- Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, China
Aim: To analyze the learning curve (LC) for robotic natural orifice specimen extraction surgery (NOSES) for colorectal neoplasms and evaluate safety and feasibility during the initial LC.
Method: Patients who consecutively underwent robotic NOSES performed by two surgeons between March 2016 and October 2019 were analyzed retrospectively. The operation time was evaluated using the cumulative sum method to analyze the LC. The clinicopathological data before and after the completion of LC were extracted and compared to evaluate safety and feasibility.
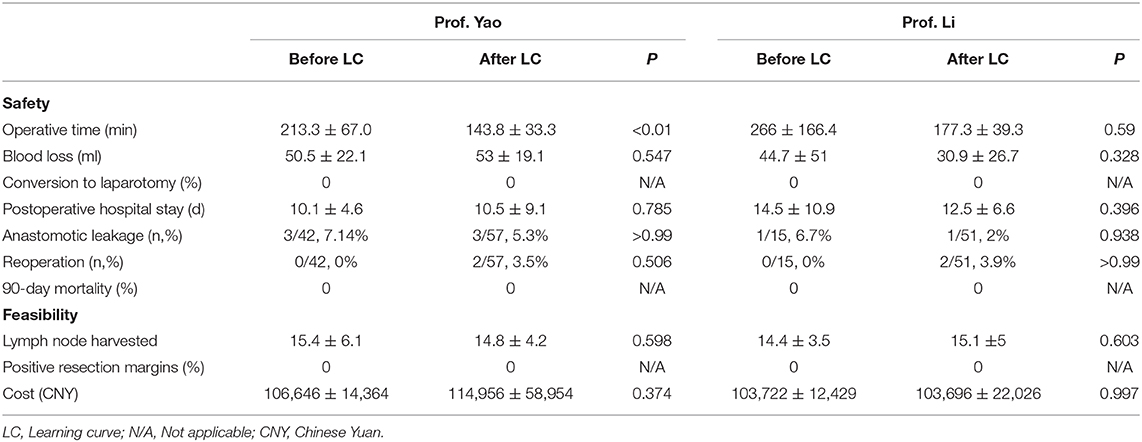
Results: In total, 99 and 66 cases were scheduled for robotic NOSES by Prof. Yao and Prof. Li, respectively. The peak points of LC were observed at the 42nd and 15th cases of Yao and Li, respectively, then operation time began to decrease. Only the operation time for Yao before the completion of LC (213.3 ± 67.0 min) was longer than that after the completion of LC (143.8 ± 33.3 min). For Yao nor for Li, other indices, such as postoperative hospital stay, intraoperative blood loss, conversion to laparotomy, incidence of anastomotic leakage, reoperation rate, and 90-day mortality rate lacked significant statistical differences(P > 0.05). In terms of feasibility, the number of lymph nodes harvested, positive resection margin rate, and total cost before and after the completion of LC had no significant statistical difference (P > 0.05).
Conclusion: The cases before the completion of LC for robotic NOSES in colorectal neoplasms varied from 15 cases to 42 cases. Robotic NOSES is safe and feasible during the initial LC.
Statement
Laparoscopic NOSES is mature, but robotic NOSES should have a faster LC and some advantages. How many cases needed to complete LC of robotic NOSES? Is it safe during the initial LC? This is an analysis of LC on the world's maximum cases of robotic NOSES in colorectal neoplasms.
Introduction
Minimally invasive surgery is the developmental tendency of colorectal surgery. After decades of development, laparoscopic colorectal surgery has become mature, and its safety and effectiveness are no less than those of open surgery. Moreover, laparoscopic surgery is with the virtue of fast recovery and minimal trauma. Although laparoscopic surgery does not require large incisions unlike open surgery, it still requires a small incision to extract specimens. This requirement leads to abdominal incision pain and wound complications, including infection, hernia formation, and scarring.
NOSES is a kind of operation that can realize the “no scar” concept to the limit and uses a soft endoscope or laparoscope to enter the abdominal or chest cavity through the mouth, gastrointestinal tract, vagina, bladder, or other natural orifice to conduct medical procedures, including exploration, biopsy, appendectomy, hysterectomy, and cystectomy, without any auxiliary incision on the body surface (1). In 2007, the French doctor Marescaux has completed transvaginal cholecystectomy, the first truly scar-free operation in the world. Thus, the minimally invasive requirements of surgery have entered a new era (2).
NOSES is especially suitable for colorectal surgery. Incisions in the oral cavity, rectum, vagina, and other natural orifices must be made to remove the specimens from the natural lumen for appendectomy, cholecystectomy, and nephrotomy. For colorectal neoplasms, however, the rectum needs to be disconnected during colorectal surgery, and then the rectum and anus can act as natural orifices for specimen extraction. This approach can avoid making any artificial incision and has natural advantages.
The Da Vinci surgical robot has been approved by the FDA since 2000. Compared with laparoscopic surgery, robotic surgery is more advanced with several benefits, such as superior three-dimensional vision and EndoWrist instruments which can achieve a wide range of motion, and a shorter learning curve (LC). However, the safety and feasibility of robotic NOSES during the initial LC and the possibility for shortening the LC remain unclear. Therefore, this work addressed the LC of robotic NOSES in our hospital through a retrospective analysis. At the same time, the clinicopathological data before and after the completion of LC were compared to analyze the safety and feasibility of robotic NOSES during the initial LC.
Methods
This work has been reported in line with the STROCSS criteria.
Patients Selection
This is a retrospective study on robotic NOSES for patients with sigmoid and rectal neoplasms performed by two surgeons, Prof. Yao and Prof. Li. Yao and Li had performed more than 1,000 open radical resections of colorectal cancer before October 2015. In October 2015, the Da Vinci Si Robot Surgical System was installed in the hospital. All patients diagnosed with sigmoid and rectal neoplasm between March 2016 and October 2019 were confirmed for resectability before operation. The exclusion criteria for robotic NOSES were as below: (1) age <18 years old; (2) emergency operation due to gastrointestinal obstruction, perforation, or bleeding; (3) any suspicious invasion of the pelvic wall, bladder, or any other perirectal tissue or organs; (4) written informed consent of patients cannot be obtained; (5) specimen was unexpectedly removed through the anus by preoperative evaluation; (6) body mass index ≥ 28 kg/m2; (7) metastasis of lung, bone, or liver that cannot be removed simultaneously; and (8) patients with contraindication that cannot tolerate robotic NOSES. Given that our study focuses on the LC of robotic NOSES for patients with sigmoid and rectal neoplasms, cases combining the resection of other organs, such as ovariectomy and hysterectomy, were excluded. This work is in accordance with the declaration of Helsinki and is approved by the Ethics Committee of Hospital.
Surgical Technique
After successful general anesthesia, the patient assumed the Trendelenburg position. Five trocars were needed (one for the robotic camera, three for the robot operation arms, and one for the assistant). First, in accordance with the principle of total mesorectal resection or complete mesocolon resection, the blood vessels were ligated at the root of the inferior mesenteric artery, and the rectum or sigmoid colon and its mesentery were completely free, whereas the left ureter was protected properly. The left colonic artery was preserved. At 10 cm from the proximal and 2–5 cm from the distal of the tumor, the rectum was ligated with a self-locking nylon bandage and then amputated. After the assistant had fully dilated the anus, an endoscope sterile sleeve was placed into the pelvic cavity through the anus. One end of the sterile sleeve was kept outside the anus, and the other end was kept inside the pelvic cavity. Sponge forceps were placed into the pelvic cavity through the sterile sleeve, and the proximal end of the removed sigmoid colon or rectum was clamped and slightly dragged outward. After the specimens were all in the sterile sleeve, the inner end of the sleeve was ligated such that the specimens would not slide out from the sterile sleeve. The assistant pulled the sponge forceps to pull out the sterile sleeve and the specimens together through the anus. Then, the assistant situated the orvil of the stapler into the pelvic cavity from the anus. The operator sutured the stump of the sigmoid colon and placed the orvil in it. After the operator sutured the stump of the rectum, the assistant placed the stapler through the anus to complete the anastomosis. Then, air was pumped into the rectum via the anus to determine the presence of air leakage from the anastomosis. If air leakage or serous membrane eversion was present, additional sutures can be performed (Figure 1). The perioperative management followed international guidelines (3).
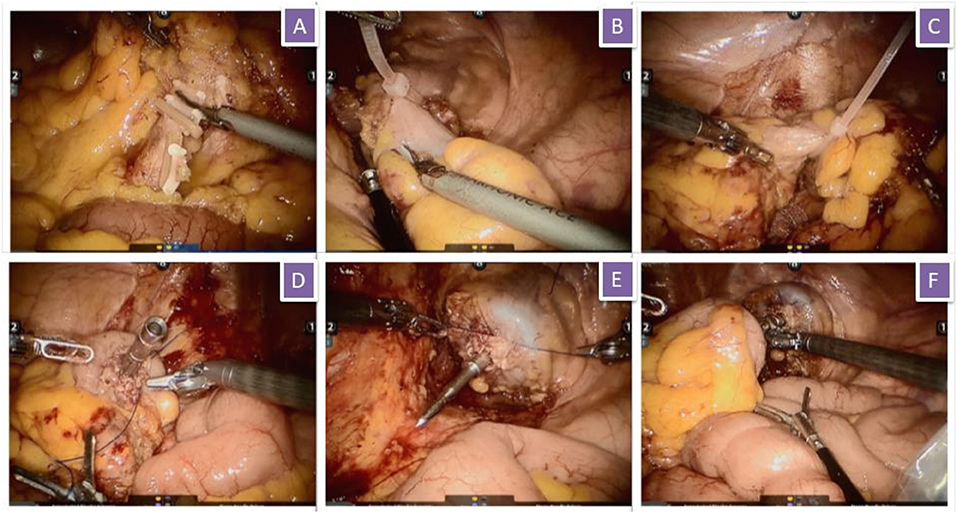
Figure 1. Surgical procedure. (A) The inferior mesenteric artery and vein were isolated and clipped by absorbable vascular clamps; (B) Rectum was ligated with self-locking nylon bandage; (C) Sigmoid colon was ligated with self-locking nylon bandage; (D) Suture the stump of sigmoid colon and put the orvil into the sigmoid colon; (E) Suture the stump of rectum; (F) Complete the anastomosis.
Date Collection
All perioperative data, including the following variables, were collected retrospectively for analysis.
1. General information of patients: gender, age, chief complaints, comorbidities, American Society of Anesthesiology (ASA) classification;
2. Perioperative data: operative time (OT), intraoperative blood loss, laparotomy conversion rate;
3. Postoperative data: postoperative hospital stay, pathological results, short-term complications, anastomotic leakage, 90-day mortality, reoperation rates, total medical expenses,
Pathological information was recorded on the recommendation of AJCC 8th Edition Cancer Staging Form. Postoperative complications were classified using the Clavien–Dindo classification of surgical complications (4).
Statistical Analysis
SPSS (version 25.0, Chicago, USA) was used for analyzing. Measurement data expressed as mean ± standard deviation, and count data were presented as numbers and percentages. The measurement data and count data were statistically analyzed with two-tailed Student's t-test and Pearson's χ2 test, respectively. P < 0.05 was considered to demonstrate statistically significant differences.
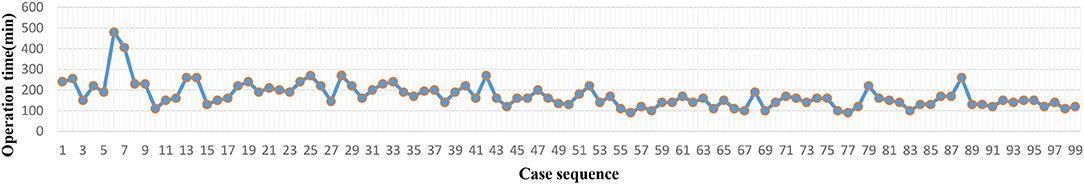
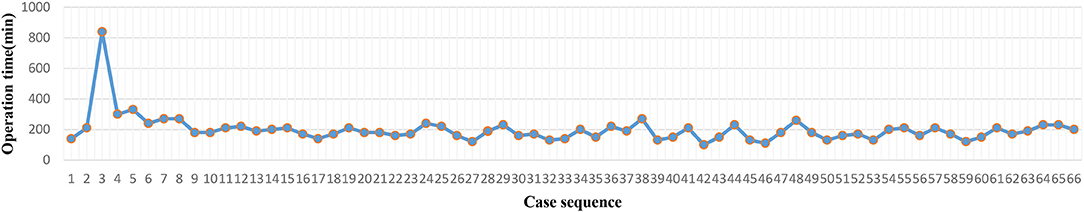
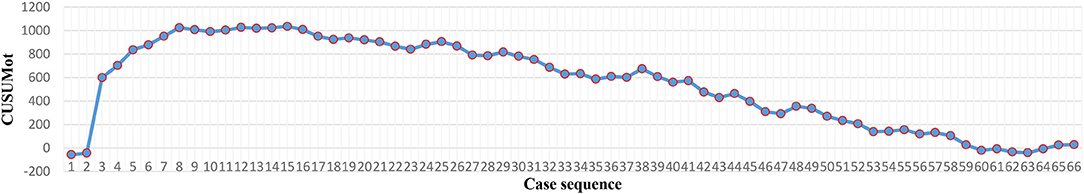
The cumulative sum (CUSUM) technique was used to detect a shift in the trend of OT. Robotic NOSES cases were arranged in chronological order. CUSUMOT1st is the difference between OT1st and the mean OT, i.e., OT1st-OTmean. CUSUMOT2nd is calculated as CUSUMOT 1st + (OT2nd-OTmean). CUSUMOT 3rd is calculated as CUSUMOT 2nd + (OT3rd-OTmean). So, CUSUMχ = CUSUMχ−1 + (OTχ- OT mean). Then notable OT change points could be observed at the peak in the CUSUM curve.
Results
Details of Patients and Clinicopathological Characteristics
In total, 99 and 66 cases of robotic NOSES were performed by Yao and Li, respectively, between March 2016 and October 2019. Details are provided in Table 1.
For Yao, in terms of gender, men and women accounted for 60.6% (60/99) and 39.4% (39/99), respectively. The mean age was 57.7 ± 12.8 years. The chief complaints were hematochezia (85.9%, 146/180), increased frequency of defecation (10.1%, 10/99), abdominal discomfort (2%, 2/99), and anal distention (2%, 2/99). The distance from the lower edge of the tumor to the anus was based on the colonoscopy report, with an average of 8.9 ± 3.7 cm, 4% (4/99) for <5 cm, 53.5% (53/99) for 5–10 cm, and 42.4% (42/99) for ≥ 10 cm. The proportion of patients who received neoadjuvant radiotherapy and chemotherapy was 17.2% (17/99). A total of 14 patients had abdominal or pelvic surgery history, accounting for 14.1%. ASA was classified as 4% (4/99), 47.4% (47/99), 47.4% (47/99), and 1% (1/99) in levels 1, 2, and 4. Cases for protective ileostomy accounted for 4% (4/99). A total of 93.9% (93/99) of histological types were tubular adenocarcinoma, 3 adenomas, 2 mucinous adenocarcinoma, and 1 neuroendocrine tumor. The average and the maximum CDmax (maximum circumferential diameter) of specimens were 3.5 ± 1.7 and 12 cm, respectively. The number of lymph nodes harvested ≥ 12 accounted for 74.7% (74/99), and the average number of lymph nodes harvested was 15 ± 5.1. According to the depth of tumor invasion, the Tis, T1, T2, T3, and T4 stages were 0% (0/99), 7.4% (7/99), 31.6% (30/99), 28.4% (27/99), and 32.6% (31/99), respectively.
For Li, in terms of gender, men accounted for 66.7% (44/66) and women accounted for 33.3% (22/66). The mean age was 57 ± 13.2 years. The chief complaints were hematochezia (74.2%, 49/66), increased frequency of defecation (22.7%, 15/66), and routine examination (3%, 2/66). The distance from the lower edge of the tumor to the anus was based on the colonoscopy report with an average of 8.17 ± 3.18 cm, 10.6% (7/66) for <5 cm, 59.1% (39/66) for 5–10 cm, and 30.3% (20/66) for ≥ 10 cm. The proportion of patients who received neoadjuvant radiotherapy and chemotherapy was 7.6% (5/66). Ten patients had abdominal or pelvic surgery history, accounting for 15.2%. ASA was classified as 4.5% (3/66), 51.5% (34/66), 42.4% (28/66), and 1.5% (1/66) in levels 1, 2, and 4. Cases for protective ileostomy accounted for 3% (2/66). A total of 95.5% (63/66) of histological types were tubular adenocarcinoma, 2 adenomas, and 1 mucinous adenocarcinoma. The average and maximum CDmax of the specimen were 3.71 ± 1.43 and 7 cm, respectively. The number of lymph nodes harvested ≥ 12 accounted for 78.8% (52/66), and the average number of lymph nodes harvested for each case was 15 ± 4.7. According to the depth of tumor invasion, the Tis, T1, T2, T3, and T4 stages were 1.6% (1/66), 9.4% (6/66), 20.3% (13/66), 50% (32/66), and 18.8% (12/66), respectively.
LC Analysis
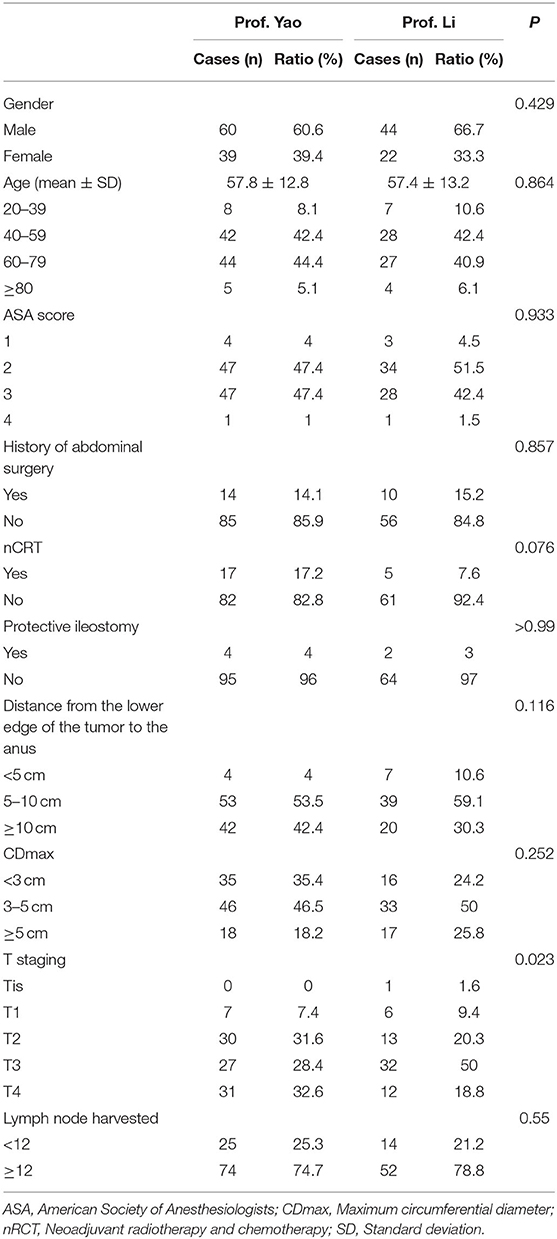
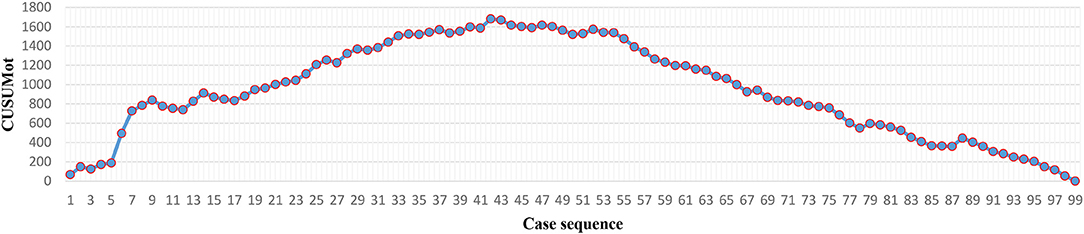
The operation time of each case was recorded (Figures 2, 4). The LC was assessed via the CUSUM method. As the CUSUMOT graph showed (Figures 3, 5), the peak point was observed at the 42nd and 15th cases for Yao and Li, respectively, after then, the operation time was decreased gradually. The beginning 42 and 15 robotic NOSES cases were considered as the initial LC for Yao and Li, respectively.
Comparison of Safety and Feasibility Before and After the Completion of LC
The cases performed by Prof. Yao and Prof. Li can be divided into two parts (by the 42nd and 15th cases, respectively), in accordance with the completion of LC. The safety and feasibility of the two parts were compared (Table 2). For Yao, in terms of safety, only the operation time before the completion of LC (213.3 ± 67 min) was longer than after the completion of LC (143.8 ± 33.3 min). Other indices, such as postoperative hospital stay, intraoperative blood loss, conversion to laparotomy, incidence of anastomotic leakage, reoperation rate, and 90-day mortality rate had no significant statistical difference. In terms of feasibility, the dissected lymph node number, positive resection margin rate, and total cost between two parts had no significant statistical difference (P > 0.05). For Li, no significant statistical difference for all indices between two parts were observed (P > 0.05).
Discussion
The incidence of colorectal cancers per 100,000 population has decreased from 60.5 in 1976 to 46.4 in 2005 (5) and continued to decrease at a rate of ~2.9% per year or greater between 2005 and 2014 (6). In 2011, the reported incidence rate for colorectal cancer is 40.0 per 100,000 persons (7). In addition, mortality from colorectal cancer has decreased by almost 35% from 1990 to 2007 (8). The improvements in the incidence and mortality from colorectal cancer are thought to be a result of cancer prevention and early diagnoses through screening and improved treatment modalities. Nevertheless, colorectal cancer remains the fourth most frequently diagnosed cancer and the second leading cause of cancer death. In 2018, an estimated 43,030 new cases of rectal cancer will occur in the United States (25,920 cases in men, 17,110 cases in women) (6). Our study showed that the incidence for males, which is ~63% (104/165), is slightly higher than that for females and similar to that in the United States. Most of the patients complained of hematochezia and were administered and diagnosed by colonoscopy and biopsy. The sensitivity of the tumor markers was unsatisfactory. The sensitivity of the carcinoembryonic antigen was only 18.4% (28/152), whereas that of the carbohydrate antigens 242 and 199 were only 4.6% (7/152) and 5.7% (7/122), respectively.
Several randomized studies have identified that the conventional laparoscopic surgery for the treatment of patients with colorectal cancer has been maturing in recent years (9–13). Compared with open surgery, short-term endpoints with patients in the laparoscopic team show advantages, such as reduced blood loss and hospital stays and quick return of bowel function, but have prolonged operation times. No difference was seen in the completeness of resection and the percentage of patients with a positive circumferential resection margin, morbidity, or mortality. Moreover, the 3- and 5-year follow-up have shown no statistically significant difference in local recurrence, progression-free survival (PFS), or overall survival (OS) (9, 14). Many reports on laparoscopic NOSES for colorectal cancer are available (15–18), and this method has been proven to be safe and effective. In addition, numerous reports comparing laparoscopic NOSES with conventional laparoscopic surgery in colorectal surgery exist. Laparoscopic NOSES is safe and feasible with numerous advantages, including reduced pain and tissue trauma, fast recovery of intestinal function, and short postoperative hospital stay duration (19–23). Two meta-analyses involving 1,435 and 837 patients have also shown that compared with conventional laparoscopic surgery, NOSES may be a safe procedure that can significantly reduce the duration of hospital stay, accelerate postoperative recovery with improved cosmetic results, and in particular, minimize postoperative pain and complications while achieving similar oncological outcomes (24, 25). In June 2017, the China NOSES Alliance is established and released the Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2017 edition) to promote the application of NOSES (26). In addition, the International Alliance of NOSES issued the International consensus on natural orifice specimen extraction surgery for colorectal cancer in 2019 (27).
The Da Vinci robot system has been approved by the FDA in 2000, and many reports of the robotic radical resection of colorectal cancer are available. However, in these reports, the specimens have been extracted through a small abdominal incision. Reports about robotic NOSES, particularly the retrospective analysis of small samples and case reports, are rare (28–31). According to our experience, especially in ultralow rectal cancer, robotic NOSES has advantages over laparoscopic NOSES. After all, the NOSES for colorectal neoplasm is a reconstruction surgery. No matter the reinforcement of the anastomosis or the suturing of the pelvic peritoneum, it needs to be sutured and knotted. Compared with conventional laparoscopic surgery, robotic surgery has more advantages in suturing and knotting, especially in a narrow pelvic cavity. Moreover, recent research shows robotics to have a faster LC in rectal cancer surgery than laparoscopy (32, 33).
In this study, the LC was assessed via the CUSUM method. Results showed that the estimated LC for robotic NOSES was achieved after the 42nd and 15th cases for Yao and Li, respectively. The LCs of the two surgeons were quite different. In our opinion, these differences can be ascribed to two reasons. First, the LC can be drastically different due to the different operative styles and experience of each surgeon. Shaw et al. have shown that the complex robotic colorectal surgery can be performed with reduced operative time and complications after 15 robotic cases (34). Foo et al. have shown that the LC for robotic-assisted total mesorectal excisions for a novice rectal surgeon is 25 cases (35). The data obtained by Jimenez et al. suggest that the estimated LC for robotic-assisted rectal cancer surgery is achieved after 21–23 cases (36). Park et al. have shown that the primary technical competence was achieved at the initial learning period of the 44th case in accordance with the robotic low anterior resection for rectal cancer (37). Second, Prof. Yao was the first surgeon to develop the robotic NOSES in our Department in March 2016, whereas Prof. Li started to develop robotic surgery in April 2017 with the support and guidance of Prof. Yao. Thus, the LC of Prof. Li can be shortened.
We also compared the outcomes of two surgeons before and after the completion of learning curve. The operation time of Yao before the completion of LC (213.3 ± 67 min) was longer than that after completion of LC (143.8 ± 33.3 min). The difference was statistically significant. Other indices in terms of safety, such as postoperative hospital stay, intraoperative blood loss, conversion to laparotomy, incidence of anastomotic leakage, reoperation rate, and 90-day mortality, had no significant statistical difference for Yao and Li. In terms of feasibility, the dissected lymph node number, positive resection margin rate, and total cost before and after the completion of LC lacked significant statistical difference (P > 0.05). Our study demonstrated that robotic NOSES in colorectal neoplasms is safe and feasible during the initial LC.
Our analysis has several limitations. First, the follow-up should be extended, and oncological outcomes, such as PFS and OS need to be verified. However, due to various reasons, many patients refused to return to hospital for adjuvant therapy or regular follow-up as our wishes. Insufficient compliance of patients leads to oncological outcomes could not be truthfully reflected. Second, this is a retrospective research. So, the integrity and accuracy of clinical data could not be guaranteed.
Conclusions
Cases before the completion of LC for robotic NOSES in colorectal neoplasms varied from 15 cases to 42 cases. Robotic NOSES is safe and feasible during the initial LC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was approved by the Ethics Committee of The Second Xiangya Hospital. The written informed consents of patients were been obtained.
Author Contributions
HY: investigation, resources, writing-reviewing and editing, and supervision. TL, SL, and WC: investigation and resources. KL: methodology, software, and formal analysis. XJ: software and data curation. JZ: conceptualization, writing-original draft preparation, software, and data curation. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to the colleagues from anesthesia department and operating room for their help during the processure of robotic NOSES.
References
1. China Natural Orifice Specimen Extraction Surgery. [Consensus of natural orifice specimen extraction surgery in gastric cancer (2019)]. Zhonghua Wei Chang Wai Ke Za Zhi. (2019) 22:711–4. doi: 10.3760/cma.j.issn.1671-0274.2019.08.002
2. Zorron R, Filgueiras M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A. Transvaginal cholecystectomy: report of the first case. Surg Innov. (2007) 14:279–83. doi: 10.1177/1553350607311090
3. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS((R))) society recommendations: 2018. World J Surg. (2019) 43:659–95. doi: 10.1007/s00268-018-4844-y
4. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
5. Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. (2011) 34:573–80. doi: 10.1097/COC.0b013e3181fe41ed
6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
7. Henley SJ, Singh SD, King J, Wilson R, O'Neil ME, Ryerson AB. Centers for disease, and prevention, invasive cancer incidence and survival–United States, 2011. MMWR Morb Mortal Wkly Rep. (2015) 64:237–42. doi: 10.15585/mmwr.mm6449a1
8. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. (2011) 61:212–36. doi: 10.3322/caac.20121
9. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. (2015) 372:1324–32. doi: 10.1056/NEJMoa1414882
10. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III Rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. (2015) 314:1346–55. doi: 10.1001/jama.2015.10529
11. Haghighatdoost F, Najafabadi MM, Bellissimo N, Azadbakht L. Association of dietary acid load with cardiovascular disease risk factors in patients with diabetic nephropathy. Nutrition. (2015) 31:697–702. doi: 10.1016/j.nut.2014.11.012
12. Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. (2015) 314:1356–63. doi: 10.1001/jama.2015.12009
13. Jepsen D. Childhood agricultural injuries. Commentary. Curr Probl Pediatr Adolesc Health Care. (2013) 43:45–6. doi: 10.1016/j.cppeds.2012.11.001
14. Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the medical research council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. (2010) 97:1638–45. doi: 10.1002/bjs.7160
15. Han FH, Hua LX, Zhao Z, Wu JH, Zhan WH. Transanal natural orifice specimen extraction for laparoscopic anterior resection in rectal cancer. World J Gastroenterol. (2013) 19:7751–7. doi: 10.3748/wjg.v19.i43.7751
16. Yuliuming W, Qian Z, Lei Y, Qingchao T, Rui H, Yinggang C, et al. Retrospective study of 203 cases of colorectal neoplasms treated by natural orifice specimen extraction surgery. Chin J Colorec Dis. (2019) 29:32–7.
17. Park JS, Kang H, Park SY, Kim HJ, Lee IT, Choi GS. Long-term outcomes after Natural orifice specimen extrac tion versus conventional laparoscopy-assisted surgery for rectal cancer: a matched case-control study. Ann Surg Treat Res. (2018) 94:26–35. doi: 10.4174/astr.2018.94.1.26
18. Karagul S, Kayaalp C, Sumer F, Ertugrul I, Kirmizi S, Tardu A, et al. Success rate of natural orifice specimen extraction after laparoscopic colorectal resections. Tech Coloproctol. (2017) 21:295–300. doi: 10.1007/s10151-017-1611-2
19. Ng HI, Sun WQ, Zhao XM, Jin L, Shen XX, Zhang ZT, et al. Outcomes of trans-anal natural orifice specimen extraction combined with laparoscopic anterior resection for sigmoid and rectal carcinoma: An observational study. Medicine. (2018) 97:e12347. doi: 10.1097/MD.0000000000012347
20. Zhou S, Wang X, Zhao C, Pei W, Zhou H, Liu Q, et al. Comparison of short-term and survival outcomes for transanal natural orifice specimen extraction with conventional mini-laparotomy after laparoscopic anterior resection for colorectal cancer. Cancer Manage Res. (2019) 11:5939–48. doi: 10.2147/CMAR.S209194
21. Wolthuis AM, Fieuws S, Van Den Bosch A, de Buck van Overstraeten A, D'Hoore A. Randomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extraction. Br J Surg. (2015) 102:630–7. doi: 10.1002/bjs.9757
22. Liu Z, Efetov S, Guan X, Zhou H, Tulina I, Wang G, et al. A multicenter study evaluating natural orifice specimen extraction surgery for rectal cancer. J Surg Res. (2019) 243:236–41. doi: 10.1016/j.jss.2019.05.034
23. Xu G, Qingchao T, Guiyu W, Junmin S, Miao W, Jungang K, et al. Retrospective study of 718 colorectal neoplasms treated by natural orifice specimen extraction surgery in 79 hospitals. Chin J Colorec Dis. (2017) 6:469–77. doi: 10.3877/cma.j.issn.2095-3224.2017.06.006
24. Liu RJ, Zhang CD, Fan YC, Pei JP, Zhang C, Dai DQ. Safety and oncological outcomes of laparoscopic NOSE surgery compared with conventional laparoscopic surgery for colorectal diseases: a meta-analysis. Front Oncol. (2019) 9:597. doi: 10.3389/fonc.2019.00597
25. Ma B, Huang XZ, Gao P, Zhao JH, Song YX, Sun JX, et al. Laparoscopic resection with natural orifice specimen extraction versus conventional laparoscopy for colorectal disease: a meta-analysis. Int J Colorectal Dis. (2015) 30:1479–88. doi: 10.1007/s00384-015-2337-0
26. Alliance CN. Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2017 edition). Chin J Colorec Dis. (2017) 6:266–73. doi: 10.3877/cma.j.issn.2095-3224.2017.04.001
27. Guan X, Liu Z, Longo A, Cai JC, Tzu-Liang Chen W, Chen LC, et al. International alliance of, international consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep. (2019) 7:24–31. doi: 10.1093/gastro/goy055
28. Xuefeng Z, Chi L, Cheng Z, Huiyong J, Guangrong G, Da L, et al. Totally robotic surgery for rectal cancer with transanal specimen extraction. Chin J Pract Surg. (2013) 33:871–3.
29. Hu R, Jiang L, Kun Z, Gang W, Zhiwei J. Trans-natural orifice transuminal specimen extraction in robotic rectal cancer surgery: An analysis of 21 patients. Chin J Pract Surg. (2014) 34:252–4.
30. Efetov SK, Tulina IA, Kim VD, Kitsenko Y, Picciariello A, Tsarkov PV. Natural orifice specimen extraction (NOSE) surgery with rectal eversion and total extra-abdominal resection. Techn Coloproctol. (2019) 23:899–902. doi: 10.1007/s10151-019-02058-y
31. Minjares-Granillo RO, Dimas BA, LeFave JJ, Haas EM. Robotic left-sided colorectal resection with natural orifice intracorporeal anastomosis with extraction of specimen: the NICE procedure. A pilot study of consecutive cases. Am J Surg. (2019) 217:670–6. doi: 10.1016/j.amjsurg.2018.11.048
32. de'Angelis N, Lizzi V, Azoulay D, Brunetti F. Robotic versus laparoscopic right colectomy for colon cancer: analysis of the initial simultaneous learning curve of a surgical fellow. J Laparoendosc Adv Surg Tech A. (2016) 26:882–92. doi: 10.1089/lap.2016.0321
33. Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, et al. Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc. (2015) 29:558–68. doi: 10.1007/s00464-014-3698-0
34. Shaw DD, Wright M, Taylor L, Bertelson NL, Shashidharan M, Menon P, et al. Robotic colorectal surgery learning curve and case complexity. J Laparoendosc Adv Surg Tech A. (2018) 28:1163–8. doi: 10.1089/lap.2016.0411
35. Foo CC, Law WL. The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg. (2016) 40:456–62. doi: 10.1007/s00268-015-3251-x
36. Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis. (2013) 28:815–21. doi: 10.1007/s00384-012-1620-6
Keywords: colorectal neoplasms, learning curve, natural orifice specimen extraction surgery (NOSES), robotic surgery, safety and feasibility
Citation: Yao H, Li T, Chen W, Lei S, Liu K, Jin X and Zhou J (2020) Safety and Feasibility of Robotic Natural Orifice Specimen Extraction Surgery in Colorectal Neoplasms During the Initial Learning Curve. Front. Oncol. 10:1355. doi: 10.3389/fonc.2020.01355
Received: 09 May 2020; Accepted: 29 June 2020;
Published: 11 September 2020.
Edited by:
Jaw-Yuan Wang, Kaohsiung Medical University, TaiwanReviewed by:
Imerio Angriman, University of Padova, ItalyGiovanni Battista Levi Sandri, San Camillo-Forlanini Hospital, Italy
Copyright © 2020 Yao, Li, Chen, Lei, Liu, Jin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangjiao Zhou, emhvdWppYW5namlhb0Bjc3UuZWR1LmNu
 Hongliang Yao
Hongliang Yao Jiangjiao Zhou
Jiangjiao Zhou