- 1Department of Interventional Ultrasound, Chinese PLA General Hospital, Beijing, China
- 2Special Clinic Department, The 985th Hospital of Chinese People's Liberation Army Joint Logistic Support Force, Taiyuan, China
- 3Ultrasonic Diagnosis Department, The 991st Hospital of Chinese People's Liberation Army Joint Logistic Support Force, Xiangyang, China
- 4Ultrasound Department, Zunhua People's Hospital, Zunhua, China
- 5Tianjin Institute of Urology, The Second Hospital of Tianjin Medical University, Tianjin, China
- 6Department of Diagnostic Ultrasound, PLA Strategic Support Force Characteristic Medical Center, Beijing, China
- 7Ultrasound Department, Hohhot Mongolian Hospital of Traditional Chinese Medicine, Hohhot, China
- 8Department of Ultrasonography, Nanhai Hospital of Southern Medical University, Foshan, China
Objectives: Acute kidney injury (AKI) is a recently observed side effect in patients after microwave ablation (MWA) of hepatocellular carcinoma (HCC) and is associated with negative outcomes. The aim of this study is to explore the risk factors of affecting the occurrence of AKI (stages 1b, 2, and 3), because they have a higher mortality rate than patients with AKI (stage 1a) and without AKI.
Materials and methods: In this retrospective study, a total of 1,214 patients with HCC who were treated with MWA under ultrasound (US) guidance in our department between January 2005 and November 2017 were enrolled. We evaluated the influence of 20 risk factors. Univariate and multivariate analysis were used for statistical analysis. The possible risk factors of AKI after MWA for HCC were summarized.
Results: AKI, AKI (stage 1a), and AKI (stages 1b, 2, and 3) after MWA were found in 34, 15, and 19 patients (2.80, 1.24, and 1.57%), respectively. Among 34 patients with AKI, 10 cases with AKI (stage 1a) and 6 cases with AKI (stages 1b, 2, and 3) recovered before their discharge without any treatment for AKI and 9 cases with AKI (stages 1b, 2, and 3) with further treatment. Four cases who had chronic renal failure before MWA of liver accepted renal dialysis. By univariate analysis, the number of antenna insertions (P = 0.027, OR = 3.3), MWA time ≥20 min (P = 0.029, OR = 4.3), creatinine (Cr)-pre above the upper limit of the reference value (P < 0.001, OR = 35.5), albumin (Alb)-pre (P = 0.030, OR = 0.9), and red blood cell (RBC)-pre (P < 0.001, OR = 0.3) were significant risk factors. By multivariate analysis, Cr-pre ≥ 110 μmol/L (P < 0.001, OR = 31.4) and MWA time ≥20 min (P = 0.043 OR = 9.9) were the independent risk factors.
Conclusion: AKI (stages 1b, 2, and 3) is a relatively serious complication after MWA for HCC, which is related to MWA time and Cr-pre. It requires attention by clinicians. So it is of great necessity to assess the Cr-pre level and reduce the MWA time to <20 min to minimize the risk of AKI after MWA for HCC.
Introduction
Microwave ablation (MWA) is an important therapy for the focal HCC with single or up to three nodules (<3 cm), which is recommended by the 2018 Barcelona Clinic Liver Cancer (BCLC) system (1). As a minimally invasive therapy, MWA is safe with a low incidence of major complications, which was 0–2.7% (2–4), including 1.7% pleural effusion requiring thoracentesis, 1.4% tumor seeding, 0.4% liver abscess and empyema, 0.1% hemorrhage requiring arterial embolization, and 0.1% bile duct injury (5). Acute kidney injury (AKI) after MWA of HCC has been a recently observed complication, which is diagnosed by the following criteria—Stage 1: Creatinine ≥1.5 times baseline or increase of ≥0.3 mg/dl within any 48-h period (1a: Creatinine <1.5 mg/dl, 1b: Creatinine ≥ 1.5 mg/dl); Stage 2: Creatinine ≥ 2.0 times baseline; Stage 3: Creatinine ≥ 3.0 times baseline or increase to ≥4.0 mg/dl or acute dialysis (6–9). Ding et al. reported that the accidence of AKI is 23.6% for the large liver tumor (>5 cm) after MWA (10). Most importantly, the patients with AKI and creatinine >1.5 mg/dl (stages 1b, 2, and 3) present a worse clinical outcome. They had a higher mortality rate than patients with AKI stage 1a (Cr < 1.5 mg/dl) and without AKI. This fact reinforces that small elevations in the value of creatinine, especially when they exceed 1.5 mg/dl, have a great impact on the morbidity and mortality of patients with cirrhosis (6, 11). Furthermore, despite most of them having recovered, a few cases after RFA of metastasis liver cancer developed the renal failure, requiring intensive care unit admission and a prolonged hospital stay (12). Ong et al. summarized that the accidence of renal failure after MWA of liver tumors was 1.7% (13). Although there are reports in the existing literature regarding AKI after MWA of liver tumors including HCC, metastasis, and hemangioma, there are seldom exclusive reports on AKI for HCC in particular. Therefore, we determined the risk factors of AKI after MWA of HCC to prevent the occurrence of severe complications.
Patients
The clinical data of 1,214 adult patients admitted for HCC with histopathological diagnosis and treated with ultrasound-guided percutaneous MWA from January 2005 to November 2017 were reviewed in this study.
Evaluation Methods
Blood tests including routine, biochemistry, and coagulation function tests were conducted before and after MWA. In 1,214 patients, hepatic and renal functions were tested on the first day after MWA. Data of the MWA maximum diameter, the MWA parameter, and all blood test results of patients in this study were obtained from our departmental database. MWA energy was calculated by the equation E = P * T, where P and T were ablation power and time, respectively. The MWA zone was spherical in shape. The maximum diameter in the three dimensions of the tumor was measured using ultrasound. The comorbidity score was the pre-operation assessment of other diseases except for HCC such as diabetes, cardiovascular diseases, or AIDS using the Charlson comorbidity index (CCI) that included 19 diseases as well as the age of the patient (14). According to the anatomical segment of the liver, the location of the tumor was separated into four sections including the left lateral lobe, the left inner lobe (including the caudate lobe), the right anterior segment, and the right posterior segment. Because there were just nine patients whose tumor located in the caudate lobe, we attributed the caudate lobe into the left inner lobe to narrow deviation. The number of antenna insertions was defined as the total number of antenna placements in each patient during ablation.
MWA Equipment and Technology
The MWA unit used was a 100 W two-cooled-shaft system (KY-2000, Kangyou Medical, Nanjing, China) with frequencies of 2,450 and 915 MHz. The antennae (KY-2450-T11b, KY-2450B-T3, 89 KY-2450B-T7 and KY-2450B-QT) were percutaneously inserted into the tumor and placed at a designated location under ultrasound guidance. When the distance of tumor to the important structure such as the main bile, gallbladder, and bowel was <5 mm, the thermocouple needles can be inserted at the margin of those structure to monitor temperature in real time. To reduce the risk of the bleeding and the seeding of tumor, the MW emission was continued until the antennae were withdrawn to below the skin entrance site after the MWA of the tumor (4).
Statistical Analysis
Data analysis was performed using EmpowerStats (Version 3.4.3) for Windows, and the continuous data were expressed as β (95%CI) P-value/OR (95%CI) P-value. All of the analyses were performed with statistical software package R (http://R-project.org, The R-foundation) and EmpowerStats (http://empowerstats.com, X&Y Solutions. Inc., Boston, MA). Data of two groups were analyzed between the group A (no AKI and AKI 1a) and group B (AKI stage 1b,2,3) by using the Student t test for unpaired data and Fisher exact test as appropriate. Twenty related risk factors, including gender, age, comorbidity scores, biochemical parameters, and blood routine before treatment like alanine transaminase (ALT)-pre, glutamic oxaloacetic transaminase (AST)-pre, ALB-pre, STB-pre, Cr-pre, Hb-pre, platelet (PLT)-pre, white blood cell (WBC)-pre, lymphocyte (LY)-pre, red blood cell (RBC)-pre, the location of tumor, the maximum diameter of tumor, MWA energy, MWA time, and the number of antenna insertions, were analyzed using the univariate and multivariate logistic regression model method.
Results
Basic Analysis
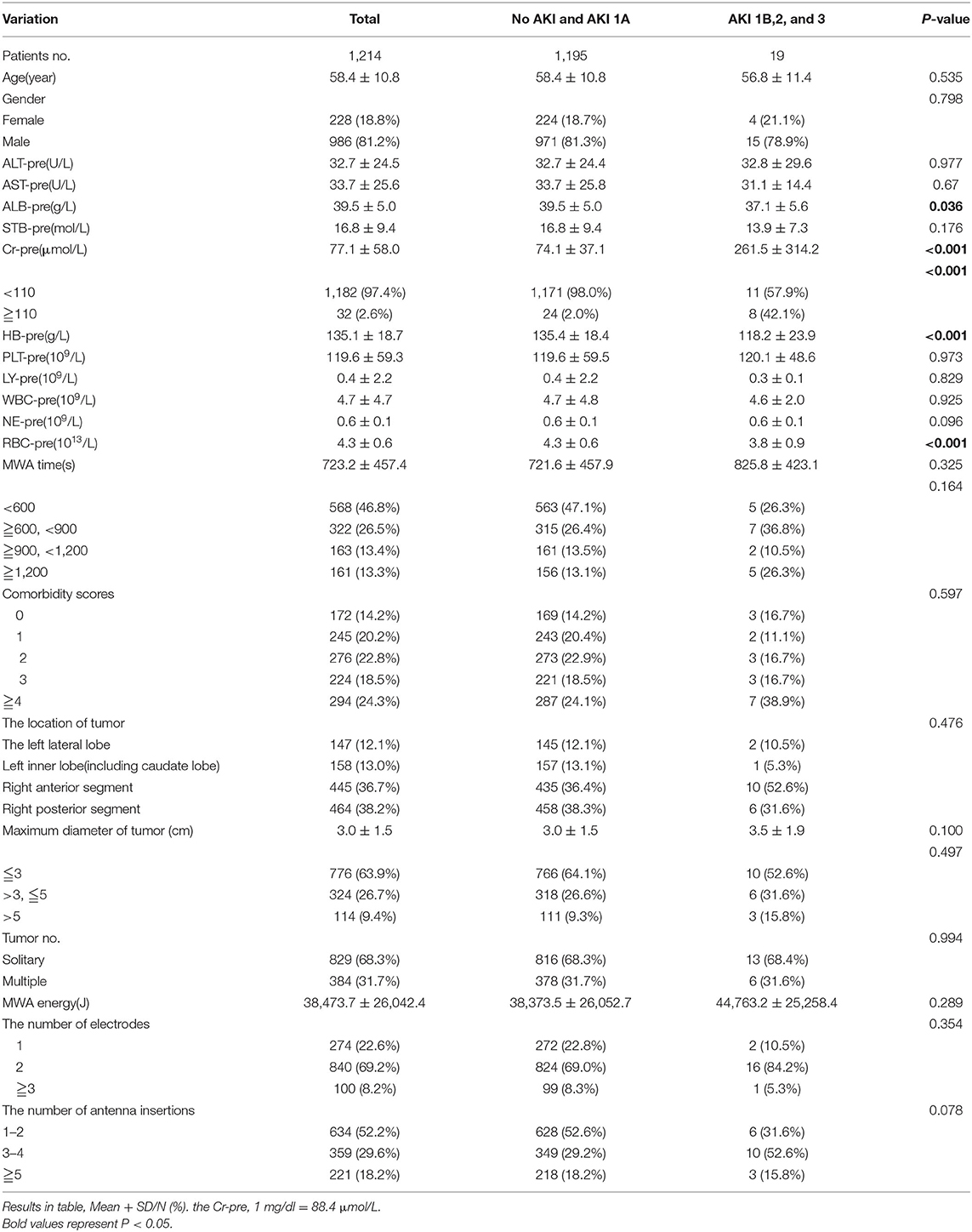
Among all 1,214 patients after MWA of HCC, 19 patients had AKI (stages 1b, 2, and 3), and the accidence was 1.57%. The biochemical parameters and blood routine before treatment of all patients, tumor characteristic, and MWA parameters were described. The mean maximum diameter of tumor for 829 patients with solitary tumor with AKI vs. without AKI was 3.0 vs. 3.8 cm, respectively. The mean ALT, AST, and STB for the 19 patients were 32.8 U/L, 31.1 U/L, and 13.9 μmol/L, respectively. The mean ALT, AST, and STB for 1,195 patients without AKI were 32.7 U/L, 33.7 U/L, and 16.8 μmol/L. Most P-values were >0.05 except Hb-pre, RBC-pre, and Alb-pre. There were no significant differences between the groups. The basic characteristic is shown in Table 1.
Risk Factors of AKI
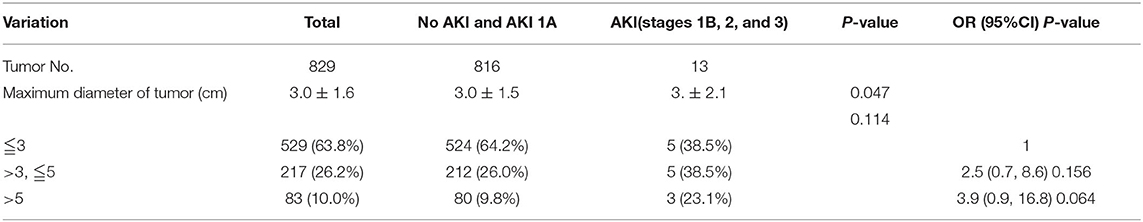
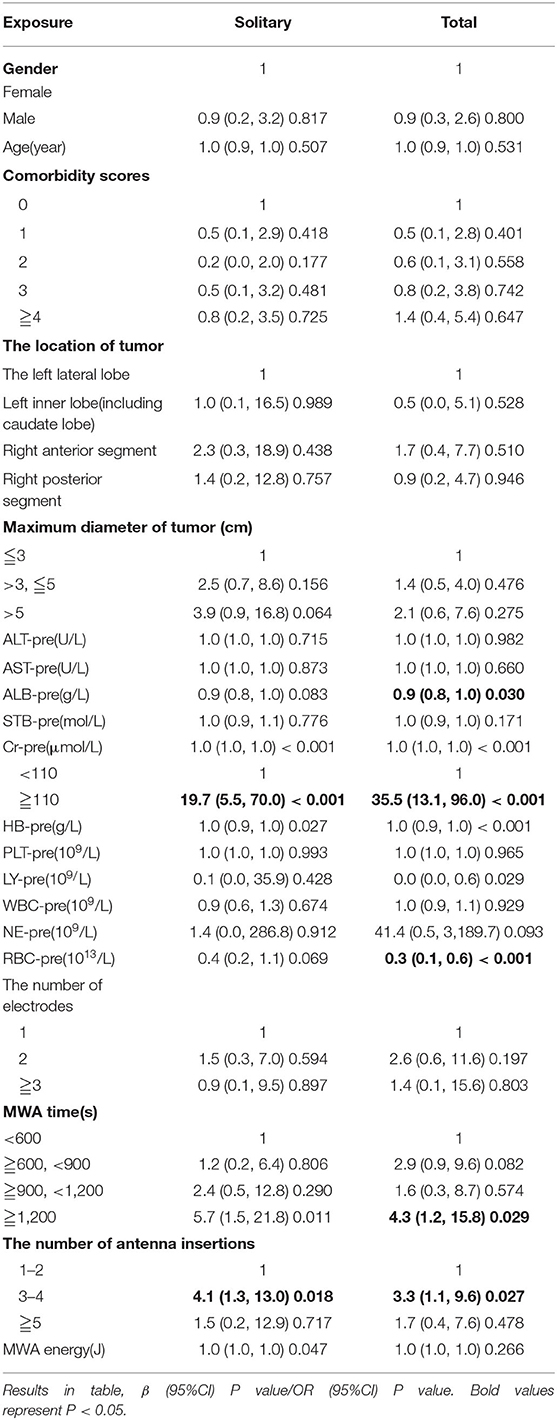
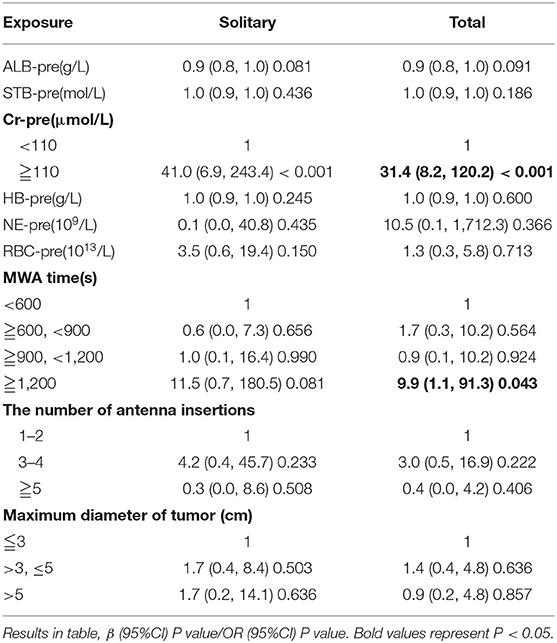
By univariate analysis, the number of antenna insertions (P = 0.027, OR = 3.3), MWA time ≥ 20 min (P = 0.029, OR = 4.3), Cr-pre above the upper limit of the reference value (P < 0.001, OR = 35.5), Alb-pre (P = 0.030, OR = 0.9), and RBC-pre (P < 0.001, OR = 0.3) were significant risk factors. The maximum diameter of tumor for patients with solitary tumor was analyzed by univariate analysis separately (Table 2). While by univariate and multivariate analysis, Cr-pre ≥ 110 μmol/L (P < 0.001, OR = 31.4) and MWA time ≥20 min (P = 0.043 OR = 9.9) were the independent risk factors associated with AKI (stages 1b, 2, and 3) (Tables 3, 4).
Discussion
AKI (stage 1a) is a transient and controlled complication for most patients after MWA of HCC, but AKI (stages 1b, 2, and 3) needs further treatment. According to our research, among 34 patients with AKI after MWA, 10 cases with AKI (stage 1a) and 6 cases with AKI (stages 1b, 2, and 3) recovered before their discharge without any treatment for AKI whose mean post-operation Cr level was 123.2 μmol/L. Five cases with AKI (stage 1a) and nine cases with AKI (stages 1b, 2, and 3) recovered after further treatment of renal conservation: (1) the diuretic-furosemide or/and spironolactone and (2) sodium bicarbonate injection. Their mean post-operation Cr was 169.8 μmol/L. Then, four cases accepted the renal dialysis who had the chronic renal failure before MWA of liver whose mean post-operation Cr level was 947.4 μmol/L. Hence, most cases of AKI after MWA for HCC had a good recovery unless with chronic kidney disease (CKD), but the hospital stay was prolonged (10). What is worse, the patients with AKI (stages 1b, 2, and 3) had a higher mortality rate than patients with AKI stage 1a (Cr < 1.5 mg/dl) and without AKI. Lins et al. reported that the mortalities of the cirrhotic patient group with (A) no AKI, (B) AKI (stage 1a), (C) AKI (stage 1b), and (D) AKI (stages 2 and 3) were 11.8, 12.5, 33.3, and 52.4%, respectively (6). Fagundes et al. presented that the survival rates of groups B, C, and D were 84, 68, and 36%, respectively (p < 0.001) (11). Furthermore, Rodriguez et al. reported that three patients after radiofrequency ablation (RFA) of metastasis liver cancer developed renal failure, requiring intensive care unit admission and a prolonged hospital stay (12), even though there was no report about acute renal failure after MWA of HCC without preexisting CKD. It is still of great necessity to pay much attention to the risk factor of AKI (stages 1b, 2, and 3) after MWA to prevent the severe complication happening and improve the survival.
This study shows that the statistically significant risk factors for AKI (stages 1b, 2, and 3) after MWA for HCC were 3 ≤ the number of antenna insertions ≤ 4 (P = 0.027, OR = 3.3) RBC-pre (P < 0.001, OR = 0.3). For the patients with multiple tumors, Hb-pre (P < 0.001, OR = 0.9) were also significant risk factors. MWA time ≥ 20 min (P = 0.029, OR = 4.3) was the independent risk factor by multivariate analysis. As we know, the thermal effect of ablation can lead to the destruction of RBC and the release of Hb to the circulation system. When the quantity of cell-free Hb exceeds the liver detoxification threshold, some Hb will cross the glomerular filtration membrane, reach the renal tubules, and cause renal tubular necrosis that ultimately leads to AKI (15–18). The ablation of large tumors took longer time and more antenna insertions, and more Hb was released into the circulation system. Therefore, patients who had a high HB-pre level and RBC-pre and took longer time were more prone to having AKI than those who have a small tumor. Hence, the number of antenna insertions should be reduced for treating large and multiple tumors. It is recommended that the MWA time is controlled within 20 min.
Additionally, another independent risk factor was Cr-pre above the upper limit of the reference value (P < 0.001, OR = 31.4). In our analysis, there were six patients with AKI after MWA for HCC with CKD, and the preoperative Cr in these patients were higher than the upper level of the reference range (110 μmol/l). Multivariate analysis showed that Cr-pre > 110 μmol/L was an independent risk factor. The present literature showed that the mortality of people who had CKD with AKI was higher than those without AKI (19, 20), especially for critically ill patients with CKD (21). Therefore, when Cr-pre is above the upper limit of the reference value, the clinician should assess the patient's magnitude of benefit from MWA and then decide whether to do it. It was noted that the Alb-pre (P = 0.030, OR = 0.9) was also a significant risk factor. It was revealed that the infection before MWA could make it easier to AKI for patients. Then, the low levels of STB-pre and Alb-pre were also significant risk factors. The mechanism was still unclear.
Therefore, to avoid complications of AKI (stages 1b, 2, and 3), the independent risk factors reported in this study should be emphasized in the preoperative evaluation. Clinicians should pay attention to the Cr-pre and the need for long MWA time. Appropriate measures should be taken including preoperative evaluation of renal function, intraoperative and postoperative administration of fluid, appropriate timing of diuresis, and urine alkalization (10, 17, 22). Furthermore, patient survival should actively involve not only the preservation of their kidney health but also postdischarge follow-up of kidney function because severe AKI predisposes patients to faster progression of CKD later on—especially when they had multiple hits of AKI or preexisted CKD (23).
The innovation of the study is that the risk factor of the maximum diameter of tumor in patients with single nodule was analyzed separately, and the interference of cases with multiple tumors on this factor was excluded. It is because the maximum diameter is just referred to the largest one among multiple tumors for them. That cannot reflect the relation of the size of tumor to the AKI accurately.
There are three limitations of this research. First, this is a retrospective study. To provide further evidence for the conclusion, prospective studies are still needed. Then, the clinical data were just collected from a single institution. Finally, we designed this study to assess the Cr only on the first day after MWA in the hospital; we did not evaluate the occurrence of late AKI. So the occurrence incidence of the AKI after MWA of HCC we obtained may be lower than the true value.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YY, FL, and PL made substantial contributions to the research design, analysis, and interpretation of data. FL contributed a lot to the data analysis and appropriate method to the research. ZC and ZH took part in the building of the clinical database. JY provided the instruction for statistical analysis by EmpowerStats (Version 2019-11-18). JD, JH, ZW, HG, QY, JT, YX, XB, and LL performed the clinical collection. YY and FL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Grants 81627803, 81971625, 91859201, and 81871374 from the National Scientific Foundation Committee of China, Grant JQ18021 from the National Scientific Foundation Committee of Beijing, Fostering Funds for National Distinguished Young Scholar Science Fund, and the National Clinical Research Center for Geriatric Diseases (NCRCG-PLAGH-2019011) of Chinese PLA General Hospital, and Grant 2018ZX10723-204 from the National Key R&D Program of Ministry of Science and Technology of China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CA declared a past co-authorship with several of the authors ZH, JD, JY, PL, ZC, and FL to the handling Editor.
Acknowledgments
This work was supported by NHC Key Laboratory of Echinococcosis Prevention and Control (Xizang Center for Disease Control and Prevention).
References
1. Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, et al. Diagnosis and staging of hepatocellular carcinoma (Hcc): current guidelines. Eur J Radiol. (2018) 101:72–81. doi: 10.1016/j.ejrad.2018.01.025
2. Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. (2013) 82:1379–84. doi: 10.1016/j.ejrad.2013.04.025
3. Francica G, Meloni MF, Riccardi L, de Sio I, Terracciano F, Caturelli E, et al. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. (2018) 105:96–101. doi: 10.1016/j.ejrad.2018.05.030
4. Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. (2009) 251:933–40. doi: 10.1148/radiol.2513081740
5. Wang XH, Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: analysis of major complications in 693 patients. Chin J Oncol. (2012) 34:945–49. doi: 10.3760/cma.j.issn.0253-3766.2012.12.014
6. Lins PR, Padilha WS, Pimentel CF, Batista MC, de Gois AF. Risk factors, mortality and acute kidney injury outcomes in cirrhotic patients in the emergency department. BMC Nephrol. (2018) 19:277–77. doi: 10.1186/s12882-018-1061-8
7. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a Kdigo summary (part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
8. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. (2007) 11:R31. doi: 10.1186/cc5713
9. Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, et al. Evaluation of the acute kidney injury network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. (2013) 59:482–89. doi: 10.1016/j.jhep.2013.03.039
10. Ding M, Ma S, Tang X, Wang T, Qi X, Chi J, et al. Oliguric acute kidney injury after microwave ablation of large liver tumors: incidence and preventive measures. Int J Hyperthermia. (2019) 35:141–9. doi: 10.1080/02656736.2018.1487589
11. Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. (2013) 59:474–81. doi: 10.1016/j.jhep.2013.04.036
12. Rodriguez J, Tellioglu G, Siperstein A, Berber E. Myoglobinuria after laparoscopic radiofrequency ablation of liver tumors. J Gastrointest Surg. (2010) 14:664–7. doi: 10.1007/s11605-009-1118-x
13. Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. (2009) 21:599–605. doi: 10.1097/MEG.0b013e328318ed04
14. Dias A, Teixeira-Lopes F, Miranda A, Alves M, Narciso M, Mieiro L, et al. Comorbidity burden assessment in older people admitted to a Portuguese University Hospital. Aging Clin Exp Res. (2015) 27:323–28. doi: 10.1007/s40520-014-0280-5
15. Billings IV FT, Ball SK, Roberts II LJ, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. (2011) 50:1480–7. doi: 10.1016/j.freeradbiomed.2011.02.011
16. Deuel JW, Schaer CA, Boretti FS, Opitz L, García-Rubio I, Baek JH, et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. (2016) 7:e2064. doi: 10.1038/cddis.2015.392
17. Qi K, Zhang XG, Liu SW, Yin Z, Chen XM, Wu D. Reversible acute kidney injury caused by paroxysmal nocturnal hemoglobinuria. Am J Med Sci. (2011) 341:68–70. doi: 10.1097/MAJ.0b013e3181f515b9
18. Verbeek DE, Waanders F, Mulder AB, Bierman WF, Croles FN. Quiz page may 2014: acute kidney injury with red urine. Am J Kidney Dis. (2014) 63:A21–4. doi: 10.1053/j.ajkd.2013.09.024
19. Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. (2011) 79:1361–9. doi: 10.1038/ki.2011.42
20. Wang HE, Jain G, Glassock RJ, Warnock DG. Comparison of absolute serum creatinine changes versus kidney disease: improving global outcomes consensus definitions for characterizing stages of acute kidney injury. Nephrol Dial Transpl. (2013) 28:1447–54. doi: 10.1093/ndt/gfs533
21. Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. (2008) 15:297–307. doi: 10.1053/j.ackd.2008.04.009
22. Rewa O, Bagshaw SM. Acute kidney injury–epidemiology, outcomes and economics. J Nat Rev Nephrol. (2014) 10:193. doi: 10.1038/nrneph.2013.282
Keywords: acute kidney injury, microwave ablation, hepatocellular carcinoma, complication, risk factor analysis
Citation: Yang Y, Liu F, Yu J, Cheng Z, Han Z, Dou J, Hu J, Wang Z, Gao H, Yang Q, Tian J, Xu Y, Bai X, Lu L and Liang P (2020) Risk Factor Analysis of Acute Kidney Injury After Microwave Ablation of Hepatocellular Carcinoma: A Retrospective Study. Front. Oncol. 10:1408. doi: 10.3389/fonc.2020.01408
Received: 05 March 2020; Accepted: 03 July 2020;
Published: 04 September 2020.
Edited by:
Fu Wang, Xidian University, ChinaReviewed by:
Xiang Jing, Tianjin Third Central Hospital, ChinaChao An, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2020 Yang, Liu, Yu, Cheng, Han, Dou, Hu, Wang, Gao, Yang, Tian, Xu, Bai, Lu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Liang, bGlhbmdwaW5nMzAxQDEyNi5jb20=
†These authors have contributed equally to this work
 Yongfeng Yang
Yongfeng Yang Fangyi Liu
Fangyi Liu Jie Yu
Jie Yu Zhigang Cheng1
Zhigang Cheng1