- 1Division of Surgical Oncology, Department of Surgery, University of Colorado, Anschutz Medical Campus, Denver, CO, United States
- 2Department of Hepatobiliary and Pancreatic Surgery, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan
- 3Department of Surgery, Oncology, and Gastroenterology, University of Padua, Padua, Italy
- 4Clinical Trials Office, Department of Surgery, University of Colorado, Anschutz Medical Campus, Denver, CO, United States
Thanks to the development of modern chemotherapeutic regimens, survival after surgery for pancreatic ductal adenocarcinoma (PDAC) has improved and pancreatologists worldwide agree that the treatment of PDAC demands a multidisciplinary approach. Neoadjuvant treatment (NAT) plays a major role in the treatment of PDAC since only about 20% of patients are considered resectable at the time of diagnosis. Moreover, increasing data demonstrating the benefits of NAT for borderline resectable/locally advanced PDAC are driving a shift from up-front surgery to NAT in the multidisciplinary treatment of even resectable PDAC. Our understanding of the role of NAT in PDAC has evolved from tumor shrinkage to controlling potential micrometastases and selecting patients who may benefit from radical resection. The present review gives an overview on the current literature of NAT concepts for BR/LA PDAC and resectable PDAC.
Introduction
The role of neoadjuvant treatment (NAT) in pancreatic adenocarcinoma (PDAC) is still under debate due to a relative lack of robust data compared with other gastrointestinal cancers, in which the role of NAT is more well-defined. For example, in esophageal cancer neoadjuvant chemoradiotherapy is standard of care for resectable disease, and is associated with improved OS, DFS, pathological Complete Response (pCR), and R0 resection rate as shown in phase III RCT (1–3). The survival benefit of neoadjuvant chemotherapy has also been reported in phase III RCT in resectable gastric cancer (4–6). Moreover, neoadjuvant chemoradiotherapy has become widely accepted as standard of care for resectable rectal cancer in the last decades, as up to 50–60% of patients are downstaged after neoadjuvant chemoradiotherapy (7–9), and up to 25% of the patients have a pCR (10). While these encouraging data have led to the development of new therapeutic approaches, permitting even organ-sparing treatments for rectal cancer (11), this has not carried over to PDAC. Several barriers have limited the application of NAT in pancreatic cancer. First, historically, the response rate to chemotherapy for pancreatic cancer was very low (12). The persistence of this perspective has contributed to low patient compliance in accepting NAT in clinical trials, particularly earlier ones (13). This has been mitigated somewhat by improved chemotherapy regimens. Another barrier is the inability of current radiological modalities to adequately define the level of response to NAT, because restaging after NAT is based on imaging findings on CT and MRI scan, which are not predictive of resectability or pathological response (14, 15). Furthermore, retrospective cohort studies report a lower pCR rate for PDAC than other GI malignancies, ranging between 2 and 15% (16–18). Median OS in patients who do attain pCR appears to be longer, but it is difficult to assess the impact on survival of pCR with such low pCR rates.
According to 2019 NCCN guidelines, NAT is now the accepted approach for borderline resectable (BR) disease, while upfront surgery is still the recommendation for resectable disease except in cases with high risk features (19). Since only about 20% of patients are considered resectable at diagnosis (20), the use of NAT plays a major role in the treatment of PDAC. The preferred regimens in the neoadjuvant/adjuvant setting, the first-line therapy for locally advanced (LA) and metastatic disease, are FOLFIRINOX (or modified FOLFIRINOX) or gemcitabine + nab-paclitaxel (GnP). In 2003, a French group first reported on the feasibility of FOLFIRINOX in metastatic solid tumor in a phase I trial, including two metastatic PDAC patients (21). Since then, phase II RCTs have studied the effects of FOLFIRINOX in metastatic PDAC, showing a response rate >30% (22, 23). In the phase III PRODIGE RCT, patients with metastatic PDAC treated with FOLFIRINOX were reported to have a 11 months median OS, vs. 6.8 months in gemcitabine group, and a PFS 6.4 vs. 3.3 months, respectively (24). In 2011, phase I/II trial results with 67 patients with advanced PDAC treated with GnP were published, showing a 12.2 months OS, and 48% partial response rate (25). Based on these results, the phase III MPACT trial compared GnP vs. gemcitabine alone in 861 metastatic PDAC patients. Primary endpoint was reached, showing a 8.7 vs. 6.6 months OS (26), and GnP group was also associated with a improved 1- and 2-year survival, response rate and PFS (27). These encouraging data reported in recent years has led to an increasing number of patients treated with NAT using FOLFIRINOX and GnP regimens, even in resectable disease.
Another important advantage of treatment with NAT is an increase in the proportion of patients who receive chemotherapy. This is based on the rationale that adjuvant chemotherapy (AC) has been shown to improve survival in phase III RCTs. The CONKO-001 trial randomized 369 patients without prior chemotherapy into gemcitabine AC group vs. observation groups. This study showed a statistically significant difference in survival (median OS 22.8 vs. 20.2 months, respectively, median DFS 13.4 vs. 6.9 months) (28). More recently, the PRODIGE-24 trial randomized 493 patients to receive mFOLFIRINOX or gemcitabine in adjuvant setting. The mFOLFIRINOX regimen showed a longer survival than gemcitabine (median OS was 54.4 vs. 35.0 months, median DFS 21.6 vs. 12.8 months, respectively) (29). Traditionally, only patients with a good performance status and a good recovery after surgery are treated with AC. About 45% of patients do not receive AC after resection due to poor performance status, postoperative morbidity, or early progression of disease (30, 31). This may lead to a decreased survival in these patients. Given the large amount of data showing the survival benefit of chemotherapy both in the neoadjuvant and adjuvant setting when compared with no chemotherapy, it has become clear that chemotherapy, either before or after surgery, is a crucial component in the treatment in PDAC.
Neoadjuvant Therapy in Borderline Resectable and Locally Advanced PDAC
The purpose of NAT in BR/LA PDAC is not necessarily to decrease tumor size to facilitate an easier resection, but to select those candidates for radical resection who do not have tumor progression that would indicate biologically-aggressive disease. Indeed, several papers have shown favorable outcomes after resections following NAT, despite post-NAT imaging suggesting persistent unresectability (14, 15). A few specialized centers have even reported favorable long term survival in patients who undergo pancreatectomy with concomitant arterial resection and reconstruction following NAT (32, 33). Tee et al. reported 2-year OS of 62.3% in 65 patients who underwent pancreatectomy with arterial resection following NAT, which was superior to upfront resection (25.8%, p = 0.038, log-rank test) (32). Del Chiaro et al. reported 5-year survival of 23.4% in 34 patients who underwent pancreatectomy with arterial resection (half of whom had undergone NAT) compared with 0% in 39 patients with BR/LA disease who underwent exploration with curative intent but ultimately were treated palliatively due to technical unresectability (0%, P = 0.003). The surgical complication rate was feasible at 38.2% and mortality rate was low at 2.9% (33). These favorable results can be attributed not only to improved surgical skills and perioperative management, but also to modern chemotherapeutics controlling potential micrometastases and selecting patients who may benefit from radical resection after NAT (33).
Surgical resection for LA disease following NAT continues to be debated. Michelakos et al. analyzed 110 resected BR/LA patients after FOLFIRINOX, and in the absence of reliable predictors of resectability advocated that all BR/LA patients with no progression on NAT should be offered surgical exploration (34). Similarly, Rangelova et al. analyzed 154 resected BR/LA patients after NAT and suggested that every patient who receives NAT for BR/LA PDAC without radiological signs of disease progression should undergo exploration with intent of resection because it is not possible radiologically to define regression criteria (35). Moreover, they showed that surgical resection had a positive impact on survival for all values of CA 19-9 despite the fact that higher levels of CA 19-9 have been associated with worse prognosis (35). On the other hand, Satoi et al. describe a relatively high early recurrence rate of 30% within 6 months after surgical resection for LA disease following NAT, highlighting a need for more judicious use of surgery in this setting. The decision process should include a multidisciplinary discussion and consideration of radiologic findings (e.g., reassuring findings include stable disease or partial response) as well as CA 19-9 levels (e.g., decreased CA 19-9 <100 U/ml) (34, 36, 37).
One main marker of effectiveness of NAT in BR/LA patients is the proportion of patients who proceed to resection, but the best regimen for BR/LA patients is still controversial. Based on the results from RCTs in metastatic patients, FOLFIRINOX and GnP are currently considered the two best chemotherapy regimens for BR/LA patients. The Heidelberg group for example reported 125 patients with locally advanced PDAC treated by FOLFIRINOX in NA setting. Resection rate was 61% and the median OS after resection was 16.0 months, and FOLFIRINOX was confirmed to be independently associated with a favorable prognosis (38). More recently, the Karolinska group reported on 156 patients treated with NAT for BR/LA PDAC, including 34.6% with FOLFIRINOX and 15.4% with GnP. Exploration was attempted in 76 patients (48.7%), and resection was performed in 52 patients. Median survival after resection was 22.4 vs. 12.7 months in non-resected group. Interestingly, while dose reductions of other regimens were associated with impaired OS, dose reduction in FOLFIRINOX did not impact overall survival (35). Macedo et al. compared resected BR/LA patients who received FOLFIRINOX vs. GnP retrospectively and revealed there was no difference between the two regimens for median local recurrence-free survival (FOLFIRINOX 23.7 months vs. GnP 17.8 months), median metastasis-free survival (23 vs. 21.2 months), overall survival (37.3 vs. 31.9 months), R0 resection rate (82.8 vs. 81.8%), ypN0 (48.9 vs. 45.6%), and normalization of CA19-9 after NAT (35.9 vs. 35.2%) (18).
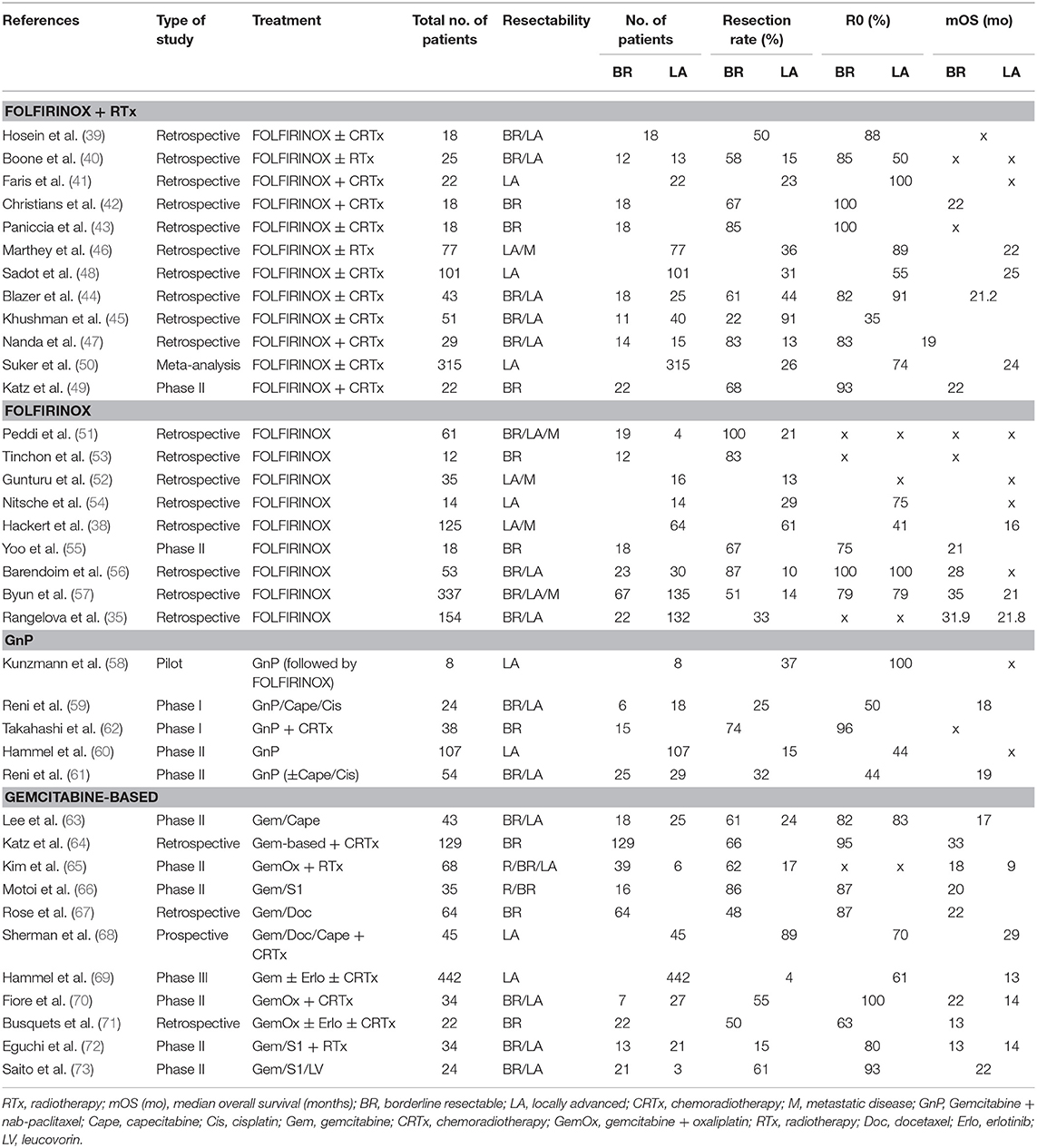
FOLFIRINOX is the most commonly used chemotherapy, but there are many reports of using radiation therapy concurrently or following chemotherapy for BR/LA patients. The resection rate of FOLFIRINOX with radiotherapy for BR/LA patients has been reported to be 58–85% for BR and 13–44% for LA (39–50). compared with resection rates 51–100% for BR, 13–61% for LA when treated with FOLFIRINOX without radiotherapy (35, 38, 51–57). Although the addition of radiotherapy does not appear to make a significant difference in resectability rates and survival (refer to tables), these results are primarily from retrospective studies and may be biased, as patients who received radiation may have had more advanced disease.
Regarding GnP, there are fewer reports than with FOLFIRINOX (58–62). As many papers on GnP combined with other chemotherapy or radiation therapy, it may be considered difficult to convert LA to resectable by GnP alone (58, 59, 61, 62). In the largest phase II study (LAPACT), 107 LA patients received GnP alone and the resection rate was only 15% and R0 resection rate was 44% (60).
Other treatments are summarized in Table 1. There are many variations of regimens based on gemcitabine, with resection rates ranging from 48 to 86% for BR and 4–89% for LA, respectively (63–73).
Due to lack of evidence from RCTs, the additional value of neoadjuvant radiotherapy is still under debate. The results of the ESPAC-1 trial, which showed a significant survival benefit with adjuvant chemotherapy but negative effect with adjuvant chemoradiotherapy, have largely led European centers to minimize the use of neoadjuvant radiotherapy. However, neoadjuvant radiotherapy continues to be commonly used in the United States (74). In addition to traditional external-beam radiotherapy, other modalities of radiotherapy have been developed and utilized in this setting. Keane et al. reported that the use of intraoperative radiotherapy after NAT was well-tolerated, and associated with encouraging median survival rates when incorporated into treatment of patients with unresectable disease or close or positive margins after resection for BR PDAC (75). Furthermore, newer techniques to minimize dose to the radiosensitive tissues in the abdomen including stereotactic body radiation therapy (SBRT) and intensity-modulated radiation therapy (IMRT) are increasingly used in the neoadjuvant setting for patients with BR/LA PDAC (76). However, there are few studies evaluating the impact of these modalities on surgical resection. Well-planned clinical trials to evaluate the efficacy of modern radiation therapy for BR/LA PDAC are needed.
Neoadjuvant Treatment in Resectable PDAC
While BR/LA PDAC is increasingly treated with neoadjuvant chemotherapy, the standard treatment for resectable PDAC currently remains upfront surgery followed by AC (77). This is based on multiple trials, including the CONKO-001, ESPAC-4, and Prodige 24 studies, which have demonstrated that adjuvant systemic therapy increases disease free survival and long term survival (29, 78, 79). In the Prodige study, a RCT comparing a modified FOLFIRINOX regimen with gemcitabine in the adjuvant setting found an impressive median survival of 54 months with mFOLFIRINOX (29). Unfortunately, there is a significant fraction of patients who undergo upfront surgery but do not recover adequate functional status to receive adjuvant therapy and its treatment benefits (80).
The success of neoadjuvant chemotherapy in BR/LA has led some to raise the question of whether administering chemotherapy before surgery might confer similar benefits for resectable disease. A similar precedent exists in other gastrointestinal malignancies. One notable example is esophageal cancer, in which the impact of neoadjuvant therapy on improved survival and conversion to resectability for locally advanced and initially unresectable disease has led to increasing application of NAT for even resectable disease (1–3). The argument in favor of neoadjuvant chemotherapy in resectable PDAC is multifaceted. One proposed benefit is that it allows for earlier treatment of micrometastatic disease, which is likely responsible for the high failure rate after surgical resection for radiologically resectable disease. Secondly, by timing chemotherapy before the physiologic stress of surgery, it may allow more patients to receive a full course of cytotoxic chemotherapy. Finally, as with BR/LA disease, it may allow for better surgical selection of patients, as more aggressive disease will “declare itself” by progressing during chemotherapy thus sparing the patient the morbidity of a surgical operation that may be of limited utility.
On the other hand, there are also several arguments against neoadjuvant therapy for resectable disease. It delays surgery, especially when patients experience significant complications such as biliary occlusion, potentially allowing the cancer to progress to a point that becomes unresectable. Another issue is that unlike surgery, the initiation of chemotherapy requires a positive biopsy. This can be elusive given the cancer's anatomic location as well as its structure—which often consists of low cellularity and high stromal content—and can thus postpone therapy (81). Endoscopic ultrasound guided biopsy has a reported specificity of 96–98% but sensitivity of only 85–92%, with repeat procedure required in up to 11% of cases (82). The cellular structure of pancreatic cancer may also have implications that limit the effectiveness of neoadjuvant chemotherapy, as it is possible that the reduction of positive resection margins after NAT may be due to reduction in the density of cancer cells rather than tumor shrinkage (83). Indeed, excluding patients with a complete pathologic response, there does not appear to be a clear correlation between histologic tumor regression and survival (84).
Multiple trials have been conducted to assess neoadjuvant chemotherapy for resectable disease (13, 85–94). Most early trials were cohort studies and involved older single agent chemotherapy regimens such as gemcitabine and 5-fluorouracil, plus or minus radiation. A summary of select studies can be found in Table 2. Heinrich et al. studied the effect of neoadjuvant gemcitabine and cisplatin in 28 patients. Ninety-three percent underwent resection, and median overall survival was 26.5 months (87). Varadhachary et al. found a higher median overall survival rate of 31 months when radiation was added to neoadjuvant gemcitabine/cisplatin in a cohort of 90 patients; however, only 58% of patients underwent resection (95). In a larger study by Takahashi et al., 87% of 188 patients with resectable disease who were treated with preoperative gemcitabine and radiation underwent resection; of these patients, 99% had a R0 resection, and 5-year overall survival was 57% (90). Other studies of gemcitabine-based neoadjuvant therapy show resection rate of 74–86% and median overall survival of 17.4–27.2 months (13, 85, 86, 89, 91).
At least two early phase II randomized controlled trials comparing gemcitabine-based neoadjuvant chemoradiation with surgery for primary resectable cancer found NAT to be safe, feasible, and efficacious, but were terminated early due to slow enrollment and did not obtain statistically significant results due to lack of power (13, 100). After Okano et al. studied the effect of S1 (an oral fluoropyrimidine derivative) plus radiation in 57 patients, and found a 2-year survival rate of 83% (93), one prospective randomized trial in Japan comparing neoadjuvant chemotherapy using gemcitabine and S1 with upfront surgery showed significant survival benefits with NAT. In this phase III trial from January 2013 to January 2016, 362 patients were enrolled across 57 centers and randomly assigned to neoadjuvant chemotherapy using gemcitabine and S1 or upfront surgery. The median overall survival in the NAT group was 36.7 months compared with 26.6 months in the upfront surgery group (p = 0.015) (94).
At the recent annual ASCO meeting in 2018, van Tienhoven et al. reported preliminary findings in their trial PREOPANC-1, a Dutch prospective randomized phase III trial comparing preoperative gemcitabine-based chemoradiotherapy vs. immediate surgery in resectable and borderline resectable pancreatic adenocarcinoma (101). Key significant findings included a longer disease free interval (9.9 vs. 7.9 months) and higher median overall survival rate in the preoperative treatment group, particularly among patients in whom the tumor was removed successfully (42.1 months with preoperative treatment vs. 16.8 months with immediate surgery) (101). Of note, these preliminary results were not stratified by primary resectable vs. borderline resectable disease. Nevertheless, the findings are intriguing. There are several other ongoing prospective trials evaluating various chemotherapy regimens in the neoadjuvant setting for resectable disease, including FOLFIRINOX vs. upfront surgery (NEPAFOX, NorPACT-1), and gemcitabine + oxaliplatin vs. upfront surgery (NEOPAC) (96–98). Another group is comparing neoadjuvant mFOLFIRINOX vs. GnP (Table 2) (99). The results of these trials should soon shed more light on this issue.
Immunotherapies and Targeted Therapies
There are currently limited data available to support the use of immunotherapy for PDAC. The immune checkpoint inhibitor trial unfortunately failed to show efficacy of anti-PD-L1 therapy in advanced PDAC patients, which has been attributed to the poor immunogenicity and immunosuppressive tumor microenvironment of pancreas cancer (102, 103). However, comprehensive genomic profiling has found deficiencies in small subsets of patients that may be targets for intervention, notably BRCA 1/2 and mismatch repair (MMR). Although incidence of BRCA 1/2 mutation or MMR deficiencies in patients with PDAC is low (7 or 1%, respectively) (104, 105), the clinical trials of novel therapies against these targets have shown some promise (106, 107). For example, MMR deficient tumors were found to be susceptible to immunotherapy across multiple solid tumors including PDAC, which led to FDA approval of pembrolizumab for patients with advanced disease that have this mutation (107). Indeed, recent treatment guidelines for the management of advanced PDAC now recommend testing for mismatch repair deficiencies despite its low prevalence, due to the potential for sustained disease remission, and recommend pembrolizumab as a second line treatment in patients who test positive for MMR deficiency (108). Meanwhile, the POLO phase III trial showed the efficacy of olaparib, a PARP inhibitor, as maintenance therapy in patients who had a germline BRCA1/2 mutation (PFS; 7.4 vs. 3.8 months with placebo, p = 0.004) (109). As a result, olaparib is currently under FDA review as a maintenance therapy in this subset of patients. Comprehensive genomic profiling has the potential to enable the identification of patients with specific alterations who may be candidates for immunotherapy and targeted therapies in the future. Finally, combination therapies that aim to reprogram the immunosuppressive tumor microenvironment in conjunction with immunotherapy are also being investigated, and have yielded some encouraging preliminary results (110).
Conclusions
Thanks to the development of modern chemotherapeutic regimens, survival after surgery for PDAC has improved and pancreatologists worldwide believe that the treatment of PDAC demands a multidisciplinary approach. Today the role of NAT in PDAC is shifting from tumor shrinkage to controlling potential micrometastases and selecting patients who may benefit from radical resection. Given the absence of reliable predictors of resectability and some evidence supporting radical pancreatectomy with arterial resection, several papers advocate that all BR/LA patients with no progression on NAT should be offered surgical exploration. Increasing data demonstrating the benefits of NAT for BR/LA PDAC are driving a shift from up-front surgery to NAT in the multidisciplinary treatment of even potentially resectable PDAC. Although FOLFIRINOX is currently the most commonly used regimen in NAT, the jury is still out on the optimal approach in this setting—namely whether or not radiation therapy should be included, and if the chemotherapy should be FOLFIRINOX or gemcitabine-based. Several ongoing prospective trials will soon contribute further to our knowledge of the role of NAT for PDAC. Despite initial poor results with immunotherapy, comprehensive genomic profiling to identify cancers with specific deficits and combination therapies that aim to increase the immunogenicity of pancreas cancer may give immunotherapy a role in NAT for PDAC in the future.
Author Contributions
AO, FH, QB, and MD: study conception and design and drafting of manuscript. AO, FH, and QB: acquisition of data. AO and FH: analysis and interpretation of data. MA-M, RS, and MD: critical revision. AO, FH, QB, MA-M, RS, and MD: final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
2. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. (2014) 32:385–91. doi: 10.1200/JCO.2013.51.2186
3. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355:11–20. doi: 10.1056/NEJMoa055531
5. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. (2016) 17:1697–708. doi: 10.1016/S1470-2045(16)30531-9
6. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393:1948–57. doi: 10.1016/S0140-6736(18)32557-1
7. Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. (2007) 109:1750–5. doi: 10.1002/cncr.22625
8. Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. (2007) 25:4379–86. doi: 10.1200/JCO.2007.11.9685
9. Smith KD, Tan D, Das P, Chang GJ, Kattepogu K, Feig BW, et al. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. (2010) 251:261–4. doi: 10.1097/SLA.0b013e3181bdfc27
10. Shivnani AT, Small W Jr, Stryker SJ, Kiel KD, Lim S, Halverson AL, et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg. (2007) 193:389–93; discussion: 93–4. doi: 10.1016/j.amjsurg.2006.09.030
11. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. (2004) 240:711–7; discussion: 7–8. doi: 10.1097/01.sla.0000141194.27992.32
12. Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. (1997) 15:2403–13. doi: 10.1200/JCO.1997.15.6.2403
13. Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. (2015) 191:7–16. doi: 10.1007/s00066-014-0737-7
14. Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. (2015) 261:12–7. doi: 10.1097/SLA.0000000000000867
15. Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol. (2017) 27:3104–16. doi: 10.1007/s00330-016-4632-8
16. He J, Blair AB, Groot VP, Javed AA, Burkhart RA, Gemenetzis G, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. (2018) 268:1–8. doi: 10.1097/SLA.0000000000002672
17. Cloyd JM, Wang H, Egger ME, Tzeng CD, Prakash LR, Maitra A, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. (2017) 152:1048–56. doi: 10.1001/jamasurg.2017.2227
18. Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or gemcitabine/Nab-paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg. (2019) 270:400-13. doi: 10.1097/SLA.0000000000003468
19. Network NCC. Pancreatic Adenocarcinoma (Version 3.2019). (2019). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
20. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. (2004) 363:1049–57. doi: 10.1016/S0140-6736(04)15841-8
21. Ychou M, Conroy T, Seitz JF, Gourgou S, Hua A, Mery-Mignard D, et al. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/ 5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol. (2003) 14:481–9. doi: 10.1093/annonc/mdg119
22. Conroy T, Paillot B, Francois E, Bugat R, Jacob JH, Stein U, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer–a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. (2005) 23:1228–36. doi: 10.1200/JCO.2005.06.050
23. Ychou M, Desseigne F, Guimbaud R, Ducreux M, Bouché O, Bécouarn Y, et al. Randomized phase II trial comparing folfirinox (5FU/leucovorin [LV], irinotecan [I] and oxaliplatin [O]) vs gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA). First results of the ACCORD 11 trial. J Clin Oncol. (2007) 25(18_suppl):4516. doi: 10.1200/jco.2007.25.18_suppl.4516
24. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
25. Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. (2011) 29:4548–54. doi: 10.1200/JCO.2011.36.5742
26. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
27. Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG, et al. CA19–9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. (2016) 27:654–60. doi: 10.1093/annonc/mdw006
28. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. (2013) 310:1473–81. doi: 10.1001/jama.2013.279201
29. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. (2018) 379:2395–406. doi: 10.1056/NEJMoa1809775
30. Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. (2012) 214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022
31. Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. (2014) 260:372–7. doi: 10.1097/SLA.0000000000000378
32. Tee MC, Krajewski AC, Groeschl RT, Farnell MB, Nagorney DM, Kendrick ML, et al. Indications and perioperative outcomes for pancreatectomy with arterial resection. J Am Coll Surg. (2018) 227:255–69. doi: 10.1016/j.jamcollsurg.2018.05.001
33. Del Chiaro M, Rangelova E, Halimi A, Ateeb Z, Scandavini C, Valente R, et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB. (2019) 21:219–25. doi: 10.1016/j.hpb.2018.07.017
34. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. (2019) 269:733–40. doi: 10.1097/SLA.0000000000002600
35. Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. (2019). doi: 10.1097/SLA.0000000000003301. [Epub ahead of print].
36. Yoo C, Shin SH, Kim K-P, Jeong JH, Chang H-M, Kang JH, et al. Clinical outcomes of conversion surgery after neoadjuvant chemotherapy in patients with borderline resectable and locally advanced unresectable pancreatic cancer: a single-center, retrospective analysis. Cancers. (2019) 11:278. doi: 10.3390/cancers11030278
37. Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. (2019) 4:6–13. doi: 10.1002/ags3.12295
38. Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with Folfirinox results in resectability in 60% of the patients. Ann Surg. (2016) 264:457–63. doi: 10.1097/SLA.0000000000001850
39. Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. (2012) 12:199. doi: 10.1186/1471-2407-12-199
40. Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. (2013) 108:236–41. doi: 10.1002/jso.23392
41. Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. (2013) 18:543–8. doi: 10.1634/theoncologist.2012-0435
42. Christians KK, Tsai S, Mahmoud A, Ritch P, Thomas JP, Wiebe L, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist. (2014) 19:266–74. doi: 10.1634/theoncologist.2013-0273
43. Paniccia A, Edil BH, Schulick RD, Byers JT, Meguid C, Gajdos C, et al. Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective cohort study. Medicine. (2014) 93:e198. doi: 10.1097/MD.0000000000000198
44. Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. (2015) 22:1153–9. doi: 10.1245/s10434-014-4225-1
45. Khushman M, Dempsey N, Maldonado JC, Loaiza-Bonilla A, Velez M, Carcas L, et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology. (2015) 15:667–73. doi: 10.1016/j.pan.2015.08.010
46. Marthey L, Sa-Cunha A, Blanc JF, Gauthier M, Cueff A, Francois E, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. (2015) 22:295–301. doi: 10.1245/s10434-014-3898-9
47. Nanda RH, El-Rayes B, Maithel SK, Landry J. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol. (2015) 111:1028–34. doi: 10.1002/jso.23921
48. Sadot E, Doussot A, O'Reilly EM, Lowery MA, Goodman KA, Do RK, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. (2015) 22:3512–21. doi: 10.1245/s10434-015-4647-4
49. Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. (2016) 151:e161137. doi: 10.1001/jamasurg.2016.1137
50. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. (2016) 17:801–10. doi: 10.1016/S1470-2045(16)00172-8
51. Peddi PF, Lubner S, McWilliams R, Tan BR, Picus J, Sorscher SM, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. J Pancreas. (2012) 13:497–501. doi: 10.6092/1590-8577/913
52. Gunturu KS, Yao X, Cong X, Thumar JR, Hochster HS, Stein SM, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. (2013) 30:361. doi: 10.1007/s12032-012-0361-2
53. Tinchon C, Hubmann E, Pichler A, Keil F, Pichler M, Rabl H, et al. Safety and efficacy of neoadjuvant FOLFIRINOX treatment in a series of patients with borderline resectable pancreatic ductal adenocarcinoma. Acta Oncol. (2013) 52:1231–3. doi: 10.3109/0284186X.2013.771821
54. Nitsche U, Wenzel P, Siveke JT, Braren R, Holzapfel K, Schlitter AM, et al. Resectability after first-line FOLFIRINOX in initially unresectable locally advanced pancreatic cancer: a single-center experience. Ann Surg Oncol. (2015) 22(Suppl 3):S1212–20. doi: 10.1245/s10434-015-4851-2
55. Yoo C, Kang J, Kim KP, Lee JL, Ryoo BY, Chang HM, et al. Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: improved efficacy compared with gemcitabine-based regimen. Oncotarget. (2017) 8:46337–47. doi: 10.18632/oncotarget.17940
56. Barenboim A, Lahat G, Geva R, Nachmany I, Nakache R, Goykhman Y, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: an intention to treat analysis. Eur J Surg Oncol. (2018) 44:1619–23. doi: 10.1016/j.ejso.2018.07.057
57. Byun Y, Han Y, Kang JS, Choi YJ, Kim H, Kwon W, et al. Role of surgical resection in the era of FOLFIRINOX for advanced pancreatic cancer. J Hepato Biliary Pancreatic Sci. (2019) 26:416-25. doi: 10.1016/j.hpb.2019.10.1708
58. Kunzmann V, Hartlapp I, Scheurlen M, Einsele H, Mueller J, Kenn W, et al. Sequential neoadjuvant chemotherapy with nab-paclitaxel plus gemcitabine and FOLFIRINOX in locally advanced pancreatic cancer (LAPC): A PILOT study. J Clin Oncol. (2013) 31(15_suppl):e15193. doi: 10.1200/jco.2013.31.15_suppl.e15193
59. Reni M, Balzano G, Zanon S, Passoni P, Nicoletti R, Arcidiacono PG, et al. Phase 1B trial of Nab-paclitaxel plus gemcitabine, capecitabine, and cisplatin (PAXG regimen) in patients with unresectable or borderline resectable pancreatic adenocarcinoma. Br J Cancer. (2016) 115:290–6. doi: 10.1038/bjc.2016.209
60. Hammel P, Lacy J, Portales F, Sobrero AF, Cid RAP, Mozo JLM, et al. Phase II LAPACT trial of nab-paclitaxel (nab-P) plus gemcitabine (G) for patients with locally advanced pancreatic cancer (LAPC). J Clin Oncol. (2018) 36(4_suppl):204. doi: 10.1200/JCO.2018.36.4_suppl.204
61. Reni M, Zanon S, Balzano G, Passoni P, Pircher C, Chiaravalli M, et al. A randomised phase 2 trial of nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in locally advanced or borderline resectable pancreatic adenocarcinoma. Eur J Cancer. (2018) 102:95–102. doi: 10.1016/j.ejca.2018.07.007
62. Takahashi H, Akita H, Ioka T, Wada H, Tomokoni A, Asukai K, et al. Phase I trial evaluating the safety of preoperative gemcitabine/nab-paclitaxel with concurrent radiation therapy for borderline resectable pancreatic cancer. Pancreas. (2018) 47:1135–41. doi: 10.1097/MPA.0000000000001140
63. Lee JL, Kim SC, Kim JH, Lee SS, Kim TW, Park DH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery. (2012) 152:851–62. doi: 10.1016/j.surg.2012.03.010
64. Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. (2012) 118:5749–56. doi: 10.1002/cncr.27636
65. Kim EJ, Ben-Josef E, Herman JM, Bekaii-Saab T, Dawson LA, Griffith KA, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. (2013) 119:2692–700. doi: 10.1002/cncr.28117
66. Motoi F, Ishida K, Fujishima F, Ottomo S, Oikawa M, Okada T, et al. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. (2013) 20:3794–801. doi: 10.1245/s10434-013-3129-9
67. Rose JB, Rocha FG, Alseidi A, Biehl T, Moonka R, Ryan JA, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. (2014) 21:1530–7. doi: 10.1245/s10434-014-3486-z
68. Sherman WH, Chu K, Chabot J, Allendorf J, Schrope BA, Hecht E, et al. Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer. (2015) 121:673–80. doi: 10.1002/cncr.29112
69. Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without Erlotinib: The LAP07 randomized clinical trial. JAMA. (2016) 315:1844–53. doi: 10.1001/jama.2016.4324
70. Fiore M, Ramella S, Valeri S, Caputo D, Floreno B, Trecca P, et al. Phase II study of induction chemotherapy followed by chemoradiotherapy in patients with borderline resectable and unresectable locally advanced pancreatic cancer. Sci Rep. (2017) 7:45845. doi: 10.1038/srep45845
71. Busquets J, Fabregat J, Verdaguer H, Laquente B, Pelaez N, Secanella L, et al. Initial experience in the treatment of “borderline resectable” pancreatic adenocarcinoma. Cirugia Espanola. (2017) 95:447–56. doi: 10.1016/j.cireng.2017.10.005
72. Eguchi H, Yamada D, Iwagami Y, Gotoh K, Kawamoto K, Wada H, et al. Prolonged neoadjuvant therapy for locally advanced pancreatic cancer. Digestive Surg. (2018) 35:70–6. doi: 10.1159/000475477
73. Saito K, Isayama H, Sakamoto Y, Nakai Y, Ishigaki K, Tanaka M, et al. A phase II trial of gemcitabine, S-1 and LV combination (GSL) neoadjuvant chemotherapy for patients with borderline resectable and locally advanced pancreatic cancer. Med Oncol. (2018) 35:100. doi: 10.1007/s12032-018-1158-8
74. Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. (2001) 358:1576–85. doi: 10.1016/S0140-6736(01)06651-X
75. Keane FK, Wo JY, Ferrone CR, Clark JW, Blaszkowsky LS, Allen JN, et al. Intraoperative radiotherapy in the Era of intensive neoadjuvant chemotherapy and chemoradiotherapy for pancreatic adenocarcinoma. Am J Clin Oncol. (2018) 41:607–12. doi: 10.1097/COC.0000000000000336
76. Chapman BC, Gleisner A, Rigg D, Meguid C, Goodman K, Brauer B, et al. Perioperative outcomes and survival following neoadjuvant stereotactic body radiation therapy (SBRT) versus intensity-modulated radiation therapy (IMRT) in pancreatic adenocarcinoma. J Surg Oncol. (2018) 117:1073–83. doi: 10.1002/jso.25004
77. Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. (2017) 35:2324–8. doi: 10.1200/JCO.2017.72.4948
78. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. (2007) 297:267–77. doi: 10.1001/jama.297.3.267
79. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. (2017) 389:1011–24. doi: 10.1016/S0140-6736(16)32409-6
80. Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch OR, et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB. (2019). doi: 10.1016/j.hpb.2019.06.019. [Epub ahead of print].
81. Haeberle L, Esposito I. Pathology of pancreatic cancer. Transl Gastroenterol Hepatol. (2019) 4:50. doi: 10.21037/tgh.2019.06.02
82. Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. (2019) 54:19–32. doi: 10.1007/s00535-018-1519-2
83. Verbeke C, Lohr M, Karlsson JS, Del Chiaro M. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev. (2015) 41:17–26. doi: 10.1016/j.ctrv.2014.11.002
84. Mellon EA, Jin WH, Frakes JM, Centeno BA, Strom TJ, Springett GM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol. (2017) 56:391–7. doi: 10.1080/0284186X.2016.1256497
85. Talamonti MS, Small W Jr, Mulcahy MF, Wayne JD, Attaluri V, Colletti LM, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. (2006) 13:150–8. doi: 10.1245/ASO.2006.03.039
86. Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. (2008) 26:3496–502. doi: 10.1200/JCO.2007.15.8634
87. Heinrich S, Pestalozzi BC, Schafer M, Weber A, Bauerfeind P, Knuth A, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. (2008) 26:2526–31. doi: 10.1200/JCO.2007.15.5556
88. Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, et al. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology. (2009) 76:413–9. doi: 10.1159/000215928
89. Tajima H, Ohta T, Kitagawa H, Okamoto K, Sakai S, Makino I, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. (2012) 3:787–92. doi: 10.3892/etm.2012.482
90. Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Fernandez-del Castillo C. Diagnosis and management of cystic pancreatic lesions. Am J Roentgenol. (2013) 200:343–54. doi: 10.2214/AJR.12.8862
91. O'Reilly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. (2014) 260:142–8. doi: 10.1097/SLA.0000000000000251
92. Grose D, McIntosh D, Jamieson N, Carter R, Dickson E, Chang D, et al. The role of induction chemotherapy + chemoradiotherapy in localised pancreatic cancer: initial experience in Scotland. J Gastrointest Oncol. (2017) 8:683–95. doi: 10.21037/jgo.2017.04.01
93. Okano K, Suto H, Oshima M, Maeda E, Yamamoto N, Kakinoki K, et al. A prospective phase II trial of neoadjuvant S-1 with concurrent hypofractionated radiotherapy in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. (2017) 24:2777–84. doi: 10.1245/s10434-017-5921-4
94. Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. (2019) 37(4_suppl):189. doi: 10.1200/JCO.2019.37.4_suppl.189
95. Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. (2008) 26:3487–95. doi: 10.1200/jco.2008.26.15_suppl.4630
96. Hozaeel W, Pauligk C, Homann N, Luley K, Kraus TW, Trojan J, et al. Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: the NEPAFOX trial. J Clin Oncol. (2015) 33(15_suppl):TPS4152-TPS. doi: 10.1200/jco.2015.33.15_suppl.tps4152
97. Labori KJ, Lassen K, Hoem D, Gronbech JE, Soreide JA, Mortensen K, et al. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) - study protocol for a national multicentre randomized controlled trial. BMC Surg. (2017) 17:94. doi: 10.1186/s12893-017-0291-1
98. Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. (2011) 11:346. doi: 10.1186/1471-2407-11-346
99. Sohal D, McDonough SL, Ahmad SA, Gandhi N, Beg MS, Wang-Gillam A, et al. SWOG S1505: a randomized phase II study of perioperative mFOLFIRINOX vs. gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. (2017) 35(15_suppl):TPS4152-TPS. doi: 10.1200/JCO.2017.35.15_suppl.TPS4152
100. Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg. (2015) 19:1802–12. doi: 10.1007/s11605-015-2890-4
101. Tienhoven GV, Versteijne E, Suker M, Groothuis KBC, Busch OR, Bonsing BA, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. J Clin Oncol. (2018) 36(18_suppl):LBA4002-LBA. doi: 10.1200/JCO.2018.36.18_suppl.LBA4002
102. Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
103. Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. (2015) 6:e1792. doi: 10.1038/cddis.2015.162
104. Luo G, Lu Y, Jin K, Cheng H, Guo M, Liu Z, et al. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther. (2015) 15:1223–31. doi: 10.1586/14737140.2015.1086271
105. Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S, et al. Hypermutation In pancreatic cancer. Gastroenterology. (2017) 152:68–74. e2. doi: 10.1053/j.gastro.2016.09.060
106. Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. (2019) 2020 17:108–23. doi: 10.1038/s41571-019-0281-6
107. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
108. Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. (2018) 36:2545–56. doi: 10.1200/JCO.2018.78.9636
109. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. (2019) 381:317–27. doi: 10.1056/NEJMoa1903387
Keywords: neoadjuvant therapy, neoadjuvant chemotherapy, neoadjuvant chemoradiotherapy, borderline resectable, locally advanced, FOLFIRINOX, gemcitabine, nab-paclitaxel
Citation: Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD and Del Chiaro M (2020) Neoadjuvant Treatment in Pancreatic Cancer. Front. Oncol. 10:245. doi: 10.3389/fonc.2020.00245
Received: 21 October 2019; Accepted: 13 February 2020;
Published: 28 February 2020.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Malin Sund, Umeå University, SwedenJoseph M. Herman, University of Texas MD Anderson Cancer Center, United States
Copyright © 2020 Oba, Ho, Bao, Al-Musawi, Schulick and Del Chiaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Del Chiaro, bWFyY28uZGVsY2hpYXJvQGN1YW5zY2h1dHouZWR1
 Atsushi Oba
Atsushi Oba Felix Ho
Felix Ho Quoc Riccardo Bao
Quoc Riccardo Bao Mohammed H. Al-Musawi4
Mohammed H. Al-Musawi4