- 1College of Bioscience and Technology, Weifang Medical University, Weifang, China
- 2Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John's University, Queens, NY, United States
- 3First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4College of Integrated Chinese and Western Medicine, Hebei Medical University, Shijiazhuang, China
ABCB1 overexpression is known to contribute to multidrug resistance (MDR) in cancers. Therefore, it is critical to find effective drugs to target ABCB1 and overcome MDR. Erdafitinib is a tyrosine kinase inhibitor (TKI) of fibroblast growth factor receptor (FGFR) that is approved by the FDA to treat urothelial carcinoma. Previous studies have demonstrated that some TKIs exhibit MDR reversal effect. In this work, we examined whether erdafitinib could reverse MDR mediated by ABCB1. The results of reversal experiments showed that erdafitinib remarkably reversed ABCB1-mediated MDR without affecting ABCG2-mediated MDR. The results of immunofluorescence and Western blot analysis demonstrated that erdafitinib did not affect the expression of ABCB1 or its cellular localization. Further study revealed that erdafitinib inhibited ABCB1 efflux function leading to increasing intracellular drug accumulation, thereby reversing MDR. Furthermore, ATPase assay indicated that erdafitinib activated the ABCB1 ATPase activity. Docking study suggested that erdafitinib interacted with ABCB1 on the drug-binding sites. In summary, this study demonstrated that erdafitinib can reverse MDR mediated by ABCB1, suggesting that combination of erdafitinib and ABCB1-substrate conventional chemotherapeutic drugs could potentially be used to overcome MDR mediated by ABCB1.
Introduction
Multidrug resistance (MDR) is one of the main reasons leading to failure of chemotherapy in cancers (1, 2). Several mechanisms of MDR have been elucidated, including the increase of intracellular drug efflux (3), cell apoptosis inhibition (4), DNA repair enhancement (5) oncogene mutations (6) and others. Among them, ATP-binding cassette (ABC) transporters-mediated drug resistance is one of the most important factor causing MDR in cancer (7).
ABC transporters are a superfamily which consists of seven subfamilies from A to G, located on cell membranes to execute the pharmacological and physiological functions (8). Among them, ABCB1 (P-gp, MDR1) and ABCG2 (breast cancer resistance protein, BCRP) have been identified as the major contributor to MDR in variety of cancers (3, 9–11). Many studies suggested that the overexpression of ABCB1 and ABCG2 cause reduction of intracellular drugs (12–18). The well-known substrates for ABCB1 transporters are doxorubicin, paclitaxel, colchicine, and vincristine et al. (19). ABCG2 transporter substrates include doxorubicin, mitoxantrone, SN-38, and topotecan et al. (20). Currently, there is no single drug which is approved by the FDA as a reversal agent for overcoming MDR mediated by ABC transporters. It would be important to find effective drugs to overcome MDR mediated by these transporters (21).
In recent years, some tyrosine kinase inhibitors (TKIs), including dasatinib, glesatinib, and imatinib, have been demonstrated to exhibit MDR reversal effect (15, 22). Although there is no drug approved by FDA for reversing MDR, using FDA-approved drug combination has become an alternative strategy to overcome MDR (8, 23). Erdafitinib (Figure 1A) is a TKI which affect the fibroblast growth factor receptor (FGFR) to inhibit the tyrosine kinase activities of FGFR1-4 (24). It is the first FGFR kinase inhibitor approved by the FDA in 2019 to treat urothelial carcinoma. Additionally, several clinical trials evaluating the effect of erdafitinib on hepatocellular carcinoma (NCT02421185), breast cancer (NCT03238196), non-small-cell lung cancer (NCT02699606), and prostate cancer (NCT03999515) have been initiated. The present study evaluates if erdafitinib could reverse MDR mediated by ABCB1 in drug resistant cancer cells.
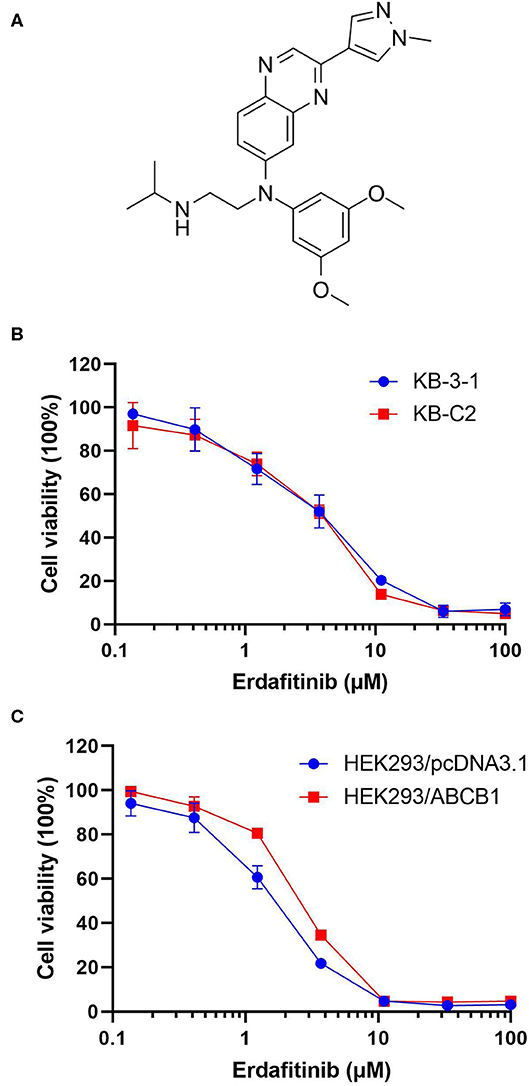
Figure 1. The cytotoxicity of erdafitinib in different cells. (A) The chemical structure of erdafitinib. (B) The cell viability curve of KB-C2 and KB-3-1 cells. (C) The cell viability curve of HEK293/ABCB1 and HEK293/pcDNA3.1 cells. The results are representative of three independent experiments. Error bars indicate SD.
Materials and Methods
Chemicals
Erdafitinib was kindly offered as a gift from ChemieTek (Indianapolis, IN). Fetal bovine serum (FBS) and Dulbecco's modified Eagle's Medium (DMEM) were purchased from Corning Incorporated (Corning, NY). The monoclonal antibodies against GAPDH and ABCB1 were bought from Thermo Fisher Inc. (Rockford, IL). MTT {3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide}, Triton X-100, 4′,6-diamidino-2-phenylindole (DAPI), paraformaldehyde, vincristine, paclitaxel, cisplatin, verapamil, Ko143, and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
ABCB1-overexpressing cell line KB-C2 was selected by colchicine with human epidermoid carcinoma cell line KB-3-1 (25). ABCG2-overexpressing cell line NCI-H460/MX20 was selected by mitoxantrone with human lung cancer cell line NCI-H460 (26). HEK293/pcDNA3.1 and HEK293/ABCB1 were obtained by transfecting HEK293 cells with empty pcDNA3.1 or ABCB1 expressing vector (27). All cell lines were cultured in DMEM supplemented with 10% FBS.
Cytotoxicity and MDR Reversal Experiments
The cytotoxicity and MDR reversal experiments were performed by the MTT assay as previously reported (17). In brief, for erdaftinib cytotoxicity study, cells were seeded overnight in 96-well plates (5 × 103 cells/well). Different concentrations of erdafitinib were added. For reversal study, different concentrations of paclitaxel, vincristine, or cisplatin were added into the wells after cells pre-incubated with different concentrations (0.06, 0.2, or 0.6 μM) of erdafitinib for 2 h. The cells were incubated for 72 h. Then 20 μL MTT (4 mg/mL) was added to each well for 4 h. After that, the medium with MTT was removed and then 100 μL DMSO was added to each well. The light absorbance values were measured using the accuSkanTM GO UV/Vis Microplate Spectrophotometer (Fisher Sci., Fair Lawn, NJ). Resistance fold (RF) was calculated by dividing the IC50 values of resistant cells by the IC50 of parental cells in the presence or absence of erdafitinib or positive control inhibitor. The known ABCB1 inhibitor, verapamil, was used as a positive control inhibitor. Cisplatin, a non-substrate drug of ABCB1, was used as a negative control anticancer drug for reversal study.
Western Blot and Immunofluorescence Analysis on ABCB1 Expression
We carried out the Western blot analysis as described previously (28). In brief, cells were treated with 0.6 μM of erdafitinib for 0, 24, 48, or 72 h at 37°C, and lysed. Equal amounts (10 μg) of proteins were subjected to SDS-PAGE and then transferred to PVDF membranes. The membranes were incubated with anti-ABCB1 (1:1000) and anti-GAPDH (1:1000) overnight at 4°C. After incubating with secondary antibody (1:2000), the bands were analyzed using the enhanced chemiluminescence reaction kit (Amersham, NJ).
Immunofluorescent staining was performed as reported previously (18). In brief, cells were seeded in 24-well plate, and then treated with 0.6 μM of erdafitinib for 0, 24, 48, or 72 h. Briefly, the cells were fixed using 4% formaldehyde for 15 min and then incubated with ABCB1 antibody (1:500) overnight. Subsequently, cells were incubated with Alexa Fluor 488 conjugated IgG secondary antibody (1:2000) and DAPI. The cells were visualized using a Nikon TE-2000S fluorescence microscope (Nikon Instruments Inc., Melville, NY) and photographs were taken.
[3H]-Paclitaxel Accumulation and Efflux Assay
As described in the previous study (15), the drug resistant cells and parental cells were seeded into 24-well plates. Cells were cultured overnight to allow for attachment. Each well was pre-treated with or without a reversal agent erdafitinib or verapamil for 2 h. After that, cells were incubated in complete DMEM medium containing 5 nM of [3H]-paclitaxel with or without a reversal agent for another 2 h. After that, the cells were rinsed with PBS, trypsinized, harvested, and placed in liquid scintillation cocktail (VWR Chemicals, Solon, OH, USA), and then collected into scintillation vials. To further assess the efflux function mediated by ABCB1, the [3H-paclitaxel efflux assay was conducted based on established protocol (17). In short, cells were prepared in the same way as the accumulation assay. Then, cells were cultured with or without an inhibitor (erdafitinib or verapamil) at indicated concentrations at 37°C. Following 2 h incubation period, cells were cultured in the presence or absence of an inhibitor for serial time frames (0, 30, 60, and 120 min). Subsequently, cells were trypsinized, harvested, and transferred into liquid scintillation solution.
The radioactivity was read using the Packard TRICARB 1900CA liquid scintillation analyzer (Packard Instrument, Downers Grove, IL).
ATPase Assay
The ATPase activity of ABCB1 upon erdafitinib (0–1 μM) treatment was measured by PREDEASY ATPase Kits (TEBU-BIO nv, Boechout, Belgium) according to manufacturer's instructions (29). In short, different concentrations of erdafitinib were incubated with ABCB1 over expressing cell membranes for 3 min, and then 5 mM Mg2+ ATP was added to start the ATPase reaction. After incubation at 37°C for 40 min, luminescence signals of Pi were measured. The changes of relative light units were determined by comparing Na3VO4-treated samples with erdafitinib-treated groups.
Docking Analysis
Molecular docking on erdafitinib with human ABCB1 model was performed as previously described (30). Briefly, human ABCB1 protein model (6QEX) was obtained from RCSB Protein Data Bank. Docking calculation was performed in AutoDock Vina (version 1.1.2) (31). Receptor/ligand preparation and docking simulation were performed with default parameters.
Statistical Analysis
The data were expressed as mean ± SD. Data was analyzed by ANOVA followed by the Dunnett's test. Curve plotting and statistical analysis were achieved using GraphPad Prism 8.00 (La Jolla, CA, USA). P < 0.05 represents statistical significance. All experiments were repeated at least three times.
Results
Erdafitinib Increased the Sensitivity of Chemotherapeutic Drugs in ABCB1-Overexpressing Cells
The cytotoxicity of erdafitinib was determined by MTT assay. The non-cytotoxic concentrations (lower than IC20 values), 0.06, 0.2, and 0.6 μM of erdafitinib were selected and applied for the following experiments (Figures 1B,C).
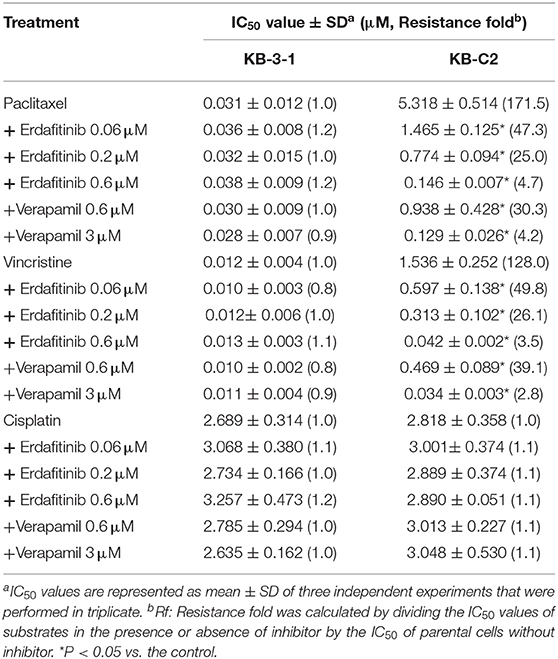
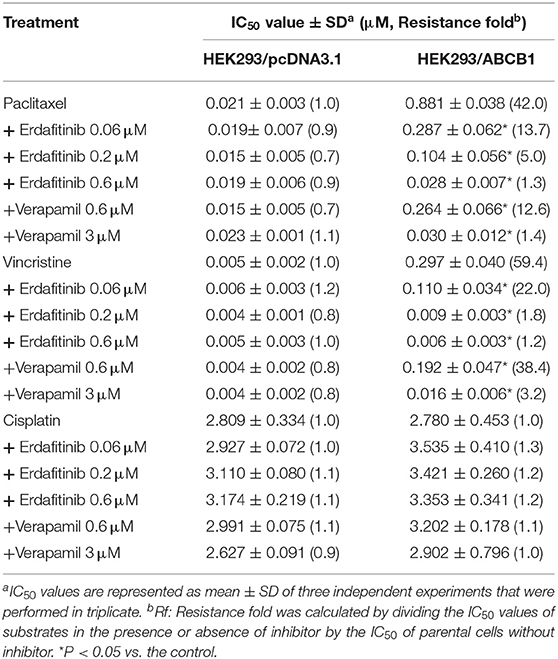
The cytotoxicity of several ABCB1 substrate drugs, including vincristine and paclitaxel, with or without co-treatment with erdafitinib at 0.06, 0.2, and 0.6 μM was tested. As an inhibitor of ABCB1, verapamil was used as a positive control. Cisplatin, which is not a substrate drug of ABCB1, was used as a negative control. As shown in Table 1, ABCB1 overexpressing KB-C2 cells exhibited greater drug resistant fold compared to KB-3-1 cells, by 171.5- and 128.0-fold to paclitaxel and vincristine, respectively. Erdafitinib significantly sensitized the drug resistant cells to paclitaxel and vincristine in a concentration-dependent manner. More importantly, erdafitinib showed stronger sensitizing effect than verapamil at the same concentration. Erdafitinib (0.6 μM) could sensitize KB-C2 cells and the reversal effect was similar to that of verapamil at 3 μM. Meanwhile, the reversal effect of erdafitinib was assessed in ABCB1-transfected cells. As shown in Table 2, erdafitinib showed similar sensitizing effect to ABCB1-transfected HEK293/ABCB1 cells, and 0.6 μM of erdafitinib could completely reverse the drug resistance to paclitaxel and vincristine.
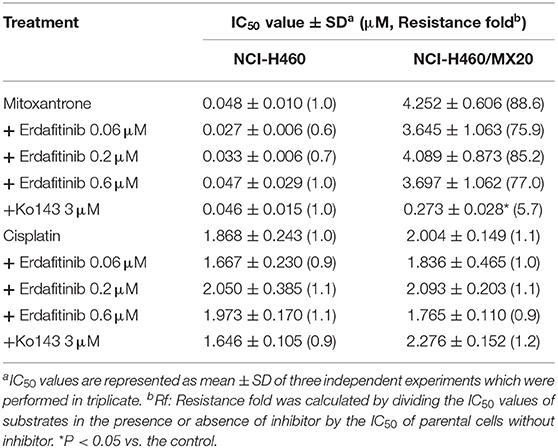
In addition, we also evaluated if erdafitinib could reverse ABCG2-mediated MDR. Mitoxantrone is a substrate of ABCG2 and was used as a positive control. Cisplatin is not a substrate of ABCG2 and was used as a negative control. As shown in Table 3, erdafitinib failed to reverse MDR mediated by ABCG2 in NCI-H460/MX20 cells. This result suggested that erdafitinib specifically reverses MDR mediated by ABCB1.
Erdafitinib Did Not Modify the Expression of ABCB1 or Its Cellular Localization
Previous studies reported that the mechanisms of reversal effect included down-regulating expression or altering cellular localization of ABCB1 and inhibition of the ABCB1 efflux activity. In order to explore the potential mechanisms, Western blot and immunofluorescence were carried out. Western blot analysis showed that at 0.6 μM, erdafitinib did not change the ABCB1 expression after 72 h treatment period (Figure 2A). Also, the immunofluorescence staining showed that at 0.6 μM, erdafitinib did not change the cellular localization of ABCB1 transporter (Figure 2B). These results revealed that erdafitinib modified neither the expression nor the localization of ABCB1.
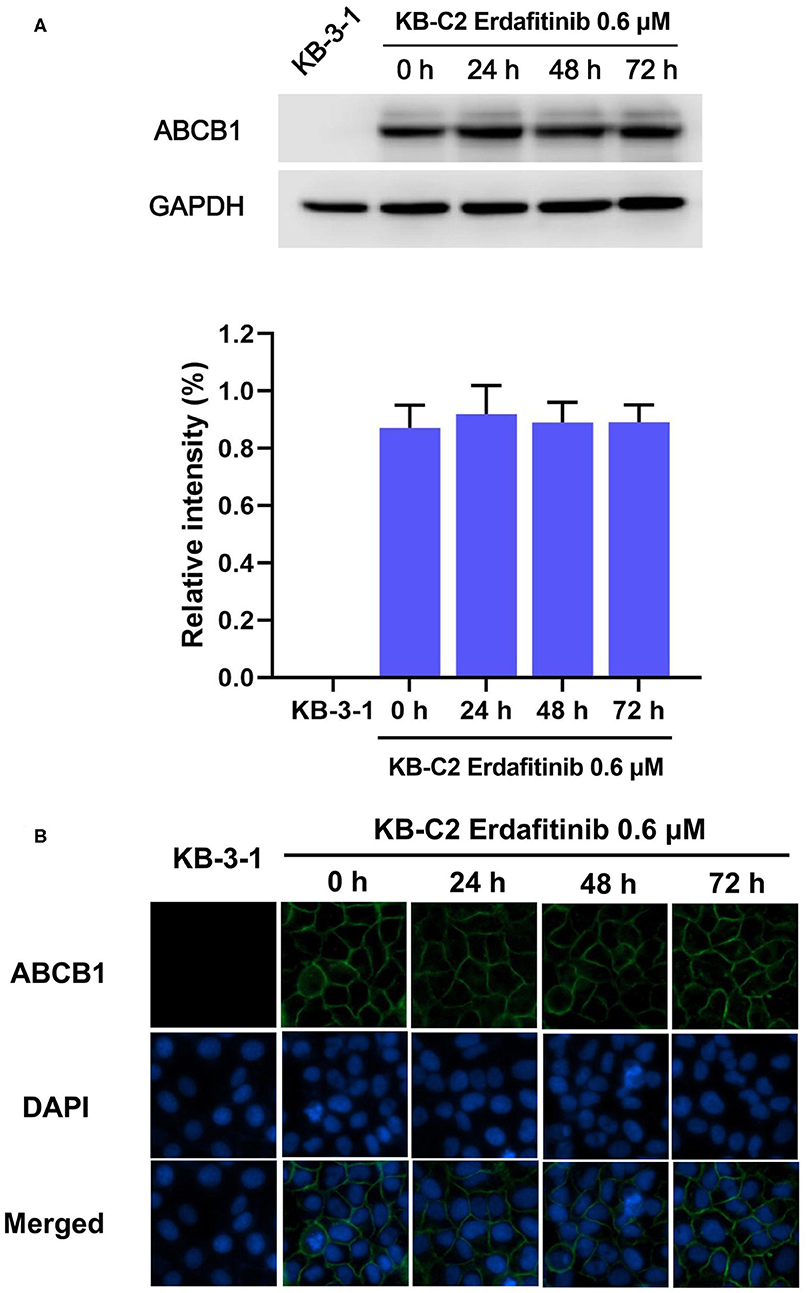
Figure 2. Erdafitinib modified neither the expression nor the cellular localization of ABCB1 in KB-C2 cells. (A) The effect of erdafitinib on ABCB1 expression in KB-C2 cells. (B) The effect of erdafitinib on the cellular localization of ABCB1 in KB-C2 cells. The results are representative of three independent experiments. Error bars indicate SD. Green: ABCB1. Blue: nuclei.
Erdafitinib Increased the Intracellular [3H]-Paclitaxel Accumulation and Inhibited [3H]-Paclitaxel Efflux in Cancer Cells Overexpressing ABCB1
Since the results mentioned above revealed that erdafitinib modified neither the expression nor the cellular localization of ABCB1, we expected that erdatinib might inhibit the function of ABCB1. To evaluate the ABCB1 activity, the intracellular concentration of tritium-labeled paclitaxel was quantified in parental and ABCB1 overexpressing cell lines with or without an inhibitor of ABCB1. By performing [3H]-paclitaxel accumulation assay, we observed that erdafitinib increased the paclitaxel accumulation in drug-resistant KB-C2 cells in a concentration-dependent manner with no change in drug-sensitive KB-3-1 cells (Figure 3A). Therefore, the reversal effect of erdafitinib, at least in part, can be attributed to increased paclitaxel accumulation. Subsequently, [3H]-paclitaxel efflux assay was performed to investigate whether erdafitinib enhances paclitaxel accumulation by increasing paclitaxel uptake and/or inhibiting paclitaxel efflux. As shown in Figures 3B,C, erdafitinib did not affect paclitaxel efflux in drug-sensitive KB-3-1 cells, which is consistent with the result of paclitaxel accumulation (Figure 3A). In drug-resistant KB-C2 cells, erdafitinib treatment significantly hindered paclitaxel efflux. Hence, this result showed that erdafitinib could inhibit the efflux activity of ABCB1 resulted in increasing paclitaxel accumulation.
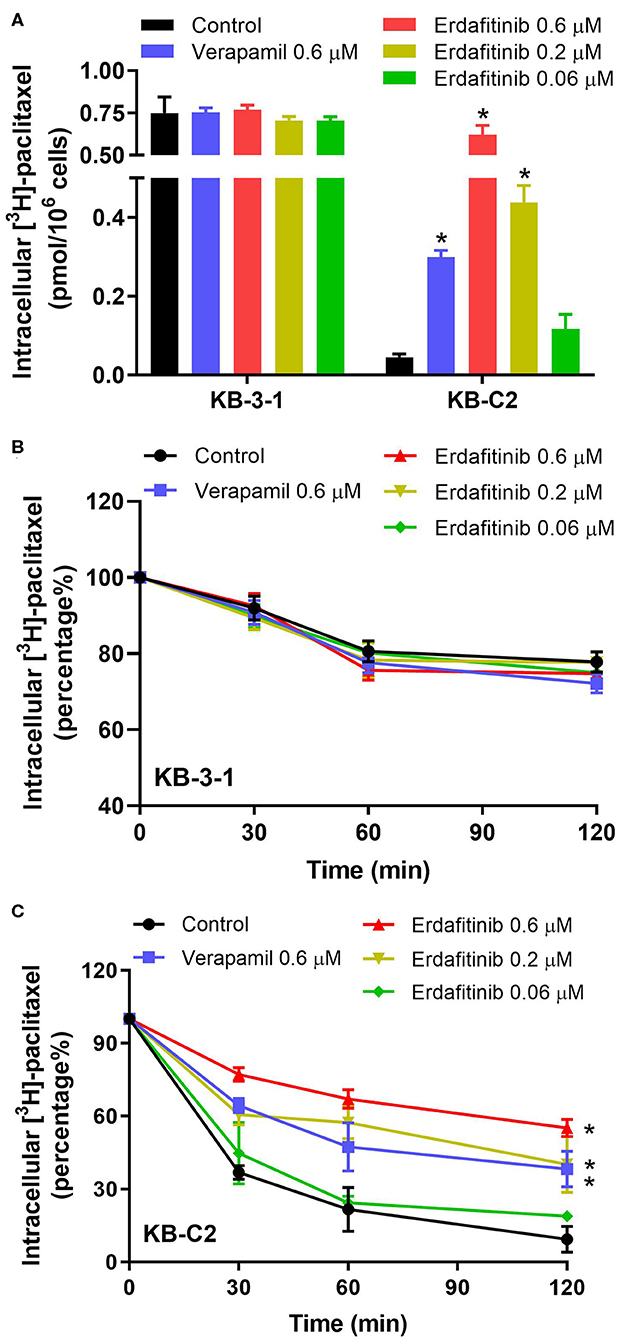
Figure 3. The effect of erdafitinib on the accumulation and efflux of [3H]-paclitaxel in KB-3-1 and KB-C2 cells. (A) The effect of erdafitinib on the accumulation of [3H]-paclitaxel in KB-3-1 and KB-C2 cells. (B) The effect of erdafitinib on the efflux of [3H]-paclitaxel in KB-3-1 cells. (C) The effect of erdafitinib on the efflux of [3H]-paclitaxel in KB-C2 cells. The results are representative of three independent experiments. Error bars indicate SD. *P < 0.05 vs. control. Positive control: the group of verapamil 0.6 μM.
Erdafitinib Activated the ABCB1-Associated ATPase
It is reported that ATP hydrolysis is the energy source of ABC transporter to pump out endogenous and exogenous toxicants (32). Hence, we evaluated whether erdafitinib affects the ATPase activity of ABCB1. The vanadate-sensitive ABCB1-associated ATPase activity at different concentrations of erdafitinib (0–1 μM) was measured. As shown in Figure 4, erdafitinib concentration-dependently activated the ABCB1-associated ATPase to a maximum of 140.8% of the basal activity. The stimulatory effect of erdafitinib reached 50% maximal (EC50) at 0.07 μM.
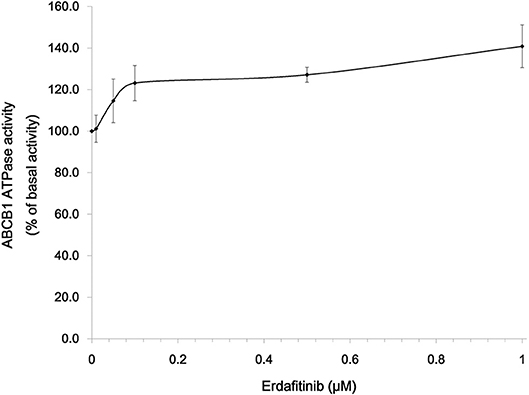
Figure 4. Erdafitinib increased the ATPase of ABCB1. Data are expressed as mean ± SD. The results are representative of three independent experiments. Error bars indicate SD.
Docking Simulation of Erdafitinib in Human ABCB1
The interaction between erdafitinib and the human ABCB1 model was determined by docking simulation. Erdafitinib was docked into the ABCB1 drug-binding sites with a high affinity score of −8.5 kcal/mol. Details of the ligand-receptor interaction was displayed in Figure 5. According to the docked complex, hydrophobic interactions were the major factor in the binding of erdafitinib to ABCB1 protein. Specifically, the pyrazole ring of erdafitinib was stabilized by the phenyl rings of Tyr307 and Phe728 of ABCB1 through π-π stacking interactions. Similarly, the center quinoxaline of erdafitinib was also stabilized by π-π stacking interactions with the phenyl rings of Phe303 and Trp232. Additionally, erdafitinib is positioned in a hydrophobic cavity formed by Trp323, Phe303, Ala302, Phe343, and Ile340.
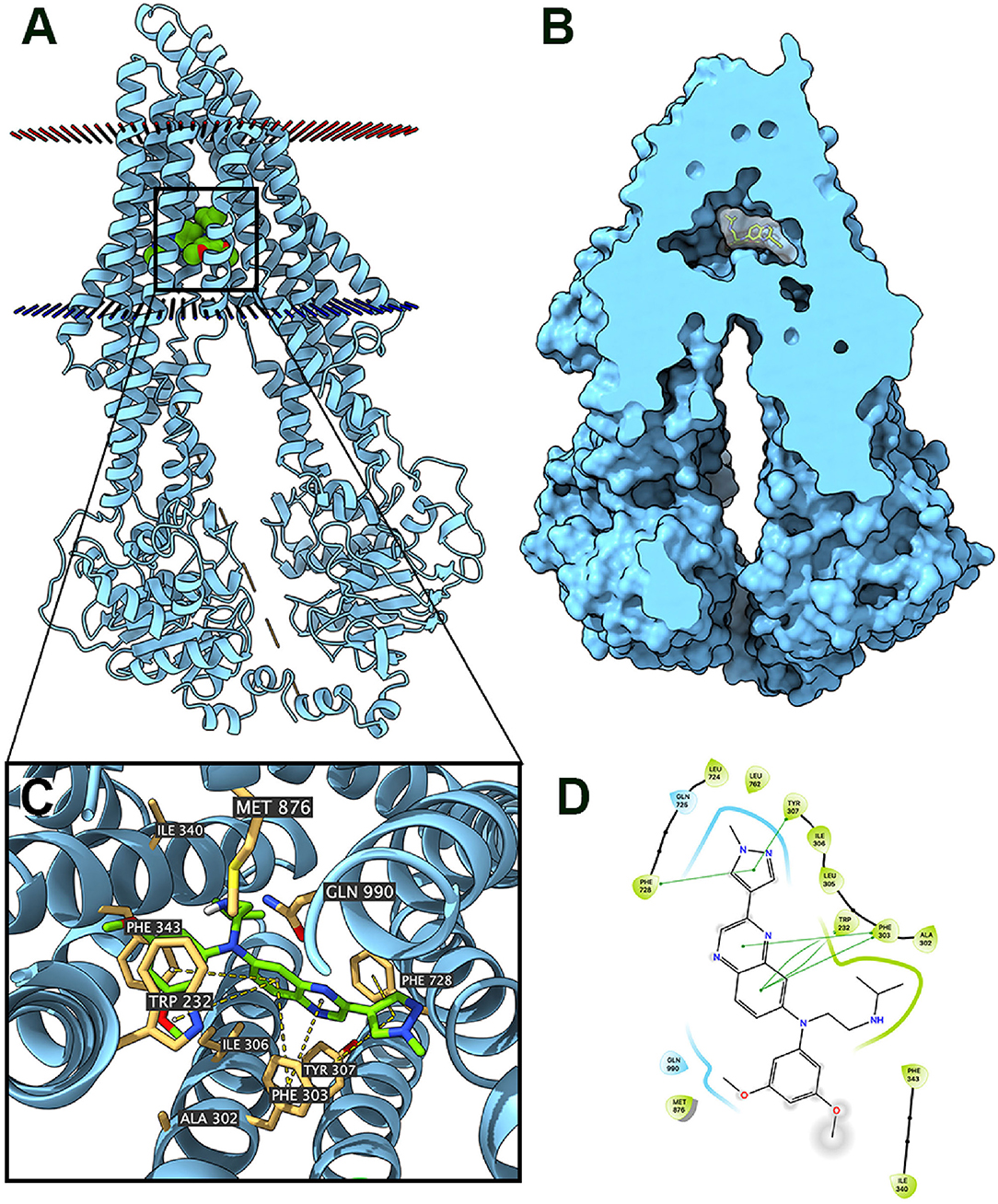
Figure 5. Interaction between erdafitinib and human ABCB1 protein. (A) Overview of the best-scoring pose of erdafitinib in the drug binding pocket of ABCB1 protein. Cytoplasmic membrane was depicted as dotted planes where red or blue plane indicate extracellular or intracellular side, respectively. ABCB1 was displayed as blue ribbons. Erdafitinib was displayed as colored spheres. Green: carbon; red: oxygen; blue: nitrogen. (B) Docked complex displayed with protein surface and ligand surface. Erdafinitib was displayed as colored sticks with white surface. (C) Details of interactions between erdafitinib and ABCB1 binding pocket. ABCB1 was displayed as blue ribbons. Important residues were displayed as colored sticks (wheat yellow: carbon; yellow: sulfur; blue: nitrogen; red: oxygen). Erdafitinib was displayed as colored sticks (lime green: carbon; blue: nitrogen; red: oxygen). π-π stackings were displayed as yellow dash lines. (D) 2D erdafitinib-ABCB1 interaction. Amino acids with 4.0 Å were displayed as color bubbles, cyan indicates polar residues, and green indicates hydrophobic residues. π-π stacking interactions are indicated with green lines.
Discussion
It was known that ABCB1 overexpression could cause MDR, leading to chemotherapy failure (3, 33, 34). It is important to search for agents that could overcome MDR mediated by ABCB1. However, no ABCB1 inhibitor has been approved by FDA owing to severely adverse effect of candidate drugs (35–37). Recently, many studies suggested that combinations of chemotherapeutic drugs and a TKI could reverse ABCB1-mediated MDR (38). Erdafitinib is an FGFR kinase inhibitor for urothelial carcinoma approved by the FDA. In this study, we evaluate whether erdafitinib could reverse ABCB1-mediated MDR.
We found that erdafitinib could significantly reverse MDR mediated by ABCB1. The reversal effect of erdafitinib was stronger than that of verapamil, which is a known ABCB1 inhibitor. It is worth noting that similar results were obtained in both drug selected ABCB1 overexpressing KB-C2 cells and HEK293/ABCB1 cells that were transfected with ABCB1 gene. Therefore, our data suggested that erdafitinib specifically reversed MDR mediated by ABCB1.
There was reports that a few TKIs may conduct reversal effect by down regulation of ABCB1 expression or altering its cellular localization (39). Therefore, Western blot and immunofluorescence for ABCB1 with or without erdafitinib treatment were carried out to test these possibilities. It was found that erdafitinib changed neither the expression nor the localization of ABCB1. It should be noted that cells were treated by 0.6 μM of erdafitinib for 72 h in this study. Thus, further research is needed to test if erdafitinib with higher concentrations or longer incubation time could affect ABCB1 expression or cellular localization.
As the reversal effect of erdafitinib may be attributed to several mechanisms, [3H]-paclitaxel accumulation assay was performed to investigate if erdafitinib could enhance the paclitaxel accumulation level. The result suggested that the intracellular level of paclitaxel increased in drug-resistant KB-C2 cells with no change in parental KB-3-1 cells in the presence of erdafitinib. Since ABCB1 transporter is the major factor that confers resistance to paclitaxel in KB-C2 cells, erdafitinib may interact with ABCB1 to increase accumulation of ABCB1 substrate drug, such as paclitaxel. There are two possibilities by which erdafitinib can enhance ABCB1 substrate accumulation, either increase substrate uptake or decrease substrate efflux. The [3H]-paclitaxel efflux assay was carried out to investigate if erdafitinib is able to inhibit the efflux of paclitaxel. It was found that erdafitinib could inhibit the efflux of paclitaxel in drug-resistant KB-C2 cells. Hence, erdafitinib could increase the intracellular accumulation of substrate drug by hindering the efflux function of ABCB1, thereby reversing MDR. Of note, the effect of erdafitinib was stronger than the known ABCB1 inhibitor verapamil, suggesting erdafitinib is a potent ABCB1 inhibitor.
ABCB1 pumps out xenobiotics by consuming ATP (40, 41). Therefore, ATPase assay of ABCB1 was performed to test if erdafitinib could alter the ATPase activity of ABCB1. Our results showed that erdafitinib concentration-dependently activated the ABCB1-associated ATPase, consisting with previous studies (42, 43). Some ABC transporter substrate drugs can act as competitive inhibitors that bind to a distinct substrate-binding site and inhibit the efflux of a particular class of substrates (44). We found that erdafitinib could stimulate the function of ATPase of ABCB1. It should be noted that, although several ABCB1 inhibitors were identified as stimulators of ATPase of ABCB1, ABCB1 overexpression might not necessarily cause drug resistance to those inhibitors (15, 45). Further study is needed to determine if erdafitinib is an ABCB1 competitive inhibitor. Furthermore, docking analysis was performed. We found that erdafitinib was docked into drug-binding pocket of ABCB1 with a high affinity, thereby inhibiting the binding of other substrate drugs of ABCB1. Our result was in consistent with other reports. For example, several TKIs, including glesatinib, TMP195, ulixertinib, and olmutinib, could bind to the drug-binding sites of ABC transporters, including ABCB1 and ABCG2, to reverse MDR (13, 15, 45, 46).
By performing the mechanistic studies, we found that erdafitinib can inhibit ABCB1 substrate efflux without affecting the ABCB1 transporter expression level or subcellular localization. At the same time, erdafitinib stimulated the ABCB1 ATPase activity, suggesting it can interact with the substrate-binding site of ABCB1 transporter. If erdafitinib binds to the substrate-binding site, it may act as a substrate to compete with other substrates for efflux pump. However, the cytotoxicity of erdafitinib showed no significant difference in parental and ABCB1-overexpressing cells, suggesting it may not be a good substrate. Our recent work showed that an inhibitor may cause a conformational change at the binding pocket and hinder the binding of other substrates (47). We suppose erdafitinib may act at the same way, stimulating a conformational change at the substrate-binding site to inhibit substrate efflux and reverse MDR. Future studies may focus on exploring the possibility of this mechanism of action.
In conclusion, our study demonstrated that as an anti-cancer drug approved by FDA, erdafitinib could antagonize ABCB1-mediated MDR. Combination of erdafitinib and a substrate of ABCB1 could improve the therapeutic effect for drug resistant cancers which overexpress ABCB1 transporter. This study result warrants evaluation in pre-clinical and clinical studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
WF and D-HY designed the experiments. WF, MZ, Z-XW, J-QW, X-DD, and Q-XT performed the experiments. YY, X-YC, and QC analyzed the data. WF and MZ drafted the paper. D-HY edited the article. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Medical and Health Development Plan of Shandong Province (2017WS058), Research and Development Plan of University in Shandong Province (J18KA120), and Shandong Province Government-Sponsored Overseas Study Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Susan Bates and Robert Robey (NCI, NIH, Bethesda, MD) for kindly providing us the cell lines such as NCI-H460 and NCI-H460/MX20. We thank Dr. Shin-Inchi Akiyama (Kagoshima University, Kagoshima, Japan) for the gift of KB-3-1 and KB-C2 cell lines. We thank ChemieTek (Indianapolis, IN) for the free sample of erdafitinib used for this study. We thank the partial support from Department of Pharmaceutical Sciences, St. John's University (Queens, NY).
References
1. Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. (2006) 5:219–34. doi: 10.1038/nrd1984
2. Kartal-Yandim M, Adan-Gokbulut A, Baran Y. Molecular mechanisms of drug resistance and its reversal in cancer. Critic Rev Biotechnol. (2016) 36:716–26. doi: 10.3109/07388551.2015.1015957
3. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. (2018) 18:452–64. doi: 10.1038/s41568-018-0005-8
4. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. (2014) 347:159–66. doi: 10.1016/j.canlet.2014.03.013
5. Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. (2015) 6:157. doi: 10.3389/fgene.2015.00157
6. Xue M, Cheng J, Zhao J, Zhang S, Jian J, Qiao Y, et al. Outcomes of 219 chronic myeloid leukaemia patients with additional chromosomal abnormalities and/or tyrosine kinase domain mutations. Int J Lab Hematol. (2019) 41:94–101. doi: 10.1111/ijlh.12928
7. Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. (2009) 109:2989–3011. doi: 10.1021/cr9000226
8. Beretta GL, Cassinelli G, Pennati M, Zuco V, Gatti L. Overcoming ABC transporter-mediated multidrug resistance: the dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem. (2017) 142:271–89. doi: 10.1016/j.ejmech.2017.07.062
9. Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, et al. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updates. (2016) 27:14–29. doi: 10.1016/j.drup.2016.05.001
10. De Vera AA, Gupta P, Lei Z, Liao D, Narayanan S, Teng Q, et al. Immuno-oncology agent IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1, ABCB1)-mediated multidrug resistance (MDR) in cancer: in vitro and in vivo. Cancer Lett. (2019) 442:91–103. doi: 10.1016/j.canlet.2018.10.020
11. Liao D, Zhang W, Gupta P, Lei Z-N, Wang J-Q, Cai C-Y, et al. Tetrandrine interaction with ABCB1 reverses multidrug resistance in cancer cells through competition with anti-cancer drugs followed by downregulation of ABCB1 expression. Molecules. (2019) 24:4383. doi: 10.3390/molecules24234383
12. Wu C-P, Ambudkar SV. The pharmacological impact of ATP-binding cassette drug transporters on vemurafenib-based therapy. Acta Pharmaceutica Sinica B. (2014) 4:105–11. doi: 10.1016/j.apsb.2013.12.001
13. Ji N, Yang Y, Lei Z-N, Cai C-Y, Wang J-Q, Gupta P, et al. Ulixertinib (BVD-523) antagonizes ABCB1-and ABCG2-mediated chemotherapeutic drug resistance. Biochem Pharmacol. (2018) 158:274–85. doi: 10.1016/j.bcp.2018.10.028
14. Zhang G-N, Zhang Y-K, Wang Y-J, Gupta P, Ashby CR Jr, Alqahtani S, et al. Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: in vitro and in vivo. Cancer Lett. (2018) 424:19–29. doi: 10.1016/j.canlet.2018.02.040
15. Cui Q, Cai C-Y, Gao H-L, Ren L, Ji N, Gupta P, et al. Glesatinib, a c-MET/SMO dual inhibitor, antagonizes P-glycoprotein mediated multidrug resistance in cancer cells. Front Oncol. (2019) 9:313. doi: 10.3389/fonc.2019.00313
16. Gupta P, Gao H-L, Ashar YV, Karadkhelkar NM, Yoganathan S, Chen Z-S. Ciprofloxacin enhances the chemosensitivity of cancer cells to ABCB1 substrates. Int J Mol Sci. (2019) 20:268. doi: 10.3390/ijms20020268
17. Wu Z-X, Teng Q-X, Cai C-Y, Wang J-Q, Lei Z-N, Yang Y, et al. Tepotinib reverses ABCB1-mediated multidrug resistance in cancer cells. Biochem Pharmacol. (2019) 166:120–7. doi: 10.1016/j.bcp.2019.05.015
18. Cai C-Y, Zhang W, Wang J-Q, Lei Z-N, Zhang Y-K, Wang Y-J, et al. Biological evaluation of non-basic chalcone CYB-2 as a dual ABCG2/ABCB1 inhibitor. Biochem Pharmacol. (2020) 175:113848. doi: 10.1016/j.bcp.2020.113848
19. Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer control. (2003) 10:159–65. doi: 10.1177/107327480301000207
20. Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. (2003) 22:7340–58. doi: 10.1038/sj.onc.1206938
21. Narayanan S, Cai C-Y, Assaraf YG, Guo H-Q, Cui Q, Wei L, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resistance Updates. (2020) 48:100663. doi: 10.1016/j.drup.2019.100663
22. Eadie L, Hughes T, White D. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Therap. (2014) 95:294–306. doi: 10.1038/clpt.2013.208
23. Wu S, Fu L. Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells. Mol Cancer. (2018) 17:25. doi: 10.1186/s12943-018-0775-3
24. Perera TP, Jovcheva E, Mevellec L, Vialard J, De Lange D, Verhulst T, et al. Discovery and pharmacological characterization of JNJ-42756493 (Erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol Cancer Therap. (2017) 16:1010–20. doi: 10.1158/1535-7163.MCT-16-0589
25. Akiyama S-I, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somatic Cell Mol Genet. (1985) 11:117–26. doi: 10.1007/BF01534700
26. Robey RW., Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim Biophys Acta. (2001). 1512:171–82. doi: 10.1016/S0005-2736(01)00308-X
27. Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. (2014) 74:598–608. doi: 10.1158/0008-5472.CAN-13-2064
28. Zhang Q, Wang G, Xie Y, Gao Z, Liang Z, Pan Z, et al. Mechanical changes and microfilament reorganization involved in microcystin-LR-promoted cell invasion in DU145 and WPMY cells. Front Pharmacol. (2020) 11:89. doi: 10.3389/fphar.2020.00089
29. Wang J-Q, Wang B, Lei Z-N, Teng Q-X, Li JY, Zhang W, et al. Derivative of 5-cyano-6-phenylpyrimidin antagonizes ABCB1-and ABCG2-mediated multidrug resistance. Eur J Pharmacol. (2019) 863:172611. doi: 10.1016/j.ejphar.2019.172611
30. Wang J-Q, Li JY, Teng Q-X, Lei Z-N, Ji N, Cui Q, et al. Venetoclax, a BCL-2 inhibitor, enhances the efficacy of chemotherapeutic agents in wild-type ABCG2-Overexpression-mediated MDR cancer cells. Cancers. (2020) 12:466. doi: 10.3390/cancers12020466
31. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. (2010) 31:455–61. doi: 10.1002/jcc.21334
32. Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr Drug Targets. (2006) 7:893–909. doi: 10.2174/138945006777709520
33. Litviakov NV, Cherdyntseva NV, Tsyganov MM, Denisov EV, Garbukov EY, Merzliakova MK, et al. Changing the expression vector of multidrug resistance genes is related to neoadjuvant chemotherapy response. Cancer Chemotherapy Pharmacol. (2013) 71:153–63. doi: 10.1007/s00280-012-1992-x
34. Bartholomae S, Gruhn B, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D, et al. Coexpression of multiple ABC-transporters is strongly associated with treatment response in childhood acute myeloid leukemia. Pediatr Blood Cancer. (2016) 63:242–7. doi: 10.1002/pbc.25785
35. Chung FS, Santiago JS, De Jesus MFM, Trinidad CV, See MFE. Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter? Am J Cancer Res. (2016) 6:1583–98.
36. Toyoda Y, Takada T, Suzuki H. Inhibitors of human ABCG2: from technical background to recent updates with clinical implications. Front Pharmacol. (2019) 10:208. doi: 10.3389/fphar.2019.00208
37. Jinyun D, Zuodong Q, Wei-Dong Z, Gang C, Yehuda GA, Charles Jr RA, et al. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: an update. Drug Resistance Updates. (2020) 2020:100681. doi: 10.1016/j.drup.2020.100681
38. Ji N, Yang Y, Cai C-Y, Lei Z-N, Wang J-Q, Gupta P, et al. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1-and ABCG2-overexpressing cancer cells. Cancer Lett. (2019) 440:82–93. doi: 10.1016/j.canlet.2018.10.007
39. Zhang L, Li Y, Wang Q, Chen Z, Li X, Wu Z, et al. The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer. Mol Cancer. (2020) 19:1–18. doi: 10.1186/s12943-019-1112-1
40. Linton KJ. Structure and function of ABC transporters. Physiology. (2007) 22:122–30. doi: 10.1152/physiol.00046.2006
41. Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Eur J Physiol. (2007) 453:555–67. doi: 10.1007/s00424-006-0126-x
42. Hsiao S-H, Lu Y-J, Li Y-Q, Huang Y-H, Hsieh C-H, Wu C-P. Osimertinib (AZD9291) attenuates the function of multidrug resistance-linked ATP-binding cassette transporter ABCB1 in vitro. Mol Pharm. (2016) 13:2117–25. doi: 10.1021/acs.molpharmaceut.6b00249
43. Wu C-P, Lusvarghi S, Wang J-C, Hsiao S-H, Huang Y-H, Hung T-H, et al. Avapritinib: a selective inhibitor of KIT and PDGFRα that reverses ABCB1 and ABCG2-mediated multidrug resistance in cancer cell lines. Mol Pharma. (2019) 16:3040–52. doi: 10.1021/acs.molpharmaceut.9b00274
44. Giri N, Agarwal S, Shaik N, Pan G, Chen Y, Elmquist WF. Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in in vitro assay design. Drug Metab Disposit. (2009) 37:560–70. doi: 10.1124/dmd.108.022046
45. Wu C-P, Lusvarghi S, Wang J-C, Hsiao S-H, Huang Y-H, Hung T-H, et al. The selective class iia histone deacetylase inhibitor TMP195 resensitizes ABCB1-and ABCG2-overexpressing multidrug-resistant cancer cells to cytotoxic anticancer drugs. Int J Mol Sci. (2020) 21:238. doi: 10.3390/ijms21010238
46. Zhang W, Fan Y-F, Cai C-Y, Wang J-Q, Teng Q-X, Lei Z-N, et al. Olmutinib (BI1482694/HM61713), a novel epidermal growth factor receptor tyrosine kinase inhibitor, reverses ABCG2-mediated multidrug resistance in cancer cells. Front Pharmacol. (2018) 9:1097. doi: 10.3389/fphar.2018.01097
Keywords: erdafitinib, ABCB1, multidrug resistance, reversal effect, cancer
Citation: Feng W, Zhang M, Wu Z-X, Wang J-Q, Dong X-D, Yang Y, Teng Q-X, Chen X-Y, Cui Q and Yang D-H (2020) Erdafitinib Antagonizes ABCB1-Mediated Multidrug Resistance in Cancer Cells. Front. Oncol. 10:955. doi: 10.3389/fonc.2020.00955
Received: 22 April 2020; Accepted: 15 May 2020;
Published: 25 June 2020.
Edited by:
Jiang-Jiang Qin, Zhejiang Chinese Medical University, ChinaReviewed by:
Junjiang Chen, Hong Kong Polytechnic University, Hong KongJun Lin, Stony Brook Medicine, United States
Copyright © 2020 Feng, Zhang, Wu, Wang, Dong, Yang, Teng, Chen, Cui and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Hua Yang, eWFuZ2QxQHN0am9obnMuZWR1
†These authors have contributed equally to this work
 Weiguo Feng
Weiguo Feng Meng Zhang
Meng Zhang Zhuo-Xun Wu
Zhuo-Xun Wu Jing-Quan Wang2
Jing-Quan Wang2 Qingbin Cui
Qingbin Cui Dong-Hua Yang
Dong-Hua Yang