenous lesion. In addition, the presence of capsule or vessel invasion for diagnosing FC cannot be evaluated by FNA (19). One FC in this study was suspicious for a follicular neoplasm, and the other FC exhibited only thyroid follicular epithelial cells and no tumor cells. Another lesion that was suspicious for FC by FNA was a nodular goiter with adenomatous hyperplasia confirmed by surgical pathology.
A limitation of our study is selective bias. This study is a retrospective study, and the subjects included were patients undergoing surgery. Whether the results are applicable to all clinical conditions remains to be verified. In addition, the strain ratio was not evaluated with ES. Further large studies using more indicators are needed to obtain a more accurate value.
Conclusions
The CEUS enhancement pattern is helpful for differentiating between benign and malignant nodules with an intermediate or low suspicion. Heterogenous enhancement is associated with malignant nodules, a finding that could modify the clinical decision to avoid the misdiagnosis of FC in some patients. CUS characteristics, other qualitative CEUS indices, ES, and FNA have limited value.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
This prospective study was approved by the ethics committee of Peking Union Medical College Hospital, and informed consent was obtained. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BZ and YJ conceived and designed the study. JX, RL, and RZ collected the data. XY and SZ performed the analysis. XL and XZ prepared all the figures and tables. XX, LG and QW were major contributors in writing the manuscript. SF edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81541131), the Capital Development Research Foundation (CD 2016-2-40110), and the International Science and Technology Cooperation Program of China (2015DFA30440).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
2. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol. (2014) 811:1–122. doi: 10.1111/cen.12515
3. Giusti M, Campomenosi C, Gay S, Massa B, Silvestri E, Monti E, et al. The use of semi- quantitative ultrasound elastosonography in combination with conventional ultrasonography and contrast-enhanced ultrasonography in the assessment of malignancy risk of thyroid nodules with indeterminate cytology. Thyroid Res. (2014) 7:9. doi: 10.1186/s13044-014-0009-8
4. Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, et al. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol. (2013) 82:1892–8. doi: 10.1016/j.ejrad.2013.07.005
5. Liang XN, Guo RJ, Li S, Zheng ZM, Liang HD. Binary logistic regression analysis of solid thyroid nodules imaged by high-frequency ultrasonography, acoustic radiation force impulse, and contrast-enhanced ultrasonography. Eur Rev Med Pharmacol Sci. (2014) 18:3601–10.
6. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: white paper of the ACR thyroid imaging, reporting and data system (TIRADS) committee. J Am Coll Radiol. (2015) 12:1272–9. doi: 10.1016/j.jacr.2015.07.011
7. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. (2003) 22:127–31. doi: 10.7863/jum.2003.22.2.127
8. Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US- elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. (2008) 18:523–31. doi: 10.1089/thy.2007.0323
9. Paramo JC, Mesko T. Age, tumor size, and in-office ultrasonography are predictive parameters of malignancy in follicular neoplasms of the thyroid. Endocr Pract. (2008) 14:447–51. doi: 10.4158/EP.14.4.447
10. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. (2002) 26:41–4. doi: 10.1002/dc.10043
11. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. (2011) 260:892–9. doi: 10.1148/radiol.11110206
12. Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J. (2009) 56:383–9. doi: 10.1507/endocrj.K08E-310
13. Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. (2001) 136:334–7. doi: 10.1001/archsurg.136.3.334
14. Yu D, Han Y, Chen T. Contrast-enhanced ultrasound for differentiation of benign and malignant thyroid lesions: meta-analysis. Otolaryngol Head Neck Surg. (2014) 151:909–15. doi: 10.1177/0194599814555838
15. Ma X, Zhang B, Ling W, Liu R, Jia H, Zhu F, et al. Contrast-enhanced sonography for the identification of benign and malignant thyroid nodules: systematic review and meta-analysis. J Clin Ultrasound. (2016) 44:199–209. doi: 10.1002/jcu.22311
16. Sun B, Lang L, Zhu X, Jiang F, Hong Y, He L. Accuracy of contrast-enhanced ultrasound in the identification of thyroid nodules: a meta-analysis. Int J Clin Exp Med. (2015) 8:12882−9.
17. Olson MT, Boonyaarunnate T, Aragon Han P, Umbricht CB, Ali SZ, Zeiger MA. A tertiary center's experience with second review of 3885 thyroid cytopathology specimens. J Clin Endocrinol Metab. (2013) 98:1450–7. doi: 10.1210/jc.2012-3898
18. Olson MT, Clark DP, Erozan YS, Ali SZ. Spectrum of risk of malignancy in subcategories of atypia of undetermined significance. Acta Cytol. (2011) 55:518–25. doi: 10.1159/000333232
Keywords: thyroid carcinoma, contrast-enhanced ultrasonography, elastosonography, American Thyroid Association, intermediate- and low-suspicion patterns
Citation: Xi X, Gao L, Wu Q, Fang S, Xu J, Liu R, Yang X, Zhu S, Zhao R, Lai X, Zhang X, Zhang B and Jiang Y (2020) Differentiation of Thyroid Nodules Difficult to Diagnose With Contrast-Enhanced Ultrasonography and Real-Time Elastography. Front. Oncol. 10:112. doi: 10.3389/fonc.2020.00112
Received: 07 September 2019; Accepted: 21 January 2020;
Published: 27 February 2020.
Edited by:
Jorge A. R. Salvador, University of Coimbra, PortugalReviewed by:
Prasanth Penumadu, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaShilpi Sharma, Narayana Superspeciality Hospital, Gurugram, India
Copyright © 2020 Xi, Gao, Wu, Fang, Xu, Liu, Yang, Zhu, Zhao, Lai, Zhang, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, dGh5cm9pZHVzQDE2My5jb20=; Yuxin Jiang, amlhbmd5dXhpbnhoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xuehua Xi
Xuehua Xi Luying Gao
Luying Gao Qiong Wu2,3†
Qiong Wu2,3† Bo Zhang
Bo Zhang