- 1Department of Hematology and Medical Oncology, Faculty of Medicine, Saint-Joseph University, Beirut, Lebanon
- 2Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Radiology Department, Columbia University Medical Center, New York Presbyterian Hospital, New York, NY, United States
- 4Department of Hematology, CHU de Rennes, Université de Rennes, Rennes, France
- 5Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
- 6Radiology Department, Gustave Roussy Cancer Campus, Villejuif, France
- 7BIOMAPS, UMR1281, INSERM.CEA.CNRS, Université Paris-Saclay, Paris, France
COVID-19 has been declared a pandemic by the world health organization. Patients with cancer, and particularly hematologic malignancies may be at higher risk for severe complications due to their malignancy, immune dysregulation, therapy, and associated comorbidities. The oncology community has been proactive in issuing practice guidelines to help optimize management, and limit infection risk and complications from SARS-COV-2. Although hematologic malignancies account for only 10% of all cancers, their management is particularly complex, especially in the time of COVID-19. Screening or early detection of COVID-19 are central for preventative/mitigation strategy, which is the best current strategy in our battle against COVID-19. Herein, we provide an overview of COVID-19 screening strategies and highlight the unique aspects of treating patients with hematologic malignancies.
Introduction
In December 2019, infection with a novel betacoronavirus, subsequently named SARS-CoV-2, has been reported in a cluster of pneumonia cases in the Wuhan region of China (1). The pathogen showed a rapid spread that led to a global pandemic and a public health emergency of international concern due to the burden on the healthcare system and lack of specific treatments. By April 26th 2020, the COVID-19 outbreak has affected more than 2.8 million people and claimed the lives of more than 190,000 patients around the globe (2). Patients with cancer are regarded as a more vulnerable population because of their immunosuppressive state induced by the malignancy, therapy, and associated comorbidities (3, 4). As a result, despite the limited available data, the oncology community has been pro-actively engaged in issuing guidelines to help clinical practice with the goal of decreasing exposure and complication risks from COVID-19 among patients with cancer. For instance, recommendations have emerged to consider on a case-by-case basis, whenever possible, options of delaying/dose reducing treatment, using lower-intensity therapy, postponing unnecessary radiological evaluations, and prioritizing telemedicine (5–9).
Hematologic malignancies encompass a wide range of neoplasms, including leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome and myeloproliferative neoplasms, which all account for 10% of all malignancies (10). Although some patients present with indolent diseases that may not require immediate therapy, many patients harbor aggressive diseases, often life-threatening, which necessitate rapid intervention with intensive chemotherapy, high dose radiation and/or hematopoietic stem cell transplant (HSCT). Prior studies have shown that patients with hematologic malignancies are at higher risk for severe lower respiratory tract infections, notably due to lymphopenia, neutropenia, hypogammaglobinemia, steroid administration, and graft-versus-host disease, it is unclear how this may be applicable to COVID-19 (11–13).
The triage of patients according to their COVID-19 status (confirmed, high- or low level of suspicion) has been the key measure in limiting in-hospital contaminations (14). The absence of a rapid screening system for assessing COVID-19 status in patients is problematic, and the current recommendations are only based on anecdotal and theoretical evidence extrapolated from the management of other infectious diseases. Herein, we provide an overview of COVID-19 screening strategies and highlight the complex aspects of caring for patients with hematologic malignancies.
Risk Factors for COVID-19 In Patients With Hematologic Malignancies
Literature on COVID-19 in hematologic malignancies is sparse and mainly derived from case series that are focused on patients with solid tumors. Two cases series from China showed a higher incidence (1 vs. 0.3%), and a higher case-fatality rate (5 vs. 1%) in patients with cancer compared with the overall population (4, 15). However, the relative contribution of cancer or cancer therapy to their infectious risk of COVID-19 is uncertain given that the majority of patients either had a remote history of cancer, were not on active anticancer therapy, and/or had multiple comorbidities. Notably, none of the patients had a hematologic malignancy (4, 15).
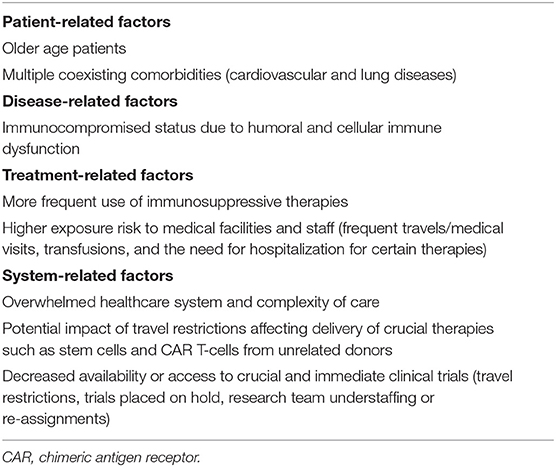
Patients with hematologic malignancies might be at higher risk of contracting and experiencing complications from COVID-19, such as hospitalization, intensive care unit (ICU)/invasive ventilation, sepsis, cytokine dysregulation, multiorgan failure and/or death (16). Reasons for this higher risk are multifactorial (Table 1). First, hematologic malignancies are directly tied to an immunocompromised status due to humoral and cellular immune dysfunction (17–20). Second, a large number of therapies employed for hematologic malignancies are highly immunosuppressive, even more than standard chemotherapies used for solid tumors. Examples include myelosuppressive chemotherapy for acute leukemia, conditioning regimens for hematopoietic stem cell transplant (HSCT), prolonged use of steroids, lymphodepleting agents used for chimeric antigen receptor (CAR) T-cell therapy, and radiation therapy. Third, a high proportion of patients with active hematologic malignancies, especially when on active therapy, are constantly exposed to medical facilities and healthcare staff, which puts them at higher exposure risk. This includes frequent travels and visits for outpatient infusional therapies, laboratory checks or transfusions, longer infusions of certain therapies (anti-CD20 antibodies in lymphoma, daratumumab in multiple myeloma), and the need for hospitalization for other therapies (e.g., induction chemotherapy for acute leukemia). Fourth, many hematologic malignancies occur in older patients (median ages in multiple myeloma, CLL, acute myeloid leukemia, follicular lymphoma, and diffuse large B-cell lymphoma are 72, 70, 68, 65, and 64 years, respectively) with multiple coexisting comorbidities (cardiovascular and lung diseases), which are among the highest risk factors for COVID-19-related morbidity and mortality in large Chinese cohorts (4, 15, 21). Fifth, clinical care for patients with hematologic malignancies requires more medical resources (equipment and staff) than for other patients due to the high risk nature of their diseases such as the need for frequent and close monitoring, transfusion burden, high rate of elective and urgent hospital admissions, and crucial need for clinical trial enrollment. Therefore, as clusters of outbreaks can overwhelm the healthcare system capacity, the detrimental effect may be even more pronounced on patients with hematologic malignancies. Sixth and last, many patients with hematologic malignancies may be directly harmed by travel restrictions affecting delivery of crucial therapies such as stem cells and CAR products from unrelated donors (22, 23). On the other hand, a large proportion of patients with hematologic malignancies, especially acute leukemia or transplant candidates/recipients have been adopting social distancing and preventative precautious measures regardless of the COVID-19 crisis, which may have reduced their exposure risk of COVID-19. Moreover, some targeted therapies commonly used in hematologic malignancies, particularly JAK-STAT pathway inhibitors and bruton tyrosine kinase inhibitors (e.g., ibrutinib), have been postulated to have a protective effect in decreasing virus infectivity (24) and abrogating cytokine-mediated lung injury, respectively (25). Clinical trials are ongoing to further investigate the role of such therapies in patients with COVID-19 (NCT04375397, NCT04320277).
COVID-19 in Patients With Hematologic Malignancies
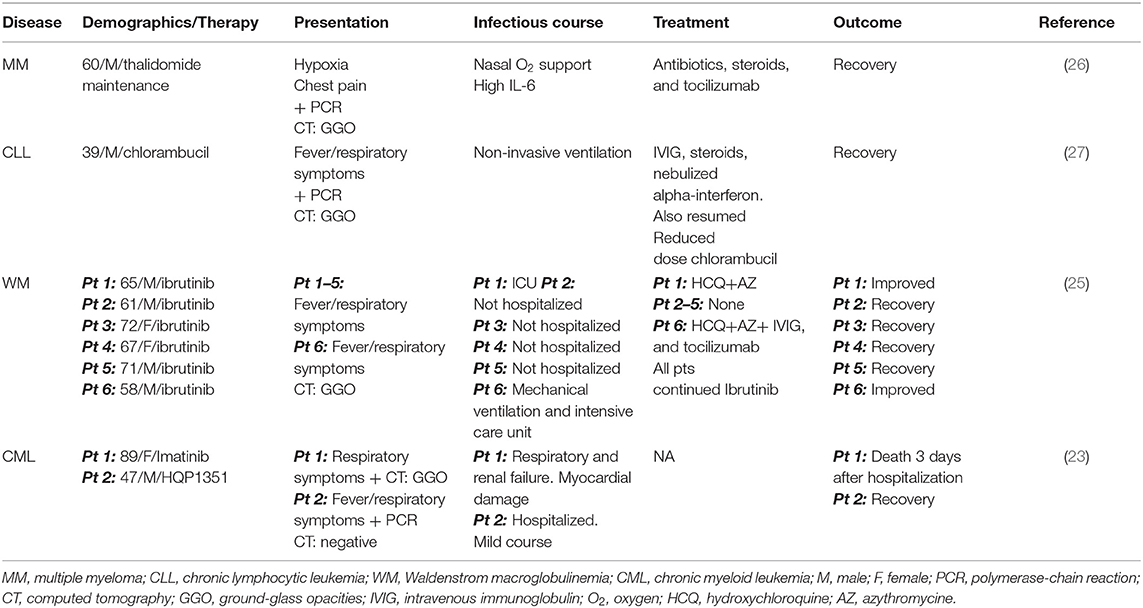
To our knowledge, at the time of writing this review, only 10 cases of COVID-19 have been published to date in the English literature in patients with hematologic malignancies. Details are summarized in Table 2 (23, 25–27). Data on screening for COVID-19 in hematologic malignancies are limited. A Chinese cross-sectional survey aimed to evaluate the incidence and outcome of COVID-19 among patients with chronic myeloid leukemia (CML) conducted over 1 week in February 2020 (23). Among 392 patients who took the survey, 12 patients were suspected to have COVID-19 infection based on their clinical presentation, but only 2 cases were confirmed using PCR and CT for an incidence of 0.6%. When the authors classified patients according to CML response milestones (according to the 2020 European Leukemia Net guidelines), the incidence of COVID-19 was 0.3% (1 of 299) among patients with optimal response as opposed to 2% (1 of 50) among patients with poor response raising the possibility that good disease control may be related to a lower incidence of COVID-19 (23). These findings must be interpreted with caution given that the relative contribution of CML diagnosis and therapy (imatinib) as a risk-factor may be questionable. The patient with “suboptimal response” to CML therapy was 89 years old with cardiac history, in hematologic remission and only residual molecular disease on Imatinib and succumbed from COVID-19 due to multiorgan failure including myocardial damage. In addition, tyrosine kinase inhibitors (e.g., imatinib) are not myelosuppressive and not known to increase infectious risks especially in the setting of hematologic and cytogenetic remission such as this case.
The American Society of Hematology Research Collaborative has recently launched a COVID-19 international registry for patients with hematologic malignancies (28). By April 23rd, 2020, there were 64 patients reported to have COVID-19. Underlying malignancies were as follows: acute leukemia (31%), non-Hodgkin lymphoma (22%), chronic lymphocytic leukemias (16%), myeloproliferative neoplasms (16), plasma cell neoplasms (11%), and Hodgkin's lymphoma (6%). Sixty percent of these patients were undergoing active anticancer therapy at the time of COVID-19; among whom 60% were undergoing induction therapy, and 20% were on maintenance/consolidation therapy.
An age-matched comparison of patients with cancer (n = 105) and without cancer (n = 536) was presented at the American Association for Cancer Research 2020 virtual meeting (29). Multivariable analysis showed that cancer diagnosis was associated with higher rates of death and ICU (intensive care unit) admissions from COVID-19. Patients with hematologic malignancies had the highest severity of symptoms (6 out 9 patients [66.67%]) high risk of ICU admission or use of invasive mechanical ventilation [4 and 2 out 9 patients, respectively [44.4 and 22.2%] and death rate (3 out 9 patients [33%]). Although authors reported that 4 out of the 9 patients with hematologic malignancies had severe immunosuppression, no information was provided on disease status or treatment details. Therefore, the small sample number and lack of detailed clinical data on patients with hematologic malignancies limit generalizability of this observation.
Screening Strategies
The COVID-19 pandemic has added another major burden to cancer care of all specialty's units, especially hematology and HSCT units. Due to limited testing and screening, little information is known about the incidence of asymptomatic carriers, who maybe as contagious as symptomatic patients (30–32). Efforts have been made to triage COVID-19 patients to separate units in order to avoid inpatient cross-contamination to other patients, thus, highlighting the importance of screening strategies. Additional testing strategies have been rapidly developed to better identify patients with COVID-19. Since SARS-CoV-2 is an RNA-virus, reverse transcriptase-polymerase chain reaction (RT-PCR) is the obvious diagnostic test to confirm virus shedding (33). However, the sub-optimal sensitivity has limited its reliability in clinical practice. The test can be obtained from nasopharyngeal swabs or sputum samples, and results are generally reported within 4–48 h (34). Nasopharyngeal swabs may lack sensitivity after the first week of COVID-19 infection due to the natural history of the disease. The virus was shown to transiently infect the upper respiratory system until it spreads more toward the lungs. This is extrapolated from data showing a higher rate of RT-PCR positivity >90% on days 1–3 of illness, vs. <80% at day 6, and vs. <50% after day 14 (35). False negatives can also be attributed to the poor quality of the specimen and type of specimen obtained (36). Another appealing screening strategy is serologic testing of SARS-CoV-2 antibodies to identify persons that are potentially “immune” and thus at minimal risk of contagion or complications, both at individual and public health levels (37). This also has important therapeutic implications by identifying potential candidates to donate blood for the preparation of convalescent plasma, an investigational product in the management of severely ill COVID-19 patients (38).
Due to limitations associated with PCR testing, including limited testing capacity, high false-negative rates, and delays in having the results, chest imaging has emerged as a potent diagnostic tool for COVID-19. Chest radiographs have a low sensitivity in early or mild disease and therefore, are not ideal screening tools. In one study, twenty percent of patients had normal chest radiographs at any point during the course of their illness (39). In contrast, chest computed tomography (CT) scan is more sensitive, especially in the presence of typical findings, such as bilateral, peripheral patchy ground-glass opacities, predominantly in the lower lobes (33, 40, 41). Although the American College of Radiology recommends that chest CT scan be reserved for symptomatic patients with suspected COVID-19-related complications and discourages its use for screening purposes, these guidelines may not be applicable for patients with cancer, especially hematologic malignancies undergoing immunosuppressive therapies (42). Since many radiological findings may be seen with other etiologies such as opportunistic infections or drug-induced pneumonitis, the role of radiologists is primordial in confirming diagnosis of COVID-19 (43). Vascular thickening, and peripheral distribution seem to be more characteristic of COVID-19 than other viral infections (44). The concordance between PCR and CT scan has been addressed in a Chinese cohort from the Wuhan region of 1,014 patients with suspected COVID-19. Ninety-seven percent of patients had positive CT scans. However, important observations were notable. Initially, CT scan was more sensitive in detecting early infections (88%, compared with 59% with PCR). Moreover, CT scan was better in assessing recovery; 42% had signs of radiological improvement before RT-PCR turned negative (33). Therefore, CT scan may have a higher yield than PCR for diagnosis and monitoring. Similar findings were shown by Fang et al. (40) (chest CT sensitivity of 98 vs. 71% with PCR; p < 0.001). One meta-analysis showed a positive predictive value reaching 90.4% for chest CT scan. Taken together, these findings support the superiority of CT over PCR for detection and screening of COVID-19 especially in endemic areas and for higher-risk patients such as those with hematologic malignancies. Nonetheless, many challenges remain before implementation to screen for asymptomatic carriers (Table 3) (45). Artificial intelligence (AI) techniques may increase the sensitivity and specificity of imaging tools to screen for COVID-19 infections (46). Deep learning techniques have been successfully used to differentiate bacterial and viral infections with specific lung injuries, suggesting that it could be a promising tool for the diagnosis and screening of COVID-19 (47, 48). AI can improve image acquisition through automation of the scanning procedure and reshaping of the workflow, which would limit the human-to-human contact and avoid virus transmission. These techniques can also increase the accuracy of the COVID-19 diagnosis through the delineation of suspected infection on chest CT scan and radiography with different segmentation methods (49). Deep learning tools for the detection of COVID-19 infections are currently being developed with promising results such as the COVID-19 detection neural network (COVNet) (50).
Published Guidelines for COVID-19 Screening
Despite the paucity of current available data, several medical societies have provided clinical practice guidelines for management of patients with hematologic malignancies in order to optimize therapy and limit infection risk and complications (9, 51). Consensus recommendations have been based on extrapolated information from other coronavirus epidemics and expert opinions based on educated assumptions. Social distancing, quarantine measures, clear guidance on the importance of hand hygiene, and avoiding high-risk exposures are recommended to halt the virus transmission (52). Preventative strategies also include screening for COVID-19 symptoms or fever at the hospital entrance for all patients, caregiver/visitors, and healthcare workers (53). Serial RT-PCR screening of healthcare workers, once every week or every 2 weeks can be another proposed measure for infection control (54). Initial evaluation of patients with hematologic malignancies or undergoing hematopoietic cell transplantation should at least include clinical history and examination, and laboratory and radiological evaluation to document active respiratory viral infections (55, 56). In patients with acute myeloid leukemia, up to 50–75% of patients present with fever and thus are at risk of delayed or missed diagnosis (57, 58). The European Hematology Association recommends testing for COVID-19 with RT-PCR in all newly diagnosed patients with acute and chronic leukemia undergoing intensive chemotherapies and before every cycle of therapy. No recommendations regarding the screening role of chest CT scan have been issued (5). According to the American Society of Hematology guidelines, screening of patients with chronic lymphocytic leukemias presenting with mild symptoms depends on the availability of testing and the need to isolate those who test positive (59). Regarding multiple myeloma, it is generally recommended to screen all newly diagnosed patients for COVID-19 in the inpatient and outpatient settings (60, 61). Screening is widely used and strongly recommended before autologous and allogeneic HSCT (6, 62).
According to the European Society for Blood and Marrow Transplantation guidelines, COVID-19 should be ruled-out in all patients, including asymptomatic ones, before undergoing conditioning chemotherapy (62). Additionally, patients planned to receive CAR-T cell therapy or HSCT should abide by home isolation for at least 2 weeks before lymphodepleting or conditioning chemotherapy. In case of close exposure to a patient with confirmed COVID-19, any elective therapy should be deferred for at least 2–3 weeks before undergoing a new RT-PCR screening to confirm negativity (62, 63). As for donors, COVID-19 positive patients should be excluded while those in contact should be isolated for 4 weeks unless the patient is asymptomatic and the procedure is urgent, then it can be considered after negative testing.
Screening measures in the pre-CAR T cell phase should include screening for symptoms for all patients before apheresis and CAR T cell infusion. RT-PCR testing is considered within 48–72 h before apheresis and recommended within 48–72 h before lymphodepleting chemotherapy and 7 days within CAR T cell infusion. A repeat evaluation of RT-PCR within 72 h of CAR T cell infusion to detect interim COVID-19 infection and perform serologic testing for COVID-19 seroconversion once available (22, 64). That being said, stem cell and CAR-T cell recipient patients residing in high-endemic areas or who have been in contact with suspected COVID-19 patients should undergo RT-PCR testing while those who tested positive should undergo a CT scan and oxygen need evaluation (62). The American Society for Transplantation and Cellular Therapy recommends that asymptomatic patients whose cellular therapy cannot be delayed should be tested within 72 h before their admission or initiation of the conditioning regimen. Also, asymptomatic allogenic donors should be screened 72 h before collection while autologous donors with chemo-mobilization should be screened 72 h before chemotherapy and cell collection and those on GCSF and plerixafor on the day of GCSF start, respectively (6).
Last, blood transfusions should be carefully monitored during this outbreak. Blood donors should be screened for any suspicious signs or close exposure to COVID-19 present in at least the past 2 weeks (65). The WHO has issued specific guidelines on maintenance and safety of blood products supply during the COVID-19 pandemic (66).
Conclusion
Patients with hematologic malignancies are thought to be particularly vulnerable for severe illness from COVID-19. The outbreak has added a large burden to the complexity of cancer care in hematologic malignancies on many aspects. Balancing the management of COVID-19 and its complications and management of cancer is particularly challenging in patients with hematologic malignancies, especially when treating with curative intent, which is often the case in many blood cancers. In the context of limited available scientific data in a such young and rapidly evolving field, physicians are left for now with their clinical judgement, educated guesses and consensual expert opinions (Figure 1). Until the advent of effective anti-viral therapy or vaccines, preventative behavioral strategy remains our best model to help protect our patients. Screening for asymptomatic carriers and immune persons has a central role in optimizing mitigation strategy to maintain safety at individual and public health levels. Despite the availability of RT-PCR, antibody serology, and CT scan as complementary diagnostic tools, several limitations exist in implementing a cost-effective screening strategy that can be applicable on a large scale. A tiered approach targeting patients at pre-defined higher risk (pre-conditioning or other intensive chemotherapy regimens, before prolonged use of steroids or anti-CD20 antibodies etc.) may be a better way and warrants more exploration. Despite the numerous difficulties during the pandemic, the oncology community has been very adaptive in adjusting to the new challenges. In addition, scientific and technological progress is evolving rapidly, which gives hope to the oncology, and international communities.
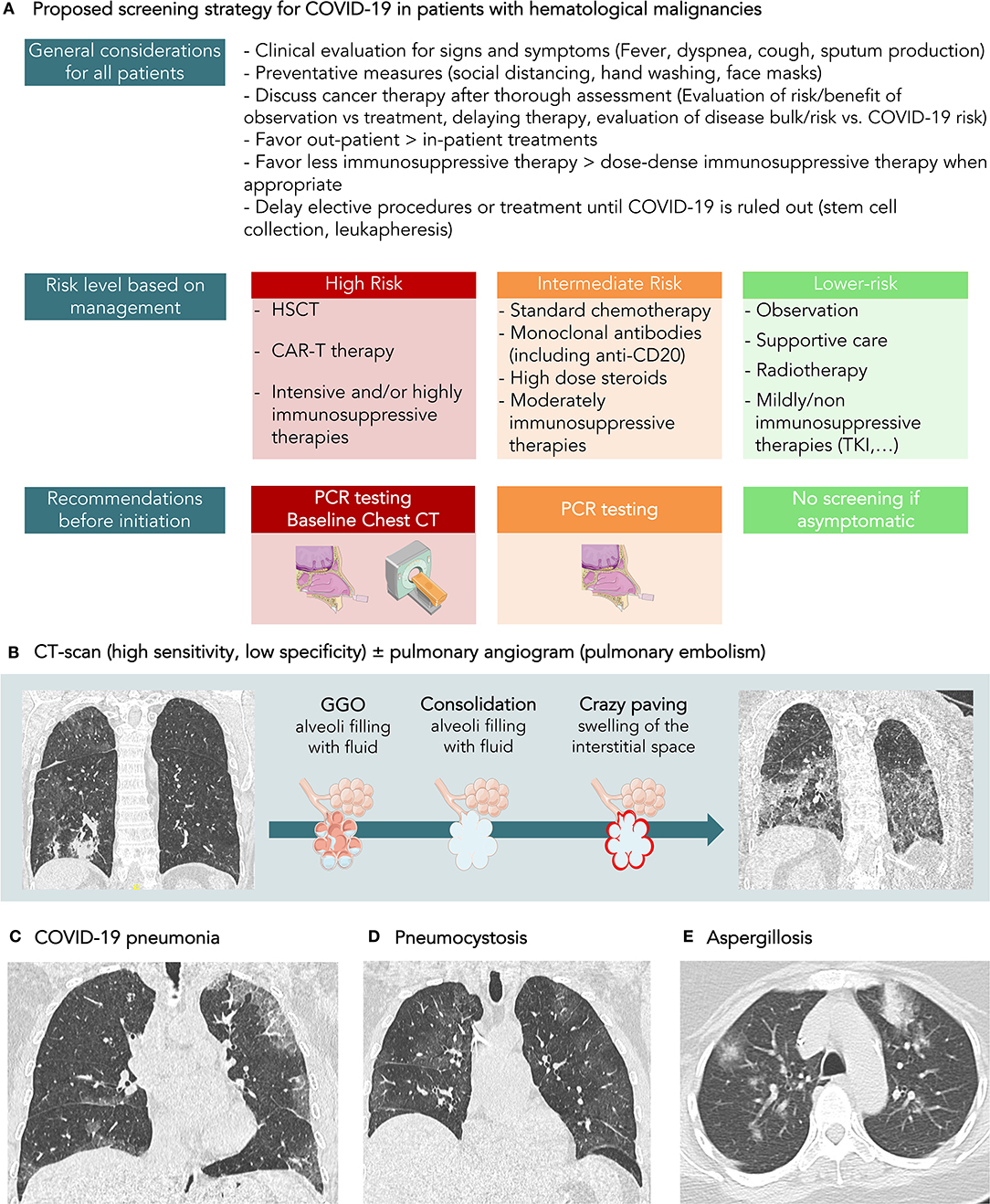
Figure 1. (A) Proposed screening strategy for COVID-19 in patients with hematological malignancies. (B) CT-scan is a sensitive tool, but its specificity for the diagnosis of COVID-19 is around 37%, according to recent meta-analysis. It has not been evaluated in patients with hematological malignancies but could be even lower due to a higher rate of infection caused by immune suppression. (C) COVID-19 pneumonia in a 58-year-old patient with diffuse B lymphoma treated with R ACVBP. Symptoms were cough and fever. SARS-CoV-2 rt-PCR was positive. On CT-scan, the CT score CORADS was 5. The axial CT image showed ordinary COVID-19 pneumonia with multiple regions of subpleural GGO (Ground Glass opacity) with superimposed inter and intralobular septal thickening. (D) Pneumocystis in a 58-year-old patient followed for acute leukemia. The clinical exam revealed a cough and fever. The patient was lymphopenic. CT-scan showed diffused ground-glass opacities. SARS-CoV-2 rt-PCR was negative. The final diagnosis was pneumocystis. (E) Diffuse pulmonary condensation in a 55-year-old patient with AML in febrile aplasia. SARS-CoV-2 rt-PCR was negative. A positive aspergillus antigenemia was confirmed by culture of bronchoalveolar lavage fluid.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors contributed equally to the manuscript writing and review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. WHO. Coronavirus Disease 2019 (COVID-19) Situation Report – 97. (2019). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200426-sitrep-97-covid-19.pdf?sfvrsn=d1c3e800_2 (accessed May 05, 2020).
3. Garnica M, Maiolino A. COVID and Hematology: special considerations regarding patient safety, gold standard therapies and safety for health care professionals. Hematol Transfus Cell Ther. (2020) 42:111–2. doi: 10.1016/j.htct.2020.04.001
4. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. (2020) 21:335–7. doi: 10.1016/S1470-2045(20)30096-6
5. EHA guidelines. Recommendations for Specific Hematologic Malignancies. (2020). Available online at: https://ehaweb.org/covid-19/covid-19-recommendations/recommendations-for-specific-hematologic-malignancies/ (accessed May 05, 2020).
6. ASTCT (American Society for Transplantation and Cellular Therapy). Guidance for BMT Center Functioning During COVID-19 Pandemic. (2020). Available online at: https://www.astct.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=5d66683f-c808-4eaf-faa1-782a5bfd687b&forceDialog=0 (accessed May 05, 2020).
7. Lambertini M, Toss A, Passaro A, Criscitiello C, Cremolini C, Cardone C, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists' perspective. EMSO Open. (2020) 5:e000759. doi: 10.1136/esmoopen-2020-000759
8. Portnoy J, Waller M, Elliott T. Telemedicine in the Era of COVID-19. J Allergy Clin Immunol Pract. (2020) 8:1489–91. doi: 10.1016/j.jaip.2020.03.008
9. Perini GF, Fischer T, Gaiolla RD, Rocha TB, Bellesso M, Teixeira LLC, et al. How to manage lymphoid malignancies during novel 2019 coronavirus (CoVid-19) outbreak: a Brazilian task force recommendation. Hematol Transfus Cell Ther. (2020) 42:103–10. doi: 10.1016/j.htct.2020.04.002
10. Leukemia and lymphoma society. Facts and Statistics. (2020). Available online at: https://www.lls.org/facts-and-statistics/facts-and-statistics-overview/facts-and-statistics (accessed May 05, 2020).
11. Hakki M, Rattray RM, Press RD. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol. (2015) 68:1–5. doi: 10.1016/j.jcv.2015.04.012
12. Weinkove R, McQuilten Z, Adler J, Agar M, Blyth E, Cheng A, et al. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance. Med J Aust. (2020) 212:481–9. doi: 10.5694/mja2.50607
13. NHS. Clinical Guide for the Management of Cancer Patients During the Coronavirus Pandemic (2020).
14. Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. (2020) 368:m1165. doi: 10.1136/bmj.m1165
15. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. (2020) 31:894–901. doi: 10.1016/j.annonc.2020.03.296
16. Willan J, King AJ, Hayes S, Collins GP, Peniket A. Care of haematology patients in a COVID-19 epidemic. Br J Haematol. (2020) 189:241–3. doi: 10.1111/bjh.16620
17. Griggio V, Mandili G, Vitale C, Capello M, Macor P, Serra S, et al. Humoral immune responses toward tumor-derived antigens in previously untreated patients with chronic lymphocytic leukemia. Oncotarget. (2017) 8:3274–88. doi: 10.18632/oncotarget.13712
18. Advani SH, Dinshaw KA, Nair CN, Gopal R, Talwalkar GV, Iyyer YS, et al. Immune dysfunction in non-Hodgkin's lymphoma. Cancer. (1980) 45:2843–8. doi: 10.1002/1097-0142(19800601)45:11<2843::AID-CNCR2820451121>3.0.CO;2-C
19. Kennedy-Nasser AA, Hanley P, Bollard CM. Hodgkin disease and the role of the immune system. Pediatr Hematol Oncol. (2011) 28:176–86. doi: 10.3109/08880018.2011.557261
20. Noonan K, Borrello I. The immune microenvironment of myeloma. Cancer Microenviron Off J Int Cancer Microenviron Soc. (2011) 4:313–23. doi: 10.1007/s12307-011-0086-3
21. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
22. Bachanova V, Bishop MR, Dahi P, Dholaria B, Grupp SA, Hayes-Lattin B, et al. Chimeric antigen receptor T cell therapy during the COVID-19 pandemic. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. (2020) 26:1239–46. doi: 10.1016/j.bbmt.2020.04.008
23. The First Report of the Prevalence of COVID-19 in Chronic Myelogenous Leukemia Patients in the Core Epidemic Area of China:Multicentre Cross-Sectional Survey | medRxiv. (2020). Available online at: https://www.medrxiv.org/content/10.1101/2020.03.12.20034876v2 (accessed May 05, 2020).
24. Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet Lond Engl. (2020) 395:e30. doi: 10.1016/S0140-6736(20)30304-4
25. Treon SP, Castillo J, Skarbnik AP, Soumerai JD, Ghobrial IM, Guerrera ML, et al. The BTK-inhibitor ibrutinib may protect against pulmonary injury in COVID-19 infected patients. Blood. (2020) 135:1912–5. doi: 10.1182/blood.2020006288
26. Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. (2020) 4:1307–10. doi: 10.1182/bloodadvances.2020001907
27. Jin XH, Zheng KI, Pan KH, Xie YP, Zheng MH. COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. (2020) 7:e351–2. doi: 10.1016/S2352-3026(20)30074-0
28. COVID-19 Registry. (2020). Available online at: https://www.ashresearchcollaborative.org/covid-19-registry (accessed May 05, 2020).
29. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. (2020) 10:783–91. doi: 10.1158/2159-8290.CD-20-0422
30. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. doi: 10.1056/NEJMc2001468
31. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. doi: 10.1001/jama.2020.2565
32. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control COVID-19. Mass Medical Soc. (2020) 382:2158–60. doi: 10.1056/NEJMe2009758
33. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. (2020). doi: 10.1148/radiol.2020200642. [Epub ahead of print].
34. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
35. Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis Off Publ Infect Dis Soc Am. (2020). doi: 10.1093/cid/ciaa310. [Epub ahead of print].
36. Liang T. Handbook of COVID-19 Prevention and Treatment. Zhejiang University School of Medicine (2020).
37. FDA. Coronavirus (COVID-19) Update: Serological Test Validation and Education Efforts. (2020). Available online at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-serological-test-validation-and-education-efforts (accessed May 05, 2020).
38. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. (2020) 20:398–400. doi: 10.1016/S1473-3099(20)30141-9
39. Wong HYF, Lam HYS, Fong AHT, Leung ST, Chin TWY, Lo CSY, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. (2020) 2:201160. doi: 10.1148/radiol.2020201160
40. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. (2020) 2:200432. doi: 10.1148/radiol.2020200432
41. Raptis CA, Hammer MM, Short RG, Shah A, Bhalla S, Bierhals AJ, et al. Chest CT and Coronavirus disease (COVID-19): a critical review of the literature to date. Am J Roentgenol. (2020) 1–4. doi: 10.2214/AJR.20.23202. [Epub ahead of print].
42. American College of Radiology (ACR). ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. (2020) Available online at: www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CTfor-Suspected-COVID19-Infection (accessed May 05, 2020).
43. Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. (2013) 33:1951–75. doi: 10.1148/rg.337130057
44. Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. (2020). doi: 10.1148/radiol.2020200823. [Epub ahead of print].
45. Bao C, Liu X, Zhang H, Li Y, Liu J. COVID-19 Computed tomography findings: a systematic review and meta-analysis. J Am Coll Radiol. (2020) 17:701–9. doi: 10.1016/j.jacr.2020.03.006
46. Bullock J, Pham KH, Lam CSN, Luengo-Oroz M. Mapping the landscape of artificial intelligence applications against COVID-19. ArXiv Prepr. (2020).
47. Kermany DS, Goldbaum M, Cai W, Valentim CC, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. (2018) 172:1122–31. doi: 10.1016/j.cell.2018.02.010
48. Anthimopoulos M, Christodoulidis S, Ebner L, Christe A, Mougiakakou S. Lung pattern classification for interstitial lung diseases using a deep convolutional neural network. IEEE Trans Med Imaging. (2016) 35:1207–16. doi: 10.1109/TMI.2016.2535865
49. Shi F, Wang J, Shi J, Wu Z, Wang Q, Tang Z, et al. Review of artificial intelligence techniques in imaging data acquisition, segmentation and diagnosis for covid-19. ArXiv Prepr. (2020). doi: 10.1109/RBME.2020.2987975. [Epub ahead of print].
50. Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, et al. Artificial intelligence distinguishes covid-19 from community acquired pneumonia on chest ct. Radiology. (2020) 284:574–82. doi: 10.1148/radiol.2020200905
51. Zic JA, Ai W, Akilov OE, Carter JB, Duvic M, Foss F, et al. United States Cutaneous lymphoma consortium recommendations for treatment of cutaneous lymphomas during the COVID-19 pandemic. J Am Acad Dermatol. (2020). doi: 10.1016/j.jaad.2020.04.049. [Epub ahead of print].
52. Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. (2020) 25:e936–45. doi: 10.1634/theoncologist.2020-0213
53. van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. (2020) 26:665–71. doi: 10.1038/s41591-020-0874-8
54. ecancer. What to Expect: Oncology's Response to Coronavirus in Italy - Ecancer. (2020). Available online at: http://ecancer.org/en/news/17495-what-to-expect-oncologys-response-to-coronavirus-in-italy (accessed May 05, 2020).
55. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. (2013) 56:258–66. doi: 10.1093/cid/cis844
56. Dignan FL, Clark A, Aitken C, Gilleece M, Jayakar V, Krishnamurthy P, et al. BCSH/BSBMT/UK clinical virology network guideline: diagnosis and management of common respiratory viral infections in patients undergoing treatment for haematological malignancies or stem cell transplantation. Br J Haematol. (2016) 173:380–93. doi: 10.1111/bjh.14027
57. Gavillet M, Carr Klappert J, Spertini O, Blum S. Acute leukemia in the time of COVID-19. Leuk Res. (2020) 92:106353. doi: 10.1016/j.leukres.2020.106353
58. Burke PJ, Braine HG, Rathbun HK, Owens JA. The clinical significance and management of fever in acute myelocytic leukemia. Johns Hopkins Med J. (1976) 139:1–12.
59. ASH Guidelines. COVID-19 and CLL: Frequently Asked Questions. (2020). Available online at: https://www.hematology.org/covid-19/covid-19-and-cll (accessed May 05, 2020).
60. Al Saleh AS, Sher T, Gertz MA. Multiple myeloma in the time of COVID-19. Acta Haematol. (2020). doi: 10.1159/000507690. [Epub ahead of print].
61. Mian H, Grant SJ, Engelhardt M, Pawlyn C, Bringhen S, Zweegman S, et al. Caring for older adults with multiple myeloma during the COVID-19 Pandemic: Perspective from the International Forum for Optimizing Care of Older Adults with Myeloma. J Geriatr Oncol. (2020) 11:764–8. doi: 10.1016/j.jgo.2020.04.008
62. Styczynski JMM, Ljungman P. EBMT recommendation on: CORONAVIRUS DISEASE COVID-19: EBMT (2020). Available online at: https://www.ebmt.org/sites/default/files/2020-03/EBMT%20COVID19%20guidelines%20v.3.2%20%282020-03-16%29.pdf
63. Dholaria B, Savani BN. How do we plan hematopoietic cell transplant and cellular therapy with the looming COVID-19 threat? Br J Haematol. (2020) 189:239–40. doi: 10.1111/bjh.16597
64. Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. (2020) 38:415–22. doi: 10.1200/JCO.19.01892
65. Mascaretti L, De Angelis V, Berti P. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and Transfusion Medicine: reflections from Italy. Blood Transfus. (2020) 18:77–8. doi: 10.2450/2020.0071-20
66. WHO. Maintaining a Safe and Adequate Blood Supply During the Pandemic Outbreak of Coronavirus Disease (COVID-19). (2020). Available online at: https://www.who.int/publications-detail/maintaining-a-safe-and-adequate-blood-supply-during-the-pandemic-outbreak-of-coronavirus-disease-(covid-19) (accessed May 05, 2020).
Keywords: hematologic malignancies, hematology, COVID-19, coronavirus, screening, polymerase chain reaction, CT scan
Citation: Assi T, Samra B, Dercle L, Rassy E, Kattan J, Ghosn M, Houot R and Ammari S (2020) Screening Strategies for COVID-19 in Patients With Hematologic Malignancies. Front. Oncol. 10:1267. doi: 10.3389/fonc.2020.01267
Received: 06 May 2020; Accepted: 18 June 2020;
Published: 03 July 2020.
Edited by:
Samata Kakkad, Johns Hopkins School of Medicine, United StatesReviewed by:
Dennis Lawrence Cooper, Rutgers Cancer Institute of New Jersey, United StatesFrancesca Gorini, National Research Council (CNR), Italy
Copyright © 2020 Assi, Samra, Dercle, Rassy, Kattan, Ghosn, Houot and Ammari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samy Ammari, c2FteS5hbW1hcmlAZ3VzdGF2ZXJvdXNzeS5mcg==
 Tarek Assi
Tarek Assi Bachar Samra
Bachar Samra Laurent Dercle
Laurent Dercle Elie Rassy1
Elie Rassy1 Samy Ammari
Samy Ammari