- 1School of Medicine, Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University, Hangzhou, China
- 2Research Center for Air Pollution and Health, School of Medicine, Zhejiang University, Hangzhou, China
- 3Department of Surgical Oncology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Since the beginning of the COVID-19 global pandemic, there has been insufficient evidence and experience to help oncologists understand how to deal with infected and non-infected cancer patients. Many hospitals worldwide have shared their experiences of managing such patients by using the internet to reach non-infected cancer patients. However, for infected or suspected infected cancer patients, their experiences in terms COVID-19 diagnosis, anticancer treatment and prognosis are largely unknown and controversial. Here, we summarize the incidence, severe illness rate and mortality according to the published clinical data of COVID-19 in cancer patients and discuss the diagnostic difficulties, anticancer treatment and prognosis of COVID-19-infected cancer patients.
Introduction
Since the outbreak of COVID-19 in December 2019, the resulting pandemic has overwhelmed medical care facilities worldwide and attracted tremendous public and social media attention. In such conditions, it is difficult for cancer patients to obtain timely and high-quality medical services as is usual due to social distancing and the utilization of medical resources for the COVID-19 crisis. A large number of cancer patients are subject to a high COVID-19 infection risk due to decreased immunity caused by cancer itself, anticancer treatments, such as chemotherapy, radiotherapy or surgery (1) and frequent visits to hospitals. Although the capacity limitations of hospitals necessitate delays, oncologists worldwide are using the power of the internet to reach millions of patients located in different geographic regions and minimize the harm caused by the interruption of anticancer treatment. Many of these oncologists have shared their valuable experience of the management and continuing education of their patients without COVID-19 (2–4). On May 19, 2020, ASCO issued a special report: A Guide to Cancer Care Delivery During the COVID-19 Pandemic (5). ESMO (European Society of Medical Oncology) also issued guidelines for patients and oncologists to deal with COVID-19 (6, 7). There are some special issues that should draw our attention when cancer patients encounter COVID-19. This short review aims to summarize the incidence and diagnostic difficulties of COVID-19 in cancer patients and discuss the anticancer treatment and prognosis of COVID-19-infected cancer patients.
Methods
PubMed database was searched by using COVID-19 and SARS-CoV2 as the main terms to identify papers published through April 26, 2020 and the available clinical data was summarized in Table 1.
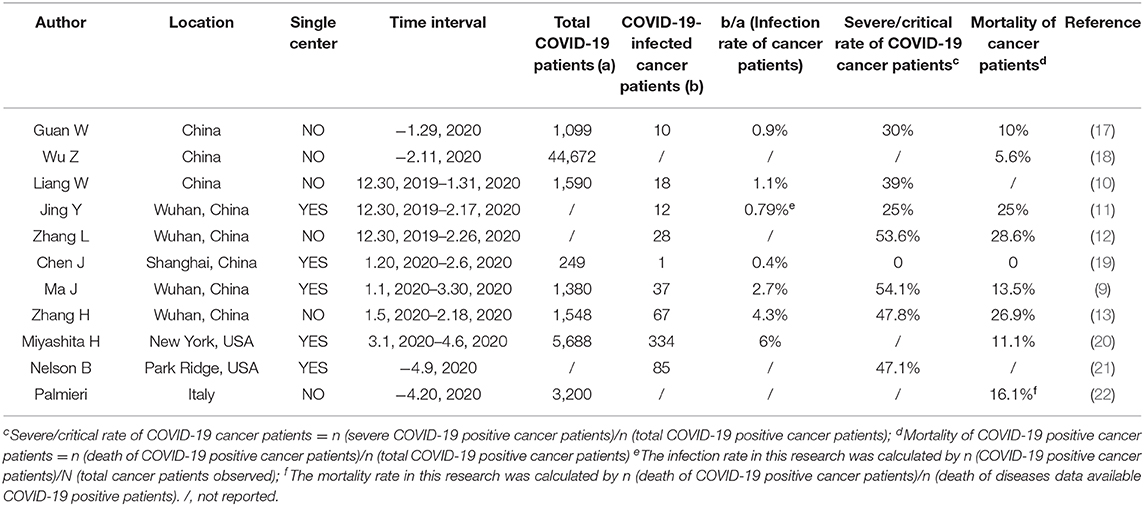
Table 1. Clinical data reporting the infection rate, severe illness rate and mortality of COVID-19-infected cancer patients.
Results
Is There an Increase in the Susceptibility, Critical Illness Rate and Mortality Due to COVID-19 in Cancer Patients?
Since cancer is associated with the overexpression of immunosuppressive cytokines, a reduction in proinflammatory danger signals, an increase in the functional immunosuppressive leukocyte population (8) and increased toxicity due to anticancer treatment, cancer patients are a potentially vulnerable group. However, are cancer patients truly more vulnerable to COVID-19? The groups in China, the USA and Italy reported that cancer patients are more likely to be infected with the COVID-19 and develop severe symptoms than the general population. The infection rate for cancer patients ranged from 0.4 to 4.3% in China and was 6% in the USA according to the current published data (Table 1). In Wuhan, the infection rate for cancer patients was 2.7% (37 of 1,380 patients), which was 6 times higher than the general population in Wuhan until Mar 30, 2020 (0.45%, 50,006 of 11,081,000) (9). As for the severe illness rate, it ranged from 0 to 54.1% in China and was 47.1% in the USA (Table 1). The mortality of COVID-19 positive cancer patients is much higher than the 6.95% mortality of the entire population (Data from COVID-19 map of John Hopkins University on April 26, 2020). The mortality ranged from 0 to 28.6% in China according to the current reports. The mortality was 11.1% in the USA and 16.1% in Italy (Table 1). In Shanghai China, since the only 1 infected cancer patients didn't develop severe illness, the severe illness rate and mortality for cancer patients in this center was 0.
Interestingly, the most common cancer type in patients differs in different studies; in some studies, the most common type was lung cancer (10–13), and in one study, the most common type was colorectal cancer (9). In addition, patients who underwent chemotherapy or surgery in the past month had a numerically increased risk (three [75%] of four patients) of clinically severe events compared to those who did not receive chemotherapy or surgery (10). However, clinical data from Ma J showed no difference between these two groups (9). Otherwise, the studies did not report the relationship between the tumor stage and the susceptibility or severe illness rate. What's more, biomarkers from peripheral blood, such as lactic dehydrogenase (LDH), lymphocyte, high-sensitivity C-reactive protein (hs-CRP) and d-dimer >1 μg/mL, and clinical features, such as older age and high Sequential Organ Failure Assessment (SOFA) score, were reported to be crucial predictive biomarkers of disease mortality (14, 15).
Ling Peng holds the view that these data are insufficient to conclude that patients with cancer have an increased risk because of the small sample sizes and high heterogeneity (16). Frequent visits to the hospital and advanced age are also high-risk factors in cancer populations. Although multicenter retrospective studies with larger sample sizes and adjustment of confounding factors are needed to determine if cancer patients have increased susceptibility and critical illness rates, we should pay greater attention to protecting cancer patients from COVID-19.
Diagnostic Difficulties in Distinguishing Between COVID-19 and Cancer: COVID-19 or Not?
Fever and respiratory symptoms are the most common symptoms of COVID-19, which may occur more frequently in cancer patients than in the healthy population. These overlapping symptoms confound the COVID-19 diagnosis. During the SARS epidemic in 2004, 11 cancer patients who met the WHO diagnostic criteria for probable SARS were admitted to hospitals, but only 1 of them was finally confirmed as having SARS (23). For patients with lung cancers, cough and fever caused by pneumonia are common initial clinical symptoms (24). Interstitial infiltrate pneumonitis exhibited by patients with primary lung cancer (25) caused by radiotherapy and checkpoint inhibitor therapy overlaps with the symptoms and CT features of COVID-19 (26). For all cancer types, patients may develop fever because of bacterial infections after chemotherapy and surgery or tumor necrosis after TACE/ultrasound ablation. Due to these specific issues, it is difficult to determine whether cancer patients are infected by COVID-19 based on clinical symptoms and CT images. Oncologists may first perform differential diagnosis of patients online based on medical history and travel history.
In some cases, symptoms may last for days, and it is difficult to exclude COVID-19. Approximately 30% of infections in cancer patients are suspected to be due to hospital-associated transmissions (12). Cancer patients with suspected COVID have to go to the hospital for nucleic or serum testing and will be exposed to a high risk of infection. Household test kits should be developed for these special groups, who may show similar symptoms because of their primary disease, to reduce nosocomial infection. Physicians at the Gustave Roussy Cancer Institute (GRCI) set up the CAPRI telemedicine program to help COVID-19-positive cancer patients. Philippe E. Spiess proposed a simple 5-part strategy to address COVID-19 in patients with cancer (27). In brief, hospitals, local health authorities, and state and national leaders will need to work much more closely together to expand the screening test quickly to all cancer patients at high risk without pretesting criteria such as travel history and fever: the elderly, patients receiving anti-cancer therapy and advanced-stage cancer patients. What's more, they hold the view that chemotherapy and surgery should be temporarily postponed for COVID-19 positive cancer patients.
Additional contactless diagnosis and treatment procedures should be established for cancer patients to reduce nosocomial infections. The guideline for cancer patients issued by ESMO recommended an untouched food delivery and medical follow-up way to prevent infection for cancer patients and reminded them to pay attention to their mental health (6).
Continuing Anticancer Therapy or Not in COVID-19 Infected Cancer Patients?
There is no doubt that higher priority should be given if the patient's condition is immediately life threatening or clinically unstable. For those patients who are in stable or mild condition, it remains controversial whether their anticancer therapy should be stopped. Concerning deterioration due to infection, the official French guidelines recommended that pneumonia should be treated first rather than cancer (28). During chemotherapy, cytotoxic treatments diminish lymphocyte populations temporarily, potentially making patients more susceptible to infection. The report by Liang revealed that patients who underwent chemotherapy in the past month had a numerically higher risk of clinically severe events than those who did not (10). For immune therapy, pneumonitis and cytokine release syndrome are possible side effects of immune checkpoint inhibitor therapy, which may worsen COVID-19 (29). Severe adverse events are also associated with immune checkpoint inhibitor therapy (30). A lung cancer patient treated with nivolumab, a PD-1 checkpoint inhibitor, showed a rapid worsening of their condition and died 5 days after diagnosis (31). Consequently, patients on immunotherapy could be at increased risk from COVID-19. During radiotherapy, pneumonia caused by radiotherapy in lung cancer patients was also an additional risk factor for the deterioration of lung injury. According to one survey, 86.3% of COVID-19 patients were discharged after 16 (12–20) days of hospitalization (19). A one-month interruption of anticancer treatments seems to be acceptable.
However, Ma J reported that anticancer therapy did not affect the severity of COVID-19 among cancer patients (9). Another case report showed that a 57-year-old lung cancer patient infected with mild COVID-19 continued targeted therapy with stable cancer control and recovered from pneumonia after Kaletra (lopinavir/ritonavir) treatment (32). Studies of cancer patients coinfected with another virus, such as HIV, HBV or HPV, may be encouraging. Interestingly, HIV-1 and HBV are not reactivated during chemotherapy in cancer patients (33), indicating the feasibility of continuing anticancer treatment in patients with COVID-19.
In summary, we should not underestimate the risk of a more severe course of COVID-19 in cancer patients, since it will not be known if severe pneumonia is more likely to be fatal than cancer until there is enough clinical and basic research data to support continuing anticancer treatment of cancer patients co-infected with COVID-19. The guideline issued by ESMO also recommended that treatment for COVID-19 infection should be given priority over cancer. And cancer treatment will resume once patients have recovered sufficiently from COVID-19 (6).
Favorable or Unfavorable Impact on Cancer After Survival From COVID-19
If a cancer patient survives COVID-19, the impact of coronavirus infection on the cancer prognosis is currently unknown. The UK launched the Coronavirus Cancer Monitoring Project on March 18, 2020 (34). The project will collect data on COVID-19-positive cancer patients, including data on the tumor type, stage, age, present cancer treatment, and clinical outcomes. Here, we discuss the potential favorable and unfavorable factors impacting infected cancer patients.
In addition to infecting the lungs, the new coronavirus will attack any part of the body and cause serious harm to the human body (35). The ACE2 protein is an important target of coronavirus to infect the human body and enter cells (36) and it is overexpressed in some cancers, including cervical, pancreatic and renal carcinomas, and is expressed at low levels in breast, liver and prostate cancer (16). However, it remains unknown whether overexpression of ACE2 in solid tumors will encourage viral attack and whether the virus will promote or inhibit tumor progression or metastasis.
Solid evidence is currently lacking for an effective antiviral therapy. There are 306 ongoing registered trials for the treatment of COVID-19 as of 4 April 2020, including trials of IL-6- and IL-6-receptor (IL-6R)-blocking antibodies, as severe COVID-19 causes strong expression of chemokines and inflammatory cytokines, especially IL-6 and IL1RA (37). Sarilumab is approved for treatment of Castleman syndrome by the FDA (38). The clinical trial of sarilumab showed positive trends in the “critical” group (39). Tocilizumab was listed in the latest version of the diagnosis and treatment guidelines for COVID-19 in China (40). The IL6/JAK/STAT3 pathway was reported to promote the metastasis of colorectal cancer (41). In addition, there is an ongoing clinical trial of camrelizumab, an anti-PD-1 antibody, in China for treatment of COVID-19 (ChiCTR200002806). The application of these drugs theoretically plays a role in inhibiting specific cancers.
For COVID-19-positive cancer patients with mild disease, in addition to the impact of delayed cancer treatment, the impact of the virus on prognosis is similar to that in the healthy population. For recovered patients who had severe COVID-19, the cytokine storm and the application of several anticancer or antivirus drugs may have a complex effect on the body and tumors. At present, the effects of COVID-19 and drug treatment on tumor prognosis are unknown, and we hope the UK cancer monitoring project will be discussed in the future.
Discussion
When the general epidemic situation is improved, an online medical treatment mode can be gradually developed for cancer patients to relieve stress and delay medical treatment. Although sufficient clinical data are lacking that indicate that cancer patients are more susceptible to COVID-19, we should pay more attention to protecting them from coronavirus. In terms of the effects of cancer itself and anticancer treatment, cancer patients are more likely to develop fever or cough than the healthy population, which increases the difficulty of COVID-19 diagnosis. We call on hospitals to set up special outpatient facilities for suspected cancer patients to reduce the risk of nosocomial infection. For the unfortunately infected cancer patients, whether to continue chemotherapy, immune therapy or radiotherapy remains controversial. Since it is difficult to predict the outcome of patients with COVID-19, the recommendation of antitumor treatment still needs to be very cautious. To better assess the impact of coronavirus infection on the prognosis of cancer patients, we hope the UK cancer monitoring program will be helpful in the future.
Thank you to all the medical staff and researchers who fought hard in this unprecedented situation. We hope that all human beings will overcome this pandemic as soon as possible and that the consequences will not be too devastating for patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LQ conceived and wrote this manuscript. KW collected the clinical data from published studies. CY provided inspiration for this article. SZ revised and polished the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81802883) and Fundamental Research Funds for the Central Universities (grant number 2018FZA7012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. (2009) 10:589–97. doi: 10.1016/S1470-2045(09)70069-5
2. Rosenbaum L. The untold toll - the Pandemic's effects on patients without Covid-19. N Engl J Med. (2020) 382:2368–71. doi: 10.1056/NEJMms2009984
3. Tan J, Yang C. Prevention and control strategies for the diagnosis and treatment of cancer patients during the COVID-19 pandemic. Br J Cancer. (2020) 123:5–6. doi: 10.1038/s41416-020-0854-2
4. England NHS. Clinical Guide for the Management of Noncoronavirus Patients Requiring Acute Treatment: Cancer. (2020). Available online at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf (accessed May 18, 2020).
5. ASCO. (2020). Available online at: https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdf (accessed May 19, 2020).
6. ESMO. (2020). Available online at: https://www.esmo.org/for-patients/patient-guides/cancer-care-during-the-covid-19-pandemic (accessed April 08, 2020).
7. ESMO. (2020). Available online at: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
8. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
9. Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: A single center's retrospective study. J Infect. (2020) 81:318–56. doi: 10.1016/j.jinf.2020.04.006
10. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. (2020) 21:335–7. doi: 10.1016/S1470-2045(20)30096-6
11. Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. (2020) 6:1108–10. doi: 10.1101/2020.02.22.20025320
12. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. (2020) 31:894–901. doi: 10.1016/j.annonc.2020.03.296
13. Zhang H, Wang L, Chen Y, Shen X, Wang Q, Yan Y, et al. A multicentre study of 2019 novel coronavirus disease outcomes of cancer patients in Wuhan, China. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.21.20037127
14. Yan L, Zhang H, Goncalves J, Xiao Y, Wang M, Guo Y, et al., An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. (2020) 2:283–8. doi: 10.1038/s42256-020-0180-7
15. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
16. Peng L, Zagorac S, Stebbing J. Managing patients with cancer in the COVID-19 era. Eur J Cancer. (2020) 132:5–7. doi: 10.1016/j.ejca.2020.03.028
17. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
18. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. (2020). doi: 10.1001/jama.2020.2648
19. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. doi: 10.1016/j.jinf.2020.03.004
20. Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. (2020) 31:1088–9. doi: 10.1016/j.annonc.2020.04.006
22. COVID-19 Surveillance Group. Characteristics of COVID-19 Patients Dying in Italy. (2020). Available online at: https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf (accessed March 20, 2020).
23. Gu J, Wu A, Li J, Zhang X, Fang J, Li M, et al. An assessment of World Health Organization criteria for severe acute respiratory syndrome in patients with cancer. Cancer. (2004) 100:1374–8. doi: 10.1002/cncr.20141
24. Kvale PA. Chronic cough due to lung tumors: ACCP evidence-based clinical practice guidelines. Chest. (2006) 129(Suppl. 1):147s−53. doi: 10.1378/chest.129.1_suppl.147S
25. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143(Suppl. 5):e142S−65S. doi: 10.1378/chest.12-2353
26. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. (2020) 20:425–34. doi: 10.1016/S1473-3099(20)30086-4
27. Spiess PE, Greene J, Keenan RJ, Paculdo D, Letson GD, Peabody JW. Meeting the challenge of the 2019 novel coronavirus disease in patients with cancer. Cancer. (2020) 126:3174–5. doi: 10.1002/cncr.32919
28. You B, Ravaud A, Canivet A, Ganem G, Giraud P, Guimbaud R, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. (2020) 21:619–21. doi: 10.1016/S1470-2045(20)30204-7
29. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall R, Manson J, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
30. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:1–11. doi: 10.1001/jamainternmed.2020.0994
31. Bonomi L, Ghilardi L, Arnoldi E, Tondini CA, Bettini AC. A rapid fatal evolution of coronavirus disease-19 in a patient with advanced lung cancer with a long-time response to nivolumab. J Thorac Oncol. (2020) 15:e83–5. doi: 10.1016/j.jtho.2020.03.021
32. Zhang H, Xie C, Huang Y. Treatment and outcome of a patient with lung cancer infected with severe acute respiratory syndrome coronavirus-2. J Thorac Oncol. (2020) 15:e63–4. doi: 10.1016/j.jtho.2020.02.025
33. Stebbing J, Atkins M, Nelson M, Rajpopat S, Davis T, Gazzard B, et al. Hepatitis B reactivation during combination chemotherapy for AIDS-related lymphoma is uncommon and does not adversely affect outcome. Blood. (2004) 103:2431–2. doi: 10.1182/blood-2003-12-4222
34. UK Coronavirus Cancer Monitoring Project team. The UK coronavirus cancer monitoring project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. (2020) 21:622–4. doi: 10.1016/S1470-2045(20)30230-8
35. Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science. (2020) 368:356–60. doi: 10.1126/science.368.6489.356
36. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
37. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. (2020) 368:473–4. doi: 10.1126/science.abb8925
38. Ascierto PA, Fox BA, Urba WJ, Anderson AC, Atkins MB, Borden EC, et al. Insights from immuno-oncology: the society for immunotherapy of cancer statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer. (2020) 8:e000878. doi: 10.1136/jitc-2020-000878corr1
39. Regeneron and Sanofi Provide Update on US Phase 2/3 Adaptive-Designed Trial of Kevzara® (Sarilumab) in Hospitalized COVID-19 Patients. (2020). Available online at: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive (accessed April 27, 2020).
40. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) 117:10970–5. doi: 10.1073/pnas.2005615117
Keywords: COVID-19, cancer, diagnosis, anticancer treatment, prognosis
Citation: Qi L, Wang K, Ye C and Zheng S (2020) Special Issues Encountered When Cancer Patients Confront COVID-19. Front. Oncol. 10:1380. doi: 10.3389/fonc.2020.01380
Received: 02 May 2020; Accepted: 30 June 2020;
Published: 07 August 2020.
Edited by:
Antonio Russo, Paolo Giaccone University Hospital in Palermo, ItalyReviewed by:
Emerson Carraro, State University of Midwest Paraná, BrazilLuca Falzone, Istituto Nazionale Tumori Fondazione G. Pascale (IRCCS), Italy
Ye Yuan, Huazhong University of Science and Technology, China
Copyright © 2020 Qi, Wang, Ye and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Zheng, emhlbmdzaHVAemp1LmVkdS5jbg==
 Lina Qi
Lina Qi Kailai Wang
Kailai Wang Chenyang Ye
Chenyang Ye Shu Zheng1,2,3*
Shu Zheng1,2,3*